Abstract
Parental prostacyclin is the only therapy with a proven survival benefit in pulmonary arterial hypertension (PAH). However, some patients are unable to tolerate continuous prostacyclin infusion because of central line infection, side effects, or sociocultural factors. Selexipag is a recently approved prostacyclin receptor agonist that is able to blunt PAH disease progression. Although in the same molecular pathway, the interchangeability of selexipag with prostacyclin infusions is relatively unexplored. Here, we present a case series of five stable PAH patients who were functional class (FC) I or II that were transitioned from prostacyclin infusion to selexipag using a standardized protocol in the inpatient setting. We show that the transition to selexipag in five highly selected patients was tolerated with no significant changes in FC, minimal changes in pulmonary vascular disease severity, and no significant PAH-related complications. However, there was a trend for a reduction in cardiac index after transition to selexipag. These data suggest that a transition from prostacyclin infusion to selexipag can be achieved in clinically stable PAH patients who are unable to tolerate continuous prostacyclin infusion. However, this approach should only be selectively implemented at specialized centers with close follow-up due to the trend for a reduction in cardiac index after transition to selexipag.
Keywords: hemodynamics, cardiac index, pulmonary vascular resistance
Introduction
Pulmonary arterial hypertension (PAH) is a progressive and lethal pulmonary vasculopathy with a median survival of only 5–7 years after diagnosis.1 Current pharmaceuticals for PAH target the nitric oxide, endothelin, and prostacyclin pathways, and most drugs were approved by the Food and Drug Administration due to improvements in exercise capacity and reduction in morbidity.2 Parental prostacyclin is the only currently available therapy with a proven survival benefit in PAH in a clinical trial setting.3 However, some patients are not able to take continuous prostacyclin therapy due to central line infection, prostanoid side effects, or social and cultural factors. In addition, parenteral prostacyclin has drawbacks including a significant patient burden with need to mix the drug at home, limiting its widespread use.
Selexipag is an oral prostacyclin IP receptor agonist that gained approval for PAH treatment due to its ability to slow disease progression.4 While selexipag and parental prostacyclin potentially work in the same molecular pathway,2 the ability to transition between the two drugs is not well studied. This is an important knowledge gap as PAH patients that experience complications with parental prostacyclin face few options for substitution therapy.
Here, we provide the algorithm we used to transition the patients from prostacyclin to selexipag in the inpatient setting and describe a series of five PAH patients that were successfully transitioned from parental prostacyclin to selexipag. A summary of the five cases is shown in Table 1.
Table 1.
Summary of five cases of transition.
| Case no. | PAH etiology | WHO FC pre | Other PAH therapy | Reason for transition | Prostacyclin dose | Final selexipag dose | WHO FC post | Complications |
|---|---|---|---|---|---|---|---|---|
| 1 | Mixed connective tissue | II | Macitentan (10 mg) Sildenafil (80 mg TID) | Line infection | 80 ng/kg/min (treprostinil) | 1600 mcg BID | I | None |
| 2 | Congenital heart disease | II | None | Line infection | 66 ng/kg/min (treprostinil) | 1600 mcg BID | II | None |
| 3 | Portopulmonary hypertension | II | Tadalafil (40 mg) Macitentan (10 mg) | Inability to mix at home | 50 ng/kg/min (treprostinil) | 1200 mcg BID | II | None |
| 4 | Idiopathic | I | Ambrisentan (5 mg) | Patient preference | 42 ng/kg/min (treprostinil) | 1000 mcg BID | I | None |
| 5 | Idiopathic | II | Tadalafil (40 mg) Bosentan (125 mg BID) | Favorable hemodynamic response to prostacylin | 96 ng/kg/min (treprostinil) | 1600 mcg BID | II | None |
FC, functional class; PAH, pulmonary arterial hypertension.
