Abstract
Objective
Antibodies to the citrullinated protein antigens (ACPAs) are important in the diagnosis and pathogenesis of rheumatoid arthritis (RA). However, the prevalence of ACPAs with different fine specificities in different populations is unclear. This study sought to examine the fine specificity of the antibody responses toward citrullinated proteins in RA patients from Malaysia, an area where genetic and environmental determinants of RA are different from those in more frequently studied cohorts of Caucasian subjects.
Methods
A multiplex analytic microarray system was used to analyze the occurrence of antibodies to 10 different citrullinated peptides (filaggrin [fil307–324], vimentin [Vim2–17, Vim60–75], fibrinogen [Fibα563–583, Fibα580–600, Fibβ36–52, Fibβ62–81a, Fibβ62–81b], enolase [Eno5–21], and type II collagen [CitCII355–378]) in serum samples from 4,089 RA patients (1,231 Malaysian and 2,858 Swedish) and 827 healthy control subjects (249 Malaysian and 578 Swedish). The positive reaction threshold for each peptide was set separately for each population based on a specificity of 98%.
Results
Distinct differences in the frequencies of 5 ACPA fine specificities (Vim60–75, Vim2–17, Fibβ62–81b, Eno5–21, and CitCII355–378) were found between the Malaysian and Swedish RA populations, despite a nearly identical percentage of patients in each population who were positive for anti–cyclic citrullinated peptide 2 antibodies. In Malaysian RA patients compared with Swedish RA patients, the frequencies of antibodies to Vim60–75 (54% versus 44%, corrected P [P corr] = 1.06 × 10−8) and CitCII355–378 (17% versus 13%, P corr = 0.02) were significantly higher, while the frequencies of antibodies to Vim2–17 (25% versus 32%, P corr = 1.91 × 10−4), Fibβ62–81b (15% versus 30%, P corr = 2.47 × 10−22), and Eno5–21 (23% versus 50%, P corr = 3.64 × 10−57) were significantly lower.
Conclusion
Serum ACPA fine specificities differ between RA patients in different populations, although the total proportions of individuals positive for ACPAs are similar. Differing patterns of ACPA fine specificity could be attributed to variations in genetic and/or environmental factors.
There is overwhelming evidence to indicate that rheumatoid arthritis (RA) is not a single disease entity, but rather can be subclassified on the basis of autoantibodies, such as rheumatoid factor (RF) and anti–citrullinated peptide/protein antibodies (ACPAs) 1, 2, 3, 4, 5, 6, 7. ACPAs are highly specific and universal diagnostic biomarkers for RA across different populations 8, 9, 10, 11, 12. Furthermore, ACPAs can be detected several years before the onset of RA 13.
ACPAs are detected by commercial anti–cyclic citrullinated peptide 2 (anti–CCP‐2) assays, which use mixed cyclic citrullinated peptides as an artificial mimic of the true autoantigens. Although the anti–CCP‐2 assay is of high diagnostic importance and is highly specific for RA 14, it does not provide information about which citrullinated proteins are recognized by the anti–CCP‐2 antibodies. Moreover, these artificial citrullinated peptides are probably not expressed in the synovial joints. Therefore, the anti–CCP‐2 assay may not detect all antibody reactivity with citrullinated antigens known to exist in the joints of patients with RA. Circumstantial evidence from previous studies has suggested that ACPAs with different fine specificities are triggered in different genetic and environmental contexts 15, and that ACPAs with different fine specificities may have different effector functions 16, 17. It is therefore of interest to understand the determinants that trigger ACPAs, to elucidate the relationship between patterns of ACPAs and clinical symptoms, and to analyze the patterns of ACPAs in different genetic and environmental contexts.
The importance of determining the fine specificity of ACPAs in RA is supported by a number of studies in which different citrullinated antigens in different organs, as well as different effects of antibodies against these citrullinated antigens, have been noted. Thus, for example, it was recently shown that certain citrullinated proteins (particularly the citrullinated vimentin peptides CitVim60–75 and CitVim435–455) are present both in the lungs and in the joints of patients with RA 18. Moreover, antibodies with changed glycosylation patterns, such as RFs and ACPAs, differentiate osteoclasts and promote bone loss 19. Further citrullination of the C1 epitope on type II collagen (CitCII) has been identified in RA joints, and murine antibodies to this epitope have been found to induce arthritis in mice 20. Antibodies to several citrullinated peptides are commonly found in the serum of patients with RA, including, but not limited to, peptides from filaggrin (Fil) 21, vimentin (Vim) 22, α‐enolase (Eno) 23, α‐ and β‐fibrin (Fib) 24, and CII 25. Interestingly, the frequency of reactivity with certain citrullinated peptides was also found to be significantly increased in RA patients carrying the HLA–DRB1 shared epitope (SE) alleles 26.
Most studies of ACPA fine specificities and their relationship to genetic and environmental determinants in RA have so far been performed in Caucasian populations. In order to investigate the effects of different genetic and environmental determinants on ACPA fine specificity in different RA populations, we utilized a recently developed multiplex analytic microarray system to examine in parallel the antibody reactivity against different peptides derived from citrullinated proteins in RA patients investigated in a case–control study from Malaysia (the Malaysian Epidemiological Investigation of Rheumatoid Arthritis [MyEIRA] study) in comparison with RA patients in a case–control study from Sweden (the Swedish EIRA study), and assessed the relationship between the occurrence of different ACPA patterns and the different genetic backgrounds of the patients in the Malaysian and Swedish populations.
PATIENTS AND METHODS
Study populations
The MyEIRA multicenter case–control study is a sister study to the Swedish EIRA study. The study subjects were recruited between August 2005 and December 2009 throughout peninsular Malaysia 7, 27, 28. For the present study, we analyzed a total of 1,231 RA cases (Malay, n = 514 [41.8%]; Chinese, n = 255 [20.7%]; Indian, n = 404 [32.8%]; mixed ethnicities, n = 58 [4.7%]) and 1,625 healthy controls (Malay, n = 1,024 [63.0%]; Chinese, n = 208 [12.8%]; Indian, n = 298 [18.4%]; mixed ethnicities, n = 95 [5.8%]).
The Swedish EIRA study is a large population‐based case–control study on early RA in which concomitant information on both environmental/lifestyle factors and genetics was obtained 6, 29. For the serologic analyses in the present study, we analyzed data generated between May 1996 and May 2006 from a total of 2,858 RA cases and 578 healthy controls in the Swedish EIRA population. The vast majority of RA patients from the EIRA study were European Caucasians, while fewer than 2% of the study subjects reported having non‐Caucasian ancestry.
The design and selection criteria utilized in the MyEIRA and EIRA studies were similar. All patients were individuals who were newly diagnosed as having RA by a rheumatologist, based on the American College of Rheumatology 1987 classification criteria for RA 30. In the MyEIRA study, ∼90% of the patients had a diagnosis of early RA (within 3 years of disease onset, n = 1,090), while 10% of the patients had longstanding RA (>3 years from disease onset, n = 141). The mean ± SD disease duration in the MyEIRA study was 1.0 ± 2.0 years, 1.2 ± 1.9 years, and 1.1 ± 1.5 years in the Malay, Chinese, and Indian RA patients, respectively. All Swedish RA patients in the EIRA study were enrolled within 12 months after onset of their first symptoms.
All subjects were ages 18–70 years at the time of recruitment. Healthy controls were randomly selected from the respective national populations, with matching for sex, age, and residential area for both studies. Additional information about the MyEIRA and EIRA studies is available in the literature 6, 7, 27, 28, 29, 31, 32, 33, 34, 35. The characteristics of the study populations are summarized in Table 1.
