Summary
The recent interest in plant pigment betalains as bioactive compounds and chemopreventive agents has led to the search for a reliable and scalable process to obtain them. The cloning of the novel and efficient enzyme 4,5‐DOPA‐extradiol dioxygenase from Gluconacetobacter diazotrophicus in an expression vector, and the subsequent heterologous expression in Escherichia coli cultures has led to the start‐up of a biotechnological production system of individual pigments. The aim of this study was to search for the optimal conditions for the production of betalamic acid in microbial factories and the scaled‐up obtention of the derived pigments. Four different betaxanthins and two betacyanins were obtained after the addition of non‐transformable amines and amino acids and their condensation with the betalamic acid produced by the dioxygenase. The scaled‐up obtention and purification of betalains improved the yields of the previous methodologies reaching quantities by up to 150 mg of pure compounds.
Introduction
Betalains are nitrogen‐containing pigments responsible for the coloration of plants of the order Caryophyllales. Betalains are classified into two groups: betaxanthins and betacyanins. Both groups have betalamic acid as a chromophoric and structural unit and condense with different molecules (Gandía‐Herrero et al., 2013). Betaxanthins are derived from the condensation of betalamic acid with amines and amino acids. These compounds have a maximum absorbance spectrum centred on a 480 nm wavelength and have a yellow coloration. In contrast, betacyanins are violet and present absorbance spectra centred at wavelengths around λm = 536 nm. Betacyanins are the result of the condensation of betalamic acid with indoline‐type cycled amines. In plants, betalains are secondary metabolites derived from L‐DOPA, and their biosynthetic pathway involves two main activities: the hydroxylation of L‐tyrosine to L‐DOPA and the aromatic ring opening oxidation of L‐DOPA catalysed by 4,5‐DOPA‐extradiol‐dioxygenase (4,5‐DODA) (Fig. 1). The monophenolase activity of tyrosinase (Steiner et al., 1999; Gandía‐Herrero et al., 2005) or cytochrome P450 activity (Sunnadeniya et al., 2016) is responsible for the hydroxylation of l‐tyrosine to L‐DOPA. This L‐DOPA is the substrate for the enzyme 4,5‐DODA (Christinet et al., 2004; Sasaki et al., 2009), which gives rise to 4,5‐seco‐DOPA, which by spontaneous intramolecular cyclization converts to betalamic acid. Although traditionally the presence of betalamic acid‐forming activity was considered to be restricted to plants of the order Caryophyllales and to the fungi Amanita (Musso, 1979) and Hygrocybe (Von‐Ardenne et al., 1974), prokaryotic organisms capable of forming betalamic acid by enzymes with dioxygenase activity have been recently described (Gandía‐Herrero and García‐Carmona, 2014; Contreras‐Llano et al., 2019). Betalains are compounds of great interest because of their bioactive properties. They have a health‐promoting effect that has been demonstrated in the in vivo model Caenorhabditis elegans, where they reduce oxidative stress and increase lifespan (Guerrero‐Rubio et al., 2019). Their action in the elimination of free radicals and their role as antioxidants have been described (Cai et al., 2003; Gliszczyńska‐Świgło et al., 2006). Their chemopreventive power has also been shown in the dose‐dependent inhibition of the growth and proliferation of cancer cells (Sreekanth et al., 2007; Khan et al., 2012; Ahmadian et al., 2018), and their administration in the diet has been shown to inhibit the formation of tumours in vivo (Lechner et al., 2010). Betalains are also of great interest as food colourants in industrial applications. The renewed interest in betalains contrasts with the limited number of biotechnological tools to produce them. The obtention of individual pigments is necessary to characterize and apply the potent health‐promoting activities described for betalains (Gandía‐Herrero et al., 2016).
Figure 1.
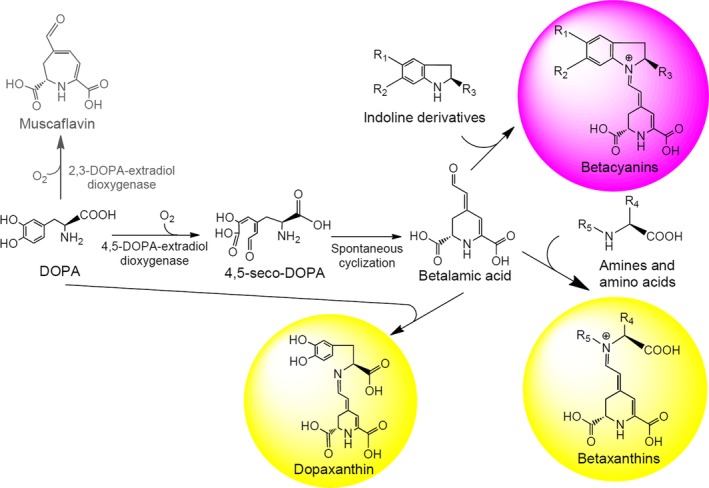
Key steps in the biosynthesis of betalamic acid and betalains. R1 and R2 can be hydroxyl groups with or without sugar derivatives. R3 can be a carboxylic acid group. R4 can be side‐chains of amino acids.
To date, obtaining betalains and purifying them from whole plants have depended directly on availability throughout the year and on environmental variables that affect their production (Khan et al., 2012). Other methods used are by a semi‐synthesis method, which requires obtaining betalamic acid from the degradation of betanin (Gandía‐Herrero et al., 2006), or the production of betalains in plant cell cultures (Guadarrama‐Flores et al., 2015; Milech et al., 2017; Henarejos‐Escudero et al., 2018). However, the main problem these techniques present is their low efficiency in the production of pure betalains. The aim of this work was to produce betalains through the development of microbial factories as a controllable system, using the key enzyme of the biosynthetic pathway, a novel 4,5‐DOPA‐extradiol‐dioxygenase of bacterial origin and superior activity, and its subsequent scaling‐up to the bioreactor level for pigment production.
