Abstract
The health benefits of extra virgin olive oil (EVOO) are related to its chemical composition and the presence of bioactive compounds with antioxidant properties. The aim of this study was to evaluate antioxidant compounds (pigments, coenzyme Q10 (CoQ10) and phenolic compounds) and antioxidant properties of EVOO from the same region comparing different cultivars (Hojiblanca and Arbequina), harvest year and crop stage. Antioxidant properties of oils were studied before and after a gastrointestinal digestion process, by in vitro assays (DPPH, ABTS and FRAP) and antioxidant markers in Caco-2 cells (reactive oxygen species production). The content of bioactive compounds measured was significantly affected by cultivar and harvest year (except for carotenoids) and by the crop stage (except for coenzyme Q10). Higher amounts of coenzyme Q10 were observed in Hojiblanca than in Arbequina EVOO. Total phenol content and antioxidant properties were also different depending on cultivar and harvest year and the in vitro digestion process strongly improved antioxidant marker values. Antioxidant potential in bioaccessible fractions was mainly related to the content of coenzyme Q10 and phenolic compounds in EVOO. Chemometric analysis showed that the oils were clearly classified by cultivars, harvest and crop stage, according to the chemical composition and antioxidant activity analyzed in the present study.
Keywords: extra virgin olive oil, Hojiblanca, Arbequina, antioxidant properties, polyphenols, chemometric analysis
1. Introduction
The health benefits of extra virgin olive oil (EVOO) are significantly attributed to its high antioxidant potential, which, in turn, is deeply linked to its chemical composition [1]. Some of the bioactive compounds with antioxidant activity identified in EVOO are carotenoids, coenzymes and phenolic compounds, among others [2,3]. Multiple health benefits have been ascribed to phenolic compounds, such as prevention of cardiovascular disease; anti-inflamatory, antimicrobial and antiviral activities; and general protection against oxidative damage [4,5].
Antioxidants present in EVOO delay oxidative stress by inhibiting the formation or preventing the propagation of free radicals by several mechanisms; methods usually used to evaluate the antioxidant capacity are mainly focused on assessing the free radical scavenging ability, such as 2,2-azinobis (3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) or the ferric reducing antioxidant power (FRAP) and are commonly applied in chemical extracts of the oils [6]. These methods may be useful to determine the oil oxidative stability of the oil, but the biological effect of antioxidant compounds in vivo will depend on their bioavailability [7]. In this sense, it is accepted that the primary requisite for a bioactive compound to exert an antioxidant activity in vivo is to be bioaccessible, i.e., be released from the from matrix during the digestive process and, moreover, maintain its properties after the biotransformations caused by the gastrointestinal digestion [8]. The determination of antioxidant activity after in vitro digestion, considering all the transformations of the EVOO matrix, and consequently of its bioactive compounds has been investigated in previous works [6,8,9,10,11].
It is widely known that composition and antioxidant properties of EVOO may be affected by several agronomic factors, such as cultivar, fruit ripening stage and agroclimatic conditions [5,12,13]. The olive ripeness and the cultivar have been described as the most important factors affecting the EVOO phenolic profile [14,15], while organoleptic characteristic such flavour have been linked to geographic and climatic factors more than to cultivar or ripening stage [13,16]. Nevertheless, studies about how these aspects could affect the bioaccessible fraction of EVOO are still very scarce.
In the present work, we aimed to evaluate some relevant aspects of chemical composition (pigments, coenzyme Q10 (CoQ10) and phenolic compounds) and the antioxidant potential of Spanish EVOO from the same geographic region, comparing different cultivars, harvest years and crop stages (early and late). As the content of bioactive compounds may also influence the oil colour, colour coordinates were also measured. The antioxidant activity was studied after and before an in vitro gastrointestinal digestion, and methods based on H-atom and single-electron transfer (DPPH, ABTS, FRAP) together with cell culture markers (generation of reactive oxygen species (ROS)) were applied.
2. Materials and Methods
2.1. Chemicals
The chemicals used were all of high purity or analytical reagent grade. Bidistilled deionized water (Milli-Q purification system, Millipore, Bedford, MA, USA) was used. Ethanol and methanol were provided by VWR (Barcelona, Spain), sodium bicarbonate, acetate sodium, sodium carbonate, hydrochloric acid (37%), caffeic acid, hydrochloric acid, anhydrous sodium carbonate and potassium hexacyanoferrate (III) were acquired to Merck (Darmstad, Germany). Folin–Ciocalteau reagent, 6-hydroxy-2,5,7,8-tetramethyl-chroman-2-carboxylic acid (Trolox), DPPH, Pepsin, Pancreatin, bile salts, (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (HEPES) and tert-butylhydroperoxide (t-BOOH), the cell culture media, cell culture-grade chemicals, standards for individual phenolic compounds and. CoQ10 analysis were purchased to Sigma (Sigma-Aldrich, St, Louis, MO, USA). The ABTS was obtained from Amresco (Solon, USA). 2,4,6-Tri(2-pyridyl)-s-triazine (TPTZ) and iron (III) chloride for the ferric reducing power (FRAP) assay were from Fluka Chemicals (Fluka Chemicals, Madrid, Spain).
2.2. Samples
EVOO from two cultivars (Arbequina and Hojiblanca) from the South of Spain (Estepa, Sevilla; latitude 30°17′N, longitude 4°52′W) were analyzed. The olives were harvested in two years (2014 and 2015) at two crop stages: early stage (September–October, stage 1) and late stage (November–December, stage 2). The oil was extracted within 24 hours under a two-phase extraction system. The samples (n = 3 from each stage and season) were directly donated by the producers from the same cooperative and sent to the Spanish National Research Council laboratories (CSIC, Granada, Spain) protected from light and high temperature until analysis. All the samples meet standards of quality established by the European Union regulation n° 2568/91 for EVOO The description of samples and the climatic conditions of seasons are presented in Table 1.
Table 1.
Description of the extra virgin olive oil (EVOO) samples.
| Cultivar | Year | Stage | Mean Temperature (°C) | Mean Rainfall (mm) |
|---|---|---|---|---|
| Arbequina/Hojiblanca | 2014 | 1 | 22.2 | 102.5 |
| 2 | 16.2 | 140.7 | ||
| Arbequina/Hojiblanca | 2015 | 1 | 20.6 | 125.5 |
| 2 | 16.2 | 45.2 |
Mean temperature and rainfall were supplied by the Spanish Meteoroly Agency, (Aemet, http://www.aemet.es), and corresponds to the province of Seville.