Table 1.
Characteristics of the RA patients and healthy controls in the MyEIRA and EIRA study populationsa
| RA patients | Healthy controls | |
|---|---|---|
| Total study sample | ||
| Malaysian (MyEIRA) | ||
| All | 1,231 | 1,625 |
| Ethnic groupb | ||
| Malay | 514 | 1,029 |
| Chinese | 255 | 208 |
| Indian | 404 | 293 |
| Swedish (EIRA), all SE positive | 2,858 | 578 |
| Malaysian (MyEIRA) | ||
| All | 491 (40.0) | 263 (16.2) |
| Ethnic group | ||
| Malay | 182 (35.4) | 133 (13.0) |
| Chinese | 93 (36.5) | 26 (12.5) |
| Indian | 194 (48.0) | 88 (30.0) |
| Swedish (EIRA), allc | 2,084 (73.6) | 296 (52.3) |
| Anti–CCP‐2 positive | ||
| Malaysian (MyEIRA) | ||
| All | 792 (64.3) | 39 (2.4) |
| Ethnic group | ||
| Malay | 311 (60.5) | 25 (2.4) |
| Chinese | 169 (66.3) | 7 (3.4) |
| Indian | 270 (66.8) | 6 (2.0) |
| Swedish (EIRA), all | 1,841 (64.4) | 9 (1.6) |
| ACPA positive (with anti–CCP‐2 positive)d | ||
| Malaysian (MyEIRA) | ||
| All | 901 (73.2) | 42 (16.9) |
| Ethnic group | ||
| Malay | 353 (68.7) | 17 (11.9) |
| Chinese | 185 (72.5) | 9 (22.0) |
| Indian | 321 (79.5) | 12 (24.0) |
| Swedish (EIRA), all | 2,112 (73.9) | 87 (15.1) |
| ACPA positive (without anti–CCP‐2 positive)e | ||
| Malaysian (MyEIRA) | ||
| All | 844 (68.6) | 40 (16.1) |
| Ethnic group | ||
| Malay | 332 (64.6) | 17 (11.9) |
| Chinese | 169 (66.3) | 8 (19.5) |
| Indian | 304 (75.2) | 11 (22.0) |
| Swedish (EIRA), all | 2,041 (71.4) | 82 (14.2) |
| Anti–CCP‐2 positive (without SE alleles) | ||
| Malaysian (MyEIRA) | ||
| All | 399 (50.4) | 31 (2.3) |
| Ethnic group | ||
| Malay | 170 (51.4) | 22 (2.5) |
| Chinese | 91 (56.2) | 6 (3.3) |
| Indian | 118 (56.2) | 2 (1.0) |
| Swedish (EIRA), all | 274 (36.7) | 7 (2.6) |
Values are the number (%) of subjects. SE = HLA–DRB1 shared epitope.
The total sample size for the Malaysian Epidemiological Investigation of Rheumatoid Arthritis (MyEIRA) study sample included a fourth ethnic group, subjects of mixed ethnicity (n = 58 patients with rheumatoid arthritis [RA] and n = 95 healthy controls) (data not shown).
In the Swedish Epidemiological Investigation of Rheumatoid Arthritis (EIRA) study, serum samples for the HLA–DRB1 genotyping analyses were available from 2,831 RA patients and 566 healthy controls.
Anti–citrullinated protein antibody (ACPA) positive (with anti–cyclic citrullinated peptide 2 [anti–CCP‐2] positive) refers to the frequency of subjects with antibody reactivity against any citrullinated peptide and/or antibody reactivity against CCP‐2 (Malaysian, n = 1,231 RA patients and n = 249 healthy controls; Swedish, n = 2,858 RA patients and n = 578 healthy controls).
ACPA positive (without anti–CCP‐2 positive) refers to the frequency of subjects with antibody reactivity against any of the investigated citrullinated peptides but lacking antibody reactivity against CCP‐2 (Malaysian, n = 1,231 RA patients and n = 249 healthy controls; Swedish, n = 2,858 RA patients and n = 578 healthy controls).
All participants were informed about the research and gave their written informed consent. Sera for serologic analysis and cells for DNA preparation were collected from all participants at the time of diagnosis in the MyEIRA and EIRA studies. Samples were stored at −80°C until used.
Ethics approval
Both studies were approved by the local ethics committees at each institution (the Medical Research and Ethics Committee of the Ministry of Health Malaysia, and the Stockholm Regional Ethics Committee, Sweden).
Anti–CCP‐2 assay
In both the MyEIRA and EIRA studies, anti–CCP‐2 levels in the patients' serum were measured using a standard anti–CCP‐2 antibody enzyme‐linked immunosorbent assay (ELISA) (Immunoscan‐RA Mark 2 ELISA test; EuroDiagnostica). Samples with a value of >25 units/ml were considered anti–CCP‐2 positive, in accordance with the manufacturer's instructions.
ACPA multiplex assay
Serum samples were analyzed for the presence of IgG‐specific ACPAs, using a custom‐made microarray based on the ImmunoCAP ISAC system (Phadia AB), which contains 10 different citrullinated peptides and their native arginine‐containing counterparts. These citrullinated peptides include Fil307–324 (filaggrin), Vim60–75 (vimentin), Vim2–17 (vimentin), Fibβ36–52 (fibrinogen) 36, Fibα563–583 (fibrinogen) 36, Fibα580–600 (fibrinogen) 36, Fibβ62–81a (fibrinogen) 36, Fibβ62–81b (fibrinogen) 36, Eno5–21 (α‐enolase), and the triple‐helical C1 epitope on CII with citrulline at positions 355 and 378 (GPO)5GLOGA[Cit]OGDAGPQGKVGPS (GPO)2 (CitCII355–378) (details on the investigated peptides are available from the corresponding author upon request). A full description of this technology, with extensive validation of the chip‐based technique in comparison with ELISA‐based technology, and a review of the diagnostic performance of this microarray system was recently published 37. Overall, microarray results were available from a total of 1,231 RA patients in the Malaysian study and 2,858 RA patients in the Swedish study.
In addition, a total of 249 Malaysian healthy control subjects (143 Malays, 41 Chinese, 49 Indian, and 16 of mixed ethnicity) and 578 Swedish healthy control subjects were randomly selected, and their serum samples were tested with the multiplex assay to establish a cutoff value for diagnostic specificity at 98% for each of the 10 citrullinated peptides. Cutoff values were determined separately for the Malaysian and Swedish cohorts, because we have previously found that there are differences between Malaysian and Swedish subjects with regard to the expression patterns of other antibodies, such as RF isotypes 34 and antibodies against the full CII molecule (Too CL and Manivel VA: unpublished observations), and also because the EIRA and MyEIRA subjects were investigated at different time points.
HLA–DRB1 genotyping
HLA–DRB1 genotyping of DNA from the MyEIRA study subjects was performed as previously described 7. Briefly, high‐resolution genotyping of HLA–DRB1 alleles was performed using polymerase chain reaction (PCR) and the sequence‐specific oligonucleotide probe hybridization method (LABType HD Class II DRB1*; One Lambda Inc.) with a Luminex Multi‐Analyte Profiling System (xMAP), in accordance with the manufacturer's instructions. In the EIRA study subjects, HLA typing was performed using sequence‐specific priming–PCR (Olerup SSP) as described elsewhere 38. The HLA–DRB1*01, *04:01, *04:04, *04:05, *04:08, *04:10, and *10 alleles were classified as the SE. HLA–DRB1 information was available for all subjects from the MyEIRA study and for 2,831 RA patients and 566 controls from the EIRA study.
