Abstract
Background:
Autoimmune disorders including nephropathies have been reported more frequently in alemtuzumab-treated multiple sclerosis (MS) patients than in the general population.
Objective:
Describe instances of autoimmune nephropathy in alemtuzumab-treated MS patients.
Methods:
Cases were identified from safety monitoring within the alemtuzumab relapsing-remitting multiple sclerosis (RRMS) clinical development program (CDP) or post-marketing, or following off-label use.
Results:
As of 16 June 2017, 16 autoimmune nephropathies have occurred following alemtuzumab treatment for MS. The incidence of autoimmune nephropathies was 0.34% within the CDP (5/1485 patients). The five CDP cases (one of anti-glomerular basement membrane (anti-GBM) disease, two of membranous glomerulonephropathy, and two of serum anti-GBM antibody without typical anti-GBM disease) were identified early, responded to conventional therapy (where needed), and had favorable outcomes. Three of 11 cases outside the CDP occurred following off-label alemtuzumab use prior to approval for RRMS and were all anti-GBM disease. Diagnosis was delayed in one of these three cases and another did not receive appropriate treatment; all three cases resulted in end-stage renal failure. All anti-GBM disease cases with documented urinalysis demonstrated prior microscopic hematuria.
Conclusion:
Close monitoring of alemtuzumab-treated MS patients facilitates diagnosis and treatment early in the nephropathy course when preservation of renal function is more likely.
Keywords: Alemtuzumab, anti-glomerular basement membrane disease, disease-modifying therapy, membranous glomerulonephropathy, multiple sclerosis, nephropathy
Introduction
Anti-glomerular basement membrane (anti-GBM) disease following alemtuzumab therapy in multiple sclerosis (MS) patients was first reported in 2006.1 Subsequently, there has been intense renal surveillance during clinical trial2–6 and non-trial treatments.
Although drug-related nephropathies are well described with several mechanisms recognized,7 alemtuzumab-related nephropathies occur in the context of an increased risk of various de novo autoimmune disorders following alemtuzumab treatment for MS, most commonly thyroid autoimmune conditions (5-year incidences: all thyroid disorders 40%–45%; serious thyroid adverse events 4%–5%)8,9 and immune thrombocytopenia (1%–3%).8–10
Alemtuzumab was initially approved (Campath®/MabCampath®) for treatment of B-cell chronic lymphocytic leukemia;11 it has also been used in off-label applications, including MS (prior to approval for this indication),1 refractory vasculitis,12 solid organ transplantation,13 and as a conditioning regimen for hematopoietic stem cell transplantation.14 Alemtuzumab (LEMTRADA®) has been approved in more than 70 countries for use in adults with relapsing forms of MS, including countries of the European Union for patients with active relapsing-remitting multiple sclerosis (RRMS) defined by clinical and imaging features, and in the US for patients who generally have had an inadequate response to two or more prior therapies.15,16 For RRMS, alemtuzumab is dosed as two initial treatment courses at baseline and 12 months later, and additional courses (up to two in Europe; no limit in the United States) as needed, administered ⩾12 months after the previous dose.15,16 The drug is detectable in serum for ~30 days after administration.15
De novo autoimmunity may relate to the proposed mechanism of action of alemtuzumab. This humanized monoclonal antibody targets B and T lymphocytes (both CD4+ and CD8+ T cells) for depletion,17,18 followed by a distinctive pattern of B- and T-cell repopulation. B cells return to baseline levels within approximately 6 months; T cells recover more slowly, with levels generally reaching lower limits of normal by 12 months.19 Relative increases in memory and regulatory T-cell levels occur during immune reconstitution, resulting in a shift from pro- to anti-inflammatory cytokine profiles.20 Decreases in circulating B and T cells and their altered behavior upon subsequent repopulation may explain the efficacy of alemtuzumab in reducing the risk of relapses, delaying disease progression, and providing efficacy in the absence of continuous treatment.19,21,22 Additional mechanistic studies are required to establish this hypothesis.
Clinical trials have established alemtuzumab as a highly efficacious disease-modifying therapy in patients with active RRMS.2–4 Effects on disease activity include improvements in relapse and disability outcomes, reduction in the occurrence of new brain magnetic resonance imaging (MRI) lesions, and reduction of brain volume loss to levels similar to those in people without MS.8,9,23–27
The purpose of this article is to review the cases of de novo renal autoimmunity in MS patients treated with alemtuzumab within or outside of the clinical development program (CDP) to better understand their natural history, and to assess implications for monitoring and treatment.
Patients and methods
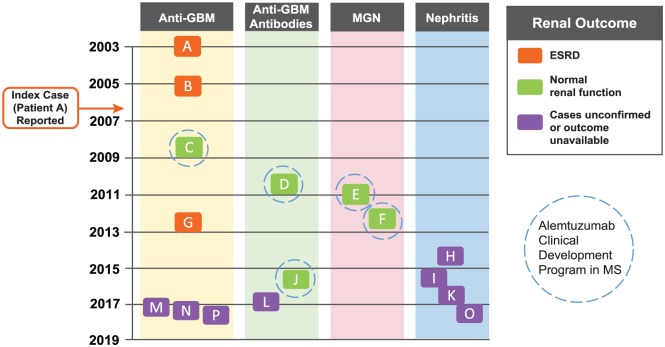
Cases of autoimmune nephropathy arising in alemtuzumab-treated MS patients are reported as of 16 June 2017. Cases occurring within the CDP were identified under a safety monitoring program run by the manufacturer that included renal monitoring (Table 1). Nephropathy cases are described alphabetically based on overall chronology (Figure 1). Five cases occurred within the CDP (patients C–F and J), comprising the phase 2 CAMMS223 (NCT00050778) trial,2 phase 3 CARE-MS I (NCT00530348) and CARE-MS II (NCT00548405) trials,3,4 4-year open-label CAMMS03409 extension study (NCT00930553),8,9 and ongoing TOPAZ extension study (NCT02255656).29 Patients provided written informed consent for the analyses described in this study. Surveillance in these trials included measurements of serum creatinine, urine dipstick tests for proteinuria and hematuria, and urine microscopy (monitoring frequency was initially quarterly, but increased to monthly after the first CDP case (patient C) was reported). Additional nephropathy cases have occurred post-marketing, and outside of either the CDP or post-marketing setting (patients A, B, and G) following off-label use of alemtuzumab (Campath®/MabCampath®). A Medline search revealed no additional reported cases.
Table 1.
Summary of routine renal safety monitoring within the alemtuzumab CDP and post-marketing.
| Renal monitoring within the CDP |
|---|
| ● Monitoring of renal function in CAMMS223 initially included
quarterly serum creatinine testing2
● Following the detection of anti-GBM disease in patient C in CAMMS223, renal monitoring for all trials was enhanced to include the following:6,10 - Monthly evaluation of serum creatinine levels and urinalysis with microscopy in all study patients who received alemtuzumab - Intensified surveillance in any patient with abnormal renal results, with criteria for urgent or non-urgent referral to a nephrologist - Education for investigators and patients about anti-GBM disease - Monthly patient questionnaires that queried for the presence of hematuria, offset by 2 weeks from laboratory collections |
| Renal monitoring post-marketing |
| ● Risk Evaluation and Mitigation Strategy in the United States
and Risk Management Plan in the European Union and other
regions, including healthcare professional and patient
education15,28
● Alemtuzumab labeling recommends (a requirement in the United States) that serum creatinine levels and urinalysis with cell counts are obtained prior to initiation of treatment and at monthly intervals thereafter until 48 months after the last infusion15,16 |
CDP: clinical development program; GBM: glomerular basement membrane.
