Abstract
Background and objective:
Endoscopic valve therapy is a treatment modality in patients with advanced emphysema and absent interlobar collateral ventilation (CV). So far, long-term outcome following valve implantation has been insufficiently evaluated. The aim of this study was to investigate the real-world efficacy of this interventional therapy over 3 years.
Methods:
From 2006 to 2013, 256 patients with severe emphysema in whom absent CV was confirmed underwent valve therapy. The 3-year effectiveness was evaluated by pulmonary function testing (VC, FEV1, RV, TLC), 6-minute-walk test (6-MWT) and dyspnea questionnaire (mMRC). Long-term outcome was also assessed according to the radiological outcome following valve placement.
Results:
Of 256 patients treated with valves, 220, 200, 187, 100 and 66 patients completed the 3-month, 6-month, 1-year, 2-year and 3-year follow-up (FU) visit, respectively. All lung function parameters, 6-MWT and mMRC were significantly improved at 3- and 6-month FU. At 1-year FU, patients still experienced a significant improvement of all outcome parameters expect VC (L) and TLC (%). At 2 years, RV (L and %) and TLC (L and %) remained significantly improved compared to baseline. Three years after valve therapy, sustained significant improvement in mMRC was observed and the proportion of patients achieving a minimal clinically important difference from baseline in RV and 6-MWT was still 71% and 46%, respectively. Overall, patients with complete lobar atelectasis exhibited superior treatment outcome with 3-year responder rates to FEV1, RV and 6-MWT of 10%, 79% and 53%, respectively.
Conclusions:
Patients treated by valves experienced clinical improvement over 1 year following valve therapy. Afterwards, clinical benefit gradually declines more likely due to COPD progression.
Keywords: bronchoscopy, COPD, emphysema, endoscopic lung volume reduction, interventional pneumology
Introduction
Chronic obstructive pulmonary disease (COPD) and emphysema are significant contributors to disability, morbidity and mortality around the world.1 Therapeutic options are limited and focus on symptom relief and disease control. Despite optimal pharmacologic therapy and rehabilitation, the majority of patients with significantly reduced lung function still have symptoms that impair quality of life. For these patients, lung volume reduction that reduces hyperinflation and optimizes respiratory muscle function may present an additional therapy. Besides lung volume reduction surgery (LVRS), various endoscopic lung volume reduction (ELVR) techniques have emerged as effective treatment approaches in selected patient cohorts, thus extending the therapeutic spectrum for patients with advanced emphysema. Endoscopic valve therapy, as the best studied ELVR method, has been shown to improve lung function parameters, exercise capacity and quality of life in patients with emphysema and absent interlobar collateral ventilation (CV) in various randomized controlled trials (RCTs),2–6 and is mentioned as additive therapy in the GOLD recommendations.1 In these RCTs, however, the benefit of valve therapy is confirmed up to 12 months after treatment. So far, only small cohort studies investigated a longer follow-up time after valve placement that demonstrated encouraging results and also proposed valve therapy as survival-enhancing therapy.7,8 Therefore, we evaluated the real-world efficacy of valve therapy up to 3 years post-treatment in patients with advanced emphysema and absent CV.
Materials and methods
This retrospective analysis evaluated the clinical long-term follow up of patients with severe emphysema and absent CV after endoscopic valve therapy. All patients gave general consent for the scientific use of the data acquired during hospitalization. The protocol of this study was approved by the local ethics committee, medical faculty, University Heidelberg (S-609/2012).
Patients and valve therapy
The database comprises 447 patients with severe emphysema who underwent endoscopic valve therapy in the Thoraxklinik at University of Heidelberg from 2006 to 2013. All patients had severe COPD with a significantly reduced forced expiratory volume in 1 s (FEV1) and severe hyperinflation. A multi-detector computed tomography (MDCT) confirmed the presence of advanced emphysema and a CT-based software analysis (YACTA, Yet Another CT Analyzer9) was used to identify the most emphysematous lobe as the target lobe for valve placement. As this database was started in 2006, when the knowledge of an absent CV as a predictor for successful therapy was not yet known, the database also includes patients with evidence of interlobar CV. In the current analysis, the clinical data of 256 patients in whom absent CV was confirmed by MDCT fissure analysis and/or catheter-based Chartis® measurement were extracted from this database and analyzed.10,11 Thereby, CT fissure analysis was performed visually by different radiologists. In cases of significant parenchymal bridges between the target lobe and the adjacent lobe, Chartis® measurement was added. As some patients were treated within prospective clinical trials, CV assessment was performed according to the study protocol.
The endoscopic valve placement has been described previously.12,13 In brief, all patients received a complete occlusion of the most emphysematous destroyed lobe by endobronchial (EBV; Zephyr®, Pulmonx, Inc., Palo Alto, CA, USA) or intrabronchial valves (IBV, Spiration®, Olympus, Tokyo, Japan) under general anesthesia or less often under deep sedation. Prior to valve placement, CV was excluded by CV assessment using the Chartis® Pulmonary Assessment system in a subgroup of patients.10 After measurement of the airway diameter of the targeted lobe, the appropriate valves were delivered to the airways using a dedicated delivery catheter.