Results and discussion
Expression of 4,5‐DOPA‐extradiol‐dioxygenase from Gluconacetobacter diazotrophicus in Escherichia coli cultures
The novel 4,5‐DOPA‐extradiol‐dioxygenase (DODA) from Gluconacetobacter diazotrophicus, the first betalain‐producing bacteria described, was used in this work. G. diazotrophicus DODA enzyme (GdDODA) presents higher activity, more affinity towards L‐DOPA substrate and better stability than other dioxygenases previously described, as Beta vulgaris DODA or E. coli YgiD (Contreras‐Llano et al., 2019). The gene sequence of G. diazotrophicus (sequence WP_012222467.1, GI:501179334) was used as a template to obtain the DODA sequence for protein expression in E. coli (Contreras‐Llano et al., 2019) and it was inserted into the expression vector pET28a. Escherichia coli Rossetta 2 (DE3) cells were transformed with the vector pET28a‐GdDODA and selected in the nutrient medium Luria‐Bertani (LB) supplemented with chloramphenicol (Cm) and kanamycin (Km). Escherichia coli was cultured as small drops of 10 μL in LB plates supplemented with IPTG, L‐DOPA and sodium ascorbate to check the ability of E. coli expressing DODA to form betalamic acid. After incubation for 24 h at 20°C plates with IPTG, L‐DOPA and sodium ascorbate showed a yellow coloration around the drops. Plates without IPTG were used as a control and showed no change of colour (Fig. 2). The yellow halos around the drops confirmed the expression and activity of heterologous DODA protein induced by IPTG. The use of L‐DOPA as a precursor in the synthesis of betaxanthins and the excretion of pigments thus resulted in yellow coloration. The positive results in the plate assays promoted the transfer of the production to E. coli liquid cultures.
Figure 2.
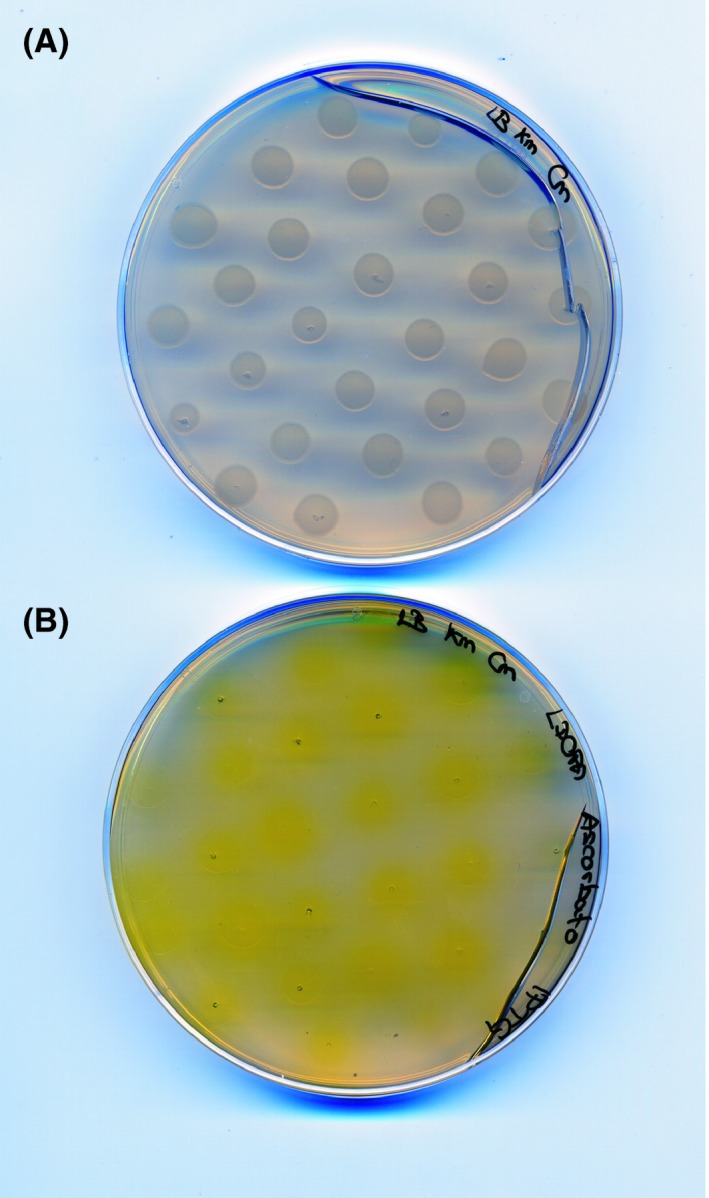
Expression of GdDODA activity in Escherichia coli cells grown in agar solid medium supplemented with L‐DOPA.
A. Control plate without IPTG.
B. Yellow halos formed around the bacterial colonies in the presence of L‐DOPA 7.6 mM indicate the formation and excretion of betalain‐related compounds. A single image contains both conditions and was taken in the same shot. Standard 60 mm Petri dishes were used.
Production of the structural unit of betalains
The production of betalamic acid was attempted with E. coli Rossetta 2 (DE3) cells transformed with pET28a‐GdDODA. Cells were grown and selected in medium LB supplemented with Cm and Km. After the induction of the expression of the gene with IPTG, L‐DOPA was added as the substrate of the reaction and precursor of betalains. L‐DOPA concentration in the medium was optimized, varying it in the range 0.76 to 22.32 mM, resulting in the highest production at 7.6 mM (Fig. S1A), the solubility limit of the substrate. Sodium ascorbate was added to prevent spontaneous oxidation. After addition of any L‐DOPA concentration assayed, the culture began to show a yellow coloration. HPLC analyses showed that the yellow coloration was due to the presence of betalamic acid in the medium and a compound with lower retention time and spectrum compatible with betaxanthins. Using real standards (Gandía‐Herrero et al., 2005), this betalain was identified as dopaxanthin (Fig. S1D), which was first described in the plant Glottiphylum longum (Impellizzeri et al., 1973) and was shown to have a strong antiradical capacity (Gandía‐Herrero et al., 2010a). Betalamic acid was detected at λm = 405 nm with a retention time of 14.9 min. To confirm its nature, betalamic acid obtained from the degradation of betanin was used as standard (Fig. S1C) (Gandía‐Herrero et al., 2009). Muscaflavin was also detected at 403 nm and retention time of 16.6 min. Dopaxanthin was detected with a maximum wavelength at λm = 471 nm and a retention time of 13.9 min. The presence of dopaxanthin is the result of the condensation of betalamic acid with L‐DOPA used as a substrate. Therefore, this betalain is obtained in cell cultures of E. coli due to the expression of the enzyme 4,5‐DODA in one‐pot experiments. The evolution of the concentration of betalamic acid and dopaxanthin was followed over time by HPLC analysis. Both products were monitored for 200 h after adding the substrate (Fig. S1B). In the first hours, a peak with retention time of 8.54 min was obtained at λmax = 361 nm. This peak corresponded to 4,5‐seco‐DOPA (Contreras‐Llano et al., 2019), the intermediate product of 4,5 DODA activity that transforms in betalamic acid by spontaneous cyclization. The maximum concentration of betalamic acid was obtained at 24–30 h, after which it drastically decreased. However, dopaxanthin increases progressively until a maximum production at 96 h. This lag period is due to the condensation of betalamic acid with the L‐DOPA used as a substrate for its production. On the other hand, the analysis of the cell pellet obtained by centrifugation did not show accumulation of betalamic acid or dopaxanthin after washing and sonication, indicating that the L‐DOPA transformation products are excreted to the culture medium, as 4,5‐seco‐DOPA intermediate or as betalamic acid.
