Abstract
Background:
We explored if age affects quality of life (QOL) in survivors of locally advanced human papillomavirus (HPV)-related oropharyngeal squamous cell carcinoma (SCC).
Methods:
In a cross-sectional survey of 185 patients, at least 12 months from radiation, we evaluated generic (EuroQOL-5D questionnaire [EQ-5D]) and head and neck specific (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Head and Neck 35-questions [EORTC-QLQ-H&N35]) QOL questionnaires and compared differences between younger (<65) and older (≥65) patients.
Results:
The median age was 57.0 years (range 25–77 years), and 31 patients (16.8%) were ≥65 years old. There was no significant difference in EQ-5D global QOL scores by age (P = .53). Patients ≥65 years reported more immobility (P < .01), problems with social eating (P < .0001), and coughing (P < .01). Patients ≥65 years were not more likely to ever require a gastrostomy (P = .24) but were more likely to remain gastrostomy-dependent at the time of the survey (P = .02).
Conclusion:
Despite similar generic QOL, older survivors may have more mobility problems and issues with social eating compared with younger survivors deserving of further evaluation.
Keywords: geriatrics, human papillomavirus, oropharyngeal cancer, quality of life
1 |. INTRODUCTION
Head and neck squamous cell carcinoma of the oropharynx (oropharyngeal SCC) includes malignancies arising from the base of the tongue, soft palate, tonsils, and pharyngeal wall and has one of the fastest growing incidence rates among all cancers.1 This increase is attributed to the growing number of cases of human papillomavirus (HPV)-associated oropharyngeal SCC, which made up about 70% of all oropharyngeal SCC diagnoses between 2008 and 2012 compared to 63% between 2004 and 2008.1,2 These patients have a better prognosis and higher survival rates compared with patients with HPV-negative oropharyngeal SCC.3 They tend to be men, white, nonsmokers, nondrinkers, healthy, and young, with a mean age of 57 years at diagnosis, about 5 years younger than historical patients with head and neck cancer.3–5 However, there is a subset of patients with HPV-related oropharyngeal SCC who are older, with 20% of all patients diagnosed between 2008 and 2012 being over 70 years of age.1 With an aging population and a rise in the number of cases of HPV-related oropharyngeal SCC in general, the number of older patients with this diagnosis will also increase over time.5–7
Combined modality, definitive concurrent chemotherapy and radiotherapy (CRT), is often utilized in the management of locally advanced oropharyngeal SCC. Although highly curative, CRT can leave survivors with acute and chronic effects of treatment, including the need for percutaneous endoscopic gastrostomy (PEG) feeding tubes.8 After CRT, patients with HPV-related oropharyngeal SCC experience a steeper decline in health-related quality of life (HR-QOL) acutely after treatment followed by a better recovery when compared with patients with HPV-negative oropharyngeal SCC.9,10 Studies have also shown that, in general, older patients with head and neck cancer are more at risk for the acute toxicities associated with CRT compared with younger patients.8,11–13 However, little data exist that describe the long-term outcomes in older patients with HPV-positive oropharyngeal SCC treated with CRT. In this study, we sought to describe the difference in health status and head and neck specific QOL between older and younger long-term survivors of HPV-positive oropharyngeal SCC treated with concurrent CRT.
2 |. MATERIALS AND METHODS
We performed a cross-sectional QOL survey among long-term survivors of HPV-positive oropharyngeal SCC who were treated and continue to be followed at a large urban cancer center. The study was approved by the institutional review board, and all study participants provided informed consent.
2.1 |. Participants
Patients were recruited at follow-up visits in outpatient medical oncology or radiation oncology clinics from 2010 through 2016. Eligible patients had pathologically confirmed locally advanced oropharyngeal SCC, which was positive for HPV (either by HPV in situ hybridization or p16 on immunohistochemistry in a Clinical Laboratory Improvement Amendment approved laboratory). We included any patient who completed definitive CRT or surgery followed by CRT or radiotherapy (RT), and was between 1 and 5 years postcompletion of all treatment, never had a documented recurrence, and remained cancer free. We approached consecutive eligible patients with approval from their treating physicians as they were seen in clinics. Of the 216 patients approached to participate, 190 agreed, and 185 of those who consented completed the study survey. Four patients refused or withdrew consent and 7 patients never returned a completed survey. Twenty patients were found to be ineligible (tumor HPV status was negative or could not be confirmed).