Statistical analysis
The net ACPA results were calculated by subtracting the fluorescence intensity of the arginine‐containing control peptide from that of each citrullinated peptide in the serum of all RA patients and controls. These net values were then used to construct receiver operating characteristic (ROC) curves and to compare their diagnostic performance (details available from the corresponding author upon request) 37. This calculation of net ACPA reactivity was performed for all peptides.
To allow stringent comparison of the diagnostic performances of each of the different citrullinated peptides, the cutoff point for each investigation was defined as the fluorescence intensity corresponding to a diagnostic specificity of 98%, calculated from the fluorescence intensity values observed in the 249 randomly selected control subjects in the MyEIRA study and 578 randomly selected control subjects in the EIRA study.
Data evaluation and statistical analysis were performed using SPSS software, version 20.0. Categorical data were analyzed by chi‐square test. The 2 × 2 association test with Yates' correction was used to analyze potential associations between the different autoantibodies and the presence of SE alleles and/or the HLA–DRB1*09:01 allele. Results were expressed as the odds ratio (OR) with 95% confidence interval (95% CI). P values less than 0.05 were considered significant. Spearman's rank correlation analyses and Fisher's r‐to‐z transformation for the comparison of rho values between Malaysian and Swedish patients were performed using the statistical software package MedCalc (version 15.2.2; MedCalc Software). For comparison between the MyEIRA and EIRA study cohorts, the corrected P values (P corr) were obtained by multiplying the observed P values by the number of investigated peptides (total of 11 peptides). ROC curves were constructed using SPSS software, version 20.0.
RESULTS
Basic characteristics of the study populations
The characteristics of the RA patients and healthy controls in the MyEIRA and EIRA studies are presented in Table 1. In the MyEIRA study, 86% of RA patients and 88% of controls were female, while in the EIRA study, the proportion of female subjects was 72% in each group. Anti–CCP‐2 antibodies were found in the serum of 64% of RA patients in the Malaysian and Swedish populations. Stratification analysis by ethnicity in the MyEIRA study revealed comparable proportions of anti–CCP‐2–positive RA patients among those of Malay, Chinese, or Indian ethnicity (P = 0.095) (Table 1).
Small, but consistent, differences in the distributions of anti–CCP‐2 and ACPA reactivity were observed between the control populations in each study, with higher proportions of healthy controls in the MyEIRA study showing serum positivity for anti–CCP‐2 and/or ACPAs. Reactivity with any single ACPA peptide was not uncommon in the control subjects (16.9% of MyEIRA controls and 15.1% of EIRA controls), but in most of these individuals, anti–CCP‐2 reactivity did not reach a diagnostically meaningful level, being observed in only 2.4% of anti–CCP‐2–positive controls in the MyEIRA study and in 1.6% of anti–CCP‐2–positive controls in the Swedish EIRA study (Table 1).
The cumulative frequency of any ACPA, including anti–CCP‐2 and/or antibodies to citrullinated peptides from filaggrin, vimentin, fibrinogen, and CII, was recognized to be close to identical between the Malaysian and Swedish RA cases (73.2% versus 73.9%). The mean number of fine specificities of ACPAs in the MyEIRA and EIRA study cohorts was 3.0 and 3.4, respectively. Furthermore, the frequency of at least 1 ACPA fine specificity, without consideration of the results of the anti–CCP‐2 test, was comparable between the RA patients in the MyEIRA study and those in the EIRA study (68.6% versus 71.4%) (Table 1). The high level of overlap between anti–CCP‐2 reactivity and the sum of reactivity with individual peptides in RA patients confirms the diagnostic performance of the anti–CCP‐2 ELISA test as a good proxy for natural citrullinated epitopes. However, we detected ACPAs in 23 Malaysian RA patients and 210 Swedish RA patients whose serum tested negative on the anti–CCP‐2 test, which corresponds to a frequency of 2% of the total patients cohort in the MyEIRA study and 7% of the total patients cohort in the EIRA study.
In this study, HLA–DRB1 SE alleles were detected in 40% of the MyEIRA study patients with RA and in 16.2% of the MyEIRA healthy controls, with a preponderance of HLA–DRB1*04:05 and HLA–DRB1*10 (7). In the EIRA study, HLA–DRB1 SE alleles were detected in 73.6% of the RA patients and 52.3% of the control subjects, with a predominance of HLA–DRB1*04:01 and HLA–DRB1*04:04 among the SE alleles 38. Thus, our findings revealed significant differences in the percentage of individuals carrying HLA–DRB1 SE alleles between the MyEIRA study and the EIRA study, both among RA cases and among healthy controls (each P < 0.0001).
It is noteworthy that more than 50% of the Malaysian anti–CCP‐2–positive RA patients (regardless of their ethnicity) did not carry any SE alleles (Table 1). Furthermore, a remarkably high frequency of the HLA–DRB1*09:01 allele has been reported in individuals of Asian ancestry (7–15%) (39), but is less common in Europeans. In the current study, 9.9% of the Malaysian RA patients and 8.1% of the Malaysian healthy controls carried the HLA–DRB1*09:01 allele (P > 0.05), compared with only 2.9% of the Swedish RA patients and 3.3% of the Swedish healthy controls (P > 0.05). However, it is noteworthy that there was no statistically significant difference between the Malaysian population and the Swedish population with regard to the frequency of HLA–DRB1*09:01 in either RA patients or healthy controls.
Features of the ACPA fine specificity profile in the Malaysian and Swedish RA populations
The numbers of RA patients who were positive for autoantibodies against the citrullinated Vim60–75 peptide and CII peptide were significantly increased in the MyEIRA study as compared with the EIRA study (P corr = 1.06 × 10−8 for Vim60–75; P corr = 0.02 for CitCII355–378) (Figure 1). Conversely, the frequencies of positive reactivity against the citrullinated peptides Vim2–17 (P corr = 1.91 × 10−4), Fibβ62–81b (P corr = 2.47 × 10−22), and Eno5–21 (P corr = 3.64 × 10−57) were significantly decreased in the Malaysian RA population when compared with the Swedish RA population. The percentages of RA patients with antibodies against the other investigated citrullinated peptides, such as Fil307–324, Fibβ36–52, Fibα563–583, Fibα580–600, and Fibβ62–81a, were comparable between the MyEIRA and EIRA studies (Figure 1).
Figure 1.
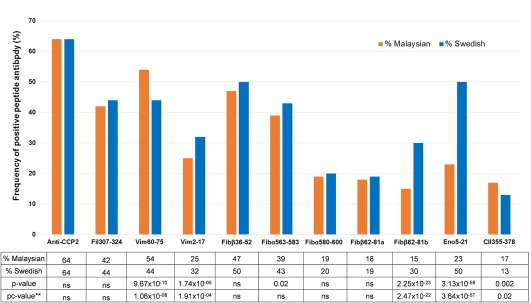
Frequencies of anti–citrullinated protein antibodies (ACPAs) to specific peptides among patients with rheumatoid arthritis in the Malaysian Epidemiological Investigation of Rheumatoid Arthritis study (n = 1,231) and the Swedish Epidemiological Investigation of Rheumatoid Arthritis study (n = 2,858). The corrected P (pc) values were calculated by multiplying the observed P values by the number of investigated peptides (total of 11). Anti–CCP‐2 = anti–cyclic citrullinated peptide 2; Fil = filaggrin; Vim = vimentin; Fib = fibrinogen; Eno = enolase; CII = type II collagen; NS = not significant.