Clinical and radiological data
Lung function parameters [FEV1, vital capacity (VC), residual volume (RV), total lung capacity (TLC)] and exercise capacity [6-minute walk test (6-MWT)] taken at baseline prior to the valve placement and 3 months, 6 months, 12 months, 24 months and 36 months following valve implantation were extracted from the database. All lung function tests and exercise tests were performed in the Thoraxklinik at University of Heidelberg according to the European Respiratory Society/American Thoracic Society guidelines.14–16 Dyspnea severity was assessed by using the modified Medical Research Council (mMRC) scale.17 Moreover, all chest X-rays and MDCT scans taken following the valve therapy were reviewed for each patient and long-term outcome was assessed according to the radiological outcome following valve placement.
Statistical analysis
Baseline characteristics are described as mean values ± standard deviations. To compare the clinical data at 3-, 6-, 12-, 24- and 36-month follow up against baseline measurements, paired t test was used for continuous and ordinal data; p-values < 0.05 were considered statistically significant. An adjustment for multiple comparisons was not performed. In addition, to handle missing data and make use of all available data, the mixed model for repeated measurements (MMRM) was also applied, assuming missing at random. Furthermore, response rates were calculated by counting the number of patients who met the minimal important difference (MID) of >100 ml improvement in FEV1, >430 ml reduction in RV and >26 m improvement in 6-MWT.18–20 All statistical analyses were performed using the open-source R software version 3.4.2.
Results
Patient characteristics
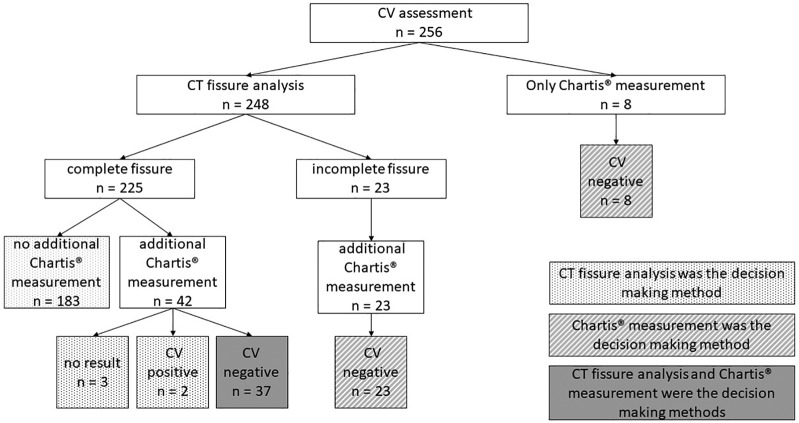
The baseline characteristics of the 256 emphysema patients (male 49.2%, mean age 64.5 ± 7 years) are presented in Table 1. Visual CT fissure analysis, Chartis® measurement or both were performed in 183, 8 and 65 patients respectively in order to assess interlobar CV (Figure 1). In 46.9% (120/256) of the patients, the left lower lobe was occluded by valves, in 22.7% (58/256) the left upper lobe, in 16.4% (42/256) the right lower lobe and in 9.8% (25/256) the right upper lobe. In 3.9% (10/256) the right upper lobe and the middle lobe and in 0.4% (1/256) the right lower lobe and the middle lobe were the target lung lobes. During the course of time, valves were removed and replaced in the contralateral lung lobe as an alternative target lobe in three patients (from the right upper lobe to the left upper lobe, right lower lobe to the left lower lobe and from the left lower lobe to the right lower lobe).
Table 1.
Baseline characteristics of all 256 emphysema patients with absent collateral ventilation who underwent valve therapy.
| n | Mean ± SD | |
|---|---|---|
| Demographics | ||
| Age (years) | 256 | 64.5 ± 7.0 |
| Weight (kg) | 256 | 66.5 ± 14.9 |
| Height (m) | 256 | 1.68 ± 0.09 |
| BMI (kg/m²) | 256 | 23.6 ± 4.4 |
| Lung function | ||
| VC (L) | 256 | 2.32 ± 0.77 |
| VC (%) | 254 | 68.5 ± 18.4 |
| FEV1 (L) | 256 | 0.79 ± 0.25 |
| FEV1 (%) | 255 | 30.4 ± 8.3 |
| RV (L) | 254 | 5.70 ± 1.34 |
| RV (%) | 255 | 260.8 ± 54.8 |
| TLC (L) | 254 | 8.07 ± 1.66 |
| TLC (%) | 256 | 139.6 ± 20.5 |
| TLCO SB (%) | 206 | 32.6 ± 10.5 |
| TLCO/VA (%) | 212 | 47.5 ± 15.8 |
| Exercise capacity and symptoms | ||
| 6-MWT (m) | 236 | 270.0 ± 107.6 |
| mMRC | 220 | 2.8 ± 1.1 |
6-MWT, 6-minute walk test; BMI, body mass index; FEV1, forced expiratory volume in 1 s; mMRC, modified Medical Research Council; RV, residual volume; TLC, total lung capacity; TLCO SB, transfer factor for carbon monoxide, single breath. TLCO/VA, transfer factor for carbon monoxide, adjusted for alveolar volume; VC, vital capacity.