Figure 1.
Cases of nephropathy associated with alemtuzumab treatment in MS patients. The nephropathy cases are shown chronologically based on date of diagnosis, and divided according to disease type (anti-GBM disease, anti-GBM antibodies, MGN, and nephritis) and renal outcome. The five cases occurring within the CDP resolved with normal renal function (patients C–F and J). The three cases of anti-GBM disease occurring during off-label use (patients A, B, and G) resulted in end-stage renal disease (ESRD; highlighted in orange). The eight post-marketing cases (patients H, I, and K–P) require further follow-up to determine outcomes; four of these eight cases (patients H, I, K, and O) involved possible immunological renal disease where full information could not be obtained; patients I and O had probable anti-GBM disease, but insufficient information was available to confirm this diagnosis. Patient A (index case) was diagnosed in April 2003 and reported in 2006.1 Dates of diagnosis for the other cases were: patient B, March 2005; patient C, September 2008; patient D, October 2010; patient E, January 2011; patient F, April 2012; patient G, July 2012; patient H, July 2014; patient I, during 2015; patient J, August 2015; patient K, September 2016; patient L, December 2016; patient M, March 2017; patient N, April 2017; patient O, date unknown (reported May 2017); patient P, date unknown (reported June 2017).
CDP: clinical development program; GBM: glomerular basement membrane; MGN: membranous glomerulonephropathy.
Details of each case were obtained from MedWatch reports and the treating physicians. When available, these included clinical histories, laboratory analyses, and pathology data. Anti-GBM disease cases were classified as those in which a renal biopsy demonstrated crescentic glomerulonephritis with linear deposition of immunoglobulin G (IgG), or there was severe nephritis without interpretable immunofluorescence imaging, but high levels of circulating anti-GBM antibodies. Biopsies from patients C, E, and F were available for review by a renal pathologist (G.S.M.). The status of patients’ MS was also evaluated for cases occurring in the CDP.
Results
Sixteen cases of autoimmune nephropathy have been reported. The first two occurred in an investigator-sponsored pilot study in MS patients1 before commencement of the alemtuzumab CDP in RRMS. Five cases occurred within the CDP, and one case occurred during independent, physician off-label use of alemtuzumab before its approval for RRMS. Eight further cases have been reported post-marketing. The CDP and post-marketing settings included close monitoring of renal function as part of the trial protocols6 and Risk Management Plan/Risk Evaluation and Mitigation Strategy,15,28 respectively (Table 1). All cases of autoimmune nephropathy in MS patients treated with alemtuzumab occurred (where known) in patients aged 25–58 years, and with onset within 39 months of the last alemtuzumab administration in all but one patient. The exception was a male patient who, 60 months after treatment, developed a transitory and self-remitting elevation in serum creatinine with detectable serum anti-GBM antibodies, possibly in relation to a mild infectious illness (patient J).
The incidence of autoimmune nephropathies (including anti-GBM disease) with alemtuzumab within the CDP was 0.34%, with a total of 1485 patients at risk and 8632 patient-years of follow-up (median follow-up 6.1 years (range, 0.7–12.7 years)). Through 16 June 2017, approximately 15,000 patients were treated worldwide for MS with alemtuzumab (LEMTRADA®). Based on the nephropathy cases reported so far, this corresponds to an estimated, point in time, post-marketing frequency rate of approximately 0.05%.
Anti-GBM disease cases
Seven patients developed anti-GBM disease, verified in most cases by biopsy showing the presence of crescentic glomerulonephritis and linear deposition of immunoglobulin along the GBM (Table 2). Two of these (patients A (index case) and B) were treated with alemtuzumab at doses higher than those currently approved for treatment of RRMS and with a different treatment regimen. Six cases exhibited rapid loss of renal function between 9 and 39 months after last receiving alemtuzumab (time to event onset not available for patient P). In five cases, there was a period of at least 1 month during which hematuria and/or elevated serum creatinine was detected before accelerating loss of renal function (pre-onset urinalysis not available for patients A, N, and P).
Table 2.
Cases of anti-GBM disease.
| Patient | Age at diagnosis; gender | Context | Cumulative alemtuzumab dose (mg) |
Onset from last alemtuzumab dose (months) | First symptoms to treatment for nephropathy (weeks) | Symptoms | Serum creatinine, µmol/L (mg/dL)a | Hematuria | Serum anti-GBM antibody | Renal pathology | Treatment for nephropathy | Renal outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 42; F | Investigator-sponsored pilot study | 100 | 9 | < 1 | Peripheral edema, fever, malaise, reduced urine output | 495 (5.6) | Yes | Yes | Crescentic glomerulonephritis (95% crescents) and linear IgG deposition along GBM | Plasmapheresis, corticosteroids, cyclophosphamide | Renal transplant |
| B | 34; F | Investigator-sponsored pilot study | 160 | 24 | ~4b | Fatigue, weight gain, influenza-like illness, dark urine, edema | 972 (11) | Yes | Yes | Severe crescentic glomerulonephritis and linear IgG deposition along GBM | Plasmapheresis (5 months), steroids, mycophenolate mofetil | Dialysis; renal transplant 12 months after diagnosis |
| C | 35; F | CAMMS223 | 96 | 39 | 5 | Proteinuria detected by screening in context of upper respiratory tract infection and rash on thighs | 230 (2.6)c,5 | Yes | Not detected | Necrotizing and crescentic glomerulonephritis (16% crescents) and linear IgG deposition along GBM | Plasmapheresis (every other day for 3 weeks), cyclophosphamide (cumulative dose of approximately 13.5 g over 6 months), and prednisone (for 6 months) | GFR was normal (>60 mL/min) at last follow-up (26 months after diagnosis)5 |
| G | 30; F | Independent, off-label physician use | 168 | ~24 | 16 | Proteinuria detected by screening | 62 (0.7) | Yes | Yes | Crescentic glomerulonephritis (15% crescents at diagnosis) and linear IgG along GBM. A subsequent biopsy found ⩾ 50% glomeruli globally sclerotic | Plasmapheresis (3×/week), prednisone, corticotropin, cyclophosphamide (1268 mg; 5 cycles) | On dialysis (6 months after diagnosis), followed by renal transplant; normal renal function post-transplant |
| M | 38; M | Post-marketing | 96 | 3 | < 2 | Weight loss, anuria, peripheral edema, elevated serum creatinine, hematuria, proteinuria, hemoptysis, pleural effusion | 948 (10.7) | Yes | Yes | Severe nephritis; hemorrhagic infarction of the renal cortex; no glomeruli on IF imaging | Dialysis, plasmapheresis, daily cyclophosphamide, steroids; subsequently, cyclophosphamide (oral, 75 mg/day), methylprednisolone (IV, 1 g x3), then prednisone (60 mg/day) and plasmapheresis daily (10 doses total). Prednisone and cyclophosphamide were continued; other drugs included ergocalciferol and ranitidine | At most recent follow-up (7 weeks after diagnosis), patient receiving dialysis 3x/week; serum creatinine elevated |
| N | 30; F | Post-marketing | 96 | 6 | < 2 | Headache, nausea, lethargy, renal failure | N/A | N/A | Yes | Extensive renal injury; 98% crescentic glomerulonephritis | Plasma exchange, dialysis, rituximab | At most recent follow-up (3 weeks after diagnosis) symptoms of renal failure ongoing |
| P | N/A; N/A | Post-marketing | N/A | N/A | N/A | Anti-GBM disease (no prior symptoms reported) | N/A | N/A | Yes | Significant renal damage | Hemodialysis | Awaiting further follow-up |
F: female; GBM: glomerular basement membrane; GFR: glomerular filtration rate; IF: immunofluorescence; IgG: immunoglobulin G; M: male; N/A: not available.
