Abstract
Background and objectives: XX male syndrome is part of the disorders of sex development (DSD). The patients generally have normal external genitalia and discover their pathology in adulthood because of infertility. There are no guidelines regarding XX male syndrome, so the aim of our study was to evaluate the literature evidence in order to guide the physicians in the management of these type of patients. Materials and Methods: We performed a systematic review of the available literature in September 2018, using MEDLINE, Web of Science, Embase and Google Scholar database to search for all published studies regarding XX male syndrome according to PRISMA guidelines. The following search terms were used: “46 XX male”, “DSD”, “infertility”, “hypogonadism”. Results: After appropriate screening we selected 37 papers. Mean (SD) age was 33.14 (11.4) years. Hair distribution was normal in 29/39 patients (74.3%), gynecomastia was absent in 22/39 cases (56.4%), normal testes volume was reported in 0/14, penis size was normal in 26/32 cases (81.2%), pubic hair had a normal development in 6/7 patients (85.7%), normal erectile function was present in 27/30 cases (90%) and libido was preserved in 20/20 patients (100%). The data revealed the common presence of hypergonadotropic hypogonadism. All patients had a 46,XX karyotype. The sex-determining region Y (SRY) gene was detected in 51/57 cases. The position of the SRY was on the Xp in the 97% of the cases. Conclusions: An appropriate physical examination should include the evaluation of genitalia to detect cryptorchidism, hypospadias, penis size, and gynecomastia; it is important to use a validated questionnaire to evaluate erectile dysfunction, such as the International Index of Erectile Function (IIEF). Semen analysis is mandatory and so is the karyotype test. Abdominal ultrasound is useful in order to exclude residual Müllerian structures. Genetic and endocrine consultations are necessary to assess a possible hypergonadotropic hypogonadism. Testicular sperm extraction is not recommended, and adoption or in vitro fertilization with a sperm donor are fertility options.
Keywords: 46,XX; XX male syndrome; disorders of sex development; De la Chapelle; infertility; hypogonadism
1. Introduction
Disorders of sex development (DSD) is used as an umbrella term [1] for various rare conditions that are characterized by an incongruence of chromosomal, gonadal, and genital sex development. XX male syndrome is part of DSD, so it is also called 46,XX testicular disorder of sex development. Patients generally have normal external genitalia and discover their pathology in adulthood because of infertility. Typical features of this condition are: 46,XX karyotype, normal male phenotype, small testes, azoospermia, and hypergonadotropic hypogonadism [2].
It is a rare disease occurring in about 1:20,000 males [3]. The first case was described in 1964 by De la Chapelle [4]; since then, several other cases were reported. In this study we present the case report of a 36-year-old man suffering from XX male syndrome, presented as infertility, and perform a systematic review of the available evidences about the topic.
Case Report
A 36-year-old man came to our clinic complaining about infertility; he engaged in regular, unprotected sexual intercourse during the last 20 months without his wife becoming pregnant. His 30-year-old wife underwent a gynecological consultation and no remarkable diseases were diagnosed. He reported no familiar history of endocrine diseases, genetic syndromes or infertility and his medical history revealed only carpal tunnel release surgery; furthermore, no history of testicular trauma or cryptorchidism was present. The patient’s job did not expose him to radiation or cytotoxic agents, and he did not take any medication. He had normal libido, good erectile function (International Index of Erectile Function (IIEF)-5 score: 22 points), normal morning erections, and no genital or urinary troubles. The patient complained of mild asthenia, impaired concentration, and breast growth in the last 2 years.
The height and weight of patient were 165 cm and 74 kg, respectively, with a BMI (body mass index) of 27.1 kg/m2), sagittal abdominal diameter of 29 cm, and his blood pressure was 110/70 mmHg. He presented sparse body hair and bilateral gynecomastia (grade II). The genital examination showed symmetrical male genitalia, stretched penis length of 8 cm, small testes (both 6 mL), and sparse pubic hair (Tanner stage II). No clinical varicocele was found. Digital rectal examination revealed a normal prostate gland. Standard abdominal ultrasound showed no significant disorders. Normal prostate gland and normal seminal vesicles, with no Müllerian derivates, were found with pelvic ultrasound. No varicocele was diagnosed with testicular ultrasound.
Hormone analysis revealed hypergonadotropic hypogonadism: follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were 24.7 mIU/mL (1–13 mIU/mL) and 9.4 mIU/mL (1–9 mIU/mL) respectively, whereas early morning total testosterone (TT) was 235 ng/dL (300–1200 ng/dL) and free testosterone (FT) calculated by formula was 3.5 pg/mL (9–30 pg/mL). Estradiol (E2) and prolactin (PRL) levels were 14 pg/mL (10–40 pg/mL) and 12.2 ng/mL (4–23 ng/mL) respectively, prostate-specific antigen (PSA) was 0.6 ng/mL, blood sugar was 88 mg/dL, total cholesterol was 213 mg/dL (<200 mg/dL), hematocrit was 44% (41–50%) and hemoglobin was 15.4 g/dL (14–17.5 g/dL). The laboratory parameters were confirmed by a second dosage.
The semen analysis, according to the guidelines of the World Health Organization (WHO) Laboratory Manual for the Examination and Processing of Human Semen (5th edition), was performed after four days of abstinence and showed normal ejaculated volume (2.4 mL) and azoospermia after centrifugation. The semen collection was performed in the laboratory and it was analyzed within the following 30 min by two expert biologists. A second and a third sample confirmed similar values.
Karyotyping was performed on peripheral blood lymphocytes and showed a 46,XX karyotype. Fluorescent in situ hybridization (FISH) was carried out using the Vysis SRY probe revealing the sex-determining region Y (SRY) on the short (p) arm of the X chromosome. The patient underwent genetic consultation that confirmed the diagnosis of 46,XX (SRY-positive) DSD. A testicular biopsy was proposed to get a histological diagnosis, but the patient refused. Artificial insemination with sperm donation and psychological support were offered to the couple, and the patient is on clinical and laboratoristic follow-up.
2. Materials and Methods
2.1. Search Strategy
We performed a systematic review of the available literature in September 2018, using MEDLINE, Web of Science, Embase, and Google Scholar database to search for all published studies regarding XX male syndrome. The following search terms were used: “46 XX male”, “DSD”, “infertility”, “hypogonadism”.
2.2. Inclusion and Exclusion Criteria
We included papers that met the following criteria: English language, human studies, adult patients (≥18 years old), full-text availability, completeness of clinical and laboratory data. No filters were applied for the date of publication.
Case reports of patients of pediatric age group and studies not having primary data (i.e., reviews not including case report, commentaries, and letters) were excluded, however they were examined to include any possible relevant citations.
2.3. Data Extraction
Reference lists in relevant studies were used to search for additional studies. After a first screening based on study titles and abstracts, all selected papers were assessed based on the full-text to choose the relevant publications to be included in the analysis.
Two authors carried out this review independently (M.T., M.S.), and disagreements regarding the inclusion of some studies were resolved by discussion including all authors. An informed consent was obtained from the patient for the publication of the case report.
