Abstract
Many chemotherapeutic drugs have been used for the treatment of cancer, for instance, doxorubicin, irinotecan, 5-fluorouracil, cisplatin, and paclitaxel. However, the effectiveness of chemotherapy is limited in cancer therapy due to drug resistance, therapeutic selectivity, and undesirable side effects. The combination of therapies with natural compounds is likely to increase the effectiveness of drug treatment as well as reduce the adverse outcomes. Curcumin, a polyphenolic isolated from Curcuma longa, belongs to the rhizome of Zingiberaceae plants. Studies from in vitro and in vivo revealed that curcumin exerts many pharmacological activities with less toxic effects. The biological mechanisms underlying the anticancer activity of co-treatment curcumin and chemotherapy are complex and worth to discuss further. Therefore, this review aimed to address the molecular mechanisms of combined curcumin and chemotherapy in the treatment of cancer. The anticancer activity of combined nanoformulation of curcumin and chemotherapy was also discussed in this study. Taken together, a better understanding of the implication and underlying mechanisms of action of combined curcumin and chemotherapy may provide a useful approach to combat cancer diseases.
Keywords: cancer, chemotherapy, curcumin, inflammation, nanoparticle, 5-fluorouracil
1. Introduction
Cancer is a major challenge worldwide, contributing to nearly 9.6 million deaths in 2018 [1]. Liver, stomach, colorectal, prostate, and lung cancers are the most common types of cancer among men; while thyroid, cervix, lung, colorectal, and breast cancers are the most common among women [1]. Despite tremendous improvements in treatment modalities have been made in recent decades, millions of cancer-associated deaths continue to escalate as a public health problem [2].
The most common therapeutic approaches for cancer treatment include immunotherapy and targeted therapy, chemotherapy, radiation, and surgery. Of these modalities, chemotherapy remains one of the most effective methods [3]. However, the efficacy and application of available anticancer chemotherapeutic drugs often fail to achieve complete cancer remission owing to the heterogeneity of cancer cells, show limited efficacy due to dose-limiting toxicity to the patients, and development of multidrug resistance [4]. Data from cell culture and animal models revealed that celecoxib, a specific cyclooxygenase-2 (COX-2) inhibitor, may suppress cancer cells such as colorectal cancer [5]. Nevertheless, the long-term use of celecoxib may increase the risk of cardiovascular toxicity [6]. Another chemotherapeutic drug, 5-fluorouracil (5-FU), has been used for the treatment of several cancers, including gastric [7], breast [8], liver [9], and prostate [10]. Notably, the study found that the anticancer efficacy of 5-FU was enhanced when its dosage was increased [11]. Unfortunately, the cytotoxicity of 5-FU was also increased in normal cells, and thus causes unacceptable toxicity to the patients [12]. Therefore, chemotherapy regimen that could enhance clinical outcomes is required for cancer patients. In order to overcome these issues, an ideal approach is to combine conventional chemotherapeutic with natural compounds to provide synergistic antitumor efficacy.
Natural products containing secondary metabolites have emerged as convincing candidate compounds for cancer treatment [13]. Curcumin, an active component with yellow phenolic pigment derived from dietary spice turmeric (Curcuma longa) rhizome, belongs to the Zingiberaceae plant family indigenous to south-eastern and southern tropical Asia [14]. In addition to its coloring, flavoring, and preservative properties in the diet, turmeric has been widely used for the treatment of many disorders and metabolic ailments such as certain cancer diseases, cough, skin wounds, and inflammation [15]. Emerging evidence has demonstrated that curcumin exerts many pharmacological activities, such as anti-inflammatory, antioxidant, and antitumor properties [16,17]. The safety of turmeric has been evaluated in several animal studies. Intriguingly, the data revealed that curcumin has advantages over traditional chemotherapeutic drugs, including broad anticancer activity and less toxic adverse outcome [18,19]. In a recent study, the data showed that pre-treatment with curcumin followed by 5-FU increased the susceptibility of the colon cancer cells/xenograft to the cytotoxicity of 5-FU [20]. Indeed, the molecular mechanisms underlying the anticancer activity of co-treatment curcumin and chemotherapy are complex and worth to discuss further. Therefore, this review aimed to address the molecular mechanisms of combined curcumin and chemotherapy in the treatment of cancer. The anticancer activity of combined nanoformulation of curcumin and chemotherapy was also discussed in this study.
2. Mechanisms of Action of Oxidative Stress-Induced Cancer
Reactive oxygen species (ROS) are produced continuously in the body by mediating oxidative metabolism, immune activity, and mitochondrial bioenergetics [21]. However, when the ROS levels are elevated under stress, it may cause a detrimental impact on health [22]. The most frequent forms of ROS such as lipid peroxides, hydroxyl radical, hypochlorite, singlet oxygen, hydrogen peroxide, hyphochlorous acid, and superoxide anion are involved in the differentiation, death, growth, and progression of cells [22]. They can interact with proteins, membrane lipids, enzymes, and nucleic acids [23]. Short-term postprandial mitochondrial oxidative stress causes inflammation, which is predominantly mediated by nuclear factor-kappa B (NF-κB) [24].
Inflammation is an essential immune response to injury or infection in the body to regulate tissue homeostasis under certain circumstances such as swelling, redness, injury, infection, and irritation [25]. Among all chronic diseases, cancer is one of the prominent diseases contributed by chronic inflammation [26]. Inflammation is the seventh hallmark of cancer [27]. Both cancer and inflammation are linked through extrinsic and intrinsic pathways. For instance, oncogenes mediate the inflammatory microenvironments intrinsically and thus facilitate the progression and development of cancer extrinsically [28]. Indeed, the inflammation response is correlated to the tumor progression and the risk of malignancy [29]. Nearly 15% of cancer cases are activated through chronic inflammation and infection [30]. NF-κB is constitutively stimulated in many cancer diseases including pancreas, lung, liver, colon, and breast in response to carcinogens, for example, alcohol and tobacco [31].
The previous studies have demonstrated that transcription factors, for instance, NF-κB and signal transducer and activator of transcription 3 (STAT3), inflammatory enzymes including matrix metalloproteinase-9 (MMP-9) and cyclooxygenase-2 (COX-2), and inflammatory cytokines such as interleukins (IL)-1, -6, -8, and tumor necrosis factor alpha (TNF-α) are the key molecular mediators for inflammation-induced cancer cell proliferation, metastasis, angiogenesis, invasion, and inhibition of apoptosis [32]. Among these mediators, the transcriptional factor NF-κB is the main mediator of inflammation as it involves in the regulation of large arrays of cell adhesion molecules, cytokine receptors, and genes encoding cytokines [33]. The activity of NF-κB is triggered in response to proinflammatory cytokines and infectious agents by mediating IκB kinase (IKK) complex [34], suggesting a molecular link between cancer and inflammation [35]. NF-κB plays a pivotal role in the stimulation of some proinflammatory cytokines in several cell types such as epithelial cells, T cells, and macrophages [36]. Further, stimulation of NF-κB also triggers chemoresistance and radioresistance [7]. This finding suggests that NF-κB plays a crucial role in cancer and inflammation. Thus, anti-inflammatory agent targeting NF-κB and other associated products are potential in the treatment and prevention of cancers.
Given its tight association to NF-κB, IL-6 is an early candidate for the myeloid-derived factor that stimulates tumorigenesis [37]. As an example, patients with colon cancer demonstrated a high level of IL-6 [38]. Several studies reported by Bromberg and Wang [37] have also found the crucial role of IL-6 family of proinflammatory cytokines and their downstream effector STAT3 in colitis-associated colon cancer. Upregulation of STAT3 has been demonstrated in cancer patients, in which the STAT3 activity was positively linked to poor prognosis [39].
IL-6 is a predominant NF-κB-dependent cytokine that triggers the STAT3 activity. STAT3 is a cytoplasmic protein that serves as a transcriptional factor to trigger the inflammatory and immune response [32]. The stimulation of STAT3 involves a nuclear translocation, homodimerization, and tyrosine phosphorylation, where it interacts with DNA and modulates gene transcription [40,41]. Additionally, protein kinases, for example Janus-activated kinase (JAK) 3, 2, and 1 can induce the phosphorylation of STAT3 and subsequently trigger nuclear translocation [40].
An overexpression of TNF-α, the most potent proinflammatory cytokine, can stimulate cancer through activation of NF-κB expression [42]. In this regard, the blockage of TNF-α might have potential in the management and prevention of chronic disease such as cancer. Interleukins are another group of cytokines produced by macrophages. Several interleukins, for example IL-8, IL-6, and IL-1β play a vitally important role in the induction of proinflammatory response [43]. Aberrant expression of IL-1β, IL-6, IL-8, and TNF-α and activation of inducible nitric oxide synthase (iNOS) and COX-2 activity may contribute to oxidative stress, which in turn leads to inflammation [44]. Taken together, dysregulation of inflammation pathways appears to be involved in the pathogenesis of cancer.
3. Curcumin
Curcumin, a yellow pigment from Curcuma longa, is a major component of turmeric and is commonly used as a food-coloring agent and spice [45]. Curcumin is a low molecular weight polyphenol, is first isolated from turmeric in 1815, and the structure was delineated in 1910 as diferuloylmethane [46]. It is generally considered as the most active compound and contains about 2–8% of most turmeric preparations [47]. Curcumin is hydrophobic in nature and usually soluble in oil, ethanol, acetone, and dimethyl sulfoxide [48].
Curcumin has been recognized to protect biomembranes against peroxidative damage [49]. In general, lipid peroxidation is a free radical-mediated chain reaction that increased the damage of the cell membranes. Previous findings revealed that curcumin can inhibit the peroxidation through scavenging of reactive free radicals [50,51,52]. The ability of curcumin confers greater protection against oxidative damage has been attributed to their functional groups, such as phenyl rings, carbon-carbon double bonds, and β-diketone group [53].
