Abstract
Right ventricular (RV) failure (RVF) has garnered significant attention in recent years because of its negative impact on clinical outcomes in patients with pulmonary hypertension (PH). PH triggers a series of events, including activation of several signaling pathways that regulate cell growth, metabolism, extracellular matrix remodeling, and energy production. These processes render the RV adaptive to PH. However, RVF develops when PH persists, accompanied by RV ischemia, alterations in substrate and mitochondrial energy metabolism, increased free oxygen radicals, increased cell loss, downregulation of adrenergic receptors, increased inflammation and fibrosis, and pathologic microRNAs. Diastolic dysfunction is also an integral part of RVF. Emerging non-invasive technologies such as molecular or metallic imaging, cardiac MRI, and ultrafast Doppler coronary flow mapping will be valuable tools to monitor RVF, especially the transition to RVF. Most PH therapies cannot treat RVF once it has occurred. A variety of therapies are available to treat acute and chronic RVF, but they are mainly supportive, and no effective therapy directly targets the failing RV. Therapies that target cell growth, cellular metabolism, oxidative stress, and myocyte regeneration are being tested preclinically. Future research should include establishing novel RVF models based on existing models, increasing use of human samples, creating human stem cell-based in vitro models, and characterizing alterations in cardiac excitation–contraction coupling during transition from adaptive RV to RVF. More successful strategies to manage RVF will likely be developed as we learn more about the transition from adaptive remodeling to maladaptive RVF in the future.
Keywords: pulmonary hypertension, right ventricular failure, cardiac hypertrophy
Right ventricular (RV) failure (RVF; also referred to as right heart failure) that develops as a result of pulmonary hypertension (PH) has been increasingly recognized as a determiner of outcome in many patients who have PH with cardiac involvement.1 RVF not only decreases RV cardiac output but also reduces left ventricular (LV) diastolic filling due to the RV dilates and the septum becomes flattened (or even convex), adversely affecting RV function.2 In addition, a severely dilated RV will cause RV ischemia and systemic congestion, thus further impairing the functions of vital organs (e.g. RV, liver, kidney, gastrointestinal tract) and negatively affecting patient outcomes. Medical management with Ca2+ channel blockers, endothelin receptor antagonists (ERA), phosphodiesterase (PDE) type 5 inhibitors/guanylate cyclase stimulators, and prostacyclin analogs/prostacyclin receptor agonists has been aggressively implemented to treat PH,3 but these drugs have shown limited ability to improve survival of patients with RV dysfunction because they mainly target pulmonary circulation. Recently, therapies that target RV dysfunction in PH patients have garnered great interest but achieved little success, primarily because the mechanism(s) underlying right heart failure, especially the transition from compensated RV to failing RV, is poorly understood.4 Nevertheless, continuous research efforts have improved our understanding of many changes at the cellular and molecular levels during development of RVF, as witnessed by a few recent excellent reviews on the subject.4–10 This review, however, will highlight current research on the molecular mechanism that underlies RV adaptation to pressure overload and the transition to maladaptive RVF. These recent studies have helped to improve our understanding of PH-induced RVF.
Methods
We searched MEDLINE through January 2019, using keywords such as right heart failure, right heart dysfunction, right heart hypertrophy, RVF, RV dysfunction, and RV hypertrophy (RVH). We then combined each key word with pulmonary hypertension, experimental, molecular mechanisms, and treatment to identify more relevant papers. We focused our review on original studies of presented topics. However, systematic reviews, society statements, and guidelines were also reviewed. Primarily, we reviewed original preclinical studies that used various models of PH, RVH, and RVF.
The right ventricle
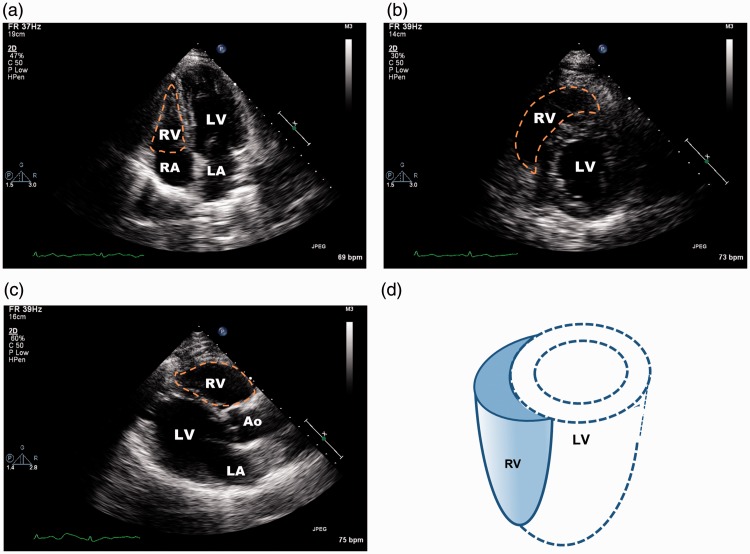
The unique anatomy of the RV's anatomy determines its responses to physiologic and pathophysiologic changes in afterload and interaction with the LV.11,12 The RV appears triangular when viewed from the apex on transthoracic echocardiography (Fig. 1). Under normal conditions, pulmonary arterial pressure (PAP) is low and the RV has low wall tension and low energy consumption, though it ejects the same amount of blood as the LV during the cardiac cycle. RV contractility decreases abruptly with an acute increase in PAP > 15 mmHg.2 The RV has a greater ability than the LV to adapt to chronic loading: the RV wall thickens to decrease wall tension and maintain (or increase) contractility. As a whole chamber, the RV is more compliant than the LV because it has a relatively smaller extracellular tissue network and much greater endothelial coverage. Thus, the RV usually dilates in response to increases in afterload (i.e. PAP). However, RV dilatation is usually limited because of increased RV wall thickness and mass and augmented contractility during adaptation.12 RVF is characterized by a severely dilated RV, decreased RV contractility, and elevated RV diastolic pressure. The dilated RV with increased pressure pushes the interventricular septum leftward, which flattens the septum and disrupts the normal interventricular relationship.2 The loss of the septal (contraction) contribution to RV stroke volume (SV) and the decreased LV performance caused by the leftward septal shift and limited diastolic filling further impair RV contractility.
Fig. 1.
Transthoracic echocardiographic images of right ventricle (RV). (a) Apical four-chamber view showing RV, left ventricle (LV), right atrium (RA), and left atrium (LA). The RV cavity appears triangular (orange dashed line). (b) Subcostal mid-papillary view showing circular LV and crescent-shaped RV. (c) Parasternal long-axis view showing RV, LV, and aortic root (Ao). (d) Three-dimensional drawing of the RV and its relationship to the LV.
At the cellular level, RV myocytes have unique features. In animal studies, RV myocytes exhibit less unloaded shortening and smaller intracellular Ca2+ transients than do LV myocytes.13 These findings are consistent with observations that force development is lower in cardiac muscles from the RV than in those from the LV.14 Moreover, RV myocytes seem to have a shorter action potential owing to larger K+ current density. However, the functions of all the major Ca2+ handling components do not differ between myocytes from the left and right ventricles,13 suggesting other underlying mechanism(s) for these differences. Metabolism of glucose and fatty acids, mitochondrial energy production processes, and volume fraction of mitochondria are similar in right and left ventricles.15
The so-called endocardial endothelial–myocyte coupling, which regulates a variety of cardiac functions such as growth, contraction, and rhythmicity,16 seems to be more prominent for myocytes in the RV. In the RV, extensive trabeculations can make up > 50% of the wall thickness. These trabeculae, together with projecting (into the RV cavity) microappendages or microvilli, augment the surface area of endothelial coverage in the RV by a factor of 100).16 Thus, nearly 25% of RV myocytes are in contact with endocardial endothelial cells. Contraction and force development of the myocytes in the RV are more profoundly modulated by a few well-established endothelial-derived signal transduction pathways activated by nitric oxide, endothelin, prostaglandins, angiotensin II, etc.16–19
RV hypertrophy
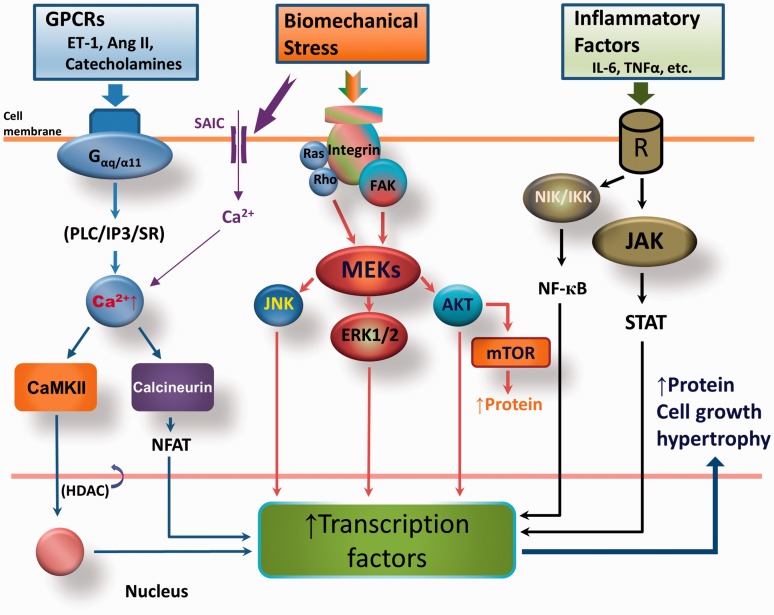
Elevations in PAP increase RV wall stress, which triggers a series of responses that eventually lead to RV remodeling.1 The increase in afterload sensed by cardiac cells generates biochemical or electrical signals that initiate structural and functional changes in the cells and tissues (i.e. mechanosensing). An initial, transient, redistribution of cytoplasmic microtubules parallel to sarcomeres is followed by onset of sarcomere growth.20 These microtubules continue to multiply and distribute along myofibrils during the onset of hypertrophy to keep the growing myofibrils in registry.21 Total protein in the myocyte increases because of increased protein synthesis/accumulation. Hypertrophy develops from the activation of several signaling pathways (Fig. 2).22–25 Stress (i.e. increased afterload) induces activation of G protein-coupled receptor (GPCR)-mediated CaMKII and calcineurin-Nuclear factor of activated T-cells (NFAT) signaling. Mechanical stress also activates integrin-dependent cytoskeletal protein focal adhesion kinase (FAK) and Ras/Rho–intracellular mitogen-activated protein kinase/extracellular regulated kinase (MEK) signaling pathways. Mechanical stretch causes activation of stretch-activated membrane ion channels, allowing Ca2+ to flow into the cell26 and promote hypertrophy development. Last, but not least, increased angiogenesis accompanies development of hypertrophy.27
Fig. 2.
Molecular mechanism by which RV hypertrophy (RVH) develops in response to pressure overload. G-protein-coupled receptors (GPCRs) are activated during stress, leading to activation of Gα-dependent calmodulin kinase II (CaMKII) and the calcineurin/ nuclear factor of activated T-cells (NFAT) pathway. Mechanical stress also directly stimulates the membrane integrin-associated mitogen-activated protein kinase kinase (MEK) pathway. In addition, inflammatory factors activate respective receptors to induce nuclear factor kappa-light-chain-enhancer of activated B cell (NF-кB) and Janus kinase (JAK)–signal transducer and activator of transcription protein (STAT) signaling. Activation of these three pathways increases transcription factors, thus promoting the cell proliferation, protein synthesis, and growth that lead to hypertrophy. Ang II, angiotensin II; ERK1/2, extracellular-signal regulated kinase; ET-1, endothelin-1; FAK, focal adhesion kinase; HDAC, histone deacetylase; IKK, IкB kinase; IL-6, interleukin 6; IP3, inositol trisphosphate; JNK, c-Jun N-terminal kinase; mTOR, mammalian target of rapamycin; NIK, NF-кB inducing kinase; PLC, phospholipase C; SAIC, stretch-activated ion channels; SR, sarcoplasmic reticulum.
RV adaptation and mechanisms
The RV responds to stress or higher afterload by increasing its mass/wall thickness. Changes include increases in myocyte size and hyperplasia of the non-muscular cells to mitigate wall stress (thus conserving energy expenditure) and maintain contractility (thus ensuring normal cardiac output). This hypertrophic remodeling with normal (or higher) contractility is called adaptive hypertrophy. When evaluated by cardiac magnetic resonance (CMR), the adapted RV has increased wall thickness but a normal chamber size.28 Several metabolic changes also occur during this adaptive phase to meet the energy demand for a normal cardiac output. This RV adaptive process is dependent on the degree, time course, and time of onset of pressure overload. Sometimes, RV adaptation can last for years without progressing to RVF, such as in patients with surgically repaired congenital heart disease29 and those with Eisenmenger syndrome,30 because contractility of the hypertrophied RV is maintained. Several cellular- and molecular-level mechanisms work together to maintain the RV in an adaptive state during overload.
