Abstract
Purpose
Angiopoietin-like protein 2 (ANGPTL2) is a secretory glycoprotein with various functions in vascular biology, inflammation and tumor development. As shown in our previous studies, ANGPTL2 expression positively correlates with liver fibrosis stages in chronic hepatitis B (CHB) patients. The aim of this study was further to assess whether ANGPTL2 represents a potential biomarker for detecting hepatocellular carcinoma (HCC).
Patients and methods
This study enrolled 361 participants including healthy controls (HCs) and patients with CHB, liver cirrhosis (LC) and HCC. A discovery cohort consisted of 35 HCs and 55 patients with HCC. A total of 271 participants, including 45 HCs, 125 patients with CHB, 38 patients with LC, and 63 patients with HCC were enrolled in a validation cohort. Serum ANGPTL2 levels were detected using a human ANGPTL2 assay kit, and hepatic expression of ANGPTL2 was analyzed using immunohistochemistry.
Results
In the discovery cohort, a significantly higher serum ANGPTL2 level was detected in HCC than in HCs (73.49±33.87 vs 30.54±9.86; p<0.001). The results of the receiver operating characteristic curve indicated a significantly higher area under the curve for the ability of the ANGPTL2 to predict HCC than alpha fetoprotein (AFP). In the validation cohort, serum ANGPTL2 level gradually increased with the progression of chronic hepatitis B virus infection and reached the highest level in HCC. Immunohistochemical staining also confirmed these findings. The serum ANGPTL2 displayed better diagnostic efficiency not only for differentiating HCC from HC but also for differentiating HCC from high-risk controls (CHB+LC). Furthermore, the combination of ANGPTL2 and AFP may increase the diagnostic accuracy for HCC compared to ANGPTL2 or AFP alone. Importantly, ANGPTL2 levels also correlated with the detection of AFP-negative HCC.
Conclusions
ANGPTL2 may be used as a promising biomarker for the diagnosis of CHB-related HCC.
Keywords: ANGPTL2, CHB, HCC, diagnosis
Introduction
Hepatocellular carcinoma (HCC) is the most common malignant tumor of the liver and is mainly caused by chronic hepatitis B or C infection in underdeveloped and developing countries.1,2 China is a major region with a high incidence of HCC, where approximately 383,000 individuals die from this disease annually, accounting for 51% of all HCC deaths worldwide.3 The early diagnosis of HCC is crucial for the implementation of therapeutic intervention to improve the prognosis and long-term survival of patients.4 The serum alpha fetoprotein (AFP) level is the most commonly marker to detect HCC in clinical practice.5 However, the diagnostic efficiency of AFP is limited due to its poor sensitivity and specificity, and it is no longer recommended by the American Association for the Study of Liver Diseases (AASLD) and European Association for the Study of the Liver (EASL).5–7
Angiopoietin-like protein 2 (ANGPTL2) is a member of the angiopoietin-like protein family that has various functions in inflammation, vascular biology and tumor metastasis.8 In HCC cell lines, the overexpression of ANGPTL2 promotes tumor formation and drives HCC metastasis.9 As shown in our previous studies, the serum ANGPTL2 level positively correlates with the stages of liver fibrosis in patients with chronic hepatitis B (CHB).10 However, few studies have investigated serum ANGPTL2 levels in patients with HCC, particularly in patients with AFP-negative HCC.
Thus, the current study was designed to assess the ANGPTL2 levels during the progression from CHB to HCC and to evaluate the diagnostic value of ANGPTL2 as a potential biomarker for the detection of HCC.
Patients and methods
Patients
This study enrolled 361 participants including healthy controls (HCs) and patients with CHB, liver cirrhosis (LC) and hepatocellular carcinoma (HCC). A discovery cohort consisted of 35 HCs and 55 patients with HCC who were enrolled at Peking University Shenzhen hospital between September 2017 and November 2017. Two hundred seventy-one participants were enrolled in a validation cohort between January 2018 and March 2019 including 45 HCs, 38 patients with LC, and 63 patients with HCC from Peking University Shenzhen Hospital as well as 125 patients with CHB from Peking University First Hospital.
HCs were individuals who visited the hospital for an annual physical examination, had normal liver biochemistry, and were negative for hepatitis virus serology. All HCs had no history of liver disease, gastrointestinal diseases or malignant tumors. Patients with CHB were randomly selected from the China HepB-related Fibrosis Assessment Research cohort supported by the China Mega-project for Infectious Diseases. Patients with CHB who were recruited to participate in this study met the following criteria: (1) age 18–75 years; (2) HBsAg seropositive status for greater than 6 months; (3) treatment naïve; (4) negative serum levels of anti-HAV IgM, anti-HCV, anti-HEV IgM/IgG, anti-EBV IgM, and anti-CMV IgM; and (5) were not treated with potential transaminase-lowering agents, such as bicyclol, for at least 2 weeks prior to blood sampling for measurements of biochemical parameters. Exclusion criteria for this study included patients with LC or HCC, and patients with overlapping etiologies for hepatitis including HCV, human immunodeficiency virus (HIV), alcohol abuse, autoimmune, genetic, drug-induced and nonalcoholic fatty liver disease.11 The diagnosis of LC was based on a history of CHB infection and confirmed by liver biopsy or imaging techniques, ie, computed tomography (CT), or magnetic resonance imaging (MRI). Patients with HCC were diagnosed based on ultrasound, CT or MRI and AFP serology, and the diagnosis was ultimately confirmed by a liver biopsy, according to the Practice Guidelines.12
All patients provided written informed consent for the scientific use of their clinical data and samples, and the study was approved by the local ethics committee of Peking University Shenzhen Hospital. The study conformed to the principles of the Helsinki Declaration.