4. Anticarcinogenic Potency and Molecular Mechanisms Induced by Curcumin
Growing evidence showed that curcumin and its analogs exert numerous pharmacological properties, for instance, antioxidant, anti-inflammation, and anticancer properties [45]. Of these properties, the anticancer activity of curcumin is known as one of the crucial effects of it. Many studies have demonstrated that curcumin (12 g/day for 3 months) induces apoptosis and antiproliferative against several types of cancer cell lines including prostate, breast, colorectal, pancreatic, and kidney [54,55]. Curcumin (0.1–3 mg/kg body weight) was shown to suppress telomerase reverse transcriptase enzyme [55] and reduce Bcl-2 expression [56]. Curcumin (2 µM) interacts with various proteins involved in angiogenesis, metastasis, and cell survival, as well as interferes with dysregulated signaling pathways in cancer cells, for instance phosphoinositide 3-kinase (PI3K)/Akt and NF-κB [57]. Curcumin exerts numerous effects by targeting several molecular and cellular pathways such as cell death, p53, Akt, mitogen-activating protein kinases (MAPK), microRNAs, and PTEN [45,58]. In this regard, these targets play a critical role in cancer pathogenesis and dysregulation of this pathway may lead to cancer progression and initiation [59]. The expression of NF-κB has been associated with inflammatory conditions as well as inducing a series of pathologic events involved in certain cancer [60]. NF-κB can be induced by endotoxin, ionizing radiation, carcinogen, free radical, and cytokine. Subsequently, these molecules trigger the activation of TNF that is linked to the upregulation of NF-κB expression [61]. Curcumin is well-recognized as an important regulator of NF-κB. For example, curcumin suppresses the activation of IKK that results in the translocation of NF-κB to the nucleus [62]. Furthermore, data from in vitro and in vivo studies have also shown a potent cytotoxic effect of curcumin on several pancreatic cancer cells through the inhibition of oxidative stress and angiogenesis as well as via the induction of apoptosis [63]. Dose-escalating study has demonstrated the safety of curcumin at doses up to 12 g/day over 3 months [64].
In addition to the targets mentioned above, microRNAs (miRNAs) are known as small non-coding RNA that plays a crucial role in various physiological conditions, including differentiation, growth, angiogenesis, and apoptosis [65,66]. Dysregulation of these molecules can upregulate and downregulate several cellular and molecular targets that lead to the progression of cancer [67]. Compelling evidence suggests that curcumin exerts its anticancer properties by targeting different miRNA expression such as miR-181b, miR-203, miR-9, miR-19, miR-21, miR203, miR-9, and miR-208 expression [58,68,69]. A study reported by Jin et al. [70] evaluated the curcumin (5-40 µM) in relation to miRNA levels. The data showed that curcumin (10 and 20 µM) upregulates miR-192-5p by modulating the PI3K/Akt signaling pathway in non-small cell lung cancer cells. The data reported by Schwertheim et al. [71] further demonstrated that curcumin (50 µM) upregulates miR-21 expression in thyroid carcinoma. Notably, phase I clinical trial for Bowen’s disease (squamous cell carcinoma in situ) conducted by Cheng et al. [72] showed that no treatment-associated toxicity up to 8000 mg/day for 3 months. The histological analysis further revealed that the precancerous lesions were reduced by 33% in patients with Bowen’s disease [72]. Despite extensive studies have shown that curcumin induces cytotoxicity against cancer cells by targeting different mechanisms, curcumin combination chemotherapy is more likely to enhance the synergistic effect of cancer cells to treatment. Data from in vitro study showed that curcumin (20 µM) sensitizes human gastric cancer cells to 5-FU (100 µM) via suppression of NF-κB signaling pathway [73]. In this regard, this can reduce the concentrations of chemotherapeutic drug and decrease the adverse effects of drugs [56].
5. The Synergistic Effect of Curcumin Combination Chemotherapy
5.1. In Vitro and In Vivo
Emerging preclinical evidence indicates that combination therapies promote anticancer efficacy without elevating toxicity [74]. Docetaxel (30 or 75 mg) has been clinically approved and widely used for the treatment of metastatic castration-resistant prostate cancer [75]. However, prolonged treatment with docetaxel could cause severe toxicity in patients [76]. A study by Banerjee et al. [77] found that the combined treatment of docetaxel (10 nM) and curcumin (20 µM) for 48 h significantly inhibited the proliferation and induced apoptosis in prostate cancer (PC-3) (DU145 and PC3) cells compared to the curcumin and docetaxel alone. The data further demonstrated that curcumin enhances the efficacy of docetaxel in PC-3 cells via modulation of COX-2, p53, NF-κB, phospho-Akt, PI3K, and receptor tyrosine kinase (RTK) [77]. In this regard, this finding implies that combining curcumin with conventional chemotherapy may act as an effective treatment regimen for patients with prostate cancer to reduce cytotoxicity and overcome drug-resistant induced by docetaxel.
Metformin (1500–3000 mg/day for 6 months) is recognized as a well-tolerated anti-diabetic drug [78]. Importantly, several studies revealed that metformin decreases the risk of many cancers including hepatocellular carcinoma and prostate cancer [79,80]. Clinical evaluation of metformin (500 mg daily for 1 week followed by 1 g twice daily for a week) for antineoplastic and chemopreventive effects has bypassed the phase I assessment and directly entered to phase II/III trials in several cancers due to less toxicity records in diabetic patients [81]. Data from an in vitro study revealed that combined metformin (10 mM) and curcumin (5 and 10 µM) can induce apoptosis and inhibit metastasis and invasion in HepG2 and PLC/PRF/5 cells. The anticancer effects could be attributed to the vascular endothelial growth factor (VEGF), MMP2/9, and vascular endothelial growth factor receptor 2 (VEGFR-2) inhibition, PTEN and p53 activation, and epidermal growth factor receptor (EGFR)/STAT3 and NF-κB/mTOR/Akt/PI3K suppression [82]. Data from an in vivo study further showed that co-treatment with metformin and curcumin significantly suppressed hepatocellular carcinoma compared to curcumin (60 mg/kg) and metformin (150 mg/kg) alone in a xenograft mouse model [82].
5-FU alone (10 μM) or in combination with other chemotherapeutic drugs has been widely applied for the treatment of colorectal cancer [83,84]. However, multidrug resistance was often developed in patients with colorectal cancer administered with 5-FU-based regimen [85]. The previous findings suggest that the combination of 5-FU and curcumin may overcome the drug-resistant induced by 5-FU. Pre-treatment with curcumin (5 µM) enhanced the chemosensitization of 5-FU (0.1 µM) and reversed the multidrug resistance in resistant mismatch repair (MMR)-deficient human colon cancer cells compared to 5-FU alone [86]. Combination of curcumin (10 μM) and 5-FU (0.1 mM)/oxaliplatin (5 μM) enhanced the synergistic antitumor activity in gastric cancer (BGC-823) cell lines compared to curcumin or 5-FU/oxaliplatin alone by downregulating the Bcl-2 mRNA and protein expression and activating the Bax and caspases-3, 8, and 9 expressions [87]. The study further demonstrated that the combination of curcumin (10 mg/kg) and 5-FU (33 mg/kg)/oxaliplatin (10 mg/kg) show potent growth inhibition of BGC-823 xenograft tumors compared to folinic acid, 5-FU, oxaliplatin (FOLFOX) or curcumin alone [87]. In addition, a combination of curcumin (50 mg/kg/day for 40 days) and 5-FU (20 mg/kg once every 2 days for 40 days) also inhibits the cell proliferation against 5-FU resistant cells via suppression of epithelial-to-mesenchymal transition (EMT) compared to 5FU alone [88]. In the context of breast cancer, a combination of curcumin (10 µM) and 5-FU (10 µM) significantly inhibited the cell viability and enhanced apoptosis compared to 5-FU alone in vitro [89]. In addition to the effects mentioned above, a study reported by Yang et al. [90] has shown that combination of 5-FU (50 µmol/L) and curcumin (25 µmol/L) can enhance the cytotoxicity against human gastric cancer (AGS) cells compared to 5-FU or curcumin alone. In a further study focused on inflammation outcomes, Yang et al. [90] found that the protein expression of COX-2 and NF-κB in human gastric cancer (MKN45) cells were diminished after co-treatment with 5-FU (50 µmol/L) and curcumin (25 µmol/L). This finding implies that curcumin sensitizes gastric cancer cells to 5-FU by modulating inflammatory molecules. The anti-gastric cancer activity is not only shown in in vitro study, data from an animal study further demonstrated that curcumin enhanced the anticancer activity of 5-FU (52 mg/kg 5-FU + curcumin 74 mg/kg, every 3 days for 6 times in total) compared to 5-FU or curcumin alone, and without increasing the toxicity in nude mice bearing MKN45 tumor xenografts [90].
Doxorubicin, one of the active single-agent drugs, is widely used for the treatment of cancers, including leukemia, lung, brain, prostate, ovarian, and breast. However, the clinical use of doxorubicin often led to critical cardiotoxicity and developed multidrug resistance [91]. Substantial evidence revealed that curcumin (4 mg/kg every 2 days for a total of 7 injections) exhibits a better treatment efficacy of doxorubicin (0.4 mg/kg) in cancer due to its efflux inhibitory effect of curcumin [92,93,94]. A study conducted by Guorgui et al. [95] has shown that combination of curcumin (5 µM) and doxorubicin (0.4 mg/mL) demonstrated a stronger additive effect by reducing the proliferation of Hodgkin lymphoma (L-540) cells by 79%. The pharmacokinetic study also revealed that curcumin (5 mg/kg) could enhance the absorption of doxorubicin (5 mg/kg) and decrease drug efflux in vivo, suggesting that curcumin downregulates the intracellular levels of ATP-binding cassette (ABC) drug transporters [96].
Cisplatin-based combination therapy has emerged as a standard therapy for metastatic and advanced bladder cancer [97], demonstrating 15–20% improved survival and 50–70% response rate. However, nearly 30% of patients do not respond to initial chemotherapy and show recurrence within 1 year [98]. Cisplatin is an inorganic platinum agent which can induce DNA-protein and interstrand and intrastrand DNA crosslinks [99]. Despite this crosslink can induce apoptosis and inhibit cell proliferation [100], the efficacy of cisplatin is limited by the development of cell resistance. Co-treatment with curcumin (10 µM) and cisplatin (10 µM) has shown a potent synergistic effect by activating caspase-3 and upregulating phospho-mitogen-activated protein kinase (p-MEK) and phospho-extracellular signal-regulated kinase 1/2 (p-ERK1/2) signaling in bladder cancer cell lines (253J-Bv and T24) compared to curcumin or cisplatin alone [101]. In addition to the effects observed on bladder cancer, the combination of curcumin and cisplatin was shown to upregulate the expression of miR-186 via modulation of Twist1 in ovarian cancer compared to cisplatin alone [102].