3. Results
3.1. Search Results
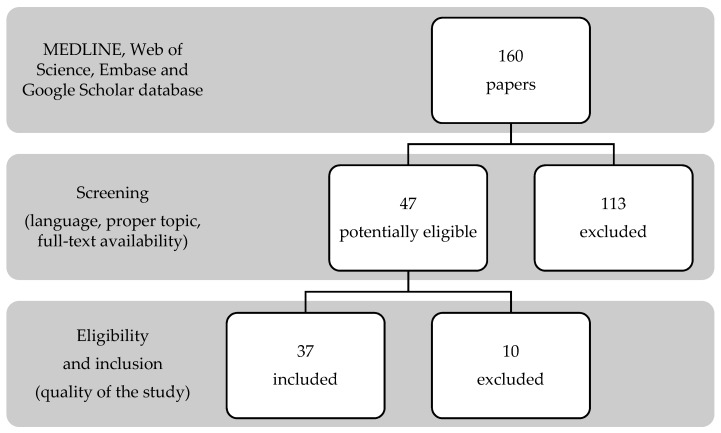
The search strategy generated 160 papers. Screening of study titles and abstracts revealed 47 articles potentially eligible for inclusion; a further assessment based on the full-text led to the exclusion of 10 papers (Figure 1). The 37 selected studies described 64 adult patients with XX male syndrome [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41].
Figure 1.
Search strategy according to PRISMA guidelines.
3.2. Synthesis of Results
The clinical data of the patients are summarized in Table 1. Mean (SD) age was 33.14 (11.4) years, mean (SD) weight was 70.3 (10.2) kg and mean (SD) height was 165.3 (7.2) cm. Hair distribution was normal in 29/39 patients (74.3%), gynecomastia was absent in 22/39 cases (56.4%), normal testes volume was reported in 0/14 patients (in 50 patients this data was not available), penis size was normal in 26/32 cases (81.2%), pubic hair had a normal development in 6/7 patients (85.7%), normal erectile function was present in 27/30 cases (90%) and libido was preserved in 20/20 patients (100%).
Table 1.
Clinical data of 46, XX male adults.
| Authors | Age Years |
Weight kg |
Height cm |
HD | GM | Testes Volume | Penis Size | Pubic Hair | ED | Libido |
|---|---|---|---|---|---|---|---|---|---|---|
| Guzman et al. [5] | 20 | 76 | 169 | Normal | No | Small | Normal | Normal | No | Normal |
| Gunes et al. [6] | 30 | 70 | 155 | Poor | Yes | Small | Normal | Normal | No | Normal |
| Gunes et al. [6] | 16 | 65 | 152 | Normal | No | Small | Normal | Normal | No | Normal |
| Valetto et al. [7] | 35 | 48 | 152 | Normal | No | NA | Normal | NA | No | Normal |
| Kim et al. [8] | 29 | 62 | 165 | Normal | No | NA | NA | NA | Yes | NA |
| Xiao et al. [9] | 27 | NA | 170 | NA | NA | NA | Small | NA | No | NA |
| Queralt et al. [10] | 31 | 58 | 170 | Normal | No | NA | NA | NA | NA | NA |
| Baziz et al. [11] | 44 | NA | NA | Normal | Yes | Small | Normal | NA | No | Normal |
| Tomomasa et al. [12] | 25 | 55 | 177 | Normal | No | Small | NA | Normal | NA | Normal |
| Chung Jung et al. [13] | 17 | NA | 154 | Normal | No | Small | Normal | Normal | No | Normal |
| Wang et al. [14] | 20 | NA | NA | Poor | No | Small | Normal | Inverted | No | Normal |
| Ahsan T et al. [15] | 24 | NA | NA | Poor | Yes | Small | Normal | Normal | No | Normal |
| Jain et al. [16] | 38 | 63 | 162 | Normal | Yes | NA | Normal | NA | Yes | NA |
| Yencilek et al. [17] | 26 | 72 | 165 | Normal | No | NA | Small | NA | No | NA |
| Pepene et al. [18] | 28 | 65 | 167 | Normal | Yes | Small | Normal | NA | No | Normal |
| Mustafa et al. [19] | 30 | 75 | 170 | Normal | Yes | NA | Normal | NA | NA | Normal |
| Majzoub et al. [20] | 40 | 84 | 175 | Normal | No | NA | Normal | NA | No | Normal |
| Majzoub et al. [20] | 31 | NA | NA | Normal | No | NA | Normal | NA | No | Normal |
| Majzoub et al. [20] | 35 | NA | NA | Poor | Yes | NA | Normal | NA | Yes | Normal |
| Majzoub et al. [20] | 39 | 74 | 160 | Normal | No | Small | Normal | NA | No | Normal |
| Majzoub et al. [20] | 29 | 77 | 181 | Normal | No | NA | Normal | NA | No | Normal |
| Majzoub et al. [20] | 32 | 86 | 170 | Normal | Yes | NA | Normal | NA | No | Normal |
| Onrat et al. [21] | 23 | NA | NA | Normal | No | Small | Normal | NA | No | Normal |
| Hado et al. [22] | 76 | NA | 157 | Normal | Yes | NA | NA | NA | No | Normal |
| Rigola et al. [23] | 33 | NA | NA | Normal | No | NA | Normal | NA | NA | NA |
| Dauwerse et al. [24] | 61 | NA | 171 | NA | No | Small | Normal | NA | NA | NA |
| Ryan et al. [25] | 40 | NA | NA | Poor | No | NA | 3.6 | NA | No | NA |
| Gao et al. [26] | NA | NA | 163 | NA | NA | NA | NA | NA | NA | NA |
| Gao et al. [26] | NA | NA | 163 | NA | NA | NA | NA | NA | NA | NA |
| Gao et al. [26] | NA | NA | 162 | NA | NA | NA | NA | NA | NA | NA |
| Gao et al. [26] | NA | NA | 161 | NA | NA | NA | NA | NA | NA | NA |
| Gao et al. [26] | NA | NA | 158 | NA | NA | NA | NA | NA | NA | NA |
| Gao et al. [26] | NA | NA | 162 | NA | NA | NA | NA | NA | NA | NA |
| Gao et al. [26] | NA | NA | 162 | NA | NA | NA | NA | NA | NA | NA |
| Gao et al. [26] | NA | NA | 161 | NA | NA | NA | NA | NA | NA | NA |
| Gao et al. [26] | NA | NA | 160 | NA | NA | NA | NA | NA | NA | NA |
| Gao et al. [26] | NA | NA | 160 | NA | NA | NA | NA | NA | NA | NA |
| Gao et al. [26] | NA | NA | 161 | NA | NA | NA | NA | NA | NA | NA |
| Rizvi et al. [27] | 33 | 85.8 | 177 | NA | NA | NA | Normal | NA | No | NA |
| Minor et al. [28] | 24 | NA | NA | NA | Yes | NA | NA | NA | NA | NA |
| Rajender et al. [29] | 34 | 64 | 156 | Normal | No | NA | Normal | NA | NA | NA |
| Tan et al. [30] | NA | NA | 176 | Normal | Yes | NA | Small | NA | No | NA |
| Zakharia et al. [31] | 65 | 65 | 165 | NA | Yes | NA | Normal | NA | No | NA |
| Chiang et al. [32] | 33 | NA | NA | NA | NA | NA | NA | NA | No | NA |
| Chiang et al. [32] | 34 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Chiang et al. [32] | 52 | NA | NA | NA | NA | NA | Small | NA | NA | NA |
| Wu et al. [33] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Wu et al. [33] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Wu et al. [33] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Wu et al. [33] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Wu et al. [33] | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Chernykh et al. [34] | 37 | 74 | 160 | Poor | Yes | NA | NA | NA | NA | NA |
| Butler et al. [35] | 31 | 72 | 169 | Normal | Yes | NA | Normal | NA | No | NA |
| Castineyra et al. [36] | 28 | NA | 180 | Normal | Yes | NA | NA | NA | NA | NA |
| Castineyra et al. [36] | 35 | NA | 170 | Normal | NA | Small | NA | NA | NA | NA |
| Castineyra et al. [36] | 28 | NA | 160 | Poor | NA | NA | NA | NA | NA | NA |
| Castineyra et al. [36] | 39 | NA | 174 | Poor | NA | NA | NA | NA | NA | NA |
| Castineyra et al. [36] | 24 | NA | 172 | Normal | NA | NA | NA | NA | NA | NA |
| Fuse et al. [37] | 30 | 90 | 172 | Normal | No | NA | NA | NA | NA | NA |
| Pais et al. [38] | 29 | 82 | 170 | Normal | Yes | NA | Small | NA | No | NA |
| Wegner et al. [39] | 35 | 81 | 167 | Normal | No | NA | Normal | NA | NA | NA |
| Micic et al. [40] | 25 | 63 | 171 | Poor | No | NA | NA | NA | No | NA |
| Matthews et al. [41] | 27 | 68 | 166 | Poor | No | NA | NA | NA | NA | |
| Our case | 36 | 74 | 165 | NA | Yes | Small | Normal | NA | No | Normal |
NA: not available; HD: hair distribution; GM: gynecomastia; ED: erectile disfunction.