Laboratory measurements
Biochemical tests, blood cell and coagulation tests were performed using routine automated analyzers. Serum AFP levels were determined using an electrochemical luminescence immunoassay (Cobas E601) (Roche, Mannheim, Germany). Serum levels of HBsAg and HBeAg were quantified using commercially available enzyme immunoassays (Roche Diagnostics, Penzberg, Germany). Serum HBV DNA levels (range 2.0×101–1.7x108 IU/ml) were measured using a COBAS AmpliPrep/COBAS TaqMan method, as previously described.13
Measurement of ANGPTL2 levels
Serum ANGPTL2 levels were measured using the Human ANGPTL2 Assay kit (Immuno-Biological Laboratories Co., Ltd., Japan) according to the manufacturer’s instructions.10 Serum sample were aliquoted into small volumes and stored at −80 °C to ensure that all samples only underwent one freeze-thaw cycle. All angiopoietin-like protein 2 tests were performed within 2 weeks. The samples were retested if the coefficient of variation between the duplicate wells was higher than 10% or if the R squared value of the standard curve was less than 0.99.
Immunohistochemistry
Immunohistochemical staining was performed on paraffin-embedded liver tissue sections cut to a thickness of 5 µm to determine the expression and distribution of ANGPTL2 in the liver tissue. Tissue were placed in an oven (at 74 °C) for 24 min and then dewaxed with automatic dewaxing equipment (through xylene, anhydrous alcohol, 90% alcohol, 80% alcohol, 70% alcohol, and water). Dewaxed tissue sections were microwaved in 10 mM citric acid buffer (pH 6.0) for 20 min and washed three times with PBS. Sections were incubated with rabbit anti-ANGPTL2 antibodies (ab199133, 1:100 dilution; Abcam, Cambridge, UK) for 1 h at 37 °C, exposed to an endogenous catalase blocker for 8 min, and then incubated with Universal anti-Mouse/Rabbit-HRP (MXB Biotechnologies) for 30 min at room temperature.
The immunoreactivity of ANGPTL2 was graded according to the average percentage of ANGPTL2-positive cells in each tissue sample as follows: <25% (1), 25–50% (2), >50–75% (3) and >75% (4).
Statistical analysis
SPSS version 16.0 (Chicago, USA) and GraphPad Prism version 8.0 (San Diego, USA) were used for the statistical analyses. Data are reported as counts and percentages for categorical variables and as the means and standard deviations (SD) for continuous variables. Comparisons between different groups were performed with Student’s t-test or the Mann–Whitney test (two groups) and one-way ANOVA test (more than two groups) for quantitative variables. Comparisons of categorical variables between different groups were performed with Fisher’s exact test. Multivariate analysis was performed to analyze whether ANGPTL2 was independent predictor of HCC using binary logistic regression with forward conditional method. An algorithm combining ANGPTL2 and AFP levels to detect HCC was also derived from the binary logistic regression analysis with enter method. The analysis and comparison of the receiver operating characteristics (ROC) curve of ANGPTL2, AFP, and the combination of ANGPTL2 and AFP level for diagnosing HCC were performed using MedCalc version 15.6 (Ostend, Belgium). Additionally, the sensitivity, specificity, positive predictive values (PPV) and negative predictive value (NPV) for cut-off values were calculated. The AUROCs were compared using the Delong test. Statistical significance was defined as p<0.05 (two-tailed).
Results
Clinical characteristics of the study population
Three hundred sixty-one participants were enrolled in this study. The characteristics of the enrolled population are summarized in Table 1. In the discovery cohort, patients with HCC were significantly older than the HCs (p<0.001). The proportion of males was higher among patients with HCC among HCs (p<0.001). The HC and HCC groups had significantly different laboratory results for alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin (TBil), direct bilirubin (DBI), albumin (ALB) and platelet (PLT) levels (all p<0.01). In the validation cohort, age and ALT, AST, TBil, DBI, ALB and PLT levels were significantly different among the HC, CHB, LC and HCC groups (p<0.001). In addition, 26 (47.27%) patients were negative for AFP (<13.4 ng/ml) in the discovery cohort whereas 30 (47.62%) patients were negative for AFP in the validation cohort.
Table 1.
Demographic and clinical characteristics of all enrolled populations
| Discovery cohort | p-value | Validation cohort | p-value | |||||
|---|---|---|---|---|---|---|---|---|
| HC (n=35) | HCC (n=55) | HC (n=45) | CHB (n=125) | LC (n=38) | HCC (n=63) | |||
| Age (years) | 41.14±9.24 | 51.09±13.65 | <0.001 | 45.41±12.95 | 35.41±10.55 | 44.92±11.30 | 51.48±13.07 | <0.001 |
| Gender (male, %) | 15, 42.86% | 47, 85.45% | <0.001 | 23, 51.11% | 111, 88.8% | 32, 84.21% | 52, 82.54% | 0.14 |
| ALT (U/L) | 20.34±12.40 | 49.56±32.01 | <0.001 | 20.45±14.17 | 77.32±84.23 | 84.81±95.83 | 48.19±30.84 | <0.001 |
| AST (U/L) | 19.74±8.00 | 67.36±52.09 | <0.001 | 19.38±6.09 | 45.76±37.38 | 62.62±51.60 | 64.28±49.51 | <0.001 |
| TBil (μmol/L) | 14.26±4.28 | 25.66±23.37 | 0.001 | 13.95±4.53 | 15.85±20.23 | 25.88±22.18 | 25.29±21.14 | <0.001 |
| DBI (μmol/L) | 2.83±0.96 | 8.35±12.79 | 0.002 | 2.77±0.96 | 4.78±3.05 | 10.25±14.32 | 8.06±12.0 | <0.001 |
| ALB (g/L) | 43.60±2.62 | 36.14±6.53 | <0.001 | 44.09±2.65 | 45.10±5.13 | 39.92±7.08 | 36.22±6.55 | <0.001 |
| PLT(/L) | 239.17±50.96 | 182.6±134.45 | 0.006 | 238.1±51.80 | 191.5±54.27 | 127.6±62.26 | 173.8±129.0 | <0.001 |
| HBV DNA (log10 IU/ml) | 1.91±1.30 | 6.81±1.99 | 4.33±3.03 | 1.70±2.10 | <0.001 | |||
| HBeAg (positive, %) | 8, 14.55% | 96, 76.8% | 13, 34.21% | 10, 15.87% | 0.91 | |||
| AFP (<13.4 ng/ml, %) | 26, 47.27% | 30, 47.62% | ||||||
Abbreviations: HC, healthy control; HCC, hepatocellular carcinoma; CHB, chronic hepatitis B; LC, liver cirrhosis; ALT, alanine transaminase, AST, aspartate transaminase; TBil, total bilirubin; DBI, direct bilirubin; ALB, albumin; PLT, platelet; HBeAg, hepatitis B e antigen; AFP, alpha-fetoprotein.
