Abstract
Simple Summary
Chinese indigenous cattle are economically important cattle breeds for animal husbandry development. The promotion and development of Chinese cattle breeds is essential. A previous study found that a nonsense mutation (rs378652941, c.483C > A, p.Cys161X) in bovine transmembrane protein 95 gene (TMEM95) seriously reduced reproductive performance in male Fleckvieh cattle; therefore, this locus was considered a candidate genetic marker in bovine marker-assisted selection (MAS) breeding. Until now, no study has identified this mutation in Chinese cattle breeds. Herein, we detected this c.483C > A mutation in 13 Chinese cattle breeds. Importantly, we found that this mutation did not exist at this locus in our analyzed breeds. Interestingly, we first identified a frameshift insertion/deletion (indel) mutation (NC_037346.1: g.27056998_27057000delCT) in the bovine TMEM95 gene in 11 cattle breeds. Together, the results of this study suggest that the mutation c.483C > A cannot serve as a genetic marker for molecular breeding among Chinese indigenous cattle breeds.
Abstract
Chinese indigenous cattle breeds have abundant genetic resources, which are valuable for the molecular breeding of cattle around the world. Thus, identifying important candidate genes and their genetic markers is very important for cattle molecular breeding. A previous study found that a nonsense mutation (rs378652941, c.483C > A, p.Cys161X) in the bovine transmembrane protein 95 gene (TMEM95) seriously reduced the reproductive performance in bulls, but few studies have detected this mutation in Chinese indigenous cattle breeds. Since the mutation c.483C > A may serve as a potential genetic marker for selecting higher fertility bulls, in the present study, using tetra-primer amplification refractory mutation system PCR (T-ARMS-PCR), forced PCR restriction fragment length polymorphism (forced PCR-RFLP), and DNA sequencing methods, the mutation c.483C > A was detected in 765 individuals from 13 Chinese cattle breeds. However, the results showed that this mutation did not exist at this locus in our analyzed breeds. Interestingly, we identified a newly frameshift insertion/deletion (indel) mutation (NC_037346.1: g.27056998_27057000delCT) in the bovine TMEM95 gene in 11 cattle breeds, which changed the location of the termination codon and changed the 16 amino acids in the C-terminal to 21 amino acids. Combined with previous studies, our study provides evidence that in Chinese cattle breeds the mutation c.483C > A cannot be used as a genetic marker in molecular breeding.
Keywords: Chinese indigenous cattle, molecular breeding, transmembrane protein 95 gene (TMEM95), male reproductive performance
1. Introduction
Cattle are among the most important domestic animals. China, vast in size and diverse in topography, has abundant cattle breeding resources. East Asian cattle are mainly composed of three distinct ancestries: East Asian taurine ancestry, Eurasian taurine ancestry, and Chinese indicine ancestry [1]. The earlier East Asian taurine ancestry reached China at least 3.9 thousand years ago [1]. China’s abundant cattle breeding resources are helpful for breeding new species or improving the reproductive performance of cattle around the world. Traditional crossbreeding depends on appearance identification and phenotypic selection, which are costly and time-consuming [2]. Nowadays, molecular breeding has brought about great changes in the field of animal breeding, such as marker-assisted selection (MAS) breeding [3]. MAS breeding is based on the functional genes or molecular markers, thus identifying genes or genetic variations is essential to the breeding of cattle [3].