The hormone profile of the patients is reported in Table 2. The data revealed the common presence of hypergonadotropic hypogonadism. Mean (SD) FSH was 40.00 (19.7) mIU/mL, mean (SD) LH was 26.5 (16.7) mIU/mL and mean (SD) total testosterone was 2.6 (1.4) ng/mL.
Table 2.
Hormone profile of 46,XX male adults.
| Authors | FSH mIU/mL |
LH mIU/mL |
TT ng/mL |
E2 pg/mL |
PRL ng/mL |
|---|---|---|---|---|---|
| Guzman et al. [5] | 27.9 | 16.5 | 2.3 | 24.8 | 24.5 |
| Gunes et al. [6] | 37.88 | 18.96 | 0.51 | 17.57 | 17.54 |
| Gunes et al. [6] | 41.05 | 14.55 | 2.16 | 32 | 24.11 |
| Valetto et al. [7] | 23.9 | 17.7 | 3.06 | NA | 7.13 |
| Kim et al. [8] | 76 | 41 | 1.79 | NA | NA |
| Xiao et al. [9] | 47 | 18.7 | 1.80 | NA | 14.6 |
| Queralt et al. [10] | 62.2 | 25.8 | 3.23 | 17 | NA |
| Baziz et al. [11] | 51 | 11.71 | NA | NA | NA |
| Tomomasa et al. [12] | 19.7 | 10.3 | 4.28 | NA | NA |
| Chung Jung et al. [13] | 25.1 | 11.4 | 4.3 | 30.1 | 16.8 |
| Wang et al. [14] | 77.5 | 40.75 | 4.64 | NA | 15.59 |
| Ahsan T et al. [15] | 35 | 21 | 1.8 | NA | NA |
| Jain et al. [16] | 76.6 | 36.3 | 1.20 | NA | NA |
| Yencilek et al. [17] | 45.6 | 48.9 | 2.70 | NA | 9.4 |
| Pepene et al. [18] | 43.9 | 25.3 | 3.33 | NA | NA |
| Mustafa et al. [19] | NA | 40.7 | 2.11 | 16.6 | 8.5 |
| Majzoub et al. [20] | 38 | 12 | 3.35 | 29 | 13.6 |
| Majzoub et al. [20] | 14 | 6 | 1.29 | 25 | 3.2 |
| Majzoub et al. [20] | 10 | 23 | 0.74 | 5.7 | NA |
| Majzoub et al. [20] | 28 | 15 | 0.74 | NA | NA |
| Majzoub et al. [20] | 13.4 | 12 | 2.46 | 19 | NA |
| Majzoub et al. [20] | 29.7 | 16.9 | 0.95 | NA | 12 |
| Onrat et al. [21] | 9.95 | 17.3 | 0.20 | NA | NA |
| Hado et al. [22] | 27.8 | 21 | 2.59 | NA | NA |
| Rigola et al. [23] | NA | NA | NA | NA | NA |
| Dauwerse et al. [24] | 13 | 10 | 3.25 | 31.3 | NA |
| Ryan et al. [25] | 1 | NA | NA | 10 | NA |
| Gao et al. [26] | 93.6 | 19.4 | 3.08 | 33 | 17.9 |
| Gao et al. [26] | 24.7 | 14.4 | 2.77 | 43 | 18.5 |
| Gao et al. [26] | NA | NA | 1.29 | NA | NA |
| Gao et al. [26] | 81.6 | 27.7 | 1.37 | 19.8 | 22.9 |
| Gao et al. [26] | 13.1 | 3.61 | 2.44 | 34 | 9.67 |
| Gao et al. [26] | 54.7 | 19.4 | 1.72 | 27 | 10.08 |
| Gao et al. [26] | 37.1 | 16.5 | 3.19 | 28 | 9.88 |
| Gao et al. [26] | 43 | 33.9 | 2.16 | 22 | 7.28 |
| Gao et al. [26] | 72 | 34.6 | 3.36 | 19.8 | 10 |
| Gao et al. [26] | 49 | 26.8 | 1.80 | 19.8 | 15.8 |
| Gao et al. [26] | 87.7 | 31.4 | 5.21 | 30.5 | 49.6 |
| Rizvi et al. [27] | 46 | 23 | 2.07 | NA | NA |
| Minor et al. [28] | 55.4 | 28.4 | 0.119 | NA | NA |
| Rajender et al. [29] | 25.8 | 15.8 | 5.8 | NA | NA |
| Tan et al. [30] | 21 | 34 | 2.63 | 25 | NA |
| Zakharia et al. [31] | 72 | 61 | 2.40 | NA | 16.3 |
| Chiang et al. [32] | 46.5 | 17.6 | 2.03 | NA | 27.05 |
| Chiang et al. [32] | 54.3 | 19.6 | 2.17 | NA | 8.15 |
| Chiang et al. [32] | 64.3 | 20.2 | 1.44 | NA | 16.08 |
| Wu et al. [33] | 35.5 | 13.8 | 1.95 | 30.5 | 4.6 |
| Wu et al. [33] | 29.2 | 12.9 | 1.55 | 19.1 | 3.6 |
| Wu et al. [33] | 45.9 | 25.1 | 2.56 | 26.7 | 7.8 |
| Wu et al. [33] | 33.7 | 22.3 | 2.41 | 29.1 | 10.9 |
| Wu et al. [33] | 31.4 | 19.6 | 2.01 | 22.1 | 7.8 |
| Chernykh et al. [34] | 26.9 | 13.5 | 2.90 | NA | NA |
| Butler et al. [35] | 51 | NA | 4.77 | NA | NA |
| Castineyra et al. [36] | 50 | 16 | 3.00 | 28 | 14 |
| Castineyra et al. [36] | 3.5 | 6.2 | 7.00 | 38 | 3.4 |
| Castineyra et al. [36] | 21 | 5.2 | 1.40 | 19 | 8.1 |
| Castineyra et al. [36] | 6.7 | 4.2 | 5.60 | 30 | 6.2 |
| Castineyra et al. [36] | 45 | 40 | 3.00 | 20 | 5.4 |
| Fuse et al. [37] | 47 | 60 | 1.60 | NA | NA |
| Pais et al. [38] | 53 | 45 | 2.67 | NA | NA |
| Wegner et al. [39] | 23.7 | 37.1 | 6.30 | NA | 3.8 |
| Micic et al. [40] | 31 | 18 | 3.19 | 47 | 6.8 |
| Matthews et al. [41] | 46 | 19 | 2.82 | 33 | 9.87 |
| Our case | 24.7 | 9.4 | 2.7 | 14 | 12.2 |
NA: not available; FSH: follicle-stimulating hormone; LH: luteinizing hormone; TT: total testosterone; E2: estradiol; PRL: prolactin.
