Abstract
Frankincense, the oleogum resin from members of Boswellia, has been used as medicine and incense for thousands of years, and essential oils derived from frankincense are important articles of commerce today. A new source of frankincense resin, Boswellia dalzielii from West Africa has been presented as a new, alternative source of frankincense. In this work, the oleogum resins from 20 different Boswellia dalzielii trees growing in Burkina Faso, West Africa were collected. Hydrodistillation of the resins gave essential oils that were analyzed by GC-MS and GC-FID. The essential oils were dominated by α-pinene (21.0%–56.0%), followed by carvone (2.1%–5.4%) and α-copaene (1.8%–5.0%). Interestingly, there was one individual tree that, although rich in α-pinene (21.0%), also had a substantial concentration of myrcene (19.2%) and α-thujene (9.8%). In conclusion, the oleogum resin essential oil compositions of B. dalzielii, rich in α-pinene, are comparable in composition to other frankincense essential oils, including B. sacra, B. carteri, and B. frereana. Additionally, the differences in composition between samples from Burkina Faso and those from Nigeria are very slight. There is, however, a rare chemotype of B. dalzielii that is dominated by myrcene, found both in Burkina Faso as well as Nigeria.
Keywords: frankincense, olibanum, essential oil composition, α-pinene
1. Introduction
Frankincense is an aromatic oleoresin with a volatile fraction typically composed primarily of terpenoids and more rarely ethers or fatty esters/alcohols [1]. The oleoresin is produced by the 20 members of the genus Boswellia (Burseraceae: Sapindales), which are distributed across sub-Saharan Africa, Arabia, and the Indian subcontinent [1,2,3]. In nature, the oleoresins defend the trees against infection and pests such as boring beetles, while humans have used them for up to 5000 years for medicine and incense [4]. Today, the oleoresins of many species are traded internationally and distilled into essential oil for aromatherapy and perfumery. The oleoresin essential oils have been characterized for most of the Boswellia genus, with the exceptions of B. microphylla Chivo., B. ogadensis Vollesen, and B. globosa Thulin [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21].
Despite being one of the most wide-ranging species, the oleoresin essential oil of Boswellia dalzielii has only recently been examined [21]. Boswellia dalzielii (see Figure 1) inhabits wooded to open savannahs from Chad to Mali; the most significant populations appear to be in Burkina Faso, Nigeria, and Mali. The trees are 4–13 m tall, typically with papery or scaly bark, fragrant white flowers, serrated compound leaves, and aromatic resin [22]. Although resin harvesting has only recently increased, the bark is harvested extensively for its use in tinctures to treat malaria, toothaches, sores, and snakebites [23,24,25,26,27,28].
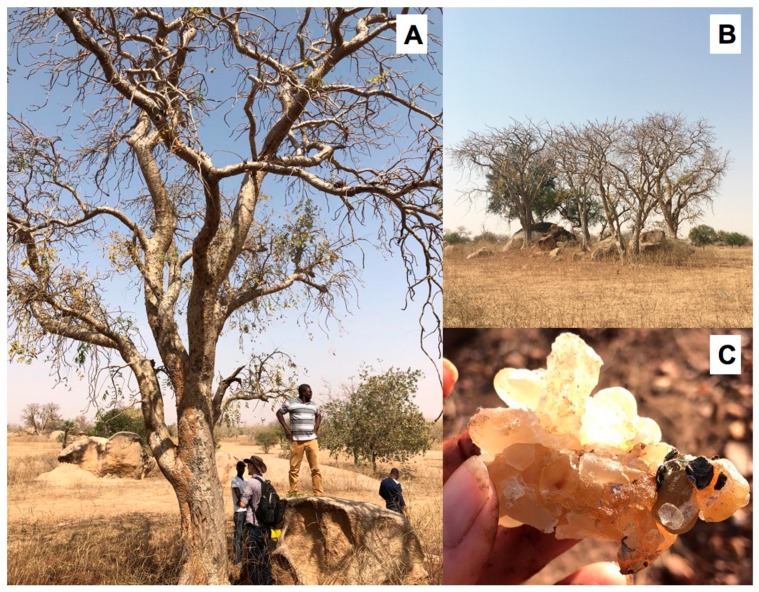
Figure 1.
Boswellia dalzielii tree in situ (A); Grove of B. dalzielii trees (B); Oleogum resin from self-exuded by a B. dalzielii tree (C).
Although there have been a number of studies on the compounds in the bark, there has been little work on the volatile compounds in this species. Two studies have examined the hydrodistilled leaf essential oils of B. dalzielii; Kohoude et al. found that the oil was dominated by δ-3-carene (27.7%) and α-pinene (15.2%) with smaller amounts of p-cymene (9.5%), β-phellandrene (8.5%), isolongifolene (6.2%), and myrcene (5.7%) [29], while Kubmarawa et al. found oils dominated by α-pinene (45.7%) and α-terpinene (11.5%) [30]. Recent work by DeCarlo et al. provided the first study on B. dalzielii oleoresin essential oil, examining single-tree oleoresin samples from northern Nigeria [21]. They found that the majority of essential oils were dominated by α-pinene (21.7%–76.6%), some with lower levels of α-thujene (2.0%–17.6%) and p-cymene (0.3%–15.6%); a second, much rarer chemotype was rich in myrcene (up to 35.2%), sometimes with a significant level of limonene (up to 32.9%). These samples were found to be rich in monterpenes but almost devoid of sesquiterpenes.
Along with Nigeria, Burkina Faso hosts one of the largest populations of B. dalzielii. The species is widespread across three of the four phytogeographic zones of the country (from 500 to 900 mm annual rainfall range). Populations are gregarious and colonize more often rocky hills and glacis. Mean density of trees in natural stands ranges between 7 and 10 trees per 1000 m2 according to phytogeographic zones in Burkina Faso [31]. The regeneration is very poor due to human disturbances (bushfire, pasture, agriculture) and climate pejoration (drought, rain fluctuations) [32,33].
The prior work on B. dalzielii oleoresin essential oil from Nigeria found multiple chemotypes as well as intrachemotypic compositional variation [21], and several other Boswellia species are known to display multiple chemotypes [1,5,11,19] (unpublished results from our laboratory). Given the extensive geographical range of B. dalzielii, it’s likely that additional chemical diversity is present beyond that captured in the study from Nigeria. Therefore, in this study, we examine the essential oils from oleoresins taken directly from individual trees in Burkina Faso to determine if additional chemical variation is present. Each oleoresin sample was hydrodistilled using the same apparatus (Clevenger) and analyzed by GC-MS and GC-FID by the same operators under the same conditions.
2. Results
Essential oils were obtained by hydrodistillation of the B. dalzielii oleogum resin samples in yields of 1.69%–17.0% (v/w) as pale-yellow essential oils. The chemical compositions of the essential oils are compiled in Table 1. Nineteen of the twenty samples were dominated by α-pinene (26.3%–56.0%), with minor levels of α-copaene (1.8%–5.0%), carvone (2.1%–5.0%), bornyl acetate (1.6%–3.5%), α-cubebene (1.2%–3.4%), myrcene (0.4%–5.5%), α-thujene (0.5%–9.2%), and γ-terpinene (0.8%–2.6%). One sample contained almost equal levels of myrcene (19.2%) and α-pinene (21.0%), with moderate α-thujene (9.8%) and α-copaene (3.8%).
Table 1.
Chemical compositions of Boswellia dalzielii oleogum resin essential oils from Burkina Faso.