Neurohormonal activation
Neurohormonal activation involves the activation of sympathetic and renin-angiotensin-aldosterone systems to maintain (or increase) contractility for normal cardiac output during pressure overload. A number of other agents also increase, such as endothelin-1, apelin, angiotensins, renin, transforming growth factor (TGF)-β1, insulin-like growth factor, and vascular endothelial growth factor (VEGF).1,31,32 Neurohormonal activation is important for hypertrophy development, but the pathways involved are not well-known at present. Neurohormonal signaling pathways (which involve GPCRs), mechanical stress-mediated, integrin-dependent signaling and inflammation-mediated pathways, are activated when the heart is stressed (Fig. 1). Because the RV is able to maintain contractility, hypertrophy from these signaling pathways is considered adaptive. Although it is beneficial, signaling for adaptive hypertrophy differs from that for the physiologic hypertrophy that occurs in response to exercise, growth, and pregnancy,33 and it becomes harmful if activated persistently. Another mediator in RV adaptation is the angiotensin II–TGF-β1 interaction, which promotes hypertrophy and cell growth.34 Vascular proliferation and angiogenesis mediated by endothelin-1 and VEGF35,36 are also involved.
Metabolic adaptation
When the RV is under stress, a shift occurs from fatty acid metabolism to glucose utilization for adenosine triphosphate (ATP) production, as further supported by downregulation of genes associated with fatty acid transport for mitochondrial oxidation.37 This adaptation offers more economical energy production, as glucose metabolism uses less ATP to generate energy. Oxidation of fatty acids to provide substrate for the Krebs cycle requires oxygen for β-oxidation, which also inhibits glucose oxidation (i.e. Randle cycle). As a result, more oxygen is required for the same amount of ATP production from fatty acid. Thus, inhibiting fatty acid metabolism, or promoting glucose oxidation, improves RV function/cardiac output.38,39 A shift from glucose oxidation to glycolysis has also been found in compensated RV myocardium.32 The increased glycolysis in hypertrophy results from activation of phosphofructokinase by several activators (ADP, AMP, Pi, and fructose-2,6-bisphosphate) and increased insulin-independent uptake of glucose.40 It remains debatable whether increased glycolysis is beneficial, however, because glycolysis is much less energy-efficient and causes accumulation of lactate.
Mitochondria also undergo functional remodeling. In hypertrophied RV, mitochondrial membrane potential is increased (i.e. hyperpolarization) owing to increased intracellular Ca2+ and activation of transcriptional factor NFAT.39 Paradoxically, this depression of mitochondrial function seems to be beneficial because reactive oxygen species (ROS) and mitochondrial-dependent apoptosis decrease.32 Despite the potential for decreased energy production, mitochondrial hyperpolarization does not seem to impact contractility at this adaptive stage, likely because energy production from glycolysis is increased.41 Increased mitochondrial fission and decreased mitochondrial fusion (i.e. increased mitochondrial fragmentation) have been shown in hypertrophied RV but are associated with decreased RV function.42 Thus, whether these changes in mitochondria are adaptive is controversial.
Hypoxia-inducible factor 1α (HIF-1α), which plays a major role in the pathogenesis of PH by promoting proliferation and contraction of vascular smooth muscles in the lung,43 is increased in hypertrophied RV as a result of mitochondrial remodeling (see above). Activation of HIF-1α is associated with angiogenesis and increased glucose uptake. The role of HIF-1α activation in preserving adapted RV is controversial because this pathway promotes aerobic glycolysis other than glucose oxidation by activating pyruvate dehydrogenase kinase (PDK), which uncouples glycolysis from glucose oxidation, leading to generation of lactate.44 However, the failed RV is associated with inhibited HIF-1α, suggesting a supporting role for HIF-1α in adapted RV during pressure overload.32
PDK activation (by several factors including HIF-1α during stress) has been postulated to play a key role in metabolic remodeling during adapted RVH.5,32 Inhibition of pyruvate dehydrogenase by PDK suppresses glucose oxidation via Krebs cycle and mitochondrial respiratory chain, thus diverting glucose oxidation to aerobic glycolysis. Reduced mitochondrial respiration leads to less ROS production and further activates HIF-1α, which stimulates angiogenesis and exerts a positive feedback stimulus to PDK. However, the claim that increased PDK activation is beneficial to the adapted RV is challenged by studies in which dichloroacetate, a small molecule inhibitor of PDK, reversed the RV dysfunction induced by PH.5 Whether activation of PDK is beneficial or harmful seems to be dependent on the time course of RV adaptation.
Maintained RV contractile reserve and underlying molecular and cellular mechanisms
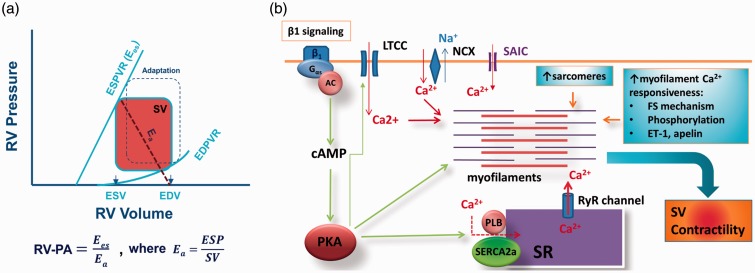
Clinically, maintained RV functional reserve (reflected by maintained systolic and diastolic reserve from the pressure–volume relationship, maintained force–frequency relationship, and maintained RV–pulmonary artery [PA] coupling) predicts better outcomes in patients with severe pulmonary arterial hypertension (PAH).45,46 Thus, preserved RV functional reserve can be viewed as the signature of RV adaptation/compensation. RV–PA coupling, the ratio of end-systolic elastance (Ees, a measure of cardiac contractility originally described by Suga et al.47), and effective arterial elastance (Ea, determined by dividing end-systolic pressure by SV) are optimally maintained (i.e. close to 1) because of maintained (or slightly increased) contractility. As a result, RV cardiac output (or SV) is guaranteed owing to preserved RV functional reserve and RV–PA coupling during adaptation (Fig. 3a). At a cellular level, several potential mechanisms may underlie the maintenance of (or increase in) RV SV and contractility (Fig. 3b).
Fig. 3.
(a) Schematic illustration of pressure-volume relationship of the right ventricle (RV) and RV–pulmonary artery (PA) coupling. An adapted RV pressure–volume relationship is also shown (defined by dotted square). Ea, arterial elastance; EDPVR, end-diastolic pressure-volume relationship; EDV, end-diastolic volume; ESD, end-systolic volume; ESPVR, end-systolic pressure-volume relationship, defined as contractility and known as Ees; SV, stroke volume. (b) Mechanism by which contractility is maintained in adapted or compensated RV during PH. An increase in afterload activates three fundamental mechanisms that increase contractility within the RV. These mechanisms remain active throughout the adaptive/compensated phase. See text for details. AC, adenylate cyclase; FS, Frank-Starling; ET-1, endothelin 1; LTCC, L-type calcium channel; PKA, protein kinase A; RyR, ryanodine receptor; SAIC, stretch-activated ion channels; SR, sarcoplasmic reticulum.
Preload and sarcomere length-dependent increase in cardiac output
Frank-Starling's mechanism describes the dependence of force development on sarcomere length.48 In the 1950s, it was demonstrated that acute increases in PAP led to increases in cardiac output as a result of increased end-diastolic RV volume.49 When afterload increases, SV decreases because of increased resistance at a given contractility. Reduced SV results in increased diastolic volume, leading to lengthening of sarcomeres and thus higher force development, as recently shown by Borgdoff et al.50 Although we believe that Frank-Starling's law is functioning, especially when the chamber is dilating, its operation could be limited in the hypertrophied/adapted RV because: (1) there is no clear evidence that the adapted RV myocardium has a longer sarcomere length to account for increased contractility at baseline. It has also been shown that after acute pulmonary arterial occlusion, cardiac output, and SV are maintained with normal (not dilated) RV end-diastolic volume,51 indicating additional mechanism(s) for the increased RV performance (i.e. cardiac contractility, rather than preload-dependent Frank-Starling mechanism) in response to increased pulmonary pressure; (2) the increase in extracellular matrix and fibrosis would limit the stretch of sarcomeres;52 and (3) possible changes in titin, a large molecule that contributes to the resting tension of cardiac muscle, would also offset this mechanism during hypertrophy (i.e. decreased phosphorylation) owing to increased stiffness.53
Increased Ca2+ availability and altered myofilament protein properties increase contractility
Intracellular Ca2+, the activator of contraction, can be increased in a number of ways in adapted RV. Stretch can activate stretch-activated membrane channels to allow Ca2+ influx into the cell.26 Additionally, activation of β-adrenergic receptors increases intracellular Ca2+ via G-protein-dependent pathways, leading to activation of protein kinase A.54 This pathway represents a major mechanism by which intracellular Ca2+ is increased, especially during early adaptation. Increased release of endothelin-1 as a result of angiotensin receptor activation by angiotensin II stimulates sarcolemmal Na+/H+ exchange, leading to elevation of intracellular Na+.55 Increased intracellular Na+ stimulates sarcolemmal Na+/Ca2+ exchange in the reversed mode, resulting in intracellular Ca2+ increases.56 Other potential mechanisms for the increased intracellular Ca2+ include nitrosylation of sarcoplasmic reticulum Ca2+ release channel and activation of protein kinase C. A number of active vascular agents such as endothelin-1, apelin, and angiotensin can also increase intracellular Ca2+ by stimulating Ca2+ release from the sarcoplasmic reticulum.57,58
Myosin isoform switching is a key feature of the adapted RV. The switch from α-myosin heavy chain (MHC) to β-MHC has been considered an adaptive effort to maintain contraction economy because cross-bridges formed by β-MHC generate relatively more force while using less ATP.59 Nevertheless, β-MHC is less powerful overall.59 Thus, whether this switch leads to increased contraction or contractility is questionable. In addition, in the adult heart, most myosin (>70–90%) is already β-MHC. Recently, Hanft et al.60 showed that there was no change in β-MHC in compensated hypertrophy when contractility was increased. These findings suggest that the β-MHC switch is not responsible for increased contractility in the adapted RV.
Sympathetic activation upon pressure overload maintains contractility and cardiac output by activating β-adrenergic receptor-PKA signaling, which can lead to phosphorylation of contractile proteins.61 Phosphorylation of myosin binding protein C is particularly influential in increasing contraction.62 Recently, myosin binding protein C was shown to be phosphorylated in hypertrophic adapted heart.60 Phosphorylation of regulatory myosin light chain (MLC) 1 can also increase force development.63 In compensated, hypertrophied, and hypercontractile regions of the LV after myocardial infarction, phosphorylation of regulatory MLC was found to be increased by 50%.64 Regulatory MLC is phosphorylated by myosin light chain kinase, which is required for adaptation to pressure overload.65 Phosphorylation of myosin binding protein C and regulatory MLC increase force development by altering the behavior of cross-bridges.63 Other active agents such as endothelin-1 and apelin, which are increased in adapted RV, also increase contractility by sensitizing the myofilament to Ca2+.58,66
RV diastolic function in RV adaptation
Conceivably, RV diastolic stiffness would increase in adapted RV owing to hypertrophy-induced increases in RV wall thickness. However, in animal models of PAH, RVH and maintained function were not associated with increased end-diastolic pressure during adaptation.32,67 Another animal study showed that end-diastolic elastance (Eed) was not increased in adapted RV either.50 In patients with PAH whose RV was well adapted, diastolic stiffness (i.e. Eed) was modestly increased but was normalized when corrected for RVH.68 Thus, it appears that normal or near normal diastolic function is another feature of adapted RV.
