Abstract
The optimal therapy for advanced thymic carcinoma has long been controversial. Despite that complete (R0) resection is recommend as the first-line treatment, multidisciplinary approach including chemotherapy and radiotherapy should be considered for patients who lost the operation chance or received incomplete resection. Here, we present a case who received concurrent chemoradiotherapy (CCRT) after cytoreductive surgery. A complete response was observed and the patient has remained disease free for over 4 years. To our knowledge, this is the first report to demonstrate the efficacy of CCRT with cisplatin plus etoposide after incomplete surgery for advanced thymic carcinoma.
INTRODUCTION
Thymic carcinoma is a malignant mediastinal neoplasm with poor prognosis [1]. Due to its rarity, little information is available about managing patients with advanced stage. Although multidisciplinary treatment strategy including surgery, chemotherapy and radiotherapy is suggested, the value of concurrent chemoradiotherapy (CCRT) after incomplete surgery for advanced thymic carcinoma has long been unknown. Here, we present a case of advanced thymic carcinoma which had complete response to CCRT with cisplatin plus etoposide after incomplete resection.
CASE REPORT
A previously healthy 34-year-old man was admitted to a local hospital with the main complain of chest tightness in September 2014. Computed tomography (CT) revealed an abnormal mass in his anterior and middle mediastinum. Subsequent 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) showed positive uptake in a large mediastinal mass with no boundary between adjacent blood vessels and pericardium, the size of which was 7.9 × 4.8 × 6.9 cm (Fig. 1a). Besides, multiple swollen lymph nodes were seen on the supraclavicular fossa and mediastinum (the possibility of lymph node metastasis is not excluded) (Fig. 1b and c). Needle aspiration was performed for the mediastinal mass. The histological findings revealed non-keratinized squamous cell carcinoma originated from thymus (Fig. 2). The patient received operation in October 2014 to relieve his chest tightness. Mediastinal lymph nodes were swept while left supraclavicular lymph nodes were spared. Postoperative histologic report was as follows: non-keratinzing squamous cell carcinoma invades the lung tissue, the right ventricular wall and the local vessel and the margin cannot be evaluated; no clear neurological invasion and positive lymph node are observed. More than 3 weeks after surgery, the patient had a CT scan, which displayed multiple swollen lymph nodes on his bilateral clavicle and mediastinum, considering the possibility of metastasis (Fig. 1d and e). On November 14th and December 7th in 2014, the patient received cisplatin (30 mg, Day 1–3), pyridoxine (THP-ADM, 80 mg, D1) and cyclophosphamide (800 mg, D1) for two cycles. Subsequent chest CT showed that there was no obvious decrease in the volume of swollen lymph nodes compared to previous CT pictures (Fig. 1f and g). When the patient came to our department of radiation oncology for help, we selected CCRT as his treatment. The intensity-modulated radiotherapy technique was used in thoracic radiation and the plan was designed as follows: 2.14 Gy × 28 fractions, total 60 Gy for tumor bed and positive lymph node; 1.79 Gy × 28 fractions, total 50 Gy for regional lymph node. As to concurrent chemotherapy regimens, we cautiously excluded doxorubicin and paclitaxel, which have cardiotoxicity or pulmonary toxicity that may aggravate the heart and lung damage derived by thoracic radiation. Cisplatin (30 mg, day 1–3) and etoposide (100 mg, day 1–5) every 3 weeks were selected as our combined chemotherapy regimens. The patient received CCRT from 5 January 2015 to 11 February in 2015. During the treatment, the patient showed grade 3 granulocytopenia and was cured by recombinant human granulocyte colony-stimulating factor. A month after the ending of whole treatment, FDG-PET revealed he had complete response (Fig. 1h–j). No obvious toxicities of heart and lung were observed. He was in good condition with no signs of recurrence at the 48-month follow-up.
Figure 1.
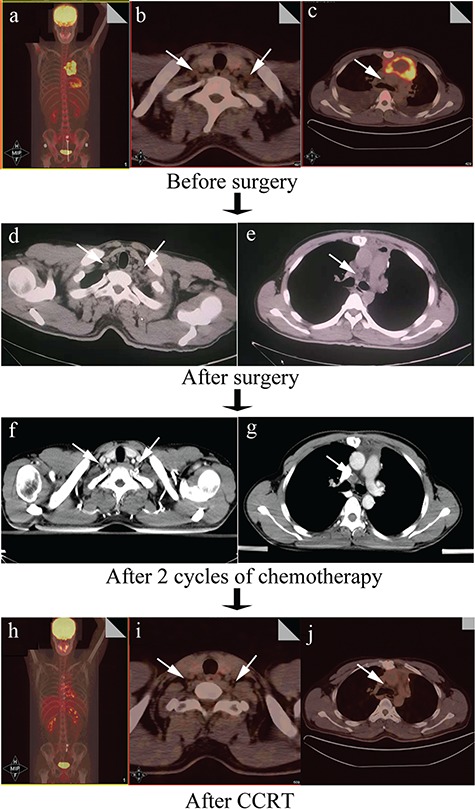
Imaging changes during the whole treatment. An anterior mediastinal mass and pericardial effusion can be seen (a); the suspicious swollen lymph nodes on the supraclavicular fossa were indicated by white arrows (b, c); chest CT showed swollen lymph nodes in the supraclavicular fossa (white arrow, d) and 4R region (white arrow, e); chest contrast-enhanced CT showed swollen lymph nodes in the supraclavicular fossa (white arrow, d) and 4R region (white arrow, e) after chemotherapy; chest contrast-enhanced CT showed swollen lymph nodes on the supraclavicular fossa (white arrow, f) and before the trachea (white arrow, g); complete response were achieved after CCRT (h); shrunken lymph nodes in the supraclavicular fossa (white arrow, i) and 4R region (white arrow, j).
