Abstract
Inflammation is a ubiquitous but poorly understood consequence of spinal cord injury (SCI). The mechanisms controlling this response are unclear but culminate in the sequential activation of resident and recruited immune cells. Collectively, these cells can exert divergent effects on cell survival and tissue repair. HMGB1 is a ubiquitously expressed DNA binding protein and also a potent inflammatory stimulus. Necrotic cells release HGMB1, but HMGB1 also is actively secreted by inflammatory macrophages. A goal of this study was to quantify spatio-temporal patterns of cellular HMGB1 expression in a controlled mouse model of experimental SCI then determine the effects of HMGB1 on post-SCI neuroinflammation and recovery of function. We documented SCI-induced changes in nuclear and cytoplasmic distribution of HMGB1 in various cell types after SCI. The data reveal a time-dependent increase in HMGB1 mRNA and protein with protein reaching maximal levels 24–72 hours post-injury then declining toward baseline 14–28 days post-SCI. Although most cells expressed nuclear HMGB1, reduced nuclear labeling with increased cytoplasmic expression was found in a subset of CNS macrophages suggesting that those cells begin to secrete HMGB1 at the injury site. In vitro data indicate that extracelluar HMGB1 helps promote the development of macrophages with a neurotoxic phenotype. The ability of HMGB1 to elicit neurotoxic macrophage functions was confirmed in vivo; 72h after injecting 500ng of recombinant HMGB1 into intact spinal cord ventral horn, inflammatory CNS macrophages co-localized with focal areas of neuronal killing. However, attempts to confer neuroprotection after SCI by blocking HMGB1 with a neutralizing antibody were unsuccessful. Collectively, these data implicate HMGB1 as a novel regulator of post-SCI inflammation and suggest that inhibition of HMGB1 could be a novel therapeutic target after SCI. Future studies will need to identify better methods to deliver optimal concentrations of HMGB1 antagonists to the injured spinal cord.
Keywords: neuroinflammation, microglia, macrophage, spinal cord injury
INTRODUCTION
Microglial and macrophage activation is a prominent feature of many central nervous system (CNS) disorders including spinal cord injury (SCI). The net effect of this activation is debatable, however, several studies show benefits from blocking the migration of or depleting monocytes and macrophages early after SCI (Blight, 1994; Giulian and Robertson, 1990; Gris et al., 2004; Popovich et al., 1999). Data from our laboratory and others indicate that intraspinal macrophage phenotype predicts their effector functions (Gensel et al., 2017; Gensel et al., 2015; Kigerl et al., 2009; Kroner et al., 2014; Miron et al., 2013; Shechter et al., 2013). In vivo, macrophages that adopt phenotypes that mimic in vitro classically activated (M1) macrophages are neurotoxic while those that resemble alternatively activated (M2) macrophages promote axon growth and repair (Kigerl et al., 2009). The signals that polarize CNS macrophages to become M1 macrophages after SCI are unknown.
HMGB1 is a highly conserved ubiquitous nuclear protein that aids in transcription factor initiation (Javaherian et al., 1978; Read et al., 1994; Sutrias-Grau et al., 1999). HMGB1 is also a potent regulator of inflammation. Originally described as a serum biomarker of macrophage activation in a mouse model of sepsis (Wang et al., 1999), HMGB1 has since been found to mediate many inflammatory disease processes (Andersson and Tracey, 2011; Harris et al., 2012). HMGB1 is passively released from necrotic cells (Rovere-Querini et al., 2004; Scaffidi et al., 2002) and also can be secreted by activated macrophages (Jiang and Pisetsky, 2006; Lamkanfi et al., 2010; Rendon-Mitchell et al., 2003; Tang et al., 2007 et al). Once released from a cell, HMGB1 can activate innate immune receptors including TLR4, TLR2, and the receptor for advanced glycation end products (RAGE) (Hori et al., 1995; Kokkola et al., 2005; Park et al., 2006; Park et al., 2004; Yu et al., 2006). These receptors are expressed by macrophages and glia and are important in regulating inflammation, gliosis, and demyelination after SCI (Church et al., 2016; Church et al., 2017; Gensel et al., 2015; Kigerl et al., 2014; Kigerl et al., 2007; Stirling et al., 2014; Stivers et al., 2017).
In the diseased and injured brain, HMGB1 contributes to neuron death. In the ischemic brain HMGB1 is released from necrotic neurons and drives neuroinflammation by acting on microglia and macrophage RAGE and Mac-1 receptors (Faraco et al., 2007; Gao et al., 2011; Muhammad et al., 2008). shRNA-mediated blockade of HMGB1 (Kim et al., 2006) and neutralizing antibodies against HMGB1 (Liu et al., 2007; Muhammad et al., 2008; Zhang et al., 2011) reduce brain infarct size and blood-brain barrier disruption in stroke models. HMGB1 also is involved in epilepsy pathogenesis (Maroso et al., 2010) and becomes concentrated at sites of trauma or inflammation in other CNS pathologies including multiple sclerosis and SCI (Andersson et al., 2008; Kawabata et al., 2010; Sun et al., 2015).
In rat models of traumatic and ischemic SCI, acute but transient increases in HMGB1 occur together with increases in proinflammatory cytokines (e.g., TNFa), TLRs and RAGE (Chen et al., 2011; Gong et al., 2012; Kawabata et al., 2010). High concentrations of extracellular HMGB1 also become enriched in the extracellular matrix of chronically-injured rat spinal cord (Didangelos et al., 2016). In humans, HMGB1 is increased in the blood after SCI and may contribute to systemic inflammatory complications (Papatheodorou et al., 2017). Thus, post-SCI regulation of HMGB1 appears to be conserved across species, although the sources and functional significance of these changes are unknown.
Here, we extended previous studies by characterizing the temporal distribution and cellular localization of SCI-induced HMGB1 (mRNA and protein) in a mouse model of controlled moderate contusion SCI. New data indicate that HMGB1 levels increase in the injured spinal cord, likely from multiple cellular sources including dying cells and secretion from activated CNS macrophages. Data from in vivo and ex vivo assays show that extracellular HMGB1 elicits neurotoxic inflammation and restricts axonal growth/plasticity. Intraperitoneal injections of an HMGB1 blocking antibody were used in an effort to block HMGB1 during the time of peak HMGB1 expression after SCI. Neutralizing HMGB1 via this route of injection had no effect; quantitative measures of intraspinal pathology and locomotor recovery were not different from SCI mice receiving control injections (vehicle or non-specific antibodies). Together, these data indicate that HMGB1 promotes intraspinal inflammation and is an important regulator of macrophage-mediated neurotoxicity and axon die-back after SCI. Future attempts to inhibit these deleterious effects of extracellular HMGB1 will likely require that antagonists are delivered directly into the spinal cord (intraparenchymal or intrathecal) or that new inhibitors are created that easily bypass the blood-spinal cord barrier.
METHODS
Animals and spinal cord injury
All surgical and post-operative care procedures were performed in accordance with the Ohio State University Institutional Animal Care and Use Committee. Animals were housed in ventilated cages and were maintained on a 12-hour light/dark cycle with ad libitum access to food and water. Ambient room temperature was maintained at 70° F +/− 4° with 30–70% humidity. Female C57BL/6 mice were anesthetized with an i.p. cocktail of ketamine (80 mg/kg)/xylazine (10 mg/ kg) after which a partial laminectomy was performed at T9. All mice received a spinal contusion injury (75kdyn) using the Infinite Horizons injury device. Post-operatively, animals were hydrated with 2 mL Ringer’s solution (s.c.) and were given prophylactic antibiotics (0.1 mL Gentacin/s.c.) for 5 days. Bladders were voided manually at least twice daily for the duration of the study.
Anti-HMGB1 neutralizing antibodies
Anti-HMGB1 neutralizing antibody was generated as previously described (Qin et al., 2006) kindly provided by Dr. Kevin Tracey (anti-HMGB1 2G7). The specificity of this antibody has been characterized elsewhere (Andersson and Tracey, 2011; Gao et al., 2010; Qin et al., 2006; Yang et al., 2010). Mice were randomly assigned to treatment or control groups using a random number generator provided by QuickCalcs on the GraphPad Software website. Antibody was injected daily via i.p. injection (50μg/day; n=6/group) starting 1 day prior to SCI and continuing for 7 days. Antibody dose was based off effective dosing in a murine sepsis model (Valdés-Ferrer et al., 2015). Equivalent amounts of PBS or IgG2a antibodies (isotype control) were also delivered via daily i.p. injection. All experimenters were unaware of group designations.