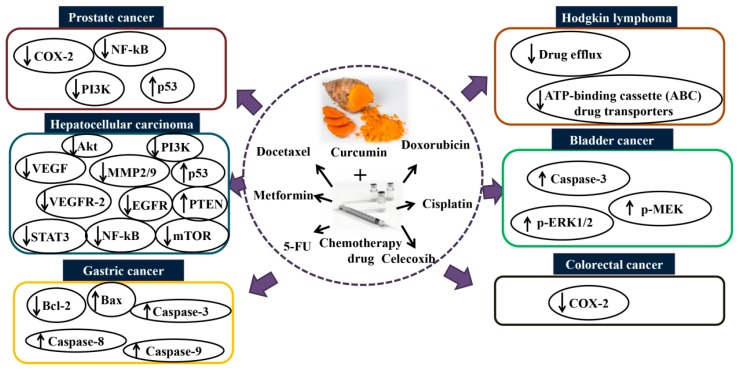
Besides the effects mentioned above, celecoxib is another selective inhibitor of COX-2, an enzyme induced by different stimuli including inflammation [103]. Celecoxib (75 µM for 16 h) has shown an ability to induce apoptosis and suppress tumor angiogenesis in several types of cancer [103]. However, the long-term use of celecoxib leads to an adverse outcome such as cardiovascular toxicity [104]. Combination of curcumin and celecoxib was shown to reduce cancer cell growth in vitro compared to celecoxib alone. A study reported by Lev-Ari et al. [105] revealed that curcumin (10–15 µmol/L) and physiological dosage of celecoxib (5 µmol/L) exhibited a synergistic inhibitory effect against human colorectal cancer (HT-29) cells. The study showed that the combination of curcumin and celecoxib induces apoptosis in HT-29 cells via downregulation of COX-2 expression, suggesting that curcumin synergistically augments the growth inhibitory effects of celecoxib in human colon cancer cell lines in vitro. Collectively, the synergistic effects of curcumin and chemotherapy on cancer are important and worth attention, particularly in patients receiving antineoplastic or anti-inflammatory drugs. Figure 1 summarizes the mechanisms of action of combination curcumin and chemotherapeutic drugs in vitro and in vivo.
Figure 1.
Mechanisms of action of combination curcumin and chemotherapy drugs in vitro and in vivo. Co-treatment with curcumin and chemotherapy drugs such as docetaxel, metformin, 5-fluorouracil, doxorubicin, cisplatin, and celecoxib enhance the synergistic effect via modulating several signaling pathways and thus inhibit cancers such as prostate, hepatocellular, gastric, Hodgkin lymphoma, bladder, and colorectal. Akt: protein kinase B; COX-2: cyclooxygenase-2; EGFR: epidermal growth factor receptor; MMP2/9: matrix metalloproteinase-2/9; mTOR: mammalian target of rapamycin; NF-κB: nuclear factor kappa B; p-ERK1/2: phospho-extracellular signal-regulated kinase 1/2; PI3K: phosphoinositide 3-kinase; p-MEK: phospho-mitogen-activated protein kinase; STAT3: signal transducer and activator of transcription 3; VEGF: vascular endothelial growth factor; VEGFR2: vascular endothelial growth factor receptor 2.
5.2. Clinical Trials
In addition to the effects observed in both in vitro and in vivo models, the implication of curcumin combination chemotherapy has been reported in several clinical trials (Table 1). The activity and feasibility of gemcitabine and curcumin were assessed in patients with pancreatic cancer. Previous studies suggest that utilization of curcumin at different dosage is safe in animal and human models [106,107]. Indeed, several studies reported by Waghela et al. [106] and Shankar et al. [107] have demonstrated the concentration of curcumin could be tolerated even at very high doses. However, low doses of curcumin are related to the therapeutic effects of several cancers [108].
Table 1.
Clinical trials conducted in a combination of curcumin and chemotherapy.
| Types of Cancer | Treatment | Participants | Findings | References |
|---|---|---|---|---|
| Advanced or metastatic breast cancer | Curcumin (500 mg/day) and escalated until a dose-limiting toxicity + docetaxel (100 mg/m2) for 7 days every 3 weeks | 14 patients | Improves biological and clinical responses | [109] |
| Pancreatic cancer | Curcumin (8000 mg/day) + gemcitabine (1000 mg/m2) weekly | 17 patients | Time to tumor progression was 1-12 months and overall survival was 1-24 months | [110] |
| Chronic myeloid leukemia | Imatinib (400 mg twice a day for 6 weeks) Group B [Turmeric powder (5 g three times/day) + imatinib (400 mg twice a day)] for 6 weeks |
50 patients | The suppressive effect of nitric oxide levels was noted at Group B | [111] |
| Pancreatic cancer | Curcumin (1000 mg/day) + gemcitabine (1000 mg/m2 on day 1 and 8) and 60 mg/m2 of S-1 orally for 14 consecutive days every 3 weeks | 21 patients | Median survival time after initiation of curcumin was 161 days and 1-year survival rate was 19% | [112] |
| Colorectal liver metastases | 5 µM curcumin + 2 µM oxaliplatin + 5 µM 5-FU | 12 patients | Curcumin enhanced the FOLFOX-based chemotherapy | [113] |
| Pancreatic cancer | Curcumin (2000 mg/die continuously (4 capsules, each of 500 mg, every day) + gemcitabine (10 mg/m2) | 44 patients (13 locally advanced and 31 metastatic) |
Median progression-free survival and overall survival were 8.4 and 10.2 months, respectively | [114] |
A phase-I escalated clinical trial evaluated the feasibility and tolerability of the docetaxel and curcumin in 14 patients with advanced or metastatic breast cancer. Curcumin was orally given at a dosage of 500 mg/day for seven days and increased until dose-limiting toxicity. The data revealed that administration of docetaxel (100 mg/m2) and curcumin (500 mg/day) show promising biologic response in the chemoprevention by reducing carcinoembryonic antigen (CEA) tumor marker [109]. The maximum tolerated dose for curcumin is 6000 mg/day for seven consecutive days every three weeks in combination with a standard dosage of docetaxel [109]. In a study reported by Kanai et al. [112] focusing on the safety of combination therapy using 8 g oral curcumin daily with gemcitabine-based chemotherapy (1000 mg/m2 on day 1 and 8) and (60 mg/m2 of S-1 orally for 14 consecutive days every 3 weeks) in 21 patients with gemcitabine-resistant pancreatic cancer, the patients were shown no dose-limiting toxicities in the phase I study, suggesting that oral curcumin 8 g/day is safe and feasible in patients with pancreatic cancer. In a prospective phase II trial evaluated the safety and efficacy of curcumin (2000 mg/die continuously (4 capsules, each of 500 mg, every day) and gemcitabine (10 mg/m2) on 44 advanced and metastatic pancreatic cancer patients, the data showed that the median progression-free survival and overall survival were 8.4 and 10.2 months, respectively. This finding implies that complementary therapy to gemcitabine with phytosome complex of curcumin is safe and efficient to translate in a good response rate in first-line therapy of advanced pancreatic cancer [114]. Moreover, curcumin (5 µM) was found to enhance FOLFOX-based chemotherapy (2 µM oxaliplatin + 5 µM 5-FU) in patient-derived colorectal liver metastases cultures [113]. The phase I escalation study demonstrated that curcumin is safe and tolerable in combination with FOLFOX chemotherapy in patients with colorectal liver metastases at a dosage up to 2 g daily [113]. There are several limitations for drawing conclusions from the clinical trials include (1) small sample size; (2) different daily dosage of curcumin; (3) short duration of treatment; (4) other factors such as age may confound the results; and (5) possible bias from the interpreting pathologist. Additionally, assessment of dose-response relationship and cancer has not been evaluated. It would be useful to have moderate and high curcumin combination chemotherapy compared in the same study. Based on the evidence, the synergistic role played by curcumin and chemotherapeutic drugs worth study in-depth in a large clinical trial.
6. Nanoformulation of Curcumin Combination Chemotherapy
Despite curcumin pharmacological properties were reported in vivo and in vitro models, poor aqueous solubility and low stability may limit the clinical usefulness of curcumin combination chemotherapy. These unfavorable effects have hampered the quantity of curcumin absorbed, and thus severely limit its bioavailability. Nanoformulation-based combination therapy has emerged as a potent approach for drug delivery system [115]. It has received attention as it can resolve problems associated with conventional therapeutic agents by improving intracellular drug concentrations and enhancing the synergistic activity for cancer therapy [116]. Thus, the application of nanotechnology could improve the efficacy and enhance bioavailability by increasing permeation in the small intestine, preventing degradation in the intestinal environment, increasing plasma half-life, and enhancing the efficacy [117].
Some attempts have been made to improve the bioavailability of curcumin. Formulation of curcumin with d-α-tocopheryl polyethylene glycol 1000 succinate-stabilized curcumin (TPGS-curc) has been widely studied [118,119,120]. The data revealed that TPGS-curc (10 mg/kg body weight) shows a better in vivo kinetic profile than curcumin alone, suggesting that TPGS an ideal formulation to improve curcumin bioavailability in vivo. In addition, formulating curcumin in solid lipid nanoparticles (SLN-curc) (100 mg/kg of curcumin, 5 days per week for 18 days) exhibited a strong anticancer activity compared to curcumin alone (100 mg/kg, 5 days per week for 18 days) [95]. The data showed that SLN-curc increased cell cycle regulator (p21) and diminished the anti-apoptotic (XIAP and Mcl-1) levels [95]. In support of the efficacy of nanoformulation-based curcumin, in vitro study has demonstrated that the efficacy of cell proliferation in dendrosomal curcumin (8.31-13.45 µM) is superior compared to curcumin alone (13 to 30 µM) [120]. The data further showed that dendrosomal curcumin therapy (13.45 and 11.66 µM) significantly increased the GAS5 and TUSC7 levels, a tumor suppressor gene expression, in human breast cancer (MCF-7, SKBR3, and MDA-MB231) cell lines in a dose-dependent manner [120]. In this regard, this finding implies that the overexpression of GAS5 might reduce chemotherapy resistance. This finding is in line with the previous study reported in in vitro model for bladder cancer cells, in which the overexpression of GAS5 enhances bladder cancer cells sensitivity to doxorubicin [121]. In the context of a clinical trial, a phase I study evaluated the safety and tolerability of increasing dose of liposomal curcumin (100-300 mg/m2) in patients with metastatic or advanced cancer [122]. The data showed that 300 mg/m2 liposomal curcumin over 6 h is the maximum tolerated dose in these heavily pre-treated patients, suggesting the recommended starting dose for anticancer trials [122].
Nanodrug delivery system has been found to be an efficient approach to enhance the synergistic effect (Table 2 and Table 3). Co-delivery micelles of doxorubicin and curcumin (8 µg/mL doxorubicin and 8 µg/mL curcumin) significantly reversed multidrug resistance compared to doxorubicin (8 μg/mL) by targeting CD44. The data from an animal study revealed that co-delivery micelles of doxorubicin and curcumin inhibited the growth of tumor in 4T1 tumor-bearing mice [123]. Polymeric micelles, a core-shell structure with hydrophilic shell and hydrophobic core of micelles, can effectively accumulate in the tumor by enhancing the permeability and retention, and subsequently increasing the therapeutic effects of chemotherapeutic drugs [124]. A study by Xiao et al. [125] further supported the role of nanodrug co-delivery system in the modulation of cancer cell sensitivity. Combined delivery of chitosan-functionalized camptothecin/curcumin-loaded poly(lactic acid/glycolic acid) polymeric nanoparticle (6 mg) can enhance the synergistic effects against colon-26 cells compared to camptothecin or curcumin alone [125]. In another study, Nguyen et al. [126] evaluated a combined nanodrug delivery system by incorporating a chemotherapeutic agent, methotrexate (MTX) (2.5 mg/kg), and a photosensitizer material (polyaniline) (5 mg/kg) into hybrid polymer nanoparticles. The data from both in vitro and in vivo studies showed that chemo-phototherapy enhanced the synergistic effect and inhibited somatostatin-positive cancer compared to MTX or polyaniline nanoparticle alone [126]. MTX, an antimetabolite drug, is one of the effective therapeutic agents for disease associated with aberrant cell growth [127]. Dendritic chitosan grafted methoxy polyethylene glycol (mPEG) coated with magnetic nanoparticle is another magnetic nanocarrier that used for co-delivery doxorubicin and MTX. Data from in vitro study demonstrated that combination drug delivery could enhance the synergistic effects and thereby better alleviate the adverse outcomes compared to doxorubicin or MTX alone [128]. Taken together, this newly developed nanoformulation by encapsulating curcumin and chemotherapeutic drugs hold a great promising as a future therapy for cancer.