| RI a | RI b | Compound | Re190227M | Re190125O | Re190125Q | Re190125R | Re190125S | Re190125T | Re190125U | Re190125V | Re190125W | Re190125X |
| 906 | 906 | Santolina triene c | 1.2 ± 0.0 d | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.8 ± 0.0 | 1.2 ± 0.0 | 1.5 ± 0.0 | 1.1 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.3 ± 0.0 |
| 918 | 919 | 5,5-Dimethyl-1-vinylbicyclo [2.1.1] hexane | 1.1 ± 0.0 | 1.1 ± 0.0 | 0.6 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.0 | 1.0 ± 0.0 | 1.4 ± 0.0 | 0.9 ± 0.0 | 0.7 ± 0.0 | 1.5 ± 0.0 |
| 920 | 921 | Tricyclene | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| 923 | 924 | α-Thujene | 1.4 ± 0.0 | 3.1 ± 0.0 | 9.2 ± 0.0 | 0.7 ± 0.0 | 3.0 ± 0.2 | 5.0 ± 0.1 | 0.7 ± 0.0 | 1.1 ± 0.0 | 0.5 ± 0.0 | 9.8 ± 0.2 |
| 932 | 932 | α-Pinene | 39.6 ± 1.5 | 56.0 ± 0.0 | 41.3 ± 0.1 | 34.9 ± 0.6 | 43.3 ± 2.3 | 26.3 ± 0.3 | 44.1 ± 0.6 | 43.5 ± 0.1 | 49.7 ± 0.0 | 21.0 ± 0.4 |
| 945 | 945 | α-Fenchene | tr e | tr | tr | --- | tr | tr | tr | tr | tr | tr |
| 947 | 946 | Camphene | 0.9 ± 0.0 | 1.1 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | 1.3 ± 0.1 | 1.1 ± 0.0 | 0.7 ± 0.0 | 1.0 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 |
| 951 | 953 | Thuja-2, 4(10)-diene | 0.8 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 1.0 ± 0.0 | 0.8 ± 0.0 | 1.0 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.4 ± 0.0 | 0.8 ± 0.0 |
| 953 | 954 | β-Fenchene | 0.2 ± 0.0 | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.5 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | tr |
| 970 | 969 | Sabinene | 1.3 ± 0.0 | 1.3 ± 0.0 | 1.5 ± 0.0 | 1.1 ± 0.0 | 1.2 ± 0.0 | 1.4 ± 0.0 | 0.9 ± 0.0 | 0.1 ± 0.0 | 0.8 ± 0.0 | 1.9 ± 0.0 |
| 975 | 974 | β-Pinene | 1.4 ± 0.0 | 2.0 ± 0.0 | 1.6 ± 0.0 | 0.8 ± 0.0 | 1.5 ± 0.1 | 1.4 ± 0.0 | 1.7 ± 0.0 | 1.3 ± 0.0 | 1.4 ± 0.0 | 1.1 ± 0.0 |
| 985 | 984 | trans-p-Mentha-2, 8-diene | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 986 | 988 | Myrcene | 1.0 ± 0.0 | 0.9 ± 0.0 | 0.5 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.1 | 1.1 ± 0.0 | 1.6 ± 0.1 | 1.0 ± 0.0 | 0.4 ± 0.0 | 19.2 ± 0.3 |
| 988 | 989 | 3, 3, 7-Trimethylcyclohepta-1, 3, 5-triene | 0.1 ± 0.0 | tr | tr | 0.2 ± 0.0 | --- | tr | --- | 0.1 ± 0.0 | tr | --- |
| 996 | 997 | (E)-2,6-Dimethyl-2, 6-octadiene | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 998 | 1001 | δ-2-Carene | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 999 | 995 | cis-p-Menth-8-ene | 0.7 ± 0.0 | 0.9 ± 0.0 | 0.6 ± 0.0 | 0.8 ± 0.0 | 0.6 ± 0.0 | 0.8 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 0.0 |
| 1002 | 1003 | p-Mentha-1(7), 8-diene | --- | --- | --- | --- | --- | --- | --- | --- | --- | tr |
| 1003 | 1002 | α-Phellandrene | tr | 0.1 ± 0.0 | tr | --- | tr | tr | tr | tr | 0.1 ± 0.0 | --- |
| 1004 | 1006 | 1,5,8-p-Menthatriene | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 | 0.7 ± 0.0 |
| 1006 | 1005 | o-Cresol methyl ether | tr | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | tr | 0.1 ± 0.0 | 0.1 ± 0.0 | --- | tr |
| 1006 | 1008 | δ-3-Carene | --- | 0.2 ± 0.0 | tr | 0.2 ± 0.0 | tr | tr | tr | tr | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 1015 | 1014 | α-Terpinene | 0.5 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.0 | 0.5 ± 0.0 |
| 1017 | 1022 | m-Cymene | 0.6 ± 0.0 | 0.9 ± 0.0 | 0.6 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.3 ± 0.0 | 0.5 ± 0.0 |
| 1021 | 1021 | p-Menth-1-ene | --- | --- | --- | --- | --- | --- | --- | --- | tr | tr |
| 1022 | 1024 | p-Cymene | 0.5 ± 0.0 | 1.2 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.0 | 0.3 ± 0.0 | 1.1 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 |
| 1024 | 1026 | 2-Acetyl-5-methylfuran | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.0 | tr | 0.1 ± 0.0 | tr | tr |
| 1027 | 1024 | Limonene | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | tr | 0.1 ± 0.0 | 0.2 ± 0.0 |
| 1028 | 1025 | β-Phellandrene | tr | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | tr | tr | tr | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.0 |
| 1029 | 1026 | 1,8-Cineole | 0.9 ± 0.0 | 1.8 ± 0.0 | 1.0 ± 0.0 | 0.6 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.6 ± 0.0 | 1.0 ± 0.0 | 0.9 ± 0.0 | 1.3 ± 0.0 |
| 1031 | 1032 | (Z)-β-Ocimene | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 1032 | 1039 | o-Cymene | 1.2 ± 0.0 | 2.0 ± 0.0 | 1.1 ± 0.0 | 1.5 ± 0.0 | 1.1 ± 0.1 | 1.4 ± 0.0 | 1.2 ± 0.0 | 1.1 ± 0.0 | 1.0 ± 0.0 | 1.5 ± 0.0 |
| 1043 | 1044 | (E)-β-Ocimene | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.0 | --- | 0.1 ± 0.0 | tr | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 |
| 1055 | 1054 | γ-Terpinene | 1.9 ± 0.0 | 0.8 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.1 | 1.9 ± 0.1 | 2.6 ± 0.0 | 1.8 ± 0.0 | 2.0 ± 0.0 | 1.5 ± 0.0 | 1.9 ± 0.0 |
| 1068 | 1065 | cis-Sabinene hydrate | tr | tr | tr | 0.1 | tr | 0.1 ± 0.0 | tr | tr | tr | tr |
| 1083 | 1086 | Terpinolene | 1.2 ± 0.0 | 0.5 ± 0.0 | 1.1 ± 0.0 | 1.5 ± 0.0 | 1.3 ± 0.1 | 1.3 ± 0.0 | 1.4 ± 0.0 | 1.5 ± 0.0 | 1.0 ± 0.0 | 1.3 ± 0.0 |
| 1088 | 1089 | p-Cymenene | 0.1 ± 0.0 | --- | --- | --- | --- | --- | 0.1 ± 0.0 | 0.1 ± 0.0 | --- | --- |
| 1088 | 1090 | 6,7-Epoxymyrcene | --- | --- | --- | --- | --- | --- | --- | --- | --- | tr |
| 1090 | 1095 | 6-Camphenone | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1094 | 1091 | Rosefuran | tr | --- | --- | --- | --- | --- | --- | tr | --- | --- |
| 1096 | 1102 | Perillene | tr | tr | --- | --- | --- | --- | 0.1 ± 0.0 | tr | --- | tr |
| 1097 | 1095 | Linalool | 1.3 ± 0.0 | 0.6 ± 0.0 | 1.0 ± 0.0 | 1.4 ± 0.0 | 1.2 ± 0.0 | 1.8 ± 0.0 | 1.3 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.0 |
| 1097 | 1099 | α-Pinene oxide | tr | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | --- |
| 1099 | 1098 | trans-Sabinene hydrate | --- | --- | tr | 0.1 ± 0.0 | --- | tr | --- | tr | tr | 0.1 ± 0.0 |
| 1103 | 1101 | cis-Thujone | 1.2 ± 0.0 | 0.6 ± 0.0 | 1.2 ± 0.0 | 1.4 ± 0.0 | 1.6 ± 0.0 | 1.8 ± 0.0 | 1.7 ± 0.0 | 1.3 ± 0.0 | 1.2 ± 0.0 | 1.4 ± 0.0 |
| 1110 | 1112 | (E)-2, 4-Dimethylhepta-2, 4-dienal | --- | --- | tr | --- | --- | tr | tr | --- | --- | --- |
| 1116 | 1112 | trans-Thujone | 0.6 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.7 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 |
| 1117 | 1119 | Myrcenol | --- | --- | --- | --- | --- | --- | 0.1 ± 0.0 | --- | --- | tr |