The standard treatment with CRT includes intravenous chemotherapy given at the discretion of the treating medical oncologists, dependent on comorbid illnesses and functional and performance status, administered concurrently with intensity-modulated radiotherapy given at doses from 66 to 70 Gy, dependent on the stage and location of the tumor. Induction chemotherapy is offered to patients with high-risk, advanced T classification or N classification disease at the discretion of the medical oncologist. All patients are referred for speech and swallow evaluation and nutrition counseling during and after RT. It is not standard of care to place a PEG, or feeding tube, prophylactically in patients undergoing RT or CRT, but rather they are placed as needed under the guidance of treating physicians.
2.2 |. Health-related quality of life measures
Health-related quality of life (HR-QOL) was estimated using the EuroQOL-5D questionnaire (EQ-5D),14 a validated instrument of health status assessing the level of impairment or function in 5 specific domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Patients selected from answer choices of “no problems” (score of 1), “some problems” (score of 2), and inability to perform function mentioned (score of 3). We dichotomized responses to these items as any problems (scores 2 or 3) or no problems (score 1).4,15 A higher score indicated worse HR-QOL. In patients with cancer, a minimally important difference of 0.08 in the EQ-5D is considered clinically significant.16 The EQ-5D also includes a visual analog scale (VAS), which asks a participant to rate their current health on a scale from 0 to 100, with 100 being the highest level of health. A minimally important difference of VAS score ranges from 8 to 12 in patients with cancer.16
Head and neck cancer-specific QOL was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Head and Neck 35 module (EORTC-QLQ-H&N35).17 This instrument assesses pain, swallowing, senses, speech, social eating, social contact, sexuality, problems with teeth, dry mouth, sticky saliva, cough, trismus, weight loss, weight gain, nutritional supplement use, feeding tubes, and painkillers.4,18 The 35 items of the EORTC-QLQ-H&N35 yield both multi-item symptom scale scores and single-item symptom scores, for a total of 18 distinct scores scaled from 0 to 100, with higher scores representing worse levels of symptomatology or problems.4,19 Patients were also given the opportunity to list up to 5 symptoms that troubled them the most.
Data on the use of PEGs were captured from the electronic medical record. It was categorized as “ever” or “never” needing a PEG and then whether or not the survivor had a PEG at the time of the survey.
2.3 |. Covariates
Covariates included sociodemographic characteristics (including age and marital status at diagnosis, sex, and race), disease and treatment characteristics (including primary site of disease, composite stage, surgery, specific chemotherapy, and use of adjuvant vs primary CRT), and clinical characteristics (including performance and comorbidity score at diagnosis, and alcohol and tobacco history at diagnosis). This information was collected from the electronic medical record. Performance status was captured using the Karnofsky Performance Scale (KPS), with higher scores representing better performance status. A Charlson comorbidity index score, unadjusted for age, was calculated based on review of physician notes.4,20
Age at diagnosis was categorized as younger (<64 years) or older (≥65 years) for comparison purposes. The World Health Organization denotes a person of 65 years or older as an “older dependent,” whereas people between the ages of 15 and 64 are of working age.21
2.4 |. Statistical analysis
We compared the age, sex, and primary sites of disease of the study sample with the nonparticipating oropharyngeal SCC population treated at our institution during the same time period to assess the generalizability of the results to the Memorial Sloan Kettering population. Within the sample, we compared sociodemographic, disease, and treatment characteristics by age group. We used the Fisher exact tests for categorical variables and the Mann-Whitney test for continuous variables for these comparisons. We calculated mean differences and described rates of any problem reported in the EQ-5D measurement of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. We used the Fisher exact tests to compare outcomes by sociodemographic, disease, and treatment characteristics. We also report on rates of PEG placement during treatment and ongoing PEG use at the time of the survey. Using the Fisher exact tests, we compared use of PEG (ever) by sociodemographic, disease, and treatment characteristics. For EQ-5D or PEG use outcomes significantly associated with age at P < .05, we then used multivariable logistic regression to evaluate whether age was an independent predictor of worse QOL outcomes in this population of long-term survivors when controlling for other characteristics that were significant in bivariate analysis.
The Mann-Whitney test was used to compare continuous scores from the EQ-5D VAS and EORTC-QLQ-H&N35 between age groups because of the skewness of the QOL data. Multivariate analysis of EORTC-QLQ-H&N35 outcomes was not performed due to the non-normal distribution of the data and small sample size. We compared scores by the sociodemographic, disease, and treatment characteristics. To address concerns associated with multiple comparisons, we used a conservative P value of ≤ .01 as the threshold for statistical significance in analysis of EORTC-QLQ-H&N35 scores.4,19 All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
3 |. RESULTS
The 185 participants were similar to nonparticipating patients with locally advanced oropharyngeal SCC treated at our institution during the study period. The mean age for participants at diagnosis was 57.0 years (range 25–77 years) compared to 58.0 years (range 27–85 years) for nonparticipants (P = .19), with 17% (31/185) and 22% (90/418) ≥65 years, (P = .18), respectively.
