Abstract
Background and Objective: Urinary tract infections (UTIs) are common in human medicine, affecting large patient populations worldwide. The principal cause of UTIs is uropathogenic Escherichia coli (UPEC) and Klebsiella, both in community and nosocomial settings. The assessment of local data on prevalence and resistance is essential to evaluate trends over time and to reflect on the national situation, compared to international data, using the methods of analytical epidemiology. Materials and Methods: The aim of this study was to assess resistance trends and epidemiology of UTIs caused by E. coli and Klebsiella species in inpatients and outpatients at a tertiary-care hospital in Hungary, using microbiological data. To evaluate resistance trends, several antibiotics were chosen as indicator drugs, based on local utilization data. Results: E. coli was the most prevalent isolate, representing 56.75 ± 4.86% for outpatients and 42.29 ± 2.94% for inpatients. For E. coli, the ratio of resistant strains for several antibiotics was significantly higher in the inpatient group, while in Klebsiella, similar trends were only observed for gentamicin. Extended-spectrum β-lactamase (ESBL)-producing isolates were detected in 4.33–9.15% and 23.22–34.22% from outpatient, 8.85–38.97% and 10.89–36.06% from inpatient samples for E. coli and Klebsiella, respectively. Conclusions: Resistance developments in common UTI pathogens (especially to fosfomycin, sulfamethoxazole-trimethoprim, fluoroquinolones, and 3rd generation cephalosporins), seriously curb therapeutic options, especially in outpatient settings.
Keywords: urinary tract infection, infectious disease, antibiotic, resistance, indicator, epidemiology, fosfomycin, ESBL, Escherichia coli, Klebsiella
1. Introduction
Urinary tract infections (UTIs) are some of the most common infections in human medicine, affecting a large patient population (around 150 million cases/year) to various extents, irrespective of age and gender [1,2]. Both community-acquired (representing 10–30% of infections) and nosocomial UTIs (accounting for 25–50% of infections) should be considered as an important factor of morbidity (they are often associated with complications, sequelae, recurrence and decreased quality of life), a serious public health issue and an economic burden (the therapy and care of these patients alone is estimated to cost around 5 billion US$) [3,4,5]. The causative agents of UTIs are diverse, especially in nosocomial settings (where prolonged catheterization and immunosuppression facilitates the occurrence of non-conventional urinary pathogens) [6,7].
However, the most common causative agents of UTIs are the members of the Enterobacteriaceae family (or the Enterobacterales order, based on recent taxonomic changes [8]), followed by (in decreasing order of frequency) some Gram-positive bacteria (Enterococcus faecalis, Group B streptococci, Staphylococcus saprophyticus and S. aureus), atypical microorganisms (Mycoplasma, Ureaplasma species), non-fermenting Gram-negative bacteria (Pseudomonas, Acinetobacter) and Candida spp. [1,3,4,5,6,9,10]. The principal cause of UTIs (>80%) are uropathogenic Escherichia coli (UPEC) and Klebsiella species (more specifically, K. pneumoniae and K. oxytoca), both in the community and nosocomial settings. E. coli is described as the etiological agent in 60–90% of urinary tract infections, while Klebsiella species accounts for 3–20% of cases [3,11,12,13,14,15]. These species are successful pathogens in the urinary system, as they possess the relevant virulence factors required to successfully survive on and adhere to urinary epithelium, cause tissue damage and to ascend to the upper urinary tract (leading to complicated UTIs) [16,17,18,19,20,21]. These virulence factors include the lipopolysaccharide (LPS), polysaccharide capsule, outer membrane vesicles, iron-uptake (aerobactin) and siderophore receptors, adhesins, Type-1 fimbriae, cytotoxins and urease production. In fact, UPEC may be classified into several phylogroups (namely A, B1, B2 and D) based on the presence of pathogenicity islands (PAI) and virulence factor expression [16,17,18,19,20,21]. The relevance of Klebsiella species has been described in nosocomial UTIs, as they are ubiquitous in the hospital environment and are able to survive on both living (e.g., patient’s skin) and abiotic (e.g., wards, catheters) surfaces [22,23,24].
The recommended drugs for the (empiric) treatment of uncomplicated urinary tract infections include nitrofurantoin, fosfomycin and sulfamethoxazole/trimethoprim (if local resistance levels do not exceed 20%), which are all available per os; in case of resistance or hypersensitivity to these drugs, or if complicated UTIs (e.g., pyelonephritis) need to be treated, other therapeutic options, such as β-lactam antibiotics (third generation cephalosporins, e.g., ceftriaxone; carbapenems), fluoroquinolones and aminoglycosides should also be considered [3,5,25,26]. The group of β-lactam antibiotics is especially important, because in several vulnerable patient populations (children, pregnant women, patients with liver/kidney failure), there drugs are the first-choice agents, due to the debilitating side effect-profile of the alternate drugs [27]. Extended-spectrum β-lactamases (ESBLs) are plasmid-encoded enzymes, capable of hydrolyzing penicillin-derivatives and cephalosporins (including third and fourth generation cephalosporins); in contrast, AmpC-type β-lactamases are mostly chromosomally-encoded (although they have also been described on plasmids), capable of hydrolyzing penicillin-derivatives and cephalosporins (including third generation cephalosporins and aztreonam, but not fourth generation cephalosporins). ESBLs are mainly Ambler Class A enzymes, that are inhibited by “first-generation” β-lactamase-inhibitors (such as clavulanic acid or sulbactam), while AmpC enzymes are Class C enzymes that are not inhibited by these adjuvants [28,29,30,31,32,33]. Among Enterobacteriaceae, the prevalence of ESBLs and plasmid-encoded AmpCs are the highest in Klebsiella spp. and E. coli [34]. Additionally, Klebsiella species have shown to be great vectors for these plasmids and they provide a suitable genetic environment to mutations [28,29,30,31,32,33]. Since the 2000s, blaCTX-M-type ESBLs are the most prevalent around the globe, while blaTEM and blaSHV-type enzymes have become less relevant over time [28,29,30,31,32,33,35,36]. Due to their transmissibility, ESBLs are considered a serious public health/infection control issue, especially because these enzymes are encoded on larger-sized plasmids, which may additionally include resistance determinants for aminoglycosides and/or fluoroquinolones [28,37]. ESBL-producers have been associated with increased morbidity and mortality, particularly in intensive care units and in severely immunocompromised patients [38,39,40]. In UTIs with an ESBL-producing isolate (coupled with other resistance mechanisms), carbapenems essentially remain the only safe drug choice, while other “last resort” agents (e.g., tigecycline, colistin, ceftazidime-avibactam) are rarely used, due to their price, pharmacokinetic profile or side effects. Multidrug resistance (MDR) in Gram-negative bacteria is a growing concern, and the treatment of UTIs is increasingly complex challenge for clinicians [25,41,42,43,44]. These uropathogens may be resistant to a wide range of available drugs, necessitating the use of drugs that are only available in intravenous formulations, limiting the care of these patients to inpatient setting or outpatient parenteral antimicrobial therapy (OPAT), if available [45,46].
Various national- and international-level surveillance programs are evaluating and publishing the resistance trends of various Gram-positive and Gram-negative bacteria [6,9,14,47,48,49,50,51,52]. Nevertheless, the epidemiology and antibiotic-susceptibility patterns of urinary tract pathogens vary greatly by region therefore, the assessment of local data is essential to evaluate trends over time and to reflect on the national situation, compared to international data, using the methods of analytical epidemiology [6,9,14,47,48,49,50,51,52]. Additionally, knowledge of the relevant antibiotic-susceptibility patterns of the major bacterial pathogens for UTIs is of utmost importance to allow for the optimal choice for antibiotic therapy [53,54]. The aim of this study was to assess and compare the resistance trends and epidemiology of E. coli and Klebsiella species in inpatients and outpatients at the Albert Szent-Györgyi Clinical Center (Szeged, Hungary) retrospectively, during a 10-year study period.