Table 2.
Summary of nanoformulation curcumin and chemotherapeutic drugs in vitro.
| Types of Cancer | Co-Delivery System | Treatment | Cancer Cell Lines | Findings | References |
|---|---|---|---|---|---|
| Colon cancer | Cationic polymeric nanoparticles | Camptothecin and curcumin | Colon-26 cells | Enhances synergistic effects of anticancer activity | [125] |
| Liver cancer | Lipid nanoparticle | Curcumin and doxorubicin | HepG2 cells | Enhance cytotoxicity and decrease inhibitory concentration in HepG2 cells | [129] |
| Breast cancer | Liposomal | Paclitaxel and curcumin | MCF-7 cell lines | Effectively kills the cancer cells compared to individual treatment | [130] |
| Breast cancer | Dendritic chitosan grafted methoxy polyethylene glycol (mPEG) coated magnetic nanoparticles | Doxorubicin and methotrexate | MCF7 cell lines | Enhances synergistic effects and alleviates adverse outcome | [128] |
| Breast cancer | Transferrin-decorated nanoparticles | Curcumin and doxorubicin | MCF-7 cells | Combination of transferrin-poly(ethylene glycol)-curcumin (Tf-PEG-CUR) and doxorubicin nanoparticle exhibited higher cytotoxicity in MCF-7 cells compared with Tf-PEG-CUR nanoparticle alone | [131] |
| Colon cancer | PEGylated long-circulating liposomes |
Curcumin and doxorubicin | C26 murine colon cancer cells | Exerts strong antiproliferative effects via inhibition of the angiogenic/inflammatory proteins such as TNF-α, TIMP-2, and IL-6 | [132] |
| Breast cancer | Albumin nanoparticles | Curcumin and doxorubicin | MCF-7 resistant breast cancer cells | Shows lower viability than the cells treated with a combination of curcumin and albumin nanoparticle or combination of doxorubicin and albumin nanoparticle | [133] |
| Liver cancer | Biotin-/lactobionic acid–modified poly(ethylene glycol)-poly(lactic-co-glycolic acid)-poly(ethylene glycol) (BLPP) copolymer | Curcumin and 5-FU | HepG2 cells | Exhibits higher cellular uptake, strong cytotoxicity for tumor cells | [134] |
| Pancreatic cancer | Iron oxide nanoparticles | Curcumin and gemcitabine | Human pancreatic cancer (HPAF-II and Panc-1) cell lines | Effectively delivers bioactive curcumin to pancreatic cells, simultaneously enhances gemcitabine uptake | [135] |
BLPP: biotin-/lactobionic acid–modified poly(ethylene glycol)-poly(lactic-co-glycolic acid)-poly(ethylene glycol); IL–6: interleukin-6; TIMP-2: tissue inhibitor of metalloproteinases 2; TNF-α: tumor necrosis factor α; 5-FU: 5-fluorouracil.
Table 3.
Summary of nanoformulation curcumin and chemotherapeutic drugs in vivo.
| Types of Cancer | Co-Delivery System | Treatment | Animal Model | Findings | References |
|---|---|---|---|---|---|
| Lung cancer | Methoxy poly(ethylene glycol)-poly(caprolactone) (MPEG-PCL) micelles | Curcumin and doxorubicin (5 mg/kg) intravenous tail injection every 5 days until the control mice became weak | The female C57 mice (aged 6-8 weeks) (n = 40) mice were injected subcutaneously with 100 µL of LL/2 cell suspension (1 × 106) into the right flank. The tumor-bearing mice were randomly divided when the mean tumor diameter reached about 6 mm |
1. Tumors in the groups treated with curcumin and doxorubicin/MPEG-PCL were smaller than those receiving the other treatments (p < 0.05) 2. Inhibited the growth of subcutaneous LL/2 lung carcinoma (p < 0.05) 3. Induced apoptosis of tumor tissue and inhibited tumor angiogenesis, as shown in TUNEL assay and CD31 staining (p < 0.05) |
[93] |
| Liver cancer | Lipid nanoparticle | Curcumin and doxorubicin (2 mg/kg doxorubicin) for 20 weeks, intravenously injected once a week |
Diethylnitrosamine-induced hepatocellular carcinoma mice (n = 32) 24 mice were administrated by oral administration of diethylnitrosamine solution in sesame oil (0.1 g/mL) at 40 mg/kg once a week for 15 weeks. 8 mice were administrated with sesame oil only (normal mice) |
1. The liver/body weight (p < 0.05) and serum ALT and AST levels (p < 0.01) were significantly decreased in curcumin and doxorubicin-lipid nanoparticle group 2. The mRNA and protein levels of Bax/Bcl-2 (p < 0.01) were all increased in tumor tissue from curcumin and doxorubicin-lipid nanoparticle group compared to the doxorubicin-lipid nanoparticle group 3. The immunohistochemistry analysis induced expression of caspase-3 4. The expression of c-myc and PCNA decreased significantly in curcumin and doxorubicin-lipid nanoparticle (p < 0.01) compared to the control group 5. The mRNA and protein levels of VEGF in curcumin and doxorubicin-lipid nanoparticle were significantly decreased compared to the control group (p < 0.05) 6. The mRNA levels of MDR1 and Bcl-2, as well as the protein levels of P-gp and Bcl-2 were all decreased in curcumin and doxorubicin-lipid nanoparticle group compared to the doxorubicin-lipid nanoparticle group (p < 0.01) |
[136] |
| Breast cancer | Polymeric micelles | Curcumin (10 mg/kg) and doxorubicin (10 mg/kg) for 12 days | The female BALB/c mice (n = 60) were injected with 1 × 106 4T1 cells into the right axilla of mice. When the tumor reached about 100 mm3, 4T1 tumor-bearing mice were randomly divided into 6 groups (n = 10) | 1. Curcumin and doxorubicin polymeric micelles treated group exhibited a considerable tumor inhibition compared to the saline-treated group (p < 0.001) 2. The AST, LDH, CK, and CKMB were significantly reduced compared to the mice treated with doxorubicin polymeric micelles (p < 0.05) 3. No pathological damages were found in heart, liver, spleen, lung, and kidney in the mice treated with curcumin and doxorubicin polymeric micelles by using H&E staining 4. Tumors treated with curcumin and doxorubicin polymeric micelles had enhanced dark brown spots by using TUNEL assay, indicating that drug encapsulated in micelles could enhance tumor cell apoptosis and showed better antitumor effects |
[123] |
| Breast cancer | Transferrin-poly(ethylene glycol) | Curcumin (50 mg/kg) and doxorubicin (50 mg/kg) were injected into the mice by tail vein for 7 weeks | BALB/c mice were inoculated subcutaneously with 1 × 106 MCF-7 cells. MCF-7 tumor xenografts were grown in BALB/c mice and estrogen was provided as a β-estradiol pellet 1 week prior to the injection of the cells. The tumors were allowed to develop on the posterolateral side of the mice for 1 week prior to treatment to obtain the breast cancer-bearing animal model. The mice were randomly divided into 6 groups | Compared with curcumin and doxorubicin, transferrin-poly(ethylene glycol)-curcumin/doxorubicin nanoparticles presented a remarkably higher inhibition effect towards tumor growth (p < 0.05) | [131] |
| Liver cancer | Biotin-/lactobionic acid–modified poly(ethylene glycol)-poly(lactic-co-glycolic acid)-poly(ethylene glycol) (BLPP) copolymer | Curcumin (10 mg/kg) and 5-FU (4 mg/kg). The mice were injected once at an interval of 2 days of a total of 4 injections through the tail vein for 30 days | The BALB/c nude mice (n = 18) were inoculated subcutaneously with HepG2 cells (2 × 106). The mice were randomly divided into 6 groups (n = 3) when the tumor volume reached about 50 mm3 | 1. The tumors in BLPP/curcumin +5-FU nanoparticle were approximately 8 times smaller than the tumor volume observed in the control (phosphate-buffered saline) group (p < 0.001) 2. The mice treated with BLPP/curcumin +5-FU nanoparticles induced tumor apoptosis or necrosis significantly compared to the BLPP/curcumin nanoparticle (p < 0.05) 3. The western blotting analysis showed that BLPP/curcumin + 5-FU nanoparticle significantly decreases the DPYD expression compared to the BLPP/5-FU nanoparticle and the control groups (p < 0.05) 4. p53 protein expression was higher in BLPP/curcumin groups than in BLPP/5-FU nanoparticle group (p < 0.05) 5. Bcl-2 protein expression of BLPP/curcumin + 5-FU was lower than that of the BLPP/5-FU nanoparticle, BLPP/curcumin nanoparticle, and control groups; while the expression of cytochrome c was higher (p < 0.05) |
[134] |
| Pancreatic cancer | Superparamagnetic iron oxide nanoparticle (SPION) formulation of curcumin (SP-CUR) | 2 treatments groups [Curcumin (100 µg dissolved in 100 μL of 0.1% Tween 20) and gemcitabine (300 μg dissolved in 50 μL of phosphate-buffered saline)] and another two groups were treated with an intraperitoneal injection of 100 μg curcumin loaded SP-CUR and combination with gemcitabine, respectively. Treatments were administered twice weekly for 7 weeks | HPAF-II cells (1.0 × 106) and human pancreatic stromal cells (stromal component; 0.5 × 106) were suspended in 50 μL of HBSS media containing 1% (v/v) matrigel and injected into the parenchyma of the pancreas in old male athymic nude mice (6 weeks old). Five days later, mice were randomly divided into five groups (n = 8) | 1. The bioluminescence imaging results showed a significant (p < 0.05) decrease in the pancreatic tumor volume of SP-CUR + gemcitabine-treated mice compared to the vehicle-treated mice 2. SP-CUR + gemcitabine-treated mice showed a significant decrease in the tumor weight of pancreas compared to the gemcitabine alone (p < 0.0001) 3. None of the mice treated with SP-CUR + gemcitabine were recorded for distant metastasis 4. Immunoblotting and immunohistochemistry analyses showed that SP-CUR + gemcitabine inhibited SHH, NF-қB, Gli-1 and Gli-2 expression 5. SP-CUR + gemcitabine reduced the amount of α-SMA, N-cadherin, and SMO and upregulated hCNT |
[135] |
α-SMA: alpha-smooth muscle actin; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BLPP: biotin-/lactobionic acid–modified poly(ethylene glycol)-poly(lactic-co-glycolic acid)-poly(ethylene glycol); CK: creatine kinase; CKMB: creatine kinase MB; hCNT: human concentrative nucleoside transporter; H&E: hematoxylin and eosin; LDH: lactate dehydrogenase; NF-қB: nuclear factor-kappa beta; PCNA: proliferating cell nuclear antigen; SHH: sonic hedgehog; SMO: smoothened; VEGF: vascular endothelial growth factor; 5-FU: 5-fluorouracil.