Most patients in our cohort were men (91%), white (91%), married (81%), and diagnosed with stage IVA/B disease (86%). More than half (56%) of the patients had a history of tobacco use (current or former; see Table 1). Patients who were 65 years or older were more likely to have ever smoked compared with younger patients (81% vs 51%; P = .003). At diagnosis, 77% of those patients who were 65 years or older had a KPS of 90% compared to 90% of younger patients (P = .06). Older patients had higher comorbidity than younger patients (P = .004; see Table 1). The median time between completion of treatment and the survey was 23 months (interquartile range [IQR] 15–29 months). This time did not vary between younger and older patients (P = .187); younger patients had a median (IQR) of 19 months (range 16–29 months) and older patients had a median (IQR) of 21 months (range 16–29 months).
TABLE 1.
Sample characteristics of long-term survivors of HPV-positive oropharyngeal squamous cell carcinoma by age at diagnosis
| Less than 65 y | 65 y or older | ||||
|---|---|---|---|---|---|
| No. | Col% | No. | Col% | P value | |
| Total | 154 | 100 | 31 | 100 | |
| Sex | .031 | ||||
| Male | 144 | 94 | 25 | 81 | |
| Female | 10 | 6 | 6 | 19 | |
| Race | .480 | ||||
| White | 139 | 90 | 30 | 97 | |
| Other/unknown | 15 | 10 | 1 | 3 | |
| Marital status | .328 | ||||
| Married | 126 | 82 | 23 | 74 | |
| Unmarried | 28 | 18 | 8 | 26 | |
| Site | .596 | ||||
| Base of tongue | 74 | 48 | 17 | 55 | |
| Tonsil | 68 | 44 | 13 | 42 | |
| Oropharynx | 12 | 8 | 1 | 3 | |
| Stage | .777 | ||||
| II/III | 21 | 14 | 5 | 16 | |
| IV | 133 | 86 | 26 | 84 | |
| Surgery | .578 | ||||
| Yes | 23 | 15 | 3 | 10 | |
| No | 131 | 85 | 28 | 90 | |
| CRT | .745 | ||||
| Yes | 137 | 89 | 29 | 94 | |
| No | 17 | 11 | 9 | 6 | |
| KPS functional status at diagnosis | .063 | ||||
| 90% | 139 | 90 | 24 | 77 | |
| 80% | 15 | 10 | 7 | 23 | |
| Charlson comorbidity score at diagnosisa | .002 | ||||
| 2 | 142 | 92 | 22 | 71 | |
| ≥3 | 12 | 8 | 9 | 29 | |
| Smoking status | .001 | ||||
| Ever | 79 | 51 | 25 | 81 | |
| Never | 75 | 49 | 6 | 19 | |
| Alcohol consumption | .680 | ||||
| > 1/d / prior heavy | 35 | 23 | 6 | 19 | |
| None / social / 1/d | 119 | 77 | 25 | 81 | |
Abbreviations: Col, column; CRT, chemoradiotherapy; KPS, Karnofsky Performance Scale.
Charlson comorbidity score, unadjusted for age.
The majority (90%) of patients were treated with definitive CRT; 12 patients (6%) received induction chemotherapy before CRT. Twenty-six patients (14%) had any head and neck surgery of which 9 (35% [9/26]) were post-CRT salvage procedures. Sixteen patients underwent resection of the primary tumor, of which 13 were completed with a transoral robotic surgery. These 16 patients also underwent a neck dissection. An additional 10 patients underwent neck dissection. Only 2 patients (1%) received surgery followed by radiation alone. Cisplatin was the most commonly used concurrent chemotherapy, used in 91% of the patients, and a total of 18% of the patients were treated on therapeutic protocols. There were no significant differences in treatment choices between younger and older groups.
3.1 |. Outcomes and quality of life
Overall, younger and older patients reported similar generic QOL on the EQ-5D questionnaire, as measured by the global EQ-5D VAS score (P = .53; see Table 2). Patients indicated an overall average health score of 85 (SD 14) on the VAS. Older patients reported a mean score of 81 (SD 17) compared to a mean score of 86 (SD 13) reported by younger patients (P = .20). The difference of VAS score of 4 does not meet the threshold for being considered clinically significant.
