Abstract
Purpose
The aim of this study was to compare chronic fallopian tubal inflammatory disease and fibrosis between patients with general tubal pregnancy (TP) and TP with levonorgestrel (LNG) emergency contraception (EC) failure.
Methods
We retrospectively studied patients with general TP (n = 79) and TP following LNG‐EC failure (n = 81) within the same conception cycle. Information on the gynecological features of each subject was collected. Pelvic inflammatory disease and associated sequelae were assessed by the serum Chlamydia trachomatis (CT) IgG test, laparoscopic evaluation of tubal damage, and histopathological observation of tube tissues. Chi‐square and Student's t‐tests were employed to determine the difference between the two groups.
Results
Compared with general TP, cases of TP following LNG‐EC failure subjects were less likely to have a history of previous ectopic pregnancy (5.06% vs. 18.52%, p = 0.009) and adnexal surgery (6.33% vs. 22.22%, p = 0.010). Patients with TP following LNG‐EC failure were less likely to have pelvic inflammatory disease and associated sequelae than those with general TP, as revealed by positive reaction to anti‐CT IgG (18.18% vs. 35.94%, p = 0.031), assessment of tubal damage (grade I: 5.06% vs. 17.28%; grade II: 2.53% vs. 11.11%; grade III: 1.27% vs. 6.17%; p = 0.001), infiltration of chronic inflammatory cells (10.91% vs. 62.50%, p < 0.001), and positive Masson's staining (7.69% vs. 39.58%; p < 0.001).
Conclusions
Compared with cases of general TP, cases of TP following LNG‐EC failure exhibited reduced rates of CT infection, fallopian tubal inflammation, and/or fibrosis. © 2015 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons, Ltd.
Keywords: tubal pregnancy, levonorgestrel, emergency contraception, pelvic inflammatory disease, pharmacoepidemiology
Introduction
Tubal pregnancy (TP) accounts for approximately 98% of all cases of ectopic pregnancy (EP).1, 2 Over the past few decades, the incidence of TP has been increasing in many regions worldwide.3 Early diagnosis of TP is somewhat difficult, because about 9% of all tubal EPs have been reported to be asymptomatic;4 consequently, around half of all women with tubal EP undergo urgent care for emergency complications, imposing substantial economical and psychological burdens on patients and society.5
Emergency contraceptive pills (ECPs) is a safe method of preventing unwanted pregnancies including occasional EP, and ECPs have been reported to have an efficacy ranging from 52% to 94% when used within 120 h of unprotected intercourse.6, 7, 8, 9, 10, 11, 12 Due to a combination of factors (over‐the‐counter availability, easy accessibility, high efficacy, and increased knowledge), an increasing number of women who engage in unprotected sexual intercourse resort to levonorgestrel (LNG) ECPs. The United States reported an increased use of ECPs in women of reproductive age (from approximately 4.2% in 2002 to 11% in 2006–2010).13 In 2012, the two largest Chinese companies manufacturing LNG‐EC pills reported that approximately 790,000 boxes containing two pills of 1.5 mg or four pills of 0.75 mg LNG were sold in Shanghai (Cheng, unpublished observations).
With the rapid increase in LNG‐EC use, there have been many reports of cases of TP following LNG‐EC failure.6, 14, 15 According to the results of our previous study, LNG‐EC could effectively reduce the risk of unwanted pregnancy, including both ectopic and intrauterine pregnancies; however, the risk of EP increased by approximately five times once LNG‐EC failure occurred within the same cycle.16 The occurrence of TP has generally been regarded to be associated with fallopian tubal inflammation and its associated sequelae. However, through our experience in clinical practice, we have noticed an increasing number of women who undergo TP, claimed to have taken LNG‐EC in their current conception cycle, and the majority of them did not have tubal damage or periadnexal adhesions revealed by laparoscopy. Although Basu et al reported a case of EP following LNG‐EC failure without chronic salpingitis,17 no studies have determined whether women with TP following LNG‐EC failure have different etiological features compared with women who undergo general TP. Thus, this study aimed to assess whether TP following LNG‐EC failure was absent from patients with fallopian tube inflammatory disease and fibrosis and whether this differed from the incidence among women with general TP.