Quantitative analysis of spared myelin and immunohistochemistry
Eriochrome cyanine (EC) staining was used to visualize myelin. Frozen sections cut through the rostro-caudal extent of the lesion were incubated in EC for 30 min at 20°C, washed in dH2O then were differentiated in 5% iron alum then borax-ferricyanide for 5–10 min. The injury epicenter was defined visually as the spinal cord section with the smallest visible rim of spared myelin. That section and those immediately rostral/caudal were analyzed from each animal/group then were averaged. To calculate the area of spared myelin and lesion volume, digital images of equidistant EC-stained sections spanning the injury epicenter were captured using a Zeiss Axioplan 2 Imaging microscope. A point grid of known area was overlaid with random orientation onto printed digital images and myelin sparing was calculated according to the Cavalieri method using the formula: (V = T• a/p• n∑p) where T equals the distance between sections, a/p equals calculated area per point, and n∑p equals the sum of points counted across all sections (Kigerl et al., 2006).
Analysis of locomotor function
Open-field locomotor function was assessed using the Basso Mouse Scale for Locomotion (BMS; (Basso et al., 2006)) at 1, 3, 7, 14, 21, 28, 35, and 42dpi. General indices of locomotion and activity were assessed with an AccuScan activity monitor (AccuScan Intruments, Columbus OH). Mice were recorded using the AccuScan system for 30 minutes prior to SCI and then again at 42dpi.
Intraspinal Microinjection
Mice were anesthetized with a cocktail of ketamine/xylazine (80 and 10mg/kg, respectively). Using aseptic technique, a laminectomy was performed at the T12–13 vertebral level after which the spinal column was secured via the spinous processes adjacent to the laminectomy site using Adson forceps fixed in a spinal frame. Sterile glass micropipettes (pulled to an external diameter of ~25μm and pre-filled with sterile recombinant HMGB1 (R&D Systems; 500ng/mouse; n=6) or sterile PBS (n=6) were positioned at 0.4mm lateral from midline. From the meningeal surface, pipettes were lowered 0.8mm using a hydraulic micropositioner (David Kopf Instruments, Tujunga, CA). Using a PicoPump (World Precision Instruments, Sarasota, FL), 1μl of solution was injected over a period of 15min. To minimize fluid reflux, pipettes remained in place for 2 additional minutes to allow the injectate to dissipate into the parenchyma. To facilitate localization of the injection sites for anatomical analysis, a small amount of sterile charcoal was placed on the adjacent dura before closing the overlying tissues. Structural testing of recombinant HMGB1 by R&D Systems indicates that the recombinant protein does contain the disulfide bond at Cys23 and Cys45 as well as a free thiol at Cys106. This disulfide form of HMGB1 contains cytokine-stimulating activity (Yang et al., 2012).
Tissue processing
At designated times post-microinjection or post-injury, mice were anesthetized then perfused intracardially with 100 ml of 0.1M PBS (pH 7.4) followed by 100ml of 4% paraformaldehyde (PF). Perfused spinal cords were post-fixed via immersion in 4% PF for 2 hours. Fixed tissues were rinsed and stored overnight at 4°C in 0.2M PB, then cryoprotected in 30% sucrose for 48 hours. Spinal cords were blocked into 1cm segments centered on the impact site then were embedded in OCT (VWR International). Serial cross-sections (10μm) were cut through each block using a Microm cryostat (HM 505 E) then were collected on SuperFrost Plus slides (Fisher Scientific, Fair Lawn, NJ) and stored at −20°C.
Immunohistochemistry
For HMGB1 labeling, 10μm fixed frozen sections were used for immunostaining. Slides were removed from −20°C freezer then air dried for at least one hour. After PBS wash (x2), slides were blocked with BP+ (4%BSA /0.1% Tx-100/0.1M PBS) for 1h at RT. Polyclonal antibody rabbit anti-HMGB1 (1:500, Abcam ab18256) was then applied to tissue sections overnight at 4°C. AF546-conjugated goat anti-rabbit secondary antibody (1:1000) was subsequently added to the slides and incubated for 1h at RT. Slides were rinsed x3 with 0.1M PBS then coverslipped with ImmuMount. The GFAP, CC1 and HuC antibodies are mouse antibodies therefore pre-incubation of primary and secondary antibodies was necessary to minimize background labeling on mouse spinal cord tissues. The primary and secondary antibodies (AF546-conjugated goat anti-mouse) were incubated in 4% BSA/ 0.1% Triton-x/3% goat serum/0.1MPBS 37°C for 1h with 0.1% normal mouse serum added at 37°C for another hour. The antibody complex was then chilled on ice for 2h then applied to tissue sections. Antibodies used for double labeling with HMGB1 included mouse anti-GFAP (1:4000, Sigma 3893), mouse anti-CC1 (1:50, Abcam ab16794), mouse anti-HuC (1:50, Molecular Probe A21271), and rat anti-CD11b (1:200, Serotec MCA-74G). Antibody labeling was confirmed using no-primary controls to check for non-specific labeling of secondary antibodies. In addition, the specificity of the HMGB1 antibody has been verified by others using preabsorption experiments (Laronda et al., 2017; Walker et al., 2017).
Neuron and glial cell quantitation
Equidistant sections spanning the injection site (100μm section spacing) were stained with HuC/HuD and then labeled cells in the ventral horn motor neurons were counted on a Zeiss Axioplan 2 Imaging light microscope under high power. Microglial activation was quantified using proportional area measurements (Donnelly et al., 2009; Kigerl et al., 2006). Sections labeled with CD11b (Mac-1) were digitized using an MCID ELITE image analysis system (InterFocus Imaging Ltd., Cambridge, England). Three sections per animal (n=6 animal per group) were analyzed and averaged. Within each section, areas of positive immunoreactivity were quantified then expressed as a fraction of the total sample area.
Western blot
Spinal cords (0.5mm sections centered on the injury epicenter; n=2–4 per time point) were homogenized in T-PER buffer (Pierce). α-Tubulin was used as the loading control. Protein samples (20μg) were loaded onto a Nupage 12% Bis-Tris gel (Invitrogen) then separated using SDS-PAGE. Proteins were then transferred onto a nitrocellulose membrane, blocked with 5% milk and immunoblotted. Antibodies used were polyclonal rabbit anti-HMGB1 (1:2000, Abcam ab18256) and anti-α-tubulin (1:2000, Abcam ab7291). Blots were developed using SuperSignal West Pico Chemiluminescent Substrate (Pierce). Densitometry was used to quantify relative expression levels after SCI.
Real-time PCR
Spinal cord tissues (0.5mm sections centered on the injury epicenter) were homogenized in Trizol (n=3–4 per time point). Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacture’s instructions then the total RNA concentration was determined by absorbance at 260/280nm. A reverse transcription was set up using 2.5μg total RNA and random primer with Superscript II (invitrogen). Primers specific for nucleotides 414 to 572 of mouse HMGB1 coding sequence (forward 5’-GAAGTTGGACCCCAATGC-3’; reverse 5’-TCATCTGCTGCAGTGTTGTTCC-3’), nucleotides 22–93 of mouse GAPDH coding sequence (forward 5’-CCAGCCTCGTCCTGTAGACAA-3’; reverse 5’-GCCTTGACTGTGCCGTTGA-3’), and Arginase 1 (forward 5’-GAACACGGCAGTGGCTTTAAC-3’; reverse 5’TGCTTAGCTCTGTCTGCTTTGC-3’), CD206 (forward 5’-TCTTTGCCTTTCCCAGTCTCC-3’; reverse 5’-TGACACCCAGCGGAATTTC-3’), iNOS (forward 5’-CCCTTCAATGGTTGGTACATGG-3’; reverse 5’-ACATTGATCTCCGTGACAGCC-3’), and CD86 (forward 5’-TTGTGTGTGTTCTGGAAACGGAG-3’; reverse 5’-AACTTAGAGGCTGTGTTGCTGGG-3’) were used. A 7300 Real-time PCR system (Applied Biosystems) was used for amplification. The gene GAPDH was used for normalization of mRNA concentrations between the PCR reactions using the ΔΔCt method.
Cell Culture
Adult dorsal root ganglion (DRG) cells were isolated from terminally anesthetized 2–3 month old female C57BL/6 mice. Dissected DRGs were incubated in dispase 2 (5 U/ml; Roche, Penzbeg, Germany) and collagenase type 2 (200U/ml; Worthington, Lakewood, NJ) for 45 min at room temperature in calcium and magnesium-free Hanks’ Balanced Salt Solution (HBSS; Mediatech, Herndon, VA). Enzymes were removed and 250 μg/ml DNase 1 type 2 (Sigma, St. Louis, Missouri) was added to the media for 5 minutes. Cells were cultured in DRG media (Neurobasal A media with 2% B27, 1% penicillin/streptomycin, 1% glutamax) on glass coverslips, as previously described (Steinmetz et al., 2005). Coverslips were coated with poly-D-lysine for 12–24h then with laminin (10μg/ml) solutions containing recombinant HMGB1 (disulfide form; kindly provided by Kevin Tracey; 5, 10, and 20μg/ml) for 2h before DRG cells were plated in media. After 3d, DRG cells were fixed with 2% PFA and labeled with β-tubulin III to visualize neurite outgrowth. Digital images were taken with a Carl Zeiss Axioplan 2 imaging microscope using the MCID software stereology module (Imaging Research Corporation) to randomly sample the coverslip. Experimenter was blinded to treatment group throughout analysis. Automated Sholl ring analysis was conducted as previously described (Gensel et al., 2010; Kigerl et al., 2009) using MetaMorph software (Molecular Devices). Axon crossings were recorded at 50 μm intervals using concentric circles (Gensel et al., 2010; Sholl, 1953).
