Abstract
Polyporus umbellatus is a well-known and important medicinal fungus in Asia. Its polysaccharides possess interesting bioactivities such as antitumor, antioxidant, hepatoprotective and immunomodulatory effects. A qualitative and quantitative method has been established for the analysis of 12 monosaccharides comprising polysaccharides of Polyporus umbellatus based on high-performance liquid chromatography coupled with electrospray ionization–ion trap–time of flight–mass spectrometry. The hydrolysis conditions of the polysaccharides were optimized by orthogonal design. The results of optimized hydrolysis were as follows: neutral sugars and uronic acids 4 mol/L trifluoroacetic acid (TFA), 6 h, 120 °C; and amino sugars 3 mol/L TFA, 3 h, 100 °C. The resulting monosaccharides derivatized with 1-phenyl-3-methyl-5-pyrazolone have been well separated and analyzed by the established method. Identification of the monosaccharides was carried out by analyzing the mass spectral behaviors and chromatography characteristics of 1-phenyl-3-methyl-5-pyrazolone labeled monosaccharides. The results showed that polysaccharides in Polyporus umbellatus were composed of mannose, glucosamine, rhamnose, ribose, lyxose, erythrose, glucuronic acid, galacturonic acid, glucose, galactose, xylose, and fucose. Quantitative recoveries of these monosaccharides in the samples were in the range of 96.10–103.70%. This method is simple, accurate, and sensitive for the identification and quantification of monosaccharides, and can be applied to the quality control of Polyporusumbellatus as a natural medicine.
Keywords: composition analysis, HPLC–MS, monosaccharide hydrolysis, Polyporus umbellatus, polysaccharide
1. Introduction
Polyporus umbellatus (Pers.) Fries, also known as “Zhu Ling” in China, belongs to the Polyporaceae family of the Basidiomycota phylum [1,2]. It is a widely used medicinal fungus in Asia, especially in China and Japan, and has been one of the most important traditional Chinese medicines for more than 2500 years [3]. The sclerotia is the main medicinal parts of Polyporus umbellatus, and has been used as a diuretic for the treatment of diseases such as kidney, edema, scant urine, and vaginal discharge, as well as diarrhea and jaundice [4]. Numerous pharmacological effects including anticancer, immuno-enhancing, hepatoprotective, radio-protective, and antioxidative activities have been reported [5,6,7,8,9,10]. To our knowledge, the physiological activities of polysaccharides depend on the composition of monosaccharides [11], and identifying this composition is the very first step to unveil relevant physicochemical properties, and structure–activity relationships. In addition, monosaccharide composition is an essential parameter for the quality evaluation of polysaccharide [12].
A great number of analytical techniques have been used to detect monosaccharides in complex samples, including thin-layer chromatography (TLC) [13], gas chromatography (GC) [14], high-performance liquid chromatography (HPLC) [15,16,17,18,19], capillary electrophoresis (CE) [20,21,22], gas chromatography–mass spectrometry(GC–MS) [23,24], and liquid chromatography–mass spectrometry (LC–MS) [25,26,27]. HPLC has been accepted as one of the common techniques for the analysis of sugars, which was used for simultaneous determination of neutral, acidic, and basic carbohydrates [28,29]. However, the lack of chromophores or fluorophores in the structure of monosaccharides prevents direct detection by UV or fluorescence detectors. Refractive index detection and other related methods do not often meet the demands of modern analysis with regard to sensitivity and/or selectivity [30,31]. Therefore, the derivatization of monosaccharides is indispensable to obtain highly sensitive detection [32,33]. The reagent 1-phenyl-3-methyl-5-pyrazolone (PMP), first developed in 1989 by Honda’s group [32], is a popular label that reacts with reducing carbohydrate under mild conditions, requiring no acid catalyst and causing no desialylation and isomerization [32,34,35,36]. In addition, pre-column derivatization with the PMP method was first developed for the analysis of carbohydrates by HPLC [37]. The derivatized monosaccharides exhibit strong ultraviolet (UV) absorption at 245 nm and higher hydrophobicity than their native form, especially compatible with HPLC separation and UV detection. In the case of complex sugar composition, the HPLC–MS method with PMP derivatization can be applied to improve the detection sensitivity and specificity.
There are many studies on polysaccharides from Polyporus umbellatus [38,39,40,41]. However, the method of HPLC–MS with PMP precolumn derivatization has not been applied to Polyporus umbellatus. In this study, we firstly designed an orthogonal test with three factors and three levels for the optimization of the polysaccharide hydrolysis, then established a precolumn PMP derivatization HPLC–DAD–ESI–IT–TOF–MS method for the simultaneous determination of 12 sugars. In addition, the characteristic fragment ions of the 12 PMP-labeled monosaccharides were collected.
2. Results and Discussion
2.1. Identification of Monosaccharides Comprising Polysaccharide
2.1.1. HPLC Separation
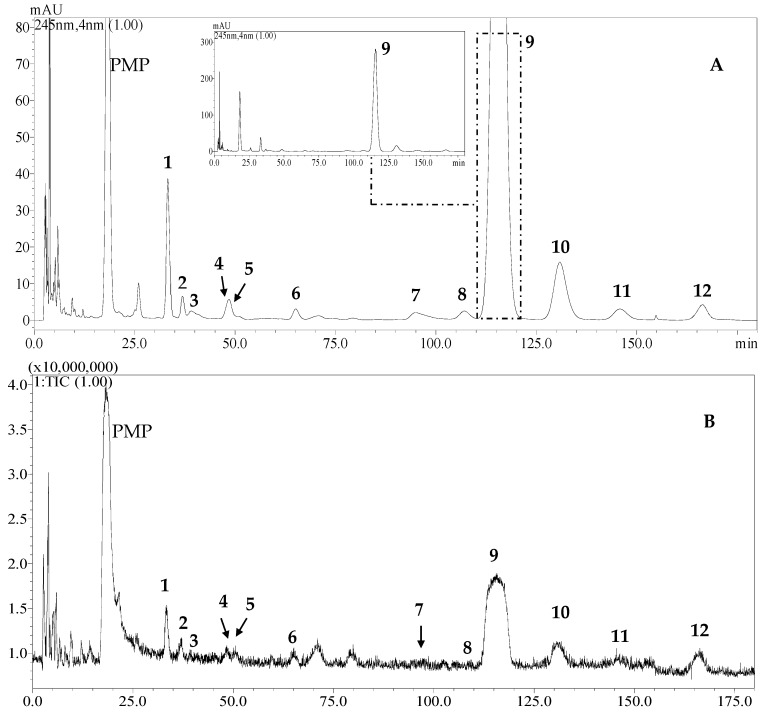
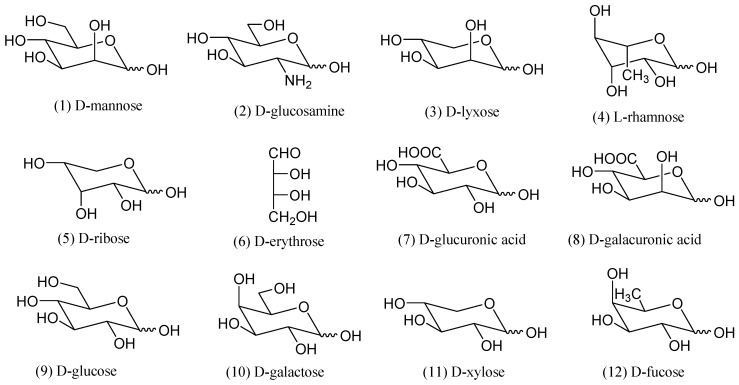
The HPLC separation conditions were adjusted and tested for a mixture of 12 PMP-labeled monosaccharides (mannose, glucosamine, lyxose, rhamnose, ribose, erythrose, glucuronic acid, galacturonic acid, glucose, galactose, xylose, and fucose). The elution system was chosen as 0.1 mol/L aqueous ammonium acetate (solvent B) (pH 4.5) and acetonitrile (solvent A), since no nonvolatile inorganic salts are allowed into the MS detector. In this study, the pH of ammoniumacetate solution and the percentage of acetonitrile were investigated to improve the separation. As a result, 12 present PMP-labeled monosaccharides were successfully separated in the order of mannose, glucosamine, rhamnose, ribose, lyxose, erythrose, glucuronic acid, galacturonic acid, glucose, galactose, xylose, and fucose within 180 min in this study (Figure 1). The structures of identified monosaccharides are displayed in Figure 2.