As discussed above, activation β-adrenergic receptor-PKA signaling in response to pressure overload leads to phosphorylation of a number of proteins, including SERCA2a and troponin I. Phosphorylated SERCA2a prevents cytosolic Ca2+ from rising during diastole by removing it into the sarcoplasmic reticulum. Moreover, phosphorylated troponin I desensitizes the myofilament to Ca2+, thus minimizing Ca2+-induced diastolic contraction (if any). Both mechanisms also accelerate relaxation. cGMP-protein kinase G (PKG)-maintained phosphorylation of the sarcomere protein titin keeps the sarcomeres in relaxed state during diastole.69 It is likely that these intracellular “relaxing” mechanisms could offset the increases in chamber stiffness in the adapted RV. Investigating the diastolic function of the adapted RV is apparently needed.
Right ventricular failure
RVF occurs when the above-mentioned stress-induced adaptive mechanisms (i.e. pressure/volume overload) fail in the presence of unrelenting stress. Clinically, RVF is characterized by a dilated RV (the shape of the RV changes from crescent to spherical with flattening and leftward shift of the ventricular septum), tricuspid regurgitation, and decreased contractility.
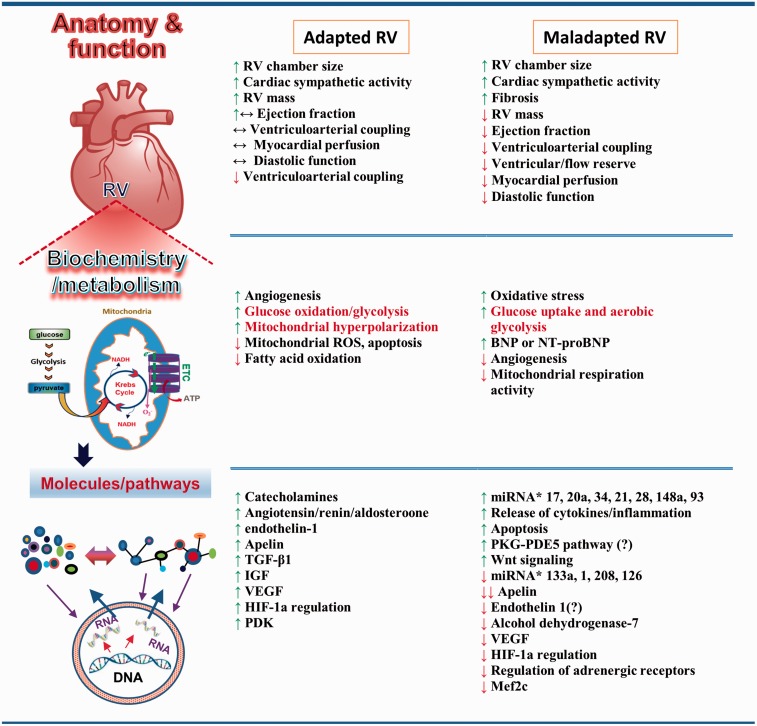
RVF has its own characteristics. Development of RVF is not purely mechanical-load-dependent. For example, patients with Eisenmenger syndrome experience increased RV afterload very early in life but have a better survival rate than patients with PH from other causes. This fact indicates that RVF also results from pressure-independent factors. The failing RV undergoes a variety of changes that differ from those of the adapted RV (Figure 4).1,5,7,8,25,70 Some of these changes may have already begun in the adaptive phase and some may be the “byproducts” of maladaptation, with little relation to RVF. The following sections focus on several studied mechanisms that underlie transition to RVF when the adaptive processes fail.
Fig. 4.
Features of RV adaptation and RVF. RV adaptation and RVF manifest at different levels of organization. At the organ level, RV adaptation is associated with RVH and maintained function. RVF presents as alterations in anatomy and function. At the cellular level, changes in metabolism and biochemistry occur in both adapted RV and RVF. Some metabolic mediators are found in both adapted and maladapted RV, and their roles in promoting each are controversial. At the molecular level, metabolites, molecules, proteins, and mediators of signaling pathways are altered or modified in adapted RV and RVF. New signaling pathways and mediators have been identified in RVF. BNP, B-type natriuretic peptide; ETC, electron transport chain; HIF-1α, hypoxia-inducible factor 1α; IGF, insulin-like growth factor; miRNA, microRNA; NT-proBNP, N-terminal pro b-type natriuretic peptide; PDE5, phosphodiesterase-5; PDK, pyruvate dehydrogenase kinase; PKG, protein kinase G; ROS, reactive oxygen species; TGF-β1, transforming growth factor beta 1; VEGF, vascular endothelial growth factor.6,73,94,98,124,125
RV ischemia
Under normal conditions, the RV receives blood during both systole and diastole, and the coronary flow is lower (than that of the LV at the same perfusion pressure) with low O2 extraction (50%). RV O2 supply/demand is sustained by autoregulation, which maintains blood flow to keep O2 extraction low. Increased pulmonary pressure will stimulate vasodilation and increase coronary blood flow in the RV.71 However, maladaptive RV is ischemic because of an imbalance between O2 demand and supply. This imbalance can be caused by several factors: (1) maladaptive, hypertrophied RV has a higher O2 demand and uses O2 inefficiently; (2) increased RV pressure reduces coronary perfusion pressure and limits coronary perfusion to diastole;72 and (3) the number of capillaries and small intramyocardial arterioles decreases when the RV is failing. Among these factors, impaired angiogenesis plays a key role in transition from a compensated to a decompensated state. Initially, RVH is accompanied by increased angiogenesis; however, as hypertrophy continues, RV angiogenesis stops.27 Studies in animals have also shown that decompensated RV has less coronary density than does compensated RV.70 The suppression of angiogenesis in RVH seems to relate to an increase in mitochondrial-derived ROS, which inhibit activation of HIF-1α. Decreased expression of microRNA (miR)-126 has also been shown to decrease RV angiogenesis and capillary density to promote RVF.73 Patients with systemic sclerosis are predisposed to RVF because they have endothelial injury and impaired angiogenesis, which causes RV ischemia.74
Metabolic derangement
The decreased utilization of glucose in hypertrophied RV is believed to mark a transition step between compensated and decompensated states. The decrease reflects inhibition of glucose oxidation through the Krebs cycle. Glycolysis, on the other hand, increases to meet the cell's need for ATP. Upregulation of PDK, as shown in patients with RVF,75 is believed to play a key role in this process. Upregulation of PDK inhibits PDE, uncouples glycolysis and glucose oxidation, and decreases cardiac contractility and cardiac output.5,44 Excessive glycolysis causes an increase in lactate production, which leads to acidosis, further impairing RV contractility. In animal studies, promoting glucose oxidation by inhibiting PDK restored RV function.44 Increased glycolysis also stimulated glucose uptake (as demonstrated by increased 18F-fludeoxy-d-glucose (18F-FDG) uptake in the RV of patients and mice with PH) and was inhibited by inhibiting PDK.44,76 However, it is controversial whether increased glycolysis alone will cause RVF, as most animal studies have shown increased aerobic glycolysis in adapted RV (i.e. no RVF).8
Coupled with decreased glucose oxidation and increased glycolysis, glutaminolysis, a pathway that promotes cell proliferation in cancer cells, was found to be augmented in maladaptive RV in both animals and humans with PAH.77 It is believed that glutaminolysis, like glycolysis, inhibits glucose oxidation, thus promoting RV dysfunction in PAH. It is, however, not clear whether increased glutaminolysis triggers transition from RV adaptation to RVF because it is induced by RV ischemia-activated cMyc-Max signaling. Nevertheless, inhibiting glutaminolysis improved RV function77 and also decreased pulmonary vascular stiffness,78 suggesting that increased glutaminolysis contributes to the development of RVF.
RVF is associated with alterations in fatty acid oxidation (FAO) and utilization. RV long chain fatty acid (LCFA) accumulation as a result of impaired FAO could lead to RVF in PH.79 This toxic effect of lipid accumulation inside RV myocytes was initially demonstrated in the RVs of bone morphogenetic protein receptor type 2 (BMPRT2)-mutant mice and patients with heritable PAH.80 Furthermore, the degree of RV lipid accumulation correlated inversely with RV function.81 In agreement with these studies, patients with PH also showed increased blood levels of LCFA and RV triglyceride, as well as ceramide accumulation.79 Although these studies provide strong evidence that abnormal lipid accumulation in myocytes has a role to play, the researchers did not examine RV function or myocyte contraction. Thus, data pertaining to the direct effect of lipid accumulation on myocardial contraction are lacking. Whether the lipotoxic effect, which likely impairs RV function to some extent, will eventually lead to RVF needs further investigation/confirmation.
The mechanism by which FAO is altered in RVF is not well understood. In both human and rat RVF, Gomez-Arroyo et al.82 found decreased expression of peroxisome proliferator-activated receptor-γ coactivator-1α (PGC1α), peroxisome proliferator-activated receptor-α, and estrogen-related receptor-α. Expression levels of multiple PGC1α target genes required for FAO, including acyl-CoA dehydrogenases, were similarly decreased (as were genes related to glucose oxidation).82 These findings suggest that FAO is inhibited at multiple levels during RVF. However, another study showed that inhibition of FAO with trietazidine increased glucose oxidation, RV ATP levels, cardiac output, and exercise tolerance.38 Thus, the role of FAO in RVF is still not well established. In addition, it is not entirely clear to what extent PGC1α-dependent metabolic alterations contribute to development of RVF and what drives downregulation of PGC1α.
Increased oxidative stress and decreased mitochondrial function
Animal models of RVH and failure exhibit increased oxidative stress as a result of stress-induced ROS production.83,84 However, in the RV, antioxidative defense mechanisms are not activated.84 This lack of antioxidant activity predisposes the RV to ROS damage and failure. Decreased expression of PGC1α, the master oxidative transcription factor regulator, also reduces cellular oxidative capacity. ROS have a wide range of negative effects on cardiac contraction and remodeling, from altering Ca2+ movements/handling to inducing arrhythmias, apoptosis, and necrosis.85 As expected, decreasing ROS production and oxidative stress improved RV function and decreased RVF in animal studies.86
Decreases in PGC1α, a key regulator of mitochondrial biogenesis, also leads to mitochondrial alterations during RVF.87 Additionally, a reduction in the expression of transcriptional factor A, mitochondria (TFAM) resulted in the inhibition of mitochondrial biogenesis, alterations in ultrastructure, and impaired respiratory function.82 Consistent with these effects, mitochondrial membrane potential is high in RVF.39 Because mitochondria are the major source of ROS,88 dysfunctional mitochondria further increase ROS and worsen oxidative stress. Thus, dysfunctional mitochondria not only reduce ATP production but also promote other deleterious processes (such as apoptosis and necrosis).
Downregulation of β1 adrenergic receptors
β1 adrenergic receptor density and adenylate cyclase catalytic subunit activity were found to be decreased in patients with PH who had RVF.89 In animals with RVF, downregulation of β1 and dopamine D1 receptors was associated with G protein-coupled receptor kinase-2 (GRK2)-mediated uncoupling of β-adrenergic receptor signaling.90 In LVF, β-adrenergic blockade improves survival by preventing sympathetic overstimulation and therefore downregulating β adrenergic receptors.91 By the same rationale, β-blockers have been investigated in both patients with RVF (caused by PH and congenital heart diseases) and animals with RVF (induced by pulmonary banding and PH). Although β blockade showed some benefits (improved RV function and survival) in experimental animals, no clear benefit was observed in patients because results among studies were contradictory.92 Consequently, β blockers are not recommended for routine management of patients with RVF. Thus, while it appears that downregulation of adrenergic and dopaminergic receptors may underlie the development of RVF, the trigger for the downregulation may not be simply limited to sympathetic overstimulation.
Inflammation, fibrosis, and apoptosis
The idea that inflammation plays a role in RVF development comes from the clinical observation that RVF is less common in patients with Eisenmenger syndrome or idiopathic PH than in those with scleroderma-associated PH. One study showed that the RVs of scleroderma patients harbored more inflammatory cells93 than those of patients with other types of PH. Inflammatory mediators such as cytokines (tumor necrosis factor [TNF]-α, interleukins) and toll-like receptors are suspected to contribute to RVF because of their known effect in left heart failure.94 Table 1 lists these mediators and their possible modes of action in RVF. Although these inflammatory responses have been studied in LVF, most have not been well studied in RVF.