2.3. Determination of CoQ10
The analysis of Co Q10 was performed as described by Borges et al. [17]. Briefly, 1-propanol was mixed with oil, vortexed and centrifuged at 11,300 g, 5 min. The supernatant was diluted (1/500) in 1-propanol before the high-performance liquid chromatography (HPLC) injection. CoQ10 in the oil extract was determined by HPLC (reversed-phase high-performance liquid chromatography, Gilson, Middleton, WI, USA) with a C18 symmetry column (3.5 μm, 4.6 × 150 mm) (Waters Chromatography, Barcelona, Spain) using methanol, ethanol, 2-propanol, glacial acetic acid (500:500:15:15) and 50 mM sodium acetate at a flow rate of 0.9 mL/min as a mobile phase. The electrochemical detector consisted of an ESA Coulochem III with the following setting: Guard cell (upstream of the injector) at +900 mV and the analytical cell at +350 mV. Reduced CoQ10 (ubiquinol) and oxidized CoQ10 (ubiquinone) could be determined by this method, although ubiquinol was not detected in our conditions of extraction and analysis. The CoQ10 concentration of the oxidized form was estimated comparing the peak areas with those of standard solutions; values of the calibration curve were reported previously [17]. The results were expressed in mg/L of sample.
2.4. Pigments (Chlorophylls and Carotenoids) and Colour
Chlorophylls and carotenoids were determined according to Minguez-Mosquera et al. [18]. The samples of oil were dissolved with cyclohexane (1.5:5 w/v) and the absorbance was measured with a UV spectrophotometer (Pharmaspec UV 1700, Shimadzu, Kyoto, Japan). The chlorophyll and the carotenoid fractions were determined at 670 and 470 nm, respectively. The results obtained are expressed as mg of pheophytin ‘‘a’’ and lutein per kg of oil, respectively.
Instrumental colour (CIE L*, a*, b*) was measured directly in the olive oil samples using a Minolta Colorimeter (CR-400, Konica Minolta Corp., Bremen, Germany) with illuminant D65, as described in Borges et al. [19].
2.5. Individual Phenolic Compounds
The individual phenolic compounds of the oil samples were determined after an extraction with methanol/water [80:20] according to the International Olive Oil Council [20]. The extracts were analyzed by UPLCTOF-MS following the method validated by Rivas et al. [21]. All the analytical parameters of the methods used are shown in Borges et al., [17].
2.6. In Vitro Digestion
The in vitro digestion was performed including sequential steps of gastric and intestinal digestion, as described by Borges et al. [6]. The EVOO samples mixed with Mili-Q water (1:10 w/v) were subjected to the two phases of digestion (2 h, 110 oscillations/min; 37 °C, each), gastric (pH 2, pepsin solution) and intestinal (pH 7, pancreatin/bile salts solution). The samples were always protected from light and were sonicated previously to each step. After centrifugation (10,000 rpm, 30 min, 4 °C, Sorvall RC 6 Plus centrifuge) the soluble or bioaccessible fraction (BF) was separated from the residual fraction (RF). Both fractions were stored at −80 °C in tubes protected from the light under a nitrogen blanket, until were used to analyze the total polyphenol content (TPC) and antioxidant activity. Aliquots of the BF were also used for assays in Caco-2 cells.
2.7. Total Phenolic Content and Antioxidant Activity
Previous to analysis of total phenolic content (TPC) and antioxidant activity, a chemical extraction was applied to the oil samples and the two fractions obtained after the in vitro digestion process (bioaccessible and residual fractions), following the procedure described by Borges et al. [11]. Samples were previously mixed with n-hexane and methanol/water (80:20 v/v) was used as extracting solvent.
The methodologies previously described by Borges et al. [6] were followed. A multilabel plate reader (Victor X3, Waltham, MA, USA) was used. TPC was determined by the Folin-Ciocalteu method and the absorbance was measured at 750 nm. The results were expressed in mg of caffeic acid equivalents (CAE) per kg of oil. The antioxidant capacity of the samples was studied following the ABTS and DPPH assays (for measuring the free radical scavenger activity) and the FRAP method (for assessing the reducing power). The final absorbance was measured at 750, 520 and 595 nm for ABTS, DPPH and FRAP assays, respectively. In each method a calibration curve of Trolox was performed and the results were expressed in mM of Trolox equivalents/kg of oil.
2.8. Cell Culture Assays
Caco-2 cells were purchased through the Cell Bank of Granada University (Granada, Spain) from the European Collection of Cell Cultures (ECACC). Culture flasks were provided by Corning Costar (Cambridge, MA, USA). The cells were maintained in 75 cm2 plastic flasks by serial passages, using as culture medium high-glucose Dulbecco’s modified minimal essential medium (DMEM), with heat-inactivated fetal bovine serum (FBS) (10%), NaHCO3 (3.7 g/L), nonessential amino acids (1%), HEPES (15 mM), bovine insulin (0.1 UI/mL) and 1% antibiotic-antimycotic solution. The cells were given fresh medium every 2–3 days and maintained under atmosphere of air/CO2 (95:5), 90% humidity and 37 °C.
The antioxidant potential of the BF from the digested oils was assessed at the cell level, by measuring the reactive oxygen species (ROS) generation. Determinations were carried out both at basal conditions and against an induced oxidative stress. Experiments were carried out with BF:FSB-free DMEM (1:2 v/v), as previous assay using the colorimetric MTT assay (3-(4,5-dime thylthiazol-2-yl)-2.5-diphenyltetrazolium bromide, Roche, Mannheim, Germany) showed that values of cell viability were never <85% at such conditions.
For determination of ROS generation we used the dichlorofluorescin (DCFH) assay as described by Seiquer et al. [8]. Briefly, cells were seeded in 24-well multiwell plates at 2 × 105 cells/well and incubated at 37 °C for 48 h. After exposition to BF of oils (2 h), cells were treated with DCFH 100 lM and incubated for 1 h. The DCFH was removed and culture medium (for basal measurements) or t-BOOH 5 mM (to induce oxidation) was added to the wells. The absorbance was measured at a wavelength of 485 nm excitation and 535 nm emission, at 37 °C for 0–90 min. DFCH in the presence of ROS is converted into dichlorofluorescein (DCH) and emits fluorescence. The data were expressed as fluorescence units from at least two independent experiments (n = 3 per experiment).