Figure 1.
The HPLC chromatogram (A) and total ion chromatogram (TIC) in positive mode (B) of 12 1-phenyl-3-methyl-5-pyrazolone (PMP)-labeled monosaccharides. 1, mannose; 2, glucosamine; 3, lyxose; 4, rhamnose; 5, ribose; 6, erythrose; 7, glucuronic acid; 8, galacturonic acid; 9, glucose; 10, galactose; 11, xylose; 12, fucose.
Figure 2.
Chemical structures of 12 monosaccharides identified from Polyporus umbellatus polysaccharide (PPS).
2.1.2. ESI–IT–TOF–MS analysis
All PMP-labeled monosaccharides were characterized by ESI–IT–TOF–MS method (positive-ion mode, Figure 1). The numerical data are listed in Table 1, which suggests the fragmentation mode for PMP-labeled monosaccharides. In the current study, 12 PMP-labeled compounds were unambiguously identified based on their chromatographic and MS fragment behaviors (Figure 1), and by comparisons with the reference standards. According to molecular weights, common monosaccharides could be divided into six groups, namely hexose, hexosamine, pentose, tetrose, methylpentose, and hexuronic acid, whose PMP derivatives gave quasimolecular ions at m/z 511, 510, 481, 451, 495 and 525, respectively.
Table 1.
Characteristic ions of MS/MS for PMP-labeled monosaccharides (m/z).
| tR (min) | Compound | [M + H]+ (Error in ppm) | Characteristic MS2 Fragments Ions of: [M + H]+ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [M + H − H2O]+ | C3-C4 Cleavage and −H2O | C2-C3 Cleavage and −H2O | [M + H − PMP]+ | [M + H − PMP − H2O]+ | [M + H − PMP − 2H2O]+ | [M + H – PMP − 3H2O]+ | C5-C6 Cleavage and − 2H2O | C4-C5 Cleavage and − 2H2O | C2-C3 Cleavage | C1-C2 Cleavage | [PMP + H]+ | |||
| 33.830 | Mannose (1) | 511.2201 (+2.74) | 493.2283 | 403.1312 | 373.1751 | 337.1331 | 319.1064 | 301.1157 | 283.1149 | 271.1069 | 241.1037 | 217.1045 | 187.086 | 175.0904 |
| 36.548 | Glucosamine (2) | 510.2350 (+0.59) | 492.2207 | 402.1679 | 372.1868 | 336.1679 | 319.1589a) | 301.0892b) | 283.1082c) | 271.1083d) | 241.0959e) | 216.1313 | 187.0923f) | 175.0904 |
| 40.298 | Lyxose (3) | 481.2061 (−4.36) | 463.2209 | - | 373.1593 | 307.1321 | 289.1476 | 271.1193 | 253.1302 | - | 241.1071 | 217.1093 | 187.0893 | 175.0873 |
| 47.701 | Rhamnose (4) | 495.2243 (+1.01) | - | - | 373.1729 | 321.1649 | 303.1136 | 285.1227 | 267.1122 | - | 241.0933 | 217.0929 | 187.0924 | 175.0902 |
| 48.832 | Ribose (5) | 481.2073 (−1.87) | 463.2216 | - | 373.1750 | 307.1325 | 289.1153 | 271.1021 | 253.1001 | - | 241.0979 | 217.1053 | 187.0901 | 175.0919 |
| 64.678 | Erythrose (6) | 451.1977 (+0.22) | 433.1797 | - | 373.1749 | 277.1583 | 259.1082 | 241.0970 | - | - | - | 217.0938 | 187.0943 | 175.0861 |
| 94.865 | Glucuronic acid (7) | 525.1972 (−1.52) | 507.1776 | - | 373.1567 | - | - | - | 297.0914 | 271.0983 | 241.0941 | 217.1039 | 187.0796 | 175.0838 |
| 107.503 | Galacuronic acid (8) | 525.2005 (+4.76) | 507.1841 | - | 373.1733 | - | - | - | 297.0766 | 271.0983 | 241.1252 | 217.1018 | 187.0809 | 175.0875 |
| 115.318 | Glucose (9) | 511.2187 (+0) | 493.1923 | 403.1419 | 373.1652 | 337.1408 | 319.1341 | 301.1345 | 283.1135 | 271.1116 | 241.0986 | 217.0986 | 187.0884 | 175.0860 |
| 130.080 | Galactose (10) | 511.2187 (+0) | 493.2219 | 403.1561 | 373.1693 | 337.1036 | 319.1108 | 301.1322 | 283.1110 | 271.1120 | 241.1001 | 217.0951 | 187.0804 | 175.0872 |
| 145.282 | Xylose (11) | 481.2072 (−2.08) | 463.1764 | - | 373.1680 | - | 289.1448 | 271.1019 | 253.0949 | - | 241.1188 | 217.0712 | 187.0896 | 175.1019 |
| 166.837 | Fucose (12) | 495.2259 (+4.24) | 477.2073 | 403.1800 | 373.1626 | 321.1555 | - | 285.1205 | 267.1102 | - | 241.1030 | 217.1096 | 187.0925 | 175.0906 |
tR: retention time; –: No detected; M: represent molecular weight of PMP-labeled monosaccharides. a) [M + H – PMP − NH3]+. b) [M + H – PMP − NH3 − H2O]+. c) [M + H – PMP − NH3 − 2H2O]+. d) [M + H – PMP − CH2O − NH3 − H2O]+. e) [M + H – PMP − 2CH2O − NH3 − H2O]+. f) [M + H – PMP − 4CH2O − CHNH2]+.