| 1117 | 1118 | exo-Fenchol | --- | --- | --- | --- | --- | --- | --- | 0.1 ± 0.0 | tr | --- |
| 1117 | 1119 | trans-p-Mentha-2, 8-dien-1-ol | tr | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1119 | 1124 | Chrysanthenone | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| 1122 | 1118 | cis-p-Menth-2-en-1-ol | --- | --- | --- | --- | --- | --- | --- | --- | --- | tr |
| 1125 | 1122 | α-Campholenal | 2.0 ± 0.0 | 1.1 ± 0.0 | 1.7 ± 0.0 | 2.3 ± 0.0 | 1.9 ± 0.0 | 2.8 ± 0.1 | 2.1 ± 0.0 | 2.2 ± 0.0 | 1.8 ± 0.0 | 1.8 ± 0.0 |
| 1130 | 1132 | cis-Limonene oxide | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1135 | 1137 | trans-Limonene oxide | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1137 | 1137 | trans-Sabinol | --- | --- | tr | --- | --- | 0.1 ± 0.0 | --- | --- | --- | --- |
| 1139 | 1135 | trans-Pinocarveol | 2.4 ± 0.0 | 1.3 ± 0.0 | 1.6 ± 0.0 | 2.7 ± 0.0 | 2.0 ± 0.1 | 2.8 ± 0.0 | 2.5 ± 0.0 | 2.3 ± 0.0 | 1.9 ± 0.0 | 1.3 ± 0.0 |
| 1139 | 1137 | cis-Verbenol | 0.6 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 1.2 ± 0.0 | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.2 ± 0.0 | tr |
| 1143 | 1140 | trans-Verbenol | 1.6 ± 0.0 | 1.4 ± 0.0 | 0.9 ± 0.0 | 2.9 ± 0.3 | 1.6 ± 0.0 | 2.2 ± 0.0 | 1.9 ± 0.0 | 2.8 ± 0.0 | 1.2 ± 0.0 | 0.6 ± 0.0 |
| 1145 | 1141 | Camphor | 0.2 ± 0.0 | tr | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 |
| 1148 | 1150 | α-Phellandren-8-ol | 0.7 ± 0.0 | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.0 | 0.7 ± 0.0 | 0.8 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.8 ± 0.0 | 0.5 ± 0.0 |
| 1158 | 1158 | trans-Pinocamphone | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.6 ± 0.0 | 0.8 ± 0.0 | 0.4 ± 0.0 | 0.8 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 |
| 1160 | 1160 | Pinocarvone | 0.5 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 |
| 1167 | 1168 | trans-Phellandrene epoxide | --- | --- | 0.1 ± 0.0 | --- | --- | 0.1 ± 0.0 | --- | tr | tr | tr |
| 1169 | 1166 | p-Mentha-1, 5-dien-8-ol | 1.5 ± 0.0 | 0.5 ± 0.0 | 1.2 ± 0.0 | 1.3 ± 0.0 | 0.9 ± 0.0 | 1.8 ± 0.0 | 1.1 ± 0.0 | 1.1 ± 0.038 | 1.3 ± 0.0 | 1.0 ± 0.0 |
| 1170 | 1165 | Borneol | --- | --- | --- | --- | --- | --- | --- | --- | --- | tr |
| 1174 | 1172 | cis-Pinocamphone | tr | tr | tr | tr | tr | 0.1 ± 0.0 | tr | 0.1 ± 0.0 | tr | --- |
| 1178 | 1174 | Terpinen-4-ol | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 |
| 1185 | 1179 | p-Cymen-8-ol | 0.8 ± 0.0 | 0.5 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.7 ± 0.0 | 1.4 ± 0.0 | 0.7 ± 0.0 | 0.8 ± 0.0 | 0.9 ± 0.0 | 0.7 ± 0.0 |
| 1192 | 1186 | α-Terpineol | 1.2 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 1.1 ± 0.0 | 0.9 ± 0.0 | 1.4 ± 0.0 | 0.9 ± 0.0 | 1.2 ± 0.0 | 1.0 ± 0.0 | 0.9 ± 0.0 |
| 1193 | 1195 | Myrtenal | 1.8 ± 0.0 | 1.1 ± 0.0 | 1.6 ± 0.0 | 2.1 ± 0.0 | 1.5 ± 0.0 | 1.8 ± 0.0 | 1.6 ± 0.0 | 1.6 ± 0.0 | 1.7 ± 0.0 | 1.1 ± 0.0 |
| 1205 | 1204 | Verbenone | 2.8 ± 0.0 | 1.2 ± 0.0 | 2.0 ± 0.0 | 3.0 ± 0.0 | 2.2 ± 0.0 | 2.9 ± 0.0 | 2.4 ± 0.0 | 2.7 ± 0.0 | 2.5 ± 0.0 | 2.1 ± 0.0 |
| 1216 | 1215 | trans-Carveol | 0.1 | tr | --- | --- | --- | tr | tr | 0.2 ± 0.0 | tr | --- |
| 1242 | 1239 | Carvone | 5.1 ± 0.1 | 2.1 ± 0.1 | 4.3 ± 0.0 | 4.4 ± 0.2 | 4.2 ± 0.1 | 5.4 ± 0.1 | 4.6 ± 0.1 | 4.7 ± 0.0 | 5.0 ± 0.0 | 4.3 ± 0.1 |
| 1246 | 1254 | Linalyl acetate | --- | --- | tr | --- | --- | tr | tr | tr | tr | --- |
| 1261 | 1265 | 3, 5-Dimethoxytoluene | --- | tr | tr | --- | --- | tr | tr | tr | --- | tr |
| 1281 | 1287 | Bornyl acetate | 3.4 ± 0.2 | 1.6 ± 0.0 | 2.9 ± 0.0 | 3.5 ± 0.3 | 2.6 ± 0.1 | 3.5 ± 0.1 | 2.6 ± 0.0 | 3.0 ± 0.0 | 2.9 ± 0.0 | 2.6 ± 0.0 |
| 1286 | 1289 | Thymol | --- | --- | tr | --- | --- | tr | --- | --- | --- | tr |
| 1294 | 1298 | Carvacrol | 2.2 ± 0.0 | 0.9 ± 0.0 | 1.6 ± 0.0 | 2.1 ± 0.0 | 1.7 ± 0.0 | 1.9 ± 0.0 | 1.7 ± 0.0 | 1.8 ± 0.0 | 1.8 ± 0.0 | 1.6 ± 0.0 |
| 1343 | 1346 | α-Terpinyl acetate | 2.1 ± 0.0 | 0.8 ± 0.0 | 1.5 ± 0.0 | 2.1 ± 0.0 | 1.6 ± 0.0 | 2.5 ± 0.0 | 1.7 ± 0.0 | 1.6 ± 0.0 | 1.9 ± 0.0 | 1.6 ± 0.0 |
| 1345 | 1345 | α-Cubebene | 3.4 ± 0.0 | 1.2 ± 0.0 | 2.7 ± 0.0 | 2.7 ± 0.1 | 2.6 ± 0.0 | 2.8 ± 0.0 | 2.4 ± 0.0 | 2.5 ± 0.0 | 2.7 ± 0.0 | 2.4 ± 0.0 |
| 1373 | 1374 | α-Copaene | 4.8 ± 0.4 | 1.8 ± 0.0 | 3.9 ± 0.0 | 4.7 ± 0.1 | 3.8 ± 0.1 | 5.0 ± 0.1 | 4.1 ± 0.1 | 3.6 ± 0.0 | 4.1 ± 0.0 | 3.8 ± 0.0 |
| 1409 | 1411 | cis-α-Bergamotene | --- | tr | tr | --- | --- | --- | tr | tr | tr | tr |
| 1416 | 1417 | β-Caryophyllene | --- | --- | --- | --- | --- | --- | tr | --- | --- | tr |
| 1429 | 1432 | trans-α-Bergamotene | --- | tr | tr | --- | --- | --- | tr | tr | 0.2 ± 0.0 | tr |
| 1441 | 1440 | (Z)-β-Farnesene | --- | --- | --- | --- | --- | --- | --- | --- | --- | tr |
| 1441 | 1449 | α-Himachalene | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1452 | 1452 | α-Humulene | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1486 | 1489 | β-Selinene | --- | --- | --- | --- | --- | --- | tr | --- | --- | tr |
| 1493 | 1498 | α-Selinene | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1579 | 1582 | Caryophyllene oxide | --- | --- | --- | --- | --- | --- | tr | tr | --- | tr |
| 1942 | 1944 | m-Camphorene | 0.1 ± 0.0 | 0.2 ± 0.0 | --- | --- | 0.1 ± 0.0 | --- | 0.1 ± 0.0 | --- | 0.1 ± 0.0 | 0.2 ± 0.0 |
| 1948 | 1947 | (3E)-Cembrene A | 0.1 ± 0.0 | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.7 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.0 |
| 1977 | 1977 | p-Camphorene | tr | tr | --- | --- | tr | --- | 0.2 ± 0.0 | --- | --- | tr |
| 1993 | 1992 | α-Pinacene | --- | tr | tr | --- | --- | --- | tr | tr | tr | tr |
| 2130 | 2138 | Cembrenol | 0.1 ± 0.0 | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.5 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| 2143 | 2144 | Incensole + Serratol | 0.4 ± 0.0 | 0.9 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.0 | 0.9 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.8 ± 0.0 |
| Monoterpene hydrocarbons | 56.6 | 76.4 | 65.1 | 52.5 | 62.4 | 49.6 | 60.5 | 59.9 | 61.5 | 65.9 | ||
| Oxygenated monoterpenoids | 33.9 | 17.8 | 27.0 | 36.7 | 29.6 | 39.8 | 31.2 | 33.1 | 30.5 | 26.1 | ||
| Sesquiterpenoids | 8.2 | 3.0 | 6.7 | 7.5 | 6.4 | 7.7 | 6.5 | 6.1 | 7.0 | 6.3 | ||
| Diterpenoids | 0.7 | 1.8 | 0.7 | 1.8 | 0.6 | 1.3 | 1.4 | 0.6 | 0.6 | 1.6 | ||
| Others | 0.1 | 0.1 | 0.4 | 0.5 | 0.3 | 0.5 | 0.1 | 0.2 | 0.0 | 0.0 | ||
| Total Identified | 99.6 | 99.1 | 99.8 | 99.0 | 99.3 | 99.0 | 99.8 | 99.9 | 99.5 | 99.9 | ||