2. Materials and Methods
2.1. Study Design, Data Collection
This retrospective study was carried out using microbiological data collected from the period between the 1st of January 2008 and 31st of December 2017 at the Institute of Clinical Microbiology (University of Szeged), which is the affiliated diagnostic microbiology laboratory of the Albert Szent-Györgyi Clinical Center, a primary- and tertiary-care teaching hospital in the Southern Great Plain of Hungary. The Clinical Center has a bed capacity of 1820-beds (1465 active and 355 chronic beds, respectively) and annually serves more than 400,000 patients in the region, according to the data of the Hungarian National Health Insurance Fund (NEAK), including GP-level care, all the way to specialized medical interventions (Figure 1) [55]. Electronic search in the records of the MedBakter laboratory information system (LIS) for urine samples positive for E. coli and Klebsiella species was conducted by the authors (M.G., Á.M. and A.L.).
Figure 1.
Study site in Hungary (Southern Great Plain of Hungary: in blue; Albert Szent-Györgyi Clinical Center, Szeged: in green).
Samples with clinically significant colony counts for the abovementioned bacteria (105 < colony forming units [CFU]/mL; however, this was subject to interpretation, based on the information provided on the request forms for microbiological analysis and relevant international guidelines, e.g., presence of underlying conditions in the genitourinary tract) were included in the data analysis. Only the first isolate per patient was included in the study, however, isolates with different antibiotic-susceptibility patterns were considered as different individual isolates. In addition, patient data was also collected, that were limited to demographic characteristics (age and sex). The study was deemed exempt from ethics review by the Institutional Review Board, and informed consent was not required as data anonymity was maintained.
2.2. Identification of Isolates
10 µL of each un-centrifuged urine sample was cultured on UriSelect chromogenic agar plates (Bio-Rad, Berkeley, CA, USA) with a calibrated loop, according to the manufacturer’s instructions and incubated at 37 °C for 24–48 h, aerobically. If the relevant pathogens presented in significant colony count, the plates were passed on for further processing. Between 2008–2012, presumptive phenotypic (biochemical reaction-based) methods and VITEK 2 ID (bioMérieux, Marcy-l’Étoile, France) were used for bacterial identification, while after 2013, this was complemented by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS; Bruker Daltonik Gmbh. Gr., Bremen, Germany). The methodology of sample preparation for MALDI-TOF MS measurements was described elsewhere [56,57,58]. Mass spectrometry was performed by the Microflex MALDI Biotyper (Bruker Daltonics, Bremen, Germany) in positive linear mode across the m/z range of 2 to 20 kDa; for each spectrum, 240 laser shots at 60 Hz in groups of 40 shots per sampling area were collected. The MALDI Biotyper RTC 3.1 software (Bruker Daltonics) and the MALDI Biotyper Library 3.1 were used for spectrum analysis.
2.3. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing (AST) was performed using the Kirby–Bauer disk diffusion method and when appropriate, E-test (Liofilchem, Abruzzo, Italy) on Mueller–Hinton agar (MHA) plates. In addition, for the verification of discrepant results, VITEK 2 AST (bioMérieux, Marcy-l’Étoile, France) was also used. The interpretation of the results was based on EUCAST breakpoints. Staphylococcus aureus ATCC 29213, Enterococcus faecalis ATCC 29212, Proteus mirabilis ATCC 35659, Escherichia coli ATCC 25922, Klebsiella pneumoniae ATCC 700603 and Pseudomonas aeruginosa ATCC 27853 were used as quality control strains.
To evaluate the resistance trends of isolated strains, ciprofloxacin (CIP), ceftriaxone (CRO), meropenem (MER), gentamicin (GEN), sulfamethoxazole/trimetoprim (SXT) and nitrofurantion (NIT; relevant in case of E. coli) were chosen as indicator antibiotics, based on local antibiotic utilization data [59,60]. In addition, susceptibility data for fosfomycin (FOS) was also available for the second half (2013–2017) of the study period. FOS susceptibility testing was not routinely performed, only per request of the clinicians or in cases of extensive drug resistance. During data analysis, intermediately-susceptible results were grouped with and reported as resistant.
If extended-spectrum beta-lactamase (ESBL)-production was suspected, detection was carried out based on EUCAST recommendations; since 2011, using AmpC-ESBL Detection Set (MAST Diagnostica GmbH, Reinfeld, Germany) and VITEK 2 AST (bioMérieux, Marcy-l’Étoile, France), according to the manufacturer’s instructions. Carbapenemase-production was suspected in case of reduced susceptibility or resistance to MER, these isolates were sent to a reference laboratory for further processing.
2.4. Statistical Analysis
Descriptive statistical analysis (including means or medians with ranges and percentages to characterize data) was performed using Microsoft Excel 2013 (Redmond, WA, USA, Microsoft Corp.). Statistical analyses were performed with SPSS software version 24 (IBM SPSS Statistics for Windows 24.0, Armonk, NY, USA, IBM Corp.), using the χ2-test, Student’s t-test and Mann–Whitney U test. The normality of variables was tested using Shapiro–Wilk tests. p values < 0.05 were considered statistically significant.
3. Results
3.1. Demographic Characteristics, Sample Types
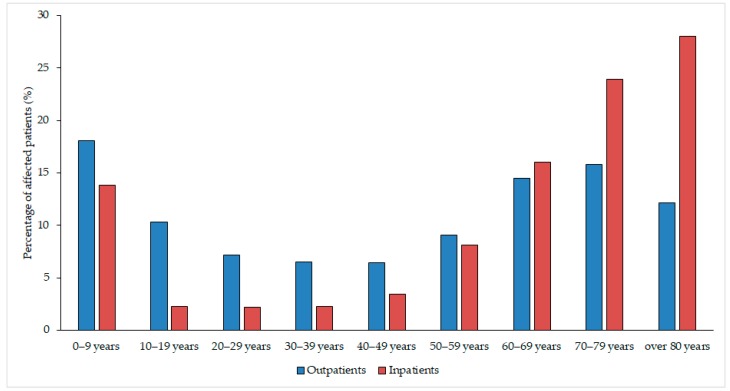
The median age of affected patients was 52 years (range: 0.3–97) in the outpatient group with a female-to-male ratio of 3.62 (78.34% female), while in the inpatient group, these values were 71 years (range: 0.4–96) and 2.42 (70.75% female), respectively. The detailed age distribution of patients in both affected patient groups is presented in Figure 2. The difference in the age distribution of the two patient groups was statistically significant (p < 0.0001). Among the affected patients, the age groups under 10 years of age (outpatients: 18.04%, inpatients: 13.80%) and over 60 years of age (outpatients: 42.44%, outpatients: 68.04%) were the most numerous. All (100%) samples received from outpatient clinics were voided (midstream) urine, while the sample distribution from the inpatient departments was the following: midstream urine (46.72%) catheter-specimen urine (45.56%), first-stream urine (7.41%) and samples obtained through suprapubic bladder aspiration (0.31%).
Figure 2.
Age distribution of the affected patients in the outpatient and inpatient groups.