Case 1
The first patient to undergo transition of therapy at the University of Minnesota Medical Center was a 36-year-old African American woman with PAH secondary to adult-onset Still’s disease. She was initiated on intravenous treprostinil in 2014 because she was functional class (FC) III with right heart failure characterized by severely reduced cardiac index and elevated right-sided filling pressures despite being on dual oral combination therapy of macitentan 10 mg daily, sildenafil 80 mg three times daily, and monthly infusions of tocilizumab. Before initiation of intravenous therapy, her hemodynamic evaluation revealed a mean right atrial pressure (RAP) of 27 mmHg, mean pulmonary arterial pressure (mPAP) of 53 mmHg, pulmonary capillary wedge pressure (PCWP) of 15 mmHg, pulmonary vascular resistance (PVR) of 14.1 Wood units (WU), and a cardiac output by thermodilution of 2.7 L/min. Intravenous treprostinil dose was gradually increased as tolerated to a maximum dose of 80 ng/kg/min and triple combination therapy was continued for 18 months. Due to recurrent line infections and a clinically stable course, the patient elected to transition to selexipag in the inpatient setting using our protocol outlined in Table 2. Before the transition, a thorough examination was performed. On Naughton-Balke exercise stress test, her maximal volume of oxygen consumption (VO2 max) was 22.4 mL/kg/min which was 71% of predicted capacity. Cardiac magnetic resonance imaging (MRI) revealed a mildly dilated right ventricle with an ejection fraction (EF) of 32%. On right heart catheterization (RHC), her RAP was 8 mmHg, mPAP was 35 mmHg, PCWP was 15 mm Hg, PVR was 2.6 WU, and cardiac output was 7.6 L/min. After completing the transition protocol (Table 2) in the inpatient setting, she was discharged on a selexipag dose of 1600 mcg twice daily. The patient underwent repeat exercise testing, cardiac MRI, and RHC three months after transition. Repeat exercise testing completed post-transition demonstrated an improved exercise capacity with a VO2 max of 25.9 mL/kg/min, which was 82% predicted capacity. Post-transition cardiac MRI showed continued right ventricle dilation but a slight increase in EF to 37%. Post-transition RHC showed a RAP of 4 mmHg, mPAP of 27 mmHg, PCWP of 10 mmHg, PVR of 3.7 WU, and cardiac output of 4.6 L/min. Since completing the transition in May of 2016, patient remains FC I and has not had any hospitalizations due to worsening of her PAH.
Table 2.
Protocol for the transition from parental prostacyclin to selexipag.
| Day | Selexipag dose | Prostacyclin dose |
|---|---|---|
| X = Total prostacyclin dose/8 | ||
| 1 | 200 mcg BID | Decrease by X |
| 2 | 400 mcg BID | Decrease by X |
| 3 | 600 mcg BID | Decrease by X |
| 4 | 800 mcg BID | Decrease by X |
| 5 | 1000 mcg BID | Decrease by X |
| 6 | 1200 mcg BID | Decrease by X |
| 7 | 1400 mcg BID | Decrease by X |
| 8 | 1600 mcg BID | Decrease by X |
Case 2
The second case involved a 22-year-old Asian woman with PAH secondary to congenital heart disease whose was status post-baffle repair of the common atrium, baffling of the anomalous veins to the left atrium, tricuspid ring repair, and cleft repair of left-sided atrioventricular canal. Before being seen at our facility, the patient was initiated on intravenous treprostinil in the setting of severe PAH and high-risk pregnancy in June 2016. A RHC was not performed before initiation of therapy, but an echocardiogram demonstrated an estimated pulmonary artery systolic pressure of 73 mmHg with right ventricular dysfunction and the patient was FC III/IV. She was also started on tadalafil 20 mg daily, but therapy was subsequently stopped due to hypotension. The patient transitioned to subcutaneous treprostinil after having repeated infections associated with her central line. Unfortunately, the patient had recurrent site infections so she was transitioned to selexipag from a subcutaneous treprostinil dose of 66 ng/kg/min as per our protocol described in Table 2. A pre-transition RHC demonstrated that her RAP was 5 mmHg, mPAP was 32 mmHg, PCWP was 5 mmHg, PVR was 4.9 WU, and cardiac output was 5.5 L/min. The patient tolerated a selexipag dose of 1600 mcg twice daily and was discharged in November 2017. RHC performed upon completion of the transition showed a mRAP of 6 mmHg, mPAP of 37 mmHg, PCWP of 7 mm Hg, PVR of 7.5 WU, and cardiac output of 4.0 L/min. She has not had any subsequent hospitalizations related to PAH since discharge, but she self-discontinued use of selexipag. She subsequently resumed selexipag and again reached the maximum dose of 1600 mcg twice daily.