Figure 1.
Collateral ventilation assessment performed by CT fissure analysis and/or Chartis® measurement.
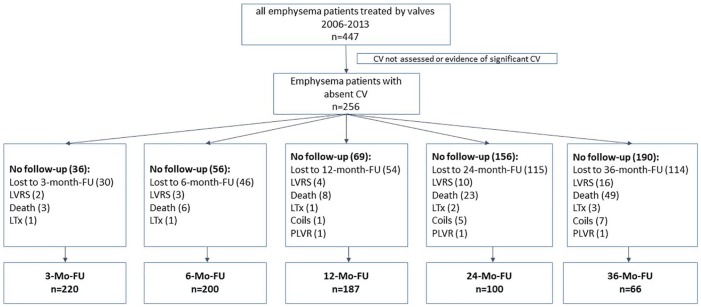
Of the 256 patients, 220, 200, 187, 100 and 66 patients completed the 3-month, 6-month, 1-year, 2-year and 3-year follow-up (FU) visit, respectively. Patients in whom valves were explanted are included in the analysis. Patients who underwent further interventional strategies (LVRS, coil therapy, polymeric lung volume reduction, lung transplantation) during the course of time were excluded after the additional therapeutic intervention. Reasons for missing data were lost to follow up or death. The flowchart of the patient enrolment criteria is presented in Figure 2.
Figure 2.
Flowchart of patients enrolled in this analysis.
CV, collateral ventilation; FU, follow up; LTx, lung transplantation; LVRS, lung volume reduction surgery; PLVR, polymeric lung volume reduction.
Long-term outcome following valve therapy
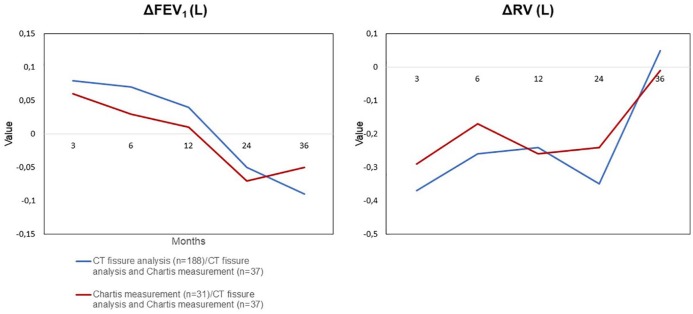
All lung function parameters, 6-MWT and mMRC were significantly improved at 3- and 6-month FU. At 1-year FU, patients still experienced a significant improvement of VC (%), FEV1 (L and %), RV (L and %), TLC (L), 6-MWT and mMRC. At 2 years, RV (L and %) and TLC (L and %) remained significantly improved compared to baseline. Three years after valve therapy, sustained significant improvement in mMRC was observed. MMRM was used as a sensitivity analysis and led to similar results. The effectiveness data are presented in Table 2. Comparing the impact of the CT fissure analysis and/or the Chartis® measurement on the outcome parameters FEV1 and RV, the CT fissure analysis appears to be slightly superior to the Chartis® measurement but of uncertain relevance (Figure 3).
Table 2.
Real-world efficacy data following valve placement at 6-, 12-, 24- and 36-month follow up. Data (mean ± SD) are based on observed data.
| 6-month FU |
1-year FU |
2- year FU |
3-year FU |
|||||
|---|---|---|---|---|---|---|---|---|
| n | n | n | n | |||||
| Δ VC (L) | 200 | 0.12 ± 0.5*# | 186 | 0.06 ± 0.55 | 100 | –0.04 ± 0.55 | 65 | –0.15 ± 0.52*# |
| Δ VC (% predicted) | 200 | 4.6 ± 15.8*# | 186 | 3.2 ± 17.9*# | 99 | –0.1 ± 17.4 | 64 | –3.5 ± 15.8# |
| Δ FEV1 (L) | 200 | 0.07 ± 0.15*# | 185 | 0.04 ± 0.18*# | 100 | –0.05 ± 0.15*# | 65 | –0.08 ± 0.14*# |
| Δ FEV1 (% predicted) | 200 | 3.2 ± 6.5*# | 186 | 2.0 ± 6.8*# | 99 | –1.3 ± 6.8# | 64 | –2.1 ± 5.4*# |
| Δ RV (L) | 197 | –0.25 ± 1.04*# | 180 | –0.25 ± 1.12*# | 95 | –0.35 ± 1.03*# | 61 | 0.03 ± 1.16 |
| Δ RV (% predicted) | 197 | –12.8 ± 47.9*# | 180 | –13.8 ± 52.4*# | 97 | –18.4 ± 46.4*# | 62 | –4.2 ± 52.6 |
| Δ TLC (L) | 198 | –0.16 ± 1.0*# | 183 | –0.16 ± 1.15*# | 98 | –0.38 ± 1.15*# | 63 | –0.2 ± 1.3 |
| Δ TLC (% predicted) | 198 | –2.8 ± 17.6*# | 183 | –2.7 ± 20.3# | 100 | –6.4 ± 18.9*# | 64 | –3.1 ± 21.2 |
| Δ 6-MWT (m) | 169 | 39.7 ± 75.2*# | 153 | 25.8 ± 82.0*# | 79 | 17.4 ± 79# | 44 | 8.6 ± 69.9 |
| Δ mMRC (points) | 148 | –0.6 ± 1.4*# | 137 | –0.6 ± 1.4*# | 70 | –0.1 ± 1.2# | 40 | –0.5 ± 1* |
p < 0.05 (paired t test); based on observed data.
p < 0.05 (MMRM); based on all 256 patients.