7. Conclusions
Chemotherapeutic drugs such as doxorubicin, irinotecan, 5-fluorouracil, cisplatin, and paclitaxel have been applied in cancer therapy. Nonetheless, the efficacy of chemotherapy is limited due to the development of drug resistance and undesirable outcomes. The combination of therapies with natural antitumor compounds was shown to enhance the effectiveness of drug treatment and decrease the toxic effect. Curcumin has found to be cytotoxic and exert chemopreventive activities in nature to a wide variety of cancer cell lines and animal tumor models via multiple molecular mechanisms targeting all stages of carcinogenesis. However, water insolubility and low stability of curcumin have hampered the quantity of curcumin absorbed and its bioavailability. Nanoencapsulation has emerged as a potent strategy to enhance the therapeutic potential of conventional drugs. Co-delivery of nanoparticle-based curcumin and chemotherapy has shown efficacy for improving intracellular drug concentration and enhancing the synergistic effect in cancer therapy by sensitizing cancer cells towards chemotherapeutic drugs. This concurrent administration enhances anticancer efficacy and reduces the use of chemotherapy drugs. Subsequently, this can reduce the adverse outcome caused by drugs. Although in vitro and in vivo studies have demonstrated the synergistic effects in co-delivery of nanoformulation of curcumin and chemotherapeutic drugs, further studies are warranted to elucidate the benefit-risk profile of curcumin as well as concurrent administration of nanoformulation of curcumin and drugs in a large clinical trial. Taken together, this evidence may pave the way for a useful approach to combat cancers, providing that this strategy involves a regimen with a low dosage of side effects.
Author Contributions
B.L.T. conceived and designed the review and wrote the manuscript. M.E.N. edited the manuscript. All authors read and approved the final manuscript.
Funding
This research was funded by Putra Grant (UPM/700-2/1/GPB/2017/9549900).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.World Health Organization Cancer. [(accessed on 25 February 2019)];2019 Available online: https://www.who.int/cancer/en/
- 2.Seyed M.A., Jantan I., Bukhari S.N.A., Vijayaraghavan K. A comprehensive review on the chemotherapeutic potential of piceatannol for cancer treatment, with mechanistic insights. J. Agric. Food Chem. 2016;64:725–737. doi: 10.1021/acs.jafc.5b05993. [DOI] [PubMed] [Google Scholar]
- 3.Liang X.-J., Chen C., Zhao Y., Wang P.C. Circumventing tumor resistance to chemotherapy by nanotechnology. Methods Mol. Biol. 2010;596:467–488. doi: 10.1007/978-1-60761-416-6_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu H.-H., Chen M.-C., Baskaran R., Lin Y.M., Day C.H., Lin Y.J., Tu C.C., Vijaya Padma V., Kuo W.W., Huang C.Y. Oxaliplatin resistance in colorectal cancer cells is mediated via activation of ABCG2 to alleviate ER stress induced apoptosis. J. Cell. Physiol. 2018;233:5458–5467. doi: 10.1002/jcp.26406. [DOI] [PubMed] [Google Scholar]
- 5.Xu X.-T., Hu W.-T., Zhou J.-Y., Tu Y. Celecoxib enhances the radiosensitivity of HCT116 cells in a COX-2 independent manner by up-regulating BCCIP. Am. J. Transl. Res. 2017;9:1088–1100. [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmad S., Panda B.P., Fahim M., Dhyani N., Dubey K. Ameliorative effect of beraprost sodium on celecoxib induced cardiotoxicity in rats. Iran. J. Pharm. Res. 2018;17:155–163. [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q., Yang G., Feng M., Zheng S., Cao Z., Qiu J., You L., Zheng L., Hu Y., Zhang T., et al. NF-κB in pancreatic cancer: Its key role in chemoresistance. Cancer Lett. 2018;421:127–134. doi: 10.1016/j.canlet.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Deveci H.A., Naziroğlu M., Nur G. 5-Fluorouracil-induced mitochondrial oxidative cytotoxicity and apoptosis are increased in MCF-7 human breast cancer cells by TRPV1 channel activation but not Hypericum perforatum treatment. Mol. Cell. Biochem. 2018;439:189–198. doi: 10.1007/s11010-017-3147-1. [DOI] [PubMed] [Google Scholar]
- 9.Cai D., He K., Chang S., Tong D., Huang C. MicroRNA-302b enhances the sensitivity of hepatocellular carcinoma cell lines to 5-FU via targeting Mcl-1 and DPYD. Int. J. Mol. Sci. 2015;16:23668–23682. doi: 10.3390/ijms161023668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z., Penet M.-F., Krishnamachary B., Banerjee S.R., Pomper M.G., Bhujwalla Z.M. PSMA-specific theranostic nanoplex for combination of TRAIL gene and 5-FC prodrug therapy of prostate cancer. Biomaterials. 2016;80:57–67. doi: 10.1016/j.biomaterials.2015.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J.J., Beumer J.H., Chu E. Therapeutic drug monitoring of 5-fluorouracil. Cancer Chemother. Pharmacol. 2016;78:447–464. doi: 10.1007/s00280-016-3054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polk A., Vaage-Nilsen M., Vistisen K., Nielsen D.L. Cardiotoxicity in cancer patients treated with 5-fluorouracil or capecitabine: A systematic review of incidence, manifestations and predisposing factors. Cancer Treat. Rev. 2013;39:974–984. doi: 10.1016/j.ctrv.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Tan B.L., Norhaizan M.E., Huynh K., Heshu S.R., Yeap S.K., Hazilawati H., Roselina K. Water extract of brewers’ rice induces apoptosis in human colorectal cancer cells via activation of caspase-3 and caspase-8 and downregulates the Wnt/β-catenin downstream signaling pathway in brewers’ rice-treated rats with azoxymethane-induced colon carcinogenesis. BMC Complement. Altern. Med. 2015;15:205. doi: 10.1186/s12906-015-0730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pulido-Moran M., Moreno-Fernandez J., Ramirez-Tortosa C., Ramirez-Tortosa M. Curcumin and health. Molecules. 2016;21:264. doi: 10.3390/molecules21030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barchitta M., Maugeri A., Favara G., Magnano San Lio R., Evola G., Agodi A., Basile G. Nutrition and wound healing: An overview focusing on the beneficial effects of curcumin. Int. J. Mol. Sci. 2019;20:1119. doi: 10.3390/ijms20051119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantzorou M., Pavlidou E., Vasios G., Tsagalioti E., Giaginis C. Effects of curcumin consumption on human chronic diseases: A narrative review of the most recent clinical data. Phytother. Res. 2018;32:957–975. doi: 10.1002/ptr.6037. [DOI] [PubMed] [Google Scholar]
- 17.Tan R.-Z., Liu J., Zhang Y.-Y., Wang H.-L., Li J.-C., Liu Y.-H., Xia Z., Zhang Y.-W., Yan Y., Lan H.-Y., et al. Curcumin relieved cisplatin-induced kidney inflammation through inhibiting Mincle-maintained M1 macrophage phenotype. Phytomedicine. 2019;52:284–294. doi: 10.1016/j.phymed.2018.09.210. [DOI] [PubMed] [Google Scholar]
- 18.Basnet P., Skalko B.N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules. 2011;16:4567–4598. doi: 10.3390/molecules16064567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Creţu E., Trifan A., Vasincu A., Miron A. Plant-derived anticancer agents-curcumin in cancer prevention and treatment. Revista MedicoChirugicala Societatii Medici Si Naturalisti Din Iasi. 2012;116:1223–1229. [PubMed] [Google Scholar]
- 20.Zhang P., Lai Z.L., Chen H.F., Zhang M., Wang A., Jia T., Sun W.Q., Zhu X.M., Chen X.F., Zhao Z., et al. Curcumin synergizes with 5-fluorouracil by impairing AMPK/ULK1-dependent autophagy, AKT activity and enhancing apoptosis in colon cancer cells with tumor growth inhibition in xenograft mice. J. Exp. Clin. Cancer Res. 2017;36:190. doi: 10.1186/s13046-017-0661-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Vakifahmetoglu-Norberg H., Ouchida A.T., Norberg E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017;482:426–431. doi: 10.1016/j.bbrc.2016.11.088. [DOI] [PubMed] [Google Scholar]
- 22.Rahal A., Kumar A., Singh V., Yadav B., Tiwari R., Chakraborty S., Dhama K. Oxidative stress, prooxidants, and antioxidants: The interplay. BioMed Res. Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajendran P., Nandakumar N., Rengarajan T., Palaniswami R., Gnanadhas E.N., Lakshminarasaiah U., Gopas J., Nishigaki I. Antioxidants and human diseases. Clin. Chim. Acta. 2014;436:332–347. doi: 10.1016/j.cca.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Jung K.J., Lee E.K., Kim J.Y., Zou Y., Sung B., Heo H.S., Kim M.K., Lee J., Kim N.D., Yu B.P., et al. Effect of short term calorie restriction on pro-inflammatory NF-kB and AP-1 in aged rat kidney. Inflamm. Res. 2009;58:143–150. doi: 10.1007/s00011-008-7227-2. [DOI] [PubMed] [Google Scholar]
- 25.Xiao T.S. Innate immunity and inflammation. Cell. Mol. Immunol. 2017;14 doi: 10.1038/cmi.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qu D., Shen L.S., Liu S., Li H., Ma Y., Zhang R., Wu K., Yao L., Li J., Zhang J. Chronic inflammation confers to the metabolic reprogramming associated with tumorigenesis of colorectal cancer. Cancer Biol. Ther. 2017;18:237–244. doi: 10.1080/15384047.2017.1294292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colotta F., Allavena P., Sica A., Garlanda C., Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 28.Raposo T.P., Beirão B.C., Pang L.Y., Queiroga F.L., Argyle D.J. Infammation and cancer: Till death tears them apart. Vet. J. 2015;205:161–174. doi: 10.1016/j.tvjl.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Rakoff-Nahoum S. Why cancer and inflammation? Yale J. Biol. Med. 2006;79:123–130. [PMC free article] [PubMed] [Google Scholar]