Case 3
The third case was a 61-year-old Caucasian woman with portopulmonary hypertension. She was treated with subcutaneous treprostinil for 13 years and was maintained on a dose of 50 ng/kg/min in combination with tadalafil 40 mg daily and macitentan 10 mg daily. The patient was followed by an outside facility for multiple years before transfer to our practice so we did not have hemodynamic and FC data before the initiation of treprostinil therapy. In light of concerns regarding the patient’s memory and ability to manage parenteral prostacyclin therapy in the home setting, she was selected to transition to selexipag as per our protocol. A RHC was performed before the transition, which showed her RAP was 3 mmHg, mPAP was 40 mmHg, PCWP was 8 mmHg, PVR was 6.3 WU, and cardiac output was 5.1 L/min. RHC three months after transition demonstrated stable hemodynamics with a RAP of 11 mmHg, mPAP of 40 mmHg, PCWP of 15 mmHg, PVR of 5.4 WU, and a cardiac output of 4.6 L/min. After discharge in December 2017, the patient was on a selexipag dose of 1600 mcg twice daily for 12 months, but due to complaints of headache during routine clinic evaluation, her selexipag dose has since been decreased to 1200 mcg twice daily. Her most recent echocardiogram completed in January of 2019 revealed normal right ventricular function with a tricuspid plane systolic excursion (TAPSE) of 3.1 cm. She has not had any PAH-related complications or hospitalization since transitioning to selexipag.
Case 4
The fourth case was a 36-year-old Indian woman with idiopathic PAH who had been on intravenous treprostinil since 2009. Before initiation of intravenous therapy, the patient was FC IV and her RHC revealed a RAP of 10 mmHg, mPAP of 65 mmHg, PCWP of 15 mmHg, PVR of 16.5 WU, and cardiac output of 3.0 L/min. An attempt to transition to oral treprostinil was made in 2015, but due to increased dyspnea and an associated decreased in exercise tolerance she was placed back on intravenous treprostinil at dose of 42 ng/kg/min along with ambrisentan 5 mg daily. She was also treated with off-label cyclosporine to provide an anti-inflammatory effect for her PAH. She was FC I before transition as her 6-min walk distance (6MWD) was 518 m and an echocardiogram showed normal right ventricular size and function. On RHC, her RAP was 3 mmHg, mPAP was 28 mmHg, PCWP was 10 mmHg, PVR was 3.7 WU, and cardiac output was 4.9 L/min. In February of 2018, she completed the transition to selexipag of 1600 mcg twice daily. Two months after discharge, a repeat RHC revealed stable hemodynamics with a RAP of 1 mmHg, mPAP of 24 mmHg, PCWP of 3 mmHg, PVR of 4.0 WU, and cardiac output of 5.2 L/min. She was maintained on 1600 mcg twice daily for eight months after discharge with no further hospitalizations, but due to concurrent pharmacological therapies inhibiting the CYP2C8 metabolism of selexipag, her dose was decreased to 1000 mcg BID during clinical evaluation in November 2018. She has remained FC I with a 6MWD of 604 m and continued normal right ventricle size and function on echocardiogram after transition.
Case 5
The final transition case was a 52-year-old Hispanic woman with idiopathic PAH. She was initially started on intravenous epoprostenol in 1997 when she was FC III and a RHC revealed her RAP was 12 mmHg, mPAP was 48 mmHg, PCWP was 9 mmHg, PVR was 15.6 WU, and cardiac output was 2.5 L/min. She was transitioned to subcutaneous treprostinil in 2008 due to recurrent line infections and finally transitioned back to intravenous treprostinil in 2014 due to pain at the subcutaneous site. Since that time, she was maintained on triple therapy with bosentan 125 mg twice daily, tadalafil 40 mg daily, and intravenous treprostinil at a dose of 96 ng/kg/min. She was felt to be a candidate for the transition after continued stable repeat RHCs and improved exercise capacity from FC III to FC I/II. RHC performed before the transition demonstrated that her RAP was 4 mmHg, mPAP was 17 mmHg, PCWP was 11 mmHg, PVR was 1.4 WU, and cardiac output was 4.3 L/min. Upon completion of the transition, the patient was discharged in June 2018 and has remained stable on goal dose of selexipag 1600 mcg twice daily without any unexpected hospitalizations. Since discharge, she remains FC I/II. Her 6MWD was 457 m and her right ventricle size and function are normal by echocardiogram. RHC repeated six months after transition showed a RAP of 9 mmHg, mPAP of 22 mmHg, PCWP of 14 mmHg, PVR of 1.9 WU, and cardiac output of 4.2 L/min.