Production of betalamic acid in different media
The HPLC analyses showed the presence of betalamic acid, dopaxanthin and muscaflavin in the above‐mentioned LB culture but also showed the formation of minor peaks at 480 nm (Fig. 3A) which had different retention times (Table 1) and evolved independently with the reaction time. The spectral analysis of the compounds was compatible with the presence of unknown betaxanthins with maximum wavelengths around 470 nm. The presence of free amines in LB medium could promote the formation of these betaxanthins. To study this phenomenon, different media were used to determine the formation of the new betaxanthins depending on the medium composition. The media used were NZCYM, fresh LB and the same LB medium where the bacteria were grown. The conditions for substrate concentration and temperature were the same in all cases. After addition of the substrate L‐DOPA, there was no difference between fresh LB and LB used in the growth of bacteria. Various betaxanthins with different retention times in the LB and NZCYM media were observed by HPLC analysis after 96 h (Fig. 3A and B). Betaxanthins present in both media corresponded to retention times of 13.91, 14.9, 21.7, 22.8, 26.1 and 26.9 min, with betaxanthins exclusive of LB medium being those of 9.2, 16.7 and 20.5 min. The mass values determined by electrospray ionization mass spectrometry (ESI‐MS) are shown in Table 1. The mass of 391.1 m/z obtained at 13.9 min and the mass of 212.1 m/z obtained at 14.9 min were compatible with the presence of dopaxanthin and betalamic acid, respectively, in LB and NZCYM media. Furthermore, the mass of 212.1 m/z obtained at 16.7 min in LB medium was compatible with muscaflavin. The rest of mass values were not compatible with any known betaxanthin. It is concluded that LB and NZCYM media have a series of elements with amino groups in their composition which condense with the betalamic acid formed by the bacterial culture, giving rise to different betaxanthins. No differences were found between fresh LB and the LB medium used in the bacterial growth. This indicates that elements with amine groups are still available for the condensation with the betalamic acid formed by the engineered bacteria. In order to simplify the final pigments obtained by the transformation of the precursor molecule L‐DOPA, water was added, instead of the amine‐containing media, after harvesting and washing of the induced cells. In this case, the production of betalamic acid (maximum wavelength at 405 nm) and dopaxanthin (maximum wavelength at 471 nm) experienced an increase, doubling the results obtained with the other media. Neither was the presence of any other derived betaxanthin observed (Fig. 3C), thus yielding dopaxanthin in a purer form. In the transformation in water, only the formation of betalamic acid and dopaxanthin was achieved. This raises the possibility of using water in the transformation phase as a way to reduce production costs and simplify future purification processes, since the culture medium is only necessary for the initial growth of the cells.
Figure 3.
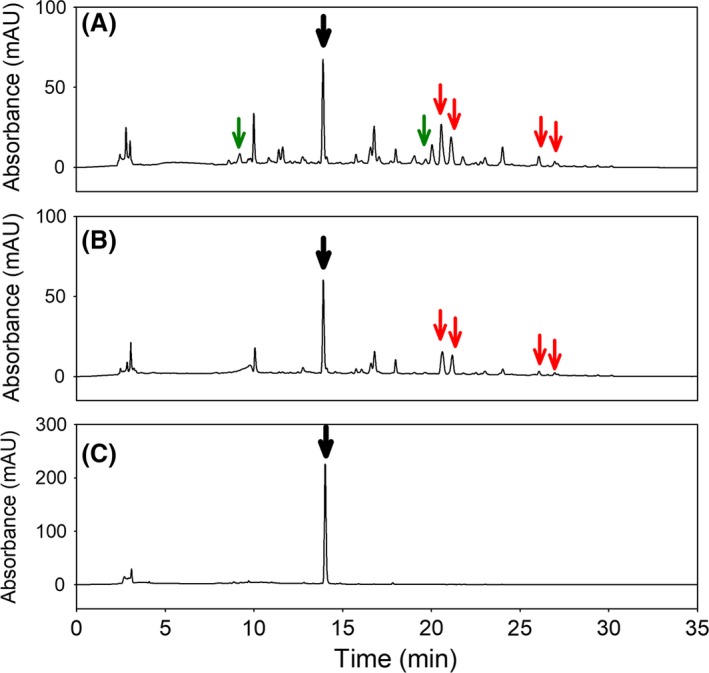
Production of dopaxanthin in microbial cultures. Chromatograms at λ = 480 nm for the detection of betaxanthins in the media LB (A), NZCYM (B) and water (C). Dopaxanthin was detected at 13.9 min with a signal intensity higher in water than in LB and NZCYM (black arrows). Betaxanthins present exclusively in LB medium are indicated with green arrows. Red arrows indicate betaxanthins present in both LB and NZCYM media.
Table 1.
HPLC and ESI‐MS/MS characteristics and identification of known and minor betalains detected after the growth of Escherichia coli expressing 4,5‐DODA in different media
| Rt (min) | Name | Media | PDA‐λm (nm) | [M+H]+ (m/z) | Main daughter ion (m/z) |
|---|---|---|---|---|---|
| 9.2 | — | LB | 471 | 235.1 | 162.0 |
| 13.9 | Dopaxanthin | LB/NZCYM | 471 | 391.1 | 347.1 |
| 14.9 | Betalamic acid | LB/NZCYM | 405 | 212.1 | 166.1 |
| 16.7 | Muscaflavin | LB | 405 | 212.1 | 166.0 |
| 20.5 | — | LB | 471 | 385.2 | 215.0 |
| 21.7 | — | LB/NZCYM | 471 | 326.8 | 244.0 |
| 22.8 | — | LB/NZCYM | 471 | 374.8 | 365.6 |
| 26.1 | — | LB/NZCYM | 471 | 288.8 | 251.1 |
| 26.9 | — | LB/NZCYM | 471 | 411.4 | 385.1 |
Oxygen effect in the production of betalains
Previous studies about the enzymatic activity of GdDODA show the importance of oxygen exchange in the formation of betalains (Contreras‐Llano et al., 2019). Thus, once water had been established as the optimum medium for the biotransformation of L‐DOPA, E. coli (pET28a‐GdDODA) was grown in LB medium and centrifuged, and cell pellets were transferred to water for the production of 4,5‐seco‐DOPA, betalamic acid and dopaxanthin. The reaction was followed under different atmospheric conditions, in order to evaluate the effect of oxygen. As Fig. S2 shows, a shaken medium, where the oxygen exchange is high, increases the production of pigments with respect to the values obtained in a non‐shaken medium where the oxygen exchange is limited by diffusion. Furthermore, deaeration and nitrogen atmosphere in the medium produce a pronounced decrease in the yield of the final product dopaxanthin (Fig. S2).