The genetic features of the patients are listed in Table 3. All patients had a 46,XX karyotype. The SRY gene was detected in 51/57 cases (89.5%) and was absent in 6/57 (10.5%) cases. It was not described in the case reports of four patients. The position of the SRY was on the Xp in 97% of the cases.
Table 3.
Genetic features of 46,XX male adults.
| Authors | Presence of SRY | Location of SRY |
|---|---|---|
| Guzman et al. [5] | + | NA |
| Gunes et al. [6] | + | NA |
| Gunes et al. [6] | + | NA |
| Valetto et al. [7] | NA | NA |
| Kim et al. [8] | NA | NA |
| Xiao et al. [9] | − | NA |
| Queralt et al. [10] | + | NA |
| Baziz et al. [11] | + | NA |
| Tomomasa et al. [12] | + | NA |
| Chung Jung et al. [13] | + | NA |
| Wang et al. [14] | + | NA |
| Ahsan T et al. [15] | NA | NA |
| Jain et al. [16] | + | NA |
| Yencilek et al. [17] | NA | NA |
| Pepene et al. [18] | + | NA |
| Mustafa et al. [19] | − | NA |
| Majzoub et al. [20] | + | NA |
| Majzoub et al. [20] | + | NA |
| Majzoub et al. [20] | + | NA |
| Majzoub et al. [20] | − | NA |
| Majzoub et al. [20] | + | NA |
| Majzoub et al. [20] | + | NA |
| Onrat et al. [21] | + | NA |
| Hado et al. [22] | + | NA |
| Rigola et al. [23] | + | Xp |
| Dauwerse et al. [24] | + | 16q |
| Ryan et al. [25] | − | NA |
| Gao et al. [26] | + | Xp |
| Gao et al. [26] | + | Xp |
| Gao et al. [26] | + | Xp |
| Gao et al. [26] | + | Xp |
| Gao et al. [26] | + | Xp |
| Gao et al. [26] | + | Xp |
| Gao et al. [26] | + | Xp |
| Gao et al. [26] | + | Xp |
| Gao et al. [26] | + | Xp |
| Gao et al. [26] | + | Xp |
| Gao et al. [26] | + | Xp |
| Rizvi et al. [27] | + | Xp |
| Minor et al. [28] | + | Xp |
| Rajender et al. [29] | − | NA |
| Tan et al. [30] | NA | NA |
| Zakharia et al. [31] | NA | NA |
| Chiang et al. [32] | + | Xp |
| Chiang et al. [32] | + | Xp |
| Chiang et al. [32] | − | NA |
| Wu et al. [33] | + | Xp |
| Wu et al. [33] | + | Xp |
| Wu et al. [33] | + | Xp |
| Wu et al. [33] | + | Xp |
| Wu et al. [33] | + | Xp |
| Chernykh et al. [34] | + | Xp |
| Butler et al. [35] | + | Xp |
| Castineyra et al. [36] | + | Xp |
| Castineyra et al. [36] | + | Xp |
| Castineyra et al. [36] | + | Xp |
| Castineyra et al. [36] | + | Xp |
| Castineyra et al. [36] | + | Xp |
| Fuse et al. [37] | + | Xp |
| Pais et al. [38] | + | Xp |
| Wegner et al. [39] | + | Xp |
| Micic et al. [40] | NA | NA |
| Matthews et al. [41] | + | Xp |
| Our case | + | Xp |
NA: not available; SRY: sex-determining region Y; Xp: short arm of X chromosome.
The patients were stratified by SRY into two groups: SRY-positive (SRY+) and SRY-negative (SRY–). A statistical analysis was performed in order to find significant clinical or hormonal differences between the two groups (Table 4).
Table 4.
Comparison of SRY-positive and SRY-negative patients.
| Patients | HD% Normal | GM% No | Penis size% Normal | ED% No | FSH mIU/mL Mean (SD) |
LH mIU/mL Mean (SD) |
TT ng/mL Mean (SD) |
PRL ng/mL Mean (SD) |
|---|---|---|---|---|---|---|---|---|
| SRY+ | 73.3 | 55.2 | 90.9 | 90.0 | 38.63 (20.85) | 29.40 (19.09) | 2.64 (1.49) | 12.93 (9.32) |
| SRY− | 66.7 | 57.1 | 60.0 | 85.7 | 51.74 (28.14) | 21.63 (9.56) | 2.49 (1.79) | 14.27 (4.07) |
| p = 0.422 | p = 0.596 | p = 0.144 | p = 0.823 | p = 0.195 | p = 0.326 | p = 0.819 | p = 0.781 |
HD: hair distribution; GM: gynecomastia; ED: erectile disfunction; FSH: follicle-stimulating hormone; LH: luteinizing hormone; TT: total testosterone; PRL: prolactin.
Hair distribution, gynecomastia, testes volume, penis size, pubic hair, erectile dysfunction, libido, FSH, LH, PRL, and E2 were compared: no statistically significant differences (p > 0.05) were found between the SRY-positive and SRY-negative patients for comparable parameters. However, it was not possible to compare all parameters between the two groups because there were few SRY-negative cases and limited available data.
4. Discussion
The term DSD, introduced by the Chicago Consensus Group in 2005, distinguishes three major groups:
DSD with atypical sex chromosome configurations, including Turner syndrome, Klinefelter syndrome, and conditions with 46,XX or 46,XY karyotypes;
XY DSD characterized by 46,XY karyotype;
XX DSD comprised of conditions characterized by 46,XX karyotype and androgen excess, such as congenital adrenal hyperplasia, P450 oxidoreductase deficiency, or exogenous causes [42].