Cases were classified as anti-GBM disease where a renal biopsy demonstrated crescentic glomerulonephritis with linear deposition of IgG, or severe nephritis without interpretable IF imaging but high levels of circulating anti-GBM antibodies.
Serum creatinine measurement at, or closest to, time of nephropathy event.
Patient B was treated in the month following diagnosis.
Value based on unscheduled lab measurement not reflected in Figure 2.
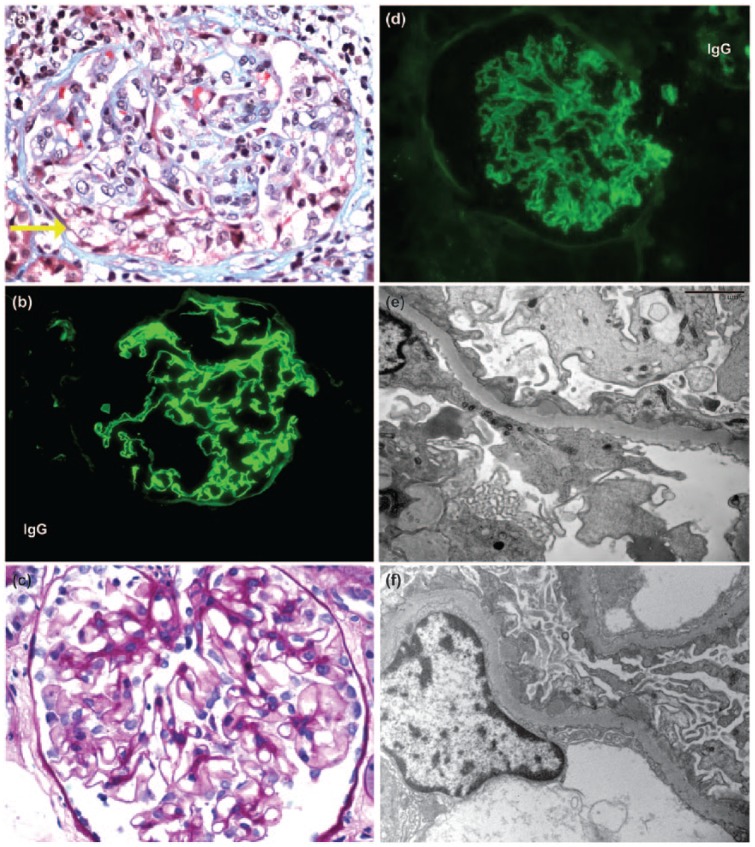
Anti-GBM antibodies were detected at the time of recognition of acute kidney failure in 6 patients (patient C tested negative). Analysis of serum taken before recognition of acute kidney failure revealed that patient A had no detectable serum anti-GBM antibodies 1 month prior.1 Patient G had detectable anti-GBM antibodies and microscopic hematuria when serum creatinine was still normal. HLA genotyping data were only available for patient A, who was found to carry a HLA DRB1*15 allele.30 Alleles of the major histocompatibility complex defined as HLA DRB1*15 are associated with an increased risk for anti-GBM disease and MS.31,32 Kidney biopsy data available for 5 patients revealed crescentic glomerulonephritis with linear IgG deposition (where measured) consistent with anti-GBM disease, including the single case (patient C) with histopathology images available for review (Figure 4(a) and (b)). The percentage of glomeruli with crescents ranged from 15%–98% (Table 2).
Figure 4.
Renal biopsy findings. (a) Patient C: by light microscopy, a glomerulus exhibits a segmental cellular crescent (yellow arrow), characteristic of anti-GBM disease (trichrome, 400× magnification). (b) Patient C: immunofluorescence reveals linear staining of the GBM for IgG, diagnostic of anti-GBM disease (300× magnification). (c) Patient E: by light microscopy, a glomerulus appears unremarkable in this patient with stage 1 MGN (400× magnification). (d) Patient E: immunofluorescence reveals granular global staining of the GBM (300× magnification). (e) Patient E: electron microscopy reveals global small subepithelial electron dense deposits, without significant intervening GBM spikes, diagnostic of stage 1 MGN (5000× magnification). (f) Patient F: similar to patient E, stage 1 changes of MGN are seen on ultrastructural evaluation of the biopsy on patient F (5000× magnification). GBM: glomerular basement membrane; MGN: membranous glomerulonephropathy.
All 7 patients received conventional treatment, typically with combinations of plasmapheresis, dialysis, cyclophosphamide, and steroids; however, there was variation in the regimens employed and timing of therapy in relation to the onset of renal failure. Patients A and B developed disease outside of a close monitoring program. Despite being diagnosed and treated (including plasmapheresis and steroids) within 3 days of first symptoms, and within the month after diagnosis, respectively, neither patient recovered renal function; however, both survived and underwent successful renal transplantation without recurrence of anti-GBM disease. Patient C was diagnosed through the CDP monitoring program and was treated with plasmapheresis, cyclophosphamide, and steroids within 6 weeks of first signs of elevated serum creatinine (Figure 2); the patient recovered and retained independent near-normal renal function. Patient G was treated within 2 months of first symptoms of hematuria when she had intact renal function and only 15% crescents on renal biopsy. The patient was treated solely with plasmapheresis (she declined cyclophosphamide), and withdrew from plasmapheresis for 1 week due to personal reasons approximately 1 month after diagnosis. She subsequently experienced a rapid deterioration in renal function. Full conventional treatment with plasmapheresis, cyclophosphamide, and steroids was introduced when renal failure was advanced (serum creatinine 530 µmol/L (6 mg/dL)). After 6 months on dialysis and disappearance of circulating anti-GBM autoantibodies, the patient underwent successful renal transplantation without recurrence of anti-GBM disease. All 3 post-marketing cases (patients M, N, and P) were dialysis-dependent at most recent follow-up based on the information available.