TABLE 2.
EuroQOL-5D questionnaire scores by age
| EQ-5D scores by age | Mean (SD) <65 y | Mean (SD) ≥65 y | P valuea |
|---|---|---|---|
| Global EQ-5D | 0.422 (0.496) | 0.484 (0.508) | .5289 |
| Mobility | 1.078 (0.269) | 1.258 (0.445) | .0033 |
| Self-care | 1.03 (0.211) | 1.000 (0) | .3656 |
| Usual activities | 1.143 (0.387) | 1.097 (0.301) | .5981 |
| Pain/discomfort | 1.281 (0.465) | 1.200 (0.407) | .5919 |
| Anxiety/depression | 1.268 (0.487) | 1.226 (0.497) | .5043 |
Abbreviation: EQ-5D, EuroQOL-5D questionnaire.
P values derived from Mann-Whitney tests.
The mean difference in scores between older and younger patients were not different for self-care (0.03; P = .37), usual activities (0.04; P = .6), pain/discomfort (0.08; P = .59), and anxiety/depression (0.04; P = .51). The only mean difference in scores that was both clinically and statistically significant was mobility with a difference of score of 0.18 (P = .003). In a univariate analysis, the mean difference in scores for usual activities (0.22; P = .02), and anxiety/depression (0.25; P = .03) were worse in patients with a higher Charlson comorbidity score at diagnosis, whereas the mean difference in scores for mobility (0.15; P = .42), self-care (0.03; P = .39) and pain/discomfort (0.13; P = .45) were not. In addition, “ever smoking” was also a risk factor for problems with mobility at the time of the survey (P = .006). When age was entered into a multivariable logistic regression with comorbidity and being an ever smoker, the adjusted odds ratio (aOR) and 95% confidence interval (CI) for problems with mobility was 3.14 (95% CI 0.93–7.64; P = .08) for age 65 years and older, 4.58 (95% CI 1.13–14.97; P = .03) for ever smoking, and 1.45 (95% CI 0.62–7.3) for higher Charlson comorbidity index. No other sociodemographic, clinical, or treatment characteristics were associated with worse mobility scores. In multivariable logistic models that controlled for smoking status, functional status, and Charlson comorbidity score at diagnosis, there was no association observed between older age and usual activity, self-care, pain/discomfort, or anxiety/depression.
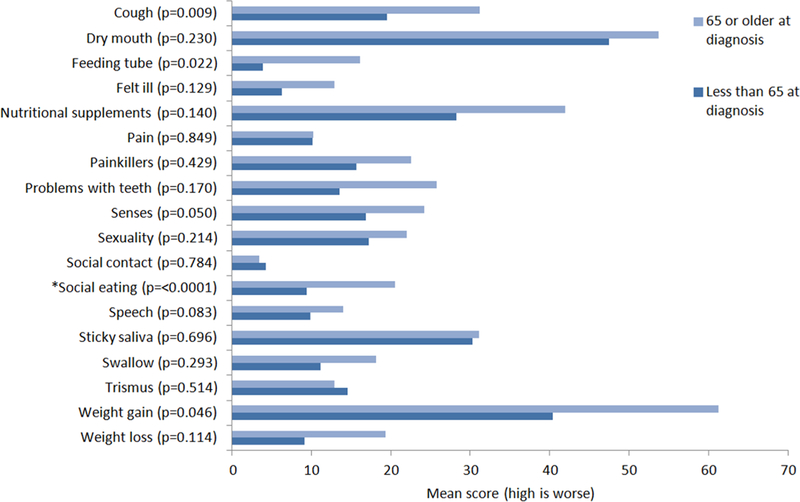
Patients responded to questions about persistent treatment toxicity and QOL using the EORTC-QLQ-H&N35 questionnaire. Patients scored the highest (worse severity) in problems with dry mouth and weight gain, followed by nutritional supplements and sticky saliva. Compared with younger patients, patients 65 years or older had significantly more problems with social eating with a mean difference in scores of 11.1 (P < .0001), and there was a trend for more coughing in older patients with a mean difference in scores of 11.7 (P = .009; see Figure 1). The difference in scores is clinically meaningful. There are other symptoms, such as use of a feeding tube at the time of the survey, use of nutritional supplements, feeling ill, problems with teeth, weight gain, and weight loss with a clinically meaningful difference in scores of >10, which did not reach statistical significance. There was not a clinically meaningful difference in EORTC-QLQ-H&N35 scores between older and younger patients in their experience of the remaining 11 symptoms.