Addition of amines and amino acids to the medium for the production of derived betalains
The addition of amines and amino acids to a medium with betalamic acid leads to the production of betalains by Schiff condensation. The spontaneous reaction between the amine group of the added molecule and the aldehyde group of betalamic acid forms the corresponding imine. Successful approaches for betalain production have been carried out in semi‐synthesis processes, where betalamic acid was obtained by basic hydrolysis from betanin extracted from red beet, and this betalamic acid was able to condense with the amines added to the medium (Gandía‐Herrero et al., 2006). In the present work, betalamic acid is obtained more efficiently by the biotechnological mean described and its ability to react with free amines is maintained. In this context, the biosynthesis of betaxanthins and betacyanins can be achieved by adding selected amino acids and amines. Addition of the compounds 2‐phenylethylamine, l‐phenylalanine, indoline, l‐indoline‐2‐carboxylic acid and l‐proline gave rise to the betalains phenylethylamine‐betaxanthin, phenylalanine‐betaxanthin, indoline‐betacyanin, indoline carboxylic acid‐betacyanin and indicaxanthin respectively. Obtention of each amine‐derived betalain was performed in flasks containing 100 ml of water and with orbital shaking at 120 rpm. The addition of these compounds was carried out at a concentration of 38 mM, five times higher than that used for L‐DOPA, to favour the condensation of betalamic acid with these molecules instead of condensation with L‐DOPA, which acts as substrate for DODA enzyme and as amine precursor in the synthesis of dopaxanthin (Fig. 1). Condensation with 2‐phenylethylamine, l‐phenylalanine and l‐proline led to the production of the betaxanthins phenylethylamine‐betaxanthin, phenylalanine‐betaxanthin and indicaxanthin, respectively, while condensation with indoline and indoline‐2‐carboxylic acid gave rise to the production of indoline‐betacyanin and indoline carboxylic acid‐betacyanin, as shown in Fig. 4. Indoline and its carboxylated derivate added at 38 mM negatively affected the activity of the dioxygenase enzyme produced. Indoline and indoline carboxylic acid formed water‐insoluble droplets in the biotransformation medium that may negatively affect the integrity of the bacterial membrane or the stability of the protein. Thus, their concentrations were lowered by several orders of magnitude, reaching a maximum betacyanin production at 3.8 mM for indoline‐2‐carboxylic acid and at 0.38 mM for indoline.
Figure 4.
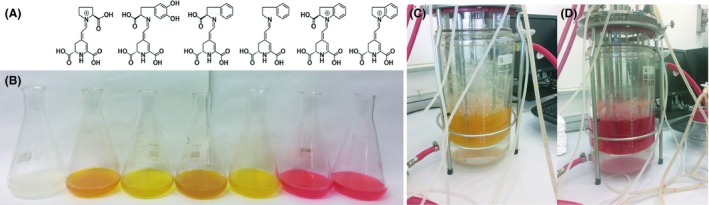
Redirection for the production of multiple individual pigments.
A: Chemical structures of the betalains produced in this study. From left to right: indicaxanthin, dopaxanthin, phenylalanine‐betaxanthin, phenylethylamine‐betaxanthin, indoline carboxylic acid‐betacyanin and indoline‐betacyanin.
B. 100‐ml cultures of Escherichia coli expressing 4,5‐DODA in the production of the individual molecules shown above.
C,D. Scaled‐up synthesis of phenylethylamine‐betaxanthin (C) and indoline‐betacyanin (D) in bioreactors with a volume of 2L.
In all cases, the compounds obtained were analysed by mass spectrometry and HPLC to confirm the structures proposed (Table 2). The values for absorption maximum wavelengths and retention times shown in Table 2 were in concordance with the values reported previously for standards obtained using a semi‐synthetic method (Gandía‐Herrero et al., 2005, 2010a). Under the optimal conditions determined for each compound in volumes of 100 ml, it was possible to obtain up to 3.1 mg in total of indoline‐betacyanin, 1.9 mg of indoline‐2‐carboxylic acid‐betacyanin, 2.6 mg of L‐phenylalanine‐betaxanthin, 1.1 mg of phenylethylamine‐betaxanthin, 2.2 mg of indicaxanthin and 4.3 mg of dopaxanthin. Once the technique was optimized and its efficiency in the synthesis of individual betalains was confirmed, the possibility of using these microbial cultures in bioreactors for a greater production of betalains was studied.
Table 2.
Production of individual betalains obtained in bacterial cultures (flasks, 100 ml) of Escherichia coli expressing the DODA enzyme of Gluconacetobacter diazotrophicus
| Betalain | Rt (min) | PDA‐λm (nm) | [M+H]+ (m/z) | Yield (mg/100 ml) | Main daughter ion (m/z) |
|---|---|---|---|---|---|
| Indicaxanthin | 12.65 | 471 | 309.1 | 2.2 | 264.0 |
| Dopaxanthin | 13.91 | 471 | 391.1 | 4.3 | 347.1 |
| Phenylethylamine‐betaxanthin | 20.08 | 471 | 315.1 | 2.6 | 271.1 |
| Phenylalanine‐betaxanthin | 22.11 | 471 | 359.2 | 1.1 | 315.1 |
| Indoline‐2‐carboxylic acid‐betacyanin | 19.17 | 524 | 357.0 | 1.9 | 313.0 |
| Indoline‐betacyanin | 22.10 | 524 | 313.0 | 3.1 | 269.0 |
Scaling‐up to bioreactor level
Indicaxanthin, dopaxanthin, phenylethylamine‐betaxanthin and indoline‐betacyanin were chosen to scaled‐up production. Indicaxanthin and dopaxanthin were chosen for their relevance in preliminary bioactivity assays (Wendel et al., 2015; Guerrero‐Rubio et al., 2019) and strong antioxidant activity (Escribano et al., 2017). Indicaxanthin is the main bioactive pigment of cactus pears (Gandía‐Herrero et al., 2010b). Dopaxanthin is the only pigment in Glottiphyllum flowers (Impellizzeri et al., 1973). Phenylethylamine‐betaxanthin and indoline‐betacyanin (Fig. 4C and D) were chosen for their close structural similarity as pigment models of betaxanthins and betacyanins. In addition, phenylethylamine‐betaxanthin is a minor pigment in cactus pears (Castellanos‐Santiago and Yahia, 2008). The biotechnological synthesis of betalains in a bioreactor was intended to allow a greater production. In addition, it made possible to control all the variables that may affect the synthesis, such