2.9. Statistical Analyses
All data are presented as the means of three independent experiments. The data obtained were analyzed applying three-way analysis of variance (ANOVA), using cultivar, year and stage of harvest as independent variables. The differences were established at P < 0.05 and the interactions were evaluated. Tukey’s test was used to compare mean values between olive oils. In addition, chemometric analysis was performed including all the variables evaluated in the present study (minor compounds, colour coordinates and parameters related with antioxidant activity). Firstly, a hierarchical clustering analysis (HCA) was performed with the aim of find eventual similarities between the EVOO samples according with cultivar, year of harvest and crop stage, by calculating the multidimensional squared Euclidean distances of scores using the nearest neighbor method. In addition, to reduce the variables into a small number of factors and explore their impact in the oil differentiation, a factor analysis (FA) using a varimax rotation was applied. For all the statistical analysis the Stat Graphics Centurion XVI software (Stat Point Technologies, Inc., Dallas, TX, USA, 2013) was used.
3. Results and Discussion
3.1. Chemical Compounds and Colour
Table 2 shows the content of CoQ10, pigments (chlorophylls and carotenoids) and phenolic compounds, as well as the instrumental colour, determined in the EVOO. Phenolic compounds are presented grouped by families: flavonoids (mainly apigenin and luteolin), phenolic acids (mainly naringenin, p-coumaric acid, gallic acid and vanillic acid) and phenol alcohol (mainly hydroxytyrosol). The content of the individual polyphenols detected is provided as Supplementary Materials (sheet 1).
Table 2.
Chemical compounds content (coenzyme Q10 (CoQ10), pigments and phenolic compounds) a and colour in EVOO.
| Item | Hojiblanca | Arbequina | P-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2014 | 2015 | |||||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | SEM | Cultivar | Year | Stage | |
| CoQ10 (mg/L) | 152A | 121A | 206B | 205B | 57.4a | 88.4b | 138c | 142c | 2.28 | ** | ** | n.s. |
| Chlorophylls (mg/kg) | 2.24B | 1.24A | 3.58C | 3.72C | 3.66a | 3.86a | 10.6c | 5.20b | 0.06 | ** | ** | ** |
| Carotenoids (mg/kg) | 4.28B | 3.74B | 2.10A | 2.28A | 1.86a | 2.20ab | 5.33c | 2.53b | 0.04 | n.s. | n.s. | ** |
| Phenolic Compounds (μg/kg) | ||||||||||||
| Flavonoids | 1054C | 1125D | 816A | 931B | 508b | 815c | 343a | 904d | 0.51 | ** | ** | ** |
| Phenolic acids | 274C | 297D | 116B | 76A | 28a | 30a | 55b | 99c | 0.51 | ** | ** | ** |
| Phenol alcohols | 519C | 640D | 97A | 135B | 81b | 55a | 294c | 464d | 0.51 | ** | ** | ** |
| L* | 22.6D | 25.0C | 19.3B | 17.9A | 26.4b | 26.3b | 18.0a | 17.6a | 0.21 | ** | ** | n.s. |
| a* | 0.53D | −0.79A | −0.04B | 0.28C | −2.19a | −2.24a | 0.24b | 0.32b | 0.01 | ** | ** | ** |
| b* | 8.15D | 12.3C | 4.36B | 2.55A | 10.9b | 12.5c | 2.54a | 2.46a | 0.02 | ** | ** | ** |
a Results are expressed per L or kg of EVOO; Means within a line are mean values for each cultivar, year and crop stage. SEM: standard deviation of mean. Within each line, capital letters and lowercase represent statistical differences (P < 0.05) in each cultivar, Hojiblanca and Arbequina, respectively. n.s: P > 0.05; * P < 0.05; ** P < 0.01.
Levels of CoQ10 were significantly affected by cultivar and harvest year, but crop stage did not have significant influence, except for Arbequina harvested in 2014. Comparing the varieties evaluated, Hojiblanca showed higher levels of CoQ10 than Arbequina (P < 0.001). Results are in accordance with a previous study [3] relating that Hojiblanca EVOO had higher levels than other commercial EVOOs from different geographic areas, including Arbequina, although with lower values than those found in the present study. Therefore, our results support that CoQ10 level in EVOO is mainly driven by genetic factors [3]. In addition, it may also be affected by climate and geographic conditions, and significant relationships with the altitude and the rainfalls of the growing areas have been found [17]. On the other hand, during the maturation process, the colour and composition of the olive fruit may change significantly and it has been suggested that EVOO harvested at early stages might be richer in CoQ levels [3]. On the contrary, we found that early or late crop stage did not affect CoQ10 content, and, as an exception, a positive effect of maturation was observed in Arbequina-2014 oils. Thus, it was confirmed that response to maturation is different depending on cultivar and may be also impacted by climatic conditions of the harvest year. Moreover, all the samples of the current study should be considered as very rich sources of CoQ10 (over than 50 mg/kg) according to the values previously established in the bibliography, which could contribute to the health benefits of the EVOO [22]. The high values of Hojiblanca-2015, over 200 mg CoQ10/L, must be highlighted among those found till the moment in the bibliography.
Regarding pigments, chlorophylls content was affected by cultivar, year and crop stage, whereas carotenoids were only impacted by stage of the harvest (P < 0.001). The highest levels of pigments were found in Arbequina-2015 from early stage, with 10.6 mg/kg and 5.35 mg/kg of chlorophylls and carotenoids, respectively, higher than those previously described for other virgin olive oil cultivars [23].
The ripening stage seemed to negatively affect pigment content, although the effect not was always significant. This influence was more consistently reported by Baccouri et al., [24] that found a significant loss of chlorophyll and carotenoids associated to the ripening degree in Tunisian monovarietal VOO. As ripening progresses in the olive fruit, photosynthetic activity decreases and the concentrations of both chlorophylls and carotenoids decrease progressively, whereas other coloured compounds, such as anthocyanins, are formed [25]. The presence of pigments has been also associated with the colour of virgin olive oils, which may vary from green-yellow to gold, depending on the variety and the stage of maturity [19,26]. It was observed that oils from the 2014 harvest were a more intense yellow (higher b*) than those from 2015, in both cultivars. Colour of oils plays an important role in the perceptions and preferences of consumers, which associate a dark green colour with high quality and pale yellow with refined and lower quality olive oils [27].