FIGURE 1.
European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire-Head and Neck 35-questions scores by age group.
*Statistically significant with P < 0.01 [Color figure can be viewed at wileyonlinelibrary.com]
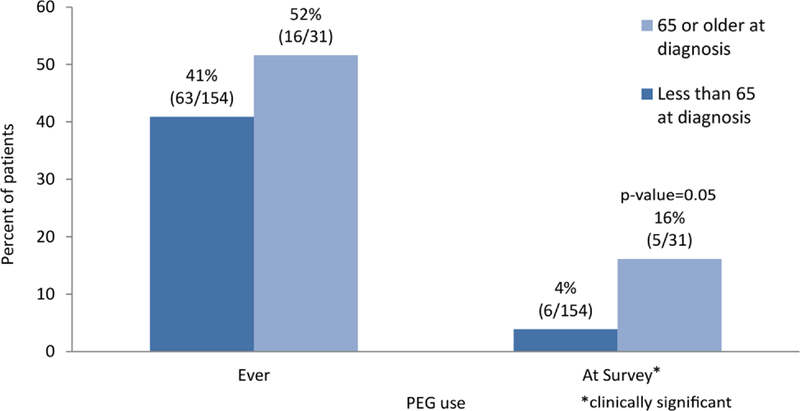
We looked at PEG use during treatment and at the time of the survey from the patient’s medical records. In this cohort, 41% of patients (63/154) younger than 65 years and 52% of patients (16/31) 65 years or older required a feeding tube at some point during the course of treatment and subsequent follow-up (P = .24). However, as shown in Figure 2, at the time of the survey, patients 65 years and older were more likely to rely on PEG feedings compared with younger patients (16%; 5/31 vs 4%; 6/154; P = .021). In addition to age at diagnosis, baseline KPS of 80% (vs 90%) was also a predictor of PEG use at the time of the survey, and both of these factors remained significant when entered together into a multivariable logistic regression (aOR 3.71; 95% CI 0.99–13.9; P = .05 for age 65 years and older, and aOR 6.32; 95% CI 1.67–23.90; P = .007 for KPS of 80%).
FIGURE 2.
The percentage of patients to have a percutaneous endoscopic gastrostomy (PEG) during treatment and at the time of the survey by age group [Color figure can be viewed at wileyonlinelibrary.com]
In response to the open-ended question about symptom burden, a total of 138 patients (75%) volunteered any symptom(s). The most commonly reported categories of symptoms were xerostomia (68%), dysphagia (27%), dysgeusia (26%), spasticity (19%), and pain (17%). There was a trend for older patients to be less likely to write in their symptoms, with 61% of age 65 years or older having reported any symptoms compared to 77% of patients younger than 65 years (P = .06). These responses only indicate what patients chose to report on the survey. Not reporting does not indicate that no symptoms were present.
4 |. DISCUSSION
Although HPV-positive oropharyngeal SCC is typically a disease of younger patients, there are a substantial number of older patients who will be diagnosed with, treated for, and survive this cancer.7 In our study, in which 17% of the patients were 65 years or older at diagnosis, we found that after treatment, these patients are at an increased risk of requiring a PEG, report trouble with social eating, poorer mobility, and marginally greater problems with coughing compared with younger patients. Despite these differences, in general, older and younger patients report similar scores on the EQ-5D VAS. Prior studies have demonstrated a more dramatic acute decline in HR-QOL after CRT in patients with HPV-related oropharyngeal SCC compared with patients who have oropharyngeal SCC that is not related to HPV.9,10 Consistent with previous reports, we found that patients with HPV-related oropharyngeal SCC who are at least 12 months from CRT can continue to experience ongoing issues that negatively impact QOL and that these might differ based on treatment, age, or other comorbid disease.9,10,22,23 However, our study confirms that age may not be the predominant predictor for worse QOL after CRT for HPV-related oropharyngeal SCC. Instead, it is possible that treatment could exaggerate issues associated with aging, such as impaired mobility, which are not unique to patients with cancer. A larger cohort of patients with additional baseline functional and QOL information would provide greater insight into the impact of CRT on older patients. Our study, looking at age as a predictor of HR-QOL, is somewhat consistent with findings observed in older patients with head and neck SCC. In terms of impaired mobility, 2 prospective studies from the Netherlands evaluating HR-QOL in patients treated for head and neck cancer, older age, defined as older than 60 years in 1 study, and as older than 70 years in the other study, predicted for decline in physical functioning.24,25 Unlike those studies with limited patients (<10%) treated with chemotherapy, all but 2 of our patients received chemotherapy, of which 91% received cisplatin. However, our patients who received more aggressive therapy with CRT reported overall high levels of QOL, which was not altogether expected. It is possible that our results are only applicable to the most robust elderly patients who are deemed candidates for concurrent cisplatin and RT. In which case, maybe age alone should not be used to exclude use of aggressive therapy in patients with HPV-related oropharyngeal SCC.