Artificial insemination (AI) is a routine breeding technique in cattle production. The application of AI in cattle began at the beginning of the 20th century, and improves the breeding efficiency of bulls [4,5]. In order to improve production efficiency, only bulls with excellent reproduction performance can be used as sires to provide high-quality semen. However, the success rate of AI varies from sire to sire [6]. This phenomenon suggests that there are some factors that affect fertilization rates without affecting sperm quality [7]. In 2014, Pausch et al. found a nonsense mutation (rs378652941, c.483C > A, p.Cys161X) in cattle transmembrane protein 95 gene (TMEM95) gene was associated with idiopathic male subfertility in Fleckvieh cattle, but did not affect semen quality [7]. Subfertility refers to any form of reduced fertility, which causes enormous economic losses in animal husbandry [7,8]. Bovine TMEM95 consists of 183 amino acids, encoding a quite conserved single-pass type I transmembrane protein (1 to 16 form a predicted extracellular signal peptide; 17 to 152 form the extracellular sequence; 153 to 175 form the transmembrane domain; 176 to 183 form the intracellular C-terminal domain) [7]. c.483C > A was identified in the transmembrane domain, introducing a premature stop-codon in TMEM95 (p.Cys161X), which truncates 22 amino acids of the TMEM95 C-terminal sequence [7]. A functional study found that TMEM95 protein localizes on the acrosomal membrane of the sperm head, and c.483C > A most likely results in disturbed anchorage of the truncated protein in the sperm plasma membrane bilayer [7]. Recently, Fernandez-Fuertes et al. revealed that c.483C > A reduced fertility by influencing the interaction with the oocyte vestments [9,10,11]. These studies indicated that c.483C > A is a deleterious mutation that should be identified and eliminated as early as possible in cattle.
Until now, no study has detected the abovementioned nonsense mutation (c.483C > A) in Chinese cattle. Considering the genetic effect of bovine TMEM95 gene mutation on male reproductive performance, testing and verifying the candidate molecular genetic markers in Chinese cattle breeds is beneficial and necessary. Herein, a total of 765 individuals from 13 Chinese representative cattle breeds were selected for this study, so as to lay the foundation for assessing the genetic variations of the bovine TMEM95 gene and evaluating the possibility of applying the genetic markers in cattle MAS breeding.
2. Materials and Methods
All animal experiments in the present study were performed in conformity to the applicable guidelines, animal welfare laws, and policies by the Ministry of Science and Technology of the People’s Republic of China (Approval Number 2006-398). Moreover, the experiments were also approved by Northwest A&F University (NWAFU), Shaanxi, P.R. China.
2.1. Animal Collection and Genomic DNA Isolation
A total of 765 cattle samples from 13 Chinese indigenous cattle breeds were analyzed: Red Steppe cattle (n = 135, Jilin Province), Qinchuan cattle (n = 60, Shaanxi Province), Nanyang cattle (n = 60, Henan Province), Jinnan cattle (n = 60, Shanxi Province), Luxi cattle (n = 30, Shandong Province), Xia’nan cattle (n = 60, Henan Province), Jiaxian Red cattle (n = 60, Henan Province), Pi’nan cattle (n = 60, Henan Province), Jinjiang cattle (n = 60, Jiangxi Province), De’nan cattle (n = 30, Henan Province), Yunling cattle (n = 60, Yunnan Province), Zaosheng cattle (n = 30, Gansu Province), and Bohai Black cattle (n = 60, Shandong Province).
All selected individuals were unrelated and healthy. By means of the phenol-chloroform method, the genomic DNA was isolated from ear tissue [12,13]. The quality of genomic DNA sample was assayed by a NanoDrop 2000 Spectrophotometer (Thermo Scientific, Waltham, MA, USA). DNA samples were then diluted to the identical standard of 50 ng/µL and stored at −20 °C [12,13].
2.2. Primer Design and Genotyping by T-ARMS-PCR and Forced PCR-RFLP Methods
A previous study found a potential nonsense mutation (rs378652941, c.483C > A, p.Cys161X) in bovine TMEM95, which introduced a premature stop-codon in the TMEM95 gene [7]. Based on the GenBank sequence of the bovine TMEM95 gene (NC_037346.1), the primers of tetra-primer amplification refractory mutation system PCR (T-ARMS-PCR), which include two allele-specific inner primers and a pair of outer primers, were designed using the Primer1 website (http://primer1.soton.ac.uk/primer1.html) [14,15]. The inner primer contained a second deliberate mismatch at position 2 at the 3′ end. The principle of the T-ARMS-PCR method is that Taq DNA polymerase lacks the 3′ → 5′ exonuclease activity, and if the primer’s 3′ end is mismatched then its amplification rate is slower. With the use of specific inner and outer primers at the exact proportion and amplification conditions, different alleles of the locus are amplified. The amplification product, which includes two homozygous and three heterozygous bands, is detected by gel electrophoresis [16]. The primers of forced PCR restriction fragment length polymorphism (forced PCR-RFLP) and the primers used for PCR amplification and sequencing were designed with Primer Premier software (version 5.0) (PREMIER Biosoft International, Palo Alto, CA) (Table 1). The forced PCR-RFLP was based on the PCR-RFLP method. Because the mutation locus could not be recognized by commonly used restriction enzymes, we designed a primer to change a base near the mutation site to induce a restriction enzyme recognition sequence. Then the mutation could be genotyped according to the bands of the PCR products digested by restriction enzymes [16].