| RI a | RI b | Compound | Re190227G | Re190227H | Re190227I | Re190227K | Re190227L | Re190227N | Re190227O | Re190227P | Re190416A | Re190416B |
| 906 | 906 | Santolina triene c | 1.2 ± 0.0 d | 1.4 ± 0.0 | 1.0 ± 0.0 | 1.2 ± 0.0 | 1.1 ± 0.0 | 1.4 ± 0.0 | 0.9 ± 0.0 | 1.3 ± 0.0 | 0.9 ± 0.0 | 0.9 ± 0.0 |
| 918 | 919 | 5, 5-Dimethyl-1-vinylbicyclo [2.1.1] hexane | 0.8 ± 0.0 | 0.9 ± 0.0 | 1.4 ± 0.0 | 1.3 ± 0.0 | 0.8 ± 0.0 | 1.4 ± 0.0 | 1.2 ± 0.0 | 0.8 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 |
| 920 | 921 | Tricyclene | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| 923 | 924 | α-Thujene | 1.4 ± 0.0 | 2.7 ± 0.0 | 0.8 ± 0.0 | 3.4 ± 0.0 | 0.9 ± 0.0 | 2.3 ± 0.0 | 0.5 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.1 ± 0.0 |
| 932 | 932 | α-Pinene | 51.0 ± 0.2 | 41.8 ± 0.3 | 44.9 ± 0.4 | 39.6 ± 0.3 | 38.2 ± 0.3 | 47.1 ± 0.5 | 40.3 ± 0.3 | 42.0 ± 0.3 | 55.2 ± 0.4 | 54.2 ± 0.2 |
| 945 | 945 | α-Fenchene | tr e | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 947 | 946 | Camphene | 0.8 ± 0.0 | 1.1 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.9 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.0 |
| 951 | 953 | Thuja-2, 4(10)-diene | 0.6 ± 0.0 | 0.9 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.4 ± 0.0 | 0.6 ± 0.0 | 0.8 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 |
| 953 | 954 | β-Fenchene | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.0 |
| 970 | 969 | Sabinene | 0.7 ± 0.0 | 1.4 ± 0.0 | 0.8 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 1.3 ± 0.0 | 1.1 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.0 | 1.1 ± 0.0 |
| 975 | 974 | β-Pinene | 1.6 ± 0.0 | 2.6 ± 0.0 | 1.8 ± 0.0 | 1.5 ± 0.0 | 1.2 ± 0.0 | 1.4 ± 0.0 | 1.4 ± 0.0 | 1.1 ± 0.0 | 2.0 ± 0.0 | 1.9 ± 0.0 |
| 985 | 984 | trans-p-Mentha-2, 8-diene | --- | --- | tr | --- | tr | tr | --- | --- | tr | tr |
| 986 | 988 | Myrcene | 0.6 ± 0.0 | 0.5 ± 0.0 | 1.9 ± 0.0 | 3.7 ± 0.0 | 0.8 ± 0.0 | 5.5 ± 0.1 | 2.7 ± 0.0 | 0.7 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 |
| 988 | 989 | 3, 3, 7-Trimethylcyclohepta-1, 3, 5-triene | --- | 0.1 ± 0.0 | tr | tr | --- | --- | 0.1 ± 0.0 | --- | tr | tr |
| 996 | 997 | (E)-2, 6-Dimethyl-2, 6-octadiene | --- | --- | tr | tr | --- | --- | --- | --- | 0.1 ± 0.0 | tr |
| 998 | 1001 | δ-2-Carene | --- | --- | tr | tr | --- | --- | --- | --- | tr | tr |
| 999 | 995 | cis-p-Menth-8-ene | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.0 | --- |
| 1002 | 1003 | p-Mentha-1(7), 8-diene | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1003 | 1002 | α-Phellandrene | tr | tr | 0.1 ± 0.0 | tr | tr | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 1004 | 1006 | 1,5,8-p-Menthatriene | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.0 | --- | 0.3 ± 0.0 |
| 1006 | 1005 | o-Cresol methyl ether | 0.1 ± 0.0 | tr | 0.1 ± 0.0 | tr | 0.1 ± 0.0 | tr | 0.3 ± 0.0 | 0.1 ± 0.0 | --- | 0.1 ± 0.0 |
| 1006 | 1008 | δ-3-Carene | tr | tr | 0.1 ± 0.0 | tr | tr | tr | tr | tr | tr | 0.1 ± 0.0 |
| 1015 | 1014 | α-Terpinene | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 |
| 1017 | 1022 | m-Cymene | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 0.0 |
| 1021 | 1021 | p-Menth-1-ene | --- | --- | --- | --- | --- | --- | 0.1 ± 0.0 | --- | 0.6 ± 0.0 | 0.6 ± 0.0 |
| 1022 | 1024 | p-Cymene | 0.5 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.4 ± 0.0 | 0.8 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 |
| 1024 | 1026 | 2-Acetyl-5-methylfuran | --- | 0.2 ± 0.0 | tr | 0.1 ± 0.0 | --- | tr | tr | --- | 0.1 ± 0.0 | tr |
| 1027 | 1024 | Limonene | tr | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 1028 | 1025 | β-Phellandrene | 0.4 ± 0.0 | --- | 0.1 ± 0.0 | tr | 0.1 ± 0.0 | 0.2 ± 0.0 | tr | 0.3 ± 0.0 | tr | tr |
| 1029 | 1026 | 1,8-Cineole | 1.3 ± 0.0 | 3. ± 0.0 | 2.1 ± 0.0 | 4.6 ± 0.1 | 5.1 ± 0.0 | 1.3 ± 0.0 | 1.2 ± 0.0 | 6.1 ± 0.1 | 1.4 ± 0.0 | 1.3 ± 0.0 |
| 1031 | 1032 | (Z)-β-Ocimene | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.1 ± 0.0 |
| 1032 | 1039 | o-Cymene | 1.3 ± 0.0 | 1.2 ± 0.0 | 1.4 ± 0.0 | 1.2 ± 0.0 | 1.3 ± 0.0 | 1.4 ± 0.0 | 1.4 ± 0.0 | 1.0 ± 0.0 | 0.7 ± 0.0 | 1.1 ± 0.0 |
| 1043 | 1044 | (E)-β-Ocimene | 0.1 ± 0.0 | tr | 0.6 ± 0.0 | 0.2 | 0.5 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.5 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| 1055 | 1054 | γ-Terpinene | 1.5 ± 0.0 | 1.8 ± 0.0 | 1.8 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 1.5 ± 0.0 | 2.4 ± 0.0 | 1.9 ± 0.0 | 1.5 ± 0.0 | 1.4 ± 0.0 |
| 1068 | 1065 | cis-Sabinene hydrate | --- | tr | tr | tr | --- | --- | tr | tr | tr | tr |
| 1083 | 1086 | Terpinolene | 1.1 ± 0.0 | 1.0 ± 0.0 | 1.3 ± 0.0 | 1.3 ± 0.0 | 1.2 ± 0.0 | 1.0 ± 0.0 | 1.5 ± 0.0 | 0.9 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.0 |
| 1088 | 1089 | p-Cymenene | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1088 | 1090 | 6,7-Epoxymyrcene | tr | --- | --- | --- | --- | --- | --- | tr | --- | --- |
| 1090 | 1095 | 6-Camphenone | --- | --- | --- | --- | --- | --- | --- | --- | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 1094 | 1091 | Rosefuran | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1096 | 1102 | Perillene | 0.1 ± 0.0 | --- | --- | --- | 0.1 ± 0.0 | 0.1 ± 0.0 | tr | tr | --- | --- |
| 1097 | 1095 | Linalool | 0.8 ± 0.0 | 1.3 ± 0.0 | 0.9 ± 0.0 | 1.1 ± 0.0 | 1.4 ± 0.0 | 0.9 ± 0.0 | 1.1 ± 0.0 | 1.2 ± 0.0 | tr | --- |
| 1097 | 1099 | α-Pinene oxide | --- | tr | tr | --- | 0.1 ± 0.0 | --- | tr | --- | --- | 0.1 ± 0.0 |
| 1099 | 1098 | trans-Sabinene hydrate | --- | tr | --- | --- | --- | --- | --- | tr | 0.6 ± 0.0 | 0.6 ± 0.0 |
| 1103 | 1101 | cis-Thujone | 1.3 ± 0.0 | 1.2 ± 0.0 | 1.3 ± 0.0 ± 0.0 | 1.3 ± 0.0 | 1.3 ± 0.0 | 1.2 ± 0.0 | 1.6 ± 0.0 | 0.9 ± 0.0 | --- | --- |
| 1110 | 1112 | (E)-2, 4-Dimethylhepta-2, 4-dienal | --- | tr | --- | --- | --- | --- | --- | --- | tr | --- |
| 1116 | 1112 | trans-Thujone | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.7 ± 0.0 | 0.5 ± 0.0 | 1.0 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 0.0 |
| 1117 | 1119 | Myrcenol | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1117 | 1118 | exo-Fenchol | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1117 | 1119 | trans-p-Mentha-2, 8-dien-1-ol | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1119 | 1124 | Chrysanthenone | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| 1122 | 1118 | cis-p-Menth-2-en-1-ol | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1125 | 1122 | α-Campholenal | 1.6 ± 0.0 | 1.7 ± 0.0 | 1.4 ± 0.0 | 1.6 ± 0.0 | 2.1 ± 0.0 | 1.4 ± 0.0 | 1.8 ± 0.0 | 1.7 ± 0.0 | 1.5 ± 0.0 | 1.5 ± 0.0 |