Table 1.
| Mediators | Actions/observations/effects |
|---|---|
| Cytokines | |
| TNF-α | Hypertrophy, fibrosis, uncoupling of β-adrenergic signaling, increasing NO and peroxyinitrite, altering Ca2+ homeostasis, negative inotropic effect, decreasing miR-208 |
| Interleukin-6 | Immune responses, stimulating T- and B-cell differentiation, activating macrophages, mediating fibrosis and hypertrophy, diastolic dysfunction, ischemia/reperfusion protection (activating PI3K/Akt pathway and ↑iNOS), activating CaMKII-dependent activation of STATs |
| Interleukin-1 | Increasing intracellular ROS production, modifying L-type Ca2+ current, decreasing collagen synthesis |
| CCL5 and CXCL 16 | Inducing fibrosis |
| Toll-like receptors (TLRs) | |
| TLR1-TLR10 | Activating NF-κB, IRFs, and MAP kinases; promoting release of chemokines, cytokines, and interferons |
| TLR9 | Promoting mitochondrial DNA-dependent inflammatory response |
CaMKII, Ca2+-calmodulin protein kinase II; iNOS, inducible nitric oxide synthase; IRFs, interferon regulatory factors; MAP, mitogen-activated protein; NO, nitric oxide; PI3K, phosphatidylinositol-3-kinase; ROS, reactive oxygen species; STATs, signal transducers and activators of transcription.
Fibrosis has been reported in PH-induced RVH,70 but the degree of fibrosis does not correlate with the degree of RV dysfunction.50 Therefore, fibrosis may be a byproduct of RVH, not a trigger of RVF. Nevertheless, treatment with a ROS scavenger was shown to attenuate PH-induced RVF and reduce RV fibrosis and apoptosis.86 In patients with PAH, RVF is highlighted by significant RV diastolic dysfunction—a result of sarcomere stiffening and fibrosis.95
Apoptosis, a feature of LVF, is also found in hypertrophied RV tissue. Levels of the proapoptotic signaling factors Bax and caspase-3 were shown to increase in the pressure-overloaded rat RV.96 It is also known that many factors (ROS, β1-adrenoreceptor agonists, angiotensin II, and proinflammatory cytokines) induce apoptosis in response to pressure overload. However, whether apoptosis signals occur during transition from the compensated to the decompensated stage is not clear. A recent study showed that significant apoptosis and fibrosis resulted from activation of p53-dependent downregulation of Bcl-XL and GATA4 but that contractility was maintained (even augmented) in a rat model of PH-induced right heart hypertrophy.97 Thus, apoptosis contributes to cell loss during RVF, but does not initiate RVF.
MicroRNAs
Small, non-coding microRNAs (miRNAs) interfere with protein translation by binding to and degrading or inhibiting mRNA. miRNAs are differentially expressed during RVH, RVF, and transition to RVF.94 Notably, miRNAs miR-34 and miR-21 are upregulated and miRNAs miR-1, miR-133, and miR-208 are downregulated during transition from compensated to decompensated stages. In addition, loss of miR-208 is accompanied by decreased expression of cardiogenic transcription factor Mef2 (which regulates expression of genes that control cardiomyocyte differentiation, proliferation, morphogenesis, and contractility) and increased expression of TNF-α (a strong inflammatory mediator).98 The differential expression of these miRNAs causes loss of genes that promote cell growth, function, and angiogenesis.94 miR-34a, miR-28, miR-93, and miR-148a, which are expressed in non-cardiac cells, act via paracrine pathways to increase oxidative stress, reduce oxidative defense, decrease angiogenesis, and activate cell death pathways, thus promoting transition to RVF.6 However, these findings only establish an association between these miRNAs and the development of RVF.
Recently, Potus et al.73 showed that capillary density (as determined with CD31+ and von Willebrand-positive staining density) was decreased by downregulation of miR-126, the proangiogenic factor downstream of VEGF receptor-2 (VEGFR2) signaling in human decompensated RV. In monocrotaline-injected rats, RV capillary density and function were preserved after two weeks of treatment with mimic-126 (to increase miR-126 expression). Thus, downregulation of miR-126 promotes transition from compensated RVH to decompensated RVF by decreasing angiogenesis and capillary density.73
Diastolic RV dysfunction
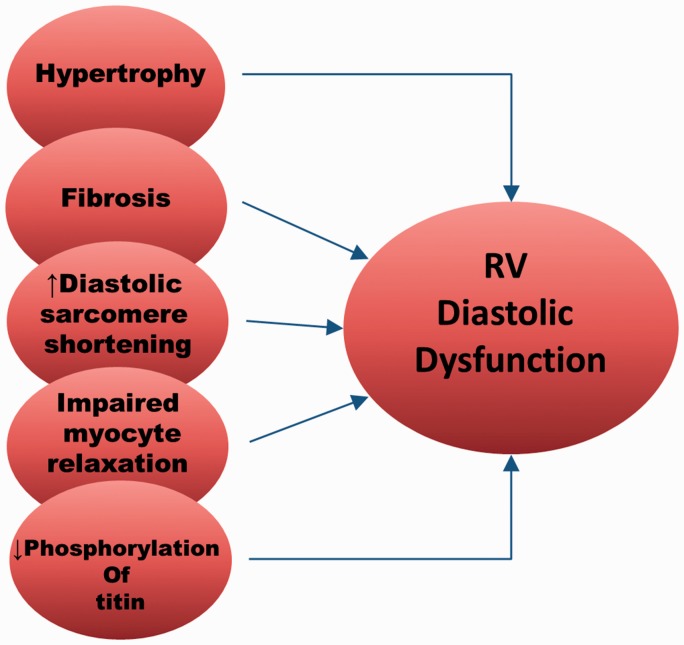
Most of the discussion so far has focused on systolic RVF. In fact, diastolic RV dysfunction (or failure) is not uncommon in patients with RVF.99 In one study, >60% of patients with PAH (with normal LV function) had RV diastolic dysfunction as determined by lower tricuspid E-A ratios, lower peak E-wave velocity, and prolonged RV isovolumic relaxation time in 2D echocardiography. Interestingly, over half of patients with LVF (normal pulmonary pressure) also had RV diastolic dysfunction. These observations suggest that PH is associated with (but not necessary for) development of diastolic RVF. In rats with monocrotaline-induced PH, diastolic RV dysfunction presented as a significantly steeper RV end-diastolic pressure volume relationship.100 Several potential factors are involved in RV diastolic dysfunction (Fig. 5). Hypertrophy and fibrosis have long been thought to make the RV stiffer and therefore cause RV diastolic dysfunction. However, hypertrophy itself does not seem to cause significant RV diastolic dysfunction in rats with PH of varied etiologies.50 Correlation between fibrosis and RV diastolic dysfunction is also difficult to show, as the amount of fibrosis and the time course of fibrosis development vary widely among experimental models. Recently, Reddy et al.101 followed disease progression in a murine model of RVF induced by a combination of pulmonary insufficiency and PH. They found that diastolic dysfunction began approximately one month after RV overloading and was accompanied by fibrosis, TGF-β signaling, and tissue expression of profibrotic miR-2. Interestingly, when systolic dysfunction began after several months, miR-2 expression decreased and RV fibrosis continued.
Fig. 5.
Factors contributing to diastolic dysfunction of the RV in PH.
Intrinsic changes in the myocytes themselves during RVF also cause diastolic dysfunction. The resting length-force relation is steeper and resting sarcomere shorter in individual isolated myocytes, suggesting impaired diastolic function.100 Furthermore, the increased resting sarcomere shortening is Ca2+-independent and believed to be due to decreased creatine kinase activity leading to an altered ratio of ADP:ATP near the sarcomere. PKG activity69 and decreased phosphorylation of the sarcomere protein titin are also associated with diastolic dysfunction in patients with PH.95 Titin is normally phosphorylated by PKG to maintain the sarcomere in a “relaxed” state and dephosphorylation leads to stiffer sarcomeres.69 If PKG is inhibited, diastolic dysfunction occurs. Targeting PKG-mediated pathways has been proposed as a treatment for diastolic dysfunction and failure.102
Imaging of the RV in PH
Molecular imaging
Molecular imaging (or metabolic imaging) is a noninvasive imaging technique that uses detectable tracers (usually radiotracers) of physiologic importance to visualize, characterize, and measure biologic processes in vivo. Thus, it enables cellular metabolism to be monitored “live” in patients with PAH. 18F-FDG positron emission tomography (PET) has shown that glucose uptake is increased in patients with PAH and RVF.103,104 Although these studies showed that glucose uptake increases in RV stress and correlates with RV ejection fraction (RVEF), it is not clear whether increased glucose uptake signals the transition from compensated RVH to decompensated RVF. Besides, 18F-FDG-PET does not distinguish glucose oxidation and glycolysis. Alterations in fatty acid metabolism have also been investigated with molecular imaging. An early study using 123I-labeled 15-(p-iodophenyl)-3-(R,S)-methylpentadecanoic acid (BMIPP) SPECT showed decreased uptake of fatty acids in RVF caused by PAH.105 However, tissue uptake of BMIPP is an energy-dependent process that sequesters fatty acid and decreased BMIPP uptake does not necessarily reflect decreased fatty acid utilization. Recently, both glucose and fatty acid metabolism were assessed using 18F-FDG-PET and 18F-fluoro-6-thioheptadecanoic acid (FTHA) PET imaging in the same patient with PAH.106 Both glucose and fatty acid uptake were positively related to pulmonary pressure and negatively to RVEF. Interestingly, in maladapted RV, increases in glucose uptake were higher than increases in fatty acid uptake, indicating that imaging of metabolic shifts between glucose and fatty acid could be used to assess transition from compensated to decompensated states.
Cardiac MRI (CMR)
CMR provides assessment of RV size and function with high resolution and has been used to follow morphologic and functional changes of RV in PH. RV volume/mass, RVEF/SV, and LV volume predict mortality during one-year follow-up in patients with PH.28 Importantly, progressive dilation of the RV, a decrease in RVEF, a decrease in SV, and a reduction in LV volume are strong predictors of mortality,28,107 indicating that MRI can be used to monitor the transition from compensated to failing RV in patients with PH. Sanz et al.108 combined CMR measurement of RV volume and RVEF with phase-contrast velocity-encoded CMR to assess RV–PA coupling non-invasively. They found that RV–PA coupling was maintained during the compensated phase and decreased gradually as RVF developed.
The tissue composition of RV can also be assessed quantitatively by measuring and comparing so-called native T1 and T2 relaxation time mapping in CMR.109 An increased T1 image usually suggests fibrosis whereas increased T2 suggests active inflammation, edema, and fat accumulation. A relatively unchanged T1 along with increasing T2 may indicate early remodeling to failure—but this needs to be substantiated with more clinical studies.
Coronary vasculature/flow imaging
Considerable experimental evidence suggests that RV ischemia as a result of decreased angiogenesis and capillary rarefaction leads to RVF. However, RV angiogenesis and coronary vasculature data from patients are lacking. Recently, a new technique called coronary ultrafast Doppler angiography (CUDA) was introduced to image micro-coronary anatomy and flow.110 CUDA allows researchers to map coronary vessels of approximately 100 μm diameter and quantify intramural coronary flows during both systole and diastole. It has been tested successfully in both animals (with myocardial infarction) and normal human subjects. It is expected that CUDA will be used to image RV microvasculature and coronary flow in compensated and decompensated stages of PH.
Treatment of RVF
Because RVF develops as a result of PH, lowering pulmonary pressure is the first necessary step in RVF management. Patients with PH are conventionally managed by targeting the pulmonary vasculature in an effort to decrease RV stress and reverse the pathologic remodeling. Drug therapy for PH includes use of Ca2+ channel blockers, ERAs, PDE5 inhibitors/guanylate cyclase stimulators, and prostacyclin analogs/prostacyclin receptor agonists.3 Because these drugs target mainly pulmonary circulation, it is conceivable that they would have limited ability to treat patients with RVF. However, protecting the RV in PH patients and aggressively treating RVF when it occurs are paramount to improving outcomes in these patients. Recently, interest has been growing in therapies that directly target the RV because RVF directly impacts patient survival.1 At present, most therapies aimed at protecting the RV are still in experimental and preclinical phases. Table 2 shows the current and emerging therapies for RVF. The following discussion highlights some controversial and emerging medical therapies.
