Abstract
BACKGROUND:
Experimental studies suggest that shallow uterine cytotrophoblastic invasion in preeclampsia may be associated with alterations in estrogen metabolism. The objective of this study was to examine the association of parent estrogens and their metabolites between preeclamptics and normotensive controls at three time points during pregnancy.
METHODS:
Parent estrogens and their metabolites were measured in urine by high-performance liquid chromatography-tandem mass spectrometry in 66 singleton preeclampsia cases and 137 matched controls. Percent change in geometric means were estimated by general linear models adjusted for gestational age at sampling, maternal age, parity, race, body mass index, and use of assisted reproductive technologies.
RESULTS:
Urinary estradiol concentrations were approximately 50% higher in early pregnancy in preeclampsia cases than controls, but similar late in pregnancy. There was an approximate 20% reduction in total 2-pathway metabolites and 4-pathway metabolites in cases compared with controls in mid- and later pregnancy that was slightly attenuated with adjustment for BMI, and a reduction in 16-pathways in mid-pregnancy but not later.
CONCLUSION(s):
Our findings show that estradiol concentrations were elevated in preeclampsia versus controls in early pregnancy. In mid-pregnancy, all three estrogen metabolism (2-, 4-, and 16-) pathways showed some reduction in preeclampsia that appeared to continue for the 2- and 4-pathways in late pregnancy. We hypothesize that this may indicate that there is a generalized reduction in estrogen metabolism in preeclampsia rather than a deficit of specific enzymes, such as those involved in the 2-hydroxylation pathway.
Keywords: estradiol-17β metabolites, estrogen metabolism pathways, preeclampsia, LC-MS/MS, urine
Introduction
Preeclampsia is characterized by new-onset or worsening hypertension and significant proteinuria after 20 weeks of gestation and remains a major cause of preterm birth and maternal morbidity and mortality worldwide.1–3 There is a growing consensus that preeclampsia is a syndrome defined by the above clinical characteristics, but the underlying pathogenesis is unclear.4 Redman and colleagues have sought to broadly classify preeclampsia into maternal and placental subtypes which either separately or in concert are sufficient to cause the clinical manifestations.5 Maternal preeclampsia is thought to be related to an exaggerated maternal inflammatory response to pregnancy and a normal functioning placenta, while placental preeclampsia arises from abnormal placentation and inadequate perfusion. While multiple factors are involved in each of these subtypes, steroid hormones have been shown to play a role in both placentation and perfusion, as well as immunomodulation.6-8
In early pregnancy, estrogens, progesterone and their metabolites participate in placental angiogenesis and in normal trophoblast development and invasion. Estradiol alters concentrations of the angiogenic markers VEGF, PlGF, and s-FLT9–11 which regulate angiogenic processes. It has been hypothesized that low levels of estradiol may lead to insufficient trophoblast development and angiogenesis, termed the estrogen deficiency hypothesis.9 Subsequently, low levels of estradiol may persist throughout pregnancy due to the impaired implantation, once the shift to placental metabolism of this hormone occurs, thus explaining the reduced estrogen values that are sometimes observed in preeclampsia.9 But while falling urinary estriol excretion was once considered a harbinger of preeclampsia,12–14 more recent studies have shown that circulating maternal parent estrogens near delivery are not necessarily lower in preeclampsia compared with uncomplicated pregnancy, and may be slightly elevated.15–17
Results from laboratory and mice studies show that cytotrophoblastic invasion of the uterus in early pregnancy requires both a hypoxic environment and low concentrations of 2-hydroxyestradiol, the estradiol-17β metabolite derived from the pathway defined by hydroxylation at the 2 position of the steroid ring.18 2-Methoxyestradiol, another metabolite in the 2-metabolism pathway, is a potent antiangiogenic factor with no proliferative activity.19 Data on estrogen metabolism and preeclampsia in humans, though scant, also suggest alterations in parent estrogen metabolism pathways, namely less metabolism along the 2-pathway than in control pregnancies.20–25 These data, however, are based on small studies, and information on the other two estrogen metabolism pathways (4- and 16-pathways) are rare.25
The objective of the current study was to comprehensively compare maternal urinary parent estrogens, estrogen metabolites, and their metabolic pathways between preeclamptic and normotensive pregnancies at three time points in pregnancy.
Methods
Study design and population
This nested case control study was based on data from an ongoing prospective cohort of women recruited early in pregnancy.26 The current analysis was limited to one of the centers in that study, the Brigham and Women’s Hospital (BWH), which included 1,601 women >18 years of age who presented for routine prenatal care at <15 weeks’ gestation, and who planned to deliver at BWH were enrolled from 2006 to 2008. The only exclusion criterion was multiple gestations (twins or greater). We selected preeclampsia cases (n=66) and matched them with approximately two controls (n=137) on gestational age at urine collection (+/− 2 weeks), maternal age (+/− 2 years), and parity (nulliparous or multiparous prior to the index pregnancy). A few of the cases and controls did not have samples available for all three time periods: the sample sizes (cases/controls) were 64/133 at time 1, 59/130 at time 2 and 61/131 at time 3.