Bone marrow-derived macrophage (BMDM) cultures were created from adult C57BL/6 mice, as described previously (Longbrake et al., 2007). Bone marrow from both tibias and femurs were collected, using aseptic techniques, and marrow cores were flushed with chilled normal RPMI into sterile tubes. Cells were triturated using 18 gauge needle 3–5x. Red blood cells were lysed in lysis buffer (0.15 m NH4Cl, 10 mm KHCO3, and 0.1 mm Na2EDTA, pH 7.4) for 3 minutes. Cells were washed in media and plated with RPMI (1% penicillin/streptomycin, 1% HEPES, 0.001% β-mercaptoethanol, 10% FBS) and 20% supernatant from sL929 (macrophage colony stimulating factor secreting) cells. At 7d, cells were scraped then replated into 6 well plates with normal RPMI containing 10% FBS/1% glutamax/1% penicillin/streptomycin. At 8d macrophages were stimulated with LPS (100ng/ml) and IFNγ (20ng/ml) for M1 macrophages, IL-4 (20ng/ml) for M2 macrophages, and HMGB1 (provided by Kevin Tracy; 1μg/ml or 5μg/ml) in DRG media for 12–24 hrs. Collected media was centrifuged (1200rpm for 5min) and the supernatant was added to fresh DRG media (1:1) to make macrophage-conditioned media (MCM). After 3d of adult DRG growth in vitro, media was replaced by the various conditions of MCM for 24h. To assess DRG neurite outgrowth, digital images from β-tubulin III-labeled DRGs were captured with a Carl Zeiss Axioplan 2 Imaging microscope from cells chosen randomly using the MCID Elite Image Analysis station (Imaging Research Corporation) stereology module. Experimenter was blinded to treatment group throughout analysis. Using the MetaMorph (Molecular Devices) image analysis system, an automated Sholl ring analysis was performed using concentric circles at 50μm intervals, and the number of crossings at each interval was recorded (Gensel et al., 2010; Sholl, 1953).
Statistical analysis
One-way analysis of variance with Bonferroni post hoc test was used to analyze PCR and Western blot data. In vitro neurite growth was analyzed using two-way analysis of variance with Bonferroni post hoc test. Student’s t test was used to analyze data for immunohistochemical experiments. An α-level of p < 0.05 was used to indicate statistical significance.
RESULTS
Intraspinal HMGB1 increases after spinal contusion injury
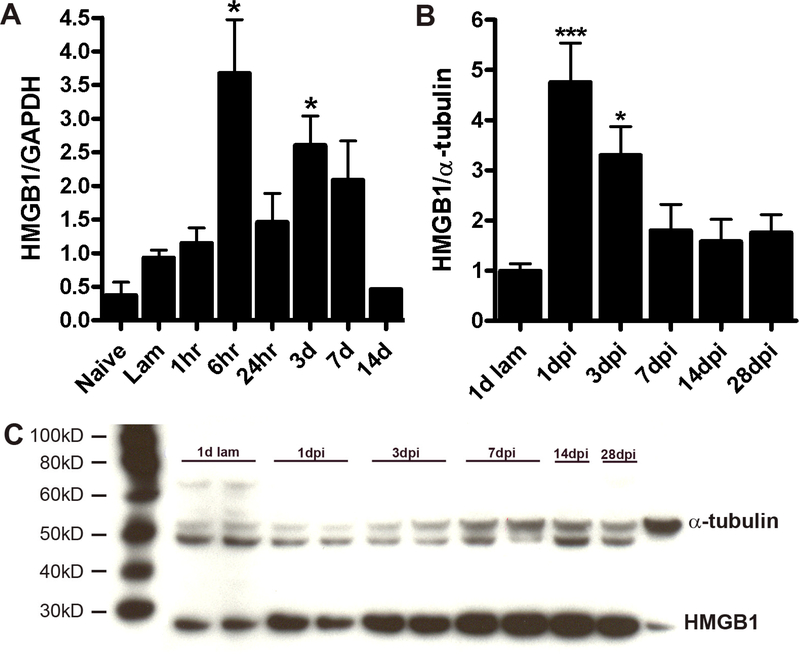
Temporal changes in HMGB1 mRNA expression were quantified in control and injured spinal cord homogenates. Expression of HMGB1 mRNA increased after 6h then returned toward baseline by 2 weeks post-injury (Figure 1A). Post-injury changes in HMGB1 protein levels followed a similar course with peak levels achieved by 1 day post-injury (dpi) (Fig. 1B&C).
Figure 1.
(A) Real-time PCR analysis of HMGB1 expression at the injury site at various times post SCI. mRNA expression was significantly increased at 6h and 3dpi. (B&C; n=3–4/time point). Western blot analysis shows that HMGB1 protein increases at 1 & 3dpi (n=3/time point; ANOVA; *p<0.05 vs. laminectomy control).
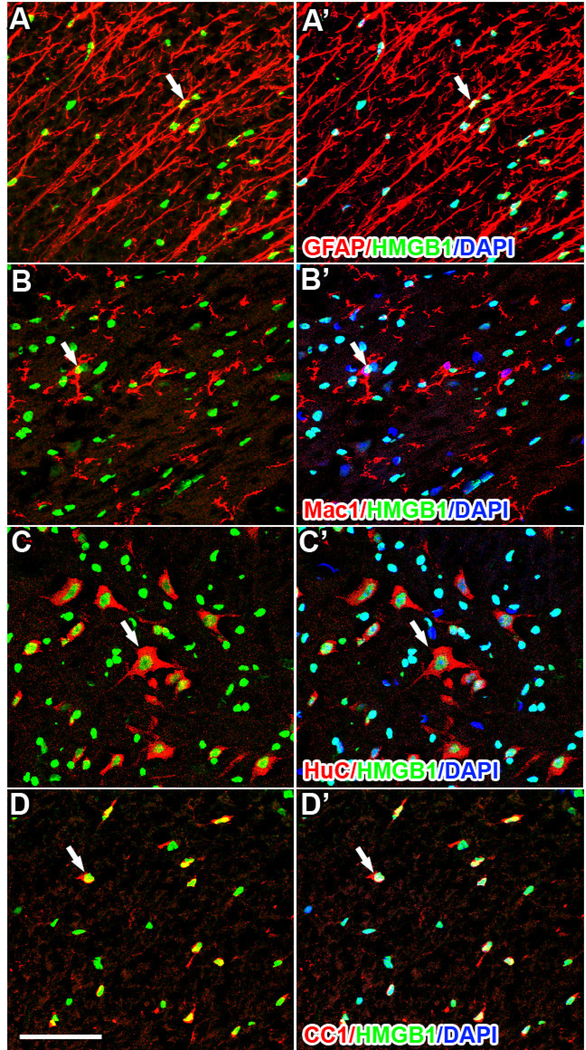
Confocal analyses of tissue sections from the lesion site and nearby rostral and caudal spinal segments revealed the distribution and phenotype of cells expressing HMGB1. HMGB1 was expressed in the nucleus of most spinal cord cells under normal conditions; HMGB1 labeling was evident in the nucleus of astrocytes (Fig. 2A), microglia, (Fig. 2B), neurons (Fig. 2C) and oligodendrocytes (Fig. 2D). After SCI the cellular localization was unchanged in astrocytes, neurons, and oligodendrocytes (Fig. 3) found in spared tissue surrounding the lesion.
Figure 2.
Immunohistochemistry of naïve spinal cord reveals nuclear HMGB1 labeling in astrocytes (A; GFAP), microglia (B; CD11b), neurons (C; HuC), and oligodendrocytes (D; CC1). Although most microglia express HMGB1 (B&B’, arrow head), some do not (B&B’, arrows). Scale = 50μm.