The data summarized in Table 1 along with the relative abundances of the peaks suggest the fragmentation pathways of PMP-labeled monosaccharides. All the observed fragments were subdivided into four groups. The first group comprises low abundance peaks which correspond to dehydration such as [M + H − H2O]+ (occurring in any monosaccharide). The second group comprises low abundance peaks corresponding to the loss of one PMP group, dehydration, and deamination such as in [M + H − PMP]+, [M + H – PMP − H2O]+, [M + H – PMP − 2H2O]+, and [M + H – PMP − 3H2O]+ (occurring in any monosaccharide), as well as [M + H − PMP − NH3]+, [M + H − PMP − NH3 − H2O]+ and [M + H – PMP − NH3 − 2H2O]+, typical for glucosamine. The third group comprises high abundance peaks which correspond to the loss of one PMP group and characteristic C-C bond cleavage of the carbohydrate skeleton. These are peaks at m/z 271 due to the loss of one PMP group and then the cleavage of the C5-C6 bond followed by loss of 2H2O or NH3 (typical for glucosamine), peaks at m/z 241 due to the loss of one PMP group and the cleavage of the C4-C5 bond followed by a loss of H2O or NH3 (typical for glucosamine), peaks at m/z 217 and 216 (only for glucosamine) due to the loss of one PMP group and the cleavage of the C2-C3 bond, and the strong peaks at m/z 187 due to the loss of one PMP group and the cleavage of the C1-C2 bond. The last group comprises high abundance peaks correspondent to base peak ions [PMP + H] + at m/z 175 and characteristic C-C bond cleavage of the carbohydrate skeleton. These are peaks at m/z 373 and 372 (only for glucosamine) due to the cleavage of the C2-C3 bond followed by loss of H2O, and the weak peaks at m/z 403 and 402 (only for glucosamine) due to the cleavage of the C3-C4 bond followed by loss of H2O (for more details, Table 1).
In the MS2 spectra, the fragments at m/z 372 (cleavage of C2-C3 in glucosamine) and m/z 402 (cleavage of C3-C4 in glucosamine) exhibit high abundance as an indication for the presence of glucosamine. Other types of monosaccharides can be identified by their fragmentation analysis.
2.2. Optimization of Hydrolysis Conditions
The PPS has a complex monosaccharide composition which may contain acidic, neutral, and basic sugars in one molecule. In the present study, the orthogonal L9 (3)4 experiments were designed to investigate optimal hydrolysis conditions of PPS. Glucosamine, glucuronic acid, and glucose were chosen on behalf of basic sugars, acidic sugars, and neutral sugars. The results of orthogonal test and extreme difference analysis are presented in Tables S2, S3 and S4. The analysis of variance was performed by statistical software SPSS 20.0 and the results are listed in Tables S5, S6 and S7. As for glucose, according to the R values, we found that hydrolysis time played the critical role in the hydrolysis process, followed by TFA concentration and hydrolysis temperature. Analysis of variance results (Tables S5, S6 and S7) indicated that hydrolysis temperature, TFA concentration, and hydrolysis time had statistically significant effects on the hydrolysis process with 95% confidence, and the order of influence factors was consistent with the results of the intuitive analysis. As for glucosamine, the hydrolysis temperature was the major factor affecting the hydrolysis process, while the minor factors were the concentration of TFA and hydrolysis time. Analysis of variance results indicated that hydrolysis temperature had statistically significant effect on hydrolysis process with 95% confidence, while TFA concentration and hydrolysis time did not have significant effect. As for glucuronic acid, it was found that the effect of factors is C > A > B based on the R values. TFA concentration played the important role in the hydrolysis process, followed by hydrolysis temperature and hydrolysis time. Analysis of variance results indicated that three factors did not have significant effect on hydrolysis process. The optimized hydrolytic conditions of PPS determined by orthogonal experiment were as follows: neutral sugars: 4 mol/L TFA, 6 h, 120 °C; amino sugars: 3 mol/L TFA, 3 h, 100 °C; uronic acids: 4 mol/L TFA, 6 h, 120 °C.
2.3. Method Validation
The analytical method was validated in terms of linearity, detection limit, precision, stability, repeatability and recovery. A total of 10 standard monosaccharides (mannose, glucosamine, lyxose, erythrose, glucuronic acid, galacturonic acid, glucose, galactose, xylose, and fucose) were used for these tests.
2.3.1. Calibration curves and limit of detection
All calibration curves were established by plotting the chromatographic peak area of monosaccharide derivatives versus the concentration of the corresponding monosaccharide solution shown in Table 2. As a consequence, the correlation coefficients (R2 >0.9991) indicate that all calibration curves had excellent linearities within the test ranges. Furthermore, the limit of detection (LOD) and limit of quantification (LOQ) of each analyte were determined as the concentration of standard solution with S/N = 3 (signal-to-noise ratio) and S/N = 10. The results showed that the LOD values of the 10 monosaccharides were in the range from 0.191 to 1.152 μmol/L (Table 2), indicating the sensitivity of the method.
Table 2.
Calibration curves, linear ranges, limits of detection (LODs), and limit of quantification (LOQs) of 10 monosaccharide derivatives.
| No. | Analyte | Calibration Curve | R2 | Linear Range (μmol/L) | LOD (μmol/L) | LOQ (μmol/L) |
|---|---|---|---|---|---|---|
| 1 | Mannose | y = 3.0804 × 104x + 6.3221 × 104 | 0.9998 | 20.20–202.04 | 0.19 | 0.63 |
| 2 | Glucosamine | y = 7.2843 × 103x − 7.3839 × 103 | 0.9999 | 8.40–151.26 | 0.83 | 2.76 |
| 3 | Lyxose | y = 2.1137 × 104x + 1.9181 × 103 | 0.9999 | 4.26–42.63 | 0.22 | 0.73 |
| 6 | Erythrose | y = 5.3561 × 103x +1.3761 × 104 | 0.9998 | 12.14–133.54 | 0.54 | 1.80 |
| 7 | Glucuronic acid | y = 2.5459 × 104x − 4.0005 × 104 | 0.9995 | 8.14–81.38 | 1.15 | 3.83 |
| 8 | Galacuronic acid | y = 3.1860 × 104x + 1.2310 × 104 | 0.9999 | 3.96–39.61 | 0.37 | 1.23 |
| 9 | Glucose | y = 2.3516 × 104x + 1.5884 × 105 | 0.9999 | 199.82–3596.80 | 0.29 | 0.97 |
| 10 | Galactose | y = 2.7091 × 104x + 7.5494 × 103 | 0.9998 | 41.33–413.33 | 0.74 | 2.47 |
| 11 | Xylose | y = 1.7797 × 104x + 5.0060 × 104 | 0.9996 | 16.95–152.51 | 0.57 | 1.90 |
| 12 | Fucose | y = 1.6309 × 104x + 5.7003 × 104 | 0.9991 | 8.07–80.67 | 0.78 | 2.60 |
2.3.2. Precision, Reproducibility, and Stability
The precision was calculated as the relative standard deviation (RSD) for consecutively analyzing the same sample for six times. The repeatability was evaluated by six repeated determination of the hydrolyzed monosaccharides from polyporus polysaccharide. The stability was assessed by analyzing the same sample six times at different times (0, 3, 6, 9, 12, and 24 h). The results of precision, reproducibility, and stability are listed in Table 3. The RSD values for precision were 0.39–1.13%, which indicated that the method precision was satisfactory. The RSD values of the stability were less than 1.30%, indicating that the sample solution was stable within 24 h. The RSD of repeatability was within 0.49–1.39%.