Serum ANGPTL2 levels and diagnostic potential in patients with HCC
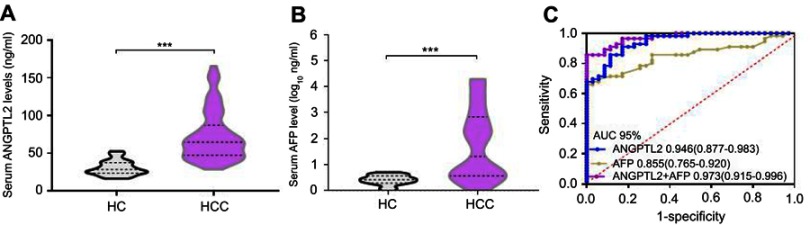
In this study, we first recruited the discovery cohort of 90 volunteers, including 35 HCs and 55 patients with HCC. The serum ANGPTL2 level was significantly higher in patients with HCC than in HCs (73.49±33.87 ng/ml vs 30.54±9.86 ng/ml; p<0.001). In addition, we further analyzed the serum AFP level (Figure 1A). Significantly higher AFP levels were detected in patients with HCC than in HCs (1.75±1.37 log10 ng/ml vs 0.39±0.20 log10 ng/ml; p<0.001) (Figure 1B). According to the multivariate analysis, the ANGPTL2 level (p<0.001), but not AFP level, was independent predictor of HCC (Table S1).
Figure 1.
Exploratory data from the discovery cohort: the ability of ANGPTL2 levels to serve as a potential serum marker for the detection of HCC, alone or in combination with AFP levels.
Notes: (A) Violin plots showing serum ANGPTL2 levels in HCs (n=35) and patients with HCC (n=55). (B) Violin plots showing serum AFP levels in HCs (n=35) and patients with HCC (n=55). (C) ROC curve analyses of the diagnostic potential of ANGPTL2, AFP, and the combination of ANGPTL2 and AFP levels. ***p<0.001.
Abbreviations: ANGPTL2, angiopoietin-like protein 2; HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein; HC, healthy control; CHB, ROC, receiver operating characteristics; AUC, area under the curve; CI, confidence interval.
Table S1.
Univariate and multivariate analysis of ANGPTL2 and clinical parameters with HC and HCC
| Univariate analysis | p-value (Univariate) | Multivariate analysis | p-value (Multivariate) | ||
|---|---|---|---|---|---|
| HC (n=35) | HCC (n=55) | Odds Ratio (95% CI) | |||
| Age (years) | 41.14±9.24 | 51.09±13.65 | <0.001 | ||
| Gender (male, %) | 15, 42.86% | 47, 85.45% | <0.001 | ||
| ALT (U/L) | 20.34±12.40 | 49.56±32.01 | <0.001 | ||
| AST (U/L) | 19.74±8.00 | 67.36±52.09 | <0.001 | ||
| TBil (μmol/L) | 14.26±4.28 | 25.66±23.37 | 0.001 | ||
| DBI (μmol/L) | 2.83±0.96 | 8.35±12.79 | 0.002 | ||
| ALB (g/L) | 43.60±2.62 | 36.14±6.53 | <0.001 | 0.608 (0.422–0.875) | 0.007 |
| PLT(/L) | 239.17±50.96 | 182.6±134.45 | 0.006 | 0.979 (0.966–0.992) | 0.001 |
| ANGPTL2 (ng/ml) | 30.54±9.86 | 73.49±33.87 | <0.001 | 1.236 (1.104–1.383) | <0.001 |
| AFP (log10 ng/ml) | 1.75±1.37 | 0.39±0.20 | <0.001 | ||
Abbreviations: HC, healthy control; HCC, hepatocellular carcinoma; ALT, alanine transaminase, AST, aspartate transaminase; TBil, total bilirubin; DBI, direct bilirubin; ALB, albumin; PLT, platelet; ANGPTL2, angiopoietin-like protein 2; AFP, alpha-fetoprotein.
The results of the ROC curve analysis indicated a significantly higher area under the curve (AUC) for the ability of ANGPTL2 levels (0.946, 95% confidence interval (CI) 0.877–0.983) to predict HCC than AFP levels (0.855, 95% CI 0.765–0.920, p=0.036) (Figure 1C). We also performed a binary logistic regression analysis combining ANGPTL2 and AFP levels, and found that the AUC for the combination of ANGPTL2 with AFP levels was significantly higher than for ANGPTL2 or AFP alone (0.973 95% CI 0.915–0.996; p=0.046 and 0.001; respectively). These data suggested that serum ANGPTL2 levels may be a potential biomarker for differentiating HCC from HC. The score for the combination of ANGPTL2 and AFP levels was calculated using the formula: logit p=1/[1+ exp (−0.148× ANGPTL2 ng/ml - 0.626× AFP ng/ml +8.469)].