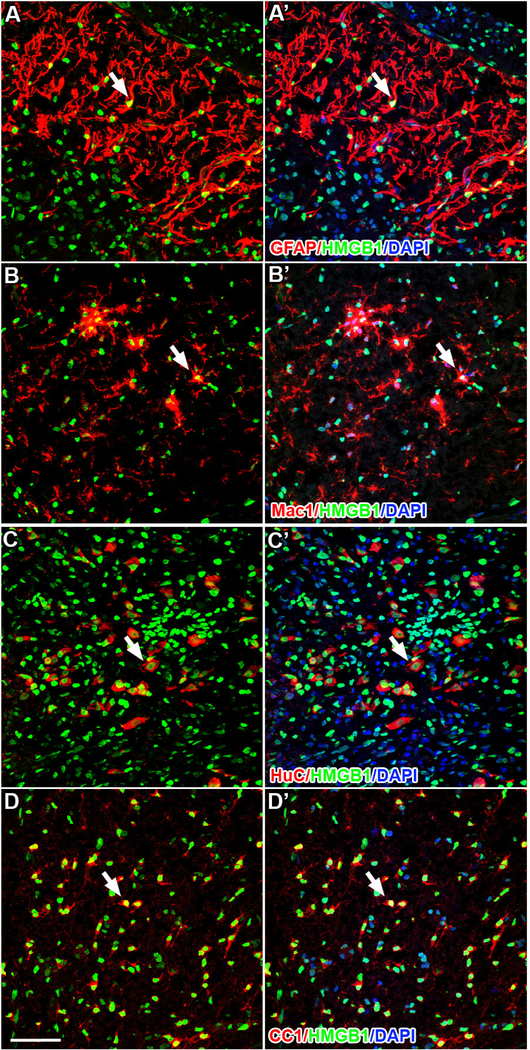
Figure 3.
At 7dpi in spared tissue surrounding the lesion site, astrocytes (A; GFAP), microglia (B; Mac1), neurons (C; HuC), and oligodendrocytes (D; CC1) continue to express nuclear HMGB1. Unlike in the naïve spinal cord, all activated microglia express HMGB1 (B&B’). Scale = 50μm.
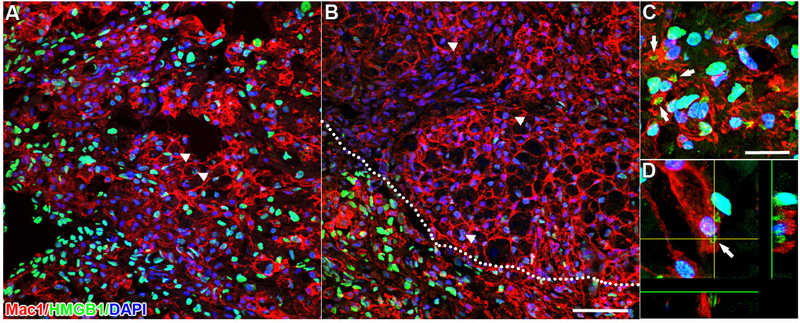
The most significant post-injury changes in HMGB1 expression were noted in CNS macrophages at the lesion epicenter and nearby lesion borders. At 7 and 14dpi, nuclear HMGB1 expression was reduced in large, round and presumably phagocytic macrophages (Fig. 4A&B, arrowheads). Closer examination of these cells revealed that HMGB1 was absent in the nucleus but was often found in small cytoplasmic vacuoles, suggesting possible intracellular packaging of HMGB1 for secretion into the extracellular space (Fig. 4C&D; arrows). Nuclear HMGB1 expression remained high in CD11b-negative cells in the surrounding spared tissue (Fig. 4B).
Figure 4.
Robust HMGB1 labeling is present in the nucleus of most cells after SCI (see Fig. 3) except CNS macrophages in the lesion center. At 7 (A) and 14dpi (B), strong nuclear HMGB1 labeling is seen in cells surrounding the lesion. However, in the lesion center, nuclear HMGB1 labeling is weak or absent in activated CD11b+ macrophages (arrowheads; lesion border - dotted line). High power confocal microscopic analysis reveals HMGB1+ vacuoles in the cytoplasm of a subset of CD11b+ macrophages (C&D; arrows), suggesting active HMGB1 secretion by these cells. Scale = 50μm (A&B) & 20μm (C).
HMGB1 triggers inflammation and neuron death in uninjured spinal cord
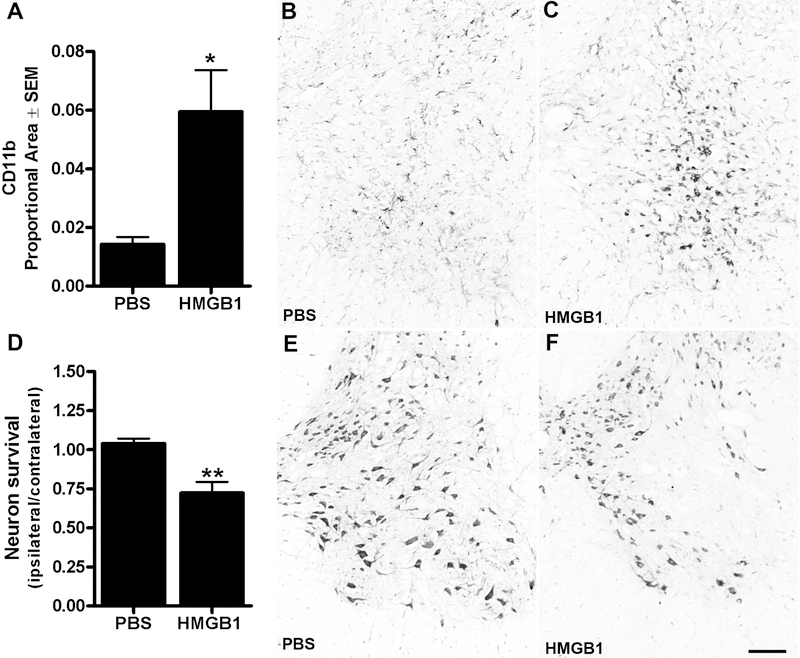
To determine whether focal increases in extracellular HMGB1 can elicit spinal cord inflammation, recombinant disulfide HMGB1 was injected unilaterally into the uninjured spinal cord. Non-traumatic intraspinal microinjections of HMGB1 (500ng in 1μl) were made into right ventral horn (L1 spinal level) then 3 days later, morphological indices of microglial activation and neuron survival were quantified. Although subtle changes in microglia morphology were detectable along the micropipette track and nearby where PBS was injected, clear foci of activated microglia were evident in the ventral horn after HMGB1 injection (Fig. 5A–C). Importantly, no neuron pathology was found in spinal cords injected with PBS. In contrast, zones of microglial activation in HMGB1-injected spinal cord co-localized with areas of pronounced neuron loss (Fig. 5D–F).
Figure 5.
(A-C) Intraspinal HMGB1 microinjection (n=6) activates microglia/macrophages in the ventral horn 3 days post inje ction. Proportional area measurements of CD11b immunoreactivity reveal a ~3-fold increase in microglial activation compared to PBS injections (n=6). (D-F) Vent ral horn motor neuron death is increased within regions occupied by HMGB1-activated macrophage s, (t-test; *p<0.05, ** p<0.001. Scale = 100μm).
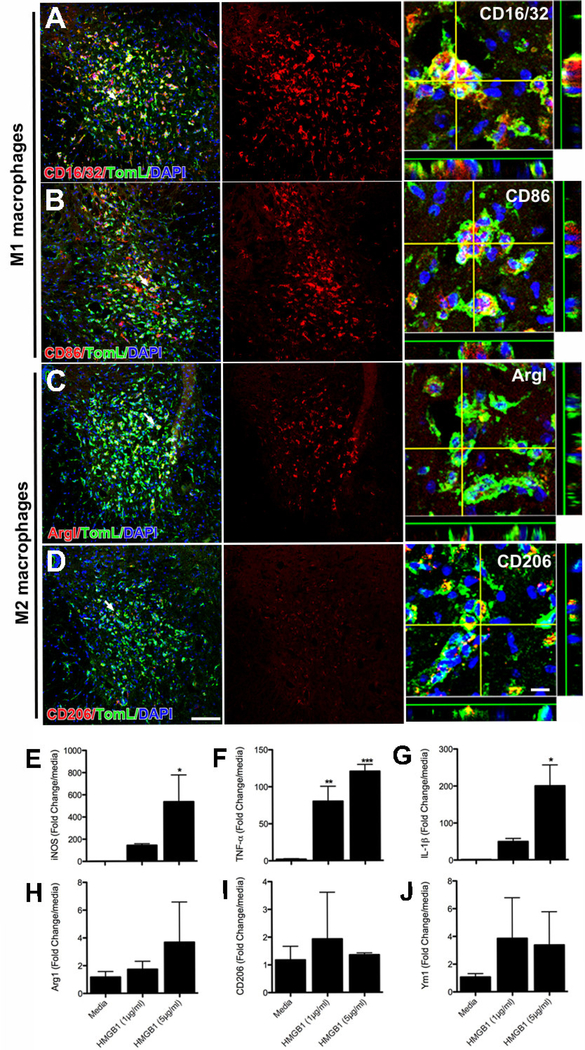
Previously, we reported that neurotoxic macrophages in the injured spinal cord adopt a phenotype that is consistent with classically activated inflammatory “M1” macrophages (Kigerl et al., 2009). After intraspinal HMGB1 injection, most activated microglia and macrophages expressed phenotypic markers associated with M1 macrophages (e.g., CD16/32 & CD86) (Fig. 6A&B). Fewer macrophages expressed markers consistent with an M2 phenotype (Arginase I & CD206) (Fig. 6C&D). To determine whether extracellular HMGB1 is sufficient to polarize macrophages toward an inflammatory (M1) phenotype, bone-marrow derived macrophages were exposed to two different concentrations (1 and 5μg/ml) of HMGB1 in vitro. In a dose-dependent manner, HMGB1 enhanced expression of iNOS (Fig. 6E), TNF-α and IL1-β, i.e., phenotypic markers and inflammatory cytokines associated with M1 macrophages (Fig. 6F&G). HMGB1 did not cause a concomitant increase in M2 markers (i.e. arginase I and CD206; Fig. 6H–J). Together, these in vivo and in vitro data suggest that extracellular HMGB1 contributes to the development of a neurotoxic inflammatory macrophage (M1) phenotype after SCI.