6-MWT, 6-minute walk test; FEV1, forced expiratory volume in 1 s; FU, follow up; mMRC, modified Medical Research Council; RV, residual volume; TLC, total lung capacity; VC, vital capacity.
Figure 3.
Impact of the CT fissure analysis and/or Chartis® measurement on the outcome parameters FEV1 and RV. Comparison of FEV1 and RV change in patients in whom CT fissure analysis and both methods confirmed CV absence to patients in whom Chartis® measurement and both methods confirmed CV absence.
CV, collateral ventilation; FEV1, forced expiratory volume in 1 s; RV, residual volume.
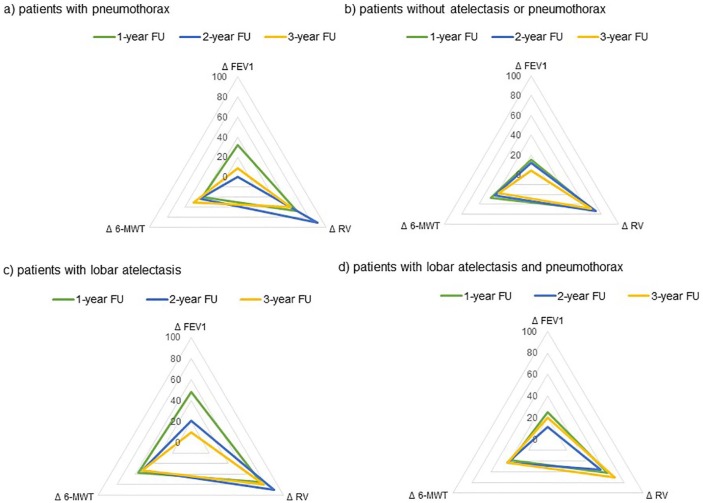
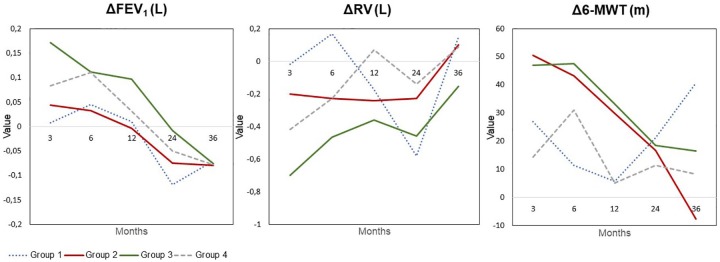
In 5 out of the 256 patients, no radiological follow up was performed. In 31.5% (79/251) of the patients, radiological examinations revealed the advent of complete lobar atelectasis following valve placement; in 46.2% (116/251) neither complete lobar atelectasis (but target lobe volume reduction or no volume change) nor pneumothorax; in 8.4% (21/251) lobar atelectasis and pneumothorax; and in 13.9% (35/251) only pneumothorax. Overall, patients with complete lobar atelectasis exhibited superior treatment responses (Figure 4). Two years following valve placement, these patients still experienced a statistically significant improvement in VC [+0.16 L ± 0.48 L (p = 0.05); 7.0% ± 18.7% predicted (p = 0.03)], RV [–0.46 L ± 0.87 L (p = 0.003); –23.8% ± 39.5% predicted (p = 0.001)], TLC [–0.46 L ± 1.18 L (p = 0.02); –7.3% ± 19.3% predicted (p = 0.02)] and mMRC [–0.6 pts ± 1.1 pts (p = 0.007)].
Figure 4.
Changes in clinical outcome measures (FEV1, RV and 6-MWT) at 3-, 6-, 12-, 24- and 36-month follow up compared to baseline according to the radiological outcome following valve placement. Group 1: patients with pneumothorax. Group 2: patients without lobar atelectasis or pneumothorax. Group 3: patients with lobar atelectasis. Group 4: patients with lobar atelectasis and pneumothorax.
6-MWT, 6-minute-walk test; CV, collateral ventilation; FEV1, forced expiratory flow in 1 s; RV, residual volume.
Responder analysis
At 6-month FU, 37% of the patients met the efficacy threshold of greater than 100 ml improvement in FEV1, 78% of the patients developed a greater than 430 ml reduction in RV and 58% of the patients experienced a greater than 26 m improvement on the 6-MWD. The number of patients reaching the MID for FEV1 and 6-MWT declines throughout the 6-month to 36-month FU, whereas the number of patients reaching the MID for RV ranging from 71% to 80% was quite stable over the course of time. At 3-year FU, the proportion of patients achieving the MID from baseline in RV and 6-MWT was still 71% and 46%, respectively. At 3-year FU, highest responder rates to FEV1, RV and 6-MWT were observed patients with lobar atelectasis with 10%, 79% and 53% respectively followed by patients who developed an atelectasis and pneumothorax with 20%, 70% and 43% respectively. Responder analysis is presented in Table 3 and Figure 5.