Table 2.
| Treatment modality | Actions/recommendations | Side effects |
|---|---|---|
| Acute RVF | ||
| Volume management | ||
| Diuretics | Action: Decreases body volume overload by promoting urine output Rec: Early aggressive high-dose strategy Combination of diuretics, use in hypotensive volume-overloaded patients | Diuretic resistance, metabolic alkalosis, hypokalemia |
| Veno-venous hemofiltration | Action: Intravascular volume removal Rec: Use in patients refractory to escalating diuretics | Hemoconcentration |
| Vasoactive therapies | ||
| Vasodilators | Action: Relaxes vascular tone, decreases PVR and SVR, relieves renal congestion Rec: Use in hypoxia and acidosis, cardiorenal disease, inhaled form use | Systemic hypotension, LVF (especially in patients with chronic LVF) |
| Inotropic agents | Action: Increases cardiac output Rec: Decreased organ perfusion | Cardiac ischemia, hypotension, arrhythmias |
| Mechanical circulatory support | ||
| IABP | Action: Augments diastolic pressure Rec: RVF refractory to drug therapy | Does not directly support RV function |
| VA-ECMO | Action: Provides cardiac output and oxygenated blood Rec: RVF in spite of maximal therapy | Bleeding, thromboembolism, infection |
| RVAD (Impella RP©, TH-RVAD©, RotaFlow©, CentriMag©, PediMag© | Action: Provides cardiac output Rec: RVF refractory to maximal medical therapy, BTT, BTR, hours to days support | Bleeding, hemolysis, thromboembolism (some devices), infection, high operative mortality |
| Chronic RVF | ||
| Diuretics and sodium restriction | Action: Fluid control Rec: Use in combination with inhibitors of neurohormonal activation | Renal dysfunction |
| RAAS inhibitors, β-blockers, hydralazine | Action: Decreases sodium retention, antihypertensive, decreases cardiac oxygen consumption | Hypotension, heart failure, renal dysfunction |
| Pulmonary vasodilators | Action: Decreases pulmonary vascular resistance Rec: Group 1 PH patients | |
| PDE5 inhibitors | Hypotension, headache | |
| ET-1 antagonists | Decreases contractility, increases liver enzymes | |
| Mechanical circulatory support | ||
| RVAD (Jarvik 2000©, HVAD©, Berlin Heart EXCOR©) | Action: Provides cardiac output Rec: BTT, BTR, long-term support | Thromboembolism, bleeding, high operative mortality |
| Emerging therapies | (Not fully tested clinically) | |
| Medical therapies | ||
| HDAC inhibitors, miRNA inhibitors, NFκ-B inhibitors | Action: Inhibits cell growth | Not tested clinically |
| Dicholoroacetate | Action: Inhibits glycolysis | Not tested clinically |
| Trimetazidine | Action: Inhibits fatty acid metabolism | Neurotoxicity? |
| Ranolazine | Action: Promotes glucose oxidation | QT prolongation |
| Metformin | Action: Maintains glucose homeostasis | Lactic acidosis (rare) |
| Stem cells | Action: Cardiac cell/tissue regeneration | No benefits in preclinical studies |
| RV pacing | Action: Improves interventricular synchrony | |
| Mechanical circulatory support | ||
| HeartWare MVAD©, DexAide©, DeltaStream DP3©, PERKAT© | Action: Provides cardiac output | Thrombus formation, infection |
BTT, bridge to transplant; BTR, bridge to recovery; ET-1, endothelin-1; HDAC, histone deacetylase; IABP, intra-aortic balloon pump; LVF, left ventricular failure; NF-кB, nuclear factor kappa-light-chain-enhancer of activated B cell; PDE5, phosphodiesterase 5; PVR, pulmonary vascular resistance; RAAS, renin-angiotensin-aldosterone system; RVAD, right ventricular assisted device; RVF, right ventricular failure; SVR, systemic vascular resistance; VA-ECMO, venous-to-arterial extracorporeal membrane oxygenation.
β-adrenergic blockade
Persistent β-adrenergic activation in the stressed heart can lead to receptor desensitization and downregulation, pathologic remodeling, and eventually heart failure.111 β-blockers are widely used in the management of LHF because they reverse pathologic LV remodeling and improve survival. However, the failing RV may not tolerate β-blockers because they have a negative inotropic effect and lower blood pressure. As a result, RV contractility can be further decreased and ischemia worsened by low blood pressure. Despite these concerns, preclinical studies in animals have shown promising results. In a rat model of PH and RVF induced by hypoxia+Sugen-5416 or monocrotaline,112 both carvedilol and metoprolol increased exercise tolerance, improved RV function, and decreased RV dilation. It was not clear whether the beneficial effect occurred directly by protecting the RV or indirectly through an effect on the lungs. Although carvedilol did not affect pulmonary vasculature or pressure in the hypoxia model, it prevented lung remodeling in the monocrotaline model. In addition, metoprolol might have decreased PH. The effect of β-adrenergic blockade was further studied with bisoprolol, a cardio-selective β-blocker, in a rat model of monocrotaline-induced PH.113 Bisoprolol delayed RVF development, improved RV function, reduced RV fibrosis and inflammation, and interestingly, restored the β-adrenergic signaling pathway. In addition, bisoprolol did not affect pulmonary pressure. It should be mentioned that, despite these significant improvements, the delay to RVF was only five days, a rather modest effect. Nevertheless, most preclinical studies in experimental animals show beneficial effects of β-adrenergic blockade in PH-induced RVF.92
A meta-analysis of clinical studies showed that β-adrenergic blockade significantly improved systemic heart failure but did not improve RV function in CHD patients with RVF.114 Recently, in the PAHTCH (Pulmonary Arterial Hypertension Treatment with Carvedilol for Heart failure) double-blinded trial, 30 patients (out of 321 with PH) were enrolled and randomly divided into treatment and placebo groups.115 After six months, carvedilol lowered heart rate, accelerated heart rate recovery from exercise, and improved RV function. These improvements were associated with decreased RV glycolytic rate and recovery of β-adrenergic receptor density. Although this trial is encouraging, most of the clinical studies so far have failed to show a clear advantage of β-blockade in reducing mortality from PH with RV dysfunction, despite the fact that some studies have shown improved RV function.92,116
Phosphodiesterase-5 inhibition
Because PDE5 is expressed in the hypertrophied human RV,117 PDE5 inhibitors are gaining attention as a possible treatment for RV dysfunction. In a rat model of RV dysfunction induced by PH, acute inhibition of PDE5 increased RV contractility in isolated hearts and myocytes. This effect was not seen in normal RV tissue where PDE5 was not expressed.117 However, chronic inhibition of PDE5 had varied results in experimental animals, from no effect on hypertrophy and fibrosis to improvement of diastolic dysfunction and little effect on contractility.8 Although PDE5 inhibitors have been used to treat patients with PH, evidence for a direct benefit to the RV is lacking.
Endothelin receptor antagonists
Endothelin receptor type A has been shown to be upregulated in patients with PH.118,119 Activation of this receptor increases cardiac contractility,120 which can be an adaptive mechanism to maintain cardiac output. Thus, antagonizing endothelin receptor function may be deleterious. However, in a hypoxia-induced PH model, bosentan attenuated RVH and fibrosis without significantly decreasing RV function.121 At present, controversy remains regarding whether endothelin receptor antagonism is beneficial to the failing RV.
Emerging therapies that specifically target the RV
Targeting myocyte growth
Histone deacetylase (HDAC) inhibition. HDACs promote cell growth (and therefore hypertrophy development) by enhancing the accessibility of transcription factors. Both specific and non-specific inhibitors of HDACs have shown success in reducing hypertrophy in animal models.122,123 Because development of hypertrophy is considered an important adaptive process, inhibiting hypertrophy may be detrimental. Thus, the use of HDAC inhibitors needs more thorough investigation.
Targeting miRNAs and Notch/NFκ-B signaling. miRNAs regulate gene expression at the post-transcription level, and several are known to be up- or downregulated (see “MicroRNAs” section above). Animal studies have shown very promising results: inhibition of miR-17 or miR-20a, both of which are upregulated in RVF, reduced hypertrophy and improved RV function.124,125 The notch-NFκ-B pathway is also involved in hypertrophy development. Kumar et al.126 reported that inactivation of NFκ-B prevented RVH whereas activation of NFκ-B promoted RVH. However, preclinical studies targeting miRNAs and the Notch-NFκ-B pathway are very limited at present.
Targeting altered metabolism
Promoting glucose oxidation by using dichloroacetate to inhibit PDK was shown to prevent hypertrophy and improve RV contractility in animals with PH induced by monocrotaline injection or pulmonary artery banding.44,127 Partial inhibition of FAO also increased cardiac function and exercise capacity.44 Improving energy metabolism/efficiency by encouraging glucose oxidation and reducing fatty acid uptake and oxidation has recently been the focus of clinical investigations in patients with chronic stable ischemic heart disease.128 Most studies have shown beneficial results in these patients. It remains to be seen if these metabolic modulators will be beneficial to PH patients with RV dysfunction.
Metformin therapy for PAH. Metformin, a well-known drug for type 2 diabetes, has been investigated in PAH and RV dysfunction. In female rats exposed to Sugen-5416+hypoxia, metformin treatment (100 mg/kg daily) attenuated development of PAH and RVH by inhibiting aromatase and phosphorylation of AMPK.129 However, a recent study showed that metformin failed to inhibit PAH and RVH in male rats subjected to the same Sugen-5416+hypoxia PAH model,130 making metformin therapy very controversial. Interestingly, metformin was shown to normalize PH via SIRT-AMPK signaling in skeletal muscle in a model of PAH-associated heart failure with preserved ejection fraction (i.e. the PH-HFpEF model).131 Based on these studies, the main benefit of metformin seems to be improved glucose homeostasis and mitochondrial energy production in PAH associated with metabolic syndrome. Nevertheless, metformin seems to have little direct effect on RV contractility. It is thus doubtful that metformin will be effective in PH-induced systolic RVF.
Ranolazine therapy for PAH-dependent RV dysfunction. Ranolazine is a new anti-ischemic drug. It is used for stable angina because it inhibits the so-called late membrane Na+ current, which promotes Ca2+ entry into myocytes, thus increasing energy consumption.132 In preclinical animal studies, ranolazine improved PAH-induced RV function by inhibiting the ability of pyruvate dehydrogenase to promote glucose oxidation and depress FAO.38 In a pilot study, patients with WHO group I PAH improved functional class, exhibited better RV function, and had a smaller RV chamber size after three months of ranolazine treatment but showed no improvement in hemodynamics.133 Despite the limited success, interest in the continued testing of ranolazine for PAH and RV dysfunction is strong.134
Targeting oxidative stress
Given the strong evidence that increased oxidative stress is central to the development of RVF, attempts have been made to use antioxidant therapy to prevent and treat RVF. ROS scavenging with superoxide dismutase and catalase decreased RVH and fibrosis in rats with RVF secondary to both monocrotaline- and hypoxia-induced PH.86,135 Resveratrol, a potent antioxidant with anti-inflammatory properties, reversed pulmonary vascular remodeling and restored RV function.136 Melatonin, another strong antioxidant, reduced fibrosis and improved cardiac function in rats with RVF.137 Despite the overwhelming experimental evidence for a beneficial effect, antioxidative therapy has not been successfully tested in the clinical setting. One concern is that such therapy would remove ROS indiscriminately such that the vital regulating effect of ROS for adaptation may be eliminated.138
Future research
Over the past few years, a wealth of information has been obtained on RV function, especially transition from compensated state to failure, but challenges and significant knowledge gaps have been identified by a group of some 20 international experts in the field.4 One of the challenges is the lack of a “perfect” RVF model; therefore, existing models must be optimized. Moreover, we lack knowledge about the molecular mechanism that underlies the transition from adaptive to maladaptive RV remodeling, including, for example, the roles of angiogenesis, cell death, inflammation, fibrosis, metabolism, and contractile signaling. Within the general framework of research priorities put forward by the experts on behalf of the American Thoracic Society,4 we select the following issues to highlight.
Study models
Currently, over a dozen RVF models are available.139 These models have helped researchers to characterize adaptive and maladaptive remodeling of the RV in PH. Monocrotaline injection, chronic hypoxia, chronic hypoxia+Suugen (i.e. angioproliferative), and pulmonary banding are four commonly used animal models of RVF. Some models may start from physiologic adaptation and some may start pathologic remodeling right away, making it difficult to study the transition in these models. An animal RVF model with clear-cut adaptive-to-maladaptive transition characteristics would be ideal but is unlikely. Nevertheless, some possible alternatives may help us meet the challenge.