Figure 2.
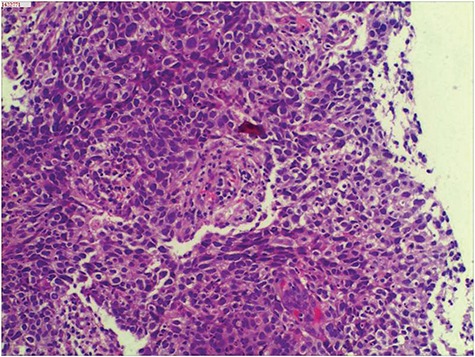
Non-keratinizing squamous cell carcinoma arised from thymus. HE. Original magnification, ×200.
DISCUSSION
Multivariate analysis from Weksler et al. [2] and Zhai et al. [3] demonstrated that complete (R0) resection is the decisive factor to cure thymic carcinoma. However, as to thymic carcinoma with lymph node metastasis or distant metastasis (stage IVb), maximal debulking surgery was suggested as a choice [4].
The impact of postoperative radiotherapy in thymic carcinoma is controversial. Jackson et al. [5] investigated 4056 patients with thymoma or thymic carcinoma and found that postoperative radiation therapy (PORT) was associated with longer overall survival, with the greatest relative benefits observed for stage IIB to III disease and positive margins. Large, randomized controlled, prospective studies are still needed to ascertain the short- and long-term efficacy of PORT.
CCRT should be selected cautiously due to its effectiveness and toxicity for advanced thymic carcinoma. Sixteen patients with unresectable thymic carcinoma who underwent CCRT showed a median survival of 82 months, which is encouraging and promising [6]. On the other hand, case reports about complete resection of advanced thymic carcinoma after CCRT suggest it may be well tolerated and useful for patients with advanced thymic cancer [7].
In this report, we present a patient with advanced thymic cancer, who had complete response to CCRT after incomplete resection. It might be an effective strategy for patients with Masaoka stage IVb thymic carcinoma with extrathoracic lymph node metastasis.
Conflict of Interest statement
The authors declare there are no potential conflicts of interest.
Funding
National Natural Science Foundation of China (8180110022).
Consent
Written consent was received for this report.
References
- 1. de Jong WK, Blaauwgeers JL, Schaapveld M, Timens W, Klinkenberg TJ, Groen HJ. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 2008;44:123–30. [DOI] [PubMed] [Google Scholar]
- 2. Weksler B, Dhupar R, Parikh V, Nason KS, Pennathur A, Ferson PF. Thymic carcinoma: a multivariate analysis of factors predictive of survival in 290 patients. Ann Thorac Surg 2013;95:299–303. [DOI] [PubMed] [Google Scholar]
- 3. Zhai Y, Hui Z, Ji W, Wang X, Liang J, Mao Y, et al. A single-center analysis of the treatment and prognosis of patients with thymic carcinoma. Ann Thorac Surg 2017;104:1718–24. [DOI] [PubMed] [Google Scholar]
- 4. Hishida T, Nomura S, Yano M, Asamura H, Yamashita M, Ohde Y, et al. Long-term outcome and prognostic factors of surgically treated thymic carcinoma: results of 306 cases from a Japanese nationwide database study. Eur J Cardiothorac Surg 2016;49:835–41. [DOI] [PubMed] [Google Scholar]
- 5. Jackson MW, Palma DA, Camidge DR, Jones BL, Robin TP, Sher DJ, et al. The impact of postoperative radiotherapy for thymoma and thymic carcinoma. J Thorac Oncol 2017;12:734–44. [DOI] [PubMed] [Google Scholar]
- 6. Chen YY, Huang CH, Tang Y, Eng HL. Concurrent chemoradiotherapy for unresectable thymic carcinoma. Chang Gung Med J 2004;27:515–22. [PubMed] [Google Scholar]
- 7. Morio A, Nakahara K, Ohse Y, Tahara M, Goto T, Yakumaru K, et al. Efficacy of induction chemoradiotherapy in thymic cancer: report of a successful case and review of the literature. Int J Clin Oncol 2002;7:201–4. [DOI] [PubMed] [Google Scholar]