Male adults with 46,XX and DSD are part of the first of these three groups. Many patients with 46,XX karyotype have external male genitalia [11], but they generally have small testes and may also have abnormalities such as cryptorchidism or hypospadias, azoospermia, hypergonadotropic hypogonadism, varying degrees of gynecomastia, poor facial hair growth, diminished libido, and normal cognitive development [43]. No extra-genital abnormalities were found in all reported cases. However, phenotypes in these patients were different, ranging from severe impairment of the external genitalia to hypospadias and/or cryptorchidism to normal male phenotype. It depends mainly, but not only, on the presence of the sex-determining region Y (SRY). In recent years a number of other genes involved in disorders of sex development have also been identified. For instance, SOX9, SOX3, DAX1, WT1, FGF9, and SF1 are also involved in the sex determination cascade [44,45]. Therefore, taking the SRY gene as the only target gene [3] may not provide enough information, and the clinical manifestations of some of the 46,XX male patients were not consistent with the expression of the SRY gene. Regardless, male sex differentiation is mostly dependent on the presence of the SRY gene, which drives the primitive gonads into testes formation during early human embryonic development [46]. This belief is held because XX SRY+ subjects are generally men with male genitalia, whereas XX SRY− subjects have ambiguous genitalia. Nevertheless, there are a few XX SRY+ subjects with ambiguous sexual characteristics and, therefore, another Y chromosome gene contributing to complete male sex differentiation has been postulated. In a small number of SRY+ cases with ambiguous genitalia, there is a small Y fragment located on inactive X in most metaphases [47,48]. Kolon [44] reports that, in general, the greater the amount of Y chromosome DNA present, the more masculinized the phenotype will be. Male adults with 46,XX and normal external genitalia generally discover their pathology in adulthood because of infertility. Infertility affects approximately 10–15% of all couples worldwide [8]. Approximately 30–40% of infertility cases can be attributed to male factors [49]. In about 15% of male infertility cases, no organic cause can be identified [8]. Idiopathic infertility found in most cases of non-obstructive azoospermia (NOA) or severe oligozoospermia is due to chromosomal abnormalities or mutations of genes involved in sex determination and spermatogenesis [50]. The incidence of cytogenetic abnormality is estimated at 5.8% in infertile men and only 0.5% in the normal population [51].
Diagnosis is based on clinical findings, endocrine testing, and cytogenetic testing [2]. Cryptorchidism is present in 15% and anterior hypospadias in around 10%. Endocrine testing normally reveals hypergonadotropic hypogonadism secondary to testicular failure [2,47]. Cytogenetic testing reveals 46,XX. Approximately 80% of 46,XX DSD individuals are SRY+, and around 20% are SRY− [2,47]. The investigation is usually based on fluorescence in situ hybridization (FISH) or polymerase chain reaction (PCR) amplification of the SRY gene.
There is no literature regarding the comparison between FISH and PCR for SRY detection and location. Considering similar studies were performed in different contexts (e.g., detection of BCR-ABL fusion gene, detection of translocation RCC), we think that FISH and RT-PCR should be used together in order to improve the sensitivity of SRY detection and location [42,52].
Various studies indicated that 80–90% of 46,XX males result from a Y to X translocation during meiosis [49,53]. 46,XX males who showed no evidence of Y specific DNA, including SRY, were reported [50]. This suggests that testicular development in these males occurred in the absence of the SRY gene. On the basis of karyotype analysis and detection of the SRY gene, 46,XX male patients can be clinically divided [3] into the SRY-positive and the SRY-negative groups.
The SRY gene is identified as the main gene regulating the testes determination cascade. The most important role of SRY is to regulate the SOX9 expression in Sertoli cell precursors. This pathway, in turn, activates testis-specific genes leading to testis determination [51].
In the absence of SRY (SRY-negative patients), the male phenotype develops probably from the gain of function in a gene downstream to the SRY pathway [29]. SOX9, SOX3, DAX1, WT1, FGF9, and SF1 are also involved in the sex determination cascade [54]. While the clinical symptoms of patients often show some degree of heterogeneity [55], usually, the development of genitalia is normal and masculinity signs are obvious in SRY+ patients. There is no abnormality in the development of penis and sex psychology as well as erection and ejaculation, and there are almost no significant positive signs except cryptorchidism before puberty. So, it is difficult to find SRY+ male DSD patients before puberty, who are often incidentally found by chromosome check for infertility or poor testicle development. On the contrary, SRY− patients could be easily discriminated due to abnormality of genitalia shortly after birth; some patients even show genital ambiguity [55]. Masculinity signs are not clear in SRY− patients; especially in adult patients. Breast development and female secondary sex characteristics can be found. For patients with the SRY− gene, due to genital ambiguity, patients’ sex psychology and physiological development should be carefully taken into consideration for treatment [3] and the patient may not be able to avoid drug-dependence for maintaining the secondary sex characteristics.
On the basis of our statistical analysis the presence/absence of SRY does not seem to affect the clinical and hormonal characteristics of the patients, however the SRY− cases are fewer than the SRY+ cases. This may have erroneously led to a no statistically significant difference between the two groups.
Individuals with the 46,XX male syndrome may have phenotypic and endocrinologic status that would be expected to be the same as in Klinefelter’s syndrome characterized by 47,XXY. Thus, this syndrome is considered as a variant form of Klinefelter’s syndrome. However, one does not always assume that a 46,XX male will be similar to individuals with Klinefelter’s syndrome [13], which has the characteristics of tall stature, gynecomastia, small testicular volume, a borderline-low intelligence quotient (IQ), and hypergonadotropic hypogonadism. Some 46,XX males do not have gynecomastia, hypogonadism, or even exaggerated LH-releasing hormone (LHRH)-stimulated gonadotropin response [56]. In 46,XX male syndrome, different from Klinefelter’s syndrome, there is short stature which is probably due to translocations of sex chromosomes or to genetic defects that affect growth hormone (GH) activity. Chung-Jung et al. [13] found exaggerated GH response after an insulin-induced hypoglycemic test. They suggested that the short stature in patients with 46,XX male syndrome should not be attributed to the syndrome itself. They speculate possible defects of GH activity as in partial GH insensitivity syndrome that involves genes controlling expression of GH receptor. Anyway, the pathogenesis of 46,XX male DSD is not clear. According to Wang et al. [3], the hypotheses are as follows:
The hypothesis of target gene mutation [57]. It supposes the structural gene that determines human gender may be located in autosome, which is regulated by the inhibition of the X chromosome and the activation of the Y chromosome. 46,XX individuals, due to defects in the inhibition of the X chromosome, which results in spontaneous activation of the downstream gene in the absence of the SRY gene, transform into 46,XX males.