Table 1.
The primers for bovine TMEM95 c.483C > A mutation identification.
| Methods | Primers (5′-3′) | a Regions | Genotype Pattern (bp) |
|---|---|---|---|
| T-ARMS-PCR | F(outer): CCTCACCCCCACCCAGATCTCTGAGCTC (1731-1758) | Intron 5 to Exon 6 | Product of outer primer = 312 CC = 312 + 196; CA = 312 + 196 + 168; AA = 312 + 168. |
| R(outer): ACCTGAGGGAAAACAGAGGGTGGGAGGC (2015-2042) | |||
| F(inner A): CTCGGATCCTGCTCCTCTTTGTGCGC (1847-1872) | |||
| R(inner C): GGGACACCCAGGAGCAGGGCAGTTTCT (1872-1898) | |||
| Forced PCR-RFLP | F: AAGCTCGGATCCTGCTCCTCTTTGTGCG (1844-1869) | Exon 6 |
Hha I (GCG↓C) AA = 253; AC = 253 + 228 + 25; CC = 228 + 25. |
| R: GGCTAGGCTCTGTCCTCGTTT (2076-2096) | |||
| Sequencing | F: GTGAGTAAGAAAGGGAAGGGGTCG (1498-1519) | Exon 4 to Exon 6 | 765 |
| R: ACCATCTGACACTGGGACTA (2243-2262) |
Note: The c.483C > A mutation was identified on the 1872nd nucleic acid of bovine TMEM95 gene (NCBI Reference Sequence: NC_037346.1). a The regions relative to the bovine TMEM95 gene mRNA sequence (XM_010815969.3). T-ARMS-PCR: tetra-primer amplification refractory mutation system PCR; PCR-RFLP: PCR restriction fragment length polymorphism.
The PCR reaction was carried out in a 13 μL reaction volume, and the PCR programs was the same as in our previous studies [17]. Using 3.0% agarose gel, the T-ARMS-PCR product and forced PCR-RFLP product were examined by electrophoresis. Using the T-ARMS-PCR program, the length of the amplified fragments would be 312 bp (outer)/196 bp (C)/168 bp (A). The product lengths of the forced PCR-RFLP amplification digested by Hha I endonuclease were 228 bp and 25 bp for the CC genotype; 253 bp, 228 bp, and 25 bp bands for genotype AC; genotype AA showed one band of 253 bp. The genotypes of individuals were classified by analyzing the electrophoresis images.