The protocol was approved by the institutional review board at BWH and written, informed consent was obtained from all participating women.
Definition of preeclampsia
Preeclampsia was defined as blood pressures ≥ 140mmHg systolic or ≥ 90mmHg diastolic after 20 weeks of gestation along with positive urinary protein testing (>300mg/24 hours or protein/creatinine ratio > 0.20) following the standard criteria available from the American College of Obstetrics and Gynecology at the time of the study. All cases of hypertensive disease were de-identified and reviewed by a panel of study principle investigators to confirm the diagnosis.
Clinical information
Maternal age, parity, and conception of the index pregnancy by assisted reproductive technologies (ART), history of and concurrent pregnancy complications, the baby’s birth anthropometrics and sex, and medical insurance were abstracted from medical records. Body mass index (weight (km)/height (m)2) was from the participants’ report of weight before pregnancy. Gestational age of pregnancy was confirmed by ultrasound scanning at ≤12 weeks gestation. If consistent with last menstrual period (LMP) dating, the LMP was used to determine the due date. If not consistent, then the due date was set by the earliest available ultrasound ≤12 weeks gestation.
Measurement of urine estrogens and estrogen metabolites
Spot urine samples were collected at 3 study visits during pregnancy: 1) median 9.8 weeks gestation (range 5.3 to 15.3 weeks); 2) median 17.7 weeks (range 15.4 to 21.7 weeks); and 3) median 25.8 weeks (range 23.1 to 29.3 weeks). Samples were stored at −80°C and then shipped on dry ice to the Laboratory of Proteomics and Analytical Technologies Leidos Biomedical Research, Inc. (Frederick, MD.) for analysis of parent estrogens and estrogen metabolites.
Stable isotope dilution liquid chromatography-tandem mass spectrometry (LC-MS/MS) was used to measure concurrently urine concentrations of 15 estrogens and estrogen metabolites in pathways defined by irreversible hydroxylation at the C-2, C-4, or C-16 positions of the steroid ring. These included the parent estrogens, estrone and estradiol, and metabolites in the 2-hydroxylation pathway (i.e. 2-hydroxyestrone, 2-methoxyestrone, 2-hydroxyestradiol, 2-methoxyestradiol, and 2-hydroxyestrone-3-methyl ether); metabolites in the 4-hydroxylation pathway (i.e. 4-hydroxyestrone, 4-methoxyestrone, and 4-methoxyestradiol); and metabolites in the 16-hydroxylation pathway (i.e. 17beta-estriol, aka estriol; 16α-hydroxyestrone, 17-epiestriol, also known as 17alpha-estriol, 16-ketoestradiol, and 16-epiestriol); henceforth referred to as the 2-, 4- and 16-pathways. Parent estrogens were the sum of estrone and estradiol; total metabolites were the sum of 2-, 4-, and 16-pathways; and total estrogens was defined as the sum of the parent estrogens and metabolites. We also created a variable (2-+4-pathway metabolites) for the sum of metabolites in the 2- and 4-pathways only. Details of the method for measuring urine estrogens and estrogen metabolites, including sample preparation and assay conditions, have been published previously.27 Case–control matched sets were assayed in the same batches, and blinded (to the laboratory) quality control samples based on a pool of urine from 5 uncomplicated pregnancies constituted 10% of each batch. No assays of estrogens or estrogen metabolites in this study resulted in non-detectable readings. Total between batch coefficients of variation based on blinded replicate samples were all ≤3.1%, and within batch were ≤2.7%. To standardize the urine estrogen and estrogen metabolite data, urine specific gravity was measured in specimens using the Digital Urine Specific Gravity Refractometer (ATAGO CO., LTD). The specific gravity (SG) correction applied to each estrogen metabolite was: SG-corrected EM = EM x (SG-reference – 1.0)/(SG-sample– 1.0), where the reference SG (SG-reference) is the mean specific gravity for each time point (1.016, 1.014 and 1.014, respectively). SG did not differ between cases and controls at any of the three time points (data not shown). While there were some differences between estrogens in the morning and afternoon at time points 1 and 2 (data not shown), the proportion of cases and controls attending the clinic was similar by time of day. All concentrations are listed as pmol/ml.
Statistical methods
We used t-tests and χ2 tests to assess differences in distributions of maternal, pregnancy and neonatal factors between preeclampsia cases and controls. At the three time points in pregnancy, estrogens and estrogen metabolites were analyzed individually, in combination, for example representing metabolic pathways (i.e. 2-, 4- and 16-pathways), and as various ratios of metabolic pathways and parent estrogens. Values for the estrogens were examined for outliers and those that were 2 interquartile ranges above the 75% percentile or below the 25% percentile were excluded for the analysis of that estrogen; results were similar with and without the outliers included (maximum of 4 cases and 8 controls removed for any one model; data not shown). Values were log- transformed to improve normality.