Table 3.
Responder analysis of all patients.
| 6-month FU | 1-year FU | 2- year FU | 3-year FU | |
|---|---|---|---|---|
| Δ FEV1 > 100 ml | 74/200 (37%) | 55/185 (29.7%) | 14/100 (14%) | 6/65 (9.2%) |
| Δ RV > –430 ml | 153/197 (77.7%) | 128/180 (71.1%) | 76/95 (80%) | 43/61 (70.5%) |
| Δ 6-MWT > 26 m | 98/169 (58.0%) | 75/153 (49%) | 37/79 (46.8%) | 20/44 (45.5%) |
6-MWT, 6-minute walk test; FEV1, forced expiratory volume in 1 s; FU, follow up; RV, residual volume.
Figure 5.
Responder analysis according to the radiological result. Response rates (%) were calculated by counting the number of patients who met the minimal important difference (MID) of >100 ml improvement in FEV1, >430 ml reduction in RV and >26 m improvement in 6-MWT.
6-MWT, 6-minute walk test; FEV1, forced expiratory volume in 1 s; FU, follow up; RV, residual volume.
Only patients who completed the 3-year FU
Of the 256 patients enrolled in this analysis, 66 patients completed the 3-year FU visit. Out of 66 patients, 20 patients developed a complete lobar atelectasis and 25 patients experienced partial atelectasis or no volume change. Pneumothorax occurred in 21 patients, and in 10 of these also a lobar atelectasis was observed. All lung function parameters (except TLC at 6-month FU), 6-MWT and mMRC were significantly improved at 3- and 6-month FU. At 1-year FU, patients experienced still a significant improvement of VC (%), FEV1 (L and %), RV (%), 6-MWT and mMRC. At 2-year FU, RV (ml and %), TLC (ml and %) and 6-MWT remained significantly improved compared to baseline. Three years after valve therapy, sustained significant improvement in mMRC was observed. The results are demonstrated in Table 4.
Table 4.
Real-world efficacy data following valve placement at 6-, 12-, 24- and 36-month follow up of patients who completed the 36-month follow up. .
| 6-month FU |
1-year FU |
2-year FU |
3-year FU |
|||||
|---|---|---|---|---|---|---|---|---|
| n | n | n | n | |||||
| Δ VC (L) | 61 | 0.16 ± 0.51* | 63 | 0.12 ± 0.5 | 50 | –0.03 ± 0.47 | 65 | –0.15 ± 0.52* |
| Δ VC (% predicted) | 61 | 5.6 ± 14* | 63 | 4.1 ± 14.6* | 49 | –0.1 ± 13.1 | 64 | –3.5 ± 15.8 |
| Δ FEV1 (L) | 61 | 0.07 ± 0.18* | 63 | 0.04 ± 0.14* | 50 | –0.04 ± 0.15 | 65 | –0.08 ± 0.14 |
| Δ FEV1 (% predicted) | 61 | 3.2 ± 7.0* | 63 | 2.3 ± 5.1* | 49 | –0.8 ± 5.5 | 64 | –2.1 ± 5.4* |
| Δ RV (L) | 58 | –0.36 ± 0.97* | 60 | –0.23 ± 1.21 | 45 | –0.43 ± 0.97* | 61 | 0.03 ± 1.16 |
| Δ RV (% predicted) | 58 | –18.8 ± 45.0* | 60 | –15.4 ± 57.1* | 47 | –20.8 ± 44.1* | 62 | –4.2 ± 52.6 |
| Δ TLC (L) | 60 | –0.3 ± 1.2 | 62 | –0.2 ± 1.3 | 47 | –0.5 ± 1.3* | 63 | –0.2 ± 1.3 |
| Δ TLC (% predicted) | 60 | –5.4 ± 20.5* | 62 | –3.3 ± 22.8 | 49 | –8.8 ± 30.4* | 64 | –3.1 ± 21.2 |
| Δ 6-MWT (m) | 40 | 47.5 ± 61.7* | 39 | 33.5 ± 67.8* | 35 | 28.3 ± 77.5* | 44 | 8.6 ± 69.9 |
| Δ mMRC (points) | 36 | –0.9 ± 1.3* | 38 | –0.9 ± 1.3* | 31 | –0.3 ± 1.2 | 40 | –0.5 ± 1.0* |
p < 0.05 (t test).
6-MWT, 6-minute walk test; FEV1, forced expiratory volume in 1 s; FU, follow up; mMRC, modified Medical Research Council; RV, residual volume; TLC, total lung capacity; VC, vital capacity.
Pneumothorax
In the patient cohort in which radiological follow up was assessed, 22% (56/251) developed a pneumothorax as an anticipated complication following valve placement. In 86% (48/56) of these patients, chest tube insertion and in 41% (23/56) valve removal was necessary for pneumothorax management.