2.3. Genomic DNA Pool Construction and DNA Sequencing
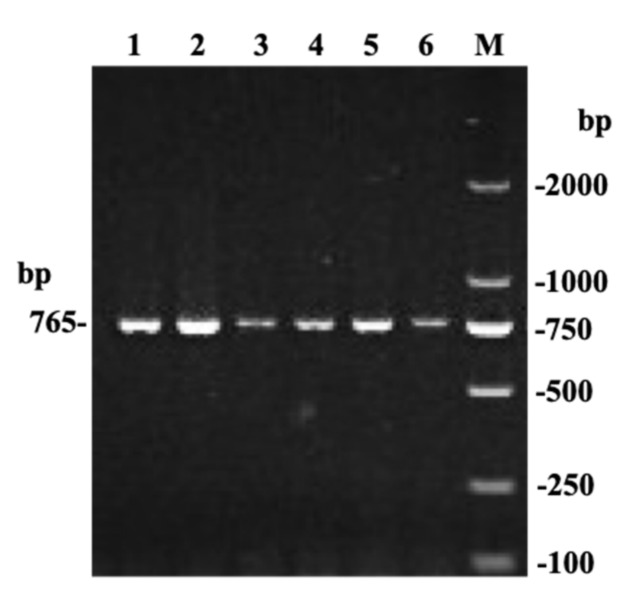
This study also verified the genotypes by sequencing; the PCR product for sequencing is 765 bp in length. Red Steppe cattle were sequenced one by one. Then, we constructed 21 DNA pools in the analyzed cattle breeds (for the following breeds, two DNA pools were constructed for each breed: Pi’nan cattle, Nanyang cattle, Jiaxian Red cattle, Xia’nan cattle, Jinnan cattle, Bohai Black cattle, Qinchuan cattle, Yunling cattle, and Jinjiang cattle; for the following breeds, one DNA pool was constructed for each breed: De’nan cattle, Zaosheng cattle, and Luxi cattle). In each DNA pool, 30 DNA samples were randomly selected, then the DNA pools or DNA were used for amplifications and sequencing. The PCR reaction was performed in a 25 μL volume, and touchdown PCR reaction was implemented according to six steps, as follows [18,19]: step 1: initial denaturation at 95 °C (5 min); step 2: 17 cycles of denaturation at 94 °C (30 s); step 3: annealing at 68 °C (30 s), with a decrease of 1 °C per cycle; step 4: extension for 1000 bp/min at 72 °C; step 5: another 30 cycles at 94 °C (30 s), at 51 °C (30 s), and at 72 °C (1000 bp/min); step 6: a final extension at 72 °C (5 min). When the PCR amplification product was the objective band, it was sequenced to screen the target c.483C > A locus (Figure 1).
Figure 1.
Agarose gel electrophoresis pattern of the bovine TMEM95 gene.
Note: 765 bp bands represent the target sequencing products, (Lane Marker) DNA Marker D2000; Lanes 1–6 are the products of six DNA pools among 13 Chinese indigenous cattle breeds.
3. Results
3.1. SNP Identification and Genotyping of the Bovine TMEM95 Gene by T-ARMS-PCR and Forced PCR-RFLP
In a previous study [7], the nonsense mutation (rs378652941, c.483C > A, p.Cys161X) was identified in the male Fleckvieh cattle TMEM95 gene. In this study, we failed to detect the different genotypes of this locus via the T-ARMS-PCR and forced PCR-RFLP methods.
3.2. Sequencing Results of the TMEM95 Gene in 13 Chinese Indigenous Cattle Breeds
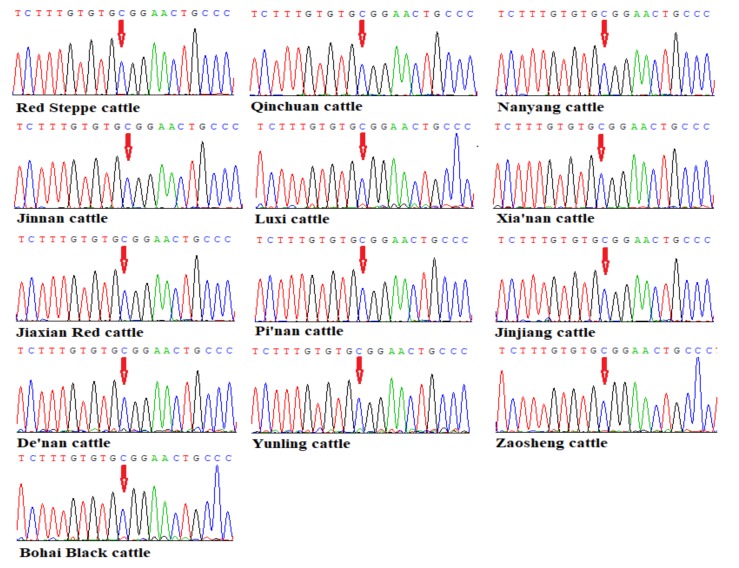
We further used the DNA sequencing method to identify the genotypes of this locus [20]. First, we sequenced the Red Steppe cattle PCR products one by one, but no mutation was found in the c.483C > A locus. Then, we constructed genomic DNA pools to identify the mutation in the other cattle breeds. The sequencing results showed that there was no mutation in our analyzed 13 Chinese cattle breeds in the c.483C > A locus, and all the individuals were CC genotype (Table 2 and Figure 2).