Methods
Study population
Women who underwent surgical treatment for TP at the inpatient Department of Gynaecology of our hospital between March 2011 and May 2013 were included into this study. All diagnoses of TP were confirmed by laparoscopy. Women diagnosed with TP who had used LNG‐EC during their current conception cycle were enlisted in group I (women with TP following LNG‐EC failure), and comparable women who did not use LNG‐EC during their current conception cycle were enlisted in group II (women with general TP). Women who had used other hormonal contraceptives within 3 months prior to the current cycle or had used long‐term contraceptive methods, such as an intrauterine device or female sterilization, or those who had been diagnosed with acute hemorrhagic shock associated with the current TP were excluded from the study.
Sample collection and serological test
Fallopian tubes were collected during surgery and fixed in 10% neutral‐buffered formaldehyde, dehydrated by passing through an upgraded ethanol series, and then embedded in paraffin.
Blood samples were collected from each subject and tested for serum anti‐Chlamydia trachomatis (CT) IgG antibodies using enzyme‐linked immunosorbent assay (ELISA; Beijing Biosynthesis Biotechnology, China) according to the manufacturer's instructions.
Assessment of pelvic inflammatory disease and associated sequelae
Previous PID and associated sequelae was diagnosed on the basis of four types of tests. The serum CT IgG test was applied to all participants for screening a previous CT infection. The severity of tubal damage was visually evaluated by laparoscopy. Histopathological evaluation of fallopian tube tissues by hematoxylin‐eosin (HE) staining and Masson's trichromatic staining (Masson's trichromatic Kit, Shanghai Seebio Biotechnology, China) was conducted in women subject to salpingectomy. HE staining revealed infiltration of chronic inflammatory cells, and Masson's staining revealed the presence of tubal fibrosis. The histopathological examinations were performed by experienced laboratory technicians, gynecologists, and pathologists.
Visual assessment of peritubal‐ovarian adhesion during laparoscopy
During laparoscopy, the degree of damage in fallopian tubes and/or ovaries was evaluated independently by two gynecologists based on the Hull & Rutherford (H&R) classification consisting of four grades.18 Normal fallopian tubes were considered grade 0. The main features of grade I tubal damage are filmy adhesions, and grades II and III are characterized by unilateral and bilateral severe damage, respectively.
Histopathological assessment
Paraffin‐embedded tissues were serially sectioned, deparaffinized, rehydrated, and stained with HE. Ten sections from each patient and four microscopic fields per section were evaluated. The chronic inflammation in the tubes was revealed by marked lymphocyte infiltration, thickened tube wall, and/or hyperplasia of the connective tissue and muscularis (Figure 1A, B, and C). A normal tubal histoarchitecture was defined as a well‐shaped structure of tube wall with no inflammatory cell infiltration or fibrosis (Figure 1D). Tubal tissue sections were also stained with Masson's trichromatic staining for detecting collagenous fibers. Four microscopic fields per section were observed to quantify the Masson's staining (positive, >2/4 fields [Figure 1E]; weakly positive, 1/4–2/4 fields; negative, <1/4 fields [Figure 1F]). Images were captured at 50× or 400× magnification by a video camera (AxioCam, Zeiss, Oberkochen, Germany). All assessments were performed independently by two experienced pathologists blinded for the source of the slides. Any disagreements in diagnosis between the two pathologists were resolved by a third pathologist, who analyzed the sections, and the results were determined by mutual consensus by the two pathologists.
Figure 1.