With regard to the overall contribution of reactivity with each peptide, the 2 study cohorts had different population profiles for these ACPA peptide reactivities, as illustrated in Figure 1, but there was also a significant overlap. It is notable that with regard to autoantibodies to 2 of the peptides, Fibβ62–81b and Eno5–21, the frequency of antibody‐positive individuals in the Swedish RA population was double or more than double that in the Malaysian RA population.
It is noteworthy that when reactivity with the different peptides from the same protein were assessed, we found a higher frequency of autoantibodies to Vim60–75, but a lower frequency of autoantibodies to Vim2–17, in the Malaysian RA population compared with the Swedish RA population. Similarly, heterogeneity in responses to different epitopes was also detected for the fibrinogen peptides. Although the frequency of autoantibodies to 4 of the 5 investigated fibrinogen peptides was almost equal between the 2 populations, it was significantly different for Fibβ62–81b (Figure 1).
We also investigated the frequencies of ACPA reactivity between the 3 ethnic groups in the MyEIRA study population. Our findings revealed that RA patients of Indian ethnicity, as compared with those of Malay or Chinese ethnicity, demonstrated increased frequencies of ACPA positivity for 5 of the 10 investigated citrullinated peptide/protein antibodies (details available from the corresponding author upon request).
Comparison of quantitative data on reactivity of each ACPA peptide and anti–CCP‐2 positivity
In the quantitative results, the frequencies of all of the ACPA peptide antibodies investigated were significantly correlated with the frequency of anti–CCP‐2 antibody positivity (P < 0.0001) in all Malaysian and Swedish RA patients; the Spearman's correlation coefficients (rho values) ranged from 0.338 to 0.752 in the MyEIRA study and 0.365 to 0.802 in the EIRA study (Table 2). Interestingly, the best correlation with anti–CCP‐2 antibody positivity was observed for Fibβ36–52, both in the Malaysian RA population and in the Swedish RA population. Comparison of the Spearman's rank correlation coefficients between the MyEIRA and EIRA studies revealed significant population differences in correlations for 3 of the 10 ACPA peptide antibodies (Fibβ36–52, Fibβ62–81b, and Eno5–21) (Table 2). These findings partly overlap with the findings of significantly different sensitivities of each peptide between Malaysian and Swedish RA patients (Figure 1).
Table 2.
Comparison of Spearman's rank correlations for reactivity with individual ACPA peptide antibodies and positivity for anti–CCP‐2 antibodies between the Malaysian and Swedish RA populationsa
| ACPA peptide antibody | Malaysian RA patients | Swedish RA patients | Comparison of Spearman's rho values between studiesb | ||||
|---|---|---|---|---|---|---|---|
| Spearman's rho | P | Spearman's rho | P | z‐statistic | P | P corr | |
| Fil307–324 | 0.733 | <0.0001 | 0.739 | <0.0001 | −0.3836 | 0.7012 | NS |
| Vim60–75 | 0.697c | <0.0001 | 0.686 | <0.0001 | 0.6178 | 0.5367 | NS |
| Vim2–17 | 0.53c | <0.0001 | 0.523 | <0.0001 | 0.2838 | 0.7766 | NS |
| Fibβ36–52 | 0.752 | <0.0001 | 0.802 | <0.0001 | −3.7113 | 0.0002 | 0.002 |
| Fibα563–583 | 0.699 | <0.0001 | 0.742 | <0.0001 | −2.6248 | 0.0087 | NS |
| Fibα580–600 | 0.564 | <0.0001 | 0.602 | <0.0001 | −1.6878 | 0.0914 | NS |
| Fibβ62–81a | 0.338 | <0.0001 | 0.398 | <0.0001 | −2.0347 | 0.0419 | NS |
| Fibβ62–81b | 0.469c | <0.0001 | 0.556 | <0.0001 | −3.4647 | 0.0005 | 0.005 |
| Eno5–21 | 0.532c | <0.0001 | 0.707 | <0.0001 | −8.4461 | <0.0001 | <0.0001 |
| CitCII355–378 | 0.427c | <0.0001 | 0.365 | <0.0001 | 2.1561 | 0.0311 | NS |
Reactivity with individual anti–citrullinated protein antibody (ACPA) peptide antibodies was assessed for correlation with positivity for anti–cyclic citrullinated peptide 2 antibodies (anti–CCP‐2) in patients with rheumatoid arthritis (RA). These ACPA peptide antibodies included antibodies against fillagrin (Fil), vimentin (Vim), fibrinogen (Fib), enolase (Eno), and citrullinated type II collagen (CitCII).
Comparison of Spearman's correlation coefficients (rho values) between the 2 studies was done with Fisher's r‐to‐z transformation. Corrected P values (P corr) were calculated by multiplying the observed P values by the number of investigated peptides (total of 10 peptides). NS = not significant.
Significant difference versus Swedish patients.
Association between the fine specificity of the ACPA response and HLA–DRB1 SE/HLA–DRB1*09:01 alleles in the Malaysian RA patients and HLA–DRB1 SE alleles in the Swedish RA patients
When we used the anti–CCP‐2 assay as a reference for ACPA positivity, we observed a consistent pattern of association between HLA–DRB1 SE alleles and ACPA reactivity. The OR for an association between the presence of HLA–DRB1 SE alleles and all ACPAs was increased in the anti–CCP‐2–positive subsets of patients with RA, whereas a modest‐to‐weak association was noted in the anti–CCP‐2–negative RA subsets (Table 3 and details available from the corresponding author upon request) . It is also noteworthy that the OR for the association of HLA–DRB1 SE alleles with anti–Eno5–21 reactivity in Malaysian RA patients (OR 11.00, 95% CI 8.22–14.73) did not overlap with the corresponding values in the Swedish RA population (OR 6.08, 95% CI 4.85–7.63) (Table 3).
Table 3.