Flavonoids represented the majority group of the phenolic compounds determined in the present assay in all the EVOO analyzed, ranging from 42% of the total detected in Hojiblanca EVOO 2014, stage 2, to 91% in Arbequina 2014, stage 2. Levels of phenolic alcohols also varied widely, reaching proportions up to 42% of the total in EVOO from Arbequina 2015, at early stage. This behavior was similar to a previous study that described flavonoids as the major group of phenols in EVOO from Cobrançosa cultivar in different harvesting times [28]. In addition, in general, the values found in the present study were between the ranges of values related by Phenol Explorer [29]. According with our results, phenolic compounds of all the groups were significantly affected by the factors analyzed, i.e., cultivar, harvest year and crop stage (P < 0.001, Table 2). In agreement, it has been already observed that the polyphenols concentration of virgin olive oils varies greatly depending on the olive cultivar, agronomic practices and degree of fruit ripening, as well as on the conditions of processing (type of olive mill, malaxation, etc.) and fruit and oil storage [30]. Variations on phenolic composition of oils associated to olives ripening stage are caused by chemical reactions and activity of enzymes such as oxidoreductases, polyphenol oxidases and peroxidases [15]. It has been described that oxidation of phenolic compounds occurs with ripening, but the oxidation rate strongly depends on the cultivar, which may be related to the different distributions of the endogenous oxidoreductases in the pulp and the seed of the olive fruit [28]. In our assay, Hojiblanca oils showed higher phenol content than Arbequina for all harvest and crop stages, and a general increase in phenolic compounds was observed from early to late oils in the same harvest. Although higher levels of phenolic compounds have been usually found in early harvested oils compared with late harvested [31], a significant increase of some polyphenols, such as vanillin or p-coumaric acid, have also been described along fruit ripening [28], which should be in agreement with our findings. Thus, it is necessary to determine the best ripening stage for each variety, in order to obtain a high-quality olive oil.
3.2. Total Phenolic Content (TPC) and Antioxidant Activity Before and After In Vitro Digestion of Oils
Results are shown in Table 3. The colorimetric assay of TPC, based on the reaction of the Folin–Ciocalteu reagent with the functional hydroxy groups of the phenolic compounds, was included as an easy and valid method for the quantification of total phenols [32]. Current bibliographic data show a large variation of phenols in EVOO samples, from a few to approximately 1200 mg/kg, depending on the cultivar and environmental variables, such as rainfalls and olives ripeness, among others [1]. In the present study, TPC content found in chemical extracts before digestion was higher in Hojiblanca (367−405 mg/kg) than in Arbequina (222−231 mg/kg) EVOO, but no effect of harvest year or crop stage was observed. In the same line, values of antioxidant activity (ABTS, DPPH and FRAP) were also increased in Hojiblanca oils compared with Arbequina samples (P < 0.01), showing than oils with more quantity of phenolic components had also higher ability of scavenging free radicals and reducing power. These results agree with previous information showing positive relationships between the phenolic content of a large number of EVOOs with their antioxidant properties [11,30]. However, the measured antioxidant markers varied greatly depending on the year of harvest and the crop stage, unlike what was observed for TPC. These findings support that antioxidant quality of EVOO could be also attributed to compounds other than polyphenols [11]. We propose that the high levels of CoQ10 of Hojibblanca-2015 EVOO have a positive role in its antioxidant power, as CoQ10 is an electron acceptor and a potent antioxidant [33]. Beside, chlorophylls display antioxidant activity in the dark, but they act as pro-oxidants in the light [27]. It was also shown that climatic conditions affect the antioxidant potential of oils, but in a different way depending on cultivars; thus, it was observed that Hojiblanca-2015 oils had higher values of ABTS and DPPH, and lower values of FRAP, than Hojiblanca-2014, but Arbequina oils behaved differently according to the harvest. Other authors have also found that the antioxidant activity of olive oils, measured by ABTS, decrease during ripening [34].
Table 3.
Total phenolic content (TPC) and antioxidant activity (ABTS, DPPH and FRAP) determinate in the chemical extracts and bioaccesible fractions in EVOO.
| Item | Hojiblanca | Arbequina | P-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2014 | 2015 | |||||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | SEM | Cultivar | Year | Stage | |
| Chemical Extracts | ||||||||||||
| TPC | 397A | 367A | 394A | 405A | 231a | 222a | 230a | 226a | 4.22 | ** | n.s. | n.s. |
| ABTS | 0.69A | 0.73B | 0.80C | 0.80C | 0.73b | 0.48a | 0.79c | 0.80 c | 0.00 | ** | ** | ** |
| DPPH | 0.67A | 0.70A | 1.39B | 1.41B | 1.52c | 0.93b | 0.70a | 0.71a | 0.01 | ** | ** | ** |
| FRAP | 3.75C | 3.44B | 1.97A | 2.10A | 1.70C | 0.98a | 1.30b | 1.36b | 0.02 | ** | ** | ** |
| Bioaccessible Fraction | ||||||||||||
| TPC | 1031B | 633A | 1018B | 893B | 371a | 451a | 1453b | 1347b | 15.8 | n.s. | ** | ** |
| ABTS | 3.90A | 4.01A | 4.41A | 4.32A | 4.33a | 4.23a | 4.37a | 4.38a | 0.04 | ** | ** | n.s. |
| DPPH | 0.98A | 0.93A | 4.97C | 3.83B | 2.28b | 1.88a | 5.44b | 4.83b | 0.06 | ** | ** | ** |
| FRAP | 8.33B | 7.77B | 4.95A | 4.95A | 3.51a | 2.98a | 3.32a | 2.86a | 0.09 | ** | ** | * |
Total phenolic content (TPC) are expressed in mg of cafeic acid equivalents per kilogram of EVOO. Antioxidant activity (ABTS, DPPH and FRAP) are expressed in mmol of Trolox per kilogram of EVOO. Means within a line are mean values for each cultivar, year and crop stage. SEM: standard deviation of mean. Within each line, capital letters and lowercase represent statistical differences (P < 0.05) in each cultivar, Hojiblanca and Arbequina, respectively. n.s: P > 0.05; * P < 0.05; ** P < 0.01.
Analysis of the bioaccesible fractions obtained after the in vitro digestion of the oils showed increased values of TPC and antioxidant properties of all the samples compared with chemical extracts (Table 3), confirming the positive effects of the digestive process in the releasing of bioactive compounds previously observed in oils [5,8] and in other foods [35]. In addition, after digestion of oils it was found that TPC was not affected by cultivar, and free radical scavenging activity (ABTS and DPPH) was higher in Arbequina than in Hojiblanca bioaccessible fractions, on the contrary to that shown in chemical extracts. Thus, results support that the digestion process is essential in defining the antioxidant properties of oils, and changes produced during digestion should be considered to predict the healthy potential of oils in vivo. It has been shown that polyphenols from EVOO undergo extensive gastrointestinal biotransformation, producing various metabolites through hydrolysis or conjugation that retain or improve the potential beneficial effect of the original compounds [36]. Hydroxytyrosol presented increased recovery during the digestive process due to the hydrolysis of secoiridoid derivatives, and has been recognized as the most efficient free radical scavenger and radical chain breaker [5,36]. Thus, depending on the profile of bioactive compounds in the oils, the related antioxidant properties will evolve differently during the digestive process.