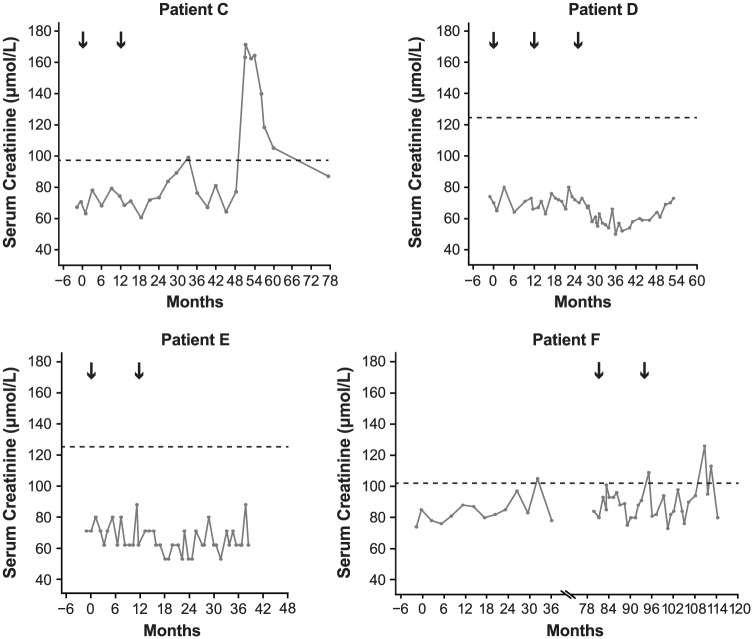
Figure 2.
Serum creatinine over time for nephropathy cases occurring during the CDP. Black arrows indicate alemtuzumab treatment courses. Horizontal dashed line indicates upper limit of normal established in each testing laboratory using differently calibrated control levels. Months shown are from first alemtuzumab treatment. Scale of horizontal axes varies due to differing timing of treatment and event onset in each patient. Patient F received subcutaneous interferon beta-1a (44 µg 3× weekly) in the CAMMS223 trial (Month 0 to Month 36) before receiving alemtuzumab.
CDP: clinical development program.
Cases in which anti-GBM antibodies occurred without typical anti-GBM disease
Three cases are reported in which anti-GBM antibodies were detected without classical anti-GBM disease (Table 3). Patient D presented as nephrotic syndrome with normal glomerular filtration rate (GFR), but the patient’s serum contained a very low titer (just above the laboratory’s reference range) of anti-GBM antibodies. The renal biopsy was reported as membranous nephropathy, but subsequent small rises in serum anti-GBM antibody titer prompted initiation of plasmapheresis, cyclophosphamide, and steroids, resulting in remission of nephrosis and disappearance of anti-GBM antibodies. At 39 months after last treatment for nephropathy, the patient was in remission requiring no medication, anti-GBM antibodies were not detectable, serum creatinine was within the normal range (Figure 2), and there was no detectable proteinuria (Figure 3). Patient J developed a transitory serum anti-GBM antibody titer in the context of a brief self-limiting disease, and patient L was diagnosed with pauci-immune vasculitis and managed accordingly.
Table 3.
Cases in which anti-GBM antibodies occurred without typical anti-GBM disease.
| Patient | Age at diagnosis; gender | Context | Cumulative dose (mg) | Onset from last alemtuzumab dose (months) | First symptoms to treatment for nephropathy (weeks) | Symptoms | Serum creatinine, µmol/L (mg/dL)a | Hematuria | Serum anti-GBM antibody | Renal pathology | Treatment for nephropathy | Renal outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | 25; F | CARE-MS extension study (previously CARE-MS I) | 132 | 4 | 14 | Hematuria, proteinuria | 58 (0.7) | Yes | Yes | Weak evidence for mild, stage 1 MGN, linear staining of GBM for IgG and lambdab | Fosinopril, plasmapheresis, methylprednisolone (500 mg IV), prednisolone (60 mg), cyclophosphamide, omeprazole | At last follow-up (39 months after diagnosis), patient in remission without clinical sequelae and with preserved renal function |
| J | 36; M | CARE-MS extension study (previously CARE-MS II) | 96 | 60 | N/A | Fever | 124 (1.4) | No | Yes | N/A | No treatment required | At last follow-up (32 months after event), kidney function and serum creatinine levels normal |
| L | 34; F | Post-marketing | 96 | 6 | <1 | Anemia; pulmonary hemorrhage, febrile episodes; elevated serum creatinine; oliguria, acute kidney injury; low GFR | 469 (5.3) | Yes | Yes | 1 early crescent and 1 cellular crescent; biopsy also suggested possible ANCA-negative pauci-immune vasculitis | Hemodialysis (3×/week) and plasma exchange (2×/week), then treatment ongoing: Hemodialysis (3x/week) and plasma exchange (2x/week), prednisolone, mycophenolate | Unknown |
GBM: glomerular basement membrane; MGN: membranous glomerulonephropathy; IgG: immunoglobulin G; F: female; M: male; GFR: glomerular filtration rate; ANCA: antineutrophil cytoplasmic antibody.
Serum creatinine measurement at, or closest to, time of nephropathy event.
Pathology findings based on limited biopsy material.
Figure 3.
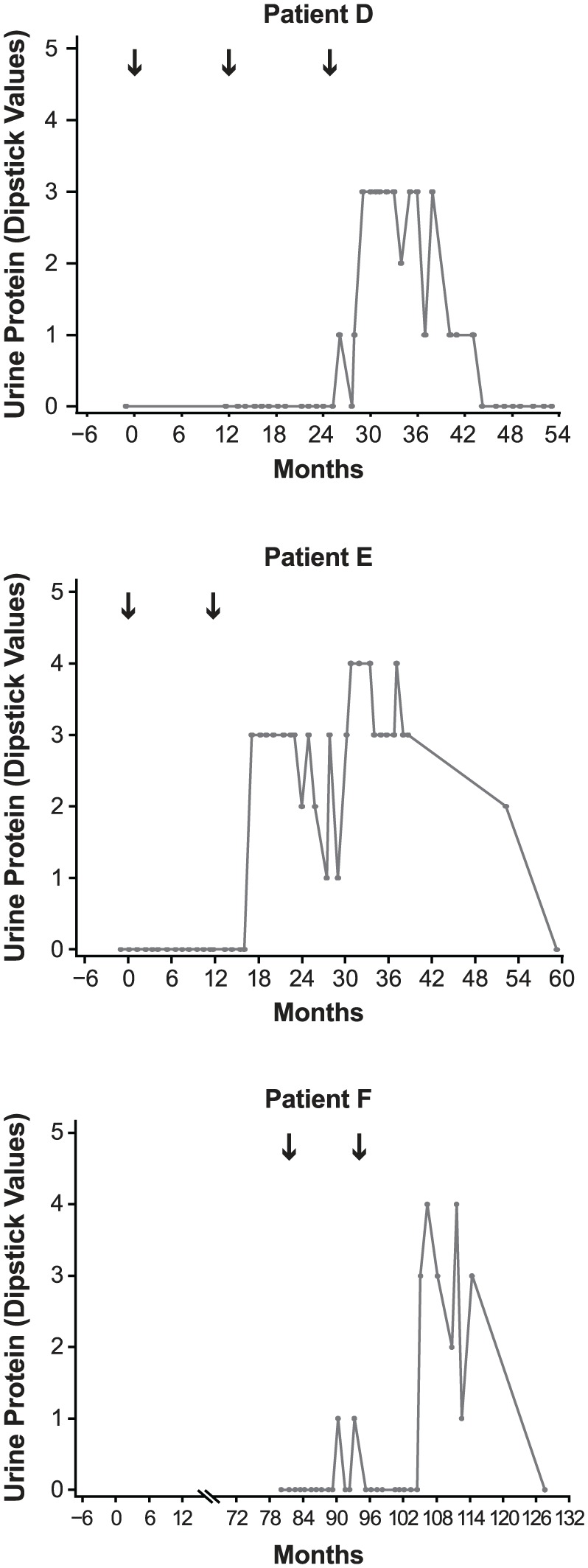
Urine protein (dipstick test) over time for nephropathy cases occurring during the CDP. Urine protein was not available for patient C (routine monitoring was modified to include urinalysis following this case). Black arrows indicate alemtuzumab treatment courses. Numbering on vertical axes refers to number of pluses from dipstick score. Months shown are from first alemtuzumab treatment. Scale of horizontal axes varies due to differing timing of treatment and event onset in each patient. Patient F received subcutaneous interferon beta-1a (44 µg 3× weekly) in the CAMMS223 trial (Month 0 to Month 36) before receiving alemtuzumab.