Among controls, correlations were calculated among the parent estrogens and the total metabolites in each of the 2- and 4- pathways. Standard linear models (Proc GLM; SAS Version 9.4) with log-estrogen (or log-ratio) as the dependent variable and case-control status as the independent variable estimated geometric means (using the LSMEANS statement). Models were adjusted for the matching factors (gestational ages at urine collections and maternal age as continuous variables, and parity vs. nulliparity). Including an additional term for gestational age squared did not change the results (data not shown). The coefficient, β, for case-control status was exponentiated and estimates the ratio of the geometric means between cases and controls. The percentage change is defined as 100 x (exp(β)-1). In separate models, we adjusted further for BMI, race/ethnicity and use of ART (referred to as the full model). P-values were based on two-sided tests and were not adjusted for multiple comparisons.
Results
The clinical and demographic characteristics of the preeclampsia cases and controls are presented in Table 1. A higher proportion of preeclampsia cases were African American (22.7% vs. 10.9%), had a higher pre-pregnancy BMI than controls (30.6 vs. 24.9 kg/m2), and used some form of assisted reproductive technology (19.7% vs. 13.1%). In addition, cases were more likely to have pre-pregnancy diabetes or chronic hypertension, concomitant gestational diabetes, and preeclampsia in a prior pregnancy than controls. There were no major differences in maternal education, insurance status, infant sex and smoking status between cases and controls. Most of the cases were diagnosed at a gestational age greater than 34 weeks gestation (n=45; 68%).
Table 1:
Baseline Characteristics of Preeclampsia and Control Pregnancies
| Preeclampsia (n=66) | Control (n=137) | ||
|---|---|---|---|
| Characteristic | Mean (SD) or n (%) | Mean (SD) or n (%) | p-valuea |
| Age (y) | 31.7 (5.2) | 31.7 (5.1) | 0.93 |
| BMI pre-pregnancy (kg/m2) | 30.6 (7.9) | 24.9 (5.2) | <0.001 |
| Race/ethnicity | 0.18 | ||
| White | 39 (59.1%) | 85 (62.0%) | |
| African-American | 15 (22.7%) | 15 (10.9%) | |
| Asian | 4 (6.1%) | 14 (10.2%) | |
| Hispanic | 6 (9.1%) | 14 (10.2%) | |
| Other race/ethnicity | 2 (3.0%) | 9 (6.6%) | |
| Maternal education | 0.10 | ||
| ≤High school | 12 (18.2%) | 14 (10.2%) | |
| >High school | 53 (80.3%) | 123 (89.8%) | |
| Missing | 1 (1.5%) | 0 (0.0%) | |
| Health Insurance | 0.76 | ||
| Self-pay or government | 12 (16.9%) | 27 (19.6%) | |
| Private/HMO | 56 (78.9%) | 109 (79.0%) | |
| Missing | 3 (4.2%) | 2 (1.4%) | |
| Nulliparous | 36 (54.5%) | 73 (53.3%) | 0.87 |
| Smoked during pregnancy | 4 (6.1%) | 10 (7.3%) | 0.74 |
| Use of ARTb | 13 (19.7%) | 18(13.1%) | 0.22 |
| History of preeclampsiac | 10 (33.3%) | 1 (1.6%) | <0.001 |
| Pre-pregnancy diabetes | 10 (15.2%) | 6 (4.4%) | 0.01 |
| History of chronic hypertension | 15 (22.7%) | 5 (3.6%) | <0.001 |
| Current diagnosis of gestational diabetes | 10 (15.2%) | 10 (7.3%) | 0.08 |
| Gestational weeks at delivery | 36.7 (3.0) | 38.8 (1.7) | <0.001 |
| Birthweight (g) | 2840.8 (823.8) | 3273.3 (577.2) | <.001 |
| Male infant | 29 (43.9%) | 70 (51.1%) | 0.34 |
Homogeneity p-values (t-test or chi-squared test)
ART = assisted reproductive technology
Restricted to multiparous women
In both the cases and controls, the 16-pathway comprised the vast amount of total estrogens at all three time points in pregnancy (cases and controls combined 77%, 80% and 76%, respectively), while the 2-pathway (11%, 8.7%, and 9.2%) and 4-pathway (0.4% at all three time points) metabolites contributed much less to total estrogens (Figures 1, 2, and 3). The parent hormones, estrone and estradiol, were highly correlated at all three time points (r=0.80, r=0.79 and r=0.79, respectively), whereas the correlations between parent estrogens and the 2-pathway (r=0.63, 0.59, 0.62) and 4-pathway metabolites (r=0.41, 0.28, 0.40) were lower.