Our study used an open-ended question to ask patients to self-report symptoms that they found bothersome in an exploratory context. Older patients were less likely to volunteer symptoms compared with their younger counterparts. We speculated that this difference could be due to older patients coping better with posttreatment symptoms secondary to modified expectations of their function. In a prospective, randomized study of patients with p16-positive oropharyngeal cancer undergoing CRT, Ringash et al10 dem-onstrated that patients with p16-positive oropharyngeal cancer demonstrated a steeper acute decline in QOL during treatment and postulated that this could be explained by a higher expectation of performance status. Given that QOL is a subjective measurement, it is possible that similar differences in expectations of posttreatment functional status could explain some of the differences seen in our cohort. Although without baseline QOL scores, we are unable to explore this further. Clear expectations for posttreatment functional status might be an important component of counseling for these patients, regardless of age. In another prospective study, Funk and colleagues26 made the observation that 5-year survivors of head and neck cancer reported similar general overall health as age-matched norms, suggesting that age was the unifying factor, rather than the cancer or its treatment. In our cross-sectional study, older patients may have experienced similar rates of symptom burden but simply did not respond to this question in the same way due to age, education level (which we did not capture), or framing of the question. Qualitative studies with follow-up questions would better clarify these observations.
Although there was no statistical difference in dysphagia as measured by the specific EORTC-QLQ-H&N35 item on swallowing, our study suggests that patients with oropharyngeal SCC over the age of 65 years treated with radiation may have greater problems swallowing compared with those patients who are younger at the time of diagnosis, as demonstrated by a clinically significant difference in report of problems with social eating and coughing. Dysphagia post-RT to the head and neck has been well described as a long-term and later developing toxicity by Hutcheson and colleagues.27 Possible explanations for worse self-reported outcomes in older patients in other studies are poorer adherence to recommended exercises, placement of PEG during or after treatment, or age-related delayed recovery, all of which have been shown to be associated with poorer long-term swallowing outcomes.28–30 Although we did not find a specific difference in swallowing scores between younger and older patients, we hypothesize that the increased rates of coughing could be part of the same swallowing difficulties in which patients are intermittently aspirating. Yet, in this retrospective study with only patient-reported measures, coughing could also reflect pulmonary disease in a group of current and former smokers.
Older patients reported clinically meaningful decreased mobility compared with younger patients, and it is likely that their decreased mobility is associated with reduced muscle mass.31,32 It is well known that muscle mass decreases with age.31,33 Moreover, weight loss and loss of lean body mass are common in patients with head and neck cancer undergoing CRT, with the loss of lean body mass associated with decreased physical performance and total physical activity level.34 It is possible that older patients do not recover their muscle mass posttreatment, suggesting an opportunity for early intervention and risk assessment. It is also possible that this difference in mobility between younger and older patients is not related to treatment but rather mirrors the expected differences between younger and older patients observed in the general adult U.S. population.35,36 Regard-less, as these patients transition to survivorship, attention to noncancer functional outcomes deserves consideration and management.
The greatest limitation of this cross-sectional survey is that we do not have longitudinal data on this cohort. Presumably, most of the toxicities reported by patients, such as dry mouth, dysgeusia, and dysphagia, are due to the cancer and its treatment but other outcomes, such as the EQ-5D QOL scores, have to be interpreted with caution. In addition, this is a select sample of our patients who continue to follow-up with our medical oncologists for surveillance. It is also a small sample size with only 31 patients over the chronological age of 65 years, and it is possible that if we had a larger sample of older patients or used functional age that we could have identified more granular differences between groups. This is particularly important given the finding that the difference between scores for many of the outcomes were clinically meaningful but did not reach statistical significance. We performed a sensitivity analysis, and although this group looked like a representative sample of our patients at diagnosis, by the time of this survey, which is at minimum 12 months since treatment, we could be selecting for a higher performing cohort who survived and is compliant with surveillance, or alternatively, those that continue to have problems seeking medical attention with an oncologist. Despite these limitations, this study highlighted some key differences, including the difference in pattern of response to open-ended questions, greater issues with mobility, and ongoing PEG-dependency, which are informative in caring for this growing cohort of head and neck cancer survivors.