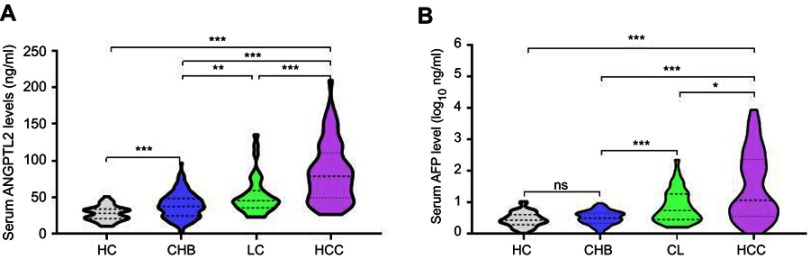
Based on the above findings, we further designed a validation cohort to confirm the potential value of ANGPTL2 levels in diagnosing HCC. Two hundred seventy-one participants were enrolled, including 45 HCs, 125 patients with CHB, 38 patients with LC and 63 patients with HCC. Serum ANGPTL2 levels in the HC, CHB, LC and HCC groups were 28.10±9.92 ng/ml, 38.94±17.79 ng/ml, 52.25±20.80 ng/ml and 83.85±40.90 ng/ml, respectively (Figure 2A). Significantly higher serum ANGPTL2 levels were detected in patients with HCC than in HCs (p<0.001). ANGPTL2 levels were increased in patients with CHB and LC, but the levels were significantly lower than in patients with HCC (all p<0.001). In addition, serum AFP levels in the HC, CHB, LC and HCC groups were 0.45±0.23 log10 ng/ml, 0.48±0.24 log10 ng/ml, 0.87±0.51 log10 ng/ml and 1.47±1.10 log10 ng/ml, respectively. Substantially higher AFP levels were observed in patients with HCC than in patients with CHB and LC (Figure 2B). Based on these results, the ANGPTL2 levels gradually increased with the progression of chronic hepatitis B and reached the highest level in patients with HCC.
Figure 2.
Serum ANGPTL2 and AFP levels in the validation cohort.
Notes: (A) Violin plots showing serum ANGPTL2 levels in the HC (n=45), CHB (n=125), CL (n=38) and HCC (n=63) groups. (B) Violin plots showing serum AFP levels in the HC (n=45), CHB (n=125), CL (n=38) and HCC (n=63) groups. * p<0.05; ** p<0.01; *** p<0.001; ns, no significance.
Abbreviations: ANGPTL2, angiopoietin-like protein 2; AFP, alpha-fetoprotein; HC, healthy control; CHB, chronic hepatitis B; LC, liver cirrhosis; HCC, hepatocellular carcinoma.
Diagnostic efficiency of ANGPTL2 levels in HCC
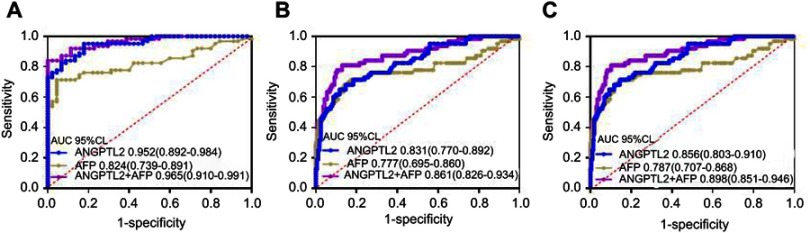
In the validation cohort, the AUC for the ability of ANGPTL2 to differentiate patients with HCC from HCs was 0.952 (95% CI 0.892–0.984), a value that was significantly higher than the value of 0.824 (95% CI 0.892–0.984) for AFP (p=0004, Figure 3A). The use of an ANGPTL2 cutoff value of 35.96 ng/ml produced a sensitivity of 95.24%, a specificity of 81.82%, and an accuracy of 90.74%. The use of an AFP cutoff value of 6 ng/ml produced a sensitivity of 71.43%, a specificity of 95.45%, and an accuracy of 81.49% (Table 2). Similar results were obtained from diagnostic efficiency analyses using the same cutoff value of ANGPTL2 and AFP in the discovery cohort. These data confirmed that ANGPTL2 exhibited better diagnostic efficiency for differentiating patients with HCC from HCs.
Figure 3.
Analysis of the ability of serum ANGPTL2 levels to diagnose HCC in the validation cohort.
Notes: (A) ROC curves for patients with HCC versus HCs; (B) ROC curves for patients with HCC versus high-risk controls (CHB + LC); (C) ROC curves for patients with HCC versus all controls (HC+CHB+LC).
Abbreviations: ANGPTL2, angiopoietin-like protein 2; HCC, hepatocellular carcinoma; ROC, receiver operating characteristics; HC, healthy control; CHB, chronic hepatitis B; LC, liver cirrhosis; ROC, receiver operating characteristics; AUC, area under the curve; CI, confidence interval.
Table 2.