Figure 1:
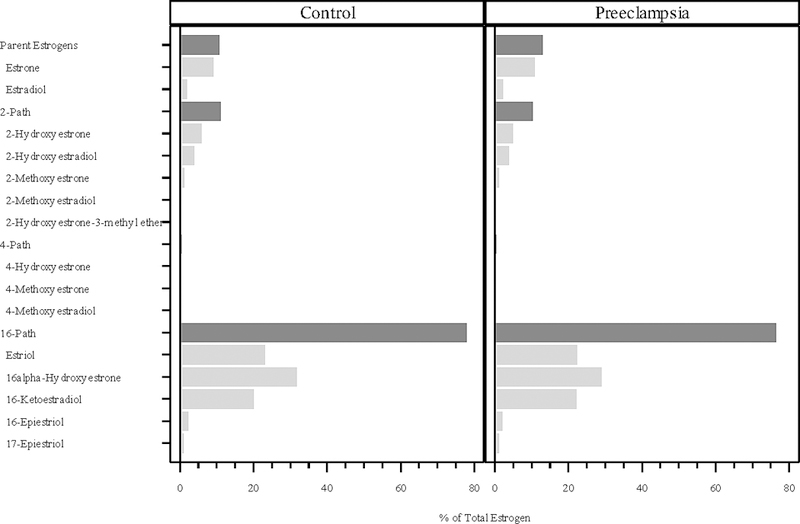
Percentage of total estrogen (sum of 15 estrogens/estrogen metabolites) in each of the metabolic pathway groups at median 9.8 weeks gestation. The proportions for the parent estrogens, 2-hydroxylation pathway (2-path), 4-hydroxylation pathway (4-path), and 16-hydroxylation pathway (16-path) estrogen metabolites sum to 100%.
Figure 2:
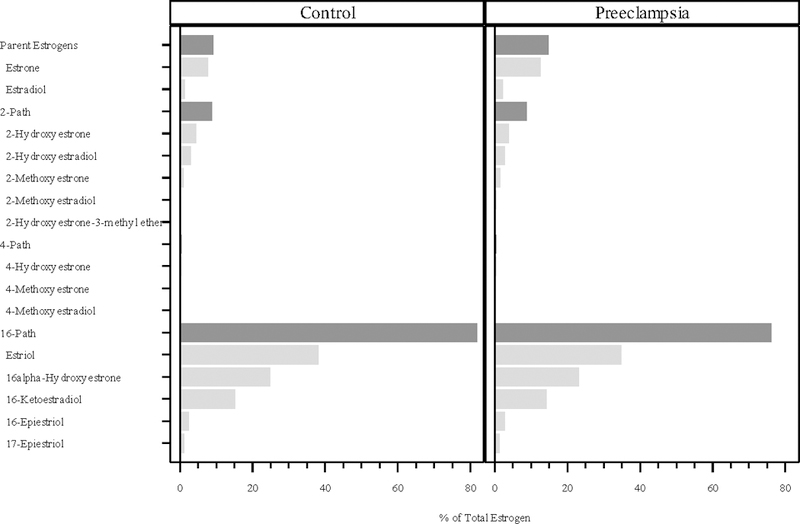
Percentage of total estrogen (sum of 15 estrogens/estrogen metabolites) in each of the metabolic pathway groups at median 17.7 weeks gestation. The proportions for the parent estrogens, 2-hydroxylation pathway (2-path), 4-hydroxylation pathway (4-path), and 16-hydroxylation pathway (16-path) estrogen metabolites sum to 100%.
Figure 3:
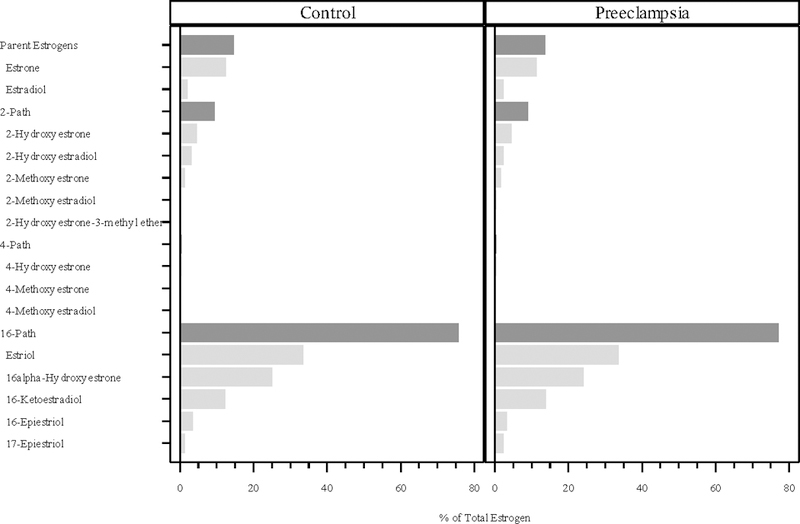
Percentage of total estrogen (sum of 15 estrogens/estrogen metabolites) in each of the metabolic pathway groups at median 25.8 weeks gestation. The proportions for the parent estrogens, 2-hydroxylation pathway (2-path), 4-hydroxylation pathway (4-path), and 16-hydroxylation pathway (16-path) estrogen metabolites sum to 100%.
Geometric and arithmetic mean concentrations of the parent hormones in cases and controls increased across the pregnancy, as did concentrations of the estrogen metabolites (Supplemental Tables S1 and S2). The ratio of the combined 2-+4-pathway metabolites to parent estrogens decreased across the three time points in pregnancy for both cases and controls. Additionally, we observed that the separate ratios of 2-pathway and 4-pathway to parent estrogens each generally decreased as well across the three time points.