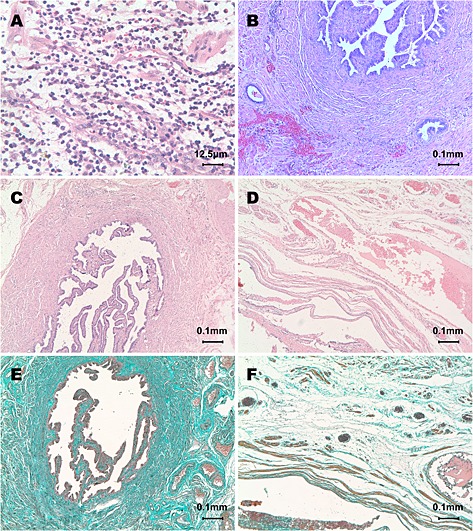
Histopathological analysis of fallopian tubes. Chronic inflammation in the fallopian tube versus normal fallopian tube as revealed by HE staining and Masson's staining. (A) Marked infiltration of lymphocytes with occasional eosinophils throughout the layers of fallopian tube tissue. (B) Fibrosis and hyalinization with collagen deposits were observed in the thickened tube wall. Representative image of mucosal epithelial cells penetrating into and throughout the tube muscularis. (C, D) Hyalinization with collagen deposition in the lamina propria and muscularis of the fallopian tube. (E, F) Normal fallopian tubal tissue with a clearly visible tube wall structure and loose connective tissue with only a few thin fibers present in the lamina propria and interstitial cells within the muscularis of the fallopian tube
Statistical analysis
Data were analyzed using SPSS 15.0 (SPSS Inc., Chicago, IL, USA). Proportions and means ± standard error were calculated. Differences between groups were compared using the chi‐square test (correction for continuity and Fisher's exact tests were used where appropriate) and Student's t test. The p‐values were estimated by two‐sided tests. Statistical significance was set at a p‐value of less than 0.05.
Ethics
The study protocol was approved by the Institutional Review Board of the International Peace Maternity and Child Health Hospital. All the study participants provided informed consent before the data collection started, in addition to blood samples and fallopian tube biopsies, according to our institutional guidelines.
Results
A total of 160 women with TP were recruited in this study. There were 79 women who had taken LNG‐EC during their current conception cycle and were placed in group I; the others (n = 81) had not taken LNG‐EC during their current conception cycle and were placed in group II. There were no significant differences in the age, marital status, and gestational days between the patients of both groups (Table 1).
Table 1.
Baseline characteristics
| Group I: LNG‐EC TP | Group II: Non‐LNG‐EC TP | p‐value | |
|---|---|---|---|
| (n = 79) | (n = 81) | ||
| Age (years)* | 31.94 ± 0.59 | 33.41 ± 0.62 | 0.087 |
| Gestational age (days)* | 48.70 ± 0.38 | 49.21 ± 0.30 | 0.285 |
| Married (proportion, %) | 67 (84.81) | 67 (82.72) | 0.720 |
Values represent mean ± standard error for age and gestational days.
LNG‐EC, Levonorgestrel‐only emergency contraception; TP, tubal pregnancy.
Table 2 presents a comparison of the history of reproduction, gynecological disease, and surgery between both the groups. There were no significant differences in the number of previous abortions (p = 0.398), history of cesarean section (p = 0.283), and previous appendectomy (p = 0.703) between the two groups. Women in group I were less likely to experience previous EP (5.06% vs. 18.52%, p = 0.009) and previous adnexal surgery (ovarian surgeries: 3.80% vs. 8.64%; tubal surgeries: 2.53% vs. 13.58%; p = 0.010) than women in group II. Furthermore, two cases (2/2) in group I and 12 cases (12/15) in group II who had previous EP exhibited and fallopian tube inflammatory disease. Among women who had undergone previous tubal surgery, both cases (2/2) in group I were treated for previous EP; in group II, five cases (5/11) were surgically treated for previous EP, four cases (4/11) were treated for previous tubal infertility, and another two cases (2/11) had undergone tubal surgeries for both previous EP and previous tubal infertility.
Table 2.
History of reproduction, gynecology, and surgery
| Group I: LNG‐EC TP | Group II: Non‐LNG‐EC TP | p‐value | |
|---|---|---|---|
| n * (%) | n * (%) | ||
| Number of previous abortions | |||
| 0 | 28 (35.44) | 25 (32.05) | 0.398 |
| 1 | 23 (29.11) | 30 (38.46) | |
| 2 | 18 (22.78) | 11 (14.10) | |
| ≥3 | 10 (12.66) | 12 (15.38) | |
| Previous EP | |||
| No | 75 (94.94) | 66 (81.48) | 0.009 |
| Yes | 4 (5.06) | 15 (18.52) | |
| Previous cesarean section † | |||
| No | 28 (45.16) | 24 (55.81) | 0.283 |
| Yes | 34 (54.84) | 19 (44.19) | |
| Previous adnexal surgery | |||
| No | 74 (93.67) | 63 (77.78) | 0.010‡ |
| Ovarian surgeries | 3 (3.80) | 7 (8.64) | |
| Tubal surgeries | 2 (2.53) | 11 (13.58) | |
| Previous appendectomy | |||
| No | 74 (93.67) | 77 (96.25) | 0.703§ |
| Yes | 5 (6.33) | 3 (3.75) | |
The sum does not necessarily equal the sample size for all variables because of missing data.