5 |. CONCLUSIONS
We explored HR-QOL in older survivors of HPV-positive oropharyngeal SCC treated with RT.25,37 At the time of the survey, at least 12 months from treatment completion, patients who were 65 years of age or older reported greater problems with social eating, were also more likely to be PEG dependent, despite not reporting more problems with swallowing. Older survivors also noted greater problems with mobility and marginally greater problems with coughing at the time of the survey. However, it is important to note that although we report on differences between age groups, survivors of HPV-related oropharyngeal SCC treated with radiation continue to experience toxicities of treatment that impact QOL. Our study explores issues that might be exaggerated in an older and growing cohort of survivors. Some of the problems identified in this hypothesis-generating study are prime for further investigation and possibly earlier interventions to mitigate long-term consequences of CRT, including routine swallowing therapy and strength training. However, given the response patterns in our study, older patients may require more direct questioning and higher attentiveness to ascertain true symptom burden.28
Funding information
National Institutes of Health (NIH); P30 Cancer Center Support Grant, Grant/Award Number: P30 CA008748; NIH, Grant/Award Number: P30 Cancer Center Support Grant (P30 CA008748).
REFERENCES
- 1.Viens LJ, Henley SJ, Watson M, et al. Human papillomavirus-associated cancers - United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2016; 65(26):661–666. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). Human papillomavirus-associated cancers - United States, 2004–2008. MMWR Morb Mortal Wkly Rep. 2012;61(15):258–261. [PubMed] [Google Scholar]
- 3.O’Rorke MA, Ellison MV, Murray LJ, Moran M, James J, Anderson LA. Human papillomavirus related head and neck cancer survival: a systematic review and meta-analysis. Oral Oncol. 2012;48(12):1191–1201. [DOI] [PubMed] [Google Scholar]
- 4.Baxi SS, Salz T, Xiao H, et al. Employment and return to work following chemoradiation in patients with HPV-related oropharyngeal cancer. Cancers Head Neck. 2016;1(4):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26(4):612–619. [DOI] [PubMed] [Google Scholar]
- 6.Michal SA, Adelstein DJ, Rybicki LA, et al. Multi-agent concurrent chemoradiotherapy for locally advanced head and neck squamous cell cancer in the elderly. Head Neck. 2012;34(8):1147–1152. [DOI] [PubMed] [Google Scholar]
- 7.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27(17):2758–2765. [DOI] [PubMed] [Google Scholar]
- 8.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26(21):3582–3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma A, Méndez E, Yueh B, et al. Human papillomavirus-positive oral cavity and oropharyngeal cancer patients do not have better quality-of-life trajectories. Otolaryngol Head Neck Surg. 2012;146(5):739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ringash J, Fisher R, Peters L, et al. Effect of p16 status on the quality-of-life experience during chemoradiation for locally advanced oropharyngeal cancer: a substudy of randomized trial Trans-Tasman Radiation Oncology Group (TROG) 02.02 (HeadSTART). Int J Radiat Oncol Biol Phys. 2017; 97(4):678–686. [DOI] [PubMed] [Google Scholar]
- 11.Allison JP, Locker D, Wood-Dauphinee S, Black M, Feine JS. Correlates of health-related quality of life in upper aerodigestive tract cancer patients. Qual Life Res. 1998;7(8):713–722. [DOI] [PubMed] [Google Scholar]
- 12.Hammerlid E, Taft C. Health-related quality of life in long-term head and neck cancer survivors: a comparison with general population norms. Br J Cancer. 2001;84(2):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Neill CB, Baxi SS, Atoria CL, et al. Treatment-related toxicities in older adults with head and neck cancer: a population-based analysis. Cancer. 2015;121(12):2083–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EuroQol Group. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. [DOI] [PubMed] [Google Scholar]
- 15.Krabbe PF, Peerenboom L, Langenhoff BS, Ruers TJ. Responsiveness of the generic EQ-5D summary measure compared to the disease-specific EORTC QLQ C-30. Qual Life Res. 2004;13(7):1247–1253. [DOI] [PubMed] [Google Scholar]