3.2. Epidemiology of Escherichia coli and Klebsiella spp. Isolates
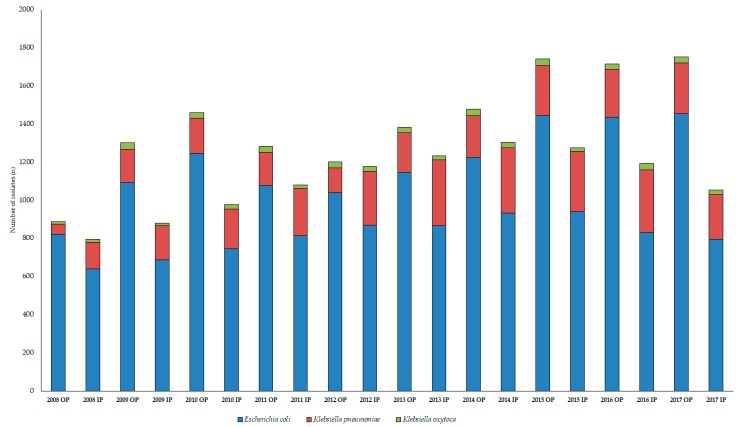
During the 10-year study period (1 January 2008–31 December 2017), the Institute of Clinical Microbiology received 21,150 urine samples from outpatient clinics and 19,325 samples from inpatient departments that turned out to be positive for a significant urinary pathogen. Out of the positive urine samples, E. coli represented the overwhelming majority of all positive urine samples; 56.75 ± 4.86% (range: 46.83–65.98%, lowest in 2008, highest in 2010) for outpatients, while 42.29 ± 2.94% (range: 37.19–45.73%, lowest in 2015, highest in 2010) for inpatients. K. pneumoniae was isolated in 8.96 ± 2.29% (range: 2.97–10.37%, lowest in 2012, highest in 2011) for outpatients, while 13.41 ± 2.20% (range: 9.54–15.25%, lowest in 2008, highest in 2011) for inpatient isolates; the isolation rate for K. oxytoca was 1.44 ± 0.34% (range: 0.74–1.77%, lowest in 2008, highest in 2011) for outpatients, and 1.16 ± 0.22% (range: 0.74–1.77%, lowest in 2008, highest in 2011) for inpatients. There was significant difference in the isolation frequency of E. coli (p = 0.0002) and K. pneumoniae (p = 0.0003) when comparing inpatient and outpatient isolates, while only a numerical tendency could be observed for K. oxytoca (p > 0.05). The epidemiology and total species distribution of outpatient and inpatient samples is presented in Figure 3.
Figure 3.
Frequency and species distribution of relevant isolates in inpatient and outpatient samples (2008–2017); IP: inpatient; OP: outpatient.
3.3. Antibiotic-Susceptibility Trends among Isolates
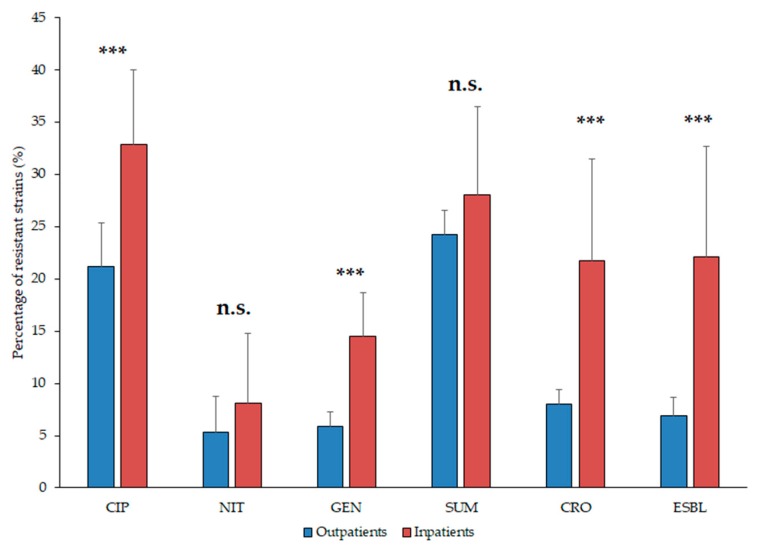
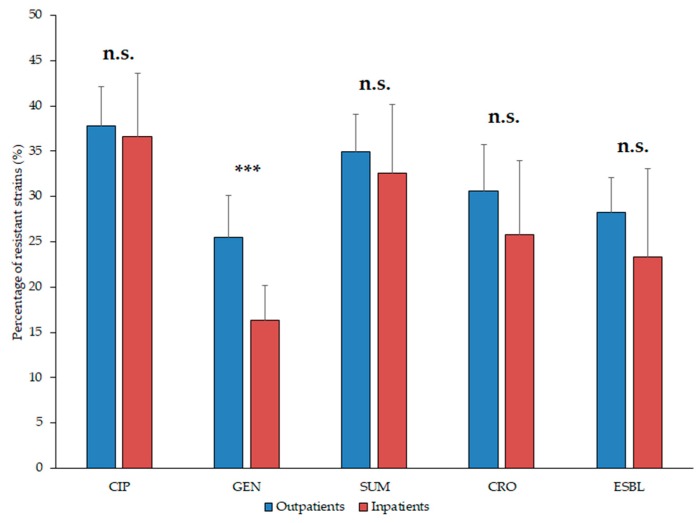
The resistance trends of the isolates E. coli and Klebsiella spp. against ciprofloxacin (CIP), ceftriaxone (CRO), gentamicin (GEN), sulfamethoxazole-trimethoprim (SXT), nitrofurantoin (NIT, relevant in case of E. coli) and the ratio of ESBL-producers (relevant since 2011) during the 10-year surveillance period are presented in Table 1 and Table 2, and Figure 4 (E. coli) and Figure 5. (Klebsiella spp.). Overall, the highest levels of resistance in E. coli and Klebsiella spp. were detected against CIP and SUM. The ratio of resistant E. coli strains in the inpatient group were significantly higher to CIP, GEN and CRO (p = 0.0003, p < 0.0001 and p = 0.0003, respectively), but not in case of NIT and FOS (p > 0.05; Figure 4). In addition, E. coli resistance levels to the indicator antibiotics were significantly higher (p < 0.05) in the second half (2013–2017) of the study period in case of every drug (apart from CRO resistance in the outpatient samples and NIT, where resistance levels were significantly higher in the first five-year-period; Table 1; Figure 4).
Table 1.
Percentage of resistant E. coli strains to indicator antibiotics from inpatient and outpatient departments (2008–2017).
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | Statistics | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIP R% | Outpatient | 13.28 | 16.12 | 21.53 | 21.80 | 19.13 | 21.64 | 21.31 | 25.57 | 25.69 | 25.95 | p = 0.0003 |
| Inpatient | 20.19 | 23.62 | 26.85 | 33.42 | 37.28 | 38.41 | 34.73 | 40.60 | 40.41 | 33.25 | ||
| NIT R% | Outpatient | 4.99 | 7.33 | 12.37 | 9.00 | 6.06 | 3.05 | 3.59 | 3.12 | 2.42 | 1.03 | n.s. (p = 0.264) |
| Inpatient | 7.98 | 12.39 | 20.81 | 17.57 | 7.36 | 3.34 | 5.04 | 2.76 | 2.05 | 1.39 | ||
| GEN R% | Outpatient | 3.17 | 5.77 | 6.35 | 7.51 | 5.58 | 4.54 | 6.37 | 7.76 | 5.16 | 6.88 | p < 0.0001 |
| Inpatient | 7.82 | 8.75 | 11.68 | 14.62 | 16.69 | 19.84 | 17.01 | 17.85 | 18.21 | 13.10 | ||
| SUM R% | Outpatient | 21.44 | 22.16 | 23.61 | 25.32 | 20.87 | 24.69 | 24.82 | 26.68 | 24.88 | 28.08 | n.s. (p = 0.189) |
| Inpatient | 25.82 | 22.45 | 21.34 | 25.55 | 11.62 | 35.29 | 34.83 | 36.24 | 39.08 | 28.21 | ||
| CRO R% | Outpatient | 8.99 | 7.74 | 7.55 | 7.33 | 5.58 | 6.46 | 8.57 | 9.56 | 8.85 | 9.70 | p = 0.0003 |
| Inpatient | 6.10 | 12.39 | 16.24 | 18.55 | 20.48 | 26.99 | 40.94 | 25.19 | 30.41 | 20.03 | ||
| ESBL% | Outpatient | 6.31 | 4.33 | 5.50 | 6.94 | 9.15 | 7.32 | 9.02 | p = 0.0028 | |||
| Inpatient | 8.85 | 10.59 | 25.72 | 38.97 | 23.06 | 29.08 | 18.39 | |||||
Values in italics represent the lowest resistance levels, boldface (peak) values correspond to the highest resistance levels in the study period; n.s.: not significant.