The number of parous women (62 in Group I and 42 in Group II) was used as the denominator to calculate the percentage.
Fisher's exact test was used.
Correction for continuity was used.
LNG‐EC, Levonorgestrel‐only emergency contraception; TP, tubal pregnancy; EP, ectopic pregnancy.
Table 3 lists the PID and associated sequelae found in the two groups. The percentage of women who showed a positive reaction to serological anti‐CT IgG antibody was significantly lower in group I than in group II (18.18% vs. 35.94%, p = 0.031). Of all the study participants, 91.14% (72/79) of group I and 65.43% (53/81) of group II patients had normal fallopian tubes (grade 0). Tubal damage was less likely to present in group I than in group II (grade I, 5.06% vs. 17.28%; grade II, 2.53% vs. 11.11%; grade III, 1.27% vs. 6.17%; p = 0.001). A total of 127 women with all three grades of tubal damage underwent salpingectomy, and HE staining evaluation was performed, and only 10.91% (6/55) of group I patients had chronic inflammation; this was significantly lower (p < 0.001) than in group II patients (62.50%, 45/72). Hyalinization with collagen deposition in the lamina propria and muscularis occurred to a lower extent in group I women than in group II women (positive: 7.69% vs. 39.58%; p < 0.001).
Table 3.
Evaluation of PID‐associated sequelae
| Group I: LNG‐EC TP | Group II: Non‐LNG‐EC TP | p‐value | |
|---|---|---|---|
| n * (%) | n * (%) | ||
| Chlamydia trachomatis IgG test | |||
| Negative | 45 (81.82) | 41 (64.06) | 0.031 |
| Positive | 10 (18.18) | 23 (35.94) | |
| Degree of fallopian tube adhesions † | |||
| Grade 0 | 72 (91.14) | 53 (65.43) | 0.001‡ |
| Grade I | 4 (5.06) | 14 (17.28) | |
| Grade II | 2 (2.53) | 9 (11.11) | |
| Grade III | 1 (1.27) | 5 (6.17) | |
| Chronic inflammation of fallopian tube as revealed by HE staining | |||
| No | 49 (89.09) | 27 (37.50) | <0.001 |
| Yes | 6 (10.91) | 45 (62.50) | |
| Fibrosis of fallopian tube as revealed by Masson Trichromatic staining | |||
| Negative | 43 (82.69) | 25 (52.08) | <0.001‡ |
| Weakly positive | 5 (9.62) | 4 (8.33) | |
| Positive | 4 (7.69) | 19 (39.58) |
The sum does not necessarily equal the sample size for all variables because of missing data.
All of the 160 women received surgical treatment. Tubal damage was scored according to the Hull & Rutherford classification for tubal damage (2002).
Fisher's exact test was used.
LNG‐EC, Levonorgestrel‐only emergency contraception; TP, tubal pregnancy; HE, hematoxylin‐eosin; PID, pelvic inflammatory disease.
Discussion
In this study, we compare chronic fallopian tubal inflammatory disease and fibrosis between patients with general TP and TP with LNG‐EC failure and found that women with TP following LNG‐EC failure showed a lower presence of chronic tubal inflammation than women with general TP.
Although the mechanism of TP is not completely understood yet, current knowledge supports that impaired embryo transport in fallopian tube and/or alterations in tubal microenvironment cause the retention of embryo in the fallopian tube, resulting in conception failure or early implantation in the fallopian tube.2, 19 Factors such as history of previous EP or adnexal surgery may be caused by previous pelvic inflammation and postoperative peritubal‐ovarian adhesions. These factors could contribute to severe tubal sequelae with abnormalities of tubal morphology and function.20, 21 Therefore, women with a history of these conditions are more likely to have an increased risk of TP. Early in 1998, Skjeldestad et al. reported that pelvic inflammation and its associated sequelae predisposed the general population to EP recurrence as a result of subacute or subclinical genital infections.22 Thus, in our study, 14 out of 17 women with previous EP and all women with a history of previous tubal surgeries had abnormalities in their fallopian tubes. Therefore, factors such as previous EP and adnexal surgeries did not contribute to the occurrence of TP following LNG‐EC failure, which differed from the etiology of general TP.