Figure 6.
Intraspinal injection of recombinant HMGB1 (500ng in 1μl) elicits neuroinflammation dominated by neurotoxic inflammatory M1 microglia/macrophages. Three days after injection, a robust CNS macrophage response is evident in the ventral horn (A-H). Immunohistochemical staining for classical M1 macrophage activation markers (CD16/32 & CD86) reveals that most macrophages exhibit an M1 phenotype (A-B). A few cells express M2 macrophage markers (C-D). In vitro, BMDMs treated with recombinant HMGB1 increase expression of M1 markers (E-G), but not M2 markers (H-J). (ANOVA; *p<0.05, **p<0.01, ***p<0.001; Scale = 100μm (10μm for orthogonal view).
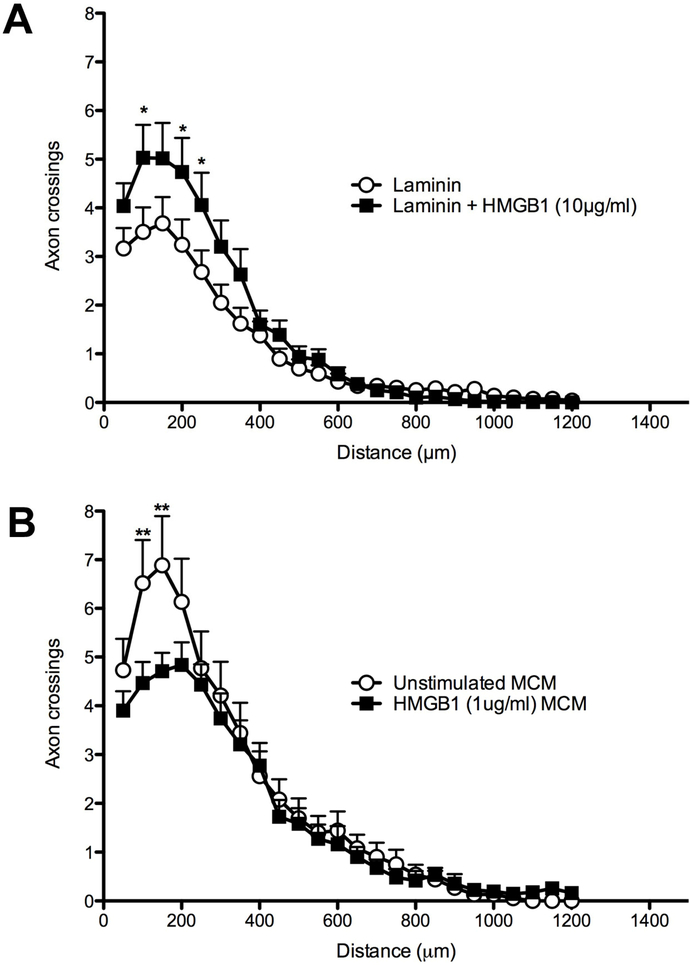
Because HMGB1 was originally described as amphoterin, a protein capable of promoting neurite outgrowth (Huttunen et al., 1999; Huttunen et al., 2000; Srikrishna et al., 2002), we determined whether HMGB1 would influence outgrowth of adult sensory axons using a well-established in vitro model. Adult DRG neurons were cultured on laminin-coated coverslips treated with different concentrations of disulfide HMGB1. Scholl analysis shows that HMGB1 (10μg/ml) significantly increases DRG axon growth (2-way ANOVA; ***p<0.0001 vs. laminin control), particularly at distances close to the neuronal soma (within 250μm) (Fig 7A).
Figure 7.
HMGB1 directly and indirectly (via BMDMs) affects DRG neurite outgrowth in vitro. Neurite outgrowth is increased when DRG neurons are grown on laminin with increasing concentrations of HMGB1 as compared to axons from DRG neurons grown on laminin alone (A; representative of n=3 independent replicates). In contrast, macrophage conditioned medium (MCM) from HMGB1-activated BMDMs impairs neurite growth from DRG neurons grown on a permissive laminin substrate (B; n=1 experiment; data represent average of triplicate wells for each group). (2-way ANOVA; *p<0.05, **p<0.01).
Although HMGB1 can promote axon growth in vitro, in order for injured spinal cord axons to regenerate in vivo they will need to navigate through pockets of inflammatory cells and an extracellular terrain in which HMGB1 is embedded in the tissue matrix (Didangelos et al., 2016). To more closely mimic the injured spinal cord environment, adult DRG neurons were grown on laminin in the presence of conditioned-media from macrophages (MCM) stimulated with disulfide HMGB1 (1 μg/ml, a concentration that induces an M1 phenotype; see Fig. 6E–G). Neurite outgrowth from DRGs exposed to HMGB1-activated MCM was reduced compared to axon growth from DRGs treated with control MCM (Fig. 7B). Previous data from our lab indicate that inflammatory M1 macrophages are neurotoxic and may limit axon growth in vitro and in vivo (Gensel et al., 2009; Gensel et al., 2015; Kigerl et al., 2009). The present data indicate that HMGB1 may be an endogenous regulator of macrophage phenotype and axon growth after SCI.
Acute intraperitoneal injections of HMGB1 neutralizing antibodies fail to provide protection after SCI
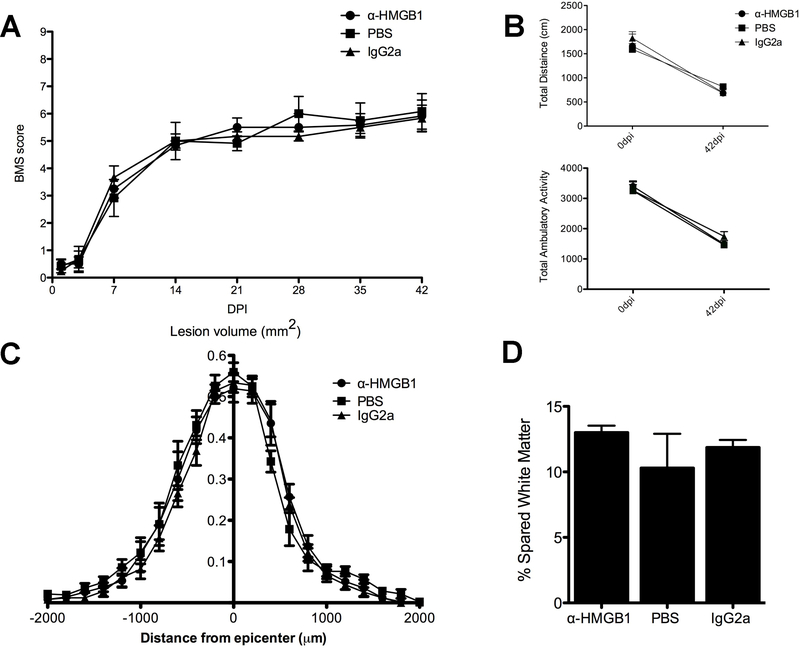
We hypothesized that inhibiting extracellular HMGB1 would decrease intraspinal inflammation and improve functional recovery after SCI. Because the blood-spinal cord barrier is compromised for at least one week post-injury, mice received daily i.p. injections of α-HMGB1 antibodies, IgG2a isotype control antibodies or PBS beginning 1 day pre-injury then each day for 7 days. Injections via this route ensured that a full dose of antibodies were delivered each day and without complications associated with intravenous injection (e.g, tail vein compromise, tail damage, incomplete or missed delivery due to poor injection). Functional recovery was analyzed using a standardized open-field locomotor test (i.e., BMS) and unbiased levels of horizontal and vertical movement were quantified using an activity box. Both types of analyses failed to reveal a therapeutic effect of α-HMGB1 antibodies out to one month post-injury (Fig. 8A&B). Quantitative analyses of lesion volume and spared tissue also were not different between groups (Fig. 8C&D). These data indicate that i.p. delivery of α-HMGB1 neutralizing antibodies is not an effective strategy to limit intraspinal inflammation after SCI.