Permanent removal of all valves
During the course of 3 years (from first valve implantation to valve removal in each individual patient), all valves were permanently removed in 24.6% (63/256) of the patients. Reasons for permanent valve removal were missing clinical benefit in 55.6% (35/63), pneumothorax in 11.1% (7/63), decision for definitive LVRS in 19% (12/63), poststenotic pneumonia in 6.3% (4/63), lung cancer in 3.2% (2/63), respiratory insufficiency in 3.2% (2/63) and recurrent pulmonary infections in 1.6% (1/63).
Discussion
Endoscopic valve therapy, which presents the best studied ELVR technique, can be considered in patients with advanced emphysema and absent CV who still have symptoms and impaired lung function despite an optimal pharmacological therapy. This interventional approach is a symptom-modifying treatment and has been demonstrated to improve lung function parameters, exercise capacity and quality of life in emphysema patients.2–5 Different retrospective studies have also confirmed that valve-induced lobar atelectasis is associated with a survival benefit.7,8,21
So far, efficacy of valve therapy has been confirmed over a 1-year period in two RCTs. The results of the ‘STELVIO’ and the ‘LIBERATE’ trials confirmed a clinically relevant benefit at 1 year follow up after valve placement.6,22 In the current analysis, similar findings were observed with a significant improvement in lung function parameters (except VC and TLC), exercise capacity and dyspnea score 1 year post-treatment. In contrast to the RCT, in whom safety and efficacy data are obtained in a narrowly defined patient population in a clinical trial setting, this analysis provides real-world data as not all patients were treated within prospective clinical trials.
So far, only small cohort studies have investigated the long-term outcome beyond 1 year after valve therapy. Venuta and colleagues reported the clinical outcome in 40 emphysema patients at 3 and 5 years after valve implantation irrespective of CV. FEV1, 6-MWT, mMRC and supplemental oxygen requirements were significantly improved at these longer time points.8 However, statistical analyses were not performed for the comparison of the outcome parameters between baseline and the different time points, making the results of this study difficult to interpret. In the current analysis, a significant decrease was still observed in RV and TLC at 2 years and a significant improvement in mMRC at 3 years post-treatment. During the course of the 3 years, VC and FEV1 declined significantly to the pre-treatment baseline more likely due to COPD progression. However, there was still a meaningful proportion of patients who had a clinically relevant response to valve therapy. It must be assumed that the responder rates beyond the time period of 6 months may be underestimated as most MIDs were calculated for short-term changes and a long-term MID is more likely lower than a short-term MID.
Radiological evidence of complete lobar atelectasis, which presents the maximum result of valve therapy, was found in 40% of the patients in this cohort with confirmed absence of CV. There are only limited data on the incidence of lobar atelectasis following valve placement. In the ‘BeLieVeR-HIFi’ study, lobar atelectasis was reported in 35% of the patients, and thus a slightly lower rate, but in that reported patient cohort, CV was not excluded in 40% of the patients treated by valves. The current study results demonstrate that particularly patients who developed a valve-induced complete lobar atelectasis seem to experience a superior long-term response to valve treatment. This sustainable improvement of lung function parameters may also explain the survival benefit in patients with lobar atelectasis following valve placement.7,21 It should be kept in mind that not only patients with complete atelectasis (target lobe volume reduction of 100%) of the treated lung lobe will benefit from the valve therapy. Also patients who develop a target lobe volume reduction of 49–54% will experience a clinically relevant improvement.23
The advent of pneumothorax was found in 22% of the patients, which is very similar to rates of 18–34% reported in other trials.2–6 Earlier studies have already demonstrated that the occurrence of pneumothorax does not appear to impair the clinical state in the majority of patients and may predict a superior outcome following valve therapy, particularly in patients with persistent atelectasis following recovery of pneumothorax.24 Also in this analysis, the responder rate in FEV1, RV and 6-MWT at 3 years post-treatment in patients who developed pneumothorax and atelectasis was still 20%, 70% and 43%, respectively.
In this study CV assessment was performed by CT fissures analysis and/or Chartis® measurement. The CT fissure analysis appeared to be slightly superior to the Chartis® measurement in predicting an improvement in the outcome measures FEV1 and RV, but this difference seems to be of uncertain clinical relevance. This result is similar to the findings of two other retrospective trials demonstrating a comparable diagnostic accuracy (77–79% for CT fissure analysis versus 74–76% for Chartis® measurement) for correctly predicting the success of valve therapy.11,25 It must be kept in mind that the data collection of the current analysis covers patients treated from 2006 to 2013, when there was only limited knowledge about the impact of interlobar CV on the outcome of valve therapy. The definition of fissure completeness was not well understood, the visual fissure analysis was moreover still a challenge at the beginning of the learning curve and there was no additional software supporting the fissure analysis.
There is also one study published on long-term follow-up data for endoscopic coil therapy that presents an alternative, irreversible therapeutic approach in patients with advanced emphysema. Similar to the findings of our study, Hartmann and colleagues found only the mMRC to be still statistically significantly improved at 3 years.26 They also found a gradual decline of the lung function parameters over time. However, the number of patients reaching the MID for 6-MWT was also satisfying, with still 40% at 3 years post-treatment, which is comparable to our result. In contrast to valve therapy, only 19% of the patients treated by coils maintained clinically relevant reduction of hyperinflation, whereas in our study 71% of patients treated with valves exhibited a clinically meaningful RV reduction at 3 years. However, these statements must be interpreted with caution as there is no trial directly comparing the two different endoscopic treatment approaches.