as pH, temperature and oxygen demand. Temperature control on a small scale was also performed in the bioreactor thanks to the use of a heating jacket that allows large temperature changes in a few hours. In preliminary assays, the synthesis of indoline‐betacyanin was carried out controlling the pH at a value of 7.0, as is done for bacterial growth. After 96 h, the culture did not show the pink coloration characteristic of the synthesis of the betacyanins (Fig. 5A) in the flask assays. It was observed that, after the addition of the reagents, the culture stopped consuming acid, but continued to consume alkali at a high rate in order to maintain pH 7 (Fig. 5C). Samples were taken over time to follow the evolution of the reaction and were analysed by HPLC. The analysis showed the accumulation of betalamic acid, but it did not condense with indoline as expected. In other experiments, the pH of the culture was allowed to evolve freely. In this way, after 48 h, a deep purple indoline‐betacyanin culture with pH = 5.8 was obtained (Fig. 5B). HPLC analysis showed that betalamic acid reacted rapidly with indoline in the synthesis of indoline‐betacyanin and that it accumulated over time, up to a maximum value at 72 h after the addition of the compounds. Thus, betalamic acid requires the medium to be slightly acidic in order to be able to condense with the amines or amino acids available in it. After observing the pronounced pH effect in the synthesis of betalains, and its favourable evolution towards acidic values in order to yield betalains, the following assays were performed without the addition of base. In this sense, the mere addition of L‐DOPA and sodium ascorbate to the bioreactor led to the synthesis of dopaxanthin. The addition, besides, of 2‐phenylethylamine or proline, gave rise to the synthesis of phenylethylamine‐betaxanthin and indicaxanthin respectively. The reaction medium for the biotransformations leading to the betaxanthins showed a final pH = 5.7. The time needed to reach maximum production of each betalain was established after the addition of reagents. Thus, time for optimal production was 48 h for phenylethylamine‐betaxanthin and indicaxanthin, 72 h for indoline‐derived betacyanin, and 96 h for dopaxanthin (Fig. S3).
Figure 5.
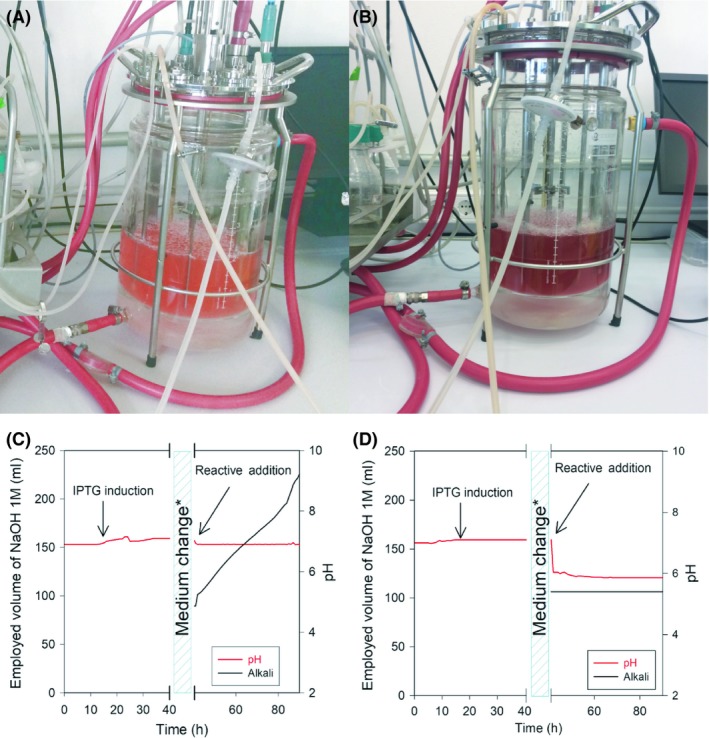
Production of indoline‐betacyanin in 2L bioreactor. Different trends in the synthesis of indoline‐betacyanin can be observed when pH is controlled to 7.0 (A) and when pH drops freely to 5.8 (B). Pictures are shown after 96‐h incubation. When carrying out the reaction at pH 7.0 (C), the consumption of alkali increases progressively due to the tendency of acidification in the synthesis of betalamic acid. D: Effect of stopping the consumption of alkali after the addition of reactives on pH when it evolves freely in the reactor. *Medium change: cells in LB medium were centrifuged and the pellet was resuspended in water.
Purification and quantification
Once the optimal production conditions for each compound had been established, all the media were collected and the pigments purified. Dopaxanthin was collected 96 h after the addition of the reagents, while the betaxanthins phenylethylamine‐betaxanthin and indicaxanthin were recovered 48 h after the addition of amines. The indoline‐derived betalain was collected 72 h after the addition of the reagents. The cultures were then centrifuged twice for 10 min at 5000 g to remove the cell pellet, and the supernatants were analysed by HPLC and submitted to purification. Although the concentration of amines was 5 times higher than L‐DOPA concentration (except for indoline), all the analysed samples showed a residual presence of dopaxanthin, at a much lower concentration than the betalain corresponding to the amine. The proportion of dopaxanthin obtained versus the major betalain was 4.3% in indoline‐betacyanin, 2.1% in phenylethylamine‐betaxanthin and 13.8% in indicaxanthin. L‐DOPA, as a precursor of betalamic acid synthesis, was present in all biotransformation media and the amount of residual dopaxanthin obtained was inversely proportional to the affinity that betalamic acid shows for the major amine.
In order to remove the substrates added and dopaxanthin, the media were purified by automatic anionic exchange chromatography (FPLC) and subsequent extraction in solid‐phase C‐18 columns (Gandía‐Herrero et al., 2006). The compounds were purified with a recovery of 92.70% for dopaxanthin, 91.24% for indoline‐betacyanin, 96.04% for phenylethylamine‐betaxanthin and 42.03% for indicaxanthin (Table S1). Pure compounds obtained in the scaled‐up bioreactors were quantified, with 148.25 mg of dopaxanthin, 112.22 mg of indoline‐betacyanin, 91.11 mg of phenylethylamine‐betaxanthin and 48.75 mg of indicaxanthin. These values are 4 orders of magnitude higher than those obtained by semi‐synthesis, where the limitations of the technique gave between 40 and 90 μg of pure betalains (Gandía‐Herrero et al., 2006).
A novel scalable bioprocess is described here to produce the plant pigment betalains in a fast and simple system. The process is carried out in bioreactors controlled by the heterologous expression of the most efficient DODA enzyme described. When biotransformations are carried out in water, it minimizes the costs of large‐scale biological production and allows the easy purification of the compounds. The number of possible betaxanthins is reduced from eight in enriched media (LB, NZCYM) to only one main pigment in costless water. The scaling‐up of this technique to large bioreactors would enable large amounts of betalains to be obtained and would increase the applicability of their health‐promoting potential in the food, pharmaceutical and cosmetic industries.