- 30.Mangino G., Chiantore M.V., Iuliano M., Fiorucci G., Romeo G. Inflammatory microenvironment and human papillomavirus-induced carcinogenesis. Cytokine Growth Factor Rev. 2016;30:103–111. doi: 10.1016/j.cytogfr.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 31.D’lgnazio L., Batie M., Rocha S. Hypoxia and inflammation in cancer, focus on HIF and NF-κB. Biomedicines. 2017;5:21. doi: 10.3390/biomedicines5020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunnumakkara A.B., Sailo B.L., Banik K., Harsha C., Prasad S., Gupta S.C., Bharti A.C., Aggarwal B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018;16:14. doi: 10.1186/s12967-018-1381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell J., Carmody R.J. NF-κB and the transcriptional control of inflammation. In: Loos F., editor. Transcriptional Gene Regulation in Health and Disease. Volume 335. Academic Press; Cambridge, MA, USA: 2018. pp. 41–84. [DOI] [PubMed] [Google Scholar]
- 34.Karin M. The IkappaB kinase—A bridge between inflammation and cancer. Cell Res. 2008;18:334–342. doi: 10.1038/cr.2008.30. [DOI] [PubMed] [Google Scholar]
- 35.Mantovani A. The inflammation-cancer connection. FEBS J. 2018;285:638–640. doi: 10.1111/febs.14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma A., Singh D., Anwar F., Bhatt P.C., Al-Abbasi F., Kumar V. Triterpenoids principle of Wedelia calendulacea attenuated diethynitrosamine-induced hepatocellular carcinoma via down-regulating oxidative stress, inflammation and pathology via NF-kB pathway. Inflammopharmacology. 2018;26:133–146. doi: 10.1007/s10787-017-0350-3. [DOI] [PubMed] [Google Scholar]
- 37.Bromberg J., Wang T.C. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo Y.Q., Xu F., Lu T.H., Duan Z.F., Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 2012;38:904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Chang Y.-C., Su C.-Y., Chen M.-H., Chen W.-S., Chen C.-L., Hsiao M. Secretory RAB GTPase 3C modulates IL6-STAT3 pathway to promote colon cancer metastasis and is associated with poor prognosis. Mol. Cancer. 2017;16:135. doi: 10.1186/s12943-017-0687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sung B., Prasad S., Yadav V.R., Aggarwal B.B. Cancer cell signaling pathways-targeted by spice-derived nutraceuticals. Nutr. Cancer. 2012;64:173–197. doi: 10.1080/01635581.2012.630551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S.J., Yoon S. Activated Rac1 regulates the degradation of IκBα and the nuclear translocation of STAT3-NFκB complexes in starved cancer cells. Exp. Mol. Med. 2016;48:e231. doi: 10.1038/emm.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung S.S., Wu Y., Okobi Q., Adekoya D., Atefi M., Clarke O., Dutta P., Vadgama J.V. Proinflammatory cytokines IL-6 and TNF-α increased telomerase activity through NF-κB/STAT1/STAT3 activation, and Withaferin A inhibited the signaling in colorectal cancer cells. Mediat. Inflamm. 2017;2017:5958429. doi: 10.1155/2017/5958429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanley J., Jua C., Irene K., Gordon W., Mieko T., Bongo S., Ashley V. Interleukin-6, a cytokine critical to mediation of inflammation, autoimmunity and allograft rejection: Therapeutic implications of IL-6 receptor blockade. Transplantation. 2017;101:32–44. doi: 10.1097/TP.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 44.Lee H.A., Koh E.K., Sung J.E., Kim J.E., Song S.H., Kim D.S., Son H.J., Lee C.Y., Lee H.S., Bae C.J., et al. Ethyl acetate extract from Asparagus cochinchinensis exerts anti-inflammatory effects in LPS-stimulated RAW264.7 macrophage cells by regulating COX-2/iNOS, inflammatory cytokine expression, MAP kinase pathways, the cell cycle and anti-oxidant activity. Mol. Med. Rep. 2017;15:1613–1623. doi: 10.3892/mmr.2017.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mirzaei H., Shakeri A., Rashidi B., Jalili A., Banikazemi Z., Sahebkar A. Phytosomal curcumin: A review of pharmacokinetic, experimental and clinical studies. Biomed. Pharmacother. 2017;85:102–112. doi: 10.1016/j.biopha.2016.11.098. [DOI] [PubMed] [Google Scholar]
- 46.Farooqui T., Farooqui A.A. Curcumin for Neurological and Psychiatric Disorders: Neurochemical and Pharmacological Properties. Academic Press; Cambridge, MA, USA: 2019. Chapter 2—Curcumin: Historical background, chemistry, pharmacological action, and potential therapeutic value; pp. 23–44. [Google Scholar]
- 47.Singh V., Pal M., Gupta S., Tiwari S.K., Malkunje L., Das S. Turmeric—A new treatment option for lichen planus: A pilot study. Natl. J. Maxillofac. Surg. 2013;4:198–201. doi: 10.4103/0975-5950.127651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shome S., Talukdar A.D., Choudhury M.D., Bhattacharya M.K., Upadhyaya H. Curcumin as potential therapeutic natural product: A nanobiotechnological perspective. J. Pharm. Pharmacol. 2016;68:1481–1500. doi: 10.1111/jphp.12611. [DOI] [PubMed] [Google Scholar]
- 49.Benzer F., Kandemir F.M., Ozkaraca M., Kucukler S., Caglayan C. Curcumin ameliorates doxorubicin-induced cardiotoxicity by abrogation of inflammation, apoptosis, oxidative DNA damage, and protein oxidation in rats. J. Biochem. Mol. Toxicol. 2018;32:e22030. doi: 10.1002/jbt.22030. [DOI] [PubMed] [Google Scholar]
- 50.Campbell M.S., Fleenor B.S. The emerging role of curcumin for improving vascular dysfunction: A review. Crit. Rev. Food Sci. Nutr. 2017;58:2790–2799. doi: 10.1080/10408398.2017.1341865. [DOI] [PubMed] [Google Scholar]
- 51.Serafini M.M., Catanzaro M., Rosini M., Racchi M., Lanni C. Curcumin in Alzheimer’s disease: Can we think to new strategies and perspectives for this molecule? Pharmacol. Res. 2017;124:146–155. doi: 10.1016/j.phrs.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Shehzad A., Qureshi M., Anwar M.N., Lee Y.S. Multifunctional curcumin mediate multitherapeutic effects. J. Food Sci. 2017;82:2006–2015. doi: 10.1111/1750-3841.13793. [DOI] [PubMed] [Google Scholar]
- 53.Khamis A.A., Sharshar A.H., Mahmoud A.H., Mohamed T.M. The antioxidant activity of curcumin extract against HepG2 cells. Int. J. Adv. Res. Sci. Technol. 2018;7:115–125. [Google Scholar]
- 54.Goel A., Kunnumakkara A.B., Aggarwal B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Byun S.-Y., Kim D.-B., Kim E. Curcumin ameliorates the tumor-enhancing effects of a high-protein diet in an azoxymethane-induced mouse model of colon carcinogenesis. Nutr. Res. 2015;35:726–735. doi: 10.1016/j.nutres.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 56.Hossain D., Bhattacharyya S., Das T., Sa G. Curcumin: The multi-targeted therapy for cancer regression. Front. Biosci. 2011;4:335–355. doi: 10.2741/272. [DOI] [PubMed] [Google Scholar]
- 57.Chiang I., Wang W.-S., Liu H.-C., Yang S.-T., Tang N.-Y., Chung J.-G. Curcumin alters gene expression-associated DNA damage, cell cycle, cell survival and cell migration and invasion in NCI-H460 human lung cancer cells in vitro. Oncol. Rep. 2015;34:1853–1874. doi: 10.3892/or.2015.4159. [DOI] [PubMed] [Google Scholar]
- 58.Momtazi A.A., Shahabipour F., Khatibi S., Johnston T.P., Pirro M., Sahebkar A. Curcumin as a MicroRNA regulator in cancer: A review. Rev. Physiol. Biochem. Pharmacol. 2016;171:1–38. doi: 10.1007/112_2016_3. [DOI] [PubMed] [Google Scholar]
- 59.Mirzaei H., Naseri G., Rezaee R., Mohammadi M., Banikazemi Z., Mirzaei H.R., Salehi H., Peyvandi M., Pawelek J.M., Sahebkar A. Curcumin: A new candidate for melanomatherapy? Int. J. Cancer. 2016;139:1683–1695. doi: 10.1002/ijc.30224. [DOI] [PubMed] [Google Scholar]
- 60.Hoesel B., Schmid J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taniguchi K., Karin M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018;18:309–324. doi: 10.1038/nri.2017.142. [DOI] [PubMed] [Google Scholar]
- 62.Mortezaee K., Salehi E., Mirtavoos-mahyari H., Motevaseli E., Najafi M., Farhood B., Rosengren R.J., Sahebkar A. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J. Cell. Physiol. 2019;234:12537–12550. doi: 10.1002/jcp.28122. [DOI] [PubMed] [Google Scholar]
- 63.Bimonte S., Barbieri A., Leongito M., Piccirillo M., Giudice A., Pivonello C., de Angelis C., Granata V., Palaia R., Izzo F., et al. Curcumin anticancer studies in pancreatic cancer. Nutrients. 2016;8:433. doi: 10.3390/nu8070433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta S.C., Patchva S., Aggarwal B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013;15:195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goradel N.H., Hour F.G., Negahdari B., Malekshahi Z.V., Hashemzehi M., Masoudifar A., Mirzaei H. Stem cell therapy: A new therapeutic option for cardiovascular diseases. J. Cell. Biochem. 2017;119:95–104. doi: 10.1002/jcb.26169. [DOI] [PubMed] [Google Scholar]
- 66.Hashemi Goradel N., Ghiyami-Hoor F., Jahangiri S., Negahdari B., Sahebkar A., Masoudifar A., Mirzaei H. Nanoparticles as new tools for inhibition of cancer angiogenesis. J. Cell. Physiol. 2018;233:2902–2910. doi: 10.1002/jcp.26029. [DOI] [PubMed] [Google Scholar]
- 67.Rupaimoole R., Slack F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 68.Mirzaei H., Masoudifar A., Sahebkar A., Zare N., Sadri Nahand J., Rashidi B., Mehrabian E., Mohammadi M., Mirzaei H.R., Jaafari M.R. MicroRNA: A novel target of curcumin in cancer therapy. J. Cell. Physiol. 2018;233:3004–3015. doi: 10.1002/jcp.26055. [DOI] [PubMed] [Google Scholar]