| 1130 | 1132 | cis-Limonene oxide | --- | 0.1 ± 0.0 | --- | tr | tr | tr | 0.1 ± 0.0 | --- | tr | tr |
| 1135 | 1137 | trans-Limonene oxide | --- | 0.2 ± 0.0 | tr | tr | tr | tr | 0.1 ± 0.0 | --- | --- | --- |
| 1137 | 1137 | trans-Sabinol | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1139 | 1135 | trans-Pinocarveol | 1.4 ± 0.0 | 2.1 ± 0.0 | 1.7 ± 0.0 | 1.7 ± 0.0 | 1.3 ± 0.0 | 1.5 ± 0.0 | 1.9 ± 0.0 | 2.5 ± 0.0 | 2.0 ± 0.0 | 1.6 ± 0.0 |
| 1139 | 1137 | cis-Verbenol | tr | 0.7 ± 0.0 | tr | 0.3 ± 0.0 | tr | tr | 0.6 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 |
| 1143 | 1140 | trans-Verbenol | 0.7 ± 0.0 | 1.3 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 | 1.7 ± 0.0 | 0.8 ± 0.0 | 0.7 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.1 | 1.3 ± 0.0 |
| 1145 | 1141 | Camphor | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| 1148 | 1150 | α-Phellandren-8-ol | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.5 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 |
| 1158 | 1158 | trans-Pinocamphone | 0.4 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 | 0.8 ± 0.0 | 0.6 ± 0.0 | 0.4 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 |
| 1160 | 1160 | Pinocarvone | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.0 | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| 1167 | 1168 | trans-Phellandrene epoxide | --- | tr | tr | tr | --- | --- | --- | --- | tr | tr |
| 1169 | 1166 | p-Mentha-1,5-dien-8-ol | 1.1 ± 0.0 | 1.3 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.0 | 1.4 ± 0.0 | 1.1 ± 0.0 | 1.3 ± 0.0 | 1.4 ± 0.0 | 1.0 ± 0.0 | 0.9 ± 0.0 |
| 1170 | 1165 | Borneol | --- | --- | --- | --- | --- | --- | --- | tr | --- | --- |
| 1174 | 1172 | cis-Pinocamphone | tr | tr | tr | tr | tr | tr | tr | tr | tr | tr |
| 1178 | 1174 | Terpinen-4-ol | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 |
| 1185 | 1179 | p-Cymen-8-ol | 0.5 ± 0.0 | 0.8 ± 0.0 | 0.7 ± 0.0 | 0.7 ± 0.0 | 0.9 ± 0.0 | 0.6 ± 0.0 | 1.0 ± 0.0 | 0.8 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 |
| 1192 | 1186 | α-Terpineol | 0.7 ± 0.0 | 0.8 ± 0.0 | 0.9 ± 0.0 | 1.1 ± 0.0 | 1.0 ± 0.0 | 0.8 ± 0.0 | 1.0 ± 0.0 | 1.0 ± 0.0 | 0.7 ± 0.0 | 0.6 ± 0.0 |
| 1193 | 1195 | Myrtenal | 1.2 ± 0.0 | 1.5 ± 0.0 | 1.2 ± 0.0 | 1.2 ± 0.0 | 1.7 ± 0.0 | 1.2 ± 0.0 | 1.5 ± 0.0 | 1.7 ± 0.0 | 1.3 ± 0.0 | 1.2 ± 0.0 |
| 1205 | 1204 | Verbenone | 1.9 ± 0.0 | 2.3 ± 0.0 | 2.2 ± 0.0 | 2.0 ± 0.0 | 2.7 ± 0.0 | 1.7 ± 0.0 | 2.6 ± 0.0 | 2.2 ± 0.0 | 1.8 ± 0.0 | 1.8 ± 0.0 |
| 1216 | 1215 | trans-Carveol | --- | 0.3 ± 0.0 | tr | 0.1 | --- | --- | 0.2 ± 0.0 | --- | 0.2 ± 0.0 | 0.1 ± 0.0 |
| 1242 | 1239 | Carvone | 4.2 ± 0.1 | 4.2 ± 0.0 | 4.3 ± 0.1 | 4.4 ± 0.1 | 3.3 ± 0.0 | 3.6 ± 0.0 | 5.1 ± 0.1 | 4.4 ± 0.0 | 3.7 ± 0.1 | 3.4 ± 0.3 |
| 1246 | 1254 | Linalyl acetate | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1261 | 1265 | 3,5-Dimethoxytoluene | tr | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1281 | 1287 | Bornyl acetate | 2.4 ± 0.0 | 2.8 ± 0.0 | 2.7 ± 0.0 | 2.6 ± 0.0 | 3.0 ± 0.0 | 2.4 ± 0.0 | 3.1 ± 0.0 | 2.4 ± 0.0 | 2.3 ± 0.0 | 2.4 ± 0.0 |
| 1286 | 1289 | Thymol | --- | tr | --- | --- | --- | --- | --- | --- | --- | --- |
| 1294 | 1298 | Carvacrol | 1.7 ± 0.0 | 1.7 ± 0.0 | 1.9 ± 0.0 | 1.6 ± 0.0 | 1.9 ± 0.0 | 1.3 ± 0.0 | 2.0 ± 0.0 | 1.9 ± 0.0 | --- | --- |
| 1343 | 1346 | α-Terpinyl acetate | 1.9 ± 0.0 | 1.6 ± 0.0 | 1.8 ± 0.0 | 1.6 ± 0.0 | 1.4 ± 0.0 | 1.4 ± 0.0 | 2.0 ± 0.0 | 1.7 ± 0.0 | 1.3 ± 0.0 | 1.5 ± 0.0 |
| 1345 | 1345 | α-Cubebene | 2.6 ± 0.0 | 2.2 ± 0.0 | 2.5 ± 0.0 | 2.4 ± 0.0 | 2.9 ± 0.0 | 2.2 ± 0.0 | 2.9 ± 0.0 | 2.5 ± 0.0 | 2.1 ± 0.0 | 2.0 ± 0.0 |
| 1373 | 1374 | α-Copaene | 3.8 ± 0.0 | 3.5 ± 0.0 | 3.7 ± 0.1 | 3.6 ± 0.1 | 2.9 ± 0.0 | 3.2 ± 0.0 | 4.1 ± 0.0 | 3.7 ± 0.0 | 2.5 ± 0.0 | 3.5 ± 0.1 |
| 1409 | 1411 | cis-α-Bergamotene | tr | tr | tr | tr | 0.2 ± 0.0 | tr | tr | tr | tr | 0.1 ± 0.0 |
| 1416 | 1417 | β-Caryophyllene | tr | tr | tr | tr | tr | tr | tr | tr | --- | tr |
| 1429 | 1432 | trans-α-Bergamotene | tr | tr | tr | tr | 0.2 ± 0.0 | tr | tr | tr | tr | 0.1 ± 0.0 |
| 1441 | 1440 | (Z)-β-Farnesene | --- | --- | --- | --- | --- | --- | --- | --- | --- | --- |
| 1441 | 1449 | α-Himachalene | --- | --- | --- | --- | tr | tr | --- | --- | --- | --- |
| 1452 | 1452 | α-Humulene | tr | --- | --- | --- | tr | --- | --- | --- | --- | --- |
| 1486 | 1489 | β-Selinene | tr | --- | --- | tr | tr | --- | --- | --- | --- | --- |
| 1493 | 1498 | α-Selinene | tr | --- | --- | --- | tr | --- | --- | --- | --- | --- |
| 1579 | 1582 | Caryophyllene oxide | tr | tr | --- | --- | tr | tr | tr | tr | --- | --- |
| 1942 | 1944 | m-Camphorene | 0.3 ± 0.0 | --- | 1.8 ± 0.0 | 0.8 ± 0.0 | 1.8 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | tr | --- | --- |
| 1948 | 1947 | (3E)-Cembrene A | 0.7 ± 0.0 | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.1 ± 0.0 | 0.9 ± 0.0 | 0.4 ± 0.0 | 0.2 ± 0.0 | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 |
| 1977 | 1977 | p-Camphorene | 0.1 ± 0.0 | --- | --- | --- | 2.1 ± 0.0 | 0.6 ± 0.0 | tr | tr | --- | --- |
| 1993 | 1992 | α-Pinacene | tr | tr | tr | --- | 0.1 ± 0.0 | tr | tr | tr | --- | 0.1 ± 0.0 |
| 2130 | 2138 | Cembrenol | 0.4 ± 0.0 | 0.1 ± 0.0 | 0.8 ± 0.0 | tr | 0.5 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.6 ± 0.0 |
| 2143 | 2144 | Incensole + Serratol | 0.7 ± 0.0 | 0.6 ± 0.0 | 0.6 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.0 | 1.0 ± 0.0 |
| Monoterpene hydrocarbons | 65.7 | 60.1 | 62.7 | 61.5 | 53.8 | 69.2 | 58.5 | 57.9 | 70.9 | 70.0 | ||
| Oxygenated monoterpenoids | 25.1 | 32.5 | 27.1 | 30.5 | 33.8 | 23.5 | 33.0 | 34.6 | 23.0 | 21.4 | ||
| Sesquiterpenoids | 6.4 | 5.7 | 6.2 | 5.9 | 6.1 | 5.4 | 7.0 | 6.1 | 4.6 | 5.7 | ||
| Diterpenoids | 2.3 | 0.9 | 3.7 | 1.1 | 5.6 | 1.6 | 0.9 | 1.0 | 0.6 | 1.9 | ||
| Others | 0.1 | 0.2 | 0.1 | 0.1 | 0.1 | 0.0 | 0.4 | 0.1 | 0.1 | 0.1 | ||
| Total Identified | 99.6 | 99.4 | 99.8 | 99.1 | 99.4 | 99.6 | 99.8 | 99.7 | 99.2 | 99.0 |
a RI: Retention Index determined in reference to a homologous series of n-alkanes on a ZB-5ms column. b RI: Retention Index from the databases. c Entries in boldface were used in the cluster analysis. d Percentages are average of three runs (±standard deviations). e tr = “trace” (<0.05%).