CDP: clinical development program.
Membranous glomerulonephropathy cases
Two cases (patients E and F) presented with nephrotic syndrome and normal serum creatinine 5 and 13 months after alemtuzumab treatment, respectively; both were diagnosed through the monitoring program established for the CDP. In both patients, renal biopsy revealed membranous glomerulonephropathy (MGN), stage 1, which was confirmed on review (Table 4 and Figure 4). Immunofluorescence staining in both biopsies was positive for IgG, complement component 3, and kappa and lambda light chains, and electron microscopy revealed global small subepithelial electron dense deposits, without significant intervening GBM spikes. In patient E, mesangial electron dense deposits were also noted. In patient F, who was considerably older than patient E, MGN was superimposed on changes of subcapsular scarring, arterionephrosclerosis, and focal renal atheroemboli.
Table 4.
Cases of MGN.
| Patient | Age at diagnosis; gender | Context | Cumulative dose (mg) | Onset from last alemtuzumab dose (months) | First symptoms to treatment for nephropathy (weeks) | Symptoms | Serum creatinine, µmol/L (mg/dL)a | Hematuria | Serum anti-GBM antibody | Renal pathology | Treatment for nephropathy | Renal outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | 27; F | CARE-MS II | 96 | 5 | 7 | Peripheral edema, hematuria, proteinuria, low serum albumin | 62 (0.7) | Yes | Not detected | Stage 1 MGN | Furosemide (20 mg at diagnosis, increased to 40 mg 2 months later) and lisinopril (5 mg/day); then received furosemide (80 mg; three times/day), valsartan (160 mg/day), metolazone (2.5 mg every other day), and oral potassium chloride for nephrotic syndrome until 45 months after diagnosis; receiving potassium chloride at last follow-up | At last follow-up (67 months after diagnosis), MGN resolved; GFR normal and minimal proteinuria |
| F | 58; F | CARE-MS extension study (previously received SC IFNB-1a in CAMMS223) | 96 | 13 | 3.5 | Leg edema, hematuria, proteinuria | 90–94 (1.0–1.1) | Yes | Not detected | MGN (mainly stage 1), subcapsular scarring consistent with arterionephrosclerosis, with superimposed renal atheroembolic disease | Diuretics and albumin for nephrotic syndrome; subsequently oral perindopril and hydrochlorthiazide (4/1.25 mg once daily) | At last follow-up (21 months after diagnosis), patient in
remission without clinical sequelae, preserved renal
function , serum creatinine levels normal (80 µmol/L), no detectable proteinuria |
MGN: membranous glomerulonephropathy; F: female; GFR: glomerular filtration rate; SC IFNB-1a: subcutaneous interferon beta-1a.
Serum creatinine measurement at, or closest to, time of nephropathy event.
Both patients were treated with angiotensin-converting enzyme inhibitors and diuretics. Patient E was in spontaneous remission 45 months after diagnosis (62 months after initiating alemtuzumab treatment) and, at most recent follow-up (67 months after diagnosis), her GFR was normal with minimal proteinuria (Figure 3). Patient F was in complete remission at last follow-up (21 months following diagnosis) with no detectable proteinuria (Figure 3). Both patients had serum creatinine levels within normal range (Figure 2).
Cases of possible immunological renal disease when full information could not be obtained
In addition to the nephropathy cases mentioned above, four further cases (patients H, I, K, and O) have been reported to the sponsor in the post-marketing setting (Table 5); however, they cannot be independently confirmed due to limitations of pharmacovigilance data collection. These include a case of focal segmental glomerulonephritis with proteinuria (patient H) and two cases of severe nephritis treated as anti-GBM disease (patients I and O). Information on renal outcomes is unavailable in all four cases and further follow-up is awaited.
Table 5.
Cases of possible immunological renal disease where full information could not be obtained.
| Patient | Age at diagnosis; gender | Context | Cumulative alemtuzumab dose (mg) | Onset from last alemtuzumab dose (months) | First symptoms to treatment for nephropathy (weeks) | Symptoms | Serum creatinine, µmol/L (mg/dL)a | Hematuria | Serum anti-GBM antibody | Renal pathology | Treatment for nephropathy | Renal outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | 30; F | Post-marketing | 96 | 29 | N/A | Elevated serum creatinine, proteinuria | 5500 (62.2) | N/A | N/A | Focal segmental glomerulosclerosis initially suggested; revised to proteinuria | N/A | N/A |
| Ib | N/A; M | Post-marketing | 60 | 11–12 | N/A | Elevated serum creatinine, chest infection (resolved), renal problems | N/A | N/A | N/A | N/A | Plasma exchange | N/A |
| K | 27; F | Post-marketing | 60 | 1 | N/A | Proteinuria, hematuria | 54 (0.6) | Yes | No | Subcapsular scar with ischemic glomeruli; no crescents | N/A | N/A |
| Ob | N/A; F | Post-marketing | N/A | 10.5 | N/A | History of urinary tract infection, raised albumen, urinalysis abnormal, dark urine | Within normal range | N/A | Yes | N/A | Plasma exchange, cyclophosphamide, dialysis | N/A |
GBM: glomerular basement membrane; F: female; M: male; N/A: not available.
Serum creatinine measurement at, or closest to, time of nephropathy event.
Patients I and O had probable anti-GBM disease, but insufficient information was available to confirm this diagnosis.