Diagnostic value of ANGPTL2 in differentiating HCC from HC, high-risk controls (CHB+LC) and all controls (HC+ CHB + LC)
| Cutoff value | Se (%) | Spe (%) | PPV (%) | NPV (%) | Accuracy (%) | AUC (95% CI) | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Discovery cohort | ||||||||
| HCC versus HC | ||||||||
| ANGPTL2 | 35.96 ng/ml | 98.18 | 68.57 | 83.08 | 96 | 86.67 | 0.946 (0.877–0.983) | 0.036* |
| AFP | 6 ng/ml | 67.27 | 100 | 100 | 69.81 | 80 | 0.855 (0.765–0.920) | 0.046# |
| ANGPTL2+AFP | 0.88 | 85.45 | 100 | 100 | 81.4 | 91.11 | 0.973 (0.915–0.996) | 0.001† |
| Validation cohort | ||||||||
| HCC versus HC | ||||||||
| ANGPTL2 | 35.96 ng/ml | 95.24 | 81.82 | 88.2 | 92.3 | 90.74 | 0.952 (0.892–0.984) | 0.004* |
| AFP | 6 ng/ml | 71.43 | 95.45 | 95.7 | 70 | 81.48 | 0.824 (0.739–0.891) | 0.351# |
| ANGPTL2+AFP | 0.92 | 84.13 | 100 | 100 | 81.5 | 91.59 | 0.965 (0.910–0.991) | 0.0001† |
| HCC versus CHB+LC | ||||||||
| ANGPTL2 | 59.1 ng/ml | 68.25 | 87.34 | 64.18 | 87.34 | 80.44 | 0.831 (0.770–0.892) | 0.267* |
| AFP | 18.5 ng/ml | 42.86 | 93.25 | 71.05 | 80.85 | 79.2 | 0.777 (0.695–0.860) | 0.208# |
| ANGPTL2+AFP | 0.99 | 77.78 | 89.57 | 74.24 | 91.2 | 82.74 | 0.861 (0.826–0.934) | 0.018† |
| HCC versus HC+CHB+LC | ||||||||
| ANGPTL2 | 59.1 ng/ml | 68.25 | 88.46 | 64.18 | 90.1 | 83.76 | 0.856 (0.803–0.910) | 0.129* |
| AFP | 18.5 ng/ml | 42.86 | 94.71 | 71.05 | 84.55 | 82.66 | 0.787 (0.707–0.868) | 0.203# |
| ANGPTL2+AFP | 0.99 | 77.78 | 91.83 | 74.24 | 93.17 | 88.56 | 0.898 (0.851–0.946) | 0.006† |
Notes: *ANGPTL2 vs AFP; #ANGPTL2+ AFP vs ANGPTL2; †ANGPTL2+ AFP vs AFP.
Abbreviations: ANGPTL2, angiopoietin-like protein 2; HCC, hepatocellular carcinoma; HC, healthy control; CHB, chronic hepatitis B; LC, liver cirrhosis; Se, Sensitivity; Spe, Specificity; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve; CI, confidence interval; AFP, alpha-fetoprotein.
The diagnostic value of ANGPTL2 in differentiating patients with HCC from high-risk controls (CHB+LC) was further assessed. According to the ROC curves analyses, the AUC of ANGPTL2 was higher than that of AFP, although a statistically significant difference was not observed between the two groups (0.831 vs 0.777; p=0.267) (Figure 3B and Table 2). The use of an ANGPTL2 cutoff value of 59.10 ng/ml produced a sensitivity of 68.25%, a specificity of 87.34% and an accuracy of 80.44%. The use of an AFP cutoff value of 18.5 ng/ml produced a sensitivity of 42.86%, a specificity of 93.25% and an accuracy of 79.2%. Similar results were obtained from ROC curve analyses when differentiating patients with HCC from all controls (HC+CHB+LC) (Figure 3C and Table 2).
Furthermore, the diagnostic value of ANGPTL2 in combination with AFP was further evaluated. The AUC of the combination of ANGPTL2 with AFP for differentiating patients with HCC from HCs was 0.965 (95% CI 0.910–0.991), whereas it was 0.861 (95% CI 0.826–0.934) for differentiating patients with HCC from high-risk controls and 0.898 (95% CI 0.851–0.946) for differentiating patients with HCC from all controls (Figure 3). The use of cut-off values of 0.92, 0.99 and 0.99 produced a sensitivity of 84.13%, 77.79% and 77.78%, a specificity of 100%, 89.57% and 91.83%, and an accuracy of 91.59%, 82.74% and 88.56% (Figure 3 and Table 2). Compared to AFP alone, the combination of ANGPTL2 with AFP showed significantly higher diagnostic efficiency for HCC (p=0.0001, 0.018 and 0.006; respectively). Together, the serum ANGPTL2 level represents a potent biomarker for the diagnosis of HCC and the combination of ANGPTL2 and AFP level exhibits a better diagnostic value.