- 69.Kronski E., Fiori M.E., Barbieri O., Astigiano S., Mirisola V., Killian P.H., Bruno A., Pagani A., Rovera F., Pfeffer U., et al. MiR181b is induced by the chemopreventive polyphenol curcumin and inhibits breast cancer metastasis via down-regulation of the inflammatory cytokines CXCL1 and -2. Mol. Oncol. 2014;8:581–595. doi: 10.1016/j.molonc.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jin H., Qiao F., Wang Y., Xu Y., Shang Y. Curcumin inhibits cell proliferation and induces apoptosis of human non-small cell lung cancer cells through the upregulation of miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol. Rep. 2015;34:2782–2789. doi: 10.3892/or.2015.4258. [DOI] [PubMed] [Google Scholar]
- 71.Schwertheim S., Wein F., Lennartz K., Worm K., Schmid K.W., Sheu-Grabellus S.Y. Curcumin induces G2/M arrest, apoptosis, NF-kappaB inhibition, and expression of differentiation genes in thyroid carcinoma cells. J. Cancer Res. Clin. Oncol. 2017;143:1143–1154. doi: 10.1007/s00432-017-2380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheng A.L., Hsu C.H., Lin J.K., Hsu M.M., Ho Y.F., Shen T.S., Ko J.Y., Lin J.T., Lin B.R., Ming-Shiang W., et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 73.Kang Y., Hu W., Bai E., Zheng H., Liu Z., Wu J., Jin R., Zhao C., Liang G. Curcumin sensitizes human gastric cancer cells to 5-fluorouracil through inhibition of the NFκB survival-signaling pathway. Onco Targets Ther. 2016;9:7373–7384. doi: 10.2147/OTT.S118272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yue Q., Gao G., Zou G., Yu H., Zheng X. Natural products as adjunctive treatment for pancreatic cancer: Recent trends and advancements. BioMed Res. Int. 2017;2017:8412508. doi: 10.1155/2017/8412508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tannock I.F., de Wit R., Berry W.R., Horti J., Pluzanska A., Chi K.N., Oudard S., Théodore C., James N.D., Turesson N.D., et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 76.Gu Z., Wang Q., Shi Y., Huang Y., Zhang J., Zhang X., Lin G. Nanotechnology-mediated immunochemotherapy combined with docetaxel and PD-L1 antibody increase therapeutic effects and decrease systemic toxicity. J. Control. Release. 2018;286:369–380. doi: 10.1016/j.jconrel.2018.08.011. [DOI] [PubMed] [Google Scholar]
- 77.Banerjee S., Singh S.K., Chowdhury I., Lillard J.W., Jr., Singh R. Combinatorial effect of curcumin with docetaxel modulates apoptotic and cell survival molecules in prostate cancer. Front. Biosci. 2017;9:235–245. doi: 10.2741/e798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Christofides E.A. Practical insights into improving adherence to metformin therapy in patients with type 2 diabetes mellitus. Clin. Diabetes. 2019;37 doi: 10.2337/cd18-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Donadon V., Balbi M., Mas M.D., Casarin P., Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750–758. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 80.Zaidi S., Gandhi J., Joshi G., Smith N.L., Khan S.A. The anticancer potential of metformin on prostate cancer. Prostate Cancer Prostatic Dis. 2019 doi: 10.1038/s41391-018-0085-2. [DOI] [PubMed] [Google Scholar]
- 81.Hadad S.M., Coates P., Jordan L.B., Dowling R.J., Chang M.C., Done S.J., Purdie C.A., Goodwin P.J., Stambolic V., Moulder-Thompson S., et al. Evidence for biological effects of metformin in operable breast cancer: Biomarker analysis in a preoperative window of opportunity randomized trial. Breast Cancer Res. Treat. 2015;150:149–155. doi: 10.1007/s10549-015-3307-5. [DOI] [PubMed] [Google Scholar]
- 82.Zhang H.-H., Zhang Y., Cheng Y.-N., Gong F.-L., Cao Z.-Q., Yu L.-G., Guo X.-L. Metformin incombination with curcumin inhibits the growth, metastasis, and angiogenesis of hepatocellular carcinoma in vitro and in vivo. Mol. Carcinog. 2018;57:44–56. doi: 10.1002/mc.22718. [DOI] [PubMed] [Google Scholar]
- 83.Xu W., Kuang M., Gong Y., Cao C., Chen J., Tang C. Survival benefit and safety of the combinations of FOLFOXIRI ± bevacizumab versus the combinations of FOLFIRI ± bevacizumab as first-line treatment for unresectable metastatic colorectal cancer: A meta-analysis. Onco Targets Ther. 2016;9:4833–4842. doi: 10.2147/OTT.S104981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Heydari K., Saidijam M., Sharifi M.R., Dermani F.K., Soleimani Asl S., Shabab N., Najafi R. The effect of miR-200c inhibition on chemosensitivity (5- FluoroUracil) in colorectal cancer. Pathol. Oncol. Res. 2018;24:145–151. doi: 10.1007/s12253-017-0222-6. [DOI] [PubMed] [Google Scholar]
- 85.Meng X., Fu R. miR-206 regulates 5-FU resistance by targeting Bcl-2 in colon cancer cells. Onco Targets Ther. 2018;11:1757–1765. doi: 10.2147/OTT.S159093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shakibaei M., Buhrmann C., Kraehe P., Shayan P., Lueders C., Goel A. Curcumin chemosensitizes 5-fluorouracil resistant MMR-deficient human colon cancer cells in high density cultures. PLoS ONE. 2014;9:e85397. doi: 10.1371/journal.pone.0085397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou X., Wang W., Li P., Zheng Z., Tu Y., Zhang Y., You T. Curcumin enhances the effects of 5-fluorouracil and oxaliplatin in inducing gastric cancer cell apoptosis both in vitro and in vivo. Oncol. Res. 2016;23:29–34. doi: 10.3727/096504015X14452563486011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Toden S., Okugawa Y., Jascur T., Wodarz D., Komarova N.L., Buhrmann C., Shakibaei M., Boland C.R., Goel A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis. 2015;36:355–367. doi: 10.1093/carcin/bgv006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vinod B.S., Antony J., Nair H.H., Puliyappadamba V.T., Saikia M., Narayanan S.S., Bevin A., Anto R.J. Mechanistic evaluation of the signaling events regulating curcumin-mediated chemosensitization of breast cancer cells to 5-fluorouracil. Cell Death Dis. 2013;4:e505. doi: 10.1038/cddis.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang H., Huang S., Wei Y., Cao S., Pi C., Feng T., Liang J., Zhao L., Ren G. Curcumin enhances the anticancer effect of 5-fluorouracil against gastric cancer through down-regulation of COX-2 and NF-κB signaling pathways. J. Cancer. 2017;8:3697–3706. doi: 10.7150/jca.20196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zong L., Cheng G., Liu S., Pi Z., Liu Z., Song F. Reversal of multidrug resistance in breast cancer cells by a combination of ursolic acid with doxorubicin. J. Pharm. Biomed. Anal. 2019;165:268–275. doi: 10.1016/j.jpba.2018.11.057. [DOI] [PubMed] [Google Scholar]
- 92.Abouzeid A.H., Pate N.R., Rachman I.M., Senn S., Torchilin V.P. Anti-cancer activity of anti-GLUT1 antibody-targeted polymeric micelles co-loaded with curcumin and doxorubicin. J. Drug Target. 2013;21:994–1000. doi: 10.3109/1061186X.2013.840639. [DOI] [PubMed] [Google Scholar]
- 93.Wang B.L., Shen Y.M., Zhang Q.W., Li Y.L., Luo M., Liu Z., Li Y., Qian Z.Y., Gao X., Shi H.S. Codelivery of curcumin and doxorubicin by MPEG-PCL results in improved efficacy of systemically administered chemotherapy in mice with lung cancer. Int. J. Nanomed. 2013;8:3521–3531. doi: 10.2147/IJN.S45250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duan J., Mansour H., Zhang Y., Deng X., Chen Y., Wang J., Pan Y., Zhao J. Reversion of multidrug resistance by co-encapsulation of doxorubicin and curcumin in chitosan/poly(butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2012;426:193–201. doi: 10.1016/j.ijpharm.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 95.Guorgui J., Wang R., Mattheolabakis G., Mackenzie G.G. Curcumin formulated in solid lipid nanoparticles has enhanced efficacy in Hodgkin’s lymphoma in mice. Arch. Biochem. Biophys. 2018;648:12–19. doi: 10.1016/j.abb.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 96.Ma W., Wang J., Guo Q., Tu P. Simultaneous determination of doxorubicin and curcumin in rat plasma by LC–MS/MS and its application to pharmacokinetic study. J. Pharm. Biomed. Anal. 2015;111:215–221. doi: 10.1016/j.jpba.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 97.Kaufman D.S. Challenges in the treatment of bladder cancer. Ann. Oncol. 2006;17:v106–v112. doi: 10.1093/annonc/mdj963. [DOI] [PubMed] [Google Scholar]
- 98.Yoon C.Y., Park M.J., Lee J.S., Lee S.C., Oh J.J., Park H., Chung C.W., Abdullajanov M.M., Jeong S.J., Hing S.K., et al. The histone deacetylase inhibitor trichostatin A synergistically resensitizes a cisplatin resistant human bladder cancer cell line. J. Urol. 2011;185:1102–1111. doi: 10.1016/j.juro.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 99.Kumar B., Yadav A., Hideg K., Kuppusamy P., Teknos T.N., Kumar P. A novel curcumin analog (H-4073) enhances the therapeutic efficacy of cisplatin treatment in head and neck cancer. PLoS ONE. 2014;9:e93208. doi: 10.1371/journal.pone.0093208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Siddik Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 101.Park B.H., Lim J.E., Jeon H.G., Seo S., II, Lee H.M., Choi H.Y., Jeon S.S., Jeong B.C. Curcumin potentiates antitumor activity of cisplatin in bladder cancer cell lines via ROS-mediated activation of ERK1/2. Oncotarget. 2016;7:63870–63886. doi: 10.18632/oncotarget.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu X., Shen H., Yin X., Long L., Xie C., Liu Y., Hui L., Lin X., Fang Y., Cao Y., et al. MiR-186 regulation of Twist1 and ovarian cancer sensitivity to cisplatin. Oncogene. 2016;35:323–332. doi: 10.1038/onc.2015.84. [DOI] [PubMed] [Google Scholar]
- 103.Kim J., Hong S.-W., Kim S., Kim D., Hur D.Y., Jin D.H., Kim B., Kim Y.S. Cyclooxygenase-2 expression is induced by celecoxib treatment in lung cancer cells and is transferred to neighbor cells via exosomes. Int. J. Oncol. 2018;52:613–620. doi: 10.3892/ijo.2017.4227. [DOI] [PubMed] [Google Scholar]