Table 2.
Genotypic and allelic frequencies of bovine TMEM95 c.483C > A (p.Cys161X) mutation.in 13 Chinese indigenous cattle breeds.
| Breeds | Total (n) | Allelic Frequency (%) | Genotypic Frequency (%) | |||
|---|---|---|---|---|---|---|
| Cys | X | Cys/Cys | Cys/X | X/X | ||
| Red Steppe cattle | 135 | 270 (100) | 0 (0) | 135 (100) | 0 (0) | 0 (0) |
| Qinchuan cattle | 60 | 120 (100) | 0 (0) | 60 (100) | 0 (0) | 0 (0) |
| Nanyang cattle | 60 | 120 (100) | 0 (0) | 60 (100) | 0 (0) | 0 (0) |
| Jinnan cattle | 60 | 120 (100) | 0 (0) | 60 (100) | 0 (0) | 0 (0) |
| Luxi cattle | 30 | 60 (100) | 0 (0) | 30 (100) | 0 (0) | 0 (0) |
| Xia’nan cattle | 60 | 120 (100) | 0 (0) | 60 (100) | 0 (0) | 0 (0) |
| Jiaxian Red cattle | 60 | 120 (100) | 0 (0) | 60 (100) | 0 (0) | 0 (0) |
| Pi’nan cattle | 60 | 120 (100) | 0 (0) | 60 (100) | 0 (0) | 0 (0) |
| Jinjiang cattle | 60 | 120 (100) | 0 (0) | 60 (100) | 0 (0) | 0 (0) |
| De’nan cattle | 30 | 60 (100) | 0 (0) | 30 (100) | 0 (0) | 0 (0) |
| Yunling cattle | 60 | 120 (100) | 0 (0) | 60 (100) | 0 (0) | 0 (0) |
| Zaosheng cattle | 30 | 60 (100) | 0 (0) | 30 (100) | 0 (0) | 0 (0) |
| Bohai Black cattle | 60 | 120 (100) | 0 (0) | 60 (100) | 0 (0) | 0 (0) |
| Total | 765 | 1530 (100) | 0 (0) | 765 (100) | 0 (0) | 0 (0) |
Figure 2.
The sequencing results of bovine TMEM95 c.483C > A mutation in 13 different cattle breeds.
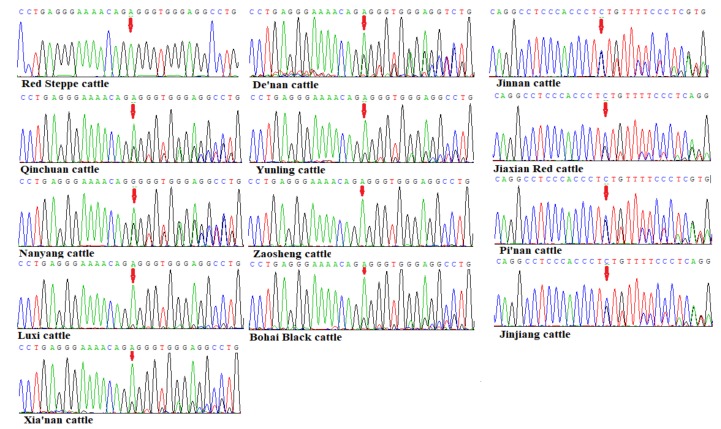
Interestingly, by amplifying and sequencing analyses of the 765 bp PCR products, a novel 2 bp indel mutation in the bovine TMEM95 gene, named NC_037346.1: g.27056998_27057000delCT, was revealed in the detected cattle breeds. This indel was found in 11 studied cattle breeds (Nanyang cattle, Jiaxian Red cattle, Xia’nan cattle, Jinnan cattle, Bohai Black cattle, Qinchuan cattle, Yunling cattle, Pi’nan cattle, Jinjiang cattle, De’nan cattle, and Luxi cattle). However, in Zaosheng cattle and Red Steppe cattle no mutation was revealed in this indel locus (Figure 3). This 2 bp indel was a frameshift mutation, which changed the location of the termination codon and changed the 16 amino acids in the C-terminal to 21 amino acids.