The hypothesis of SOX9 gene (SRY box-related gene 9) overexpression. SOX are a large gene family, in which SOX9 is located in 17q24.3–q25.1 and homologous with SRY High-Mobility Group box (HMG)-box as high as 60%. Early studies confirmed that SOX9 was mainly involved in bone formation and the regulation of Sertoli cell differentiation [58], which was also expressed in the precursor cell of supporting cells with the SRY gene expression. In addition, the fact that SOX9 can be found in spinal animals and mammals, compared with the SRY gene which can be found only in mammals, might indicate that SOX9 was a more ancient gene involved in sex differentiation than the SRY gene. Huang et al. [59] reported the case of a SRY-negative 46,XX male patient, and chromosome analysis showed the existence of overlapping of the SOX9 gene. Malki et al. [60] found that prostaglandin D2 (PGD2) could induce the expression of SOX9 in a normal female rat gonad in vitro. Thus, up-regulation of SOX9 expression caused by chromosomal abnormalities or mediated by other bypass activation (e.g., PGD2) may result in the occurrence of SRY-negative 46,XX male patients.
The hypothesis of Xp-Yp translocation. It supposes that the ends of the XY chromosomes’ abnormal exchange (Xp-Yp translocation) occurs during paternal sperm meiosis and results in X-type sperm containing the SRY gene, which could lead to 46,XX offspring when combined with eggs. PCR can detect the SRY gene, however, only to find it translocated in the X chromosome by FISH. In Brazil, Domenice et al. [61] found that 90% of 46,XX males carried Y chromosome material, including the SRY gene, in most cases. It indicated that the Y chromosome fragment containing the SRY gene translocation may be an important factor for the occurrence of SRY+ 46,XX male patients.
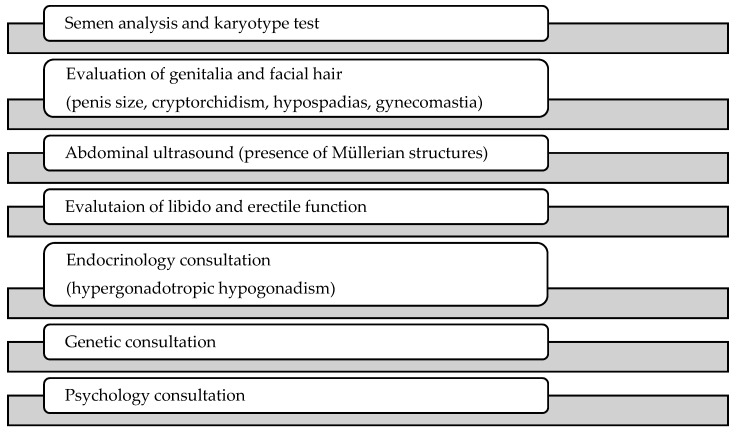
Facing a patient with 46,XX male syndrome, the clinician should keep in mind the possible abnormalities linked to the syndrome and thus perform an adequate screening: an appropriate physical examination should include evaluation and palpation of the genitalia to detect cryptorchidism, hypospadias, penis size, and gynecomastia. It is important to use a validated questionnaire to evaluate erectile dysfunction and diminished libido, such as the International Index of Erectile Function (IIEF); semen analysis is mandatory (even though this is often the reason why they come to the urologist) and so is the karyotype test. An abdominal ultrasound is useful in order to exclude residual Müllerian structures. Genetic and endocrine consultations are necessary to assess the status of the patient who often has hypergonadotropic hypogonadism (Figure 2).
Figure 2.
Diagnostic management for 46,XX male syndrome.
In SRY-negative patients, no further instrumental or blood tests are necessary, however, we suggest searching for mutations of other genes involved in the sex determination cascade such as SOX9, SOX3, DAX1, WT1, FGF9, and SF1.
5. Conclusions
In conclusion, 46,XX male DSD, characterized by mismatch of genetic, gonadal, and phenotypic sex is quite rare, and due to genetic or chromosomal abnormalities. FISH and PCR technology can quickly and accurately detect information about the SRY gene in patients, so as to provide more valuable clinical information. Although chromosomal abnormalities are rarely present in patients with apparently normal external genitalia, they should be considered in urology consultations by adolescents and adults, particularly in the investigation of gynecomastia or infertility. Finally, considering genetic abnormalities, testicular sperm extraction (TESE) is not recommended and these patients should consider adoption or in vitro fertilization with a sperm donor as fertility options.
Acknowledgments
This research did not receive any funding from agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Conceptualization, M.T. and M.S.; methodology, D.A.; software, D.A.; validation, G.B.; formal analysis, D.A.; investigation, M.S.; resources, C.Q. and F.B.; data curation, C.M.; writing—original draft preparation, M.T. and M.S.; writing—review and editing, C.M. and D.A.; visualization, D.R.G.; supervision, M.D.S.; project administration, D.A.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Nordenstrom B.C., Rohle A.R., Thyen R.U. Hormone therapy and patient satisfaction with treatment, in a large cohort of diverse disorders of sex development. Clin. Endocrinol. 2018;88:397–408. doi: 10.1111/cen.13518. [DOI] [PubMed] [Google Scholar]
- 2.Vilain E. 46, XX Testicular Disorder of Sex Development. Gene Rev. Last Updat. 2009;26:38–41. [Google Scholar]
- 3.Wang T., Bai J., Sakai S., Ohno Y., Ohno H.T. Magnetotransport studies of AlGaN/GaN heterostructures grown on sapphire substrates: Effective mass and scattering time. Appl. Phys. Lett. 2000;76:2737–2739. doi: 10.1063/1.126460. [DOI] [Google Scholar]
- 4.De la Chapelle A., Hortling H., Niemi M. XX sex chromosome in a human male, first case. Acta Med. Scand. 1964;175(Suppl. 412):25–28. doi: 10.1111/j.0954-6820.1964.tb04630.x. [DOI] [PubMed] [Google Scholar]
- 5.Guzman J.M.P., Navarro H.P., Mata Q.M., Lopez P.C., Ruiz J.M., Sanchiz C.M., Rodriguez J.A.V. 46, XX Testicular Disorder of sex development. Case report. Arch. Esp. Urol. 2011;64:468–472. [PubMed] [Google Scholar]
- 6.Gunes S., Asci R., Okten G., Atac F., Onat O.E., Ogur G., Aydin O., Ozcelik T. Two males with SRY positive 46 XX testicular disorder of sex development. Syst. Biol. Reprod. Med. 2013;59:42–47. doi: 10.3109/19396368.2012.731624. [DOI] [PubMed] [Google Scholar]
- 7.Valetto A., Bertini V., Rapalini E. A 46, XX SRY negative man with complete virilization and infertility as the main anomaly. Fertil. Steril. 2005;83:216–219. doi: 10.1016/j.fertnstert.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 8.Kim S.Y., Lee B.Y., Oh A.R., Park S.Y., Lee H.S., Seo J.T. Clinical, Hormonal, and Genetic Evaluation of Idiopathic Nonobstructive Azoospermia and Klinefelter Syndrome Patients. Cytogenet. Genome Res. 2018;153:190–197. doi: 10.1159/000487039. [DOI] [PubMed] [Google Scholar]