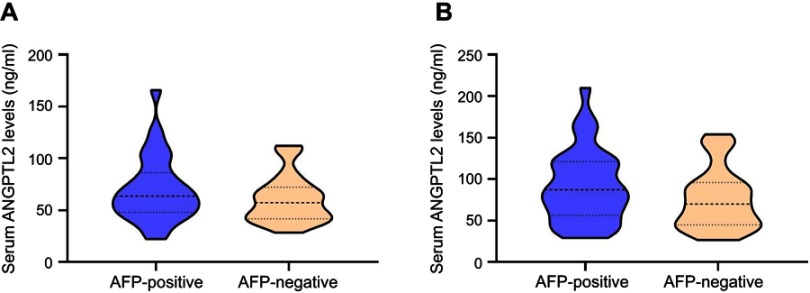
Serum ANGPTL2 levels in the detection of AFP-negative HCC
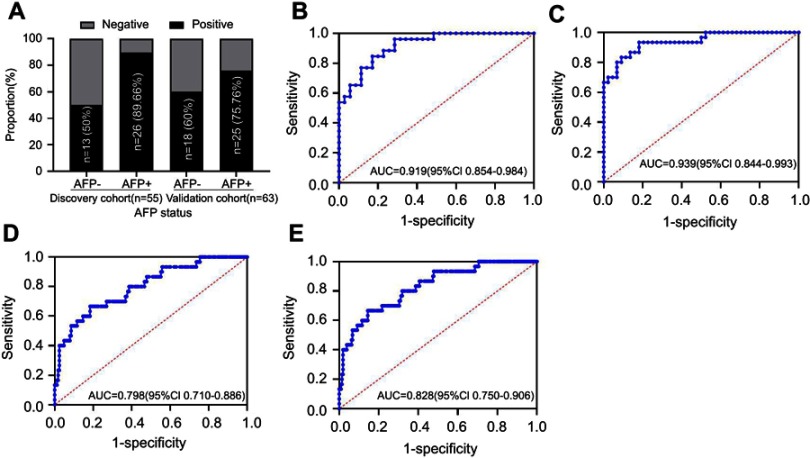
We evaluated the expression of ANGPTL2 in AFP-negative patients to determine its ability to effectively detect patients with AFP-negative HCC. The serum ANGPTL2 levels was not significantly different between AFP-positive and AFP-positive patients with HCC both in the discovery and validation cohorts (Figure S1). In the discovery cohort, 26 patients were AFP-negative, among whom 13 (50%) patients were ANGPTL2-positive (using a cutoff of 59.10 ng/ml, Figure 4A). The ROC analyses for ANGPTL2 in differentiating AFP-negative patients with HCC from HCs revealed a diagnostic value, with an AUC of 0.919 (95% CI 0.854–0.984) (Figure 4B). These findings were confirmed in the validation cohort with 30 AFP-negative patients with HCC (Figure 4A and C). Furthermore, the AUC of ANGPTL2 for differentiating AFP-negative patients with HCC from high-risk controls was 0.798 (95% CI 0.710–0.886), whereas it was 0.828 (95% CI 0.750–0.906) for differentiating AFP-negative patients with HCC from all controls (Figure 4D–E). Thus, ANGPTL2 levels have potential diagnostic value in detecting AFP-negative patients with HCC.
Figure 4.
Analysis of the ability of serum ANGPTL2 levels to diagnose AFP-negative HCC.
Notes: (A) ANGPTL2-positive rates in patients with HCC stratified by AFP status. (B) ROC curves for patients with HCC versus HCs in the discovery cohort; (C) ROC curves for patients with HCC versus HCs in the validation cohort; (D) ROC curves for patients with HCC versus high-risk controls (CHB + LC) in the validation cohort; (E) ROC curves for patients with HCC versus all controls (HC+CHB+LC) in the validation cohort. AFP <13.4 ng/ml is clinically normal and is considered as negative.
Abbreviations: ANGPTL2, angiopoietin-like protein 2; AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma; HC, healthy control; CHB, chronic hepatitis B; LC, liver cirrhosis; ROC, receiver operating characteristics; AUC, area under the curve; CI, confidence interval.
ANGPTL2 expression in the liver tissue
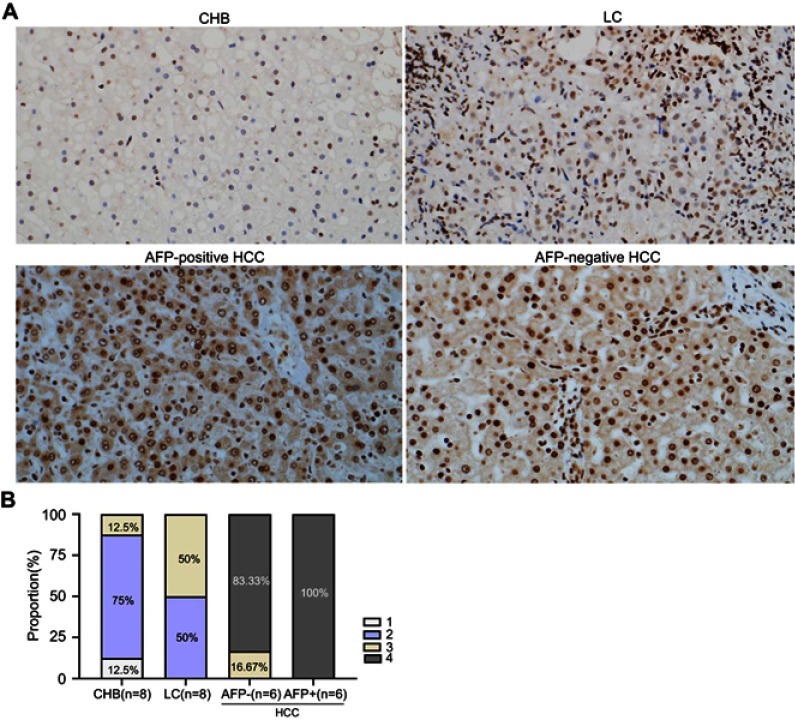
ANGPTL2 expression in the liver tissues derived from 8 patients with CHB, 8 patients with LC and 12 patients with HCC (6 AFP-positive and 6 AFP-negative patients) was visualized using immunohistochemistry to histologically examine ANGPTL2 levels during the progression of chronic hepatitis B. As shown in Figure 5, compared with high-risk controls (CHB and LC), ANGPTL2 was expressed at higher levels in patients with HCC, regardless of the AFP status. Specifically, 91.67% of liver tissues from patients with HCC received a score of 4 points, whereas liver tissues from patients with LC scored 2–3 points and 75% of liver tissues from patients with CHB scored 2 points.
Figure 5.
Immunohistochemical staining for ANGPTL2 in liver tissues from patients with CHB, LC and HCC.
