Abstract
MLC901 (NurAiDII) is used as a treatment for stroke patients. It has been shown that MLC901 improves motor and cognitive recovery in ischemic and traumatic brain‐injured rodents. The present study seeks to delineate cognitive effects induced by MLC901 in normal, noninjured mice. To this end, the behaviors of vehicle‐ and MLC901‐treated C57BL/6 mice in hippocampus‐dependent (passive avoidance, Morris water maze) and hippocampus‐independent (novel object recognition) cognitive tasks are compared. The potential influence of the compound on the anxiety level and nycthemeral rhythm of mice is also assessed. In addition, the long‐term effects of MLC901 on hippocampal neurogenesis are measured. The results clearly demonstrate that MLC901 promotes extinction in passive avoidance and reversal learning in the Morris water maze and improves the performance of mice in novel object recognition. In parallel, this study shows the long‐term proneurogenesis effects of MLC901 that result in the increase in the number of mature neurons in the hippocampus. If these observations can be extended to humans, then MLC901 could represent a promising therapeutic strategy. © 2015 The Authors. Journal of Neuroscience Research Published by Wiley Periodicals, Inc.
Keywords: anxiety disorders, cognitive flexibility, fear extinction, memory, MLC901, NeuroAiD, passive avoidance, posttraumatic stress disorder, stroke
NeuroAiD (MLC601) is used in patients to fight the deleterious effects caused by stroke (Chen et al., 2009, 2013a,b; Kong et al., 2009; Bavarsad Shahripour et al., 2011; Harandi et al., 2011; Navarro et al., 2014). NeuroAiD/MLC601 was registered with the Sino Food and Drug Administration in 2001. There have been clinical assays, including large‐scale clinical assays, of the use of this treatment for stroke patients. Limited clinical studies with nonacute stroke patients have shown that MLC601 improves recovery in terms of functional outcome and neurological disability, with a good safety profile (Gan et al., 2008; Siow, 2008; Chen et al., 2009; Kong et al., 2009; Young et al., 2010; Bavarsad Shahripour et al., 2011; Harandi et al., 2011; Navarro et al., 2013; Siddiqui et al., 2013). Moreover, a large clinical trial of more than 1,000 patients, Chinese medicine NeuroAiD efficacy on stroke recovery (CHIMES), was recently completed (Venketasubramanian et al., 2009; Chen et al., 2013a,b). The CHIMES study showed a reduction of early recurrent vascular events and vascular deaths in poststroke patients (Chen et al., 2013b). A recent post hoc analysis of more than 500 patients recruited from the Philippines with predictors of poorer outcome indicated a positive effect of MLC601 (Navarro et al., 2014).
Consistent with observations on MLC601 in humans, pharmacological data obtained from rodents have demonstrated that, in addition to infarct volume reduction, MLC601 and MLC901 (NurAidII) promote functional recovery in rodent cerebral ischemia and traumatic brain injury (TBI) models (Heurteaux et al., 2010, 2013; Quintard et al., 2011; Tsai et al., 2014). MLC901 improves motor function of mice after focal ischemia (Heurteaux et al., 2010), reduces spatial memory impairments induced by global ischemia in rats (Quintard et al., 2011), and restores the temporal order memory deficits observed in the rat lateral percussion model of TBI (Quintard et al., 2014). The efficacy of MLC601/MLC901 has also been demonstrated in vitro in models of excitotoxicity and neuronal growth (Heurteaux et al., 2010) and in a model of oxygen glucose deprivation, which mimics ischemic conditions (Moha Ou Maati et al., 2012). MLC901 activates KATP channels (Moha Ou Maati et al., 2012) and induces the protein kinase B survival pathway (Quintard et al., 2011), two crucial mechanisms involved in neuroprotection (Franke et al., 1997; Liss and Roeper, 2001). Neurophysiological and neuroanatomical modifications observed with MLC901 support both neuroprotective and neurorepair properties for the compound. MLC901 stimulates neuritogenesis and synaptogenesis‐related GAP43 and synaptotagmin 1 protein expression in cultured mouse cortical neurons (Heurteaux et al., 2010). In addition, MLC901 