Combining knockout or overexpression of a particular gene with induced PH models may be useful. For example, both compensated and decompensated RVs develop hypertrophy in response to PH, and it is difficult to distinguish between physiologic hypertrophy (adaptive) and pathologic hypertrophy (maladaptive), and their contributions to RVF development. Using a transgenic model in which the heart's physiologic hypertrophy pathway is knocked down,140 we would be able to detect the timing of pathologic RVH development and its associated pathways. Likewise, using a transgenic mouse in which the pathologic hypertrophy pathway (e.g. the calcineurin-NFAT pathway) is suppressed,141 we would be able to evaluate the relative contribution of physiologic hypertrophy to the RVF transition. Signaling of other, non-hypertrophic, pathologic processes that contribute to the RVF transition in PH can also be studied using transgenic models. Use of transgenic mice with suppressed inflammation signaling has provided us with novel information about the crucial role of early inflammation in adverse cardiac remodeling during pressure overload (see below).142
Using human RV tissue samples to study the mechanism of RVF would represent the “gold standard.” The RV functional status is usually well documented clinically in patients with PH, and the biopsied tissue presents clear clinical pictures of compensated versus decompensated states. However, studying human RV biopsy samples from PH patients still has significant limitations. First, RV biopsies are not routinely performed for every PH patient because the procedure is invasive and caries high risk. Second, the quantity of tissue biopsied is very small, as it is meant for pathology diagnosis only.
The successful generation of human cardiomyocytes from human induced pluripotent stem cells (hiPSCs) makes it possible to decipher the mechanism of cardiac disease in human myocytes.143 In addition to being used as a single myocyte model system for studying disease mechanism, these young human myocytes can be used to regenerate adult human heart-like microtissue.144 Although tremendous efforts are needed to continuously improve these hiPSC-based human cardiac models and achieve a mature, adult-like functional phenotype,145 these single-cell and tissue-level models provide new platforms for studying the mechanisms of PH-associated RVF development, especially if they are studied in an in vitro microsystem,146 which can be modified to recapitulate the structural and mechanical microenvironment of the stressed RV.
Characterization of cardiac excitation-contraction (EC) coupling
Direct studies of RV myocyte EC coupling in RVH and RVF are lacking, and we do not know how Ca2+ metabolism is altered. For example, in compensated RVH, we do not know how contractility is maintained despite significant pre- and afterload changes at the cellular level. Little information is available regarding post-translational modification of myofilament proteins in compensated myocardium. Membrane channel electrophysiology also needs to be investigated in compensated RV myocytes. More importantly, we need to determine whether these EC coupling changes set the stage for transition to failure or delay the transition process. Much of our understanding about EC coupling in RVF is based on the wealth of information obtained from LVF studies. Few studies have focused on aspects of EC coupling (membrane electrophysiology, sarcoplasmic reticular Ca2+ regulation, and myofilament properties) by using myocytes or tissues from hearts with RVF. Serial studies on changes in EC coupling will offer crucial information regarding the exact role of EC coupling in transition from compensated state to failure.
Characterization of endothelial-myocyte (EM) coupling
Endothelial dysfunction decreases angiogenesis and promotes development of RVF. The cross-talk between endothelial cells and myocytes, or EM coupling, mediates angiogenesis via VEGF signaling to meet the nutrient demand of the hypertrophied myocytes.147 Prolonged hypertrophy downregulates HIF-1 by increasing p53, thus leading to reduced VEGF signaling and angiogenesis and promoting transition from compensated to decompensated hypertrophy.148 However, the mechanism for p53 accumulation, as well as other signaling involved in the EM coupling-mediated angiogenesis, needs further investigation.
Endothelial cells (in both endocardium and coronary vasculature) also modulate myocyte contraction and force development via EM coupling.16 We showed that endocardial endothelium modifies the cardiac force-frequency response via PKA and PKC signaling.149 The impact of dysfunctional cardiac endothelium on force generation in RVF has not been investigated, nor has the impact of EM on transition from compensated RVH to decompensated RVF in PH. Deciphering the underlying mechanisms for these changes in EM coupling during RVH and RVF will enable us to identify novel pathways and offer new targets for interventions to halt the transition to RVF.
Identifying new inflammatory pathways
Evidence of inflammation in the stressed RV has been documented in patients with PH and in animal models of PH.150 The role of inflammation has been better studied in LVF,151,152 but anti-inflammatory therapy for LVF has failed so far. Recently, Suetomi et al.142 showed that Ca2+/calmodulin-dependent protein kinase II δ (CaMKIIδ) activated NOD-like receptor pyrindomain-containing protein 3 (NLRP3) inflammasome to promote adverse cardiac remodeling. Their study deepened our understanding of the role that inflammation plays in RVF development: (1) early activation of the inflammatory process is essential for later pathologic remodeling; and (2) identification of novel inflammatory pathways is necessary for the development of new targeted therapies. Research in these areas of PH-associated RVH and RVF is currently lacking.
Summary
Physiologically, the RV behaves like the LV. However, the unique anatomy of the RV enables it to become much larger and more adaptive to chronic afterload increases (than to acute afterload increases). The RV adapts to increased afterload (i.e. PH) and develops hypertrophy in similar fashion to the LV, by activating fetal gene programs, activating several neurohormonal pathways, and undergoing metabolic and mitochondrial remodeling. RV adaptation maintains contractility through the Frank-Starling mechanism and by increasing activator Ca2+ and myofilament responsiveness to Ca2+. RV maladaptation manifests as RVF, which results from RV ischemia; inhibition of glucose oxidation; deranged metabolism of energy production; and increases in ROS, cell loss, inflammation, and fibrosis. Recently, miRNAs have been implicated in promoting RVF. RV diastolic dysfunction is an integral part of RVF and is likely caused by hypertrophy, fibrosis, and altered properties of the myofilaments. Non-invasive imaging offers the opportunity to visualize the adapted and maladapted RV and will potentially guide clinical therapies. Therapies to treat RVF include conventional therapies (which are similar to those for LVF) and experimental therapies designed to protect the RV. β-blockers have been investigated, and most preclinical and animal studies have shown promising results. However, clinical studies have not shown a clear benefit so far. Traditional agents that target PH (PDE5 inhibitors, endothelin-1 antagonists) are being tested, but their benefits to the failing RV need to be established. Therapies are emerging that specifically target the RV, such as inhibiting myocyte growth to reduce hypertrophy, promoting glucose oxidation to improve energy metabolism/efficiency, and reducing oxidative stress. New research is needed that will decipher the molecular mechanism by which a compensated state transitions to decompensated state, for example by alterations in EC and EM coupling and new inflammatory pathways. Knowledge in these areas will permit the development of more successful RVF management strategies.
Acknowledgments
The authors thank Ms. Claire Levine for her editing of the manuscript.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
National Institute of Health U52HL123827 (Johns). American Heart Association 17GRNT33670387 (Gao).
References
- 1.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013; 62: D22–33. [DOI] [PubMed] [Google Scholar]
- 2.Haddad F, Doyle R, Murphy DJ, et al. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008; 117: 1717–1731. [DOI] [PubMed] [Google Scholar]
- 3.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 4.Lahm T, Douglas IS, Archer SL, et al. Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. An official American Thoracic Society Research Statement. Am J Respir Crit Care Med 2018; 198: e15–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryan JJ, Archer SL. The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ Res 2014; 115: 176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy S, Bernstein D. Molecular mechanisms of right ventricular failure. Circulation 2015; 132: 1734–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan JJ, Huston J, Kutty S, et al. Right ventricular adaptation and failure in pulmonary arterial hypertension. Can J Cardiol 2015; 31: 391–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borgdorff MA, Dickinson MG, Berger RM, et al. Right ventricular failure due to chronic pressure load: What have we learned in animal models since the NIH working group statement?. Heart Fail Rev 2015; 20: 475–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstam MA, Kiernan MS, Bernstein D, et al. Evaluation and management of right-sided heart failure: a scientific statement from the American Heart Association. Circulation 2018; 137: e578–e622. [DOI] [PubMed] [Google Scholar]
- 10.Naeije R, Vanderpool R, Peacock A, et al. The right heart-pulmonary circulation unit: physiopathology. Heart Fail Clin 2018; 14: 237–245. [DOI] [PubMed] [Google Scholar]
- 11.Haddad F, Hunt SA, Rosenthal DN, et al. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008; 117: 1436–1448. [DOI] [PubMed] [Google Scholar]
- 12.Friedberg MK, Redington AN. Right versus left ventricular failure: differences, similarities, and interactions. Circulation 2014; 129: 1033–1044. [DOI] [PubMed] [Google Scholar]
- 13.Kondo RP, Dederko DA, Teutsch C, et al. Comparison of contraction and calcium handling between right and left ventricular myocytes from adult mouse heart: a role for repolarization waveform. J Physiol 2006; 571: 131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks WW, Bing OH, Blaustein AS, et al. Comparison of contractile state and myosin isozymes of rat right and left ventricular myocardium. J Mol Cell Cardiol 1987; 19: 433–440. [DOI] [PubMed] [Google Scholar]
- 15.Phillips D, Aponte AM, Covian R, et al. Homogenous protein programming in the mammalian left and right ventricle free walls. Physiol Genomics 2011; 43: 1198–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev 2003; 83: 59–115. [DOI] [PubMed] [Google Scholar]
- 17.Winegrad S. Endothelial cell regulation of contractility of the heart. Annu Rev Physiol 1997; 59: 505–525. [DOI] [PubMed] [Google Scholar]
- 18.Sys SU, De Keulenaer GW, Brutsaert DL. Physiopharmacological evaluation of myocardial performance: how to study modulation by cardiac endothelium and related humoral factors?. Cardiovasc Res 1998; 39: 136–147. [DOI] [PubMed] [Google Scholar]
- 19.Leucker TM, Jones SP. Endothelial dysfunction as a nexus for endothelial cell-cardiomyocyte miscommunication. Front Physiol 2014; 5: 328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samuel JL, Bertier B, Bugaisky L, et al. Different distributions of microtubules, desmin filaments and isomyosins during the onset of cardiac hypertrophy in the rat. Eur J Cell Biol 1984; 34: 300–306. [PubMed] [Google Scholar]
- 21.Watkins SC, Samuel JL, Marotte F, et al. Microtubules and desmin filaments during onset of heart hypertrophy in rat: a double immunoelectron microscope study. Circ Res 1987; 60: 327–336. [DOI] [PubMed] [Google Scholar]
- 22.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 2006; 7: 589–600. [DOI] [PubMed] [Google Scholar]
- 23.Umar S, Hessel M, Steendijk P, et al. Activation of signaling molecules and matrix metalloproteinases in right ventricular myocardium of rats with pulmonary hypertension. Pathol Res Pract 2007; 203: 863–872. [DOI] [PubMed] [Google Scholar]
- 24.Watts JA, Marchick MR, Kline JA. Right ventricular heart failure from pulmonary embolism: key distinctions from chronic pulmonary hypertension. J Card Fail 2010; 16: 250–259. [DOI] [PubMed] [Google Scholar]
- 25.Poels EM, da Costa Martins PA, van Empel VP. Adaptive capacity of the right ventricle: why does it fail?. Am J Physiol Heart Circ Physiol 2015; 308: H803–813. [DOI] [PubMed] [Google Scholar]
- 26.Hu H, Sachs F. Stretch-activated ion channels in the heart. J Mol Cell Cardiol 1997; 29: 1511–1523. [DOI] [PubMed] [Google Scholar]