Pelvic inflammation is the commonly recognized factor leading to structural and functional abnormalities of the fallopian tube,23 consequently increasing the risk of TP and infertility.24, 25 Epidemiological studies have confirmed that previous PID is strongly associated with TP.26, 27, 28, 29 Although the female genital tract infection can be polymicrobial, CT has been reported as a leading pathogen associated with PID, and up to 60% women with salpingitis were found positive for urogenital infection by CT.30 Post‐infection of CT could be subclinical with positive anti‐CT antibody titres persisting in vivo for decades. Therefore, anti‐CT IgG antibodies could be used to screen for salpingitis that might have occurred in recent years with a positive predictive value of 85–90% and a negative predictive value of 30–65%.21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34 Consistent with others reports,28, 35 we found that about 35.94% of the women with general TP had a positive reaction to serological CT IgG. Furthermore, only 18.18% of the women with TP following LNG‐EC failure showed positive reactions in this study. Based on our results, women with TP following LNG‐EC failure are less likely to have a history of previous CT infection than women with general TP.
In addition to the serological anti‐CT IgG antibody test, we also conducted laparoscopic evaluation to assess the tubal damage in women who had been treated surgically. Laparoscopic examination can not only confirm the diagnosis with a specificity of 100% and a sensitivity of 81%,36 but it can also help determine the cause of tubal damage other than CT infection.37 According to the H&R classification, the severity of fallopian tube damage was assessed by observing tubal fibrosis, tubal patency, mucosal appearances, and peritubal‐ovarian adhesions.18 We previously reported that minor or moderate tubal damage increased the risk of EP, while severe tubal damage increased the risk of infertility.38 In this study, women with TP following LNG‐EC failure had significantly less tubal damage in all grades than women with general TP. It seems that women with TP following LNG‐EC failure are less likely to have tubal damage. Instead, they are more likely to have fallopian tubes with normal morphology. This creates a question why women with TP following LNG‐EC failure were less likely to have PID and associated sequelae compared with women with general TP. For this, we speculate that the unique EP following LNG‐EC failure might be associated with LNG‐EC intake rather than PID and other factors. Levenorgestrel functions by interrupting follicular development and thus delaying or inhibiting ovulation.39, 40 Our previous study established that a high dose of LNG could reduce the tubal cilia activity both in vitro and in vivo. 41 To the best of our knowledge, LNG can also reduce muscular contractility in fallopian tubes. 42 A decline of cilia beats and/or muscular contractility can cause the retention of embryo‐tubal transplantation, which has been considered to be mainly responsible for the etiology of TP.2
It is known that LNG‐EC does not affect fertilization, embryo development, or implantation.43 In our previous study on the candidate molecules involved in embryo development, we found no differences in the expression levels of these molecules in the chorionic villi in women with tubal EP exposed to LNG‐EC versus those with general tubal EP. Thus, we concluded that the etiological features but not pathophysiological or clinical features of specific tubal EP following LNG‐EC failure differed from those of general EP.44
The present study is the first to evaluate whether TP following LNG‐EC failure was associated with fallopian tube inflammation using a combination of four objective measurements, involving serum CT IgG tests, laparoscopic examination, histopathological analysis, and Masson's staining. However, this study had some limitations. As a result of the study design for the clinical comparisons, strong evidence is not provided that can address this question. Furthermore, because LNG‐EC are available over‐the‐counter, the total number of LNG‐EC users cannot be calculated; therefore, it is difficult to estimate the prevalence of TP following LNG‐EC failure. From the findings of the present study, the occurrence of TP following LNG‐EC failure was shown to be decreased in cases with chronic tubal inflammation. A future prospective cohort study is required to address whether the use of LNG‐EC is correlated with TP following LNG‐EC failure.
In summary, compared with cases of general TP, cases of TP following LNG‐EC failure exhibited lower rates of CT infections, fallopian tube inflammation, and/or fibrosis, and were less likely to have a history of previous EP and adnexal surgery.