increases dentate gyrus neurogenesis (Heurteaux et al., 2010; Quintard et al., 2011, 2014) and expression of the cortical brain‐derived neurotrophic factor (BDNF; Heurteaux et al., 2010) and vascular endothelial growth factor (Quintard et al., 2014). The beneficial effects of MLC601/901 are probably a result of the combination of neuroprotective and neurorepair mechanisms induced by compounds acting on different targets. Originally made with nine herbal and five animal extracts, the MLC601 composition was simplified, and MLC901 contains only the extracts of the plant components. We have shown that there are a number of identified molecules in the different herbs extracted to manufacture MLC901 that are neurobeneficial, including butylidenephtalide, tanshinone, salvianolic acid B, ferulic acid, and tetramethylpyrazine (Chen et al., 2012; Tang et al., 2012; Zhuang et al., 2012; Kao et al., 2013; Koh, 2013; 2013 et al., 2013). However, at present, none of these molecules, alone or in combination in vitro or in vivo, can reconstitute the overall properties of MLC901 in stroke, indicating the complexity of the therapeutic cocktail.
Brain plasticity constitutes the cornerstone of learning and memory theories (Kim and Linden, 2007; Caroni et al., 2012; Zatorre et al., 2012). Neurogenesis in the dentate gyrus subgranular zone has been proposed to optimize hippocampus‐dependent processes, such as spatial and contextual learning and memory (Garthe et al., 2009; Denny et al., 2012; Pan et al., 2012). In the Morris water maze, it has been shown that hippocampal neurogenesis suppression reduces the capacities of mice to learn and memorize the location of a platform hidden under the surface of a pool in relation to external visual cues (Rola et al., 2004; Garthe et al., 2009; Goodman et al., 2010). It has also been demonstrated that impaired neurogenesis induces alteration of both fear memory and extinction (i.e., the progressive decrease of the conditioned response with repeated exposures to the conditional stimulus alone) in the contextual fear conditioning paradigm (Imayoshi et al., 2008; Pan et al., 2013) and changes in novelty‐induced exploratory activity (Denny et al., 2012; Kalm et al., 2013). In addition, BDNF has been found to be crucially involved in a wide range of cognitive tasks (Schulz‐Klaus et al., 2013).
Because MLC901‐induced improvement of stroke and TBI functional outcomes is probably linked to stimulation of synaptic plasticity and neurogenesis (Quintard et al., 2011; Heurteaux et al., 2010, 2013), two processes known to contribute critically to cognitive performances, we decided to investigate further the effects of chronic administration of MLC901 on cognitive skills in normal mice. To this end, we compared the performances of vehicle‐ and MLC901‐treated mice in two hippocampus‐dependent tasks, the Morris water maze and the passive avoidance tests, and in the hippocampus‐independent novel object recognition task. Mice were also assessed in an actimeter and the elevated plus‐maze test to determine if MLC901 modified their spontaneous activity and unconditioned anxiety level, both of these behavioral features being able to influence all the other behaviors measured. This article describes a series of interesting behavioral effects of MLC901 on mice that add to previous observations (Heurteaux et al., 2010; Quintard et al., 2011, 2014), supporting the proposed beneficial effects of this traditional Chinese medicine in humans.
MATERIALS AND METHODS
Animals
Five‐week‐old C57BL/6 male mice (Janvier, Saint Berthevin, France) were housed five per cage under an inverse 12‐hr light/dark cycle (lights on at 8:00 pm) in an animal facility maintained at 21°C ± 1°C. Animals were provided food and water or MLC901 ad libitum. MLC901 was dissolved in drinking water (6 g/liter). All behavioral tests began after a minimum 2‐week period of acclimation of mice to the breeding conditions. All experiments were performed in accordance with European Community policies on the care and use of laboratory animals (directive 2010/63/EU) and were approved by the French Ministry of Higher Education and Scientific Research (approval No. 00735).