Notes: (A) The staining of ANGPTL2 immunohistochemistry in liver tissues (original magnification ×200). (B) The scores of hepatic ANGPTL2 staining were demonstrated as histogram. The immunoreactivity of ANGPTL2 was graded according to the average percentage of ANGPTL2-positive cells in each tissue sample as follows: <25% (1), 25–50% (2), >50–75% (3) and >75% (4).
Abbreviations: ANGPTL2, angiopoietin-like protein 2; CHB, chronic hepatitis B; LC, liver cirrhosis; HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein.
Discussion
The ANGPTL family consists of eight secreted glycoproteins that play important roles in the regulation of inflammation, angiogenesis, and different stages of tumorigenesis and metastasis.8 The expression of the ANGPTL2 gene is upregulated in various tumors, such as sarcoma, skin squamous cell carcinoma and colorectal cancer.14–16 Regarding liver diseases, ANGPTL2 is overexpressed in patients with HCC,9 and our previous studies examining liver biopsies and clinicopathological data suggested a potential role for ANGPTL2 as a novel biomarker for predicting fibrosis stages in patients with CHB.10 In the present study, we recruited 361 participants including HCs and subjects with CHB, LC and HCC. Serum and histological ANGPTL2 levels gradually increased with the progression of CHB, and the highest levels were observed in patients with HCC.
HCC is an aggressive tumor that frequently develops in patients with underlying chronic liver diseases, such as chronic hepatitis B virus infection and cirrhosis.2 Therefore, the early diagnosis of the initial stage of HCC plays important roles in controlling disease progression and prolonging the survival time. Clinically, histopathology, imaging techniques and AFP are the main methods used to screen for HCC.17 However, liver biopsy is invasive and may increase the risk of needle metastasis and imaging techniques are usually only able to detect tumors larger than 1 cm in diameter;18 AFP is the most widely used clinical noninvasive biomarker and has been used for more than 50 years, but it lacks sufficient sensitivity and specificity.7 In the present study, a markedly higher serum ANGPTL2 level was detected in patients with HCC than in HCs. The results of ROC curve analyses revealed a superior diagnostic value of ANGPTL2 levels for differentiating patients with HCC from HCs compared to AFP levels, suggesting that ANGPTL2 represents a potential biomarker for the diagnosis of HCC. In addition to HCs, patients with CHB and LC were further analyzed as high-risk control. ANGPTL2 levels exhibited better diagnostic performance for differentiating patients with HCC from high-risk controls than AFP levels, and the combination of ANGPTL2 and AFP levels significantly increased the diagnostic efficiency compared to AFP alone (cutoff of 0.99 for the combination vs 59.10 ng/ml for ANGPTL2 or 18.5 ng/ml for AFP). Thus, the data confirm that ANGPTL2 represents a potential biomarker that is able to differentiate patients with HCC from HCs, and patients with chronic hepatitis and liver cirrhosis at risk of developing HCC with high accuracy.
AFP is negative in approximately one-third of patients with HCC, and the effective diagnosis of AFP-negative HCC is currently a key problem to be solved in the diagnosis of HCC.19 In the present study, greater than half of AFP-negative patients with HCC were positive for ANGPTL2. Furthermore, serum ANGPTL2 levels had high diagnostic value for differentiating AFP-negative patients with HCC from HCs, high-risk controls and all controls. Therefore, serum ANGPTL2 levels have potential value in detecting AFP-negative HCC.
The possible mechanism underlying the roles of ANGPTL2 in HCC remains unknown. In the present study, immunohistochemistry revealed high ANGPTL2 expression in liver tissues from patients with HCC, and the protein was mainly localized around the nucleus in tumor cells. Based on these data, ANGPTL2 might be involved in the occurrence and progression of HCC.9 The leucocyte immunoglobulin-like receptor B2 (LILRB2) has been identified as a unique ANGPTL2 receptor.20 The autocrine signaling pathway involving ANGPTL2 and its receptor LILRB2 plays a key role in maintaining the epithelial-to-mesenchymal transition during the progression of pancreatic ductal carcinogenesis.21 Moreover, ANGPTL2 also promotes lung cancer cells proliferation and migration by binding to LILRB2 and thus is a novel therapeutic target.22 LILRB2 is overexpressed in HCC tissues and remarkably correlates with a poor prognosis for patients with HCC.23 Therefore, we hypothesize that the ANGPTL2-LILRB2 axis potentially plays an important role in the pathogenesis and progression of HCC.
This study has several limitations. First, the sample size was limited, and large-scale research studies examining different populations, particularly patients with different stages of HCC, are still needed to verify our results. Second, our study employed a cross-sectional design and we did not further evaluate whether ANGPTL2 has potential value in determining the prognosis of patients with HCC. Finally, a mechanism explaining the role of ANGPTL2 in the pathogenesis of HCC is lacking. The specific molecular mechanisms require further research in the future.
Conclusion
We are the first to show that serum ANGPTL2 levels accurately differentiate patients with HCC from HCs and patients with chronic hepatitis and liver cirrhosis at high risk of development HCC. The combination of ANGPTL2 and AFP levels significantly improves diagnostic accuracy for HCC. ANGPTL2 levels are also able to accurately differentiate AFP-negative patients with HCC. Therefore, ANGPTL2 is a promising biomarker that exhibits better diagnostic accuracy for HBV-related HCC.
Acknowledgments
This study was supported by China Mega-Project for Infectious Diseases (grant numbers 2013ZX10002005 and 2017ZX10203202), China Postdoctoral Science Foundation (grant number 2018M633096), the Project of Guangdong Medical Science and Technology Research Foundation (grant number A2018102) and SZSM201612071.
Author contributions
JYZ designed the study, analyzed data, and contributed to writing the manuscript; SZ and TZ did the ELISA experiments; WNY, JTL, XQH and YQL recruited patients; GQW and JHC modified the manuscript and provided overall direction. All authors contributed to data analysis, drafting and revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
Supplementary materials
Levels of ANGPTL2 in AFP-negative and AFP-positive patients with HCC.