Table 2.
Percentage of resistant Klebsiella spp. strains to indicator antibiotics from inpatient and outpatient departments (2008–2017).
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | Statistics | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIP R% | Outpatient | 37.31 | 41.48 | 45.16 | 35.47 | 40.77 | 40.38 | 33.03 | 38.78 | 31.60 | 34.08 | n.s. (p = 0.641) |
| Inpatient | 23.40 | 30.00 | 35.10 | 37.50 | 34.28 | 35.76 | 48.40 | 45.57 | 38.18 | 37.45 | ||
| GEN R% | Outpatient | 27.69 | 34.09 | 25.81 | 24.53 | 27.69 | 27.40 | 21.10 | 28.14 | 18.80 | 19.10 | p = 0.0002 |
| Inpatient | 26.24 | 13.33 | 14.42 | 13.31 | 16.96 | 15.41 | 17.99 | 16.14 | 16.36 | 13.62 | ||
| SUM R% | Outpatient | 25.38 | 39.77 | 33.33 | 35.00 | 33.08 | 35.58 | 37.61 | 39.16 | 32.80 | 37.45 | n.s. (p = 0.399) |
| Inpatient | 27.66 | 21.67 | 34.62 | 30.24 | 23.43 | 29.07 | 43.15 | 43.99 | 36.06 | 35.74 | ||
| CRO R% | Outpatient | 23.85 | 31.25 | 37.63 | 26.74 | 36.15 | 35.10 | 27.52 | 35.36 | 27.20 | 25.09 | p = 0.132 |
| Inpatient | 23.40 | 21.11 | 17.31 | 20.56 | 23.67 | 19.77 | 41.98 | 28.48 | 38.18 | 25.79 | ||
| ESBL% | Outpatient | 26.74 | 30.00 | 31.73 | 25.69 | 34.22 | 26.00 | 23.22 | p = 0.243 | |||
| Inpatient | 10.89 | 13.43 | 18.60 | 33.53 | 28.48 | 36.06 | 23.36 | |||||
Values in italics represent the lowest resistance levels, boldface (peak) values correspond to the highest resistance levels in the study period; n.s.: not significant.
Figure 4.
Resistance levels of Escherichia coli isolates from inpatient and outpatient urinary tract infections, expressed in the percentage (%) of resistant isolates. Asterisk (***): p < 0.001; n.s.: not significant; CIP: ciprofloxacin; NIT: nitrofurantoin; GEN: gentamicin; SUM: sulfamethoxazole/trimethoprim; CRO: ceftriaxone; ESBL: extended-spectrum β-lactamase-producing isolates.
Figure 5.
Resistance levels of Klebsiella spp. isolates from inpatient and outpatient urinary tract infections, expressed in the percentage (%) of resistant isolates. Asterisk (***): p < 0.001; n.s.: not significant; CIP: ciprofloxacin; NIT: nitrofurantoin; GEN: gentamicin; SUM: sulfamethoxazole/trimethoprim; CRO: ceftriaxone; ESBL: extended-spectrum β-lactamase-producing isolates.
For the statistical analysis of resistance trends, K. pneumoniae and K. oxytoca isolates were grouped together as Klebsiella spp., as K. oxytoca represented only a minority (~1–1.5%) of isolates; in addition, after a preliminary analysis of the resistance trends among these two species, no differences (statistical or otherwise) could be observed. For Klebsiella spp., ratio of resistant strains in the inpatient group were significantly higher to GEN only (p = 0.0002; Figure 5). Resistance levels to the indicator antibiotics in Klebsiella spp. of inpatient origin were significantly higher (p < 0.05) in the second half (2013–2017) of the study period in case of every drug, while no similar trend was observed for the outpatient samples (Table 2). Regarding the outpatient isolates, the resistance levels of Klebsiella spp. were significantly higher, compared to E. coli to all antibiotics, except for SXT (CIP: p < 0.0001; GEN: p < 0.0001; CRO p = 0.0003), while during the analysis of inpatient samples, no similar trends were observed (p > 0.05).
ESBL-positivity (or simultaneous ESBL and AmpC-positivity) was detected in 6.94 ± 1.76% of outpatient and 22.09 ± 10.57% of inpatient E. coli isolates (p = 0.0028); in Klebsiella spp., these ratios were 28.23 ± 3.87% for outpatient and 23.36 ± 9.73% for inpatient isolates (p = 0.243). As of 2013, FOS susceptibility testing was performed for 15.63% of E. coli and 16.34% of Klebsiella spp. isolates in the inpatient group, while for outpatient isolates, 5.67% of E. coli and 17.77% of Klebsiella spp. were tested. Regarding inpatient samples, resistance to FOS was present in 14.66% of E. coli and 23.47% of Klebsiella spp., while in outpatient samples, this was 8.89% for E. coli (p = 0.0035) and 15.92% for Klebsiella spp. (p = 0.047), respectively. In outpatient samples, six individual isolates were detected which were intermediate/resistant to MER (one in 2009, 2011, 2014 and 2016, and two isolates in 2017; representing 2 E. coli and 4 K. pneumoniae isolates): one MER-intermediate isolate was resistant to all indicator antibiotics (but susceptible to colistin), while 5 out of 6 were susceptible to GEN and FOS, and 4 out of 6 were susceptible to SXT. In inpatient samples, eight such isolates were detected (one in 2011 and 2013, and two isolates in 2015, 2016 and 2017, respectively; representing 3 E. coli and 5 K. pneumoniae isolates). All relevant isolates were susceptible to GEN, 6 out of 8 were susceptible to FOS, while 4 out of 8 were susceptible to SXT. Most isolates intermediate/resistant to carbapenems were also resistant to CIP (in both patient groups).
4. Discussion
E. coli and Klebsiella species are the most common cause of urinary tract infections (UTIs) in both community and healthcare settings [1,3,4,6,9,14,47,48,49,50,51,52]. This was further proven in the context of our study, as E. coli was isolated in ~46–66%, and Klebsiella spp. was isolated in ~3–16% of cases. Their relatively lower prevalence in inpatient isolates is attributable to the more pronounced diversity in the causative agents of nosocomial UTIs, however, their clinical relevance should not be disregarded in either settings. In line with international literature, there was a predominance of females and patients over 50 years of age, among the affected population [1,3,4,6,9,14,47,48,49,50,51,52].
Regarding the local resistance levels, the results of the 10-year survey showed that there has been a pronounced increase in the resistance rates of several antibiotics by the second half of the study period (2013–2017); in contrast, resistance levels of NIT in E. coli decreased. There was no single underlying event found that may be responsible for this local advantageous change in NIT resistance levels, although due to the extended and favoured use of fluoroquinolones and the inability to access NIT—both at the Albert Szent-Györgyi Clinical Center and in the country in the recent years—may have had a notable role. Henceforth, the role of NIT may have a renaissance in the therapy of uncomplicated UTIs [61,62]. The comparison of our results with the surveillance data of the European Antimicrobial Resistance Surveillance Network (EARS-Net, [63]) for the relevant time periods is presented in Table 3.