Drug Treatment
MLC901 combines nine herbal components with the following composition per capsule: 0.80 g Radix astragali, 0.16 g Radix salvia miltiorrhizae, 0.16 g Radix paeoniae rubra, 0.16 g Rhizoma chuanxiong, 0.16 g Radix angelicae sinensis, 0.16 g Carthamus tinctorius, 0.16 g Prunus persica, 0.16 g Radix polygalae, and 0.16 g Rhizoma acori tatarinowii. MLC901 was administered in drinking water (6 g/liter) until animals were sacrificed. In pilot studies, we analyzed whether the individual consumption of water by mice was affected by MLC901. No significant difference was induced by MLC901 (daily consumption: water 4.54 ± 0.4 ml, water with MLC901 4.48 ± 0.22 ml; n = 8 in both groups; P > 0.05). The result was that MLC901‐treated mice received about 27 mg MLC901 per day. All experiments were performed with the MLC901 batch BN112 provided by Moleac (Singapore). MLC901 and vehicle (water) were put in dark bottles to make the cages of experimental groups nonidentifiable. The flowchart illustrating the experimental design is shown in Figure 1.
Figure 1.
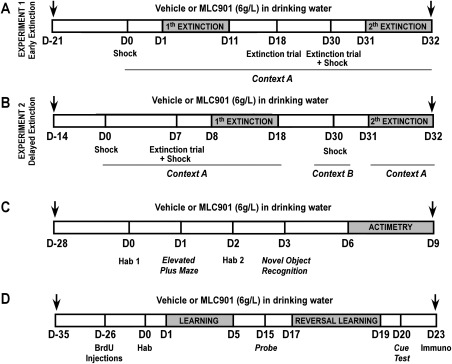
Diagram of the experimental design. A: Experimental design of the step‐through passive avoidance experiment 1: early extinction (vehicle n = 10, MLC901 n = 10). B: Experimental design of the step‐through passive avoidance experiment 2: delayed extinction (vehicle n = 15, MLC901 n = 15). C: Experimental design of the elevated plus maze, novel object recognition, and actimetry tests. Hab, habituation to the open field (vehicle n = 20, MLC901 n = 20 for elevated plus maze; vehicle n = 14, MLC901 n = 16 for novel object recognition; and vehicle n = 10, MLC901 n = 10 for actimetry). D: Experimental design of the Morris water maze test. Hab, habituation to the experimental conditions; immuno, immunohistochemistry.
Behavioral Testing
After each exposure to the experimental devices, mice were transiently placed into new cages to prevent spreading of their test‐induced stress to their untested cagemates. When the task was finished, all tested mice were returned to their original home cages. In all cases, the exposure to the “posttest cage” lasted for only a few minutes. All experiments were randomized. Vehicle‐ and MLC‐901‐treated mice were alternately tested in all procedures to avoid an order effect. All behavioral tests were monitored by one researcher blinded to mouse treatment code.
Step‐Through Passive Avoidance Test
The purpose of the passive avoidance paradigm is to assess memory of fear conditioning. The conditioned response of mice consists of avoiding an area where they have experienced a negative reinforcement (generally footshocks). Extinction protocols were developed to investigate the progressive disappearance of the conditioned response when the negative reinforcement was no longer administered. In the literature, the term “extinction” is often interchangeably used to refer to the protocols leading to decrease of the conditioned response, the decrease of the conditioned response itself, and the underlying psychological and neurobiological mechanisms (Myers and Davis, 2007). For clarity, here we use “extinction training” or “extinction trials” for our extinction protocols, “behavioral extinction” for the decrease of the conditioned response, and “extinction” alone for the underlying mechanisms.