Percent changes in geometric means for the parent estrogens, estrogen metabolites, and their ratios adjusted for maternal age, parity and gestational age at blood draw at each sampling time point are presented in Table 2. Parent estrogens, largely from estradiol, appeared higher in preeclampsia than controls in early pregnancy (Δ=22.7%, p=0.13); the change was attenuated after full adjustment for race/ethnicity, pre-pregnancy BMI and use of ART in the index pregnancy (Δ=16.8%, p=0.27). Mean urinary estradiol concentrations were approximately 50% higher in preeclampsia cases compared with controls in early pregnancy and 20% higher in mid-pregnancy, remaining higher with full adjustment, but were similar in cases and controls later in pregnancy. Total metabolite concentrations were slightly lower at the second time point but the change was attenuated with full adjustment. Total 2-pathway metabolites were approximately 20% lower in cases compared with controls in mid- and later pregnancy, but the change was attenuated with full adjustment. There were no significant changes in the 4-pathway and 16-pathways between cases and controls. When models included the factors used for full adjustment individually, it became clear that the change in results was due to BMI only (data not shown).
Table 2:
Percent Change in Urinary Estrogen Compounds and Metabolites for Preeclampsia (PE) compared with Controls
| 5.3–15.3 weeks (PE=64 Controls=133) |
15.4–21.7 weeks (PE=59 Controls=130) |
23.1–29.3 weeks (PE=61 Controls=131) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM Measures | % Δa | p | % Δb | p | % Δa | p | % Δb | p | % Δa | p | % Δb | p |
| Total Estrogens | 5.1 | 0.64 | 9.4 | 0.43 | −13.9 | 0.05 | −11.3 | 0.15 | −12.7 | 0.04 | −5.7 | 0.42 |
| Parent Estrogens | 22.7 | 0.13 | 16.8 | 0.27 | 2.6 | 0.84 | −2.5 | 0.85 | −14.3 | 0.16 | −13.4 | 0.24 |
| Estrone | 19.8 | 0.20 | 13.4 | 0.40 | −0.5 | 0.97 | −6.5 | 0.64 | −16.1 | 0.13 | −16.1 | 0.17 |
| Estradiol | 45.6 | <0.01 | 44.4 | <0.01 | 21.5 | 0.10 | 23.9 | 0.09 | −0.5 | 0.96 | 4.9 | 0.71 |
| Total Metabolites | −2.4 | 0.82 | 2.3 | 0.85 | −19.7 | <0.01 | −16.2 | 0.03 | −12.2 | 0.07 | −3.5 | 0.65 |
| 2-+4-Pathway Metabolites | −3.1 | 0.80 | 0.0 | 1.00 | −23.2 | 0.03 | −20.6 | 0.09 | −28.5 | <0.01 | −25.8 | 0.03 |
| 2-Path | −9.3 | 0.41 | −5.1 | 0.68 | −23.3 | 0.03 | −20.7 | 0.09 | −25.5 | 0.02 | −21.2 | 0.08 |
| 4-Path | −6.1 | 0.65 | −9.8 | 0.49 | −21.1 | 0.09 | −18.5 | 0.19 | −22.7 | 0.09 | −20.4 | 0.16 |
| 16-Path | −1.3 | 0.91 | 3.3 | 0.81 | −18.3 | <0.01 | −15.1 | 0.05 | −12.4 | 0.08 | −3.2 | 0.69 |
| 2-+4-Pathway Metabolites: Parent Estrogens | −23.2 | 0.02 | −18.0 | 0.07 | −25.1 | 0.01 | −18.6 | 0.09 | −14.1 | 0.16 | −8.7 | 0.43 |
| 2-Path: Parent Estrogens | −23.0 | 0.02 | −17.5 | 0.08 | −25.3 | 0.01 | −18.7 | 0.08 | −14.5 | 0.14 | −9.1 | 0.40 |
| 4-Path: Parent Estrogens | −25.5 | 0.05 | −26.1 | 0.06 | −21.4 | 0.14 | −14.1 | 0.39 | −7.2 | 0.62 | −2.0 | 0.90 |
| 2-Path: 4-Path | −0.3 | 0.98 | 9.4 | 0.36 | −4.9 | 0.60 | −5.4 | 0.60 | −10.9 | 0.19 | −6.0 | 0.52 |
Models adjusted for maternal age, parity, and gestational age at sample collection.
Models adjusted for maternal age, parity, gestational age at sample collection, race, BMI, and use of ART.
The ratio of 2-+4-pathway metabolites to parent estrogens, was generally lower in preeclamptic than control pregnancies in early and mid-pregnancy, as were the relative amounts of each of the pathways (i.e. 2- and 4-pathways) to parent estrogens (Table 2). These changes in the ratios of total and pathway specific estrogen metabolites to parent estrogens were attenuated with full adjustment later in pregnancy. The ratio 2-pathway: 4-pathway demonstrated no evidence that estrogens were preferentially metabolized in one of the two pathways.