- 27.Kolb TM, Peabody J, Baddoura P, et al. Right ventricular angiogenesis is an early adaptive response to chronic hypoxia-induced pulmonary hypertension. Microcirculation 2015; 22: 724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Wolferen SA, Marcus JT, Boonstra A, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 2007; 28: 1250–1257. [DOI] [PubMed] [Google Scholar]
- 29.Oosterhof T, Tulevski II, Vliegen HW, et al. Effects of volume and/or pressure overload secondary to congenital heart disease (tetralogy of fallot or pulmonary stenosis) on right ventricular function using cardiovascular magnetic resonance and B-type natriuretic peptide levels. Am J Cardiol 2006; 97: 1051–1055. [DOI] [PubMed] [Google Scholar]
- 30.Hopkins WE, Waggoner AD. Severe pulmonary hypertension without right ventricular failure: the unique hearts of patients with Eisenmenger syndrome. Am J Cardiol 2002; 89: 34–38. [DOI] [PubMed] [Google Scholar]
- 31.Park HK, Park SJ, Kim CS, et al. Enhanced gene expression of renin-angiotensin system, TGF-beta1, endothelin-1 and nitric oxide synthase in right-ventricular hypertrophy. Pharmacol Res 2001; 43: 265–273. [DOI] [PubMed] [Google Scholar]
- 32.Sutendra G, Dromparis P, Paulin R, et al. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med (Berl) 2013; 91: 1315–1327. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu I, Minamino T. Physiological and pathological cardiac hypertrophy. J Mol Cell Cardiol 2016; 97: 245–262. [DOI] [PubMed] [Google Scholar]
- 34.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res 2004; 63: 423–432. [DOI] [PubMed] [Google Scholar]
- 35.Michel RP, Langleben D, Dupuis J. The endothelin system in pulmonary hypertension. Can J Physiol Pharmacol 2003; 81: 542–554. [DOI] [PubMed] [Google Scholar]
- 36.Bry M, Kivela R, Leppanen VM, et al. Vascular endothelial growth factor-B in physiology and disease. Physiol Rev 2014; 94: 779–794. [DOI] [PubMed] [Google Scholar]
- 37.Buermans HP, Redout EM, Schiel AE, et al. Microarray analysis reveals pivotal divergent mRNA expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics 2005; 21: 314–323. [DOI] [PubMed] [Google Scholar]
- 38.Fang YH, Piao L, Hong Z, et al. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: exploiting Randle's cycle. J Mol Med (Berl) 2012; 90: 31–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagendran J, Gurtu V, Fu DZ, et al. A dynamic and chamber-specific mitochondrial remodeling in right ventricular hypertrophy can be therapeutically targeted. J Thorac Cardiovasc Surg 2008; 136: 168–78, 178.e1–3. [DOI] [PubMed]
- 40.Nascimben L, Ingwall JS, Lorell BH, et al. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension 2004; 44: 662–667. [DOI] [PubMed] [Google Scholar]
- 41.Piao L, Sidhu VK, Fang YH, et al. FOXO1-mediated upregulation of pyruvate dehydrogenase kinase-4 (PDK4) decreases glucose oxidation and impairs right ventricular function in pulmonary hypertension: therapeutic benefits of dichloroacetate. J Mol Med (Berl) 2013; 91: 333–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ryan JJ, Archer SL. Emerging concepts in the molecular basis of pulmonary arterial hypertension: part I: metabolic plasticity and mitochondrial dynamics in the pulmonary circulation and right ventricle in pulmonary arterial hypertension. Circulation 2015; 131: 1691–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res 2014; 115: 148–164. [DOI] [PubMed] [Google Scholar]
- 44.Piao L, Fang YH, Cadete VJ, et al. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med (Berl) 2010; 88: 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grunig E, Tiede H, Enyimayew EO, et al. Assessment and prognostic relevance of right ventricular contractile reserve in patients with severe pulmonary hypertension. Circulation 2013; 128: 2005–2015. [DOI] [PubMed] [Google Scholar]
- 46.Hsu S, Houston BA, Tampakakis E, et al. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation 2016; 133: 2413–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suga H. Ventricular energetics. Physiol Rev 1990; 70: 247–277. [DOI] [PubMed] [Google Scholar]
- 48.de Tombe PP, Mateja RD, Tachampa K, et al. Myofilament length dependent activation. J Mol Cell Cardiol 2010; 48: 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guyton AC, Lindsey AW, Gilluly JJ. The limits of right ventricular compensation following acute increase in pulmonary circulatory resistance. Circ Res 1954; 2: 326–332. [DOI] [PubMed] [Google Scholar]
- 50.Borgdorff MA, Bartelds B, Dickinson MG, et al. Distinct loading conditions reveal various patterns of right ventricular adaptation. Am J Physiol Heart Circ Physiol 2013; 305: H354–364. [DOI] [PubMed] [Google Scholar]
- 51.de Vroomen M, Cardozo RH, Steendijk P, et al. Improved contractile performance of right ventricle in response to increased RV afterload in newborn lamb. Am J Physiol Heart Circ Physiol 2000; 278: H100–105. [DOI] [PubMed] [Google Scholar]
- 52.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 2007; 87: 1285–1342. [DOI] [PubMed] [Google Scholar]
- 53.LeWinter MM, Granzier HL. Cardiac titin and heart disease. J Cardiovasc Pharmacol 2014; 63: 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capote LA, Mendez Perez R, Lymperopoulos A. GPCR signaling and cardiac function. Eur J Pharmacol 2015; 763: 143–148. [DOI] [PubMed] [Google Scholar]
- 55.Cingolani HE, Perez NG, Aiello EA, et al. Early signals after stretch leading to cardiac hypertrophy. Key role of NHE-1. Front Biosci 2008; 13: 7096–7114. [DOI] [PubMed] [Google Scholar]
- 56.Nagasaka T, Izumi M, Hori M, et al. Positive inotropic effect of endothelin-1 in the neonatal mouse right ventricle. Eur J Pharmacol 2003; 472: 197–204. [DOI] [PubMed] [Google Scholar]
- 57.Wang C, Du JF, Wu F, et al. Apelin decreases the SR Ca2 + content but enhances the amplitude of [Ca2+]i transient and contractions during twitches in isolated rat cardiac myocytes. Am J Physiol Heart Circ Physiol 2008; 294: H2540–2546. [DOI] [PubMed] [Google Scholar]
- 58.Noireaud J, Andriantsitohaina R. Recent insights in the paracrine modulation of cardiomyocyte contractility by cardiac endothelial cells. Biomed Res Int 2014; 2014: 923805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gupta MP. Factors controlling cardiac myosin-isoform shift during hypertrophy and heart failure. J Mol Cell Cardiol 2007; 43: 388–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hanft LM, Emter CA, McDonald KS. Cardiac myofibrillar contractile properties during the progression from hypertension to decompensated heart failure. Am J Physiol Heart Circ Physiol 2017; 313: H103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamdani N, de Waard M, Messer AE, et al. Myofilament dysfunction in cardiac disease from mice to men. J Muscle Res Cell Motil 2008; 29: 189–201. [DOI] [PubMed] [Google Scholar]
- 62.Moss RL, Fitzsimons DP, Ralphe JC. Cardiac MyBP-C regulates the rate and force of contraction in mammalian myocardium. Circ Res 2015; 116: 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Colson BA, Locher MR, Bekyarova T, et al. Differential roles of regulatory light chain and myosin binding protein-C phosphorylations in the modulation of cardiac force development. J Physiol 2010; 588: 981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toepfer CN, Sikkel MB, Caorsi V, et al. A post-MI power struggle: adaptations in cardiac power occur at the sarcomere level alongside MyBP-C and RLC phosphorylation. Am J Physiol Heart Circ Physiol 2016; 311: H465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Warren SA, Briggs LE, Zeng H, et al. Myosin light chain phosphorylation is critical for adaptation to cardiac stress. Circulation 2012; 126: 2575–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perjes A, Skoumal R, Tenhunen O, et al. Apelin increases cardiac contractility via protein kinase Cepsilon- and extracellular signal-regulated kinase-dependent mechanisms. PLoS One 2014; 9: e93473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Faber MJ, Dalinghaus M, Lankhuizen IM, et al. Time dependent changes in cytoplasmic proteins of the right ventricle during prolonged pressure overload. J Mol Cell Cardiol 2007; 43: 197–209. [DOI] [PubMed] [Google Scholar]
- 68.Trip P, Rain S, Handoko ML, et al. Clinical relevance of right ventricular diastolic stiffness in pulmonary hypertension. Eur Respir J 2015; 45: 1603–1312. [DOI] [PubMed] [Google Scholar]
- 69.Kruger M, Kotter S, Grutzner A, et al. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res 2009; 104: 87–94. [DOI] [PubMed] [Google Scholar]
- 70.Bogaard HJ, Natarajan R, Henderson SC, et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation 2009; 120: 1951–1960. [DOI] [PubMed] [Google Scholar]
- 71.Zong P, Tune JD, Downey HF. Mechanisms of oxygen demand/supply balance in the right ventricle. Exp Biol Med (Maywood) 2005; 230: 507–519. [DOI] [PubMed] [Google Scholar]
- 72.van Wolferen SA, Marcus JT, Westerhof N, et al. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J 2008; 29: 120–127. [DOI] [PubMed] [Google Scholar]
- 73.Potus F, Ruffenach G, Dahou A, et al. Downregulation of microRNA-126 contributes to the failing right ventricle in pulmonary arterial hypertension. Circulation 2015; 132: 932–943. [DOI] [PubMed] [Google Scholar]
- 74.Hassoun PM. The right ventricle in scleroderma (2013 Grover Conference Series). Pulm Circ 2015; 5: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rich S, Pogoriler J, Husain AN, et al. Long-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertension. Chest 2010; 138: 1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oikawa M, Kagaya Y, Otani H, et al. Increased [18F]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol 2005; 45: 1849–1855. [DOI] [PubMed] [Google Scholar]
- 77.Piao L, Fang YH, Parikh K, et al. Cardiac glutaminolysis: a maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. J Mol Med (Berl) 2013; 91: 1185–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bertero T, Oldham WM, Cottrill KA, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J Clin Invest 2016; 126: 3313–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brittain EL, Talati M, Fessel JP, et al. Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation 2016; 133: 1936–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hemnes AR, Brittain EL, Trammell AW, et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am J Respir Crit Care Med 2014; 189: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Talati MH, Brittain EL, Fessel JP, et al. Mechanisms of lipid accumulation in the bone morphogenetic protein receptor type 2 mutant right ventricle. Am J Respir Crit Care Med 2016; 194: 719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gomez-Arroyo J, Mizuno S, Szczepanek K, et al. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circ Heart Fail 2013; 6: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rungatscher A, Hallstrom S, Linardi D, et al. S-nitroso human serum albumin attenuates pulmonary hypertension, improves right ventricular-arterial coupling, and reduces oxidative stress in a chronic right ventricle volume overload model. J Heart Lung Transplant 2015; 34: 479–488. [DOI] [PubMed] [Google Scholar]
- 84.Wang X, Shults NV, Suzuki YJ. Oxidative profiling of the failing right heart in rats with pulmonary hypertension. PLoS One 2017; 12: e0176887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Munzel T, Camici GG, Maack C, et al. Impact of oxidative stress on the heart and vasculature: part 2 of a 3-part series. J Am Coll Cardiol 2017; 70: 212–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Redout EM, van der Toorn A, Zuidwijk MJ, et al. Antioxidant treatment attenuates pulmonary arterial hypertension-induced heart failure. Am J Physiol Heart Circ Physiol 2010; 298: H1038–1047. [DOI] [PubMed] [Google Scholar]
- 87.Wenz T. Regulation of mitochondrial biogenesis and PGC-1alpha under cellular stress. Mitochondrion 2013; 13: 134–142. [DOI] [PubMed] [Google Scholar]
- 88.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J 2009; 417: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bristow MR, Minobe W, Rasmussen R, et al. Beta-adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms. J Clin Invest 1992; 89: 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Piao L, Fang YH, Parikh KS, et al. GRK2-mediated inhibition of adrenergic and dopaminergic signaling in right ventricular hypertrophy: therapeutic implications in pulmonary hypertension. Circulation 2012; 126: 2859–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Triposkiadis F, Karayannis G, Giamouzis G, et al. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 2009; 54: 1747–1762. [DOI] [PubMed] [Google Scholar]
- 92.Andersen S, Andersen A, de Man FS, et al. Sympathetic nervous system activation and beta-adrenoceptor blockade in right heart failure. Eur J Heart Fail 2015; 17: 358–366. [DOI] [PubMed] [Google Scholar]