- 9.Xiao B., Ji X., Xing Y., Chen Y.W. A rare case of 46, XX SRY negative male with approximately 74 kb duplication in a region upstream of SOX9. Eur. J. Med. Genet. 2013;56:695–698. doi: 10.1016/j.ejmg.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Queralt R., Madrigal I., Vallecillos M.A., Morales C., Ballesca J.L., Oliva R., Soler A., Sánchez A., Margarit E. Atypical XX male with the SRY gene located at the long arm of chromosome 1 and a 1qter microdeletion. Am. J. Med. Genet. Part A. 2008;146:1335–1340. doi: 10.1002/ajmg.a.32284. [DOI] [PubMed] [Google Scholar]
- 11.Baziz M., Hamouli-Said Z., Ratbi I., Habel M., Guaoua S., Sbiti A., Sefiani A. Cytogenetic investigation in a group of ten infertile men with non-obstructive azoospermia: First Algerian 46, xx syndrome. Iran. J. Public Health. 2016;45:739–747. [PMC free article] [PubMed] [Google Scholar]
- 12.Tomomasa H. XX-male syndrome bearing the sex-determining region Y. Arch. Androl. 1999;42:89–96. doi: 10.1080/014850199262922. [DOI] [PubMed] [Google Scholar]
- 13.Chung Jung W., Song Y.M., Sheu W.H. Short stature in a 46 XX Male Adolescent. South Med. J. 1999;92:921–924. doi: 10.1097/00007611-199909000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Wang T., Liu J.H., Yang J., Chen J. 46, XX male sex reversal syndrome: A case report and review of the genetic basis. Andrologia. 2009;41:59–62. doi: 10.1111/j.1439-0272.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- 15.Ahsan T., Saleem M., Ahmed A. 46 XX male: A case of sex reversal syndrome. J. Pak. Med. Assoc. 1998;48:19–20. [PubMed] [Google Scholar]
- 16.Jain M., Veeramohan V., Chaudhary I. The Sertoli cell only syndrome and glaucoma in a sex—Determining region Y (SRY) positive XX infertile male. J. Clin. Diagn. Res. 2013;7:1457–1459. doi: 10.7860/JCDR/2013/5186.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yencilek F., Baykal C. 46, XX male syndrome: A case report. Clin. Exp. Obs. Gyn. 2005;32:263–264. [PubMed] [Google Scholar]
- 18.Pepene C.E., Coman I., Mihu D., Militaru M. Infertility in a new 46, XX male with positive SRY confirmed by fluorescence in situ hybridization: A case report. Clin. Exp. Obs. Gynecol. 2008;35:299–300. [PubMed] [Google Scholar]
- 19.Mustafa O.A. 46, XX SRY—Negative man with infertility, and co existing with chronic autoimmune thyroiditis. Gynecol. Endocrinol. 2010;26:413–415. doi: 10.3109/09513591003632225. [DOI] [PubMed] [Google Scholar]
- 20.Majzoub A., Arafa M., Starks C., Elbardisi H., Al Said S., Sabanegh E. 46 XX karyotype during male fertility evaluation; case series and literature review. Asian J. Androl. 2017;19:168. doi: 10.4103/1008-682X.181224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onrat S.T., Söylemez Z. 46, XX der (15), t (Y;15) (q12; p11) karyotype in an azoospermic male. Indian J. Hum. Genet. 2012;18:241–245. doi: 10.4103/0971-6866.100785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hado H.S., Helmy S.W., Klemm K., Miller P. XX male: A rare cause of short stature, infertility, gynaecomastia and carcinoma of the breast. Int. J. Clin. Pract. 2003;57:844–845. [PubMed] [Google Scholar]
- 23.Rigola M.A., Carrera M., Ribas I., Egozcue J., Miro R., Fuster C. A comparative genomic hybridization study in a 46, XX male. Fertil. Steril. 2002;78:186–188. doi: 10.1016/S0015-0282(02)03165-5. [DOI] [PubMed] [Google Scholar]
- 24.Dauwerse J.G., Hansson K.B., Brouwers A.A., Peters D.J. An XX male with the sex determining region Y gene inserted in the long arm of chromosome 16. Fertil. Steril. 2006;86:463. doi: 10.1016/j.fertnstert.2005.12.062. [DOI] [PubMed] [Google Scholar]
- 25.Ryan N.A. A case report of an incidental finding of a 46, XX, SRY negative male with masculine phenotype during standard fertility workup with review of the literature and proposed immediate and long term management guidance. Fertil. Steril. 2013;99:1273–1276. doi: 10.1016/j.fertnstert.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 26.Gao X., Chen G., Huang J., Bai Q., Zhao N., Shao M., Jiao L., Wei Y., Chang L., Li D., et al. Clinical, cytogenetic, and molecular analysis with 46, XX male sex reversal syndrome: Case reports. J. Assist. Reprod. Genet. 2013;30:431–435. doi: 10.1007/s10815-013-9939-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizvi A.A. 46, XX man with SRY gene translocation: Cytogenetic characteristics, clinical features and management. Am. J. Med. Sci. 2008;335:307–309. doi: 10.1097/MAJ.0b013e31811ec1b4. [DOI] [PubMed] [Google Scholar]
- 28.Minor A., Mohammed F., Farouk A., Hatakeyama C., Johnson K., Chow V., Ma S. Genetic characterization of two 46, XX males without gonadal ambiguities. J. Assist. Reprod. Genet. 2008;25:547–552. doi: 10.1007/s10815-008-9265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rajender S., Rajani V., Gupta N.J., Chakravarty B., Singh L., Thangaraj K. SRY negative 46, XX male with normal genitals, complete masculinization and infertility. Mol. Hum. Reprod. 2006;12:341–346. doi: 10.1093/molehr/gal030. [DOI] [PubMed] [Google Scholar]
- 30.Tan T.T. Primary infertility in a phenotypic male with 46XX chromosomal constitution. Postgrad. Med. J. 1983;69:315–317. doi: 10.1136/pgmj.69.810.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zakharia G. Sex reversal syndrome (XX male) Urology. 1990;36:322–324. doi: 10.1016/0090-4295(90)80238-I. [DOI] [PubMed] [Google Scholar]
- 32.Chiang H.S., Wu Y.N., Wu C.C. Cytogenic and molecular analyses of 46, XX male syndrome with clinical comparison to other groups with testicular azoospermia of genetic origin. J. Formos. Med. Assoc. 2013;112:72–78. doi: 10.1016/j.jfma.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Wu Q.Y., Li N., Li W.W., Li T.F., Zhang C., Cui Y.X., Xia X.Y., Zhai J.S. Clinical, molecular and cytogenetic analysis of 46, XX testicular disorder of sex development with SRY positive. BMC Urol. 2014;14:70. doi: 10.1186/1471-2490-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chernykh V.B., Kurilo L.F., Shilova N.V., Zolotukhina T.V., Ryzhkova O.P., Bliznetz E.A., Polyakov A.V. X chromosomal mosaicism in a 46, XX male. Sex Dev. 2009;3:183–187. doi: 10.1159/000228718. [DOI] [PubMed] [Google Scholar]