Discussion
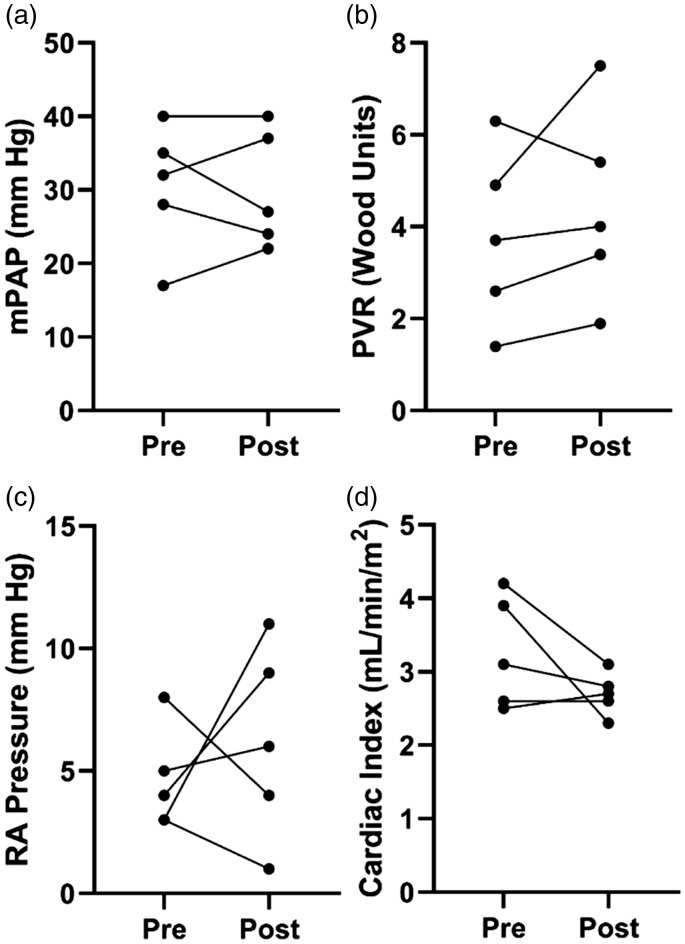
Here, we discuss the transition of five clinically stable PAH patients from parenteral prostacyclin to selexipag using a specified protocol in the inpatient setting. All patients were FC I/II with normal cardiac output and right-sided filling pressures before transition. All five patients tolerated the maximum dose of selexipag during the inpatient transition, but two patients reduced the dose in the outpatient setting due to side-effects. We showed no significant change in pulmonary vascular disease severity or FC when selexipag was substituted for prostacyclin. However, there was a trend for reduction in cardiac index after selexipag transition (Fig. 1). These highly selected cases demonstrate a rigorous protocol with close follow-up can be used to transition clinically stable PAH patients from parental prostacyclin to selexipag.
Fig. 1.
Change in invasive hemodynamics after transition to selexipag. There was no significant change in mPAP (30.4 ± 8.7 mmHg vs. 30.0 ± 8.0 mmHg, P = 0.88) (a), PVR (3.8 ± 1.9 WU vs. 4.4 ± 2.1 WU, P = 0.31) (b), or RAP (4.6 ± 2.1 mmHg vs. 6.2 ± 4.0 mmHg, P = 0.51) (c). However, there was a trend for reduced cardiac index (3.3 ± 0.8 mL/min/m2 vs. 2.7 ± 0.3 mL/min/m2, P = 0.18) (d).
While these data are promising, applying this algorithm to all PAH patients requiring parental prostacyclin should not be adopted. The highlighted five patients in our series were selected because of clinical stability. Due to our high selectivity of those eligible for the transition, we have not had any unsuccessful cases; however, only five patients have been transitioned in the past five years. Furthermore, all but one of these patients were on other PAH-specific therapy and thus there was some added safety during the transition. Although it was not statistically significant, there was a trend for a reduction in cardiac index, which strongly suggests that this protocol should be avoided in patients with impaired cardiac output. Finally, we do not have long-term follow up for these five patients to be certain whether transitioning from parenteral prostacyclin infusion to selexipag is a durable long-term option.
In conclusion, we provide the algorithm we used to transition the patients from prostacyclin to selexipag in the inpatient setting and describe a series of five PAH patients that were successfully transitioned from parental prostacyclin to selexipag. All five patients did not tolerate continuous prostacyclin therapy. The transition to selexipag was well tolerated and was not associated with a significant change in FC or pulmonary vascular disease severity. However, there was a trend for a decline in cardiac index after transition to selexipag. These highly selected cases suggest that PAH patients can be transitioned from parental prostacyclin to selexipag if patients are clinically stable and have a normal cardiac output.
Conflict of interest
TT receives modest consultation fees from Actelion and Gilead.
Funding
KWP is funded by NIH K08 HL140100. TT is funded by AHA Scientist Development Grant 15SDG25560048.
References
- 1.Prins KW, Thenappan T. World Health Organization Group I Pulmonary Hypertension: Epidemiology and Pathophysiology. Cardiol Clin 2016; 34: 363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thenappan T, Ormiston ML, Ryan JJ, et al. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ 2018; 360: j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med 1996; 334: 296–301. [DOI] [PubMed] [Google Scholar]
- 4.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. [DOI] [PubMed] [Google Scholar]