One limitation of this study is its retrospective and noncontrolled design. Moreover, there is a high number of patients who were lost to 2- and 3-year follow up that may considerably limit any evidence regarding the long-term effect of valve therapy. To show the robustness of the results in the context of missing data, MMRM was used for sensitivity analysis and led to similar results. Nevertheless, as 49 patients died over the 3-year time period, a survival bias leading to a distortion of the clinical outcome measures in a positive sense must be taken into account. A prospective trial investigating the long-term outcome of emphysema patients treated by valves is imperative to reach a final conclusion.
Summarizing, patients treated by valves experience clinical improvement over 1 year following valve therapy. Afterwards, clinical benefit gradually declines, most likely due to COPD progression, which emphasizes the importance of ongoing optimal medical treatment and physical exercise in order to minimize progression of the disease. However, there is still a high proportion of patients who experience clinically meaningful improvement of RV and 6-MWT at 3 years post-treatment, which may explain the survival-enhancing effect of successful valve therapy.
Supplemental Material
Supplemental material, Author_response for Long-term follow up after endoscopic valve therapy in patients with severe emphysema by Daniela Gompelmann, Tobias Heinhold, Matthias Rötting, Elena Bischoff, Konstantina Kontogianni, Ralf Eberhardt and Felix J. F. Herth in Therapeutic Advances in Respiratory Disease
Supplemental Material
Supplemental material, Reviewer_1_v.1_(2) for Long-term follow up after endoscopic valve therapy in patients with severe emphysema by Daniela Gompelmann, Tobias Heinhold, Matthias Rötting, Elena Bischoff, Konstantina Kontogianni, Ralf Eberhardt and Felix J. F. Herth in Therapeutic Advances in Respiratory Disease
Acknowledgments
The abstract of this article was presented at the ERS International Congress, Paris, France in 2018.
Footnotes
Author contributions: DG, TH, MR, EB, KK, RE and FH contributed to the conception or design of the work; DG, TB, MR and EB to the data acquisition and the analysis of the data for the work. All authors contributed to the interpretation of data for the work and drafting or revising the manuscript. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: The authors received no financial support for the research, authorship and/or publication of this article.
Conflict of interest statement: DG: Lecture and travel fees from Pulmonx, Olympus, Chiesi, Boehringer Ingelheim, Novartis, Astra Zeneca, Mundipharma, Berlin Chemie and Grifols.
TH: No conflicts of interest.
MR: No conflicts of interest.
EB: No conflicts of interest.
KK: No conflicts of interest.
RE: Lecture and travel fees from Olympus, Pulmonx and Uptake Medical.
FH: Fees for lectures and advisory boards from Astra, Allmirall, Berlin Chemie, Boehringer, Roche, GSK, Pulmonx, BTG, Olympus, PneumRx, Boston Scientific, Medupdate, Grifols, CSL Behring, Omniamed, Lilly, Novartis, Teva, Uptake and Vital Air.
ORCID iD: Daniela Gompelmann  https://orcid.org/0000-0002-4857-9854
https://orcid.org/0000-0002-4857-9854
Supplemental material: The reviews of this paper are available via the supplementary material section.
Contributor Information
Daniela Gompelmann, Pneumology and Critical Care Medicine, Thoraxklinik at University of Heidelberg, Roentgenstr. 1, Heidelberg, 69126, Germany Translational Lung Research Center Heidelberg (TLRCH), German Center for Lung Research, Heidelberg, Germany.
Tobias Heinhold, Pneumology and Critical Care Medicine, Thoraxklinik, University of Heidelberg, Heidelberg, Germany.
Matthias Rötting, Pneumology and Critical Care Medicine, Thoraxklinik, University of Heidelberg, Heidelberg, Germany.
Elena Bischoff, Pneumology and Critical Care Medicine, Thoraxklinik, University of Heidelberg, Heidelberg, Germany.
Konstantina Kontogianni, Pneumology and Critical Care Medicine, Thoraxklinik, University of Heidelberg, Heidelberg, Germany Translational Lung Research Center Heidelberg (TLRCH), German Center for Lung Research, Heidelberg, Germany.
Ralf Eberhardt, Pneumology and Critical Care Medicine, Thoraxklinik, University of Heidelberg, Heidelberg, Germany Translational Lung Research Center Heidelberg (TLRCH), German Center for Lung Research, Heidelberg, Germany.