Table 3.
Precision, repeatability and stability of 10 monosaccharide derivatives. RSD: relative standard deviation.
| No. | Analyte | RSD (%) | ||
|---|---|---|---|---|
| Precision | Repeatability | Stability | ||
| 1 | Mannose | 0.39 | 0.49 | 0.67 |
| 2 | Glucosamine | 1.03 | 1.09 | 1.23 |
| 3 | Lyxose | 1.13 | 1.22 | 1.30 |
| 6 | Erythrose | 0.62 | 0.69 | 0.64 |
| 7 | Glucuronic acid | 1.14 | 1.13 | 1.05 |
| 8 | Galacuronic acid | 0.53 | 0.71 | 0.75 |
| 9 | Glucose | 0.48 | 0.70 | 0.73 |
| 10 | Galactose | 0.94 | 1.40 | 1.02 |
| 11 | Xylose | 1.10 | 1.22 | 1.15 |
| 12 | Fucose | 0.84 | 0.88 | 1.10 |
2.3.3. Recovery
Each monosaccharide solution was added to the hydrolyzed polysaccharide sample at a similar concentration of the same monosaccharide in the sample (n = 3). The recovery rate was calculated as follows: recovery (%) = [(amount detected- original amount)/amount spiked] × 100%. The RSD (%) was calculated according to the following equation: RSD (%) = (standard deviation/mean) × 100%. The average recoveries of all ten monosaccharides ranged from 96.10% to 103.70%, and the RSD values fell within 0.96–2.36% (Table 4). This accuracy is within the acceptable ranges. Results of the method validation described above indicate that the method is precise and accurate for the analysis of polysaccharide from Polyporus umbellatus.
Table 4.
Recovery analysis of 10 monosaccharides of PPS (n = 5).
| No. | Analyte | Content (nmol/mg) | Spiked (nmol/mg) | Mean Found (nmol/mg) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|
| 1 | Mannose | 234.88 | 230 | 470.88 | 102.61 | 1.79 |
| 2 | Glucosamine | 111.70 | 110 | 217.41 | 96.10 | 2.01 |
| 3 | Lyxose | 291.81 | 291 | 576.87 | 97.96 | 1.25 |
| 6 | Erythrose | 271.27 | 271 | 545.06 | 101.03 | 2.36 |
| 7 | Glucuronic acid | 72.81 | 72 | 143.73 | 98.50 | 1.56 |
| 8 | Galacuronic acid | 46.14 | 46 | 90.90 | 97.30 | 1.37 |
| 9 | Glucose | 3079.52 | 3000 | 6091.52 | 100.40 | 0.96 |
| 10 | Galactose | 484.26 | 485 | 977.36 | 101.67 | 1.01 |
| 11 | Xylose | 220.69 | 220 | 439.59 | 99.50 | 1.16 |
| 12 | Fucose | 289.87 | 290 | 590.60 | 103.70 | 1.43 |
2.4. Sample Analysis
To assess the quality of Polyporus umbellatus and obtain the composition of the polysaccharides, 12 samples of Polyporus umbellatus purchased from different natural habitats in China were analyzed in this study. The results of 12 samples are shown in Table 5.
Table 5.
Determination results of the monosaccharides in PPS from 12 samples of Polyporus umbellatus.
| Sample | Origin | Contents (nmol/mg, n = 3) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mannose | Glucosamine | Lyxose | Erythrose | Glucuronic Acid | Galacuronic Acid | Glucose | Galactose | Xylose | Fucose | ||
| S1 | Shaanxi | 234.88 | 111.70 | 291.81 | 271.27 | 72.81 | 46.14 | 3079.52 | 484.26 | 220.69 | 289.87 |
| S2 | Shanxi | 145.22 | 61.86 | 302.68 | 460.78 | 37.15 | 29.94 | 2769.76 | 351.48 | 342.07 | 363.18 |
| S3 | Shanxi | 107.39 | 99.07 | 217.79 | 331.03 | 31.02 | 27.53 | 1473.43 | 239.13 | 282.08 | 289.29 |
| S4 | Yunnan | 153.97 | 106.59 | 182.08 | 520.85 | 52.42 | 55.43 | 3279.84 | 268.36 | 479.31 | 390.09 |
| S5 | Yunnan | 175.84 | 102.97 | 221.64 | 439.15 | 47.16 | 38.33 | 2761.47 | 437.67 | 306.93 | 403.57 |
| S6 | Shaanxi | 136.04 | 80.02 | 94.31 | 373.67 | 77.96 | 80.28 | 3329.49 | 516.64 | 284.02 | 291.84 |
| S7 | Shaanxi | 160.08 | 75.85 | 129.04 | 355.81 | 41.13 | 35.81 | 2814.63 | 444.62 | 289.06 | 296.59 |
| S8 | Henan | 82.81 | 63.42 | 115.88 | 153.51 | 26.26 | 16.21 | 1222.47 | 266.39 | 101.85 | 169.37 |
| S9 | Hebei | 55.98 | 69.50 | 77.42 | 157.88 | 20.88 | 15.67 | 1284.87 | 126.54 | 110.60 | 117.21 |
| S10 | Sichuan | 69.01 | 60.01 | 136.19 | 150.25 | 23.89 | 17.32 | 1379.81 | 253.40 | 129.34 | 169.23 |
| S11 | Jilin | 76.03 | 64.66 | 50.73 | 146.89 | 34.02 | 32.02 | 1094.02 | 222.16 | 117.18 | 130.78 |
| S12 | Gansu | 174.9 | 153.11 | 68.71 | 321.26 | 70.45 | 70.53 | 3474.96 | 308.13 | 261.42 | 224.71 |
All 12 batches of Polyporus umbellatus polysaccharide were found to be composed of mannose, glucosamine, rhamnose, ribose, lyxose, erythrose, glucuronic acid, galacturonic acid, glucose, galactose, xylose, and fucose. Compared to previous reports [5,39,40,41,42,43], the monosaccharide composition of PPS includes mannose, rhamnose, glucuronic acid, glucose, galactose, xylose, and fucose. However, glucosamine, ribose, lyxose, erythrose, and galacturonic acid have not been previously reported in PPS. In addition, the contents of monosaccharides were between 20.88 and 3329.49 nmol/mg. The predominant monosaccharides in PPS are glucose, galactose, xylose, fucose and erythrose. However, the contents of glucuronic acid and galacturonic acid are less than other monosaccharides. There are significant differences in the content and composition of the components across the samples. The reason for this phenomenon might be due to the differences in geographical origin and growth environment. Interestingly, the composition and order of monosaccharide contents were similar with each other. It was reported that terminal mannose, N-acetylglucosamine, or fucose in polysaccharides can be recognized by mannose receptors in macrophages, leading to elevated protective function of immune system [44,45,46,47,48]. This study showed that all PPS samples contain mannose and fucose, which may partially explain the immune-enhancement activity of the sample.