MS status of patients within the CDP
Evaluation of the MS status of the five cases (patients C‒F and J) occurring within the CDP suggested that the onset of nephropathy was not associated with exacerbation of MS. Patients C and E experienced a 2-point increase in Expanded Disability Status Scale (EDSS) score from core study baseline to most recent visit, and annualized relapse rate (ARR) remained low (Table 6). Patients D and J showed a decreased EDSS score from baseline to most recent visit and did not relapse over the same period. Patient F experienced an increased EDSS score from 3.5 at baseline to 6.0 at most recent visit and a relatively high ARR (0.45) at most recent visit; however, the patient’s EDSS score was already 6.0 over a 5-month period before the nephropathy event.
Table 6.
MS status of patients within the CDP.
| Patient | Date of alemtuzumab Course 1 |
Date of alemtuzumab Course 2 |
Date of alemtuzumab Course 3 (if applicable) |
Date of nephropathy eventa (months after final course) | EDSS score at study baseline (date) | EDSS score at most recent visit (date) | ARR from baseline to most recent visit |
|---|---|---|---|---|---|---|---|
| C | June 2004 | June 2005 | September 2008 (39) | 0 (May 2004) | 2.0 (June 2009) | 0.2 | |
| D | May 2008 | May 2009 | June 2010 | October 2010 (4) | 2.5 (May 2008) | 1.5 (September 2016) | 0 |
| E | September 2009 | August 2010 | January 2011 (5) | 1.5 (August 2009) | 3.5 (August 2016) | 0.14 | |
| F | February 2010 | March 2011 | April 2012 (13) | 3.5 (January 2010)b | 6.0 (April 2016) | 0.45 | |
| J | July 2009 | July 2010 | August 2015 (N/A) | 3.0 (July 2009) | 1.0 (May 2016) | 0 |
CDP: clinical development program; EDSS: Expanded Disability Status Scale; ARR: annualized relapse rate; SC IFNB-1a: subcutaneous interferon beta-1a.
Date when first symptoms were reported.
Baseline EDSS score prior to receiving alemtuzumab in the CARE-MS extension study (patient F previously received SC IFNB-1a in CAMMS223).
Discussion
The observation of up to 16 cases of de novo renal autoimmunity to date in MS patients treated with alemtuzumab suggests an elevated relative risk for nephropathies. The overall incidence of nephropathies as of 16 June 2017 was 0.34% within the alemtuzumab CDP for RRMS, but so far a lower (0.05%) incidence has been observed in the post-marketing setting. Post-marketing event frequencies are not directly comparable with clinical trial incidences because of differences in ascertainment methodology and follow-up duration, and the limitations of post-marketing reporting, including possible under-reporting, limited availability of clinical details, and potential for inaccurate diagnoses.33 A case of anti-GBM disease was also previously reported in a patient who received off-label alemtuzumab treatment for systemic vasculitis.1
Anti-GBM disease occurs rarely in the normal population (up to 1 case/million people/year), with a slight male predominance.34 It has been reported in MS patients35 and, although the available data do not enable estimation of the incidence rate, it may be slightly higher than in the normal population, as MS patients have an increased risk for other autoimmune diseases.36 MS and anti-GBM disease are associated with inheritance of the same HLA class II allele, HLA DRB1*15:01.31,32 In MS, the association with this allele has been reported to be female-specific,37 and it is interesting to note that the majority of cases of anti-GBM disease following alemtuzumab treatment to date have occurred in female patients, with one patient (patient A) a confirmed carrier of HLA DRB1*15.30
MGN is a common etiology of nephrotic syndrome in adults, with an incidence in the normal population of 4–10 cases/million people/year.38 MGN may be idiopathic and associated with phospholipase A2 receptor autoantibodies, or may occur secondary to other diseases such as systemic lupus erythematosus or hepatitis B and C infections.39 Like anti-GBM disease, very few cases of MGN have been reported in MS patients.40
Mechanisms underlying the increased risk of autoimmune-mediated conditions with alemtuzumab are not fully understood. However, homeostatic expansion of lymphocytes may be linked to lymphopenia-associated autoimmunity41,42 and represents a potential explanation for increased nephropathy risk in alemtuzumab-treated patients.
Evaluation of the MS status of five autoimmune nephropathy cases occurring within the CDP suggested that nephropathy onset was not associated with exacerbation of MS. Although based on a small number of patients, this finding is consistent with a study showing that another autoimmune disorder, thyroid dysfunction, did not adversely affect MS outcomes in alemtuzumab-treated patients.43
A number of preliminary conclusions may be drawn regarding the nature of the risk of renal disease with alemtuzumab and the potential for its mitigation. First, with regard to the natural history of anti-GBM disease, five of the seven patients with the disease exhibited a prodrome of at least a month with indications of renal disease in the form of microscopic hematuria and/or elevated serum creatinine, detected while asymptomatic by renal surveillance laboratory testing. Idiopathic anti-GBM disease is usually viewed as fulminant, as it was in the other two alemtuzumab cases, but an asymptomatic prodrome could easily be missed in clinical practice. There have been reports of idiopathic anti-GBM cases with such a prodromal period, notably in patients also presenting with lung hemorrhage and/or hemoptysis and hematuria.44 Although it is possible that a prodrome is a distinctive feature of anti-GBM disease related to alemtuzumab treatment for MS, more likely, anti-GBM disease (with or without lung involvement) should be reconceived as a condition that takes at least a few weeks to build through minor renal injury to the well-described fulminant presentation.
Second, at least in the alemtuzumab group, serum anti-GBM antibodies were not always detectable by conventional assays during the prodrome and hence cannot be used reliably to rule out a diagnosis of anti-GBM disease. Of particular note, in three of the reported nephropathy cases, serum anti-GBM antibodies were detected without classical anti-GBM disease. False-positives may occur due to non-specific binding in patients with inflammatory diseases.44 However, two of these three cases occurred in the presence of MGN and pauci-immune vasculitis, respectively, which is consistent with previous reports of anti-GBM antibodies occurring in combination with these diseases.34
Third, outcomes and response to treatment are not obviously different from those in patients with these conditions occurring outside the context of alemtuzumab treatment. In particular, conventional treatment of anti-GBM disease in alemtuzumab-treated patients was effective when started before substantial renal damage had occurred (patient C, within the CDP with close monitoring), and failed to preserve native renal function when treatment was interrupted (patient G) or when renal injury was very advanced (patients A, B, N, and P, all of whom were reported to have severe injury on biopsy with >90% glomerular crescents).
Finally, close observation of alemtuzumab patients seems to enable, in at least some patients, recognition of anti-GBM disease early, when treatment is most likely to preserve kidney function. Unfortunately, the earliest indication of the disease is microscopic hematuria, which has a low positive predictive value for anti-GBM disease, particularly in younger women who comprise the majority of the at-risk group.45 The Risk Management Plan/Risk Evaluation and Mitigation Strategy for alemtuzumab adopted for use in clinical practice, including monthly laboratory monitoring required for 48 months following the last infusion, and healthcare professional and patient education on signs and symptoms of autoimmune events, may serve to mitigate against significant renal damage.15,28
Conclusion
Autoimmune nephropathies occur at increased rates in MS patients treated with alemtuzumab. Close monitoring can identify new cases early in the evolution of the nephropathy when treatment is most likely to be effective in preserving renal function. These findings are of critical importance, as the treatment of RRMS with alemtuzumab is expanding, having been judged to have a favorable benefit:risk profile in the majority of patients.