Association between HLA–DRB1 SE alleles and/or the HLA–DRB1*09:01 allele and the presence of anti–CCP‐2 antibodies in Malaysian and Swedish patients with RAa
| Malaysian study | Swedish study | |||||
|---|---|---|---|---|---|---|
| No. of cases/no. of controls | OR | 95% CI | No. of cases/no. of controls | OR | 95% CI | |
| Anti–CCP‐2 positive | ||||||
| No SE | 379/1,283 | Referent | – | 274/270 | Referent | – |
| Any SE | 371/247 | 5.08 | 4.17–6.20 | 1,550/296 | 5.16 | 4.19–6.36 |
| SE− and DRB1*09:01− | 309/1,176 | Referent | – | – | – | – |
| SE+ and/or DRB1*09:01+ | 441/354 | 4.74 | 3.93–15.72 | – | – | – |
| Anti–CCP‐2 negative | ||||||
| No SE | 324/1,283 | Referent | – | 473/270 | Referent | – |
| Any SE | 98/247 | 1.57 | 1.21–2.05 | 534/296 | 1.03 | 0.84–1.27 |
| SE− and DRB1*09:01− | 295/1,176 | Referent | – | – | – | – |
| SE+ and/or DRB1*09:01+ | 127/354 | 1.43 | 1.13–1.82 | – | – | – |
| Vim60–75 positive | ||||||
| No SE | 276/1,283 | Referent | – | 131/270 | Referent | – |
| Any SE | 353/247 | 6.64 | 5.39–8.18 | 1,103/296 | 7.68 | 6.01–9.81 |
| SE− and DRB1*09:01− | 228/1,176 | Referent | – | – | – | – |
| SE+ and/or DRB1*09:01+ | 401/354 | 5.84 | 4.78–7.15 | – | – | – |
| Vim60–75 negative | ||||||
| No SE | 427/1,283 | Referent | – | 616/270 | Referent | – |
| Any SE | 116/247 | 1.41 | 1.10–1.81 | 981/296 | 1.45 | 1.20–1.76 |
| SE− and DRB1*09:01− | 376/1,176 | Referent | – | – | – | – |
| SE+ and/or DRB1*09:01+ | 167/354 | 1.48 | 1.19–1.83 | – | – | – |
| Vim2–17 positive | ||||||
| No SE | 139/1,283 | Referent | – | 120/270 | Referent | – |
| Any SE | 150/247 | 5.61 | 4.29–7.33 | 792/296 | 6.02 | 4.67–7.75 |
| SE− and DRB1*09:01− | 118/1,176 | Referent | – | – | – | – |
| SE+ and/or DRB1*09:01+ | 171/354 | 4.81 | 3.70–6.26 | – | – | – |
| Vim2–17 negative | ||||||
| No SE | 564/1,283 | Referent | – | 627/270 | Referent | – |
| Any SE | 319/247 | 2.94 | 2.42–3.56 | 1,292/296 | 1.87 | 1.55–2.27 |
| SE− and DRB1*09:01− | 486/1,176 | Referent | – | – | – | – |
| SE+ and/or DRB1*09:01+ | 397/354 | 2.71 | 2.27–3.24 | – | – | – |
| Fibβ62–81b positive | ||||||
| No SE | 79/1,283 | Referent | – | 105/270 | Referent | – |
| Any SE | 100/247 | 6.08 | 4.37–8.39 | 750/296 | 6.52 | 5.01–8.47 |
| SE− and DRB1*09:01− | 69/1,176 | Referent | – | – | – | – |
| SE+ and/or DRB1*09:01+ | 110/354 | 5.30 | 3.83–7.32 | – | – | – |
| Fibβ62–81b negative | ||||||
| No SE | 624/1,183 | Referent | – | 642/270 | Referent | – |
| Any SE | 369/247 | 2.83 | 2.35–3.42 | 1,324/296 | 1.90 | 1.57–2.29 |
| SE− and DRB1*09:01− | 535/1,176 | Referent | – | – | – | – |
| SE+ and/or DRB1*09:01+ | 458/354 | 2.84 | 2.39–3.38 | – | – | – |
| Eno5–21 positive | ||||||
| No SE | 85/1,283 | Referent | – | 184/270 | Referent | – |
| Any SE | 180/247 | 11.00 | 8.22–14.73 | 1,227/296 | 6.08 | 4.85–7.63 |
| SE− and DRB1*09:01− | 75/1,176 | Referent | – | – | – | – |
| SE+ and/or DRB1*09:01+ | 190/354 | 8.42 | 6.28–11.28 | – | – | – |
| Eno5–21 negative | ||||||
| No SE | 618/1,283 | Referent | – | 563/270 | Referent | – |
| Any SE | 289/247 | 2.43 | 2.00–2.95 | 857/296 | 1.39 | 1.14–1.69 |
| SE− and DRB1*09:01− | 529/1,176 | Referent | – | – | – | – |
| SE+ and/or DRB1*09:01+ | 378/354 | 2.37 | 1.99–2.84 | – | – | – |
| CitCII355–378 positive | ||||||
| No SE | 90/1,283 | Referent | – | 65/270 | Referent | – |
| Any SE | 103/247 | 5.94 | 4.34–8.14 | 312/296 | 4.38 | 3.20–5.99 |
| SE− and DRB1*09:01− | 80/1,176 | Referent | – | – | – | – |
| SE+ and/or DRB1*09:01+ | 113/354 | 4.69 | 3.44–6.40 | – | – | – |
| CitCII355–378 negative | ||||||
| No SE | 613/1,283 | Referent | – | 682/270 | Referent | – |
| Any SE | 366/247 | 3.10 | 2.57–3.74 | 1,772/296 | 2.37 | 1.97–2.86 |
| SE− and DRB1*09:01− | 524/1,176 | Referent | – | – | – | – |
| SE+ and/or DRB1*09:01+ | 455/354 | 2.88 | 2.43–3.43 | – | – | – |
SE = shared epitope; OR = odds ratio; 95% CI = 95% confidence interval (see Table 2 for other definitions).
Since the HLA–DRB1*09:01 allele association with RA susceptibility is known to be evident in Asian populations, we performed additional association analyses in which we assessed associations with the combined presence of HLA–DRB1 SE alleles and/or the HLA–DRB1*09:01 allele in the MyEIRA study. Overall, in the Malaysian population, irrespective of ethnicity, the combined genetic effect size of HLA–DRB1 SE alleles and/or HLA–DRB1*09:01 was comparable to that of the presence of any HLA–DRB1 SE allele alone with regard to the risk of developing ACPA‐positive RA (Table 3 and details available from the corresponding author upon request).
Furthermore, we also assessed the reactivity against CCP‐2 and reactivity against each individual citrullinated peptide in analyses stratified according to HLA–DRB1 SE status in both the MyEIRA study and the Swedish EIRA study. Antibodies against the Vim60–75 peptide (frequency of 75.3% among RA patients in the MyEIRA study) seemed to account for most of the anti–CCP‐2 reactivity in the Malaysian RA patients who were positive for the HLA–DRB1 SE, regardless of ethnicity (Table 4), whereas reactivities to other peptides showed a weaker relationship to the presence of the HLA–DRB1 SE alleles. In contrast, in the Swedish EIRA study, the presence of the majority of autoantibodies to the investigated citrullinated peptides was correlated with the presence of the HLA–DRB1 SE alleles in RA patients.
Table 4.
Frequency of ACPA peptide antibody positivity in the Malaysian and Swedish populations of RA patients who were positive or negative for the HLA–DRB1 SEa
| SE‐negative RA | SE‐positive RA | |||
|---|---|---|---|---|
| ACPA peptide | Malaysian | Swedish | Malaysian | Swedish |
| antibody | (n = 703) | (n = 747) | (n = 469) | (n = 2,084) |
| Anti–CCP‐2 | 379 (53.9) | 274 (36.7) | 371 (79.1) | 1,550 (74.4) |
| Fil307–324 | 266 (37.8) | 208 (27.8) | 230 (49.0) | 1,028 (49.3) |
| Vim60–75 | 274 (39.0) | 131 (17.5) | 353 (75.3) | 1,103 (52.9) |
| Vim2–17 | 139 (19.8) | 120 (16.1) | 150 (32.0) | 792 (38.0) |
| Fibβ36–52 | 274 (39.0) | 224 (30.0) | 267 (56.9) | 1,191 (57.1) |
| Fibα563–583 | 220 (31.3) | 166 (22.2) | 231 (49.3) | 1,045 (50.1) |
| Fibα580–600 | 96 (13.7) | 75 (10.0) | 124 (26.4) | 499 (23.9) |
| Fibβ62–81a | 113 (16.1) | 108 (14.5) | 98 (20.9) | 428 (20.5) |
| Fibβ62–81b | 79 (11.2) | 105 (14.1) | 100 (21.3) | 750 (36.0) |
| Eno5–21 | 85 (12.1) | 184 (24.6) | 180 (38.4) | 1,227 (58.9) |
| CitCII355–378 | 90 (12.8) | 65 (8.7) | 103 (22.0) | 312 (15.0) |
Values are the number (%) of RA patients who were negative or positive for the HLA–DRB1 shared epitope (SE) and whose serum showed reactivity to the listed ACPA peptide antibodies. See Table 2 for other definitions.