Notes: (A) Levels of ANGPTL2 in AFP-negative and AFP-positive patients with HCC in discovery cohort. (B) Levels of ANGPTL2 in AFP-negative and AFP-positive patients with HCC in validation cohort.
Abbreviations: ANGPTL2, angiopoietin-like protein 2; AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma.
References
- 1.Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7(8):448–458. doi: 10.1038/nrgastro.2010.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. doi: 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- 3.Wang FS, Fan JG, Zhang Z, et al. The global burden of liver disease: the major impact of China. Hepatology. 2014;60(6):2099–2108. doi: 10.1002/hep.27406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrell GC, Chan HL, Yuen MF ,et al. Prevention of hepatocellular carcinoma in the Asia-Pacific region: consensus statements. J Gastroenterol Hepatol. 2010;25(4):657–663. doi: 10.1111/j.1440-1746.2009.06167.x [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Ducreux M, Lencioni R ,et al.EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 7.Farinati F, Marino D, De Giorgio M, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol. 2006;101(3):524–532. doi: 10.1111/j.1572-0241.2006.00443.x [DOI] [PubMed] [Google Scholar]
- 8.Carbone C, Piro G, Merz V, et al. Angiopoietin-like proteins in angiogenesis, inflammation and cancer. Int J Mol Sci. 2018;19(2):431. doi: 10.3390/ijms19020431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao L, Ge C, Fang T, et al. ANGPTL2 promotes tumor metastasis in hepatocellular carcinoma. J Gastroenterol Hepatol. 2015;30(2):396–404. doi: 10.1111/jgh.12702 [DOI] [PubMed] [Google Scholar]
- 10.Deng Y, Zhao H, Zhou J, et al. Angiopoietin-like protein as a novel marker for liver fibrosis in chronic hepatitis B patients with normal to minimally raised ALT. BMC Infect Dis. 2017;17(1):650. doi: 10.1186/s12879-017-2757-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou J, Song L, Zhao H, et al. Serum hepatitis B core antibody as a biomarker of hepatic inflammation in chronic hepatitis B patients with normal alanine aminotransferase. Sci Rep. 2017;7(1):2747. doi: 10.1038/s41598-017-03102-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cong WM, Hu XQ, Sun YT, et al. Expert consensus on the scheme of pathological diagnosis of primary liver cancer. Chin Clin Oncol. 2012;1(1):12. [DOI] [PubMed] [Google Scholar]
- 13.Jia W, Song LW, Fang YQ, et al. Antibody to hepatitis B core antigen levels in the natural history of chronic hepatitis B: a prospective observational study. Medicine. 2014;93(29):e322. doi: 10.1097/MD.0000000000000322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teicher BA. Searching for molecular targets in sarcoma. Biochem Pharmacol. 2012;84(1):1–10. doi: 10.1016/j.bcp.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 15.Aoi J, Endo M, Kadomatsu T, et al. Angiopoietin-like protein 2 is an important facilitator of inflammatory carcinogenesis and metastasis. Cancer Res. 2011;71(24):7502–7512. doi: 10.1158/0008-5472.CAN-11-1758 [DOI] [PubMed] [Google Scholar]
- 16.Toiyama Y, Inoue Y, Shimura T, et al. Serum angiopoietin-like protein 2 improves preoperative detection of lymph node metastasis in colorectal cancer. Anticancer Res. 2015;35(5):2849–2856. [PubMed] [Google Scholar]
- 17.Benson AB 3rd, D’Angelica MI, Abbott DE, et al. NCCN guidelines insights: hepatobiliary cancers, version 1.2017. J Natl Compr Canc Netw. 2017;15(5):563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts LR, Sirlin CB, Zaiem F, et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology. 2018;67(1):401–421. doi: 10.1002/hep.29487 [DOI] [PubMed] [Google Scholar]
- 19.Luo P, Wu S, Yu Y, et al. Current status and perspective biomarkers in AFP negative HCC: towards screening for and diagnosing hepatocellular carcinoma at an earlier stage. Pathol Oncol Res. 2019. doi: 10.1007/s12253-019-00585-5 [DOI] [PubMed] [Google Scholar]
- 20.Zheng J, Umikawa M, Cui C, et al. Inhibitory receptors bind ANGPTLs and support blood stem cells and leukaemia development. Nature. 2012;485(7400):656–660. doi: 10.1038/nature11095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carbone C, Piro G, Fassan M, et al. An angiopoietin-like protein 2 autocrine signaling promotes EMT during pancreatic ductal carcinogenesis. Oncotarget. 2015;6(15):13822–13834. doi: 10.18632/oncotarget.2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Yu X, Xie J, et al. ANGPTL2/LILRB2 signaling promotes the propagation of lung cancer cells. Oncotarget. 2015;6(25):21004–21015. doi: 10.18632/oncotarget.4217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Wei X, Xu H, et al. Expression of leukocyte immunoglobulin-like receptor B2 in hepatocellular carcinoma and its clinical significance. J Cancer Res Ther. 2018;14(7):1655–1659. doi: 10.4103/jcrt.JCRT_542_18 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Levels of ANGPTL2 in AFP-negative and AFP-positive patients with HCC.
Notes: (A) Levels of ANGPTL2 in AFP-negative and AFP-positive patients with HCC in discovery cohort. (B) Levels of ANGPTL2 in AFP-negative and AFP-positive patients with HCC in validation cohort.
Abbreviations: ANGPTL2, angiopoietin-like protein 2; AFP, alpha-fetoprotein; HCC, hepatocellular carcinoma.