Acknowledgments
The authors and Sanofi thank the patients for their participation in the CAMMS223, CARE-MS I, CARE-MS II, CAMMS03409, and TOPAZ studies, as well as the Steering Committees and the investigators. Critical review of the manuscript was provided by Isabel Firmino, MD, and Colin Mitchell, PhD, of Sanofi. Editorial support was provided by David R Thomas, PhD, and Richard J Hogan, PhD, of Eloquent Scientific Solutions.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.P. has received consulting fees from Sanofi. J.A.W. has received personal fees from Gilead Sciences and Sanofi. D.W. has received research support and/or consulting fees from Acorda Therapeutics, Avanir Pharmaceuticals, EMD Serono, GlaxoSmithKline, Roche/Genentech, Novartis, Ono Pharmaceutical, Opexa Therapeutics, Osmotica, Pfizer, Questcor, Receptos, Sanofi, Teva, and XenoPort. M.H. has participated as clinical investigator and/or speaker for Actelion, Alexion, Alvogen, Bayer, Biogen, Merck, Novartis, Roche, Sanofi, and Teva. H.-P.H. has received honoraria for consulting, serving on steering and data monitoring committees, and speaking at symposia from Bayer Healthcare Pharmaceuticals, Biogen, CSL Behring, Merck Serono, Novartis, Octapharma, Roche, Sanofi, and Teva, with approval by the Rector of Heinrich-Heine University. E.K.H. has received honoraria and grant support from Actelion, Biogen, Merck Serono, Novartis, Receptos, Roche, Sanofi, and Teva. G.S.M. reviewed clinical histories, laboratory analyses, and pathology data for nephropathy patients C–F, and provided post hoc definitive diagnoses as a paid consultant to Sanofi; he did not receive compensation for contributing to writing and critical review of the manuscript, or for approval of the final submission draft. D.H.M., C.E.R., and D.P.B. received compensation as employees of Sanofi. A.J.C. has received consulting fees, lecture fees, and institutional grant support from Sanofi.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Prof. Eva Kubala Havrdová was supported by the Czech Ministry of Education, Project PROGRES Q27/LF1. Editorial support in the development of this paper was funded by Sanofi. The CAMMS223, CARE-MS, CAMMS03409, and TOPAZ studies were funded by Sanofi and Bayer HealthCare Pharmaceuticals.
ORCID iD: Mario Habek  https://orcid.org/0000-0002-3360-1748
https://orcid.org/0000-0002-3360-1748
Contributor Information
Richard Phelps, Centre for Inflammation Research, University of Edinburgh, Edinburgh, UK.
Jonathan A Winston, Mount Sinai School of Medicine, New York, NY, USA.
Daniel Wynn, Consultants in Neurology MS Center, Northbrook, IL, USA.
Mario Habek, Department of Neurology, School of Medicine, University of Zagreb and University Hospital Center, Zagreb, Croatia.
Hans-Peter Hartung, Department of Neurology, Medical Faculty, Heinrich-Heine University, Düsseldorf, Germany.
Eva Kubala Havrdová, Department of Neurology, First Medical Faculty, Charles University, Prague, Czech Republic.
Glen S Markowitz, Department of Pathology and Cell Biology, Columbia University, New York, NY, USA.
David H Margolin, Sanofi, Cambridge, MA, USA/Cerevance Inc., Boston, MA, USA.
Claudio E Rodriguez, Sanofi, Cambridge, MA, USA/Sunovion Pharmaceuticals, Marlborough, MA, USA.
Darren P Baker, Sanofi, Cambridge, MA, USA.
Alasdair J Coles, School of Medicine, University of Cambridge, Cambridge, UK.
References
- 1. Coles AJ, Cox A, Le Page E, et al. The window of therapeutic opportunity in multiple sclerosis: Evidence from monoclonal antibody therapy. J Neurol 2006; 253(1): 98–108. [DOI] [PubMed] [Google Scholar]
- 2. Coles AJ, Compston DA, Selmaj KW, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008; 359(17): 1786–1801. [DOI] [PubMed] [Google Scholar]
- 3. Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet 2012; 380(9856): 1819–1828. [DOI] [PubMed] [Google Scholar]
- 4. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet 2012; 380(9856): 1829–1839. [DOI] [PubMed] [Google Scholar]
- 5. Meyer D, Coles A, Oyuela P, et al. Case report of antiglomerular basement membrane disease following alemtuzumab treatment of relapsing-remitting multiple sclerosis. Mult Scler Rel Disord 2013; 2: 60–63. [DOI] [PubMed] [Google Scholar]
- 6. Wynn DR, Arnold DL, Coles A, et al. Detection, incidence, and management of glomerulonephritis in the alemtuzumab clinical development program. Mult Scler 2013; 19: P597. [Google Scholar]
- 7. Perazella MA. Drug-induced nephropathy: An update. Expert Opin Drug Saf 2005; 4: 689–706. [DOI] [PubMed] [Google Scholar]
- 8. Havrdova E, Arnold DL, Cohen JA, et al. Alemtuzumab CARE-MS I 5-year follow-up: Durable efficacy in the absence of continuous MS therapy. Neurology 2017; 89: 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: Efficacy and safety findings. Neurology 2017; 89: 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coles AJ, Fox E, Vladic A, et al. Alemtuzumab more effective than interferon beta-1a at 5-year follow-up of CAMMS223 clinical trial. Neurology 2012; 78: 1069–1078. [DOI] [PubMed] [Google Scholar]
- 11. Osterborg A, Mellstedt H, Keating M. Clinical effects of alemtuzumab (Campath-1H) in B-cell chronic lymphocytic leukemia. Med Oncol 2002; 19(Suppl.): S21–S26. [DOI] [PubMed] [Google Scholar]
- 12. Walsh M, Chaudhry A, Jayne D. Long-term follow-up of relapsing/refractory anti-neutrophil cytoplasm antibody associated vasculitis treated with the lymphocyte depleting antibody alemtuzumab (CAMPATH-1H). Ann Rheum Dis 2008; 67(9): 1322–1327. [DOI] [PubMed] [Google Scholar]
- 13. Friend PJ. Alemtuzumab induction therapy in solid organ transplantation. Transplant Res 2013; 2(Suppl. 1): S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cull GM, Haynes AP, Byrne JL, et al. Preliminary experience of allogeneic stem cell transplantation for lymphoproliferative disorders using BEAM-CAMPATH conditioning: An effective regimen with low procedure-related toxicity. Br J Haematol 2000; 108(4): 754–760. [DOI] [PubMed] [Google Scholar]
- 15. Genzyme Therapeutics Ltd. Lemtrada™ (alemtuzumab 12 mg concentrate for solution for infusion). EU summary of product characteristics, https://www.ema.europa.eu/en/documents/product-information/lemtrada-epar-product-information_en.pdf (2018, accessed 12 February 2018).