Levels of peptide reactivity in anti–CCP‐2–positive versus anti–CCP‐2–negative RA patients
The recognition of multiple ACPA specificities was found mainly among anti–CCP‐2–positive RA patients (details available from the corresponding author upon request). Anti–CCP‐2–positive RA patients were grouped according to the levels of peptide reactivity, as follows: 1) low‐to‐moderate levels (<1,600 units/ml), comprising 644 patients (81.3%) in the MyEIRA study and 1,469 patients (79.8%) in the EIRA study, and 2) high levels (≥1,600 units/ml), comprising 148 patients (18.7%) in the MyEIRA study and 372 patients (20.2%) in the EIRA study. Based on these groups, the fractions of RA patients with high levels of anti–CCP‐2 reactivity did not differ between the MyEIRA study and the EIRA study (P = 0.4).
In addition, when the Spearman's rank correlation test was performed to assess correlations between the number of detected ACPA specificities and the net reactivity value of anti–CCP‐2, we found similar correlations in each study: r2 = 0.657 (P < 0.0001) in the MyEIRA study, and r2 = 0.691 (P < 0.0001) in the EIRA study (data not shown). These findings indicate that in the EIRA study, enrichment in the level of reactivity could not be attributed to higher absolute numbers of individual autoantibodies.
Frequency of ACPA specificities in ever smokers versus nonsmokers in the Malaysian RA population
In the MyEIRA study, only 90 (8.1%) of the 1,107 Malaysian RA patients reported a history of ever smoking. The percentage of patients in the MyEIRA study who were ever smokers compared with nonsmokers among those who were positive or negative for each peptide antibody are presented in Table 5. The results demonstrated significant differences in the numbers of patients with specific ACPAs between ever smokers and nonsmokers for 6 of the 11 studied ACPA specificities, including anti–CCP‐2, Vim2–17, Fibα563–583, Fibα580–600, Fibβ62–81a, and Eno5–21 (Table 5).
Table 5.
Frequency of ACPA specificities among RA patients in the Malaysian study who were ever smokers compared with nonsmokersa
| Ever smoker (n = 90) | Nonsmoker (n = 1,017) | ||||
|---|---|---|---|---|---|
| ACPA peptide antibody | Peptide positive | Peptide negative | Peptide positive | Peptide negative | P |
| Anti–CCP‐2 | 72 (80) | 18 (20) | 629 (61.8) | 388 (38.2) | 0.0009 |
| Fil307–324 | 45 (50) | 45 (50) | 408 (40.1) | 609 (59.9) | NS |
| Vim60–75 | 26 (28.9) | 64 (71.1) | 249 (24.5) | 768 (75.5) | NS |
| Vim2–17 | 61 (67.8) | 29 (32.2) | 526 (51.7) | 491 (48.3) | 0.004 |
| Fibβ36–52 | 50 (55.6) | 40 (44.4) | 455 (44.7) | 562 (55.3) | NS |
| Fibα563–583 | 50 (55.6) | 40 (44.4) | 360 (35.4) | 657 (64.6) | 0.0002 |
| Fibα580–600 | 26 (28.9) | 64 (71.1) | 184 (18.1) | 833 (81.9) | 0.01 |
| Fibβ62–81a | 25 (27.8) | 65 (72.2) | 175 (17.2) | 842 (82.8) | 0.01 |
| Fibβ62–81b | 16 (17.8) | 74 (82.2) | 148 (14.6) | 869 (85.4) | NS |
| Eno5–21 | 36 (40) | 54 (60) | 207 (20.4) | 810 (79.6) | <0.0001 |
| CitCII355–378 | 17 (18.9) | 73 (81.1) | 171 (16.8) | 846 (83.2) | NS |
Information on smoking was missing for 124 RA patients. Values are the number (%). See Table 2 for definitions.
DISCUSSION
In the present study, we observed profound differences in aspects of ACPA fine specificity between 2 cohorts of RA patients from Malaysia and Sweden, in which the same classification criteria had been used for patient inclusion and in which identical criteria (98% specificity for each peptide, use of matched population controls) were used to establish cutoff values. Thus, despite the fact that the general ACPA multiplex assay showed results identical to those of the anti–CCP‐2 test in terms of the proportions of patients positive for ACPAs, we found different frequencies of autoantibodies for 5 of the 10 investigated peptides. We also confirmed that the occurrence of ACPAs in general was dependent on the presence of SE alleles, but also noted intriguing ethnicity‐specific differences, since we found that the relationship between the presence of HLA–DRB1 SE alleles and specific ACPAs differed between Malaysian and Swedish RA patients.
Publically available information regarding the detection of specific ACPAs in different ethnic groups is very limited, and the present study is, to our knowledge, the first to systematically compare the occurrence of ACPA fine specificities in 2 populations in which different genetic and environmental factors may influence the occurrence of ACPAs, as well as the occurrence of RA. In this context, it is interesting that these RA populations in Malaysia and Sweden, both of which were composed of patients with disease of recent onset, show almost identical frequencies of anti–CCP‐2–positive individuals.
What is the reason for differing autoantibody production in different populations? Most probably it is a consequence of both genetic and environmental factors, which most likely act in synergy 6. There are vast differences between the European and Asian lifestyles and environmental exposures. However, the outcomes of RA, expressed as the clinical manifestations of RA, the course of the disease, and the overall involvement of autoimmunity in RA, are very similar in the 2 populations, and both patient cohorts were classified according to the same disease classification criteria. The estimated prevalence of RA in different countries is ∼0.5–1%, and it is very similar in Sweden and Malaysia 40, 41, 42.
Notably, the most well–described environmental risk factor for RA—smoking—is a risk factor for ACPA‐positive RA in both the Malaysian and Swedish populations 27, 43. Whereas a status of current or, in particular, previous smoking is common in the Swedish RA population 6, only a minor fraction of Malaysian RA patients (mostly men) are smokers 27. The smoking prevalence among women in Malaysia is very low, being 1% among female patients with RA and lower than 0.4% among female control subjects, although >80% of the RA patients in the MyEIRA study are women. Therefore, we believe that smoking does not contribute to the high prevalence of ACPAs among female Malaysian RA patients.
In this study, HLA–DRB1*04:05 and HLA–DRB1*10 were found to be the most common alleles in the Malaysian RA patients, while HLA–DRB1*04:01 and HLA–DRB1*04:04 were prominent in the Swedish RA patients. Since the distribution of specific HLA–DRB1 SE alleles varied between the Malaysian and Swedish populations, we hypothesized that the associations between specific HLA–DRB1 SE alleles and ACPA specificity might also differ between populations because their allele frequencies are different. This could be one of the possible explanations for the differences in autoantibody frequencies observed in the present study. It is also noteworthy that the influence of genetic factors differed between the 2 populations, as indicated by the high occurrence of ACPAs among Malaysian RA patients who were negative for HLA–DRB1 SE alleles (Malays 51.4%, Chinese 56.2%, and Indian 56.2%). Therefore, the most interesting results of the present study are that the total frequencies of ACPAs, both in the RA populations and in the similarly matched healthy control populations, in Sweden and Malaysia are so similar, despite the fact that the respective pools of ACPAs consisted of antibodies with different fine specificities that were generated in different genetic and environmental contexts.
Our study also indicates that a significant number of anti–CCP‐2–negative RA patients were found to have ACPAs when they were detected as reactivity with individual natural peptides (2% of the MyEIRA study patients and 7% of the EIRA study patients). This observation points toward a possible source of misclassification of autoantibody‐positive and antibody‐negative RA patients, and suggests that the anti‐CCP assay could be further improved. Importantly, this may influence the diagnosis of early RA.