Table 3.
Comparison of the resistance data obtained in this study with the surveillance data of EARS-Net.
| Local Resistance Data (2008–2012) | EARS-Net Surveillance Data for Hungary; 2012 [63] | Local Resistance Data (2013–2017) | EARS-Net Surveillance Data for Hungary; 2017 [63] | ||
|---|---|---|---|---|---|
| 3rd generation cephalosporins | E. coli | 5.58–26.99% | 17.40% | 6.46–40.94% | 20.10% |
| Klebsiella spp. | 17.31–37.63% | 43.00% | 19.77–35.36% | 41.10% | |
| Fluoroquinolones | E. coli | 13.28–21.80% | 28.90% | 21.31–25.95% | 30.60% |
| Klebsiella spp. | 23.40–45.16% | 41.60% | 31.60–48.40% | 41.45% | |
| Aminoglycosides | E. coli | 3.17–16.69% | 15.10% | 4.54–19.84% | 17.10% |
| Klebsiella spp. | 13.31–34.09% | 37.80% | 18.80–27.40% | 41.50% | |
| Carbapenems | E. coli | <0.05% | <0.05% | <0.05% | 0.10% |
| Klebsiella spp. | <0.05% | 0.30% | <0.05% | 0.30% | |
Values in italics represent resistance levels that were lower than the national average.
Some of the most concerning developments is the resistance in Klebsiella species to 3rd generation cephalosporins (exemplified by ceftriaxone in this survey), where even the lowest levels of resistance were around 15% in the outpatient group and close to 40% in the inpatient group. ESBL production has been observed in large percentage of urinary isolates in this study, especially in Klebsiella species. Some difference could be observed in the ratio of ESBL-positive and CRO-resistant strains (0.42–9.90% for E. coli and 0–10.25% for Klebsiella spp., respectively); this may be due to the fact that CRO resistance may also occur due to ESBL-independent mechanisms, such as AmpCs or other β-lactamases, overexpression of efflux pumps, changes in membrane permeability or porin mutations [64,65,66]. As ESBLs were the only one with public health/infection control significance in our hospital, the exact mechanism of resistance in this minor group of CRO-resistant isolates was not characterized further [34]. There was a significant difference in the rate of ESBL-positivity in E. coli among inpatient and outpatient isolates, however, no such different was observed for Klebsiella. ESBL-producing isolates have first been detected in nosocomial settings, nevertheless, after the 2000s, this demarcation line exist less and less [29,36]. In Hungary (and specifically in the southern region of the country), the blaCTX-M group is the most prevalent, which is associated with carrying resistance determinants to quinolones and aminoglycosides in addition to the relevant β-lactam antibiotics [67]. UTIs caused by these strains cannot be treated with the “conventional” β-lactam antibiotics (penicillin, cephalosporins), but carbapenems remain as a part of the antibiotic armamentarium [68,69]. However, the overuse of these drugs will inevitably lead to selection pressure (the prevalence of carbapenemase-producing Enterobacteriaceae (or CRE) is steadily increasing worldwide). Hungary is currently known to be a low-prevalence country for CRE (most of the isolates carry VIM- or OXA-48-like enzymes), however, most of the isolates originate from UTIs [70,71].
Similarly, high levels of resistance to fluoroquinolone antibiotics have been reported for Enterobacteriaceae in regions where no restrictions were introduced to their use in the community and they are still considered to be first-line agents. If the Hungarian antibiotic utilization trends are considered, the country is doing well quantitatively (i.e., the amount of antibiotics consumed), however, a qualitative analysis (i.e., targeted therapy) reveals a much bleaker picture: the use of broad-spectrum antimicrobials (including fluoroquinolones) is significantly higher than in Scandinavian countries, which may also correspond to the development of local resistance trends (based on the data of a Hungarian study group and European Surveillance of Antimicrobial Consumption Network; ESAC-Net) [59,60,72,73]. Some novel antibiotics, that recently received marketing authorization (for example, delafloxacin, ceftolozane-tazobactam, ceftazidime-avibactam) may aid the therapy of resistant Gram-negative infections; nevertheless, due to their prohibitive price and the limited clinical experience associated with these drugs, it is unknown when will they be considered in routine therapeutic protocols [42,74,75]. The increase in resistance is not only forcing changes in treatment guidelines and issues in the clinic, but also to poor prognoses, decreased QoL and an increase in the mortality rate of patients, especially in nosocomial settings [5]. E. coli and K. pneumoniae are major pathogens of nosocomial infections, including UTIs, that is frequently associated with resistance to the critically important antimicrobials. The WHO has recently published a priority list of resistant pathogens, where Escherichia coli and Klebsiella species resistant to third generation cephalosporins and carbapenems represent top-priority [76].
Some limitations of this study must be acknowledged: firstly, the design of the study is retrospective and there has been an inability to access the medical records of the individual patients affected by these infections. For this reason, the correlation between the existence of relevant risk factors and underlying illnesses (apart from age and inpatient/outpatient status) and the E. coli/Klebsiella spp. UTIs could not be assessed. The age-associated incidence in isolation of these pathogens may also reflect (at least in part) the high rate of bacteriuria in the elderly population (especially in catheterized patients) [77]. There is a risk of selection bias, as studies describing the prevalence of infectious diseases and resistance trends are mainly tertiary-care centers, which generally correspond to patients with more severe conditions or underlying illnesses. Finally, the molecular characterization of the resistance determinants in the individual isolates was not performed, only to the extent of presence/absence of ESBLs [78,79].
5. Conclusions
This study presents the epidemiological trends and resistance levels of E. coli and Klebsiella species, the main pathogens associated with urinary tract infections (UTIs) in Hungary, over a long surveillance period (10 years), mainly demonstrating an increasing tendency regarding the resistance levels to various antibiotics. To the best of our knowledge, this is the longest-spanning study reporting on the prevalence and susceptibility patterns of these pathogens (and UTIs caused by these uropathogens by proxy) in Hungary. Their significantly higher prevalence in female patients with advanced age and instrumentation/catheterization and is in line with the findings in the literature. The type of setting (inpatient/outpatient) also had an effect on their isolation frequency, as there was a predominance of E. coli in uncomplicated UTIs, while in the inpatient setting, more relevant pathogens may be implicated.
In addition to patient history, drug allergies and national/institutional drug availability, the choice of empiric antibiotic therapy should be selected based on local susceptibility profiles or a cumulative hospital antibiogram, based on the guidelines of the Infectious Diseases Society of America (IDSA); nonetheless, the choice of antimicrobial drugs should be revised after the specific antibiogram for the relevant urinary pathogen has become available. The data in this study may aid the creation of a local/hospital antibiogram or a national surveillance system for urinary tract pathogens in Hungary.
Acknowledgments
M.G. was supported by the National Youth Excellence Scholarship [Grant Number NTP-NTFÖ-18-C-0225] and the ESCMID Mentorship and Observership Programme.