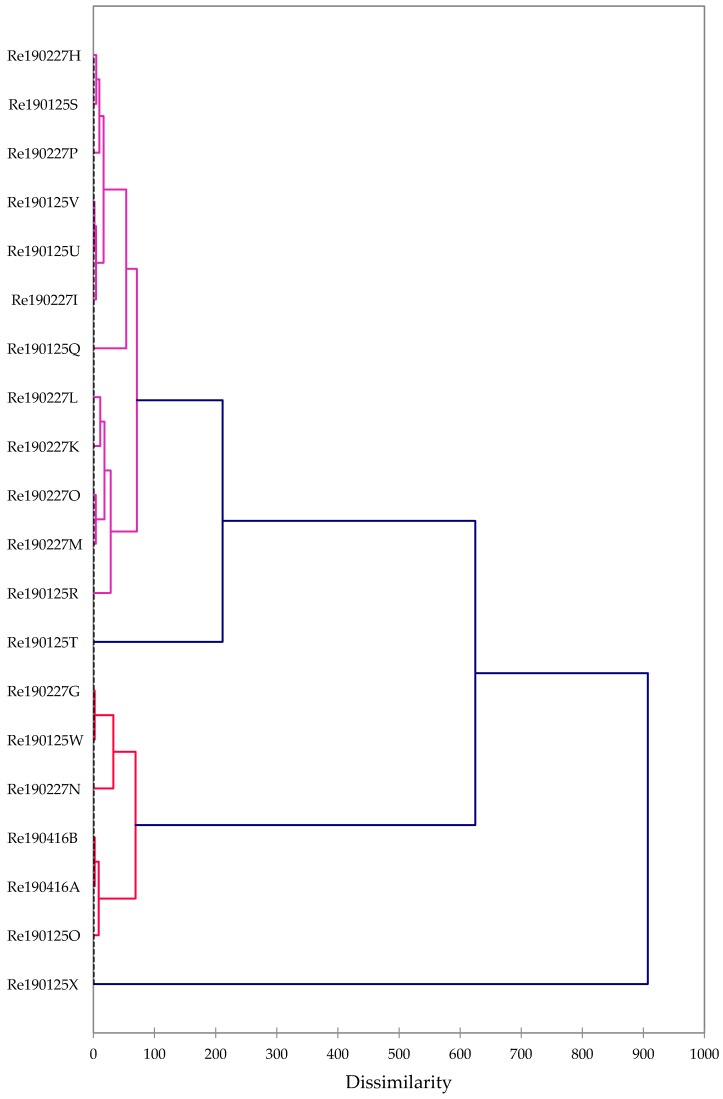
A hierarchical cluster analysis of the essential oil compositions revealed two major groups (Figure 2): One dominated by high α-pinene, and one rich in myrcene. Although unusual, the myrcene sample is only moderately dissimilar to the predominant α-pinene samples; we therefore conclude that there is a single, α-pinene dominant chemotype with a rare subchemotype rich in myrcene. The chemical compositions do not appear to correlate with the geographical locations from which they were taken, either within central Burkina Faso or between central and western Burkina Faso.
Figure 2.
Dendrogram obtained from the agglomerative hierarchical cluster analysis of 20 Boswellia dalzielii oleogum resin essential oil compositions from Burkina Faso.
3. Discussion
The oleogum resin essential oil of Boswellia dalzielii has only been described once previously, to our knowledge, in oleoresins taken from trees in northern Nigeria [21]. Our results are largely consistent with the findings of that study: In both areas, the essential oils are most commonly dominated by α-pinene, with a small number of samples showing high levels of myrcene. Levels of α-pinene were generally higher in Nigeria (42.6%–72.1%) compared to Burkina Faso (21.0%–56.0%) and α-thujene and p-cymene were less prevalent in this study. Additionally, in contrast to the samples from Nigeria, the samples from this study were found to contain an appreciable percentage of sesquiterpenes, particularly α-copaene.
The leaf essential oils of B. dalzielii have also been observed to contain a significant proportion of α-pinene, although other major components in those oils such as δ-3-carene, α-terpinene, and p-cymene were only observed in minor quantities in the oleoresin essential oils [29,30]. Boswellia dalzielii is rich in monoterpenes, particularly α-pinene, similar to many Boswellia species; Boswellia sacra and Boswellia carteri oleoresin essential oils are most commonly dominated by α-pinene, as well as lesser amounts of α-thujene, limonene, myrcene, sabinene, and p-cymene [11,13,18,34,35,36]. Many B. frereana essential oils are similarly dominated by α-pinene, with lower levels of sabinene and p-cymene [7,13,19]. A second chemotype of B. frereana is dominated by α-thujene, as are the oleoresins of B. serrata from India and B. ameero, B. dioscoridis, B. elongata, B. nana, and B. popoviana from Socotra Island, Yemen [12,17,37,38] (unpublished results from our laboratory). By contrast, several Boswellia species have completely different chemical profiles: B. papyrifera essential oils are dominated by octyl acetate and to a lesser degree octanol [8,13,39]; B. occulta oils have methoxyalkanes as the major components [11,40,41]; and B. bullata produces an unusual mix of δ-cadinene, β-caryophyllene, (E)-β-farnesene, α-cadinol, and several unidentified components [37]. The Boswellia dalzielii oleoresin essential oils are therefore fairly similar to those of several commonly traded commercial species (B. frereana, B. sacra, B. carteri).
Further work will be necessary to determine if the essential oils from Burkina Faso versus Nigerian B. dalzielii oleoresins show differential biological activities. In general, the reason for the diversity of terpenes and especially the importance of minor constituents is not clear [42]. The differential profiles likely convey some ecological benefits, though, as even essential oils from the same species, showing only modest chemical differences, do vary significantly in the degree to which they inhibit different strains of microbial pathogens [34]. This may indicate that the variation is related to variation in pathogenic threats.
The myrcene-dominated resins are intriguing as they represent only a small number of samples and differ greatly from the dominant α-pinene chemotype in both Nigeria and Burkina Faso. Environmental conditions can influence the chemical composition of plant volatiles [43,44,45]; although there was no geographic pattern observed here with regard to chemical variation, the myrcene chemotype samples taken in Nigeria were from the same geographic location. However, biotic factors also play a role: in a study of the heartwood essential oil of Santalum insulare in the Marquesas Islands, different chemotypes were observed in trees only a few meters apart, implying differences based on genetics or possibly pathogen attack history [46]; our laboratory has also observed different oleoresin chemical compositions in Boswellia carteri trees only a few meters apart in Somaliland (unpublished results from our laboratory). Further work is thus necessary to elucidate the reasons for the observed chemotypic differences in B. dalzielii.
4. Materials and Methods
4.1. Collection of Oleogum Resins
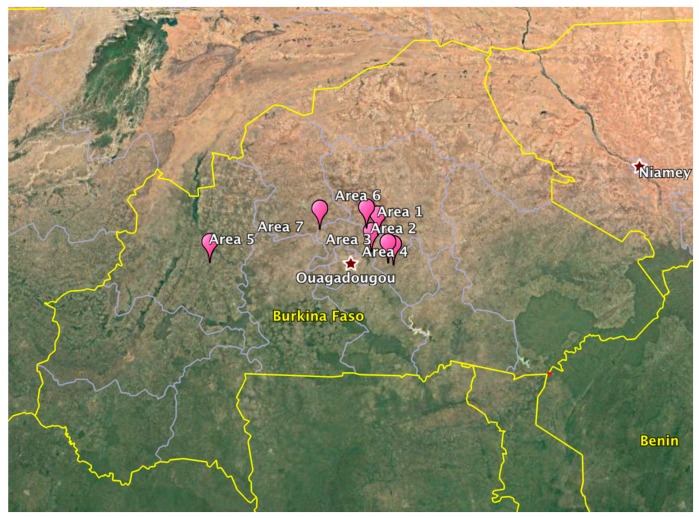
Twenty Boswellia dalzielii oleoresin samples were collected directly from source trees in six locations across central and western Burkina Faso (Figure 3). Samples were collected during the dry season (February–March 2019), and each sample location was GPS tagged (Table 2). All samples were taken from fresh resin exuding either naturally from tree branches or from wounds left by recent bark harvesting; consequently, age differences in the resin were not enough to significantly alter the resins′ chemical compositions. The trees were identified in the field by Anjanette DeCarlo and Stephen Johnson. A voucher specimen (Voucher number OUA6892) was deposited at the University of Ouagadougou herbarium and the identification confirmed by Amadé Ouédraogo, a botanist working there.
Figure 3.
Locations where B. dalzielii oleogum resin samples were collected.
Table 2.
Geographical collection locations of Boswellia dalzielii oleogum resins.
| Sample Code | GPS Coordinates | Elevation, m | |
|---|---|---|---|
| Re190227M | 12°21′13.92″ N | 3°16′57.72″ W | 290 |
| Re190125O | 12°41′43.74″ N | 1°10′48.18″ W | 291 |
| Re190125P | 12°41′43.74″ N | 1°10′48.18″ W | 291 |
| Re190125Q | 12°41′43.74″ N | 1°10′48.18″ W | 291 |
| Re190125R | 12°41′43.74″ N | 1°10′48.18″ W | 291 |
| Re190125S | 12°41′43.74″ N | 1°10′48.18″ W | 291 |
| Re190125T | 12°29′36.54″ N | 1°15′40.20″ W | 321 |
| Re190125U | 12°29′36.54″ N | 1°15′40.20″ W | 321 |
| Re190125V | 12°29′36.54″ N | 1°15′40.20″ W | 321 |
| Re190125W | 12°21′28.98″ N | 1°3′15.60″ W | 284 |
| Re190125X | 12°20′5.58″ N | 0°59′10.14″ W | 318 |
| Re190227G | 12°21′13.92″ N | 3°16′57.72″ W | 290 |
| Re190227H | 12°21′13.92″ N | 3°16′57.72″ W | 290 |
| Re190227I | 12°21′13.92″ N | 3°16′57.72″ W | 290 |
| Re190227K | 12°21′13.92″ N | 3°16′57.72″ W | 290 |
| Re190227L | 12°21′13.92″ N | 3°16′57.72″ W | 290 |
| Re190227N | 12°21′13.92″ N | 3°16′57.72″ W | 290 |
| Re190227P | 12°21′13.92″ N | 3°16′57.72″ W | 290 |
| Re190416A | 12°46′53.88″ N | 1°19′20.16″ W | 348 |
| Re190416B | 12°45′56.70″ N | 1°54′19.56″ W | 372 |
4.2. Hydrodistillation of Oleogum Resins
Hydrodistillations of the Boswellia dalzielii oleoresin samples were carried out in an all-glass Clevenger-type apparatus as previously described [21].