Conflict of Interest
The authors had no conflict of interest to disclose.
Key points.
Cases of ectopic pregnancy following levonorgestrel emergency contraception failure have been reported to be free of abnormalities in fallopian tube.
Tubal pregnancy following levonorgestrel emergency contraception failure showed a lower presence of chronic tubal inflammation.
Four tests were used to evaluate the history of pelvic inflammatory disease and associated sequelae.
Ethics Statement
This study was approved by the Institutional Review Board at International Peace Maternity and Child Health Hospital in Shanghai, China. Written informed consent was obtained from each subject before recruitment.
Acknowledgements
This work was supported by grants from the Shanghai Scientific and Technical Committee grant no. (124119a4802). The authors are grateful to Dr. Mei‐Zhen Xin (Department of Pathology, Key Laboratory of Cell Differentiation and Apoptosis of Chinese Ministry of Education, Institutes of Medical Sciences, School of Medicine, Shanghai Jiao Tong University, Shanghai, China) for her excellent technical assistance. The authors are also grateful to Dr. Liu‐Qi Zhuang (Emerita Dean of the International Peace Maternity and Child Health Hospital, Shanghai, China) for her assistance with the study design and data interpretation. The authors would also like to thank Mr. Michael McCarty (a copy editor in Grand Rapids, MI, USA) for volunteer language checking.
Li, C. , Meng, C.‐X. , Sun, L.‐L. , Zhao, W.‐H. , Zhang, M. , Zhang, J. , and Cheng, L. (2015), Reduced prevalence of chronic tubal inflammation in tubal pregnancies after levonorgestrel emergency contraception failure. Pharmacoepidemiol Drug Saf, 24, 548–554. doi: 10.1002/pds.3775.
References
- 1. Saraiya M, Berg CJ, Shulman H, Green CA, Atrash HK. Estimates of the annual number of clinically recognized pregnancies in the United States, 1981‐1991. Am J Epidemiol 1999; 149(11): 1025–1029. [DOI] [PubMed] [Google Scholar]
- 2. Shaw JL, Dey SK, Critchley HO, Horne AW. Current knowledge of the aetiology of human tubal ectopic pregnancy. Hum Reprod Update 2010; 16(4): 432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nama V, Manyonda I. Tubal ectopic pregnancy: diagnosis and management. Arch Gynecol Obstet 2009; 279(4): 443–453. [DOI] [PubMed] [Google Scholar]
- 4. Farquhar CM. Ectopic pregnancy. Lancet 2005; 366(9485): 583–591. [DOI] [PubMed] [Google Scholar]
- 5. Wheeler SR. Psychosocial needs of women during miscarriage or ectopic pregnancy. AORN J 1994; 60(2): 221‐227, 230‐231. [DOI] [PubMed] [Google Scholar]
- 6. Ho PC, Kwan MS. A prospective randomized comparison of levonorgestrel with the Yuzpe regimen in post‐coital contraception. Hum Reprod 1993; 8(3): 389–392. [DOI] [PubMed] [Google Scholar]
- 7. Wu S, Wang C, Wang Y. A randomized, double‐blind, multicentre study on comparing levonorgestrel and mifepristone for emergency contraception. Zhonghua Fu Chan Ke Za Zhi 1999; 34(6): 327–330. [PubMed] [Google Scholar]
- 8. Arowojolu AO, Okewole IA, Adekunle AO. Comparative evaluation of the effectiveness and safety of two regimens of levonorgestrel for emergency contraception in Nigerians. Contraception 2002; 66(4): 269–273. [DOI] [PubMed] [Google Scholar]
- 9. von Hertzen H, Piaggio G, Ding J, et al. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet 2002; 360(9348): 1803–1810. [DOI] [PubMed] [Google Scholar]
- 10. Hamoda H, Ashok PW, Stalder C, Flett GMM, Kennedy E, Templeton A. A randomized trial of mifepristone (10 mg) and levonorgestrel for emergency contraception. Obstet Gynecol 2004; 104(6): 1307–1313. [DOI] [PubMed] [Google Scholar]