3. Materials and Methods
3.1. Chemicals and Reagents
d-Mannose, d-galactose, d-xylose, l-rhamnose, d-glucuronic acid, and d-glucosamine were purchased from the Harveybio Gene Technology Co., Ltd. (Beijing, China). d-galacturonic acid and d-fucose were obtained from Solarbio Technology (Beijing, China). d-Glucose, d-ribose, and d-lyxose were acquired from J&K scientific Ltd. (Beijing, China). d-Erythrose was from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). The purities of all reference compounds except D-erythrose (75%) were found to be above 95% as determined by HPLC. 1-Phenyl-3-methyl-5-pyrazolone (PMP) and trifluoroacetic acid (TFA) were obtained from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). MS grade acetonitrile (Merck, Darmstadt, Germany), MS grade formic acid (FA) (Fisher Scientific), and de-ionized water of 18.2 MΩ purified by a Milli-Q system (Milford, MA, USA) were used for the mobile phases.
A total of 12 batches of crude medicinal Polyporus umbellatus from different natural habitats in China were purchased, all of which were identified by Professor Hong Wang (Department of Natural Medicines, School of Pharmaceutical Sciences, Peking University) and the voucher specimens were deposited at the School of Pharmaceutical Sciences, Peking University. Their habitats and codes were as follows: Shaanxi (S1); Shanxi (S2); Shanxi (S3); Yunnan (S4); Yunnan (S5); Shaanxi (S6); Shaanxi (S7); Henan (S8); Hebei (S9); Sichuan (S10); Jilin (S11); Gansu (S12). Sample S1 was used for the method development.
3.2. Sample Preparation
The polysaccharide was extracted from Polyporus umbellatus by hot-water extraction and ethanol precipitation [49,50]. Briefly, the finely powder of dried sclerotia of Polyporus umbellatus (10 g) was defatted with 95% alcohol and dried (3 × 100 mL), then extracted with boiling water (2 × 300 mL) for 3 h. After filtration, the water extracts were combined and concentrated to 100 mL under reduced pressure. Then, 400 mL of ethanol were slowly added to the solution under stirring to precipitate the polysaccharide, and kept at 4 °C overnight. Finally, the polysaccharide precipitation was obtained by centrifugation for 10 min at 6000 rpm and washed sequentially with minimal amounts of ethanol, acetone and ether. Residue was dried to obtain the Polyporus umbellatus polysaccharide (PPS).
3.3. Optimization of Hydrolysis Conditions
Orthogonal experiment is a common and simple method to study multiple factors and multiple levels, which can optimize the process by analyzing the typical experimental results [22,29,51,52,53]. In order to investigate optimal hydrolysis conditions of PPS, the orthogonal L9 (3)4 experiments were designed. The influencing factors were considered, including hydrolysis time (factor A), hydrolysis temperature (factor B), and TFA concentration (factor C). In addition, each factor had three levels to be optimized. Nine hydrolysis experiments were conducted in sequence at different temperatures (100, 110, and 120 °C), hydrolysis times (3, 6, and 9 h), and TFA concentrations (2, 3, and 4 mol/L). The experimental conditions for the hydrolysis of PPS were listed in Supporting Information Table S1.
3.4. Hydrolysis of Polysaccharide
Polysaccharide sample (20 mg) was infiltrated with 2 mL TFA [54] in a 10 mL ampoule that was sealed under a nitrogen atmosphere, and hydrolysis of the polysaccharide was performed. After cooling to room temperature, the resulting reaction solutions were centrifuged at 6000 rpm for 10 min, then the supernatant was evaporated to dryness under reduced pressure. The dried sample was for the following experiments.
3.5. Derivatization of Hydrolyzed Polysaccharide
The PMP derivatization of monosaccharides was carried out according to previous method [27,55,56,57] with minor modifications. The resulting monosaccharides were dissolved in 5 mL ammonia (28%). Then, 100 μL of solution were mixed with 0.5 mol/L methanolic solution of PMP (100 μL) in a 3-mL ampoule that was sealed under a nitrogen atmosphere. The mixture was allowed to react for 30 min at 70 °C in water bath, then cooled to room temperature and was concentrated to dryness under nitrogen. Water and chloroform (1.0 mL each) were added and mixed, and the organic phase was carefully removed. The extraction process was repeated three times and the aqueous layer was filtered through a 0.45-μm membrane prior to LC/MS analysis.
3.6. HPLC–MS Conditions
3.6.1. HPLC Conditions
The chromatographic analysis was performed on a Shimadzu analytical HPLC system (Kyoto, Japan), consisting of DGU-20A3 degasser, two LC-20AD pumps, an SIL-20AC auto injector, a CTO-20A column oven, and an SPD-M20A detector. Sample separation was carried on a Shimpack VP-ODS C18 column (250 mm × 4.6 mm, 5 μm, Shimadzu, Kyoto, Japan) kept at 30 °C. The mobile phase was composed of 0.1 mol/L ammonium acetate (adjusted to pH 4.5 with acetic acid): acetonitrile (83:17, v/v). The flow rate was 1.0 mL/min. The injection volume was 20 μL. The UV spectra were recorded in the range of 200–400 nm, and the DAD was set at 245 nm.
3.6.2. ESI–IT–TOF–MS Conditions
The HPLC system was coupled with ion-trap time-of flight (IT–TOF) mass spectrometer (Shimadzu LCMS–IT–TOF, Kyoto, Japan) via an ESI interface. The HPLC eluent at a flow rate of 0.2 mL/min was introduced into the ESI source after DAD detection. The MS scan range in positive mode was m/z 100–700. The temperatures of curved desolvation line (CDL) and block heater were both maintained at 200 °C. The capillary voltage, CDL voltage, and detector voltage were set at 4.5 kV, 10 V, and 1.75 kV, respectively. Nitrogen was used as the nebulizer gas at a flow rate of 1.5 L/min. The collision energy was adjusted to 70% in the HPLC–MS analysis, and the isolation width of precursor ions was 3.0 U. The data was acquired and processed by LC/MS solution software (version 3, Shimadzu, Kyoto, Japan) with a chemical formula predictor.