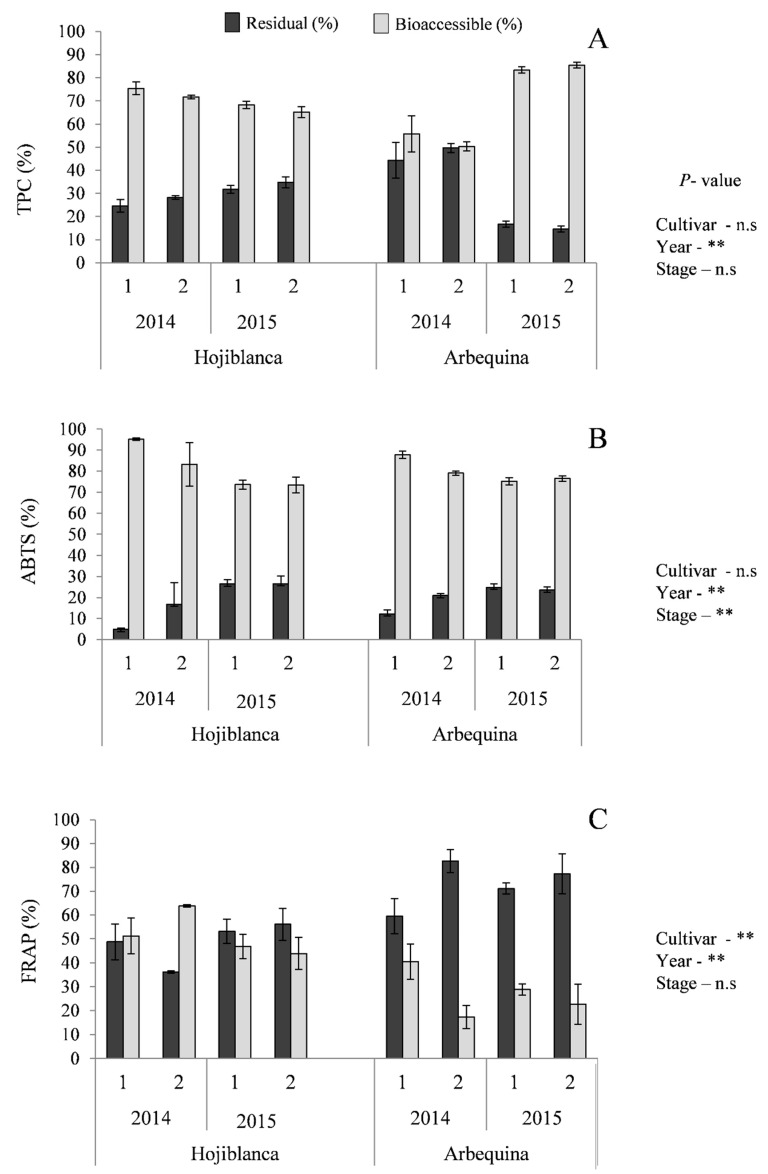
Together with the soluble or bioaccessible fraction, a residual or non-soluble fraction after the in vitro digestion was also recovered and analyzed. This fraction is usually discarded when studying bioavailability of bioactive compounds, but it still may contain a large quantity of complexes which could be metabolically active, as it has been previously described after digestion of Arbequina EVOO of different origin [11]. According to our results, a range of 18−52% of TPC from the total determined after digestion was located in the residual part (Figure 1A). These compounds may act locally exerting an antioxidant and anti-inflammatory action at the intestinal level [37]. In agreement, this fraction contained substantial ABTS activity (5−25% from the total) and reducing power in higher proportions in some cases than the bioaccessible fraction (Figure 1B,C), although no DPPH activity was observed. These findings reveal a different distribution of individual antioxidant compounds between fractions: Compounds based in electron-transfer action (FRAP assay) were mainly located in residual parts after digestion, whereas bioaccessible fractions were enriched in H-atom transfer based antioxidants (ABTS assay). Moreover, polyphenols remaining in the large intestine after digestion may interact with the intestinal microbiota and be transformed into low molecular-weight structures which are potentially absorbable [38]. It was observed that cultivar in the present assay did not affect the distribution of TPC or ABTS activity in the different fractions obtained after digestion of the oils, although the year of harvest had a significant influence.
Figure 1.
Average distribution of TPC (A) and antioxidant activity (ABTS, (B), and FRAP, (C)) evaluated after gastrointestinal digestion corresponding to residual (%) and bioaccessible (%) fractions. n.s. P > 0.05; ** P < 0.01.
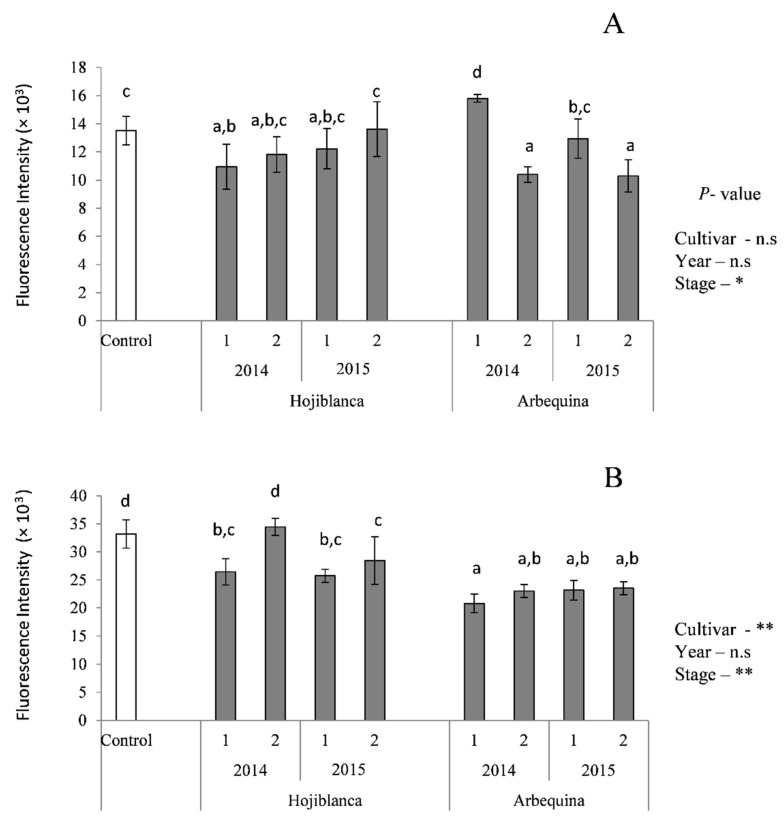
Finally, the antioxidant activity was assessed by the ROS production in Caco-2 cells (Figure 2). After incubating the cells with the BF of the oils, a modest ROS generation was observed, in most cases similar to the of the control cells, and non-affected by cultivar or harvest year of the EVOO assayed (Figure 2A). When an oxidative injury is caused, preincubation of cells with oils over 2 h was able to protect by reducing ROS generation compared with control cells (with the only exception of Hojiblanca 2014, stage 2). In this case, Arbequina oils showed a higher protecting effect than Hojiblanca. It has been described that EVOO polyphenols and their active metabolites may reach a high enough concentration in the intestinal lumen to act as antioxidant and scavenge ROS [36]. The protectant effect of EVOOs against oxidative damage in Caco-2 cells has been mainly attributed to the most abundant phenolic compounds present, hydroxytyrosol, tyrosol and oleuropein [39]. Particularly hydroxytyrosol and its metabolites have shown an efficient role in protecting Caco-2 cells from the cytotoxic effects of oxidized low-density lipoprotein (LDL) and peroxyl radicals, due to their scavenging properties [40].