Felix J. F. Herth, Pneumology and Critical Care Medicine, Thoraxklinik, University of Heidelberg, Heidelberg, Germany Translational Lung Research Center Heidelberg (TLRCH), German Center for Lung Research, Heidelberg, Germany
References
- 1. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2019 report [Google Scholar]
- 2. Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomized controlled trial. Lancet 2015; 386: 1066–1073. [DOI] [PubMed] [Google Scholar]
- 3. Klooster K, ten Hacken NH, Hartman JE, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015; 373: 2325–2335. [DOI] [PubMed] [Google Scholar]
- 4. Valipour A, Slebos DJ, Herth F, et al. Endobronchial valve therapy in patients with homogeneous emphysema: results from the IMPACT study. Am J Respir Crit Care Med 2016; 194: 1073–1082. [DOI] [PubMed] [Google Scholar]
- 5. Kemp SV, Slebos DJ, Kirk A, et al. A multicenter randomized trial of zephyr endobronchial valve treatment in heterogeneous emphysema (TRANSFORM). Am J Respir Crit Care Med 2017; 196: 1535–1543. [DOI] [PubMed] [Google Scholar]
- 6. Criner GJ, Sue R, Wright S, et al. A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (LIBERATE). Am J Respir Crit Care Med 2018; 198: 1151–1164. [DOI] [PubMed] [Google Scholar]
- 7. Hopkinson NS, Kemp SV, Toma TP, et al. Atelectasis and survival after bronchoscopic lung volume reduction for COPD. Eur Respir J 2011; 37: 1346–1351. [DOI] [PubMed] [Google Scholar]
- 8. Venuta F, Anile M, Diso D, et al. Long-term follow-up after bronchoscopic lung volume reduction in patients with emphysema. Eur Respir J 2012; 39: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 9. Achenbach T, Weinheimer O, Buschsieweke C, et al. Fully automatic detection and quantification of emphysema on thin section MD-CT of the chest by a new and dedicated software. Foro 2001; 176: 1409–1415. [DOI] [PubMed] [Google Scholar]
- 10. Herth FJ, Eberhardt R, Gompelmann D, et al. Radiological and clinical outcomes of using ChartisTM to plan endobronchial valve treatment. Eur Respir J 2013; 41: 302–308. [DOI] [PubMed] [Google Scholar]
- 11. Schuhmann M, Raffy P, Yin Y, et al. Computed tomography predictors of response to endobronchial valve lung reduction treatment: comparison with Chartis. Am J Respir Crit Care Med 2015; 191: 767–774. [DOI] [PubMed] [Google Scholar]
- 12. Sciurba FC, Ernst A, Herth FJF, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010; 363: 1233–1244. [DOI] [PubMed] [Google Scholar]
- 13. Slebos DJ, Shah PL, Herth FJ, et al. Endobronchial valves for endoscopic lung volume reduction: best practice recommendations from expert panel in endoscopic lung volume reduction. Respiration 2017; 93: 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 15. Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J 2005; 26: 511–522. [DOI] [PubMed] [Google Scholar]
- 16. Brooks D, Solway S, Gibbons WJ. ATS statement on six-minute walk test. Am J Respir Crit Care Med 2003; 167: 1287. [DOI] [PubMed] [Google Scholar]
- 17. Bestall JC, Paul EA, Garrod R, et al. Usefulness of the medical research council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54: 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005; 2: 111–124. [DOI] [PubMed] [Google Scholar]
- 19. Hartman JE, ten Hacken NHT, Klooster K, et al. The minimal important difference for residual volume in patients with severe emphysema. Eur Respir J 2012; 40: 1137–1141. [DOI] [PubMed] [Google Scholar]
- 20. Puhan MA, Chandra D, Mosenifar Z, et al. The minimal important difference of exercise tests in severe COPD. Eur Respir J 2011; 37: 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gompelmann D, Benjamin N, Bischoff E, et al. Survival after endoscopic valve therapy in patients with severe emphysema. Respiration 2019; 97: 145–152. [DOI] [PubMed] [Google Scholar]
- 22. Klooster K, Hartmann JE, ten Hacken NH, et al. One-year follow-up after endobronchial valve treatment in patients with emphysema without collateral ventilation treated in the STELVIO trial. Respiration 2017; 93: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gompelmann D, Kontogianni K, Schuhmann M, et al. The minimal important difference for target lobe volume reduction after endoscopic valve therapy. Int J Chron Obstruct Pulmon Dis 2018; 13: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gompelmann D, Benjamin N, Kontogianni K, et al. Clinical and radiological outcome following pneumothorax after endoscopic lung volume reduction with valves. Int J Chron Obstruct Pulmon Dis 2016; 11: 3093–3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gompelmann D, Eberhardt R, Slebos DJ, et al. Diagnostic performance comparison of the Chartis system and high-resolution computerized tomography fissure analysis for planning endoscopic lung volume reduction. Respirology 2014; 19: 524–530. [DOI] [PubMed] [Google Scholar]
- 26. Hartmann JE, Klooster K, Gortzak K, et al. Long-term follow-up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology 2015; 20: 319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_response for Long-term follow up after endoscopic valve therapy in patients with severe emphysema by Daniela Gompelmann, Tobias Heinhold, Matthias Rötting, Elena Bischoff, Konstantina Kontogianni, Ralf Eberhardt and Felix J. F. Herth in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1_(2) for Long-term follow up after endoscopic valve therapy in patients with severe emphysema by Daniela Gompelmann, Tobias Heinhold, Matthias Rötting, Elena Bischoff, Konstantina Kontogianni, Ralf Eberhardt and Felix J. F. Herth in Therapeutic Advances in Respiratory Disease