4.3. Gas-Chromatographic-Mass Spectral Analysis
Each of the B. dalzielii oleogum resin essential oils was analyzed by GC-MS as previously described [21]: Shimadzu GCMS-QP2010 Ultra (Shimadzu Scientific Instruments, Columbia, MD, USA), ZB-5ms capillary column (Phenomenex, Torrance, CA, USA). Identification of the essential oil components was based on their retention indices determined by reference to a homologous series of n-alkanes, and by comparison of their mass spectral fragmentation patterns with those reported in the literature [47], and our in-house library.
4.4. Gas Chromatographic-Flame Ionization Detection
Analysis of the B. dalzielii oleogum resin essential oils by GC-FID was carried out as previously described [21]: Shimadzu GC 2010 with flame ionization detector (Shimadzu Scientific Instruments, Columbia, MD, USA), ZB-5 capillary column (Phenomenex, Torrance, CA, USA). The percent compositions listed in Table 1 are averages from three separate runs of the essential oils were determined from peak areas and corrected using response factors for the different classes of chemical components [48].
4.5. Hierarchical Cluster Analysis
The chemical compositions of the B. dalzielii oleoresin essential oils were used in the hierarchical cluster analysis. The 20 essential oil compositions were treated as operational taxonomic units (OTUs), and the concentrations (percentages) of 23 major components were used to determine the chemical associations between these frankincense essential oils using agglomerative hierarchical cluster (AHC) analysis using XLSTAT Premium, version 2018.5.53172 (Addinsoft, Paris, France). Dissimilarity was determined using Euclidean distance, and clustering was defined using Ward’s method.
5. Conclusions
Boswellia dalzielii oleoresin essential oils from Burkina Faso are similar to those from Nigeria in that they are generally dominated by α-pinene, but unlike the samples from Nigeria, those in the current study contained a significant percentage of sesquiterpenes. The reason for these differences is not clear, but it potentially points to different biological and ecological activity due to different pathogenic threats. A rare chemotype or subchemotype dominated by myrcene was observed both in this study and in samples from Nigeria; this is potentially also related to specific pathogenic threats that may exist in both locations, or possibly genetic factors. This species thus offers a promising option for future work to elucidate the drivers of intraspecific chemical variation.
Acknowledgments
This work was carried out as part of the activities of the Aromatic Plant Research Center (APRC, https://aromaticplant.org/). We are grateful to Roland Traore, Denis Dipama, and Zongo Daouda, who helped collect samples.
Author Contributions
Conceptualization, A.D. and S.J.; methodology, A.D. and S.J.; validation, A.D., S.J. and W.N.S.; formal analysis, N.S.D. and W.N.S.; investigation, A.D., S.J., A.O., N.S.D., and W.N.S.; resources, A.D.; data curation, W.N.S.; writing—original draft preparation, S.J., A.O., and W.N.S.; writing—review and editing, A.D., S.J., A.O., N.S.D., and W.N.S.; supervision, A.D.; project administration, A.D.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.DeCarlo A., Dosoky N.S., Satyal P., Sorensen A., Setzer W.N. The essential oils of the Burseraceae. In: Malik S., editor. Essential Oil Research: Trends in Biosynthesis, Analytics, Industrial Applications and Biotechnological Production. Springer Nature; Cham, Switzerland: 2019. [Google Scholar]
- 2.Thulin M., DeCarlo A., Johnson S.P. Boswellia occulta (Burseraceae), a new species of frankincense tree from Somalia (Somaliland) Phytotaxa. 2019;394:219–224. doi: 10.11646/phytotaxa.394.3.3. [DOI] [Google Scholar]
- 3.Eslamieh J. Cultivation of Boswellia. 2nd ed. A Book’s Mind; Fort Collins, CO, USA: 2017. [Google Scholar]
- 4.Langenheim J.H. Plant Resins: Chemistry, Evolution, Ecology, and Ethnobotany. Timber Press, Inc.; Portland, OR, USA: 2003. [Google Scholar]
- 5.Al-Harrasi A., Al-Saidi S. Phytochemical analysis of the essential oil from botanically certified oleogum resin of Boswellia sacra (Omani Luban) Molecules. 2008;13:2181–2189. doi: 10.3390/molecules13092181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali N.A.A., Wurster M., Arnold N., Teichert A., Schmidt J., Lindequist U., Wessjohann L. Chemical composition and biological activities of essential oils from the oleogum resins of three endemic Soqotraen Boswellia species. Rec. Nat. Prod. 2008;2:6–12. [Google Scholar]
- 7.Basar S. Ph.D. Thesis. Universität Hamburg; Hamburg, Germany: 2005. Phytochemical Investigations on Boswellia Species. [Google Scholar]
- 8.Bekana D., Kebede T., Assefa M., Kassa H. Comparative phytochemical analyses of resins of Boswellia species (B. papyrifera (Del.) Hochst., B. neglecta S. Moore, and B. rivae Engl.) from northwestern, southern, and southeastern Ethiopia. ISRN Anal. Chem. 2014;2014:374678. doi: 10.1155/2014/374678. [DOI] [Google Scholar]
- 9.Benelli G., Rajeswary M., Vijayan P., Senthilmurugan S., Alharbi N.S., Kadaikunnan S., Khaled J.M., Govindarajan M. Boswellia ovalifoliolata (Burseraceae) essential oil as an eco-friendly larvicide? Toxicity against six mosquito vectors of public health importance, non-target mosquito fishes, backswimmers, and water bugs. Environ. Sci. Pollut. Res. 2017;25:10264–10271. doi: 10.1007/s11356-017-8820-0. [DOI] [PubMed] [Google Scholar]
- 10.Camarda L., Dayton T., Di Stefano V., Pitonzo R. Chemical composition and antimicrobial activity of some oleogum resin essential oils from Boswellia spp. (Burseraceae) Ann. Chim. 2007;97:837–844. doi: 10.1002/adic.200790068. [DOI] [PubMed] [Google Scholar]
- 11.DeCarlo A., Johnson S., Poudel A., Satyal P., Bangerter L., Setzer W.N. Chemical variation in essential oils from the oleo-gum resin of Boswellia carteri: A preliminary investigation. Chem. Biodivers. 2018;15:e1800047. doi: 10.1002/cbdv.201800047. [DOI] [PubMed] [Google Scholar]
- 12.Gupta M., Rout P.K., Misra L.N., Gupta P., Singh N., Darokar M.P., Saikia D., Singh S.C., Bhakuni R.S. Chemical composition and bioactivity of Boswellia serrata Roxb. essential oil in relation to geographical variation. Plant Biosyst. 2017;151:623–629. doi: 10.1080/11263504.2016.1187681. [DOI] [Google Scholar]
- 13.Hamm S., Bleton J., Connan J., Tchapla A. A chemical investigation by headspace SPME and GC–MS of volatile and semi-volatile terpenes in various olibanum samples. Phytochemistry. 2005;66:1499–1514. doi: 10.1016/j.phytochem.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Lebaka P.A.R., Ratnam K.V., Lepakshi B.M., Bhumi N.R., Lebaka V.R. Chemical profile, antioxidant and antimicrobial activity of essential oils from Boswellia ovalifoliolata Bal. et. Henry. Int. J. Pharm. Clin. Res. 2015;7:96–101. [Google Scholar]
- 15.Mothana R.A.A., Hasson S.S., Schultze W., Mowitz A., Lindequist U. Phytochemical composition and in vitro antimicrobial and antioxidant activities of essential oils of three endemic Soqotraen Boswellia species. Food Chem. 2011;126:1149–1154. doi: 10.1016/j.foodchem.2010.11.150. [DOI] [Google Scholar]
- 16.Niebler J., Buettner A. Frankincense revisited, part 1: Comparative analysis of volatiles in commercially relevant Boswellia species. Chem. Biodivers. 2016;13:613–629. doi: 10.1002/cbdv.201500329. [DOI] [PubMed] [Google Scholar]
- 17.Singh B., Kumar R., Bhandari S., Pathania S., Lal B. Volatile constituents of natural Boswellia serrata oleo-gum-resin and commercial samples. Flavour Fragr. J. 2007;22:145–147. doi: 10.1002/ffj.1772. [DOI] [Google Scholar]
- 18.Suhail M.M., Wu W., Cao A., Mondalek F.G., Fung K.-M., Shih P.-T., Fang Y.-T., Woolley C., Young G., Lin H.-K. Boswellia sacra essential oil induces tumor cell-specific apoptosis and suppresses tumor aggressiveness in cultured human breast cancer cells. BMC Complement. Altern. Med. 2011;11:129. doi: 10.1186/1472-6882-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Vuuren S.F., Kamatou G.P.P., Viljoen A.M. Volatile composition and antimicrobial activity of twenty commercial frankincense essential oil samples. S. Afr. J. Bot. 2010;76:686–691. doi: 10.1016/j.sajb.2010.06.001. [DOI] [Google Scholar]