Figure 2.
Reactive oxygen species (ROS) generation in Caco-2 cells expressed as fluorescence intensity at 90 minutes after incubation concerning the basal (A) and protective effect (B) oxidized with t-BOOH 5 mM. The control cells were incubated with culture medium only. n.s: P > 0.05; * P < 0.05; ** P < 0.01. Different letters indicate significant differences within samples and controls (P < 0.05).
3.3. Chemometric Analysis
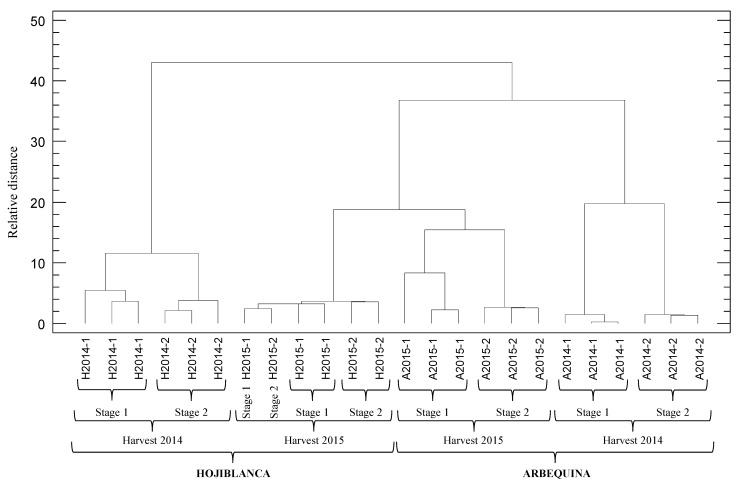
An HCA was initially applied for grouping samples that share common characteristics according to the analyzed variables and the dendogram obtained is shown in Figure 3. Four separated clusters were obtained by grouping the EVOOs by cultivar and year of harvest. According to the Euclidean distance, oils were clustered as follows: Hojiblanca-2015 < Hojiblanca-2014 < Arbequina-2015 < Arbequina-2014. In addition, all samples were well clustered by crop stage, with the exception of Hojiblanca-2015.
Figure 3.
Dendogram generated using the nearest neighbor method and squared Euclidean distance measure, showing the conglomeration of EVOO samples obtained by clustering of all the variables analyzed in the present assay.
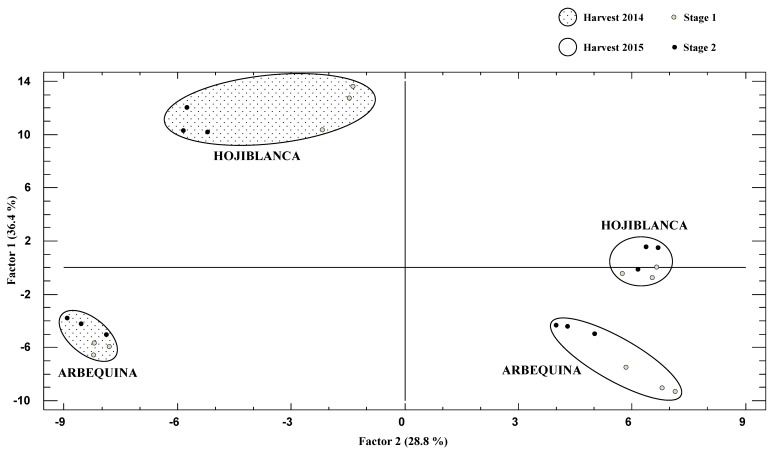
In the FA, three factors justifying 79% of total variance were obtained (F1 36%, F2 29%, F3 14%). F1 was explained by antioxidant markers (FRAP in BF, chemical extracts and residual fractions, 0.92, 0.91 and 0.77, respectively, ROS protective effect 0.73), total phenol content in chemical extracts (0.87), phenolic acids (0.85), flavonoids (0.84) and chlorophylls (−0.78). F2 was mainly governed by colour (L*-0.96, b*-0.94, a*-0.84) and CoQ10 (0.88) and F3 was explained by DPPH in chemical extracts (−0.94) and carotenoids (0.84). The spatial representation of the oils according to F1 and F2 is depicted in Figure 4 and a clear separation of the samples according with cultivar and harvest year was observed (factor scores are provided as Supplementary Materials (sheet 2). Samples were separated by harvest year especially due to F2; oils from 2014 harvest were located in the left hand of the graph and those of 2015 in the right hand, according with their different colours and CoQ10 content. In addition, Hojiblanca cultivar oils were situated on the upper side (especially Hojiblanca-2014) whereas Arbequina samples were located in the lower side, mainly due to variables affecting F1, i.e., antioxidant properties, phenolic compounds and chlorophylls. Therefore, HCA was confirmed by the factor analysis, which in turn showed that the RVOO samples can be correctly classified according with the variables analyzed in the present assay.
Figure 4.
Factor analysis score graph of the two main factors (Factor 1 vs. Factor 2), considering all the variables analyzed in the EVOO from different cultivars, years and crop stage (n = 24).
4. Conclusions
Findings of the present study confirm that composition and antioxidant properties of the EVOO strongly depend on cultivar and, within cultivars, also differ depending the year of harvest and the crop stage. The response of cultivars to climatic conditions may vary in each case and, as a consequence, different levels of bioactive compounds can increase or decrease during ripening. Antioxidant properties seem to be linked to phenolic content and profile, but other compounds, such as CoQ10, also have a significant role. Chemometric analysis showed that EVOOs may be classified by cultivars, harvest year and crop stage according the variables analyzed in the present assay.