- 11. Wai NS. A randomized trial to compare 24 h versus 12 h double dose regimen of levonorgestrel for emergency contraception. Hum Reprod 2004; 20(1): 307–311. [DOI] [PubMed] [Google Scholar]
- 12. Creinin MD, Schlaff W, Archer DF, et al. Progesterone receptor modulator for emergency contraception. Obstet Gynecol 2006; 108(5): 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daniels K, Jones J, Abma J. Use of emergency contraception among women aged 15‐44: United States, 2006‐2010. NCHS Data Brief 2013; 112: 1–8. [PubMed] [Google Scholar]
- 14. Kozinszky Z, Bakken RT, Lieng M. Ectopic pregnancy after levonorgestrel emergency contraception. Contraception 2011; 83(3): 281–283. [DOI] [PubMed] [Google Scholar]
- 15. Matsushita H, Takayanagi T, Ikarashi H. Ectopic pregnancy following emergency contraception with ethinyloestradiol‐levonorgestrel: a case report. Eur J Contracept Reprod Health Care 2007; 12(2): 184–186. [DOI] [PubMed] [Google Scholar]
- 16. Li C, Zhao WH, Meng CX, et al. Contraceptive use and the risk of ectopic pregnancy: a multi‐center case‐control study. PLoS One 2014; 9(12): e115031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Basu A, Candelier C. Ectopic pregnancy with postcoital contraception‐‐a case report. Eur J Contracept Reprod Health Care 2005; 10(1): 6–8. [DOI] [PubMed] [Google Scholar]
- 18. Rutherford AJ, Jenkins JM. Hull and Rutherford classification of infertility. Hum Fertil (Camb) 2002; 5(1 Suppl): S41–S45. [DOI] [PubMed] [Google Scholar]
- 19. Horne AW, Critchley HO. Mechanisms of disease: the endocrinology of ectopic pregnancy. Expert Rev Mol Med 2012; 14: e7. [DOI] [PubMed] [Google Scholar]
- 20. Pientong C, Ekalaksananan T, Wonglikitpanya N, Swadpanich U, Kongyingyoes B, Kleebkaow P. Chlamydia trachomatis infections and the risk of ectopic pregnancy in Khon Kaen women. J Obstet Gynaecol Res 2009; 35(4): 775–781. [DOI] [PubMed] [Google Scholar]
- 21. Abrao MS, Muzii L, Marana R. Anatomical causes of female infertility and their management. Int J Gynaecol Obstet 2013; 123(Suppl 2): S18–S24. [DOI] [PubMed] [Google Scholar]
- 22. Skjeldestad FE, Hadgu A, Eriksson N. Epidemiology of repeat ectopic pregnancy: a population‐based prospective cohort study. Obstet Gynecol 1998; 91(1): 129–135. [DOI] [PubMed] [Google Scholar]
- 23. Bjartling C, Osser S, Persson K. Deoxyribonucleic acid of Chlamydia trachomatis in fresh tissue from the fallopian tubes of patients with ectopic pregnancy. Eur J Obstet Gynecol Reprod Biol 2007; 134(1): 95–100. [DOI] [PubMed] [Google Scholar]
- 24. Cates WJ, Wasserheit JN. Genital chlamydial infections: epidemiology and reproductive sequelae. Am J Obstet Gynecol 1991; 164(6 Pt 2): 1771–1781. [DOI] [PubMed] [Google Scholar]
- 25. Scholes D, Stergachis A, Heidrich FE, Andrilla H, Holmes KK, Stamm WE. Prevention of pelvic inflammatory disease by screening for cervical chlamydial infection. N Engl J Med 1996; 334(21): 1362–1366. [DOI] [PubMed] [Google Scholar]
- 26. Karaer A, Avsar FA, Batioglu S. Risk factors for ectopic pregnancy: a case‐control study. Aust N Z J Obstet Gynaecol 2006; 46(6): 521–527. [DOI] [PubMed] [Google Scholar]
- 27. Anorlu RI, Oluwole A, Abudu OO, Adebajo S. Risk factors for ectopic pregnancy in Lagos, Nigeria. Acta Obstet Gynecol Scand 2005; 84(2): 184–188. [DOI] [PubMed] [Google Scholar]
- 28. Bouyer J, Coste J, Shojaei T, et al. Risk factors for ectopic pregnancy: a comprehensive analysis based on a large case‐control, population‐based study in France. Am J Epidemiol 2003; 157(3): 185–194. [DOI] [PubMed] [Google Scholar]