Abbreviations
AST: antimicrobial susceptibility testing; CFU: colony-forming unit; CRE: carbapenem-resistant Enterobacteriaceae; CIP: ciprofloxacin; CRO: ceftriaxone; EARS-Net: European Antimicrobial Resistance Surveillance Network; ESBL: extended-spectrum β-lactamase; ESAC-Net: European Surveillance of Antimicrobial Consumption Network; EUCAST: European Committee for Antimicrobial Susceptibility Testing; FOS: fosfomycin; GEN: gentamicin; GP: general practitioner; ID: identification; IDSA: Infectious Disease Society of America; IP: inpatient; LIS: laboratory information system; LPS: lipopolysaccharide; MALDI-TOF MS: matrix-assisted laser desorption/ionization time-of-flight mass spectrometry; MDR: multidrug-resistance; MER: meropenem; NEAK: Hungarian National Health Insurance Fund; NIT: nitrofurantoin; OP: outpatient; OPAT: outpatient parenteral antibiotic therapy; PAI: pathogenicity island; QoL: quality-of-life; SXT: sulfamethoxazole-trimethoprim; UPEC: uropathogenic Escherichia coli; UTI: urinary tract infection; WHO: World Health Organization.
Author Contributions
M.G. and M.Á. conceived and designed the study. M.Á. and A.L. were the senior microbiologists and performed the identification and of the bacterial isolates and interpreted susceptibility-testing results during the study period. M.G. and A.L. performed data collection and analysis, wrote and revised the full paper. K.B. wrote and revised the full paper.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest, monetary or otherwise.
References
- 1.Flores-Mireles A.L., Walker J.N., Caparon M., Hultgren S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sobel J.D., Kaye D. 74—Urinary Tract Infections. In: Bennett J.E., Dolin R., Blaser M.J., editors. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th ed. Elsevier; Philadelphia, PA, USA: 2015. pp. 886–913.e3. [Google Scholar]
- 3.Gupta K., Hooton T.M., Naber K.G., Wullt B., Colgan R., Miller L.G., Moran G.J., Nicolle L.E., Raz R., Schaeffer A.J., et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011;52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 4.Wiedemann B., Heisig A., Heisig P. Uncomplicated urinary tract infections and antibiotic resistance-epidemiological and mechanistic aspects. Antibiotics. 2014;3:341–352. doi: 10.3390/antibiotics3030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hooton T.M., Bradley S.F., Cardenas D.D., Colgan R., Geerlings S.E., Rice J.C., Saint S., Schaeffer A.J., Tambayh P.A., Tenke P., et al. Diagnosis, Prevention, and Treatment of Catheter-Associated Urinary Tract Infection in Adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clin. Infect. Dis. 2010;50:625–663. doi: 10.1086/650482. [DOI] [PubMed] [Google Scholar]
- 6.Stefaniuk E., Suchocka U., Bosacka K., Hryniewicz W. Etiology and antibiotic susceptibility of bacterial pathogens responsible for community-acquired urinary tract infections in Poland. Eur. J. Clin. Microbiol. Infect. Dis. 2016;35:1–7. doi: 10.1007/s10096-016-2673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbo L.M., Hooton T.M. Antimicrobial Stewardship and Urinary Tract Infections. Antibiotics (Basel) 2014;3:174–192. doi: 10.3390/antibiotics3020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adeolu M., Alnajar S., Naushad S., S Gupta R. Genome-based phylogeny and taxonomy of the “Enterobacteriales”: proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016;66:5575–5599. doi: 10.1099/ijsem.0.001485. [DOI] [PubMed] [Google Scholar]
- 9.Calzi A., Grignolo S., Caviglia I., Calevo M.G., Losurdo G., Piaggio G., Bandettini R., Castagnola E. Resistance to oral antibiotics in 4569 Gram-negative rods isolated from urinary tract infection in children. Eur. J. Pediatr. 2016;175:1219–1225. doi: 10.1007/s00431-016-2763-1. [DOI] [PubMed] [Google Scholar]
- 10.Behzadi P., Behzadi E., Ranjbar R. Urinary tract infections and Candida albicans. Cent. Eur. J. Urol. 2015;68:96–101. doi: 10.5173/ceju.2015.01.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizwan M., Akhtar M., Najmi A.K., Singh K. Escherichia coli and Klebsiella pneumoniae Sensitivity/Resistance Pattern Towards Antimicrobial Agents in Primary and Simple Urinary Tract Infection Patients Visiting University Hospital of Jamia Hamdard New Delhi. Drug Res. (Stuttg.) 2018;68:415–420. doi: 10.1055/a-0576-0079. [DOI] [PubMed] [Google Scholar]
- 12.Yüksel S., Oztürk B., Kavaz A., Ozçakar Z.B., Acar B., Güriz H., Aysev D., Ekim M., Yalçinkaya F. Antibiotic resistance of urinary tract pathogens and evaluation of empirical treatment in Turkish children with urinary tract infections. Int. J. Antimicrob. Agents. 2006;28:413–416. doi: 10.1016/j.ijantimicag.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Alexander B.T., Marschall J., Tibbetts R.J., Neuner E.A., Dunne W.M., Ritchie D.J. Treatment and clinical outcomes of urinary tract infections caused by KPC-producing Enterobacteriaceae in a retrospective cohort. Clin. Ther. 2012;34:1314–1323. doi: 10.1016/j.clinthera.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cullen I.M., Manecksha R.P., McCullagh E., Ahmad S., O’Kelly F., Flynn R., McDermott T.E.D., Murphy P., Grainger R., Fennell J.P., et al. An 11-year analysis of the prevalent uropathogens and the changing pattern of Escherichia coli antibiotic resistance in 38,530 community urinary tract infections, Dublin 1999–2009. Ir. J. Med. Sci. 2013;182:81–89. doi: 10.1007/s11845-012-0834-5. [DOI] [PubMed] [Google Scholar]
- 15.Behzadi P., Behzadi E., Yazdanbod H., Aghapour R., Akbari Cheshmeh M., Salehian Omran D. A survey on urinary tract infections associated with the three most common uropathogenic bacteria. Maedica (Buchar) 2010;5:111–115. [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Y., Ma Y., Zhao Q., Wang L., Guo L., Ye L., Zhang Y., Yang J. Similarity and divergence of phylogenies, antimicrobial susceptibilities, and virulence factor profiles of Escherichia coli isolates causing recurrent urinary tract infections that persist or result from reinfection. J. Clin. Microbiol. 2012;50:4002–4007. doi: 10.1128/JCM.02086-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terlizzi M.E., Gribaudo G., Maffei M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017;8:1566. doi: 10.3389/fmicb.2017.01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marques C., Menezes J., Belas A., Aboim C., Cavaco-Silva P., Trigueiro G., Telo Gama L., Pomba C. Klebsiella pneumoniae causing urinary tract infections in companion animals and humans: population structure, antimicrobial resistance and virulence genes. J. Antimicrob. Chemother. 2019;74:594–602. doi: 10.1093/jac/dky499. [DOI] [PubMed] [Google Scholar]
- 19.Ejrnæs K. Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli. Dan. Med. Bull. 2011;58:B4187. [PubMed] [Google Scholar]