- 35.Butler M.G., Walzak M.P., Sanger W.G. A possible etiology of the infertile 46XX male subject. J. Urol. 1983;130:154–156. doi: 10.1016/S0022-5347(17)51010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castineyra G., Copelli S. 46, XX male: Clinical, hormonal/genetic findings. Arch. Androl. 2002;48:251–257. doi: 10.1080/01485010290031556. [DOI] [PubMed] [Google Scholar]
- 37.Fuse H., Satomi S., Kazama T., Katayama T., Nagabuchi S., Tamura T., Nakahori Y., Nakagome Y. DNA hybridization study using Y specific probes in an XX male. Andrologia. 1991;23:237–239. doi: 10.1111/j.1439-0272.1991.tb02547.x. [DOI] [PubMed] [Google Scholar]
- 38.Pais V.M. Infertility in an XX male. J. Urol. 1977;118:690–691. doi: 10.1016/S0022-5347(17)58160-4. [DOI] [PubMed] [Google Scholar]
- 39.Wegner R.D. Clinical, cytological, and biochemical investigations in a case of an XX male. Andrologia. 1983;15:253–258. doi: 10.1111/j.1439-0272.1983.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 40.Micic S., Micic M. An example of a 46, XX infertile man and his permanent tooth sizes. Int. J. Fertil. 1983;28:165–168. [PubMed] [Google Scholar]
- 41.Matthews C.D., Ford J. The XX male. Clinical and theoretical aspects. Reprod. Fertil. 1983;2:207–215. [PubMed] [Google Scholar]
- 42.Lee P.A., Nordenström A., Houk C.P., Ahmed S.F., Auchus R., Baratz A., Baratz Dalke K., Liao L.M., Lin-Su K., Looijenga L.H., 3rd, et al. Global Disorders of Sex Development Update since 2006: Perceptions, Approach and Care. Horm. Res. Paediatr. 2016;85:158–180. doi: 10.1159/000442975. [DOI] [PubMed] [Google Scholar]
- 43.Li J.H., Huang T.H., Jiang X.W. 46, XX male sex reversal syndrome. Asian J. Androl. 2004;6:165–167. [PubMed] [Google Scholar]
- 44.Kolon T.T. Bases moleculares de los trastornos intersexuales. AUA Updat. Ser. 2001;34:39–50. [Google Scholar]
- 45.Zenteno-Ruiz J.C., Kofman-Alfaro S., Méndez J.P. 46, XX sex reversal. Arch. Med. Res. 2001;32:559–566. doi: 10.1016/S0188-4409(01)00322-8. [DOI] [PubMed] [Google Scholar]
- 46.Lopez M., Torres L., Mendez J.P., Cervantes A., Alfaro G., Pérez-Palacios G., Erickson R.P., Kofman-Alfaro S. SRY alone can induce normal male sexual differentiation. Am. Med. Genet. 1995;55:356–358. doi: 10.1002/ajmg.1320550321. [DOI] [PubMed] [Google Scholar]
- 47.Bouayed Abdelmoula N., Portnoi M.F., Keskes L., Recan D., Bahloul A., Boudawara T., Saad A., Rebai T. Skewed X-chromosome inactivation pattern in SRY positive XX maleness: A case report and review of literature. Ann. Genet. 2003;46:11–18. doi: 10.1016/S0003-3995(03)00011-X. [DOI] [PubMed] [Google Scholar]
- 48.Kusz K., Kotecki M., Wodja A., Szarras-Czapnik M., Latos-Bielenska A., Warenik-Szymankiewicz A., Ruszczynska-Wolskab A., Jaruzelskaa J. Incomplete masculinisation of XX subjects carrying the SRY gene on an inactive X chromosome. J. Med. Genet. 1999;36:452–456. [PMC free article] [PubMed] [Google Scholar]
- 49.Conte F.A., Grumbach M.M. In: Disorders of Sex Determination and Differentiation. Greenspan’s Basic & Clinical Endocrinology. 9th ed. David G., Dolores S., editors. McGraw-Hill; New York, NY, USA: 2011. [Google Scholar]
- 50.Ferguson-Smith N., Cooke M.A., Affara A., Boyd E., Tolmie J.L. Genotype- phenotype correlations in XX males and their bearing on current theories of sex determination. Hum. Genet. 1990;84:198–202. doi: 10.1007/BF00208942. [DOI] [PubMed] [Google Scholar]
- 51.She Z.Y., Yang W.X. Seminars in Cell & Developmental Biology. Volume 63. Academic Press; New York NY, USA: 2017. Sry and SoxE genes: How they participate in mammalian sex determination and gonadal development? pp. 13–22. [DOI] [PubMed] [Google Scholar]
- 52.Cox M.C., Maffei L., Buffolino S., Del Poeta G., Venditti A., Cantonetti M., Aronica G., Aquilina P., Masi M., Amadori S. A Comparative Analysis of FISH, RT-PCR, and Cytogenetics for the Diagnosis of bcr-abl Positive Leukemias. Am. J. Clin. Pathol. 1998;109:24–31. doi: 10.1093/ajcp/109.1.24. [DOI] [PubMed] [Google Scholar]
- 53.Petit C., de la Chapelle A., Levilliers J., Castillo S., Noë B., Weissenbach J. An abnormal XY interchange accounts for most but not all the cases of human XX maleness. Cell. 1997;49:595–602. doi: 10.1016/0092-8674(87)90535-6. [DOI] [PubMed] [Google Scholar]
- 54.Mizuno K., Kojima Y., Kamisawa H., Moritoki Y., Nishio H., Kohri K., Hayashi Y. Gene expression profile during testicular development in patients with SRY-negative 46, XX testicular disorder of sex development. Urology. 2013;82:1453. doi: 10.1016/j.urology.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 55.Ergun-Longmire N.M., Vinci G., Alonso L., Matthew S., Tansil S., Lin-Su K., McElreavey K. Clinical, hormonal and cytogenetic evaluation of 46, XX males and review of the literature. J. Pediatr. Endocrinol. Metab. 2005;18:739–748. doi: 10.1515/JPEM.2005.18.8.739. [DOI] [PubMed] [Google Scholar]
- 56.Hazama M., Kondo K. Male infertility with chromosomal abnormalities. II. XX-male syndrome. Hinyokika Kiyo Acta Urologica Japonica. 1987;33:193–203. [PubMed] [Google Scholar]
- 57.Liu L., Feng L.N. The phenotype and genetics of 46, XX male syndrome. Endocrinol. Foreign Med. Sci. 2005;25:283–285. [Google Scholar]
- 58.Foster J.W., Dominguez-Steglich M.A., Guioli S., Kowk G., Weller P.A., Stevanovic M., Weissenbach J., Mansour S., Young I.D., Goodfellow P.N., et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY- related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 59.Huang B., Wang S., Ning Y., Lamb A.N. Autosomal XX sex reversal caused by duplication of SOX9. Am. J. Med. Genet. 1999;87:349–353. doi: 10.1002/(SICI)1096-8628(19991203)87:4<349::AID-AJMG13>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 60.Malki S., Nef S., Notarnicola C., Thevenet L., Gasca S., Méjean C., Berta P., Poulat F. Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation. EMBO J. 2005;24:1798–1809. doi: 10.1038/sj.emboj.7600660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Domenice S., Correa R.V., Costa E.M., Nishi M.Y., Vilain E., Arn-Hold I.J. Mutations in the SRY, DAX1, SF1 and WNT4 genes in Brazilian sex-reversed patients. Braz. J. Med. Biol. Res. 2004;37:145–150. doi: 10.1590/S0100-879X2004000100020. [DOI] [PubMed] [Google Scholar]