Figure 3.
The sequencing results of bovine TMEM95 indel mutation (NC_037346.1: g.27056998_27057000delCT) in 13 different cattle breeds.
4. Discussion
In 2014, the nonsense mutation c.483C > A (p.Cys161X) in the bovine TMEM95 gene was first detected in the male Fleckvieh cattle population by Pausch et al. [7]. In 2017, Fernandez-Fuertes et al. [10] examined the effects of this mutation on bovine sperm function. Their findings showed that in vivo and in vitro the male reproductive performance of the CC and CA genotypes is normal, but the AA genotype exhibits extremely poor fertility. Based on these findings, it is necessary to scientifically identify this genetic variant in more cattle breeds as potential genetic markers for selecting bulls with better reproductive performance.
In the present study, we first detected the c.483C > A in 13 Chinese indigenous cattle breeds. Surprisingly, there was no mutation in the c.483C > A locus in the detected Chinese cattle breeds. So, the fact that the nonsense mutation c.483C > A only exists in Fleckvieh cattle but not in Chinese cattle breeds (at least in the breeds analyzed in this study) suggests that this locus is not suitable to be used in MAS breeding in Chinese cattle. Still, in order to understand why Chinese cattle breeds did not contain this mutation, follow-up studies are necessary to clarify the breeding discrepancy and reproduction differences between foreign and Chinese cattle breeds.
In this study, an indel mutation (NC_037346.1: g.27056998_27057000delCT) was discovered in 11 cattle breeds by direct sequencing of the bovine TMEM95 gene. This is the first study to detect the indel mutation in this gene. Multiple studies have suggested that indel mutations located in coding or non-coding regions have notable effects on gene expression and phenotypes [21]; thus, this mutation might significantly affect the function of bovine TMEM95, though that needs further verification. Furthermore, two alternative splicing variants of the TMEM95 gene have been identified in the brain and testicular tissue of fetal Qinchuan cattle [22]. Alternative splicing plays a critical role in regulating biological function and is viewed as a crucial factor in generating functional diversity proteins in metazoan organisms [23]. Taken together, this study indicates that the bovine TMEM95 gene has abundant genetic polymorphisms, but further investigation is needed to better understand the function of this gene as well as enhance the male reproductive performance of bulls.
5. Conclusions
In this study, T-ARMS-PCR, forced PCR-RFLP, and DNA sequencing methods were used to detect the c.483C > A mutation in 765 individuals from 13 Chinese cattle breeds. The c.483C > A mutation was not found in our analyzed breeds. However, we identified a new frameshift indel mutation (NC_037346.1: g.27056998_27057000delCT) in the bovine TMEM95 gene in 11 cattle breeds, which changed the location of the termination codon and changed the 16 amino acids in the C-terminal to 21 amino acids. These results suggest that the c.483C > A mutation cannot serve as a genetic marker in molecular breeding in Chinese indigenous cattle breeds.
Acknowledgments
We would like to thank the staff of each cattle breeding farm for samples and data collection.