Figure 8.
Systemic injections of neutralizing HMGB1 antibodies fail to improve locomotor function or lesion pathology after SCI. SCI C57BL/6 mice were injected (i.p.) with α-HMGB1 antibodies (n=6), IgG2a isotype antibodies (n=6), or PBS (n=6) starting 1 day pre-injury followed by daily injections for 7 days. Open-field locomotor (BMS) scores (A) and Accuscan activity box data (B) show no differences between control groups and SCI mice treated with anti-HMGB1 antibodies (repeated measures 2-way ANOVA). Analysis of lesion volume (C) and spared white matter at the injury epicenter (D) also were not different between groups (ANOVA).
DISCUSSION
HMGB1 is a nuclear protein that can also act as an inflammatory cytokine and neurotrophic factor. In the current report we describe the spatiotemporal dynamics of HMGB1 mRNA and protein expression in the acutely and chronically injured spinal cord. HMGB1 mRNA and protein expression increase during the first week post-injury – a time corresponding with the onset and peak of intraspinal inflammation. In the uninjured spinal cord, all cell types express nuclear HMGB1; however, after SCI only in microglia and macrophages was HMGB1 found localized in vacuolar or lysosomal-like structures in the cytoplasm. We proved that extracellular HMGB1, when delivered into the intact spinal cord via non-traumatic intraspinal microinjection, elicits neurotoxic inflammation. However, in the absence of inflammatory mediators, extracellular HMGB1 can also augment growth or sprouting of adult sensory axons.
HMGB1 was originally defined as a DNA binding protein that controls DNA architecture and gene transcription (Javaherian et al., 1978; Read et al., 1994; Sutrias-Grau et al., 1999). Decades later, extracellular HMGB1 was found to also be an endogenous signaling molecule with cytokine-like activity. In a model of lethal septic shock, high-dose injections of endotoxin (lipopolysaccharide; LPS) caused circulating HMGB1 levels to rise after ~8h – long after the initial release of inflammatory cytokines including IL1-β and TNF-α (Wang et al., 1999). Delayed blockade of HMGB1 protected mice from lethal septic shock (Wang et al., 1999). These data were the first to show that eliciting inflammatory signaling in macrophages causes them to release HMGB1. Since then, several inflammatory mediators have been shown to be potent regulators of HMGB1 release from macrophages. For example, IFNγ triggers HMGB1 release through a TNFα-dependent mechanism (Rendon-Mitchell et al., 2003). Reactive oxygen and reactive nitrogen species can also trigger HMGB1 release (Jiang and Pisetsky, 2006; Tang et al., 2007). In the injured spinal cord, oxidative stress and the acute release of cytokines (e.g., TNFα) from reactive glia could stimulate HMGB1 release from activated microglia and newly recruited blood monocytes.
In addition to active secretion by macrophages at the injury site, HMGB1 is also passively released from necrotic (Rovere-Querini et al., 2004; Scaffidi et al., 2002) and apoptotic cells (Bell et al., 2006). Importantly, high concentrations of extracellular HMGB1 accumulate in the extracellular matrix of injured spinal cord (Didangelos et al., 2016) and data indicate that passively released HMGB1 will elicit inflammatory signaling (Scaffidi et al., 2002). Extracellular HMGB1 activates inflammatory cells via several receptors. The receptor for advanced glycation end-products (RAGE), TLR2 and TLR4 are all activated by extracellular HMGB1 (Hori et al., 1995; Kokkola et al., 2005; Park et al., 2006; Park et al., 2004; van Beijnum et al., 2008; Yu et al., 2006). TLR2 and TLR4 are expressed by microglia/macrophages and astrocytes in the spinal cord and are upregulated at the lesion site after SCI (Kigerl et al., 2007). In the CNS, RAGE is expressed on microglia, macrophages, astrocytes and neurons (Fang et al., 2010; Hori et al., 1995; Huttunen et al., 1999; Kokkola et al., 2005; Origlia et al., 2010; Zurolo et al., 2011). Activation of any of these receptors will enhance NFκB-mediated transcription of inflammatory mediators, a process associated with adverse effects on functional recovery and axon growth in animal models of SCI (Brambilla et al., 2005; Brambilla et al., 2009). HMGB1 also exacerbates ischemic brain pathology. HMGB1 is released from necrotic neurons after ischemia and enhances neuroinflammation via activation of RAGE and Mac-1 receptors on microglia (Faraco et al., 2007; Gao et al., 2011; Muhammad et al., 2008). HMGB1-dependent activation of innate immune cells also can occur by binding to intracellular pattern recognition receptors (PRRs). As microglia and macrophages scavenge cell debris and nucleic acids from dead/dying cells, cytosolic HMGB1 acts as a chaperone, binding to these cellular remnants to enhance binding and/or signaling via intracellular PRRs (e.g., TLR3, TLR9) (Yanai et al., 2009).
In addition to its role as an inflammatory mediator, extracellular HMGB1 is also a potent regulator of axon growth. HMGB1 and RAGE are localized to the leading edge of axon growth cones in cortical neurons in vitro (Merenmies et al., 1991; Srikrishna et al., 2002), and neurons cultured on HMGB1-coated surfaces extend neurites in a RAGE-dependent manner (Huttunen et al., 1999; Huttunen et al., 2000; Srikrishna et al., 2002). This in vitro axon growth can be blocked with antibodies that target either HMGB1 or RAGE (Merenmies et al., 1991; Srikrishna et al., 2002). HMGB1-RAGE interactions also can enhance cell survival by increasing expression of the anti-apoptotic protein, Bcl-2 (Huttunen et al., 2000).
HMGB1 and RAGE also influence axon growth in vivo. Following unilateral sciatic nerve crush injury, RAGE is expressed on axons and infiltrating macrophages (Rong et al., 2004). Delivery of soluble RAGE or anti-RAGE antibodies decreases motor and sensory recovery, as well as axon regeneration (Rong et al., 2004). Data show that HMGB1 mRNA is constitutively transported into axons of DRG neurons, and that this axon-targeted mRNA can increase neurite outgrowth (Merianda et al., 2015). Indeed, HMGB1 contributes to spontaneous regeneration in lower vertebrates after CNS injury (Dong et al., 2013; Fang et al., 2014). These data suggest in addition to its role as a proinflammatory mediator, HMGB1 may also act as a key intrinsic regulator of axon growth. Thus, attempts to neutralize HMGB1 to control inflammation could have the adverse side effect of inhibiting spontaneous axon growth.
In the current study, new in vitro and in vivo data show that extracellular HMGB1 is sufficient to activate macrophages, creating a neurotoxic phenotype that could contribute to secondary injury after SCI. However, sustained delivery of an HMGB1 blocking antibody, beginning one day pre-injury then continuing daily throughout the period of maximal intraspinal inflammation (7 days), did not confer neuroprotection. Given that a similar approach has been used successfully in models of ischemic stroke (Liesz et al., 2015; Muhammad et al., 2008), our data do not rule out HMGB1 as a therapeutic target after SCI. Instead, we must question whether the intraperitoneal route of administration allows sufficient concentrations of blocking antibody to accumulate in the injury site and tissues in adjacent intact spinal cord. A more direct route of delivery may improve access (e.g., bypassing the blood-spinal cord barrier) and binding efficiency. Intravenous injection of neutralizing HMGB1 antibodies is effective in models of stroke (Liu et al., 2007; Zhang et al., 2011) and EAE (Robinson et al., 2013). Small molecule inhibitors of HMGB1 (e.g., inflachromene) may also be preferable to blocking antibodies since these molecules can prevent the secretion of HMGB1 (Lee et al., 2014). Targeting extracellular HMGB1 remains a promising target for reducing pathological inflammation following CNS trauma.
Highlights.
Intraspinal HMGB1 expression increased after spinal cord injury (SCI).
CNS macrophages have increased cytoplasmic HMGB1 and decreased nuclear HMGB1.
Extracellular HMGB1 elicits a neurotoxic macrophage phenotype in vivo an in vitro.
Intraspinal HMGB1 triggers microglial activation and neuron death in the ventral horn.
Delivery of an anti-HMGB1 antibody did not result in neuroprotection after SCI.