Acknowledgments
The authors are grateful to Moisés Caballero Páez, from Consejo Regulador de Denominación de Origen (DOP) Estepa (Sevilla, Spain) for the donation of the oil samples. We also thank to the Instituto de Estudios Giennenses, Diputación provincial de Jaén (Jaen, Spain) for the collaboration in publishing the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3921/8/7/217/s1.
Author Contributions
Formal analysis, A.S. and L.C.L.; Software, L.L.; Supervision, R.N.; Writing-original draft, T.H.B.; Writing-review & editing, I.S.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Servili M., Sordini B., Esposto S., Urbani S., Veneziani G., Di Maio I., Selvaggini R., Taticchi A. Biological activities of phenolic compounds of extra virgin olive oil. Antioxidants. 2014;3:1–23. doi: 10.3390/antiox3010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopez S., Bermudez B., Montserrat-de la Paz S., Jaramillo S., Varela L.M., Ortega-Gomez A., Muriana F.J. Membrane composition and dynamics: A target of bioactive virgin olive oil constituents. Biochim. Biophys. Acta (BBA)-Biomembr. 2014;1838:1638–1656. doi: 10.1016/j.bbamem.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Žmitek K., Rodríguez Aguilera J.C., Pravst I. Factors influencing the contents of coenzyme Q10 and Q9 in olive oils. J. Agric. Food Chem. 2014;62:3211–3216. doi: 10.1021/jf5002167. [DOI] [PubMed] [Google Scholar]
- 4.Valavanidis A., Nisiotou C., Papageorgiou Y., Kremli I., Satravelas N., Zinieris N. Comparison of the radical scavenging potential of polar and lipidic fractions of olive oil and other vegetable oils under normal conditions and after thermal treatment. J. Agric. Food Chem. 2004;52:2358–2365. doi: 10.1021/jf030491h. [DOI] [PubMed] [Google Scholar]
- 5.Quintero-Flórez A., Pereira-Caro G., Sánchez-Quezada C., Moreno-Rojas J.M., Gaforio J.J., Jimenez A., Beltrán G. Effect of olive cultivar on bioaccessibility and antioxidant activity of phenolic fraction of virgin olive oil. Eur. J. Nutr. 2018;57:1925–1946. doi: 10.1007/s00394-017-1475-2. [DOI] [PubMed] [Google Scholar]
- 6.Borges T.H., Cabrera-Vique C., Seiquer I. Antioxidant properties of chemical extracts and bioaccessible fractions obtained from six Spanish monovarietal extra virgin olive oils: Assays in Caco-2 cells. Food Funct. 2015;6:2375–2383. doi: 10.1039/C5FO00529A. [DOI] [PubMed] [Google Scholar]
- 7.Manna C., Galletti P., Maisto G., Cucciolla V., D’Angelo S., Zappia V. Transport mechanism and metabolism of olive oil hydroxytyrosol in Caco-2 cells. FEBS Lett. 2000;470:341–344. doi: 10.1016/S0014-5793(00)01350-8. [DOI] [PubMed] [Google Scholar]
- 8.Seiquer I., Rueda A., Olalla M., Cabrera-Vique C. Assessing the bioavailability of polyphenols and antioxidant properties of extra virgin argan oil by simulated digestion and Caco-2 cell assays. Comparative study with extra virgin olive oil. Food Chem. 2015;188:496–503. doi: 10.1016/j.foodchem.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Dinnella C., Minichino P., D’Andrea A.M., Monteleone E. Bioaccessibility and antioxidant activity stability of phenolic compounds from extra-virgin olive oils during in vitro digestion. J. Agric. Food Chem. 2007;55:8423–8429. doi: 10.1021/jf072244+. [DOI] [PubMed] [Google Scholar]
- 10.Soler A., Romero M.P., Macià A., Saha S., Furniss C.S., Kroon P.A., Motilva M.J. Digestion stability and evaluation of the metabolism and transport of olive oil phenols in the human small-intestinal epithelial Caco-2/TC7 cell line. Food Chem. 2010;119:703–714. doi: 10.1016/j.foodchem.2009.07.017. [DOI] [Google Scholar]
- 11.Borges T.H., Pereira J.A., Cabrera–Vique C., Seiquer I. Study of the antioxidant potential of Arbequina extra virgin olive oils from Brazil and Spain applying combined models of simulated digestion and cell culture markers. J. Funct. Foods. 2017;37:209–218. doi: 10.1016/j.jff.2017.07.059. [DOI] [Google Scholar]
- 12.Inglese P., Famiani F., Galvano F., Servili M., Esposto S., Urbani S. Factors Affecting Extra-Virgin Olive Oil Composition. Hort. Rev. 2011;38:83. [Google Scholar]
- 13.Romero N., Saavedra J., Tapia F., Sepúlveda B., Aparicio R. Influence of agroclimatic parameters on phenolic and volatile compounds of Chilean virgin olive oils and characterization based on geographical origin, cultivar and ripening stage. J. Sci. Food Agric. 2016;96:583–592. doi: 10.1002/jsfa.7127. [DOI] [PubMed] [Google Scholar]
- 14.Gómez-Alonso S., Salvador M.D., Fregapane G.J. Phenolic Compounds Profile of Cornicabra Virgin Olive Oil. Agric. Food Chem. 2002;50:6812–6817. doi: 10.1021/jf0205211. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez B., Sánchez-Ortiz A., Lorenzo M.L., Rivas A. Influence of fruit ripening on agronomic parameters, quality indices, sensory attributes and phenolic compounds of Picudo olive oils. Food Res. Int. 2013;54:1860–1867. doi: 10.1016/j.foodres.2013.08.016. [DOI] [Google Scholar]
- 16.Tura D., Failla O., Bassi D., Attilio C., Serraiocco A. Regional and cultivar comparison of Italian single cultivar olive oils according to flavor profiling. Eur. J. Lipid Sci. Technol. 2013;115:196–210. doi: 10.1002/ejlt.201200104. [DOI] [Google Scholar]
- 17.Borges T.H., López L.C., Pereira J.A., Cabrera–Vique C., Seiquer I. Comparative analysis of minor bioactive constituents (CoQ10, tocopherols and phenolic compounds) in Arbequina extra virgin olive oils from Brazil and Spain. J. Food Comp. Anal. 2017;63:47–54. doi: 10.1016/j.jfca.2017.07.036. [DOI] [Google Scholar]
- 18.Minguez-Mosquera M.I., Rejano-Navarro L., Gandul-Rojas B., Sánchez Gómez A.H., Garrido-Fernández J. Color-pigment correlation in virgin olive oil. J. Am. Oil Chem. Soc. 1991;68:332–336. doi: 10.1007/BF02657688. [DOI] [Google Scholar]