- 20.Takhar S.S., Moran G.J. Diagnosis and management of urinary tract infection in the emergency department and outpatient settings. Infect. Dis. Clin. North Am. 2014;28:33–48. doi: 10.1016/j.idc.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Wiles T.J., Kulesus R.R., Mulvey M.A. Origins and Virulence Mechanisms of Uropathogenic Escherichia coli. Exp. Mol. Pathol. 2008;85:11–19. doi: 10.1016/j.yexmp.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vading M., Nauclér P., Kalin M., Giske C.G. Invasive infection caused by Klebsiella pneumoniae is a disease affecting patients with high comorbidity and associated with high long-term mortality. PLoS ONE. 2018;13:e0195258. doi: 10.1371/journal.pone.0195258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W., Sun G., Yu Y., Li N., Chen M., Jin R., Jiao Y., Wu H. Increasing occurrence of antimicrobial-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin. Infect. Dis. 2014;58:225–232. doi: 10.1093/cid/cit675. [DOI] [PubMed] [Google Scholar]
- 24.Bassetti M., Righi E., Carnelutti A., Graziano E., Russo A. Multidrug-resistant klebsiella pneumoniae: Challenges for treatment, prevention and infection control. Expert Rev. Anti Infect. Ther. 2018;16:749–761. doi: 10.1080/14787210.2018.1522249. [DOI] [PubMed] [Google Scholar]
- 25.Bader M.S., Loeb M., Brooks A.A. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad. Med. 2017;129:242–258. doi: 10.1080/00325481.2017.1246055. [DOI] [PubMed] [Google Scholar]
- 26.Hooton T.M. The current management strategies for community-acquired urinary tract infection. Infect. Dis. Clin. North Am. 2003;17:303–332. doi: 10.1016/S0891-5520(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 27.Kong K.-F., Schneper L., Mathee K. Beta-lactam Antibiotics: From Antibiosis to Resistance and Bacteriology. APMIS. 2010;118:1–36. doi: 10.1111/j.1600-0463.2009.02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhillon R.H.-P., Clark J. ESBLs: A Clear and Present Danger? Crit. Care Res. Pract. 2012;2012:625170. doi: 10.1155/2012/625170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rupp M.E., Fey P.D. Extended spectrum beta-lactamase (ESBL)-producing Enterobacteriaceae: considerations for diagnosis, prevention and drug treatment. Drugs. 2003;63:353–365. doi: 10.2165/00003495-200363040-00002. [DOI] [PubMed] [Google Scholar]
- 30.Paterson D.L., Bonomo R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacoby G.A. AmpC beta-lactamases. Clin. Microbiol. Rev. 2009;22:161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanson N.D. AmpC β-lactamases: what do we need to know for the future? J. Antimicrob. Chemother. 2003;52:2–4. doi: 10.1093/jac/dkg284. [DOI] [PubMed] [Google Scholar]
- 33.Bush K. Past and Present Perspectives on β-Lactamases. Antimicrob. Agents Chemother. 2018;62:e01076-18. doi: 10.1128/AAC.01076-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leclercq R., Cantón R., Brown D.F.J., Giske C.G., Heisig P., MacGowan A.P., Mouton J.W., Nordmann P., Rodloff A.C., Rossolini G.M., et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2013;19:141–160. doi: 10.1111/j.1469-0691.2011.03703.x. [DOI] [PubMed] [Google Scholar]
- 35.Bilchenko A.V., Chub O.I. Prevalence of Types TEM, SHV and CTX-M βLES Among Pathogens of Chronic Pyelonephritis. Antibiot. Khimioter. 2014;59:24–26. [PubMed] [Google Scholar]
- 36.Cantón R., González-Alba J.M., Galán J.C. CTX-M Enzymes: Origin and Diffusion. Front. Microbiol. 2012;3:110. doi: 10.3389/fmicb.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonkat G., Müller G., Rieken M., Frei R., Widmer A.F., Feicke A., Wyler S., Rentsch C.A., Ebinger-Mundorff N., Subotic S., et al. Epidemiology of urinary tract infections caused by extended-spectrum beta-lactamase (ESBL) producing pathogens at a tertiary care swiss University Hospital. J. Urol. 2011;185:e545. doi: 10.1016/j.juro.2011.02.1190. [DOI] [Google Scholar]
- 38.Walker K.J., Lee Y.R., Klar A.R. Clinical Outcomes of Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae Infections with Susceptibilities among Levofloxacin, Cefepime, and Carbapenems. Can. J. Infect. Dis. Med. Microbiol. 2018;2018:3747521. doi: 10.1155/2018/3747521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker E., Lyman A., Gupta K., Mahoney M.V., Snyder G.M., Hirsch E.B. Clinical Management of an Increasing Threat: Outpatient Urinary Tract Infections Due to Multidrug-Resistant Uropathogens. Clin. Infect. Dis. 2016;63:960–965. doi: 10.1093/cid/ciw396. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh C.-J., Shen Y.-H., Hwang K.-P. Clinical implications, risk factors and mortality following community-onset bacteremia caused by extended-spectrum β-lactamase (ESBL) and non-ESBL producing Escherichia coli. J. Microbiol. Immunol. Infect. 2010;43:240–248. doi: 10.1016/S1684-1182(10)60038-2. [DOI] [PubMed] [Google Scholar]
- 41.Pallett A., Hand K. Complicated urinary tract infections: Practical solutions for the treatment of multiresistant gram-negative bacteria. J. Antimicrob. Chemother. 2010;65:iii25–iii33. doi: 10.1093/jac/dkq298. [DOI] [PubMed] [Google Scholar]
- 42.Sherry N., Howden B. Emerging Gram negative resistance to last-line antimicrobial agents fosfomycin, colistin and ceftazidime-avibactam—Epidemiology, laboratory detection and treatment implications. Expert Rev. Anti Infect. Ther. 2018;16:289–306. doi: 10.1080/14787210.2018.1453807. [DOI] [PubMed] [Google Scholar]
- 43.Beuk C., Hill C., Whitehead S., Blondel-Hill E., Wagner K., Cheeptham N. Determination of susceptibility to fosfomycin and tigecycline of Enterobacteriaceae, particularly Escherichia coli isolates, producing extended-spectrum β-lactamases from multiple regional Canadian hospitals. Can. J. Infect. Dis. Med. Microbiol. 2013;24:e80–e82. doi: 10.1155/2013/645018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gajdács M. Extra deaths due to pandrug resistant bacteria: A survey of the literature. Egészségfejlesztés. 2019;60:31–36. [Google Scholar]
- 45.Chapman A.L.N. Outpatient parenteral antimicrobial therapy. BMJ. 2013;346:f1585. doi: 10.1136/bmj.f1585. [DOI] [PubMed] [Google Scholar]
- 46.Denes E., Prouzergue J., Ducroix-Roubertou S., Aupetit C., Weinbreck P. Antibiotic prescription by general practitioners for urinary tract infections in outpatients. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:3079–3083. doi: 10.1007/s10096-012-1668-9. [DOI] [PubMed] [Google Scholar]
- 47.Bryce A., Hay A.D., Lane I.F., Thornton H.V., Wootton M., Costelloe C. Global prevalence of antibiotic resistance in paediatric urinary tract infections caused by Escherichia coli and association with routine use of antibiotics in primary care: systematic review and meta-analysis. BMJ. 2016;352:i939. doi: 10.1136/bmj.i939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fasugba O., Mitchell B.G., Mnatzaganian G., Das A., Collignon P., Gardner A. Five-Year Antimicrobial Resistance Patterns of Urinary Escherichia coli at an Australian Tertiary Hospital: Time Series Analyses of Prevalence Data. PLoS ONE. 2016;11:e0164306. doi: 10.1371/journal.pone.0164306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magyar A., Köves B., Nagy K., Dobák A., Arthanareeswaran V.K.A., Bálint P., Wagenlehner F., Tenke P. Spectrum and antibiotic resistance of uropathogens between 2004 and 2015 in a tertiary care hospital in Hungary. J. Med. Microbiol. 2017;66:788–797. doi: 10.1099/jmm.0.000498. [DOI] [PubMed] [Google Scholar]
- 50.Yang B., Yang F., Wang S., Wang Q., Liu Z., Feng W., Sun F., Xia P. Analysis of the spectrum and antibiotic resistance of uropathogens in outpatients a tertiary hospital. J. Chemother. 2018;30:145–149. doi: 10.1080/1120009X.2017.1418646. [DOI] [PubMed] [Google Scholar]