Author Contributions
Conceptualization: Y.Z., X.L., and S.Z.; formal analysis: K.P., S.Z., M.Z., and X.L.; funding acquisition: G.Z., Y.C., Y.Z., X.L., H.C., and C.L.; investigation: K.P., S.Z., and M.Z.; methodology: S.Z., M.Z., and X.L.; project administration: G.Z., Y.C., Y.Z., and S.Z.; resources: G.Z., Y.C., Y.Z., K.P., S.Z., X.L., H.C., and C.L.; software: K.P. and M.Z.; supervision: Y.Z. and X.L.; validation: K.P., S.Z., and X.L.; visualization: S.Z. and K.P.; writing—original draft: S.Z. and K.P.; writing—review and editing: S.Z., K.P., X.L., and Y.Z.
Funding
This research was funded by the Jilin Provincial Academy of Agricultural Sciences Innovation Project, Domestic Animal Germplasm Resources Platform, the National Beef Cattle and Yak Industrial Technology System under Grant Number CARS-37.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chen N., Cai Y., Chen Q., Li R., Wang K., Huang Y., Hu S., Huang S., Zhang H., Zheng Z., et al. Whole-genome resequencing reveals world-wide ancestry and adaptive introgression events of domesticated cattle in East Asia. Nat. Commun. 2018;9:2337. doi: 10.1038/s41467-018-04737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams A.R., Franke D.E., Saxton A.M., Turner J.W. Two-, three- and four-breed rotational crossbreeding of beef cattle: Reproductive traits. J. Anim. Sci. 1990;68:1536–1546. doi: 10.2527/1990.6861536x. [DOI] [PubMed] [Google Scholar]
- 3.Meuwissen T.H., Van Arendonk J.A. Potential improvements in rate of genetic gain from marker-assisted selection in dairy cattle breeding schemes. J. Dairy Sci. 1992;75:1651–1659. doi: 10.3168/jds.S0022-0302(92)77922-3. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson L.E. Artificial insemination of beef cattle. Aust. Vet. J. 1976;52:565–569. doi: 10.1111/j.1751-0813.1976.tb05422.x. [DOI] [PubMed] [Google Scholar]
- 5.Thundathil J.C., Dance A.L., Kastelic J.P. Fertility management of bulls to improve beef cattle productivity. Theriogenology. 2016;86:397–405. doi: 10.1016/j.theriogenology.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 6.Kastelic J.P., Thundathil J.C. Breeding soundness evaluation and semen analysis for predicting bull fertility. Reprod. Domest. Anim. 2008;43:368–373. doi: 10.1111/j.1439-0531.2008.01186.x. [DOI] [PubMed] [Google Scholar]
- 7.Pausch H., Kölle S., Wurmser C., Schwarzenbacher H., Emmerling R., Jansen S., Trottmann M., Fuerst C., Götz K.U., Fries R. A nonsense mutation in TMEM95 encoding a nondescript transmembrane protein causes idiopathic male subfertility in cattle. PLoS Genet. 2014;10:e1004044. doi: 10.1371/journal.pgen.1004044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurunath S., Pandian Z., Anderson R.A., Bhattacharya S. Defining infertility–a systematic review of prevalence studies. Hum. Reprod. Update. 2011;17:575–588. doi: 10.1093/humupd/dmr015. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Fuertes B., Kölle S., Lonergan P. Subfertility in bulls carrying a nonsense mutation in TMEM95 is due to failure to penetrate the zona pellucida. Reprod. Fertil. Dev. 2016;29:109–110. doi: 10.1071/RDv29n1Ab4. [DOI] [Google Scholar]
- 10.Fernandez-Fuertes B., Laguna-Barraza R., Fernandez-Gonzalez R., Gutierrez-Adan A., Blanco-Fernandez A., O’Doherty A.M., Di Fenza M., Kelly A.K., Kölle S., Lonergan P. Subfertility in bulls carrying a nonsense mutation in transmembrane protein 95 is due to failure to interact with the oocyte vestments. Biol. Reprod. 2017;97:50–60. doi: 10.1093/biolre/iox065. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Fuertes B., Lonergan P. Sperm Transmembrane Protein 95 (TMEM95) is required for sperm–oocyte interaction and successful fertilization but not mucus penetration in cattle. Reprod. Fertil. Dev. 2018;30:209–210. doi: 10.1071/RDv30n1Ab139. [DOI] [Google Scholar]