Discussion
In this study estrogen concentrations increased markedly throughout pregnancy with a preponderance of estradiol and estrone, and unlike the non-pregnant state, metabolites in the 16-pathway. Metabolites in the 16 pathway largely reflect 16 hydroxylation of androgen precursors in the fetus, which then return to the placenta for aromatization. In early pregnancy, maternal urinary parent estrogen concentrations, particularly estradiol, were elevated in preeclamptic pregnancies compared with control pregnancies. Absolute concentrations of metabolites in cases and controls, however, were generally similar during early pregnancy despite the preeclamptic mothers’ greater parent estrogen concentrations. This resulted in a lower ratio of 2- and 4-pathway metabolites to parent estrogens in preeclamptic pregnancies in early and mid-pregnancy. In later pregnancy, reduced absolute 2-pathway metabolite concentrations in preeclamptic mothers compared with controls was partly explained by higher BMI in the cases, and there were no other differences in parent estrogens, absolute metabolites or their ratios. These results indicate that despite greater parent estrogen concentrations in preeclamptic pregnancies than controls in early to mid-pregnancy, total urinary concentrations of metabolites are similar, suggesting that metabolism is reduced in preeclamptic women. A slight reduction in absolute levels of 2-pathway metabolites later in pregnancy in preeclampsia was explained by the greater BMI of women with preeclampsia.
Steroid hormone metabolism is adversely affected in preeclamptic pregnancies. Maternal androgen concentrations by the second half of pregnancy are elevated in preeclampsia,15,16,28 although whether levels differ earlier in pregnancy is unclear.29 Normal concentrations of maternal DHEA, the substrate for androgen synthesis, in the presence of elevated androstenedione and testosterone concentrations15 are consistent with in-vitro studies showing reduced conversion of androgens to estrogens in placental tissue from preeclamptic pregnancies.30 Data on estradiol and estrone concentrations in preeclampsia, though, are inconsistent, with some studies showing lower concentrations of parent estrogens15,20,25 and others showing no difference15,16 compared with control pregnancies. In our study, maternal urinary estradiol was significantly greater in preeclamptic compared with control pregnancies adjusting for gestational age at collection, parity, maternal age, race/ethnicity, BMI and use of ART, in early and mid-pregnancy, but not later in pregnancy. Most studies have assessed pregnancy estrogens later in the pregnancy only.20,25
A few studies have assessed whether concentrations of estrogen metabolites differ in preeclampsia,20–25 but comprehensive estrogen metabolite data in humans are limited. There is some evidence that 2-pathway metabolites in the maternal circulation late in pregnancy are lower in preeclamptic compared with control pregnancies.20,23,25 2-methoxyestradiol was lower in preeclampsia compared with uncomplicated pregnancy,23–25 as was 2-methoxyestrone, and the premethylated forms 2-hydroxyestrone and 2-hydroxyestradiol,25 while in another, 2-methoxyestradiol was only decreased in severe preeclampsia with HELLP syndrome.20 Only one study found higher plasma 2-methoxyestradiol concentrations at delivery in a late-onset PE group vs. control group.31 Some differences have also been observed in the 4-pathway and 16-pathways in plasma but results depended on the severity of preeclampsia.25 Reduced maternal plasma 2-methoxyestradiol concentrations in preeclamptic than control pregnancies have been shown at 22–29 weeks,21 and as early as 11–14 weeks.22 Our data showed reductions in estrogen metabolism that were greater for, but not limited to the 2-pathway, based on measurements of all three pathways and the use of ratios to assess relative metabolism of a given amount of parent estrogens. Thus, there may be a more generalized effect of placental impairment on estrogen metabolism not entirely mediated through lower levels of catechol-O-methyltransferase (COMT), the enzyme that catalyzes the methylation of catechol estrogens to methoxy estrogens, as has been suggested by others.32
Our data are based on measurements in urine instead of plasma or serum; estrogens in urine are generally conjugated, whereas those in circulation are a combination of unconjugated and conjugated. We observed high correlations (r=0.78 and r=0.83, respectively) between estradiol and estrone measured in serum and urine (per unit creatinine) in 313 premenopausal women with specimen collections made on the same day (unpublished data). Additionally, in a study of 83 pregnant women serum estriol was highly correlated (r=0.81) to creatinine corrected urine estriol.33 In pregnancy, the placenta secretes principally unconjugated estrogens into the maternal circulation where they are conjugated in the maternal liver to be excreted in urine. It has been speculated that in preeclampsia, reduced maternal hepatic blood flow lowers the proportion of estrogen conjugated in the liver, leading to lower estrogen concentrations in urine.17 Parent estrogens, when presented, were lower in preeclamptic pregnancies in some studies,22,25 which could explain the lower concentration of metabolites. In contrast, we observed higher estradiol concentrations in preeclampsia, as previously shown in serum,15 than in control pregnancies. We presented relative concentrations of 2- and 4-pathway metabolites to their parent hormones to account for these higher levels. Renal function may decline in preeclampsia compared to controls34 affecting urinary hormone concentrations, though in our study the samples were collected at <30 weeks gestation, before preeclampsia was likely to have resulted in changes to renal function.