4. Conclusions
In this study, a simple, robust, and effective HPLC–DAD–ESI–IT–TOF–MS method with PMP derivatization was established for the analysis of monosaccharide composition of polysaccharide from Polyporus umbellatus. All 12 PMP-labeled derivatives of monosaccharides displayed high chemical stability, characteristic MS fragmentations for structural identification. Among them, ribose, lyxose, erythrose, and galacturonic acid were detected for the first time in Polyporus umbellatus. In addition, the optimized hydrolysis conditions of PPS by the orthogonal L9 (3)4 experiments will greatly facilitate further development of the medicinal polysaccharide from Polyporus umbellatus. This study provides an analytical approach and chemical basis for the quality control of Polyporus umbellatus.
Acknowledgments
This work was supported by Kangmei Pharmaceutical Co. Ltd. The authors are grateful for the constructive discussion and proofreading received from Zongtao Lin in the Department of Chemistry, University of Pennsylvania.
Supplementary Materials
The following are available online, Table S1: Factors and levels of the orthogonal L9 (3)4 experiments, Table S2: The orthogonal experiment results of glucose released from PPS, Table S3: The orthogonal experiment results of glucosamine released from PPS, Table S4: The orthogonal experiment results of glucuronic acid released from PPS, Table S5: Variance analysis results of glucose released from PPS, Table S6: Variance analysis results of glucosamine released from PPS, Table S7: Variance analysis results of glucuronic acid released from PPS.
Author Contributions
N.G. and H.W. conceived and designed the experiments; N.G. performed the experiments; W.J. and W.W. analyzed the data; Z.B. and J.S. contributed reagents/materials/analysis tools; N.G. and S.C. wrote the paper.
Funding
This work was supported by the project Standardization Construction of Zhuling (No.: ZYY-2017-159) belonging to TCM Standardization Project (No.: ZYBZH-GD-13) in Emerging Industries Major Engineering Package from National Development and Reform Commission (NDRC), China.
Conflicts of Interest
The authors declare no conflicts of interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors upon request.
References
- 1.Guo W.J., Xing Y.M., Chen J., Guo S.X. Growth promoting effects of water extract of armillaria mellea rhizomorph on mycelia of Polyporus umbellatus. Cryptogamie Myco. 2011;32:171–176. doi: 10.7872/crym.v32.iss2.2011.171. [DOI] [Google Scholar]
- 2.Choi K.D., Lee K.T., Shim J.O., Lee Y.S., Lee T.S., Lee S.S., Guo S.X., Lee M.W. A new method for cultivation of sclerotium of Grifola umbellata. Mycobiology. 2003;31:105–112. doi: 10.4489/MYCO.2003.31.2.105. [DOI] [Google Scholar]
- 3.Xu J. Chinese Medicinal Fungus. 1st ed. China Medical University and Peking Union Medical College Joint Press; Beijing, China: 1997. pp. 300–303. [Google Scholar]
- 4.Pharmacopoeia Commission of PRC . Pharmacopoeia of the People’s Republic of China. Volume 1. China Medical Science Press; Beijing, China: 2015. pp. 318–319. [Google Scholar]
- 5.Li X.Q., Xu W., Chen J. Polysaccharide purified from Polyporus umbellatus (Per) Fr induces the activation and maturation of murine bone-derived dendritic cells via toll-like receptor 4. Cell. Immunol. 2010;265:50–56. doi: 10.1016/j.cellimm.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Lin Y.F., Wu G.L. Protective effect of Polyporus umbellatus polysaccharides on toxic hepatitis in mice. Acta. Pharmacologica. Sinica. 1988;9:345–348. [PubMed] [Google Scholar]
- 7.Peng K., Lan L.S., Yan W.X., Jie S.L., Wu Y.J., Hua Z.Y., Chen J.S. Polyporus umbellatus polysaccharides ameliorates carbon tetrachloride-induced hepatic injury in mice. Afr. J. Pharm. Pharmacol. 2012;6:2686–2691. [Google Scholar]
- 8.Ueno Y., Abe M., Yamauchi R., Kato K. Structural analysis of the alkali-soluble polysaccharide from the sclerotia of Grifora umbellata (Fr.) Pilat. Carbohydr. Res. 1980;87:257–264. doi: 10.1016/S0008-6215(00)85212-X. [DOI] [PubMed] [Google Scholar]
- 9.Wu H.C., Cheng Y.J., Liang J.A., Huang H.F., Wu K.Y., Chiang S.Y. Radio- and chemoprotective effects of Zhu-Ling Mushroom (Polyporus umbellatus) in human cultured cells and in mice. Toxicol. Lett. 2011;205:S37. doi: 10.1016/j.toxlet.2011.05.148. [DOI] [Google Scholar]
- 10.Zhang G.W., Zeng X., Li C.X., Li J.J., Huang Y., Han L., Wei J.A., Huang H.D. Inhibition of urinary bladder carcinogenesis by aqueous extract of sclerotia of Polyporus umbellatus fries and polyporus polysaccharide. Am. J. Chin. Med. 2011;39:135–144. doi: 10.1142/S0192415X11008701. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M., Cui S.W., Cheung P.C.K., Wang Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Tech. 2007;18:4–19. doi: 10.1016/j.tifs.2006.07.013. [DOI] [Google Scholar]
- 12.Wang X.B., Zhao Y., Wang Q.W., Wang H.F., Mei Q.B. Analysis of the monosaccharide components in Angelica polysaccharides by high performance liquid chromatography. Anal. Sci. 2005;21:1177–1180. doi: 10.2116/analsci.21.1177. [DOI] [PubMed] [Google Scholar]
- 13.Bischel M.D., Austin J.H., Kemeny M.D., Hubble C.M., Lear R.K. Separation and identification of acid polysaccharides by thin-layer chromatography. J. Chromatogr. A. 1966;21:40–45. doi: 10.1016/S0021-9673(01)91258-0. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y., Xie M.Y., Wang Y.X., Nie S.P., Li C. Analysis of the monosaccharide composition of purified polysaccharides in Ganoderma atrum by capillary gas chromatography. Phytochem. Anal. 2009;20:503–510. doi: 10.1002/pca.1153. [DOI] [PubMed] [Google Scholar]
- 15.Gomis D.B., Tamayo D.M., Alonso J.M. Determination of monosaccharides in cider by reversed-phase liquid chromatography. Anal. Chim. Acta. 2001;436:173–180. doi: 10.1016/S0003-2670(01)00889-3. [DOI] [Google Scholar]