- 12.Yang Q.X, Zhang S.H., Cao X.K., Liu L.L., Lei C.Z., Qi X.L., Lin F.P., Qu W.D., Qi X.S., Chen H., et al. Application of mathematical expectation (ME) strategy on detecting the low frequency mutation: An example for evaluating 14 bp InDel of the PRNP gene 3′UTR in four Chinese indigenous cattle breeds. Prion. 2016;10:409–419. doi: 10.1080/19336896.2016.1211593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X.Y., Yang Q., Wang K., Yan H.L., Pan C.Y., Chen H., Liu J.W., Zhu H.J., Qu L., Lan X.Y. Two strongly linked single nucleotide polymorphisms (Q320P and V397I) in GDF9 gene are associated with litter size in cashmere goats. Theriogenology. 2019;125:115–121. doi: 10.1016/j.theriogenology.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Ye S., Dhillon S., Ke X.Y., Collins A.R., Day I.N. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res. 2001;29:e88. doi: 10.1093/nar/29.17.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ke X.Y., Collins A.R., Ye S. PCR designer for restriction analysis of various types of sequence mutation. Bioinformatics. 2002;18:1688–1689. doi: 10.1093/bioinformatics/18.12.1688. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S., Dang Y., Zhang Q., Qin Q., Lei C., Chen H., Lan X. Tetra-primer amplification refractory mutation system PCR (T-ARMS-PCR) rapidly identified a critical missense mutation (P236T) of bovine ACADVL gene affecting growth traits. Gene. 2015;559:184–188. doi: 10.1016/j.gene.2015.01.043. [DOI] [PubMed] [Google Scholar]
- 17.Wang X.Y., Yang Q., Wang K., Zhang S.H., Pan C.Y., Chen H., Qu L., Yan H.L., Lan X.Y. A novel 12-bp InDel polymorphism within the GDF9 gene is significantly associated with litter size and growth traits in goats. Anim. Genet. 2017;48:735–736. doi: 10.1111/age.12617. [DOI] [PubMed] [Google Scholar]
- 18.Yang Q., Yan H.L., Li J., Xu H., Wang K., Zhu H.J., Chen H., Qu L., Lan X.Y. A novel 14-bp duplicated deletion within goat GHR gene is significantly associated with growth traits and litter size. Anim. Genet. 2017;48:499–500. doi: 10.1111/age.12551. [DOI] [PubMed] [Google Scholar]
- 19.Yang Q., Zhang S.L., Li J., Wang X., Peng K., Lan X.Y., Pan C.Y. Development of a touchdown multiplex PCR method for simultaneously rapidly detecting three novel insertion/deletions (InDels) within one gene: An example for goat GHR gene. Anim. Biotechnol. 2018 doi: 10.1080/10495398.2018.1517770. [DOI] [PubMed] [Google Scholar]
- 20.Cui Y., Yan H.L., Wang K., Xu H., Zhang X.L., Zhu H.J., Liu J.W., Qu L., Lan X.Y., Pan C.Y. Insertion/deletion within the KDM6A gene is significantly associated with litter size in goat. Front. Genet. 2018;9:91. doi: 10.3389/fgene.2018.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang Z., Jiang E., Wang K., Pan C., Chen H., Yan H., Zhu H., Liu J., Qu L., Lan X. Goat membrane associated ring-CH-type finger 1 (MARCH1) mRNA expression and association with litter size. Theriogenology. 2019;128:8–16. doi: 10.1016/j.theriogenology.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S., Cai H., Yang Q., Shi T., Pan C., Lei C., Dang R., Chen H., Lan X. Identification of novel alternative splicing transcript and expression analysis of bovine TMEM95 gene. Gene. 2016;575:531–536. doi: 10.1016/j.gene.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Kelemen O., Convertini P., Zhang Z.Y., Wen Y., Shen M.L., Falaleeva M., Stamm S. Function of alternative splicing. Gene. 2013;514:1–30. doi: 10.1016/j.gene.2012.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]