- 104.Solomon S.D., McMurray J.J., Pfeffer M.A., Wittes J., Fowler R., Finn P., Anderson W.F., Zauber A., Hawk E., Bertagnolli M., et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N. Engl. J. Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 105.Lev-Ari S., Strier L., Kazanov D., Madar-Shapiro L., Dvory-Sobol H., Pinchuk I., Marian B., Lichtenberg D., Arber N. Celecoxib and curcumin synergistically inhibit the growth of colorectal cancer cells. Clin. Cancer Res. 2005;11:6738–6744. doi: 10.1158/1078-0432.CCR-05-0171. [DOI] [PubMed] [Google Scholar]
- 106.Waghela B.N., Sharma A., Dhumale S., Pandey S.M., Pathak C. Curcumin conjugated with PLGA potentiates sustainability, anti-proliferative activity and apoptosis in human colon carcinoma cells. PLoS ONE. 2015;10:e0117526. doi: 10.1371/journal.pone.0117526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shankar T.B., Shantha N.V., Ramesh H.P., Murthy I.A., Murthy V.S. Toxicity studies on turmeric (Curcuma longa): Acute toxicity studies in rats, guineapigs and monkeys. Indian J. Exp. Biol. 1980;18:73–75. [PubMed] [Google Scholar]
- 108.Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bayet-Robert M., Kwiatowski F., Leheurteur M., Gachon F., Planchat E., Abrial C., Mouret-Reynier M.A., Durando X., Barthomeuf C., Chollet P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010;9:8–14. doi: 10.4161/cbt.9.1.10392. [DOI] [PubMed] [Google Scholar]
- 110.Epelbaum R., Schaffer M., Vizel B., Badmaev V., Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr. Cancer. 2010;62:1137–1141. doi: 10.1080/01635581.2010.513802. [DOI] [PubMed] [Google Scholar]
- 111.Ghalaut V.S., Sangwan L., Dahiya K., Ghalaut P.S., Dhankhar R., Saharan R. Effect of imatinib therapy with and without turmeric powder on nitric oxide levels in chronic myeloid leukemia. J. Oncol. Pharm. Pract. 2012;18:186–190. doi: 10.1177/1078155211416530. [DOI] [PubMed] [Google Scholar]
- 112.Kanai M., Yoshimura K., Asada M., Imaizumi A., Suzuki C., Matsumoto S., Nishimura T., Mori Y., Masui T., Kawaguchi Y., et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011;68:157–164. doi: 10.1007/s00280-010-1470-2. [DOI] [PubMed] [Google Scholar]
- 113.James M.I., Iwuji C., Irving G., Karmokar A., Higgins J.A., Griffin-Teal N., Thomas A., Greaves P., Cai H., Patel S.R., et al. Curcumin inhibits cancer stem cell phenotypes in ex vivo models of colorectal liver metastases, and is clinically safe and tolerable in combination with FOLFOX chemotherapy. Cancer Lett. 2015;364:135–141. doi: 10.1016/j.canlet.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pastorelli D., Fabricio A.S.C., Giovanis P., D’lppolito S., Fiduccia P., Soldà C., Buda A., Sperti C., Bardini R., Da Dalt G., et al. Phytosome complex of curcumin as complementary therapy of advanced pancreatic cancer improves safety and efficacy of gemcitabine: Results of a prospective phase II trial. Pharmacol. Res. 2018;132:72–79. doi: 10.1016/j.phrs.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 115.Desai P., Ann D., Wang J., Prabhu S. Pancreatic cancer: Recent advances in nanoformulation-based therapies. Crit. Rev. Ther. Drug Carr. Syst. 2019;36:59–91. doi: 10.1615/CritRevTherDrugCarrierSyst.2018025459. [DOI] [PubMed] [Google Scholar]
- 116.Duan X., Chan C., Lin W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew. Chem. Int. Ed. Engl. 2019;58:670–680. doi: 10.1002/anie.201804882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hu B., Liu X., Zhang C., Zeng X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017;25:3–15. doi: 10.1016/j.jfda.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rachmawati H., Pradana A.T., Safitri D., Adnyana I.K. Multiple functions of dalpha-tocopherol polyethylene glycol 1000 succinate (TPGS) as curcumin nanoparticle stabilizer: In vivo kinetic profile and anti-ulcerative colitis analysis in animal model. Pharmaceutics. 2017;9:24. doi: 10.3390/pharmaceutics9030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rachmawati H., Safitri D., Pradana A.T., Adnyana I.K. TPGS-stabilized curcumin nanoparticles exhibit superior effect on carrageenan-induced inflammation in wistar rat. Pharmaceutics. 2016;8:24. doi: 10.3390/pharmaceutics8030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Esmatabadi M.J.D., Motamedrad M., Sadeghizadeh M. Down-regulation of lncRNA, GAS5 decreases chemotherapeutic effect of dendrosomal curcumin (DNC) in breast cancer cells. Phytomedicine. 2018;42:56–65. doi: 10.1016/j.phymed.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 121.Zhang H., Guo Y., Song Y., Shang C. Long noncoding RNA GAS5 inhibits malignant proliferation and chemotherapy resistance to doxorubicin in bladder transitional cell carcinoma. Cancer Chemother. Pharmacol. 2017;79:49–55. doi: 10.1007/s00280-016-3194-4. [DOI] [PubMed] [Google Scholar]
- 122.Greil R., Greil-Ressler S., Weiss L., Schönlieb C., Magnes T., Radl B., Bolder G.T., Vcelar B., Sordillo P.P. A phase 1 dose-escalation study on the safety, tolerability and activity of liposomal curcumin (Lipocurc™) in patients with locally advanced or metastatic cancer. Cancer Chemother. Pharmacol. 2018;82:695–706. doi: 10.1007/s00280-018-3654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma W., Guo Q., Li Y., Wang X., Wang J., Tu P. Co-assembly of doxorubicin and curcumin targeted micelles for synergistic delivery and improving anti-tumor efficacy. Eur. J. Pharm. Biopharm. 2017;112:209–223. doi: 10.1016/j.ejpb.2016.11.033. [DOI] [PubMed] [Google Scholar]
- 124.Li P.-Y., Lai P.-S., Hung W.-C., Syu W.-J. Poly (l-lactide)-vitamin E TPGS nanoparticles enhanced the cytotoxicity of doxorubicin in drug-resistant MCF-7 breast cancer cells. Biomacromolecules. 2010;11:2576–2582. doi: 10.1021/bm1005195. [DOI] [PubMed] [Google Scholar]
- 125.Xiao B., Si X., Han M.K., Viennois E., Zhang M., Merlin D. Co-delivery of camptothecin and curcumin by cationic polymeric nanoparticles for synergistic colon cancer combination chemotherapy. J. Mater. Chem. B Mater. Biol. Med. 2015;3:7724–7733. doi: 10.1039/C5TB01245G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nguyen H.T., Phung C.D., Thapa R.K., Pham T.T., Tran T.H., Jeong J.H., Ku S.K., Choi H.G., Yong C.S., Kim J.O. Multifunctional nanoparticles as somatostatin receptor-targeting delivery system of polyaniline and methotrexate for combined chemo-photothermal therapy. Acta Biomater. 2018;68:154–167. doi: 10.1016/j.actbio.2017.12.033. [DOI] [PubMed] [Google Scholar]
- 127.Wu K.-F., Liang W.-C., Feng L., Pang J.X., Waye M.M., Zhang J.F., Fu W.M. H19 mediates methotrexate resistance in colorectal cancer through activating Wnt/β-catenin pathway. Exp. Cell Res. 2017;350:312–317. doi: 10.1016/j.yexcr.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 128.Rahimi M., Safa K.D., Salehi R. Co-delivery of doxorubicin and methotrexate by dendritic chitosan-g-mPEG as a magnetic nanocarrier for multi-drug delivery in combination chemotherapy. Polym. Chem. 2017;8:7333–7350. doi: 10.1039/C7PY01701D. [DOI] [Google Scholar]
- 129.Zhao X., Chen Q.W., Liu W., Li Y., Tang H., Liu X., Yang X. Codelivery of doxorubicin and curcumin with lipid nanoparticles results in improved efficacy of chemotherapy in liver cancer. Int. J. Nanomed. 2015;10:257–270. doi: 10.2147/IJN.S73322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ruttala H.B., Ko Y.T. Liposomal co-delivery of curcumin and albumin/paclitaxel nanoparticle for enhanced synergistic antitumor efficacy. Colloids Surf. B Biointerfaces. 2015;128:419–426. doi: 10.1016/j.colsurfb.2015.02.040. [DOI] [PubMed] [Google Scholar]
- 131.Cui T., Zhang S., Sun H. Co-delivery of doxorubicin and pH-sensitive curcumin prodrug by transferrin-targeted nanoparticles for breast cancer treatment. Oncol. Rep. 2017;37:1253–1260. doi: 10.3892/or.2017.5345. [DOI] [PubMed] [Google Scholar]
- 132.Sesarman A., Tefas L., Sylvester B., Licarete E., Rauca V., Luput L., Patras L., Banciu M., Porfire A. Anti-angiogenic and anti-inflammatory effects of long-circulating liposomes co-encapsulating curcumin and doxorubicin on C26 murine colon cancer cells. Pharmacol. Rep. 2018;70:331–339. doi: 10.1016/j.pharep.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 133.Motevalli S.M., Eltahan A.S., Liu L., Magrini A., Rosato N., Guo W., Bottini M., Liang X.-J. Co-encapsulation of curcumin and doxorubicin in albumin nanoparticles blocks the adaptive treatment tolerance of cancer cells. Biophys. Rep. 2019;5:19–30. doi: 10.1007/s41048-018-0079-6. [DOI] [Google Scholar]
- 134.Ni W., Li Z., Liu Z., Ji Y., Wu L., Sun S., Jian X., Gao X. Dual-targeting nanoparticles: Codelivery of curcumin and 5-fluorouracil for synergistic treatment of hepatocarcinoma. J. Pharm. Sci. 2019;108:1284–1295. doi: 10.1016/j.xphs.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 135.Khan S., Setua S., Kumari S., Dan N., Massey A., Hafeez B.B., Yallapu M.M., Stiles Z.E., Alabkaa A., Yue J., et al. Superparamagnetic iron oxide nanoparticles of curcumin enhance gemcitabine therapeutic response in pancreatic cancer. Biomaterials. 2019;208:83–97. doi: 10.1016/j.biomaterials.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 136.Zhao X., Chen Q., Li Y., Tang H., Liu W., Yang X. Doxorubicin and curcumin co-delivery by lipid nanoparticles for enhanced treatment of diethylnitrosamine-induced hepatocellular carcinoma in mice. Eur. J. Pharm. Biopharm. 2015;93:27–36. doi: 10.1016/j.ejpb.2015.03.003. [DOI] [PubMed] [Google Scholar]