- 20.Woolley C.L., Suhail M.M., Smith B.L., Boren K.E., Taylor L.C., Schreuder M.F., Chai J.K., Casabianca H., Haq S., Lin H.K., et al. Chemical differentiation of Boswellia sacra and Boswellia carterii essential oils by gas chromatography and chiral gas chromatography-mass spectrometry. J. Chromatogr. A. 2012;1261:158–163. doi: 10.1016/j.chroma.2012.06.073. [DOI] [PubMed] [Google Scholar]
- 21.Decarlo A., Johnson S., Okeke-Agulu K.I., Dosoky N.S., Wax S.J., Owolabi M.S., Setzer W.N. Compositional analysis of the essential oil of Boswellia dalzielii frankincense from West Africa reveals two major chemotypes. Phytochemistry. 2019;164:24–32. doi: 10.1016/j.phytochem.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Hutchinson J., Dalziel J.M. Flora of West Tropical Africa. 2nd ed. Crown Agents for Oversea Governments and Administrations; London, UK: 1954. [Google Scholar]
- 23.Arbonnier M. Arbres, Arbustes et Lianes des Zones Sèches d′Afrique de l′Ouest. Muséeum national d′histoire naturelle; Paris, France: 2009. Troisième. [Google Scholar]
- 24.Jansen O., Angenot L., Tits M., Nicolas J.P., De Mol P., Nikiéma J.B., Frédérich M. Evaluation of 13 selected medicinal plants from Burkina Faso for their antiplasmodial properties. J. Ethnopharmacol. 2010;130:143–150. doi: 10.1016/j.jep.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 25.Nadembega P., Boussim J.I., Nikiema J.B., Poli F., Antognoni F. Medicinal plants in Baskoure, Kourittenga Province, Burkina Faso: An ethnobotanical study. J. Ethnopharmacol. 2011;133:378–395. doi: 10.1016/j.jep.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Ouédraogo A., Thiombiano A., Hahn-Hadjali K., Guinko S. Régénération sexuée de Boswellia dalzielii Hutch., un arbre médicinal de grande valeur au Burkina Faso. Bois Forêts Des. Trop. 2006;289:41–48. [Google Scholar]
- 27.Tapsoba H., Deschamps J.-P. Use of medicinal plants for the treatment of oral diseases in Burkina Faso. J. Ethnopharmacol. 2006;104:68–78. doi: 10.1016/j.jep.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 28.Nacoulma-Ouédraogo G. Plantes Médicinales et Pratiques Médicinales Traditionnelles au Burkina Faso. Cas du Plateau Central. Université de Ouagadougou; Hamburg, Germany: 1996. [Google Scholar]
- 29.Kohoude M.J., Gbaguidi F., Agbani P., Ayedoun M.-A., Cazaux S., Bouajila J. Chemical composition and biological activities of extracts and essential oil of Boswellia dalzielii leaves. Pharm. Biol. 2017;55:33–42. doi: 10.1080/13880209.2016.1226356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kubmarawa D., Ogunwande I.A., Okorie D.A., Olawore N.O., Kasali A.A. Constituents of the essential oils of Boswellia dalzielii Hutch. from Nigeria. J. Essent. Oil Res. 2006;18:119–120. doi: 10.1080/10412905.2006.9699038. [DOI] [Google Scholar]
- 31.Ouédraogo A. Ph.D. Thesis. Université de Ouagadougou; Burkina Faso, Africa: 2006. Diversité et Dynamique de la Végétation Ligneuse de la Partie Orientale du Burkina Faso; p. 196. [Google Scholar]
- 32.Ouédraogo A., Thiombiano A., Guinko S. In: Utilisations, état Des Peuplements et Régénération de Cinq Espèces Ligneuses Utilitaires Dans l′EST du BURKINA FASO in homme, Plantes et Environnement au Sahel Occidental. Boussim I.J., Lykke A.M., Nombré I., Nielsen I., Guinko S., editors. University of Copenhagen; Copenhagen, Denmark: 2005. pp. 173–183. Seren Occasional Paper N° 19. [Google Scholar]
- 33.Ouédraogo A., Thiombiano A. Regeneration pattern of four threatened tree species in Sudanian savannas of Burkina Faso. Agrofor. Syst. 2012;86:35–48. doi: 10.1007/s10457-012-9505-9. [DOI] [Google Scholar]
- 34.Al-Saidi S., Rameshkumar K.B., Hisham A., Sivakumar N., Al-Kindy S. Composition and antibacterial activity of the essential oils of four commercial grades of Omani luban, the oleo-gum resin of Boswellia sacra Flueck. Chem. Biodivers. 2012;9:615–624. doi: 10.1002/cbdv.201100189. [DOI] [PubMed] [Google Scholar]
- 35.Ni X., Suhail M.M., Yang Q., Cao A., Fung K.-M., Postier R.G., Woolley C., Young G., Zhang J., Lin H.-K. Frankincense essential oil prepared from hydrodistillation of Boswellia sacra gum resins induces human pancreatic cancer cell death in cultures and in a xenograft murine model. BMC Complement. Altern. Med. 2012;12:253. doi: 10.1186/1472-6882-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niebler J., Buettner A. Identification of odorants in frankincense (Boswellia sacra Flueck.) by aroma extract dilution analysis and two-dimensional gas chromatography–mass spectrometry/olfactometry. Phytochemistry. 2015;109:66–75. doi: 10.1016/j.phytochem.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 37.Maděra P., Paschová Z., Ansorgová A., Vrškový B., Lvončík S., Habrová H. Volatile compounds in oleo-gum resin of Socotran species of Burseraceae. Acta Univ. Agric. Silvic. Mendel. Brun. 2017;65:73–90. doi: 10.11118/actaun201765010073. [DOI] [Google Scholar]
- 38.Sadhasivam S., Palanivel S., Ghosh S. Synergistic antimicrobial activity of Boswellia serrata Roxb. ex Colebr. (Burseraceae) essential oil with various azoles against pathogens associated with skin, scalp and nail infections. Lett. Appl. Microbiol. 2016;63:495–501. doi: 10.1111/lam.12683. [DOI] [PubMed] [Google Scholar]
- 39.Schillaci D., Arizza V., Dayton T., Camarda L., Di Stefano V. In vitro anti-biofilm activity of Boswellia spp. oleogum resin essential oils. Lett. Appl. Microbiol. 2008;47:433–438. doi: 10.1111/j.1472-765X.2008.02469.x. [DOI] [PubMed] [Google Scholar]
- 40.Johnson S., DeCarlo A., Satyal P., Dosoky N.S., Sorensen A., Setzer W.N. Organic certification is not enough: The case of the methoxydecane frankincense. Plants. 2019;8:88. doi: 10.3390/plants8040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Satyal P., Pappas R.S. First reporting on the chemistry and biological activity of a novel Boswellia chemotype: The methoxy alkane frankincense. Glob. J. Sci. Front. Res. B Chem. 2016;16:1–9. [Google Scholar]
- 42.Pichersky E., Raguso R.A. Why do plants produce so many terpenoid compounds? New Phytol. 2018;220:692–702. doi: 10.1111/nph.14178. [DOI] [PubMed] [Google Scholar]
- 43.Gil A., de la Fuente E.B., Lenardis A.E., López Pereira M., Suárez S.A., Bandoni A., van Baren C., Di Leo Lira P., Ghersa C.M. Coriander essential oil composition from two genotypes grown in different environmental conditions. J. Agric. Food Chem. 2002;50:2870–2877. doi: 10.1021/jf011128i. [DOI] [PubMed] [Google Scholar]
- 44.Perry N.B., Anderson R.E., Brennan N.J., Douglas M.H., Heaney A.J., McGimpsey J.A., Smallfield B.M. Essential oils from Dalmatian sage (Salvia officinalis L.): Variations among individuals, plant parts, seasons, and sites. J. Agric. Food Chem. 1999;47:2048–2054. doi: 10.1021/jf981170m. [DOI] [PubMed] [Google Scholar]
- 45.Vokou D., Kokkini S., Bessiere J.-M. Geographic variation of Greek oregano (Origanum vulgare ssp. hirtum) essential oils. Biochem. Systemat. Ecol. 1993;21:287–295. doi: 10.1016/0305-1978(93)90047-U. [DOI] [Google Scholar]
- 46.Braun N.A., Butaud J.-F., Bianchini J.-P., Kohlenberg B., Hammerschmidt F.-J., Meier M., Raharivelomanana P. Eastern Polynesian sandalwood oil (Santalum insulare Bertero ex A. DC.)–a detailed investigation. Nat. Prod. Commun. 2007;2:695–699. doi: 10.1177/1934578X0700200615. [DOI] [Google Scholar]
- 47.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing; Carol Stream, IL, USA: 2007. [Google Scholar]
- 48.Bicchi C., Liberto E., Matteodo M., Sgorbini B., Mondello L., d′Acampora Zellner B., Costa R., Rubiolo P. Quantitative analysis of essential oils: A complex task. Flavour Fragr. J. 2008;23:382–391. doi: 10.1002/ffj.1905. [DOI] [Google Scholar]