Strengths of the study included comprehensive measurement of estrogen metabolites before preeclampsia diagnosis and over much of the pregnancy, and availability of information on factors that could confound the association with preeclampsia. Because parent estrogens and estrogen metabolites increase throughout the pregnancy, careful adjustment for gestational week at sample collection is important, as preeclamptic pregnancies will generally deliver earlier. Metabolites in samples coinciding with labor may be altered by stress, type of delivery or medications administered. In addition, estrogen metabolism towards the end of a preeclamptic pregnancy might be expected to be impaired by increasing senescence of the placenta, reflecting the consequences of the disease process rather than its etiology. Our findings, however, are limited to the less severe form of preeclampsia. Few of the preeclampsia cases included in the present analysis were severe as evidenced by the limited number cases with extreme prematurity (≤34 weeks at delivery) so we were unable to assess whether estrogen metabolism differed by severity of disease. 2-methoxyestradiol concentrations were inversely correlated with preeclampsia severity, and possibly with blood pressure values in one study.20
Preeclampsia has its origin in the first stages of pregnancy, with abnormal placentation and implantation. Our data show that even in the first trimester there is evidence of estrogen metabolic changes in preeclampsia cases compared with women who do not develop preeclampsia.
Supplementary Material
Acknowledgements
This research was supported by federal funds from the Division of Cancer Epidemiology and Genetics and Center for Cancer Training, Cancer Prevention Fellowship Program, National Cancer Institute. The data from the LIFECODES cohort was funded by an unrestricted grant from Abbott Diagnostics Division (9MZ-04–06N03) and by R01 ES018872 NIEHS, NIH.
References
- 1.American College of Obstetricians and Gynecologists. Task force on Hypertension in Pregnancy; Hypertension in Pregnancy Washington, DC; 2013. [DOI] [PubMed] [Google Scholar]
- 2.Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilekis JV, Reddy UM, Roberts JM. Preeclampsia--a pressing problem: an executive summary of a National Institute of Child Health and Human Development workshop. Reproductive Sciences 2007;14:508–523. [DOI] [PubMed] [Google Scholar]
- 4.Redman CW. Preeclampsia: a multi-stress disorder. La Revue de Medecine Interne. 2011;32 Suppl 1:S41–44. [DOI] [PubMed] [Google Scholar]
- 5.Staff AC, Benton SJ, von Dadelszen P, et al. Redefining preeclampsia using placenta-derived biomarkers. Hypertension 2013;61:932–942. [DOI] [PubMed] [Google Scholar]
- 6.Patel S, Kilburn B, Imudia A, Armant DR, Skafar DF. Estradiol Elicits Proapoptotic and Antiproliferative Effects in Human Trophoblast Cells. Biology of Reproduction 2015;93:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corcoran JJ, Nicholson C, Sweeney M, Charnock JC, Robson SC, Westwood M, et al. Human uterine and placental arteries exhibit tissue-specific acute responses to 17β-estradiol and estrogen-receptor-specific agonists. Molecular Human Reproduction 2014. May;20(5):433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science 2005;308:1592–1594. [DOI] [PubMed] [Google Scholar]
- 9.Berkane N, Liere P, Oudinet JP, Hertig A, Lefèvre G, Pluchino N, et al. From Pregnancy to Preeclampsia: A Key Role for Estrogens. Endocrine Reviews 2017;38:123–144. [DOI] [PubMed] [Google Scholar]
- 10.Aberdeen GW, Bonagura TW, Harman CR, Pepe GJ, Albrecht ED. Suppression of trophoblast uterine spiral artery remodeling by estrogen during baboon pregnancy: impact on uterine and fetal blood flow dynamics. American Journal of Physiology-Heart and Circulatory Physiology 2012;302:H1936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonagura TW, Pepe GJ, Enders AC, Albrecht ED. Suppression of extravillous trophoblast vascular endothelial growth factor expression and uterine spiral artery invasion by estrogen during early baboon pregnancy. Endocrinology 2008;149:5078–5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garoff L, Seppälä M. Toxemia of pregnancy: Assessment of fetal distress by urinary estriol and circulating human placental lactogen and alpha-fetoprotein levels. American Journal of Obstetrics and Gynecology 1976;126:1027–1033. [DOI] [PubMed] [Google Scholar]
- 13.Long PA, Abell DA, Beischer NA. Fetal growth and placental function assessed by urinary estriol excretion before the onset of pre-eclampsia. American Journal of Obstetrics and Gynecology 1979;135:344–347. [DOI] [PubMed] [Google Scholar]
- 14.Long PA, Abell DA, Beischer NA. Fetal growth retardation and pre-eclampsia. British Journal of Obstetrics and Gynaecology 1980;87:13–18. [DOI] [PubMed] [Google Scholar]