- 29. Levin AA, Schoenbaum SC, Stubblefield PG, Zimicki S, Monson RR, Ryan KJ. Ectopic pregnancy and prior induced abortion. Am J Public Health 1982; 72(3): 253–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mitchell C, Prabhu M. Pelvic inflammatory disease: current concepts in pathogenesis, diagnosis and treatment. Infect Dis Clin North Am 2013; 27(4): 793–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Siassakos D, Manley K, Wardle P, Halawa S. Chlamydia screening or prophylaxis before laparoscopy and dye hydrotubation: no readmissions, no worry, or is that so? Int J Std Aids 2007; 18(12): 861–862. [DOI] [PubMed] [Google Scholar]
- 32. Lardenoije CM, Land JA. Chlamydia antibody testing for tubal factor subfertility. Ned Tijdschr Geneeskd 2007; 151(36): 1981–1985. [PubMed] [Google Scholar]
- 33. Cohen CR, Gichui J, Rukaria R, Sinei SS, Gaur LK, Brunham RC. Immunogenetic correlates for Chlamydia trachomatis‐associated tubal infertility. Obstet Gynecol 2003; 101(3): 438–444. [DOI] [PubMed] [Google Scholar]
- 34. Shibahara H, Takamizawa S, Hirano Y, et al. Relationships between Chlamydia trachomatis antibody titers and tubal pathology assessed using transvaginal hydrolaparoscopy in infertile women. Am J Reprod Immunol 2003; 50(1): 7–12. [DOI] [PubMed] [Google Scholar]
- 35. Agholor K, Omo‐Aghoja L, Okonofua F. Association of anti‐Chlamydia antibodies with ectopic pregnancy in Benin city, Nigeria: a case‐control study. Afr Health Sci 2013; 13(2): 430–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaitan H, Angel E, Diaz R, Parada A, Sanchez L, Vargas C. Accuracy of five different diagnostic techniques in mild‐to‐moderate pelvic inflammatory disease. Infect Dis Obstet Gynecol 2002; 10(4): 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Akande VA, Hunt LP, Cahill DJ, Caul EO, Ford WC, Jenkins JM. Tubal damage in infertile women: prediction using chlamydia serology. Hum Reprod 2003; 18(9): 1841–1847. [DOI] [PubMed] [Google Scholar]
- 38. Chen SF, Zhang J. Using laparoscopy combined with hysteroscopy in the diagnosis and treatment of patients with tubal infertility. Chin J Clin Med 2009; 16(6): 923–925. [Google Scholar]
- 39. Croxatto HB, Brache V, Pavez M, et al. Pituitary‐ovarian function following the standard levonorgestrel emergency contraceptive dose or a single 0.75‐mg dose given on the days preceding ovulation. Contraception 2004; 70(6): 442–450. [DOI] [PubMed] [Google Scholar]
- 40. Gemzell‐Danielsson K, Rabe T, Cheng L. Emergency contraception. Gynecol Endocrinol 2013; 29(S1): 1–14. [DOI] [PubMed] [Google Scholar]
- 41. Zhao W, Zhu Q, Yan M, et al. Levonorgestrel decreased cilia beat frequency of human fallopian tubes and rat oviducts without changing the morphological structure. Clin Exp Pharmacol Physiol 2015. doi: 10.1111/1440-1681.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wanggren K, Stavreus‐Evers A, Olsson C, Andersson E, Gemzell‐Danielsson K. Regulation of muscular contractions in the human fallopian tube through prostaglandins and progestagens. Hum Reprod 2008; 23(10): 2359–2368. [DOI] [PubMed] [Google Scholar]
- 43. Christow A, Sun X, Gemzell‐Danielsson K. Effect of mifepristone and levonorgestrel on expression of steroid receptors in the human fallopian tube. Mol Hum Reprod 2002; 8(4): 333–340. [DOI] [PubMed] [Google Scholar]
- 44. Huang C, Zhang M, Meng C, Shi W, Sun L, Zhang J. Expressions of candidate molecules in the human fallopian tube and chorionic villi of tubal pregnancy exposed to levonorgestrel emergency contraception. Reprod Biol Endocrinol 2013; 11: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]