Experimental procedures
Chemicals
3,4‐Dihydroxy‐l‐phenylalanine (L‐DOPA), sodium ascorbate, isopropyl β‐d‐1‐thiogalactopyranoside (IPTG), 2‐phenylethylamine, l‐phenylalanine, l‐indoline‐2‐carboxylic acid, indoline, l‐proline, chloramphenicol and kanamycin were obtained from Sigma‐Aldrich (St. Louis, MO, USA). HPLC‐grade acetonitrile was purchased from Fisher Scientific UK (Leicestershire, UK). Distilled water was purified using a Milli‐Q system (Millipore, Bedford, MA, USA).
Plasmids and strains
Escherichia coli Rossetta 2 (DE3) was employed and transformed with the expression vector pET28a, harbouring the coding gene of protein 4,5‐DOPA‐extradiol‐dioxygenase (DODA) from Gluconacetobacter diazotrophicus (pET28a‐GdDODA) (sequence WP_012222467.1, GI:501179334) (Contreras‐Llano et al., 2019).
Standard betalains
Betalamic acid and the betalains indicaxanthin, dopaxanthin, phenylalanine‐betaxanthin, phenylethylamine‐betaxanthin, indoline carboxylic acid‐betacyanin and indoline‐betacyanin were obtained by a semi‐synthesis method (Gandía‐Herrero et al., 2006, 2010a) and subsequently purified for use as real standard betalains for identification purposes.
Production of betalains
The enzyme 4,5‐DOPA‐extradiol dioxygenase (DODA) was chosen for biotransformation assays in E. coli because it is the key enzyme in the betalain synthesis pathway. Escherichia coli, harbouring the above‐mentioned pET28a‐GdDODA, was cultured in Luria‐Bertani (LB) or NZCYM (10 g NZ amine, 5 g NaCl, 5 g yeast extract, 1 g casamino acids and 2 g MgSO4‧7H2O in 1L deionized water, pH 7.0) (Sambrook et al., 2001) media. Both media were supplemented with chloramphenicol (Cm) 35 μg ml−1 and kanamycin (Km) 100 μg ml−1, and bacteria were cultured at 37°C until reaching an O.D.600 nm 0.8‐1.0. Next, IPTG 1 mM was added and the culture was maintained for 15 h at 20°C. After that, the culture was centrifuged 10 min at 5000 g. The pellet obtained was resuspended in Milli‐Q water and kept at 20°C and shaking at 120 rpm. Optimal conditions for the production of each betalain were established considering different concentrations of L‐DOPA and amines, varying from 0.76 to 22.32 mM and from 0.38 to 38 mM respectively. In optimal conditions, L‐DOPA 7.6 mM and sodium ascorbate 15 mM were added. In the case of the addition of amines, these were added at a final optimized concentration of 38 mM, except indoline, which was added at 0.38 mΜ, and l‐indoline‐2‐carboxylic acid, at 3.8 mM.
Scaled‐up production in bioreactor
The EZ‐Control system from Applikon Biotechnology (Delft, The Netherlands) was used to control the production in bioreactors. Escherichia coli harbouring pET28a‐GdDODA was cultured in 20 ml LB Cm Km at 37°C as starter culture. After 15 h, the starter culture was diluted in a reactor with 2 L of LB supplemented with Cm and Km and cultured at 37 °C until an O.D.600 nm 0.8–1.0 was reached. The temperature was then dropped to 20 °C and IPTG 1 mM was added. Fifteen hours later, the culture was centrifuged for 10 min at 5,000 g and the medium was replaced in the reactor by 1 L of sterile Milli‐Q water. Furthermore, 500 ml of sterile water was employed to resuspend the cell pellet and another 500 ml was employed to add L‐DOPA and sodium ascorbate and the corresponding amines, giving a reactor with a final volume of 2 L. The culture was kept in the dark and shaken at 50 rpm and 20°C for 96 h with 50‐65% dO2. dO2 was kept under control by air inlet assisted by the oxygen sensor AppliSens DO2 (Applikon Biotechnology).
Purification of betalains
Pigments were purified with a combination of chromatographic steps. First, solid‐phase extraction in C‐18 cartridges (35 cc, Waters, MA, USA). Columns were conditioned with 70 ml ethanol followed by 70 ml purified water. Betalains were injected and bound to the column and eluted later with ethanol. Afterwards, anionic exchange chromatography of betalains was performed in an Äkta purifier apparatus (General Electric Healthcare, Milwaukee, USA). The equipment was operated via a PC using UNIKORN software version 3.00. Elutions were monitored at 280, 480 and 536 nm. The solvents used were 20 mM sodium phosphate buffer, pH 6.0 (solvent A) and 20 mM sodium phosphate buffer, pH 6.0, with 2 M NaCl (solvent B). A 25 × 16 mm, 20‐ml Q‐Sepharose Fast Flow column (cross‐linked agarose with quaternary ammonium as an exchanger group) purchased from General Electric Healthcare was used. After sample injection, the elution process was as follows: 0% B from 0.0 to 10 ml; after washing, a linear gradient was performed over 120 ml from 0% B to 26% B, with 3 ml fractions being collected. Injection volume was 25 ml, and the flow rate was 2.5 ml min−1. Pigment containing fractions were pooled, and salts were removed through C‐18 solid‐phase extraction (Gandía‐Herrero et al., 2006).
HPLC analysis
A Shimadzu LC‐10A apparatus (Kyoto, Japan) equipped with a SPD‐M10A PDA detector was used for analytical HPLC separations. Reversed phase chromatography was performed with a 250 x 4.6 mm Kinetex 5μ C‐18 column (Phenomenex, Torrance, CA, USA). Gradients were formed with the following solvents: solvent A was water with 0.05% TFA, and solvent B was composed of acetonitrile with 0.05% TFA. A linear gradient was performed for 25 min from 0% B to 35% B. The flow rate was 1 ml min−1, operated at 25°C. Injection volume was 20 μL. The culture media were centrifuged 1 min at 14,000 g, and the supernatants were analysed. The resulting pellets were sonicated with a Branson Digital sonifier (Branson Ultrasonic Corporation, Connecticut, USA) with 5 pulses of 10 s (medium intensity) used to disrupt cells. The cell content was analysed under the same conditions.