- 16.Kakita H., Kamishima H., Komiya K., Kato Y. Simultaneous analysis of monosaccharides and oligosaccharides by high-performance liquid chromatography with postcolumn fluorescence derivatization. J. Chromatogr. A. 2002;961:77–82. doi: 10.1016/S0021-9673(02)00655-6. [DOI] [PubMed] [Google Scholar]
- 17.Ikegami T., Horie K., Saad N., Hosoya K., Fiehn O., Tanaka N. Highly efficient analysis of underivatized carbohydrates using monolithic-silica-based capillary hydrophilic interaction (HILIC) HPLC. Anal. Bioanal. Chem. 2008;391:2533–2542. doi: 10.1007/s00216-008-2060-6. [DOI] [PubMed] [Google Scholar]
- 18.Lv Y., Yang X.B., Zhao Y., Ruan Y., Yang Y., Wang Z.Z. Separation and quantification of component monosaccharides of the tea polysaccharides from Gynostemma pentaphyllum by HPLC with indirect UV detection. Food Chem. 2009;112:742–746. doi: 10.1016/j.foodchem.2008.06.042. [DOI] [Google Scholar]
- 19.Dai J., Wu Y., Chen S.W., Zhu S., Yin H.P., Wang M., Tang J. Sugar compositional determination of polysaccharides from Dunaliella salina by modified RP-HPLC method of precolumn derivatization with 1-phenyl-3-methyl-5-pyrazolone. Carbohydr. Polym. 2010;82:629–635. doi: 10.1016/j.carbpol.2010.05.029. [DOI] [Google Scholar]
- 20.Guttman A. Analysis of monosaccharide composition by capillary electrophoresis. J. Chromatogr. A. 1997;763:271–277. doi: 10.1016/S0021-9673(96)00750-9. [DOI] [PubMed] [Google Scholar]
- 21.Xia Y.G., Wang Q.H., Liang J., Yang B.Y., Li G.Y., Kuang H.X. Development and application of a rapid and efficient CZE method coupled with correction factors for determination of monosaccharide composition of acidic hetero-polysaccharides from Ephedra sinica. Phytochem. Anal. 2011;22:103–111. doi: 10.1002/pca.1235. [DOI] [PubMed] [Google Scholar]
- 22.Chen J.Y., Yang F.F., Guo H.Z., Wu F., Wang X.H. Optimized hydrolysis and analysis of Radix Asparagi polysaccharide monosaccharide composition by capillary zone electrophoresis. J. Sep. Sci. 2015;38:2327–2331. doi: 10.1002/jssc.201500120. [DOI] [PubMed] [Google Scholar]
- 23.Kenne L., Stromberg S. A method for the microanalysis of hexoses in glycoproteins. Carbohydr. Res. 1990;198:173–179. doi: 10.1016/0008-6215(90)84290-B. [DOI] [PubMed] [Google Scholar]
- 24.Guadalupe Z., Martínez-Pinilla O., Garrido Á., Carrillo J., Ayestarán B. Quantitative determination of wine polysaccharides by gas chromatography–mass spectrometry (GC–MS) and size exclusion chromatography (SEC) Food Chem. 2012;131:367–374. doi: 10.1016/j.foodchem.2011.08.049. [DOI] [Google Scholar]
- 25.Dye C., Yttri K. Determination of monosaccharide anhydrides in atmospheric aerosols by use of high-performance liquid chromatography combined with high-resolution mass spectrometry. Anal. Chem. 2005;77:1853–1858. doi: 10.1021/ac049461j. [DOI] [PubMed] [Google Scholar]
- 26.Hammad L.A., Derryberry D.Z., Jmeian Y.R., Mechref Y. Quantification of monosaccharides through multiple-reaction monitoring liquid chromatography/mass spectrometry using an aminopropyl column. Rapid Commun. Mass Spe. 2010;24:1565–1574. doi: 10.1002/rcm.4536. [DOI] [PubMed] [Google Scholar]
- 27.Wu X.D., Jiang W., Lu J.J., Yu Y., Wu B. Analysis of the monosaccharide composition of water-soluble polysaccharides from Sargassum fusiforme by high performance liquid chromatography/electrospray ionisation mass spectrometry. Food Chem. 2014;145:976–983. doi: 10.1016/j.foodchem.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Huie C.W., Di X. Chromatographic and electrophoretic methods for Lingzhi pharmacologically active components. J. Chromatogr. B. 2004;812:241–257. doi: 10.1016/S1570-0232(04)00678-6. [DOI] [PubMed] [Google Scholar]
- 29.Wang Q.C., Zhao X., Pu J.H., Luan X.H. Influences of acidic reaction and hydrolytic conditions on monosaccharide composition analysis of acidic, neutral and basic polysaccharides. Carbohydr. Polym. 2016;143:296–300. doi: 10.1016/j.carbpol.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q.J., Fang Y.Z. Analysis of sugars in traditional Chinese drugs. J. Chromatogr. B. 2004;812:309–324. doi: 10.1016/S1570-0232(04)00763-9. [DOI] [PubMed] [Google Scholar]
- 31.Fu X.F., Huang L., Zhai M.L., Li W., Liu H.W. Analysis of natural carbohydrate biopolymer-high molecular chitosan and carboxymethyl chitosan by capillary zone electrophoresis. Carbohydr. Polym. 2007;68:511–516. doi: 10.1016/j.carbpol.2006.11.001. [DOI] [Google Scholar]
- 32.Honda S., Iwase S., Makino A., Fujiwara S. Simultaneous determination of reducing monosaccharides by capillary zone electrophoresis as the borate complexes of N-2-pyridylglycamines. Anal. Biochem. 1989;176:72–77. doi: 10.1016/0003-2697(89)90274-1. [DOI] [PubMed] [Google Scholar]
- 33.Fu D.T., Oneill R.A. Monosaccharide composition analysis of oligosaccharides and glycoproteins by high-performance liquid chromatography. Anal. Biochem. 1995;227:377–384. doi: 10.1006/abio.1995.1294. [DOI] [PubMed] [Google Scholar]
- 34.Fu D.T., Zopf D. Analysis of sialyllactoses in blood and urine by high-performance liquid chromatography. Anal. Biochem. 1999;269 doi: 10.1006/abio.1998.3021. [DOI] [PubMed] [Google Scholar]
- 35.Strydom D.J. Chromatographic separation of 1-phenyl-3-methyl-5-pyrazolone-derivatized neutral, acidic and basic aldoses. J. Chromatogr. A. 1994;678:17–23. doi: 10.1016/0021-9673(94)87069-1. [DOI] [Google Scholar]
- 36.Zhang L.Y., Xu J., Zhang L.H., Zhang W.B., Zhang Y.K. Determination of 1-phenyl-3-methyl-5-pyrazolone-labeled carbohydrates by liquid chromatography and micellar electrokinetic chromatography. J. Chromatogr. B. 2003;793:159–165. doi: 10.1016/S1570-0232(03)00373-8. [DOI] [PubMed] [Google Scholar]