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Zhen Guan, Megan Muccigrosso, and Jack Burgeson for providing technical assistance. This work was funded by NINDS RO1 NS37846, the Craig H. Neilsen Foundation 164246, and the Ray W. Poppleton Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson A, Covacu R, Sunnemark D, Danilov AI, Dal Bianco A, Khademi M, Wallström E, Lobell A, Brundin L, Lassmann H, Harris RA, 2008. Pivotal advance: HMGB1 expression in active lesions of human and experimental multiple sclerosis. J Leukoc Biol 84, 1248–1255. [DOI] [PubMed] [Google Scholar]
- Andersson U, Tracey KJ, 2011. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol 29, 139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG, 2006. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23, 635–659. [DOI] [PubMed] [Google Scholar]
- Bell CW, Jiang W, Reich CF, Pisetsky DS, 2006. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol 291, C1318–1325. [DOI] [PubMed] [Google Scholar]
- Blight AR, 1994. Effects of silica on the outcome from experimental spinal cord injury: implication of macrophages in secondary tissue damage. Neuroscience 60, 263–273. [DOI] [PubMed] [Google Scholar]
- Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR, 2005. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med 202, 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Hurtado A, Persaud T, Esham K, Pearse DD, Oudega M, Bethea JR, 2009. Transgenic inhibition of astroglial NF-kappa B leads to increased axonal sparing and sprouting following spinal cord injury. J Neurochem 110, 765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KB, Uchida K, Nakajima H, Yayama T, Hirai T, Rodriguez Guerrero A, Kobayashi S, Ma WY, Liu SY, Zhu P, Baba H, 2011. High-mobility group box-1 and its receptors contribute to proinflammatory response in the acute phase of spinal cord injury in rats. Spine (Phila Pa 1976) 36, 2122–2129. [DOI] [PubMed] [Google Scholar]
- Church JS, Kigerl KA, Lerch JK, Popovich PG, McTigue DM, 2016. TLR4 Deficiency Impairs Oligodendrocyte Formation in the Injured Spinal Cord. J Neurosci 36, 6352–6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church JS, Milich LM, Lerch JK, Popovich PG, McTigue DM, 2017. E6020, a synthetic TLR4 agonist, accelerates myelin debris clearance, Schwann cell infiltration, and remyelination in the rat spinal cord. Glia 65, 883–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didangelos A, Puglia M, Iberl M, Sanchez-Bellot C, Roschitzki B, Bradbury EJ, 2016. High-throughput proteomics reveal alarmins as amplifiers of tissue pathology and inflammation after spinal cord injury. Sci Rep 6, 21607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Gu Y, Huan Y, Wang Y, Liu Y, Liu M, Ding F, Gu X, 2013. HMGB1 protein does not mediate the inflammatory response in spontaneous spinal cord regeneration: a hint for CNS regeneration. J Biol Chem 288, 18204–18218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly DJ, Gensel JC, Ankeny DP, van Rooijen N, Popovich PG, 2009. An efficient and reproducible method for quantifying macrophages in different experimental models of central nervous system pathology. J Neurosci Methods 181, 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D, Walker DG, Stern DM, Schmidt AM, Chen JX, Yan SS, 2010. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB J 24, 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P, Pan HC, Lin SL, Zhang WQ, Rauvala H, Schachner M, Shen YQ, 2014. HMGB1 contributes to regeneration after spinal cord injury in adult zebrafish. Mol Neurobiol 49, 472–483. [DOI] [PubMed] [Google Scholar]
- Faraco G, Fossati S, Bianchi ME, Patrone M, Pedrazzi M, Sparatore B, Moroni F, Chiarugi A, 2007. High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J Neurochem 103, 590–603. [DOI] [PubMed] [Google Scholar]
- Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS, 2011. HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration. J Neurosci 31, 1081–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q, Ma LL, Gao X, Yan W, Williams P, Yin DP, 2010. TLR4 mediates early graft failure after intraportal islet transplantation. Am J Transplant 10, 1588–1596. [DOI] [PubMed] [Google Scholar]
- Gensel JC, Kopper TJ, Zhang B, Orr MB, Bailey WM, 2017. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci Rep 7, 40144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Nakamura S, Guan Z, van Rooijen N, Ankeny DP, Popovich PG, 2009. Macrophages promote axon regeneration with concurrent neurotoxicity. J.Neurosci. 29, 3956–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Schonberg DL, Alexander JK, McTigue DM, Popovich PG, 2010. Semi-automated Sholl analysis for quantifying changes in growth and differentiation of neurons and glia. J Neurosci Methods 190, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel JC, Wang Y, Guan Z, Beckwith KA, Braun KJ, Wei P, McTigue DM, Popovich PG, 2015. Toll-Like Receptors and Dectin-1, a C-Type Lectin Receptor, Trigger Divergent Functions in CNS Macrophages. J Neurosci 35, 9966–9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D, Robertson C, 1990. Inhibition of mononuclear phagocytes reduces ischemic injury in the spinal cord. Ann Neurol 27, 33–42. [DOI] [PubMed] [Google Scholar]
- Gong G, Yuan LB, Hu L, Wu W, Yin L, Hou JL, Liu YH, Zhou LS, 2012. Glycyrrhizin attenuates rat ischemic spinal cord injury by suppressing inflammatory cytokines and HMGB1. Acta Pharmacol Sin 33, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA, Weaver LC, 2004. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci 24, 4043–4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HE, Andersson U, Pisetsky DS, 2012. HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat Rev Rheumatol 8, 195–202. [DOI] [PubMed] [Google Scholar]
- Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, Nagashima M, Lundh ER, Vijay S, Nitecki D, 1995. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. Mediation of neurite outgrowth and co-expression of rage and amphoterin in the developing nervous system. J Biol Chem 270, 25752–25761. [DOI] [PubMed] [Google Scholar]
- Huttunen HJ, Fages C, Rauvala H, 1999. Receptor for advanced glycation end products (RAGE)-mediated neurite outgrowth and activation of NF-kappaB require the cytoplasmic domain of the receptor but different downstream signaling pathways. J Biol Chem 274, 19919–19924. [DOI] [PubMed] [Google Scholar]
- Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H, 2000. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem 275, 40096–40105. [DOI] [PubMed] [Google Scholar]
- Javaherian K, Liu JF, Wang JC, 1978. Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science 199, 1345–1346. [DOI] [PubMed] [Google Scholar]
- Jiang W, Pisetsky DS, 2006. The role of IFN-alpha and nitric oxide in the release of HMGB1 by RAW 264.7 cells stimulated with polyinosinic-polycytidylic acid or lipopolysaccharide. J Immunol 177, 3337–3343. [DOI] [PubMed] [Google Scholar]
- Kawabata H, Setoguchi T, Yone K, Souda M, Yoshida H, Kawahara K, Maruyama I, Komiya S, 2010. High mobility group box 1 is upregulated after spinal cord injury and is associated with neuronal cell apoptosis. Spine (Phila Pa 1976) 35, 1109–1115. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW, 2014. Pattern recognition receptors and central nervous system repair. Exp Neurol 258, 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG, 2009. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29, 13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG, 2007. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J.Neurochemistry 102, 37–50. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, McGaughy VM, Popovich PG, 2006. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol 494, 578–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK, 2006. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci 26, 6413–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkola R, Andersson A, Mullins G, Ostberg T, Treutiger CJ, Arnold B, Nawroth P, Andersson U, Harris RA, Harris HE, 2005. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand J Immunol 61, 1–9. [DOI] [PubMed] [Google Scholar]
- Kroner A, Greenhalgh AD, Zarruk JG, Passos Dos Santos R, Gaestel M, David S, 2014. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 83, 1098–1116. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Sarkar A, Vande Walle L, Vitari AC, Amer AO, Wewers MD, Tracey KJ, Kanneganti TD, Dixit VM, 2010. Inflammasome-dependent release of the alarmin HMGB1 in endotoxemia. J Immunol 185, 4385–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laronda MM, Rutz AL, Xiao S, Whelan KA, Duncan FE, Roth EW, Woodruff TK, Shah RN, 2017. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat Commun 8, 15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Nam Y, Koo JY, Lim D, Park J, Ock J, Kim J, Suk K, Park SB, 2014. A small molecule binding HMGB1 and HMGB2 inhibits microglia-mediated neuroinflammation. Nat Chem Biol 10, 1055–1060. [DOI] [PubMed] [Google Scholar]