- 15.Troisi R, Potischman N, Roberts JM, Ness R, Crombleholme W, Lykins D, et al. Maternal serum hormone concentrations in preeclamptic and uncomplicated pregnancies. International Journal of Epidemiology 2003;32:455–460. [DOI] [PubMed] [Google Scholar]
- 16.Atamer Y, Erden AC, Demir B, Koçyigit Y, Atamer A. The relationship between plasma levels of leptin and androgen in healthy and preeclamptic pregnant women. Acta Obstetricia et Gynecologica Scandinavica 2004;83:425–430. [DOI] [PubMed] [Google Scholar]
- 17.Jobe SO, Tyler CT, Magness RR. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia implications for vascular dysfunction. Hypertension 2013;61:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SB, Wong AP, Kanasaki K, Xu Y, Shenoy VK, McElrath TF, et al. 2-Methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. American Journal of Pathology 2010;176:710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tinley TL, Leal RM, Randall-Hlubek DA, Cessac JW, Wilkens LR, Rao PN, et al. Novel 2-methoxyestradiol analogues with antitumor activity. Cancer Research 2003;63:1538–1549. [PubMed] [Google Scholar]
- 20.Hertig A, Liere P, Chabbert-Buffet N, Fort J, Pianos A, Eychenne B, et al. Steroid profiling in preeclamptic women: evidence for aromatase deficiency. American Journal of Obstetrics and Gynecology 2010;203:477e1–9. [DOI] [PubMed] [Google Scholar]
- 21.Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, Xie L, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 2008;453:1117–1121. [DOI] [PubMed] [Google Scholar]
- 22.Pérez-Sepúlveda A, Torres MJ, Valenzuela FJ, Larraín R, Figueroa-Diesel H, Galaz J, et al. Low 2-methoxyestradiol levels at the first trimester of pregnancy are associated with the development of pre-eclampsia. Prenatal Diagnosis 2012;32:1053–1058. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Wang T, Shen Y, Wang X, Baker PN, Zhao A. 2-Methoxyestradiol deficiency is strongly related to hypertension in early onset severe pre-eclampsia. Pregnancy Hypertension 2014;4:215–219. [DOI] [PubMed] [Google Scholar]
- 24.Pertegal M, Fenoy FJ, Hernández M, Mendiola J, Delgado JL, Bonacasa B, et al. Fetal Val108/158Met catechol-O-methyltransferase (COMT) polymorphism and placental COMT activity are associated with the development of preeclampsia. Fertility and Sterility 2016;105:134–143. [DOI] [PubMed] [Google Scholar]
- 25.Jobe SO, Tyler CT, Magness RR. Aberrant synthesis, metabolism, and plasma accumulation of circulating estrogens and estrogen metabolites in preeclampsia: Implications for vascular dysfunction. Hypertension 2013;61:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McElrath TF, Lim KH, Pare E, Rich-Edwards J, Pucci D, Troisi R, et al. Longitudinal evaluation of predictive value for preeclampsia of circulating angiogenic factors through pregnancy. American Journal of Obstetrics and Gynecology 2012;207:407e1–7. [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Keefer LK, Ziegler RG, Veenstra TD. A liquid chromatography-mass spectrometry method for the quantitative analysis of urinary endogenous estrogen metabolites. Nature protocols 2007;2:1350–1355. [DOI] [PubMed] [Google Scholar]
- 28.Martin JD, Hähnel E, Hähnel R. Plasma androstenedione in normotensive and hypertensive pregnancy. Steroids 1986;48:315–329. [DOI] [PubMed] [Google Scholar]
- 29.Steier JA, Ulstein M, Myking OL. Human chorionic gonadotropin and testosterone in normal and preeclamptic pregnancies in relation to fetal sex. Obstetrics and Gynecology 2002;100:552–556. [DOI] [PubMed] [Google Scholar]
- 30.Wolf M, Sandler L, Muñoz K, Hsu K, Ecker JL, Thadhani R. First trimester insulin resistance and subsequent preeclampsia: A prospective study. Journal of Clinical Endocrinology and Metabolism 2002;87:1563–1568. [DOI] [PubMed] [Google Scholar]
- 31.Seol HJ, Cho GJ, Oh MJ, Kim HJ. 2-methoxyoestradiol levels and placental catechol-O-methyltransferase expression in patients with late-onset preeclampsia. Archives of Gynecology and Obstetrics 2013;287:881–886. [DOI] [PubMed] [Google Scholar]
- 32.Dragun D, Haase-Fielitz A. Low catechol-O-methyltransferase and 2-methoxyestradiol in preeclampsia: more than a unifying hypothesis. Nephrology Dialysis Transplantion 2009;24:31–33. [DOI] [PubMed] [Google Scholar]
- 33.Dawson EB, Simmons JE, Nagamani M. A comparison of serum free estriol with spontaneous urine total estrogen and estrogen/creatinine ratio. Int J Gynaecol Obstet 1983;21:371–5. [DOI] [PubMed] [Google Scholar]
- 34.Hussein W, Lafayette RA. Renal function in normal and disordered pregnancy. Current Opinion in Nephrology and Hypertension 2014;23: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