- 51.Morrissey I., Hackel M., Badal R., Bouchillon S., Hawser S., Biedenbach D. A Review of Ten Years of the Study for Monitoring Antimicrobial Resistance Trends (SMART) from 2002 to 2011. Pharmaceuticals (Basel) 2013;6:1335–1346. doi: 10.3390/ph6111335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sader H.S., Farrell D.J., Flamm R.K., Jones R.N. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalised with pneumonia in US and European hospitals: Results from the SENTRY Antimicrobial Surveillance Program, 2009–2012. Int. J. Antimicrob. Agents. 2014;43:328–334. doi: 10.1016/j.ijantimicag.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Gajdács M., Paulik E., Szabó A. The opinions of community pharmacists related to antibiotic use and resistance. Acta Pharmaceutica Hungarica. 2018;88:249–252. (In Hungarian) [Google Scholar]
- 54.Gajdács M., Paulik E., Szabó A. The attitude of community pharmacists towards their widening roles in the prevention and treatment of infectious diseases in the southeast region of Hungary. Gyógyszerészet. 2019;63:26–30. (In Hungarian) [Google Scholar]
- 55.National Health Insurance Fund of Hungary . Hospital Bed Count and Patient Turnover Report 2017. National Health Insurance Fund of Hungary; Budapest, Hungary: 2017. [Google Scholar]
- 56.Gajdács M., Spengler G., Urbán E. Identification and Antimicrobial Susceptibility Testing of Anaerobic Bacteria: Rubik’s Cube of Clinical Microbiology? Antibiotics (Basel) 2017;6:25. doi: 10.3390/antibiotics6040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ábrók M., Lázár A., Szécsényi M., Deák J., Urbán E. Combination of MALDI-TOF MS and PBP2’ latex agglutination assay for rapid MRSA detection. J. Microbiol. Methods. 2018;144:122–124. doi: 10.1016/j.mimet.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 58.Nagy E., Becker S., Kostrzewa M., Barta N., Urban E. The value of MALDI-TOF MS for the identification of clinically relevant anaerobic bacteria in routine laboratories. J. Med. Microbiol. 2012;61:1393–1400. doi: 10.1099/jmm.0.043927-0. [DOI] [PubMed] [Google Scholar]
- 59.Benkő R., Matuz M., Hajdú E., Bor A., Doró P., Viola R., Soós G. Antibiotic use in the Hungarian hospitals in the last two decades (1996–2015) Orv. Hetil. 2016;157:1839–1846. doi: 10.1556/650.2016.30523. [DOI] [PubMed] [Google Scholar]
- 60.Matuz M., Benkő R., Hajdú E., Viola R., Soós G. Evaluation of ambulatory antibiotic use in Hungary using drug-specific quality indicators. Orv. Hetil. 2013;154:947–956. doi: 10.1556/OH.2013.29632. [DOI] [PubMed] [Google Scholar]
- 61.Garau J. Other antimicrobials of interest in the era of extended-spectrum beta-lactamases: fosfomycin, nitrofurantoin and tigecycline. Clin. Microbiol. Infect. 2008;14(Suppl. 1):198–202. doi: 10.1111/j.1469-0691.2007.01852.x. [DOI] [PubMed] [Google Scholar]
- 62.Al-Zarouni M., Senok A., Al-Zarooni N., Al-Nassay F., Panigrahi D. Extended-spectrum β-lactamase-producing Enterobacteriaceae: In vitro susceptibility to fosfomycin, nitrofurantoin and tigecycline. Med. Princ. Pract. 2012;21:543–547. doi: 10.1159/000339200. [DOI] [PubMed] [Google Scholar]
- 63.European Antimicrobial Resistance Surveillance Network (EARS-Net) [(accessed on 28 May 2019)]; Available online: https://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/ears-net.
- 64.Hsueh P.-R., Chen W.-H., Luh K.-T. Relationships between antimicrobial use and antimicrobial resistance in Gram-negative bacteria causing nosocomial infections from 1991–2003 at a university hospital in Taiwan. Int. J. Antimicrob. Agents. 2005;26:463–472. doi: 10.1016/j.ijantimicag.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spengler G., Kincses A., Gajdacs M., Amaral L. New Roads Leading to Old Destinations: Efflux Pumps as Targets to Reverse Multidrug Resistance in Bacteria. Molecules. 2017;22:468. doi: 10.3390/molecules22030468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rice L.B. Mechanisms of Resistance and Clinical Relevance of Resistance to β-Lactams, Glycopeptides, and Fluoroquinolones. Mayo Clin. Proc. 2012;87:198–208. doi: 10.1016/j.mayocp.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Melegh S., Schneider G., Horváth M., Jakab F., Emődy L., Tigyi Z. Identification and characterization of CTX-M-15 producing Klebsiella pneumoniae clone ST101 in a Hungarian university teaching hospital. Acta Microbiol. Immunol. Hung. 2015;62:233–245. doi: 10.1556/030.62.2015.3.2. [DOI] [PubMed] [Google Scholar]
- 68.Tandogdu Z., Wagenlehner F.M.E. Global epidemiology of urinary tract infections. Curr. Opin. Infect. Dis. 2016;29:73–79. doi: 10.1097/QCO.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 69.Van Duin D., Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence. 2016;8:460–469. doi: 10.1080/21505594.2016.1222343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cassini A., Högberg L.D., Plachouras D., Quattrocchi A., Hoxha A., Simonsen G.S., Colomb-Cotinat M., Kretzschmar M.E., Devleesschauwer B., Cecchini M., et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Codjoe F.S., Donkor E.S. Carbapenem Resistance: A Review. Med. Sci. (Basel) 2017;6:1. doi: 10.3390/medsci6010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Matuz M., Benko R., Doro P., Hajdu E., Nagy G., Nagy E., Monnet D.L., Soos G. Regional variations in community consumption of antibiotics in Hungary, 1996–2003. Br. J. Clin. Pharmacol. 2006;61:96–100. doi: 10.1111/j.1365-2125.2005.02525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.European Surveillance of Antimicrobial Consumption Network (ESAC-Net) [(accessed on 28 May 2019)]; Available online: http://ecdc.europa.eu/en/about-us/partnerships-and-networks/disease-and-laboratory-networks/esac-net.
- 74.Gajdács M. The Concept of an Ideal Antibiotic: Implications for Drug Design. Molecules. 2019;24:892. doi: 10.3390/molecules24050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Candel F.J., Peñuelas M. Delafloxacin: Design, development and potential place in therapy. Drug Des. Devel. Ther. 2017;11:881–891. doi: 10.2147/DDDT.S106071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.World Health Organisation . Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. WHO; Geneva, Switzerland: 2017. pp. 1–7. [Google Scholar]
- 77.Gajdács M., Dóczi I., Ábrók M., Lázár A., Burián K. Epidemiology of candiduria and Candida urinary tract infections in inpatients and outpatients: Results from a 10-year retrospective survey. Cent. Eur. J. Urol. 2019;72:209–214. doi: 10.5173/ceju.2019.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gajdács M., Ábrók M., Lázár A., Burián K. Susceptibility patterns of extended-spectrum beta-lactamase-producing (ESBL) urinary pathogens: single-center experience. Gyógyszerészet. 2019 accepted. (In Hungarian) [Google Scholar]
- 79.Gajdács M., Urbán E. Resistance Trends and Epidemiology of Citrobacter-Enterobacter-Serratia in Urinary Tract Infections of Inpatients and Outpatients (RECESUTI): A 10-Year Survey. Medicina (Kaunas) 2019;55:285. doi: 10.3390/medicina55060285. [DOI] [PMC free article] [PubMed] [Google Scholar]