- Liesz A, Dalpke A, Mracsko E, Antoine DJ, Roth S, Zhou W, Yang H, Na SY, Akhisaroglu M, Fleming T, Eigenbrod T, Nawroth PP, Tracey KJ, Veltkamp R, 2015. DAMP signaling is a key pathway inducing immune modulation after brain injury. J Neurosci 35, 583–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Mori S, Takahashi HK, Tomono Y, Wake H, Kanke T, Sato Y, Hiraga N, Adachi N, Yoshino T, Nishibori M, 2007. Anti-high mobility group box 1 monoclonal antibody ameliorates brain infarction induced by transient ischemia in rats. FASEB J 21, 3904–3916. [DOI] [PubMed] [Google Scholar]
- Longbrake EE, Lai W, Ankeny DP, Popovich PG, 2007. Characterization and modeling of monocyte-derived macrophages after spinal cord injury. J Neurochem 102, 1083–1094. [DOI] [PubMed] [Google Scholar]
- Maroso M, Balosso S, Ravizza T, Liu J, Aronica E, Iyer AM, Rossetti C, Molteni M, Casalgrandi M, Manfredi AA, Bianchi ME, Vezzani A, 2010. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat Med 16, 413–419. [DOI] [PubMed] [Google Scholar]
- Merenmies J, Pihlaskari R, Laitinen J, Wartiovaara J, Rauvala H, 1991. 30-kDa heparin-binding protein of brain (amphoterin) involved in neurite outgrowth. Amino acid sequence and localization in the filopodia of the advancing plasma membrane. J Biol Chem 266, 16722–16729. [PubMed] [Google Scholar]
- Merianda TT, Coleman J, Kim HH, Kumar Sahoo P, Gomes C, Brito-Vargas P, Rauvala H, Blesch A, Yoo S, Twiss JL, 2015. Axonal amphoterin mRNA is regulated by translational control and enhances axon outgrowth. J Neurosci 35, 5693–5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, van Wijngaarden P, Wagers AJ, Williams A, Franklin RJ, ffrench-Constant C, 2013. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci 16, 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad S, Barakat W, Stoyanov S, Murikinati S, Yang H, Tracey KJ, Bendszus M, Rossetti G, Nawroth PP, Bierhaus A, Schwaninger M, 2008. The HMGB1 receptor RAGE mediates ischemic brain damage. J Neurosci 28, 12023–12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Origlia N, Bonadonna C, Rosellini A, Leznik E, Arancio O, Yan SS, Domenici L, 2010. Microglial receptor for advanced glycation end product-dependent signal pathway drives beta-amyloid-induced synaptic depression and long-term depression impairment in entorhinal cortex. J Neurosci 30, 11414–11425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatheodorou A, Stein A, Bank M, Sison CP, Gibbs K, Davies P, Bloom O, 2017. High-Mobility Group Box 1 (HMGB1) Is Elevated Systemically in Persons with Acute or Chronic Traumatic Spinal Cord Injury. J Neurotrauma 34, 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Gamboni-Robertson F, He Q, Svetkauskaite D, Kim JY, Strassheim D, Sohn JW, Yamada S, Maruyama I, Banerjee A, Ishizaka A, Abraham E, 2006. High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol 290, C917–924. [DOI] [PubMed] [Google Scholar]
- Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E, 2004. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. Journal of Biological Chemistry 279, 7370–7377. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT, 1999. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol 158, 351–365. [DOI] [PubMed] [Google Scholar]
- Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, Lin X, Sherry B, Kumar A, Larosa G, Newman W, Tracey KJ, Yang H, 2006. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med 203, 1637–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read CM, Cary PD, Preston NS, Lnenicek-Allen M, Crane-Robinson C, 1994. The DNA sequence specificity of HMG boxes lies in the minor wing of the structure. EMBO J 13, 5639–5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendon-Mitchell B, Ochani M, Li J, Han J, Wang H, Yang H, Susarla S, Czura C, Mitchell RA, Chen G, Sama AE, Tracey KJ, 2003. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol 170, 3890–3897. [DOI] [PubMed] [Google Scholar]
- Robinson AP, Caldis MW, Harp CT, Goings GE, Miller SD, 2013. High-mobility group box 1 protein (HMGB1) neutralization ameliorates experimental autoimmune encephalomyelitis. J Autoimmun 43, 32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong LL, Trojaborg W, Qu W, Kostov K, Yan SD, Gooch C, Szabolcs M, Hays AP, Schmidt AM, 2004. Antagonism of RAGE suppresses peripheral nerve regeneration. FASEB J 18, 1812–1817. [DOI] [PubMed] [Google Scholar]
- Rovere-Querini P, Capobianco A, Scaffidi P, Valentinis B, Catalanotti F, Giazzon M, Dumitriu IE, Müller S, Iannacone M, Traversari C, Bianchi ME, Manfredi AA, 2004. HMGB1 is an endogenous immune adjuvant released by necrotic cells. EMBO Rep 5, 825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P, Misteli T, Bianchi ME, 2002. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195. [DOI] [PubMed] [Google Scholar]
- Shechter R, Miller O, Yovel G, Rosenzweig N, London A, Ruckh J, Kim KW, Klein E, Kalchenko V, Bendel P, Lira SA, Jung S, Schwartz M, 2013. Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 38, 555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA, 1953. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87, 387–406. [PMC free article] [PubMed] [Google Scholar]
- Srikrishna G, Huttunen HJ, Johansson L, Weigle B, Yamaguchi Y, Rauvala H, Freeze HH, 2002. N -Glycans on the receptor for advanced glycation end products influence amphoterin binding and neurite outgrowth. J Neurochem 80, 998–1008. [DOI] [PubMed] [Google Scholar]
- Steinmetz MP, Horn KP, Tom VJ, Miller JH, Busch SA, Nair D, Silver DJ, Silver J, 2005. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J Neurosci 25, 8066–8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling DP, Cummins K, Mishra M, Teo W, Yong VW, Stys P, 2014. Toll-like receptor 2-mediated alternative activation of microglia is protective after spinal cord injury. Brain 137, 707–723. [DOI] [PubMed] [Google Scholar]
- Stivers NS, Pelisch N, Orem BC, Williams J, Nally JM, Stirling DP, 2017. The toll-like receptor 2 agonist Pam3CSK4 is neuroprotective after spinal cord injury. Exp Neurol 294, 1–11. [DOI] [PubMed] [Google Scholar]
- Sun Y, Chen H, Dai J, Zou H, Gao M, Wu H, Ming B, Lai L, Xiao Y, Xiong P, Xu Y, Gong F, Zheng F, 2015. HMGB1 expression patterns during the progression of experimental autoimmune encephalomyelitis. J Neuroimmunol 280, 29–35. [DOI] [PubMed] [Google Scholar]
- Sutrias-Grau M, Bianchi ME, Bernués J, 1999. High mobility group protein 1 interacts specifically with the core domain of human TATA box-binding protein and interferes with transcription factor IIB within the pre-initiation complex. J Biol Chem 274, 1628–1634. [DOI] [PubMed] [Google Scholar]
- Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, Xiao X, 2007. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol 81, 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés-Ferrer SI, Papoin J, Dancho ME, Olofsson P, Li J, Lipton JM, Avancena P, Yang H, Zou YR, Chavan SS, Volpe BT, Gardenghi S, Rivella S, Diamond B, Andersson U, Steinberg BM, Blanc L, Tracey KJ, 2015. HMGB1 mediates anemia of inflammation in murine sepsis survivors. Mol Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beijnum JR, Buurman WA, Griffioen AW, 2008. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 11, 91–99. [DOI] [PubMed] [Google Scholar]
- Walker LE, Frigerio F, Ravizza T, Ricci E, Tse K, Jenkins RE, Sills GJ, Jorgensen A, Porcu L, Thippeswamy T, Alapirtti T, Peltola J, Brodie MJ, Park BK, Marson AG, Antoine DJ, Vezzani A, Pirmohamed M, 2017. Molecular isoforms of high-mobility group box 1 are mechanistic biomarkers for epilepsy. J Clin Invest 127, 2118–2132. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, Manogue KR, Faist E, Abraham E, Andersson J, Andersson U, Molina PE, Abumrad NN, Sama A, Tracey KJ, 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251. [DOI] [PubMed] [Google Scholar]
- Yanai H, Ban T, Wang Z, Choi MK, Kawamura T, Negishi H, Nakasato M, Lu Y, Hangai S, Koshiba R, Savitsky D, Ronfani L, Akira S, Bianchi ME, Honda K, Tamura T, Kodama T, Taniguchi T, 2009. HMGB proteins function as universal sentinels for nucleic-acid-mediated innate immune responses. Nature 462, 99–103. [DOI] [PubMed] [Google Scholar]
- Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ, 2010. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A 107, 11942–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Lundbäck P, Ottosson L, Erlandsson-Harris H, Venereau E, Bianchi ME, Al-Abed Y, Andersson U, Tracey KJ, Antoine DJ, 2012. Redox modification of cysteine residues regulates the cytokine activity of high mobility group box-1 (HMGB1). Mol Med 18, 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yu M, Wang H, Ding A, Golenbock DT, Latz E, Czura CJ, Fenton MJ, Tracey KJ, Yang H, 2006. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 26, 174–179. [DOI] [PubMed] [Google Scholar]
- Zhang J, Takahashi HK, Liu K, Wake H, Liu R, Maruo T, Date I, Yoshino T, Ohtsuka A, Mori S, Nishibori M, 2011. Anti-high mobility group box-1 monoclonal antibody protects the blood-brain barrier from ischemia-induced disruption in rats. Stroke 42, 1420–1428. [DOI] [PubMed] [Google Scholar]
- Zurolo E, Iyer A, Maroso M, Carbonell C, Anink JJ, Ravizza T, Fluiter K, Spliet WG, van Rijen PC, Vezzani A, Aronica E, 2011. Activation of Toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development. Brain 134, 1015–1032. [DOI] [PubMed] [Google Scholar]