Our data support previous findings that have suggested that different citrullinated proteins may be involved in autoimmune reactions in RA. Moreover, the contribution of these proteins, including different epitopes, does not follow a single pattern and is so complex that it may suggest randomness in this process, with more abundant proteins being more likely involved in the development of antibodies against citrullinated epitopes in RA. It is evident from the analyses of fibrinogen and vimentin peptides in our study that different human RA populations differ with regard to their dominant ACPA epitopes. Although our findings were based on the immune response to autoantigens, similar mechanisms could also be at work in the response to infectious agents and vaccines.
This hypothesis obviously needs much more empirical support. Nevertheless, we believe that the multiethnic and multicultural approach to the etiology and molecular pathogenesis of RA applied in the present study may be a useful complement to other, more mechanistic studies focused on the patterns of antibodies with fine specificities similar to those seen in both Malaysia and Sweden.
In conclusion, using a custom‐made peptide‐based microarray, we have demonstrated significant differences in the occurrence of specific ACPAs between 2 distinct RA populations from Malaysia and Sweden. We hypothesize that the ACPA epitope differences observed could be attributed to genetic and environmental differences between the 2 populations. Such geographically determined epitope differences may help in elucidating the putative causative disease pathways of RA.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Too had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Too, Murad, Hansson, Alm, Dhaliwal, Holmdahl, Jakobsson, Alfredsson, Klareskog, Rönnelid, Padyukov.
Acquisition of data
Too, Rönnelid, Padyukov.
Analysis and interpretation of data
Too, Murad, Hansson, Alm, Dhaliwal, Holmdahl, Jakobsson, Alfredsson, Klareskog, Rönnelid, Padyukov.
ADDITIONAL DISCLOSURE
Dr. Alm is an employee of Thermo Fisher Scientific, Uppsala, Sweden.
ACKNOWLEDGMENTS
We thank the Director General of Health, Ministry of Health Malaysia, for supporting the work described in this article, and are indebted to all of the patients and control subjects for their kind participation. We also thank the MyEIRA and EIRA study group members for conducting the data collection and providing practical help and assistance in the hospital, in the laboratory, and at the sites of investigation.
The Malaysian Epidemiological Investigation of Rheumatoid Arthritis (MyEIRA) study was supported by the Ministry of Health, Malaysia (grants MRG 7/2005, JPP‐IMR 07‐017, JPP‐IMR 08‐012, JPP‐IMR 08‐006, and JPP‐IMR 11‐005), the Swedish Medical Research Council (grant DNR 348‐2009‐6468), and the Swedish Combine Program. The Swedish EIRA study was supported by grants from the Swedish Medical Research Council, the Swedish Council for Working Life and Social Research, the King Gustav V 80‐year Foundation, the Swedish Rheumatism Foundation, the Stockholm County Council, and the BTCure IMI EU program. Additional support was provided by Phadia AB (for the serotyping and microarray analyses) and by the Swedish Strategic Research Foundation and the Swedish Science Research Council (for identification and synthesis of the triple‐helical type II collagen epitope).
Dr. Holmdahl holds a patent relating to the use of the CitC1 peptide. Dr. Rönnelid has received speaking fees from Phadia (less than $10,000).
REFERENCES
- 1. Daha NA, Toes RE. Rheumatoid arthritis: are ACPA‐positive and ACPA‐negative RA the same disease? Nat Rev Rheumatol 2011;7:202–3. [DOI] [PubMed] [Google Scholar]
- 2. Kurreeman F, Liao K, Chibnik L, Hickey B, Stahl E, Gainer V, et al. Genetic basis of autoantibody positive and negative rheumatoid arthritis risk in a multi‐ethnic cohort derived from electronic health records. Am J Hum Genet 2011;88:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Padyukov L, Seielstad M, Ong RT, Ding B, Ronnelid J, Seddighzadeh M, et al. A genome‐wide association study suggests contrasting associations in ACPA‐positive versus ACPA‐negative rheumatoid arthritis. Ann Rheum Dis 2011;70:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van der Helm‐van Mil AH, Huizinga TW. Advances in the genetics of rheumatoid arthritis point to subclassification into distinct disease subsets. Arthritis Res Ther 2008;10:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene–environment interaction between smoking and shared epitope genes in HLA–DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum 2004;50:3085–92. [DOI] [PubMed] [Google Scholar]
- 6. Klareskog L, Stolt P, Lundberg K, Kallberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA–DR (shared epitope)–restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum 2006;54:38–46. [DOI] [PubMed] [Google Scholar]
- 7. Chun‐Lai T, Padyukov L, Dhaliwal JS, Lundstrom E, Yahya A, Muhamad NA, et al. Shared epitope alleles remain a risk factor for anti‐citrullinated proteins antibody (ACPA): positive rheumatoid arthritis in three Asian ethnic groups. PLoS One 2011;6:e21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Somers K, Geusens P, Elewaut D, de Keyser F, Rummens JL, Coenen M, et al. Novel autoantibody markers for early and seronegative rheumatoid arthritis. J Autoimmun 2011;36:33–46. [DOI] [PubMed] [Google Scholar]
- 9. Van Venrooij WJ, van Beers JJ, Pruijn GJ. Anti‐CCP antibody, a marker for the early detection of rheumatoid arthritis. Ann N Y Acad Sci 2008;1143:268–85. [DOI] [PubMed] [Google Scholar]
- 10. Hamad MB, Marzouk S, Kaddour N, Masmoudi H, Fakhfakh F, Rebai A, et al. Anticyclic citrullinated peptide antibody and rheumatoid factor in south Tunisian patients with rheumatoid arthritis: association with disease activity and severity. J Clin Lab Anal 2014;28:21–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shidara K, Inoue E, Tanaka E, Hoshi D, Seto Y, Nakajima A, et al. Comparison of the second and third generation anti‐cyclic citrullinated peptide antibody assays in the diagnosis of Japanese patients with rheumatoid arthritis. Rheumatol Int 2011;31:617–22. [DOI] [PubMed] [Google Scholar]
- 12. Singwe‐Ngandeu M, Finckh A, Bas S, Tiercy JM, Gabay C. Diagnostic value of anti‐cyclic citrullinated peptides and association with HLA‐DRB1 shared epitope alleles in African rheumatoid arthritis patients. Arthritis Res Ther 2010;12:R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rantapää‐Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum 2003;48:2741–9. [DOI] [PubMed] [Google Scholar]
- 14. Whiting PF, Smidt N, Sterne JA, Harbord R, Burton A, Burke M, et al. Systematic review: accuracy of anti‐citrullinated peptide antibodies for diagnosing rheumatoid arthritis. Ann Intern Med 2010;152:456–64. [DOI] [PubMed] [Google Scholar]
- 15. Lundberg K, Bengtsson C, Kharlamova N, Reed E, Jiang X, Kallberg H, et al. Genetic and environmental determinants for disease risk in subsets of rheumatoid arthritis defined by the anticitrullinated protein/peptide antibody fine specificity profile. Ann Rheum Dis 2013;72:652–8. [DOI] [PubMed] [Google Scholar]