- 16. Sanofi Genzyme. Prescribing information. Lemtrada™ (alemtuzumab), for intravenous injection. Cambridge, MA: Sanofi Genzyme, 2017. [Google Scholar]
- 17. Hu Y, Turner MJ, Shields J, et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology 2009; 128(2): 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rao SP, Sancho J, Campos-Rivera J, et al. Human peripheral blood mononuclear cells exhibit heterogeneous CD52 expression levels and show differential sensitivity to alemtuzumab mediated cytolysis. PLoS ONE 2012; 7(6): e39416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Turner MJ, Lamorte MJ, Chretien N, et al. Immune status following alemtuzumab treatment in human CD52 transgenic mice. J Neuroimmunol 2013; 261(1–2): 29–36. [DOI] [PubMed] [Google Scholar]
- 20. De Mercanti S, Rolla S, Cucci A, et al. Alemtuzumab long-term immunologic effect: Treg suppressor function increases up to 24 months. Neurol Neuroimmunol Neuroinflamm 2016; 3: e194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fox EJ. Alemtuzumab in the treatment of relapsing-remitting multiple sclerosis. Expert Rev Neurother 2010; 10: 1789–1797. [DOI] [PubMed] [Google Scholar]
- 22. Freedman MS, Kaplan JM, Markovic-Plese S. Insights into the mechanisms of the therapeutic efficacy of alemtuzumab in multiple sclerosis. J Clin Cell Immunol 2013; 4(4): 1000152. [PMC free article] [PubMed] [Google Scholar]
- 23. Coles AJ, Boyko AN, De Seze J, et al. Alemtuzumab durably improves clinical outcomes in patients with active RRMS in the absence of continuous treatment: 7-year follow-up of CARE-MS I patients (TOPAZ study). Mult Scler 2017; 23(Suppl. 3): P1188. [Google Scholar]
- 24. Arnold DL, Barnett M, Comi G, et al. Durable reduction in MRI disease activity and slowing of brain volume loss with alemtuzumab in patients with active RRMS: 7-year follow-up of CARE-MS I patients (TOPAZ study). Mult Scler 2017; 23(Suppl. 3): P1189. [Google Scholar]
- 25. Singer BA, Alroughani R, Brassat D, et al. Durable improvements in clinical outcomes with alemtuzumab in patients with active RRMS in the absence of continuous treatment: 7-year follow-up of CARE-MS II patients (TOPAZ study). Mult Scler 2017; 23(Suppl. 3): P736. [Google Scholar]
- 26. Pelletier D, Traboulsee A, Barnett M, et al. Patients with active RRMS experience durable reductions in MRI disease activity and slowing of brain volume loss with alemtuzumab: 7-year follow-up of CARE-MS II patients (TOPAZ study). Mult Scler 2017; 23(Suppl. 3): P741. [Google Scholar]
- 27. Ziemssen T, Thomas K. Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: An update on the clinical trial evidence and data from the real world. Ther Adv Neurol Disord 2017; 10(10): 343–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sanofi Genzyme. Lemtrada™ REMS (Risk Evaluation and Mitigation Strategy) program. Cambridge, MA: Sanofi Genzyme, 2017. [Google Scholar]
- 29. Brinar V, Giovannoni G, Havrdova E, et al. A phase 3b/4 long-term study of alemtuzumab in patients with relapsing-remitting multiple sclerosis: TOPAZ study design. Neurology 2015; 84: P7219. [Google Scholar]
- 30. Clatworthy MR, Wallin EF, Jayne DR. Anti-glomerular basement membrane disease after alemtuzumab. N Engl J Med 2008; 359: 768–769. [DOI] [PubMed] [Google Scholar]
- 31. Phelps RG, Rees AJ. The HLA complex in Goodpasture’s disease: A model for analyzing susceptibility to autoimmunity. Kidney Int 1999; 56(5): 1638–1653. [DOI] [PubMed] [Google Scholar]
- 32. Hollenbach JA, Oksenberg JR. The immunogenetics of multiple sclerosis: A comprehensive review. J Autoimmun 2015; 64: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Selmaj KW, Habek M, Bass A, et al. Efficacy and safety of alemtuzumab in patients with RRMS is durable over 10 years: Follow-up from the CAMMS223 study. Neurology 2017; 88: P5338. [Google Scholar]
- 34. Turner N, Rees AJ. Antiglomerular basement disease. In: Davison AM, Cameron JS, Grunfeld JP, et al. (eds) Oxford textbook of clinical nephrology. 3rd ed. New York: Oxford University Press, 2005. [Google Scholar]
- 35. Henderson RD, Saltissi D, Pender MP. Goodpasture’s syndrome associated with multiple sclerosis. Acta Neurol Scand 1998; 98(2): 134–135. [DOI] [PubMed] [Google Scholar]
- 36. Henderson RD, Bain CJ, Pender MP. The occurrence of autoimmune diseases in patients with multiple sclerosis and their families. J Clin Neurosci 2000; 7(5): 434–437. [DOI] [PubMed] [Google Scholar]
- 37. Irizar H, Munoz-Culla M, Zuriarrain O, et al. HLA-DRB1*15:01 and multiple sclerosis: A female association? Mult Scler 2012; 18(5): 569–577. [DOI] [PubMed] [Google Scholar]
- 38. Deegens JK, Wetzels JF. Diagnosis and treatment of primary glomerular diseases. Membranous nephropathy, focal segmental glomerulosclerosis and IgA nephropathy. Minerva Urol Nefrol 2005; 57: 211–236. [PubMed] [Google Scholar]
- 39. Hofstra JM, Wetzels JF. Management of patients with membranous nephropathy. Nephrol Dial Transplant 2012; 27(1): 6–9. [DOI] [PubMed] [Google Scholar]
- 40. Campos A, Gieron MA, Gunasakeran S, et al. Membranous nephropathy associated with multiple sclerosis. Pediatr Neurol 1993; 9(1): 64–66. [DOI] [PubMed] [Google Scholar]
- 41. Jones JL, Thompson SA, Loh P, et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc Natl Acad Sci U S A 2013; 110(50): 20200–20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Krupica T, Jr, Fry TJ, Mackall CL. Autoimmunity during lymphopenia: A two-hit model. Clin Immunol 2006; 120: 121–128. [DOI] [PubMed] [Google Scholar]
- 43. Daniels GH, Vladic A, Brinar V, et al. Alemtuzumab-related thyroid dysfunction in a phase 2 trial of patients with relapsing-remitting multiple sclerosis. J Clin Endocrinol Metab 2014; 99(1): 80–89. [DOI] [PubMed] [Google Scholar]
- 44. Phelps RG, Turner AN. Antiglomerular basement membrane disease and Goodpasture’s disease. In: Floege J, Johnson R, Feehally J. (eds.) Comprehensive clinical nephrology. 4th ed. St. Louis, MO: Saunders, 2010, pp. 282–291. [Google Scholar]
- 45. Zuvich RL, McCauley JL, Pericak-Vance MA, et al. Genetics and pathogenesis of multiple sclerosis. Semin Immunol 2009; 21: 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]