Electrospray ionization mass analysis
An Agilent VL 1100 apparatus with LC/MSD Trap (Agilent Technologies, Palo Alto, CA, USA) was used for HPLC–ESI–MS analyses. Elution conditions were the same as described above (HPLC analysis section) using a Kinetex 5μ C‐18 column with a flow rate of 0.8 ml min−1. Vaporizer temperature was 350°C, and voltage was maintained at 3.5 kV. The sheath gas was nitrogen, operated at a pressure of 45 psi. Samples were ionized in positive mode. Ion monitoring mode was full scan in the range m/z 50–600. The electron multiplier voltage for detection was 1,350 V.
Conflict of interest
None declared.
Supporting information
Fig. S1. A. Production of betalamic acid at different concentrations of L‐DOPA (mM). Production is expressed in % considering the highest value as 100% production. B. Time course of the betalamic acid and dopaxanthin production. C. Chromatogram at λ=405 nm of LB medium after addition of L‐DOPA. The use of the betalamic acid standard (below) confirms its presence. D. Chromatogram at λ = 480 nm of LB medium after addition of L‐DOPA. The use of a dopaxanthin standard (below) confirms this presence as a major peak with a retention time of 13.9 min.
Fig.S2. Effect of aeration in the microbial factories. Production was followed for 4,5‐seco‐DOPA (A), betalamic acid (B) and dopaxanthin (C). D. Products accumulation was due to E. coli (pET28a‐GdDODA) biotransformation in water solution supplemented with L‐DOPA in a shaken, not shaken and anoxia media. Anoxia conditions were created with deaeration and nitrogen atmosphere.
Fig. S3. Time evolution of the scaled‐up synthesis of betalains in bioreactor (volume of 2L).
Table S1. Betalain production in 2L bioreactors.
Acknowledgements
The authors are grateful to Dr. José Rodriguez and to Dr. Almudena Gutierrez (SAI, University of Murcia, Spain) for expert technical assistance in ESI‐MS/MS mass spectrometry experiments and biomass production respectively. This work was supported by the Spanish Ministry of Economy and Competitiveness (MEC‐FEDER, Spain) (Projects AGL2014‐57431 and AGL2017‐86526) and by the ‘Programa de Ayudas a Grupos de Excelencia de la Región de Murcia, Fundación Séneca, Agencia de Ciencia y Tecnología de la Región de Murcia’ (Project 19893/GERM/15). M.A.G.‐R. holds a contract financed by MEC‐FEDER (Spain). The authors declare no competing financial interest.
Microbial Biotechnology (2019) 12(5), 993–1002
Funding Information
This work was supported by the Spanish Ministry of Economy and Competitiveness (MEC‐FEDER, Spain) (Projects AGL2014‐57431 and AGL2017‐86526) and by the ‘Programa de Ayudas a Grupos de Excelencia de la Región de Murcia, Fundación Séneca, Agencia de Ciencia y Tecnología de la Región de Murcia’ (Project 19893/GERM/15). M.A.G.‐R. holds a contract financed by MEC‐FEDER (Spain).
References
- Ahmadian, E. , Khosroushahi, A.Y. , Eghbal, M.A. , and Eftekhari, A. (2018) Betanin reduces organophosphate induced cytotoxicity in primary hepatocyte via an anti‐oxidative and mitochondrial dependent pathway. Pestic Biochem Physiol 144: 71–78. [DOI] [PubMed] [Google Scholar]
- Cai, Y. , Sun, M. , and Corke, H. (2003) Antioxidant activity of betalains from plants of the Amaranthaceae . J Agric Food Chem 51: 2288–2294. [DOI] [PubMed] [Google Scholar]
- Castellanos‐Santiago, E. , and Yahia, E.M. (2008) Identification and quantification of betalains from the fruits of 10 Mexican prickly pear cultivars by high‐performance liquid chromatography and electrospray ionization mass spectrometry. J Agric Food Chem 56: 5758–5764. [DOI] [PubMed] [Google Scholar]
- Christinet, L. , Burdet, F. , Zaiko, M. , Hinz, U. , and Zrÿd, J.P. (2004) Characterization and functional identification of a novel plant 4,5‐extradiol dioxygenase involved in betalain pigment biosynthesis in Portulaca grandiflora . Plant Physiol 134: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras‐Llano, L.E. , Guerrero‐Rubio, M.A. , Lozada‐Ramirez, J.D. , García‐Carmona, F. , and Gandía‐Herrero, F. (2019) First betalain producing bacteria break the exclusive presence of the pigments in the plant kingdom. mBio 10: e00345‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escribano, J. , Cabanes, J. , Jiménez‐Atiénzar, M. , Ibañez‐Tremolada, M. , Gómez‐Pando, L.R. , García‐Carmona, F. , and Gandía‐Herrero, F. (2017) Characterization of betalains, saponins and antioxidant power in differently colored quinoa (Chenopodium quinoa) varieties. Food Chem 234: 285–294. [DOI] [PubMed] [Google Scholar]
- Gandía‐Herrero, F. , and García‐Carmona, F. (2014) Escherichia coli protein YgiD produces the structural unit of plant pigments betalains: characterization of a prokaryotic enzyme with DOPA‐extradiol‐dioxygenase activity. Appl Microbiol Biotechnol 98: 1165–1174. [DOI] [PubMed] [Google Scholar]
- Gandia‐Herrero, F. , Escribano, J. , and García‐Carmona, F. (2005) Betaxanthins as substrates for tyrosinase. An approach to the role of tyrosinase in the biosynthetic pathway of betalains. Plant Physiol 138: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandía‐Herrero, F. , Escribano, J. , and García‐Carmona, F. (2005) Betaxanthins as pigments responsible for visible fluorescence in flowers. Planta 222: 586–593. [DOI] [PubMed] [Google Scholar]
- Gandía‐Herrero, F. , García‐Carmona, F. , and Escribano, J. (2006) Development of a protocol for the semi‐synthesis and purification of betaxanthins. Phytochem Anal 17: 262–269. [DOI] [PubMed] [Google Scholar]
- Gandía‐Herrero, F. , Jiménez‐Atiénzar, M. , Cabanes, J. , Escribano, J. , and García‐Carmona, F. (2009) Fluorescence detection of tyrosinase activity on dopamine‐betaxanthin purified from Portulaca oleracea (common purslane) flowers. J Agric Food Chem 57: 2523–2528. [DOI] [PubMed] [Google Scholar]