- 16. Krishnamurthy A, Joshua V, Haj Hensvold A, Jin T, Sun M, Vivar N, et al. Identification of a novel chemokine‐dependent molecular mechanism underlying rheumatoid arthritis‐associated autoantibody‐mediated bone loss. Ann Rheum Dis 2016;75:721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wigerblad G, Bas DB, Fernades‐Cerqueira C, Krishnamurthy A, Nandakumar KS, Rogoz K, et al. Autoantibodies to citrullinated proteins induce joint pain independent of inflammation via a chemokine‐dependent mechanism. Ann Rheum Dis 2016;75:730–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ytterberg AJ, Joshua V, Reynisdottir G, Tarasova NK, Rutishauser D, Ossipova E, et al. Shared immunological targets in the lungs and joints of patients with rheumatoid arthritis: identification and validation. Ann Rheum Dis 2015;74:1772–7. [DOI] [PubMed] [Google Scholar]
- 19. Harre U, Lang SC, Pfeifle R, Rombouts Y, Fruhbeisser S, Amara K, et al. Glycosylation of immunoglobulin G determines osteoclast differentiation and bone loss. Nat Commun 2015;6:6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uysal H, Bockermann R, Nandakumar KS, Sehnert B, Bajtner E, Engstrom A, et al. Structure and pathogenicity of antibodies specific for citrullinated collagen type II in experimental arthritis. J Exp Med 2009;206:449–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simon M, Girbal E, Sebbag M, Gomes‐Daudrix V, Vincent C, Salama G, et al. The cytokeratin filament‐aggregating protein filaggrin is the target of the so‐called “antikeratin antibodies,” autoantibodies specific for rheumatoid arthritis. J Clin Invest 1993;92:1387–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vossenaar ER, Despres N, Lapointe E, van der Heijden A, Lora M, Senshu T, et al. Rheumatoid arthritis specific anti‐Sa antibodies target citrullinated vimentin. Arthritis Res Ther 2004;6:R142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lundberg K, Kinloch A, Fisher BA, Wegner N, Wait R, Charles P, et al. Antibodies to citrullinated α‐enolase peptide 1 are specific for rheumatoid arthritis and cross‐react with bacterial enolase. Arthritis Rheum 2008;58:3009–19. [DOI] [PubMed] [Google Scholar]
- 24. Masson‐Bessiere C, Sebbag M, Girbal‐Neuhauser E, Nogueira L, Vincent C, Senshu T, et al. The major synovial targets of the rheumatoid arthritis‐specific antifilaggrin autoantibodies are deiminated forms of the α‐ and β‐chains of fibrin. J Immunol 2001;166:4177–84. [DOI] [PubMed] [Google Scholar]
- 25. Burkhardt H, Sehnert B, Bockermann R, Engstrom A, Kalden JR, Holmdahl R. Humoral immune response to citrullinated collagen type II determinants in early rheumatoid arthritis. Eur J Immunol 2005;35:1643–52. [DOI] [PubMed] [Google Scholar]
- 26. Scherer HU, van der Woude D, Willemze A, Trouw LA, Knevel R, Syversen SW, et al. Distinct ACPA fine specificities, formed under the influence of HLA shared epitope alleles, have no effect on radiographic joint damage in rheumatoid arthritis. Ann Rheum Dis 2011;70:1461–4. [DOI] [PubMed] [Google Scholar]
- 27. Too CL, Yahya A, Murad S, Dhaliwal JS, Larsson PT, Muhamad NA, et al. Smoking interacts with HLA‐DRB1 shared epitope in the development of anti‐citrullinated protein antibody‐positive rheumatoid arthritis: results from the Malaysian Epidemiological Investigation of Rheumatoid Arthritis (MyEIRA). Arthritis Res Ther 2012;14:R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yahya A, Bengtsson C, Lai TC, Larsson PT, Mustafa AN, Abdullah NA, et al. Smoking is associated with an increased risk of developing ACPA‐positive but not ACPA‐negative rheumatoid arthritis in Asian populations: evidence from the Malaysian MyEIRA case‐control study. Mod Rheumatol 2012;22:524–31. [DOI] [PubMed] [Google Scholar]
- 29. Stolt P, Bengtsson C, Nordmark B, Lindblad S, Lundberg I, Klareskog L, et al. Quantification of the influence of cigarette smoking on rheumatoid arthritis: results from a population based case‐control study, using incident cases. Ann Rheum Dis 2003;62:835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 31. Chun‐Lai T, Murad S, Erlandsson MC, Hussein H, Sulaiman W, Dhaliwal JS, et al. Recognizing rheumatoid arthritis: oncoprotein survivin opens new possibilities: a population‐based case‐control study. Medicine (Baltimore) 2015;94:e468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo J, Wu X, Too CL, Yin F, Lu X, He J, et al. A replication study confirms the association of dendritic cell immunoreceptor (DCIR) polymorphisms with ACPA‐negative RA in a large Asian cohort. PLoS One 2012;7:e41228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Too CL, Murad S, Dhaliwal JS, Larsson P, Jiang X, Ding B, et al. Polymorphisms in peptidylarginine deiminase associate with rheumatoid arthritis in diverse Asian populations: evidence from MyEIRA study and meta‐analysis. Arthritis Res Ther 2012;14:R250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Too CL, Rönnelid J, Yuslina MY, Dhaliwal JS, Nor Ashikin J, Yahya A, et al. Increased IgG rheumatoid factor‐positivity in the Asian rheumatoid arthritis patients irrespective of ethnicity. Open J Rheumatol Autoimmune Dis 2014;4:43–51. [Google Scholar]
- 35. Yahya A, Bengtsson C, Larsson P, Too CL, Mustafa AN, Abdullah NA, et al. Silica exposure is associated with an increased risk of developing ACPA‐positive rheumatoid arthritis in an Asian population: evidence from the Malaysian MyEIRA case‐control study. Mod Rheumatol 2014;24:271–4. [DOI] [PubMed] [Google Scholar]
- 36. Fernandes‐Cerqueira C, Ossipova E, Gunasekera S, Hansson M, Mathsson L, Catrina AI, et al. Targeting of anti‐citrullinated protein/peptide antibodies in rheumatoid arthritis using peptides mimicking endogenously citrullinated fibrinogen antigens. Arthritis Res Ther 2015;17:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hansson M, Mathsson L, Schlederer T, Israelsson L, Matsson P, Nogueira L, et al. Validation of a multiplex chip‐based assay for the detection of autoantibodies against citrullinated peptides. Arthritis Res Ther 2012;14:R201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lundstrom E, Kallberg H, Smolnikova M, Ding B, Ronnelid J, Alfredsson L, et al. Opposing effects of HLA–DRB1*13 alleles on the risk of developing anti–citrullinated protein antibody–positive and anti–citrullinated protein antibody–negative rheumatoid arthritis. Arthritis Rheum 2009;60:924–30. [DOI] [PubMed] [Google Scholar]
- 39. Jinam TA, Saitou N, Edo J, Mahmood A, Phipps ME. Molecular analysis of HLA class I and class II genes in four indigenous Malaysian populations. Tissue Antigens 2010;75:151–8. [DOI] [PubMed] [Google Scholar]
- 40. Cross M, Smith E, Hoy D, Carmona L, Wolfe F, Vos T, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1316–22. [DOI] [PubMed] [Google Scholar]
- 41. Mahdi H, Fisher BA, Kallberg H, Plant D, Malmstrom V, Ronnelid J, et al. Specific interaction between genotype, smoking and autoimmunity to citrullinated α‐enolase in the etiology of rheumatoid arthritis. Nat Genet 2009;41:1319–24. [DOI] [PubMed] [Google Scholar]
- 42. Snir O, Gomez‐Cabrero D, Montes A, Perez‐Pampin E, Gomez‐Reino JJ, Seddighzadeh M, et al. Non‐HLA genes PTPN22, CDK6 and PADI4 are associated with specific autoantibodies in HLA‐defined subgroups of rheumatoid arthritis. Arthritis Res Ther 2014;16:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lundstrom E, Kallberg H, Alfredsson L, Klareskog L, Padyukov L. Gene–environment interaction between the DRB1 shared epitope and smoking in the risk of anti–citrullinated protein antibody–positive rheumatoid arthritis: all alleles are important. Arthritis Rheum 2009;60:1597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]