- 37.Shen X.D., Perreault H.L. Characterization of carbohydrates using a combination of derivatization, high-performance liquid chromatography and mass spectrometry. J. Chromatogr. A. 1998;811:47–59. doi: 10.1016/S0021-9673(98)00238-6. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y., Zhou X.Y. Purification, initial characterization and immune activities of polysaccharides from the fungus, Polyporus umbellatus. Food Sci. Human Wellness. 2014;3:73–78. doi: 10.1016/j.fshw.2014.06.002. [DOI] [Google Scholar]
- 39.He P.F., Zhang A.Q., Zhang F.M., Linhardt R.J., Sun P.L. Structure and bioactivity of a polysaccharide containing uronic acid from Polyporus umbellatus sclerotia. Carbohydr. Polym. 2016;152:222–230. doi: 10.1016/j.carbpol.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 40.He P.F., Zhang A.Q., Wang X.L., Qu L., Li G.L., Li Y.P., Sun P.L. Structure elucidation and antioxidant activity of a novel polysaccharide from Polyporus umbellatus sclerotia. Int. J. Biol. Macromol. 2016;82:411–417. doi: 10.1016/j.ijbiomac.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 41.He P.F., He L., Zhang A.Q., Wang X.L., Qu L., Sun P.L. Structure and chain conformation of a neutral polysaccharide from sclerotia of Polyporus umbellatus. Carbohydr. Polym. 2017;155:61–67. doi: 10.1016/j.carbpol.2016.08.041. [DOI] [PubMed] [Google Scholar]
- 42.Dai H., Han X.Q., Gong F.Y., Dong H.L., Tu P.F., Gao X.M. Structure elucidation and immunological function analysis of a novel beta-glucan from the fruit bodies of Polyporus umbellatus (Pers.) Fries. Glycobiology. 2012;22:1673–1683. doi: 10.1093/glycob/cws099. [DOI] [PubMed] [Google Scholar]
- 43.Li X.Q., Xu W. TLR4-mediated activation of macrophages by the polysaccharide fraction from Polyporus umbellatus(pers.) Fries. J. Ethnopharmacol. 2011;135:1–6. doi: 10.1016/j.jep.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 44.Yeeprae W., Kawakami S., Yamashita F., Hashida M. Effect of mannose density on mannose receptor-mediated cellular uptake of mannosylated O/W emulsions by macrophages. J. Control. Release. 2006;114:193–201. doi: 10.1016/j.jconrel.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 45.Swain S.D., Lee S.J., Nussenzweig M.C., Harmsen A.G. Absence of the macrophage mannose receptor in mice does not increase susceptibility to pneumocystis carinii infection in vivo. Infect. Immun. 2003;71:6213–6221. doi: 10.1128/IAI.71.11.6213-6221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Magnusson S., Berg T. Extremely rapid endocytosis mediated by the mannose receptor of sinusoidal endothelial rat liver cells. Biochem. J. 1989;257 doi: 10.1042/bj2570651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Largent B.L., Walton K.M., Hoppe C.A., Lee Y.C., Schnaar R.L. Carbohydrate-specific adhesion of alveolar macrophages to mannose-derivatized surfaces. J. Biol. Chem. 1984;259:1764–1769. [PubMed] [Google Scholar]
- 48.Burgdorf S., Lukacs-Kornek V., Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J. Immunology. 2006;176:6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- 49.Yoshizawa Y., Enomoto A., Todoh H., Ametani A., Kaminogawa S. Activation of murine macrophages by polysaccharide fractions from marine algae (Porphyra yezoensis) Biosci. Biotech. Biochem. 1993;157:1862–1866. doi: 10.1271/bbb.57.1862. [DOI] [PubMed] [Google Scholar]
- 50.Wang Z.J., Luo D.H. Antioxidant activities of different fractions of polysaccharide purified from Gynostemma pentaphyllum Makino. Carbohydr. Polym. 2007;68:54–58. doi: 10.1016/j.carbpol.2006.07.022. [DOI] [Google Scholar]
- 51.Zhu X.F., Lin B.C., Jakob A., Epperlein U., Kppenhoefer B. Optimization and parameter study for chiral Separation by capillary electrophoresis. J. High Resol. Chromatogr. 1999;22:449–453. doi: 10.1002/(SICI)1521-4168(19990801)22:8<449::AID-JHRC449>3.0.CO;2-K. [DOI] [Google Scholar]
- 52.Chang Y.L., Liu B., Shen B. Orthogonal array design for the optimization of supercritical fluid extraction of baicalin from roots of Scutellaria baicalensis Georgi. J. Sep. Sci. 2007;30:1568–1574. doi: 10.1002/jssc.200700020. [DOI] [PubMed] [Google Scholar]
- 53.Li T.L., Zhang Z.M., Zhang L., Huang X.J., Lin J.W., Chen G.A. An improved facile method for extraction and determination of steroidal saponins in Tribulus terrestris by focused microwave-assisted extraction coupled with GC-MS. J. Sep. Sci. 2009;32:4167–4175. doi: 10.1002/jssc.200900483. [DOI] [PubMed] [Google Scholar]
- 54.Åman P., McNeil M., Franzén L.E., Darvill A.G., Albersheim P. Structural elucidation, using h.p.l.c.-m.s. and g.l.c.-m.s., of the acidic polysaccharide secreted by rhizobium meliloti strain 1021. Carbohydr. Res. 1981;95:263–282. doi: 10.1016/S0008-6215(00)85582-2. [DOI] [Google Scholar]
- 55.Doležalová M., Čápová H., Jobánek R. Determination of the purity of phenoxymethylpenicillin by micellar electrokinetic chromatography and reversed phase liquid chromatography on a monolithic silica column. J. Sep. Sci. 2003;26:701–708. doi: 10.1002/jssc.200301441. [DOI] [PubMed] [Google Scholar]
- 56.Zitka O., Heger Z., Kominkova M., Skalickova S., Krizkova S., Adam V., Kizek R. Preconcentration based on paramagnetic microparticles for the separation of sarcosine using hydrophilic interaction liquid chromatography coupled with coulometric detection. J. Sep. Sci. 2014;37:465–575. doi: 10.1002/jssc.201301188. [DOI] [PubMed] [Google Scholar]
- 57.Wang N.N., Wang X.P., Huang X.W., Mao Z.J., Zhang Y., Yu Y., Shou D. Monosaccharide composition analysis of immunomodulatory polysaccharides by on-line hollow fiber microextraction with high-performance liquid chromatography. J. Sep. Sci. 2016;39:818–826. doi: 10.1002/jssc.201501205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.