- Gandía‐Herrero, F. , Escribano, J. , and García‐Carmona, F. (2010a) Structural implications on color, fluorescence, and antiradical activity in betalains. Planta 232: 449–460. [DOI] [PubMed] [Google Scholar]
- Gandía‐Herrero, F. , Jiménez‐Atiénzar, M. , Cabanes, J. , García‐Carmona, F. , and Escribano, J. (2010b) Stabilization of the bioactive pigment of Opuntia fruits through maltodextrin encapsulation. J Agric Food Chem 58: 10646–10652. [DOI] [PubMed] [Google Scholar]
- Gandía‐Herrero, F. , Cabanes, J. , Escribano, J. , García‐Carmona, F. , and Jimenez‐Atienzar, M. (2013) Encapsulation of the most antioxidant betalains in edible matrices as powders of different colors. J Agric Food Chem 61: 4294–4302. [DOI] [PubMed] [Google Scholar]
- Gandía‐Herrero, F. , Escribano, J. , and García‐Carmona, F. (2016) Biological activities of plant pigments betalains. Crit Rev Food Sci Nutr 56: 937–945. [DOI] [PubMed] [Google Scholar]
- Gliszczyńska‐Świgło, A. , Szymusiak, H. , and Malinowska, P. (2006) Betanin, the main pigment of red beet: Molecular origin of its exceptionally high free radical‐scavenging activity. Food Addit Contam 23: 1079–1087. [DOI] [PubMed] [Google Scholar]
- Guadarrama‐Flores, B. , Rodriguez‐Monroy, M. , Cruz‐Sosa, F. , García‐Carmona, F. , and Gandía‐Herrero, F. (2015) Production of dihydroxylated betalains and dopamine in cell suspension cultures of Celosia argentea var. plumosa. J Agric Food Chem 63: 2741–2749. [DOI] [PubMed] [Google Scholar]
- Guerrero‐Rubio, M.A. , Hernández‐García, S. , García‐Carmona, F. , and Gandía‐Herrero, F. (2019) Extension of life‐span using a RNAi model and in vivo antioxidant effect of Opuntia fruit extracts and pure betalains in Caenorhabditis elegans . Food Chem 274: 840–847. [DOI] [PubMed] [Google Scholar]
- Henarejos‐Escudero, P. , Guadarrama‐Flores, B. , Guerrero‐Rubio, M.A. , Gómez‐Pando, L.R. , García‐Carmona, F. , and Gandía‐Herrero, F. (2018) Development of betalain producing callus lines from colored quinoa varieties (Chenopodium quinoa Willd). J Agric Food Chem 66: 467–474. [DOI] [PubMed] [Google Scholar]
- Impellizzeri, G. , Piattelli, M. , and Sciuto, S. (1973) A new betaxanthin from Glottiphyllum longum . Phytochemistry 12: 2293–2294. [Google Scholar]
- Khan, M.I. , Sri Harsha, P.S.C. , Giridhar, P. , and Ravishankar, G.A. (2012) Pigment identification, nutritional composition, bioactivity, and in vitro cancer cell cytotoxicity of Rivina humilis L. berries, potential source of betalains. LWT – Food Sci Technol 47: 315–323. [Google Scholar]
- Lechner, J.F. , Wang, L.‐S. , Rocha, C.M. , Larue, B. , Henry, C. , McIntyre, C.M. , et al (2010) Drinking water with red beetroot food color antagonizes esophageal carcinogenesis in N‐nitrosomethylbenzylamine‐treated rats. J Med Food 13: 733–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milech, C. , Lucho, S.R. , Kleinowski, A.M. , Dutra, D.B. , Soares, M.M. , Jacira, E. , and Braga, B. (2017) Production of pigments in Alternanthera sessilis calli mediated by plant growth regulators and light. Acta Sci Biol Sci Mar 39: 381–388. [Google Scholar]
- Musso, H. (1979) The pigments of fly agaric, Amanita muscaria . Tetrahedron 35: 2843–2853. [Google Scholar]
- Sambrook, J. , Fritsch, E.F. , and Maniatis, T. (2001) Molecular Cloning: A Laboratory Manual. New York, NY, USA: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Sasaki, N. , Abe, Y. , Goda, Y. , Adachi, T. , Kasahara, K. , and Ozeki, Y. (2009) Detection of DOPA 4,5‐dioxygenase (DOD) activity using recombinant protein prepared from Escherichia coli cells harboring cDNA encoding DOD from Mirabilis jalapa. Plant Cell Physiol 50: 1012–1016. [DOI] [PubMed] [Google Scholar]
- Sreekanth, D. , Arunasree, M.K. , Roy, K.R. , Chandramohan Reddy, T. , Reddy, G.V. , and Reddanna, P. (2007) Betanin a betacyanin pigment purified from fruits of Opuntia ficus‐indica induces apoptosis in human chronic myeloid leukemia cell line‐K562. Phytomedicine 14: 739–746. [DOI] [PubMed] [Google Scholar]
- Steiner, U. , Schliemann, W. , Böhm, H. , and Strack, D. (1999) Tyrosinase involved in betalain biosynthesis of higher plants. Planta 208: 114–124. [Google Scholar]
- Sunnadeniya, R. , Bean, A. , Brown, M. , Akhavan, N. , Hatlestad, G. , Gonzalez, A. , et al (2016) Tyrosine hydroxylation in betalain pigment biosynthesis is performed by cytochrome P450 enzymes in beets (Beta vulgaris). PLoS One 11: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von‐Ardenne, R. , Döpp, H. , Musso, H. , and Steglich, W. (1974) Isolation of muscaflavin from Hygrocybe species (Agaricales) and its dihydroazepine structure. Z Naturforsch 29: 637–639. [Google Scholar]
- Wendel, M. , Szot, D. , Starzak, K. , Tuwalska, D. , Prukala, D. , Pedzinski, T. , et al (2015) Photophysical properties of indicaxanthin in aqueous and alcoholic solutions. Dye Pigment 113: 634–639. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. A. Production of betalamic acid at different concentrations of L‐DOPA (mM). Production is expressed in % considering the highest value as 100% production. B. Time course of the betalamic acid and dopaxanthin production. C. Chromatogram at λ=405 nm of LB medium after addition of L‐DOPA. The use of the betalamic acid standard (below) confirms its presence. D. Chromatogram at λ = 480 nm of LB medium after addition of L‐DOPA. The use of a dopaxanthin standard (below) confirms this presence as a major peak with a retention time of 13.9 min.
Fig.S2. Effect of aeration in the microbial factories. Production was followed for 4,5‐seco‐DOPA (A), betalamic acid (B) and dopaxanthin (C). D. Products accumulation was due to E. coli (pET28a‐GdDODA) biotransformation in water solution supplemented with L‐DOPA in a shaken, not shaken and anoxia media. Anoxia conditions were created with deaeration and nitrogen atmosphere.
Fig. S3. Time evolution of the scaled‐up synthesis of betalains in bioreactor (volume of 2L).
Table S1. Betalain production in 2L bioreactors.