- 19.Borges T.H., Pereira J.A., Cabrera-Vique C., Lara L., Oliveira A., Seiquer I. Characterization of Arbequina virgin olive oils produced in diferente regions of Brazil and Spain: Physicochemical properties, oxidative stability and fatty acid profile. Food Chem. 2017;215:454–462. doi: 10.1016/j.foodchem.2016.07.162. [DOI] [PubMed] [Google Scholar]
- 20.IOOC, International Olive Oil Council. Document COI/T.20/DOC. 29; Proceedings of the International Olive Oil Council 2009; Madrid, Spain. 30 November–4 December 2009. [Google Scholar]
- 21.Rivas A., Sanchez-Ortiz A., Jimenez B., García-Moyano J., Lorenzo M.L. Phenolic acid content and sensory properties of two Spanish monovarietal virgin olive oils. Eur. J. Lipid Sci. Technol. 2013;115:621–630. doi: 10.1002/ejlt.201200371. [DOI] [Google Scholar]
- 22.Pravst I., Žmitek K., Žmitek J. Coenzyme Q10 contents in foods and fortification strategies. Crit. Rev. Food Sci. Nutr. 2010;50:269–280. doi: 10.1080/10408390902773037. [DOI] [PubMed] [Google Scholar]
- 23.Ramos-Escudero F., Morales M.T., Asuero A.G. Characterization of bioactive compounds from monovarietal virgin olive oils: Relationship between phenolic compounds-antioxidant capacities. Int. J. Food Prop. 2018;18:348–358. doi: 10.1080/10942912.2013.809542. [DOI] [Google Scholar]
- 24.Baccouri O., Guerfel M., Baccouri B., Cerretani L., Bendini A., Lercker G., Zarrouk M., Ben Miled D.D. Chemical composition and oxidative stability of Tunisian monovarietal virgin olive oils with regard to fruit ripening. Food Chem. 2008;109:743–754. doi: 10.1016/j.foodchem.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 25.Roca M., Minguez-Mosquera M.I. Change in the natural ratio between chlorophylls and carotenoids in olive fruit during processing for virgin olive oil. J. Am. Oil Chem. Soc. 2001;78:133–138. doi: 10.1007/s11746-001-0233-z. [DOI] [Google Scholar]
- 26.Moyano M.J., Meléndez-Martínez A.J., Alba J., Heredia F.J. A comprehensive study on the colour of virgin olive oils and its relationship with their chlorophylls and carotenoids indexes (II): CIELUV and CIELAB uniform colour spaces. Food Res. Int. 2008;41:513–521. doi: 10.1016/j.foodres.2008.03.006. [DOI] [Google Scholar]
- 27.Gandul-Rojas B., Gallardo-Guerrero L., Roca M., Aparicio-Ruiz R. Handbook of Olive Oil. Springer; New York, NY, USA: 2013. Chromatographic methodologies: Compounds for olive oil color issues. [Google Scholar]
- 28.Peres F., Martins L.L., Mourato M., Vitorino C., Antunes P., Ferreira-Dias S. Phenolic compounds of ‘Galega Vulgar’ and ‘Cobrançosa’ olive oils along early ripening stages. Food Chem. 2016;211:51–58. doi: 10.1016/j.foodchem.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Phenol-Explorer: Database on Polyphenol Content in Foods (2019) [(accessed on 25 June 2019)]; Available online: http://phenol-explorer.eu/compounds.
- 30.Caporaso N., Savarese M., Paduano A., Guidone G., De Marco E., Sacchi R. Nutritional quality assessment of extra virgin olive oil from the Italian retail market: Do natural antioxidants satisfy EFSA health claims? J. Food Comp. Anal. 2015;40:154–162. doi: 10.1016/j.jfca.2014.12.012. [DOI] [Google Scholar]
- 31.Franco M.N., Galeano-Díaz T., López O., Fernandez-Bolaños J.G., Sánchez J., De Miguel C., Gil M.V., Matín-Vertedor D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014;161:289–298. doi: 10.1016/j.foodchem.2014.04.091. [DOI] [PubMed] [Google Scholar]
- 32.Hrncirik K., Fritsche S. Comparability and reliability of different techniques for the determination of phenolic compounds in virgin olive oil. Eur. Lipid Sci. Technol. 2004;106:540–549. doi: 10.1002/ejlt.200400942. [DOI] [Google Scholar]
- 33.Nakajima Y., Inokuchi Y., Nishi M., Shimazawa M., Otsubo K., Hara H. Coenzyme Q10 protects retinal cells against oxidative stress in vitro and in vivo. Brain Res. 2008;1226:226–233. doi: 10.1016/j.brainres.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 34.Köseoglu O., Sevim D., Kadirog˘lu P. Quality characteristics and antioxidant properties of Turkish monovarietal olive oils regarding stages of olive ripening. Food Chem. 2016;212:628–634. doi: 10.1016/j.foodchem.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 35.Pastoriza S., Delgado-Andrade C., Haro A., Rufián-Henares J.A. A physiologic approach to test the global antioxidant response of foods. The GAR method. Food Chem. 2011;129:1926–1932. doi: 10.1016/j.foodchem.2011.06.009. [DOI] [Google Scholar]
- 36.Deiana M., Serra G., Corona G. Modulation of intestinal epithelium homeostasis by extra virgin olive oil phenolic compounds. Food Funct. 2018;9:4085–4099. doi: 10.1039/C8FO00354H. [DOI] [PubMed] [Google Scholar]
- 37.Biasi F., Astegiano M., Maina M., Leonarduzzi G., Poli G. Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Curr. Med. Chem. 2011;18:4851–4865. doi: 10.2174/092986711797535263. [DOI] [PubMed] [Google Scholar]
- 38.Cardona F., Andres-Lacuerva C., Tulipania S., Tinahonesb F.J., Queipo-Ortuno M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013;24:1415–1422. doi: 10.1016/j.jnutbio.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Incani A., Serra G., Atzeri A., Melis M.P., Serreli G., Bandino G., Sedda P., Campus M., Tuberoso C.I., Deiana M. Extra virgin olive oil phenolic extracts counteract the pro-oxidant effect of dietary oxidized lipids in human intestinal cells. Food Chem. Toxicol. 2016;90:171–180. doi: 10.1016/j.fct.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Giovannini C., Straface E., Modesti D., Coni E., Cantafora A., De Vincenzi M., Malorni W., Masella R. Tyrosol, the major olive oil biophenol, protects against oxidized-LDL-induced injury in Caco-2 cells. J. Nutr. 1999;129:1269–1277. doi: 10.1093/jn/129.7.1269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.