- 93.Overbeek MJ, Mouchaers KT, Niessen HM, et al. Characteristics of interstitial fibrosis and inflammatory cell infiltration in right ventricles of systemic sclerosis-associated pulmonary arterial hypertension. Int J Rheumatol 2010; 2010: 604615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Samson N, Paulin R. Epigenetics, inflammation and metabolism in right heart failure associated with pulmonary hypertension. Pulm Circ 2017; 7: 572–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rain S, Handoko ML, Trip P, et al. Right ventricular diastolic impairment in patients with pulmonary arterial hypertension. Circulation 2013; 128: 2016–2025. 1–10. [DOI] [PubMed] [Google Scholar]
- 96.Braun MU, Szalai P, Strasser RH, et al. Right ventricular hypertrophy and apoptosis after pulmonary artery banding: regulation of PKC isozymes. Cardiovasc Res 2003; 59: 658–667. [DOI] [PubMed] [Google Scholar]
- 97.Zungu-Edmondson M, Shults NV, Wong CM, et al. Modulators of right ventricular apoptosis and contractility in a rat model of pulmonary hypertension. Cardiovasc Res 2016; 110: 30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Paulin R, Sutendra G, Gurtu V, et al. A miR-208-Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circ Res 2014; 116: 56–69. [DOI] [PubMed] [Google Scholar]
- 99.Yun KL, Reichenspurner H, Schmoker J, et al. Heart transplantation complicated by a patent foramen ovale of the recipient atrial septum. Ann Thorac Surg 1996; 62: 897–899. [PubMed] [Google Scholar]
- 100.Fowler ED, Benoist D, Drinkhill MJ, et al. Decreased creatine kinase is linked to diastolic dysfunction in rats with right heart failure induced by pulmonary artery hypertension. J Mol Cell Cardiol 2015; 86: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reddy S, Hu DQ, Zhao M, et al. miR-21 is associated with fibrosis and right ventricular failure. JCI Insight 2017; 2: 91625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kovacs A, Alogna A, Post H, et al. Is enhancing cGMP-PKG signalling a promising therapeutic target for heart failure with preserved ejection fraction?. Neth Heart J 2016; 24: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Can MM, Kaymaz C, Tanboga IH, et al. Increased right ventricular glucose metabolism in patients with pulmonary arterial hypertension. Clin Nucl Med 2011; 36: 743–748. [DOI] [PubMed] [Google Scholar]
- 104.Mielniczuk LM, Birnie D, Ziadi MC, et al. Relation between right ventricular function and increased right ventricular [18F]fluorodeoxyglucose accumulation in patients with heart failure. Circ Cardiovasc Imaging 2011; 4: 59–66. [DOI] [PubMed] [Google Scholar]
- 105.Nagaya N, Goto Y, Satoh T, et al. Impaired regional fatty acid uptake and systolic dysfunction in hypertrophied right ventricle. J Nucl Med 1998; 39: 1676–1680. [PubMed] [Google Scholar]
- 106.Ohira H, deKemp R, Pena E, et al. Shifts in myocardial fatty acid and glucose metabolism in pulmonary arterial hypertension: a potential mechanism for a maladaptive right ventricular response. Eur Heart J Cardiovasc Imaging 2016; 17: 1424–1431. [DOI] [PubMed] [Google Scholar]
- 107.van de Veerdonk MC, Kind T, Marcus JT, et al. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol 2011; 58: 2511–2519. [DOI] [PubMed] [Google Scholar]
- 108.Sanz J, Garcia-Alvarez A, Fernandez-Friera L, et al. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart 2012; 98: 238–243. [DOI] [PubMed] [Google Scholar]
- 109.Kumar S, Wang G, Liu W, et al. Hypoxia-induced mitogenic factor promotes cardiac hypertrophy via calcium-dependent and hypoxia-inducible factor-1alpha mechanisms. Hypertension 2018; 72: 331–342. [DOI] [PubMed] [Google Scholar]
- 110.Maresca D, Correia M, Villemain O, et al. Noninvasive imaging of the coronary vasculature using ultrafast ultrasound. JACC Cardiovasc Imaging 2018; 11: 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res 2014; 114: 1815–1826. [DOI] [PubMed] [Google Scholar]
- 112.Bogaard HJ, Natarajan R, Mizuno S, et al. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med 2010; 182: 652–660. [DOI] [PubMed] [Google Scholar]
- 113.de Man FS, Handoko ML, van Ballegoij JJ, et al. Bisoprolol delays progression towards right heart failure in experimental pulmonary hypertension. Circ Heart Fail 2012; 5: 97–105. [DOI] [PubMed] [Google Scholar]
- 114.Cho MJ, Lim RK, Jung Kwak M, et al. Effects of beta-blockers for congestive heart failure in pediatric and congenital heart disease patients: a meta-analysis of published studies. Minerva Cardioangiol 2015; 63: 495–505. [PubMed] [Google Scholar]
- 115.Farha S, Saygin D, Park MM, et al. Pulmonary arterial hypertension treatment with carvedilol for heart failure: a randomized controlled trial. JCI Insight 2017; 2: 95240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ameri P, Bertero E, Meliota G, et al. Neurohormonal activation and pharmacological inhibition in pulmonary arterial hypertension and related right ventricular failure. Heart Fail Rev 2016; 21: 539–547. [DOI] [PubMed] [Google Scholar]
- 117.Nagendran J, Archer SL, Soliman D, et al. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation 2007; 116: 238–248. [DOI] [PubMed] [Google Scholar]
- 118.Nagendran J, Sutendra G, Paterson I, et al. Endothelin axis is upregulated in human and rat right ventricular hypertrophy. Circ Res 2013; 112: 347–354. [DOI] [PubMed] [Google Scholar]
- 119.Kuc RE, Carlebur M, Maguire JJ, et al. Modulation of endothelin receptors in the failing right ventricle of the heart and vasculature of the lung in human pulmonary arterial hypertension. Life Sci 2014; 118: 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Drawnel FM, Archer CR, Roderick HL. The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth. Br J Pharmacol 2013; 168: 296–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Choudhary G, Troncales F, Martin D, et al. Bosentan attenuates right ventricular hypertrophy and fibrosis in normobaric hypoxia model of pulmonary hypertension. J Heart Lung Transplant 2011; 30: 827–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cho YK, Eom GH, Kee HJ, et al. Sodium valproate, a histone deacetylase inhibitor, but not captopril, prevents right ventricular hypertrophy in rats. Circ J 2010; 74: 760–770. [DOI] [PubMed] [Google Scholar]
- 123.Cavasin MA, Demos-Davies K, Horn TR, et al. Selective class I histone deacetylase inhibition suppresses hypoxia-induced cardiopulmonary remodeling through an antiproliferative mechanism. Circ Res 2012; 110: 739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pullamsetti SS, Doebele C, Fischer A, et al. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med 2012; 185: 409–419. [DOI] [PubMed] [Google Scholar]
- 125.Brock M, Samillan VJ, Trenkmann M, et al. AntagomiR directed against miR-20a restores functional BMPR2 signalling and prevents vascular remodelling in hypoxia-induced pulmonary hypertension. Eur Heart J 2014; 35: 3203–3211. [DOI] [PubMed] [Google Scholar]
- 126.Kumar S, Wei C, Thomas CM, et al. Cardiac-specific genetic inhibition of nuclear factor-kappaB prevents right ventricular hypertrophy induced by monocrotaline. Am J Physiol Heart Circ Physiol 2012; 302: H1655–1666. [DOI] [PubMed] [Google Scholar]
- 127.Sun XQ, Zhang R, Zhang HD, et al. Reversal of right ventricular remodeling by dichloroacetate is related to inhibition of mitochondria-dependent apoptosis. Hypertens Res 2016; 39: 302–311. [DOI] [PubMed] [Google Scholar]
- 128.Guarini G, Huqi A, Morrone D, et al. Pharmacological agents targeting myocardial metabolism for the management of chronic stable angina: an update. Cardiovasc Drugs Ther 2016; 30: 379–391. [DOI] [PubMed] [Google Scholar]
- 129.Dean A, Nilsen M, Loughlin L, et al. Metformin reverses development of pulmonary hypertension via aromatase inhibition. Hypertension 2016; 68: 446–454. [DOI] [PubMed] [Google Scholar]
- 130.Goncharov DA, Goncharova EA, Tofovic SP, et al. Metformin therapy for pulmonary hypertension associated with heart failure with preserved ejection fraction versus pulmonary arterial hypertension. Am J Respir Crit Care Med 2018; 198: 681–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lai YC, Tabima DM, Dube JJ, et al. SIRT3-AMP-activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation 2016; 133: 717–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hasenfuss G, Maier LS. Mechanism of action of the new anti-ischemia drug ranolazine. Clin Res Cardiol 2008; 97: 222–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Khan SS, Cuttica MJ, Beussink-Nelson L, et al. Effects of ranolazine on exercise capacity, right ventricular indices, and hemodynamic characteristics in pulmonary arterial hypertension: a pilot study. Pulm Circ 2015; 5: 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Han Y, Forfia PR, Vaidya A, et al. Rationale and design of the ranolazine PH-RV study: a multicentred randomised and placebo-controlled study of ranolazine to improve RV function in patients with non-group 2 pulmonary hypertension. Open Heart 2018; 5: e000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ahmed Z, Tang WH. Pharmacologic strategies to target oxidative stress in heart failure. Curr Heart Fail Rep 2012; 9: 14–22. [DOI] [PubMed] [Google Scholar]
- 136.Paffett ML, Lucas SN, Campen MJ. Resveratrol reverses monocrotaline-induced pulmonary vascular and cardiac dysfunction: a potential role for atrogin-1 in smooth muscle. Vascul Pharmacol 2012; 56: 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maarman G, Blackhurst D, Thienemann F, et al. Melatonin as a preventive and curative therapy against pulmonary hypertension. J Pineal Res 2015; 59: 343–53. [DOI] [PubMed] [Google Scholar]
- 138.Wong CM, Bansal G, Pavlickova L, et al. Reactive oxygen species and antioxidants in pulmonary hypertension. Antioxid Redox Signal 2013; 18: 1789–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guihaire J, Bogaard HJ, Flecher E, et al. Experimental models of right heart failure: a window for translational research in pulmonary hypertension. Semin Respir Crit Care Med 2013; 34: 689–699. [DOI] [PubMed] [Google Scholar]
- 140.McMullen JR, Shioi T, Zhang L, et al. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci U S A 2003; 100: 12355–12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bueno OF, Wilkins BJ, Tymitz KM, et al. Impaired cardiac hypertrophic response in Calcineurin Abeta -deficient mice. Proc Natl Acad Sci U S A 2002; 99: 4586–45891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Suetomi T, Willeford A, Brand CS, et al. Inflammation and NLRP3 inflammasome activation initiated in response to pressure overload by Ca(2+)/calmodulin-dependent protein kinase ii delta signaling in cardiomyocytes are essential for adverse cardiac remodeling. Circulation 2018; 138: 2530–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Burridge PW, Matsa E, Shukla P, et al. Chemically defined generation of human cardiomyocytes. Nat Methods 2014; 11: 855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ronaldson-Bouchard K, Ma SP, Yeager K, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018; 556: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Berry JL, Zhu W, Tang YL, et al. Convergences of life sciences and engineering in understanding and treating heart failure. Circ Res 2019; 124: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McCain ML, Sheehy SP, Grosberg A, et al. Recapitulating maladaptive, multiscale remodeling of failing myocardium on a chip. Proc Natl Acad Sci U S A 2013; 110: 9770–9775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oka T, Akazawa H, Naito AT, et al. Angiogenesis and cardiac hypertrophy: maintenance of cardiac function and causative roles in heart failure. Circ Res 2014; 114: 565–571. [DOI] [PubMed] [Google Scholar]
- 148.Sano M, Izumi Y, Helenius K, et al. Menage-a-trois 1 is critical for the transcriptional function of PPARgamma coactivator 1. Cell Metab 2007; 5: 129–142. [DOI] [PubMed] [Google Scholar]
- 149.Shen X, Tan Z, Zhong X, et al. Endocardial endothelium is a key determinant of force-frequency relationship in rat ventricular myocardium. J Appl Physiol (1985) 2013; 115: 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun XQ, Abbate A, Bogaard HJ. Role of cardiac inflammation in right ventricular failure. Cardiovasc Res 2017; 113: 1441–1452. [DOI] [PubMed] [Google Scholar]
- 151.Cocco G, Jerie P, Amiet P, et al. Inflammation in Heart Failure: known knowns and unknown unknowns. Expert Opin Pharmacother 2017; 18: 1225–1233. [DOI] [PubMed] [Google Scholar]
- 152.Dick SA, Epelman S. Chronic heart failure and inflammation: what do we really know?. Circ Res 2016; 119: 159–176. [DOI] [PubMed] [Google Scholar]
- 153.Chopski SG, Murad NM, Fox CS, et al. Mechanical circulatory support of the right ventricle for adult and pediatric patients with heart failure. ASAIO J 2019; 65: 106–116. [DOI] [PubMed] [Google Scholar]
- 154.Rasmussen JT, Thenappan T, Benditt DG, et al. Is cardiac resynchronization therapy for right ventricular failure in pulmonary arterial hypertension of benefit?. Pulm Circ 2014; 4: 552–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lambert V, Gouadon E, Capderou A, et al. Right ventricular failure secondary to chronic overload in congenital heart diseases: benefits of cell therapy using human embryonic stem cell-derived cardiac progenitors. J Thorac Cardiovasc Surg 2015; 149: 708–715. [DOI] [PubMed] [Google Scholar]