- 16.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes. 2007;5:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherman AC, Simonton S, Adams DC, Vural E, Owens B, Hanna E. Assessing quality of life in patients with head and neck cancer: cross-validation of the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Head and Neck module (QLQ-H&N35). Arch Otolaryngol Head Neck Surg. 2000;126(4):459–467. [DOI] [PubMed] [Google Scholar]
- 18.Bjordal K, Freng A, Thorvik J, Kaasa S. Patient self-reported and clinician-rated quality of life in head and neck cancer patients: a cross-sectional study. Eur J Cancer B Oral Oncol. 1995;31B(4):235–241. [DOI] [PubMed] [Google Scholar]
- 19.Bjordal K, Hammerlid E, Ahlner-Elmqvist M, et al. Quality of life in head and neck cancer patients: validation of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-H&N35. J Clin Oncol. 1999;17(3):1008–1019. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. World report on ageing and health 2015. Geneva, Switzerland, World Health Organization, 2015. [Google Scholar]
- 22.Broglie MA, Soltermann A, Haile SR, et al. Quality of life of oropharyngeal cancer patients with respect to treatment strategy and p16-positivity. Laryngoscope. 2013;123(1):164–170. [DOI] [PubMed] [Google Scholar]
- 23.Maxwell JH, Mehta V, Wang H, et al. Quality of life in head and neck cancer patients: impact of HPV and primary treatment modality. Laryngoscope. 2014;124(7):1592–1597. [DOI] [PubMed] [Google Scholar]
- 24.de Graeff A, de Leeuw JR, Ros WJ, Hordijk GJ, Blijham GH, Winnubst JA. Long-term quality of life of patients with head and neck cancer. Laryngoscope. 2000;110(1):98–106. [DOI] [PubMed] [Google Scholar]
- 25.Derks W, de Leeuw RJ, Hordijk GJ, Winnubst JA. Quality of life in elderly patients with head and neck cancer one year after diagnosis. Head Neck. 2004;26(12):1045–1052. [DOI] [PubMed] [Google Scholar]
- 26.Funk GF, Karnell LH, Christensen AJ. Long-term health-related quality of life in survivors of head and neck cancer. Arch Otolaryngol Head Neck Surg. 2012;138(2):123–133. [DOI] [PubMed] [Google Scholar]
- 27.Hutcheson KA, Lewin JS, Barringer DA, et al. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer. 2012; 118(23):5793–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutcheson KA, Bhayani MK, Beadle BM, et al. Eat and exercise during radiotherapy or chemoradiotherapy for pharyngeal cancers: use it or lose it. JAMA Otolaryngol Head Neck Surg. 2013;139(11):1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akst LM, Chan J, Elson P, Saxton J, Strome M, Adelstein D. Functional outcomes following chemoradiotherapy for head and neck cancer. Otolaryngol Head Neck Surg. 2004;131(6):950–957. [DOI] [PubMed] [Google Scholar]
- 30.Terrell JE, Ronis DL, Fowler KE, et al. Clinical predictors of quality of life in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130(4):401–408. [DOI] [PubMed] [Google Scholar]
- 31.Visser M, Goodpaster BH, Kritchevsky SB, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–333. [DOI] [PubMed] [Google Scholar]
- 32.Whynes DK, McCahon RA, Ravenscroft A, Hodgkinson V, Evley R, Hardman JG. Responsiveness of the EQ-5D health-related quality-of-life instrument in assessing low back pain. Value Health. 2013;16(1): 124–132. [DOI] [PubMed] [Google Scholar]
- 33.Tsai S Importance of lean body mass in the oncologic patient. Nutr Clin Pract. 2012;27(5):593–598. [DOI] [PubMed] [Google Scholar]
- 34.Silver HJ, Dietrich MS, Murphy BA. Changes in body mass, energy balance, physical function, and inflammatory state in patients with locally advanced head and neck cancer treated with concurrent chemoradiation after low-dose induction chemotherapy. Head Neck. 2007;29(10): 893–900. [DOI] [PubMed] [Google Scholar]
- 35.Lubetkin EI, Jia H, Franks P, Gold MR. Relationship among sociodemographic factors, clinical conditions, and health-related quality of life: examining the EQ-5D in the U.S. general population. Qual Life Res. 2005;14(10): 2187–2196. [DOI] [PubMed] [Google Scholar]
- 36.Luo N, Johnson JA, Shaw JW, Feeny D, Coons SJ. Self-reported health status of the general adult U.S. population as assessed by the EQ-5D and Health Utilities Index. Med Care. 2005;43(11):1078–1086. [DOI] [PubMed] [Google Scholar]
- 37.van der Schroeff MP, Derks W, Hordijk GJ, de Leeuw RJ. The effect of age on survival and quality of life in elderly head and neck cancer patients: a long-term prospective study. Eur Arch Otorhinolaryngol. 2007;264(4):415–422. [DOI] [PubMed] [Google Scholar]