We performed two different experiments (experiments 1 and 2) with the passive avoidance test (Fig. 1A,B). The step‐through passive avoidance device (Imetronic, Pessac, France) was formed by two compartments of the same size (19.5 × 10 × 13 cm) separated by an electronically controlled guillotine door (5 × 5.5 cm). The departure compartment was illuminated with a 150‐lux light source. The other compartment was maintained in darkness to motivate animals to enter it. This dark compartment constituted the context A. For conditioning, mice were individually placed into the departure compartment, and the guillotine door was lifted 30 sec later. The latency before entering the dark compartment was noted. If mice did not enter it within 5 min, they were removed from the apparatus and assessed again a few minutes later. After they had entered the dark compartment, the door was immediately closed, and, 2 sec later, three electrical footshocks (0.3 mA, 2 sec), each separated by 3 sec, were delivered to them. For the extinction training, the dark compartment was no longer punished. The door was opened 30 sec (experiment 1) or 10 sec (experiment 2) after mice were placed into the departure compartment, and the latency before entering the dark compartment was measured. The latency before the opening of the door was shortened for experiment 2 because we observed in experiment 1 that a 10‐sec latency was sufficient to avoid early entries into the dark compartment resulting from stress. The cutoff was set at 300 sec. Each time a mouse exceeded the cutoff, the animal was gently pushed forward to enter the dark compartment. The door was immediately closed after a mouse had entered through it. The mouse was then left in the compartment for 30 sec before the test ended. The threshold and the intensity of the behavioral response (vocalizations and motor reactions) to incremental electrical footshocks (0.05, 0.1, 0.2, 0.3, and 0.4 mA, for 2 sec) were measured in 10 vehicle‐ and 10 MLC901‐treated naïve mice to verify the influence of the treatment on the nociceptive sensitivity of the animals.
Experiment 1: early extinction
10 vehicle‐ and 10 MLC901‐treated mice were used for experiment 1 (Fig. 1A). The test began after 3 weeks of treatment. For assessment of their spontaneous locomotor activity into the device, mice were allowed to move freely in both communicating compartments for 5 min on the day before conditioning. After this conditioning (D0), they underwent daily extinction trials for 11 consecutive days (from D1 to D11). On D18, they were subjected to another extinction trial to check spontaneous recovery of conditioned response. Twelve days later (D30), the animals underwent a new conditioning (reconditioning), identical to the first one. New extinction trials were performed daily on the 2 following days, D31 and D32. The goal of the reconditioning trial performed on D30 and the following extinction trials on D31 and D32 was to assess the cumulative effect of repeated stress on extinction.
Experiment 2: delayed extinction
15 vehicle‐ and 15 MLC901‐treated mice were used for experiment 2 (Fig. 1B). The conditioning (D0) was performed after 2 weeks of treatment. This treatment duration prior to the conditioning was chosen so that MLC901‐treated mice received their treatment for 3 weeks before the beginning of the extinction training (Fig. 1B). On D7 (after the conditioning), mice were subjected to a reconditioning. The reconditioning was identical to the first conditioning except the door was opened 10 sec after the introduction of animals into the departure compartment (instead of the 30‐sec delay used for the first conditioning). On the next day, the daily extinction trials began and were carried out for 11 consecutive days (from D8 to D19). On D30, mice received a single electrical footshock (0.3 mA, 2 sec) in context B (a 25 × 25 × 25 cm black box illuminated with a 5‐lux light and located in a different room from the passive avoidance device) different from context A (i.e., the context formed by the dark compartment of the passive avoidance apparatus) before being subjected to two supplementary extinction trials on D31 and D32. This step was designed to compare the extent of the reinstatement effect, i.e., the resurgence of the extinguished conditioned response caused by the administration of the reinforcement stimulus in a new context in the vehicle‐ and MLC901‐treated mice. Additional groups, including 10 vehicle‐ and 10 MLC901‐treated naïve mice, received the single shock in context B before being subjected to one extinction trial in the passive avoidance device. This was performed to check the preeminence of the reinstatement effect over the generalization of the potential fear conditioning occurring in context B.
Complementary Tests
The behavioral tests described above were planned after we had obtained the results of the passive avoidance experiments. They were carried out to complete the characterization of MLC901‐induced behavioral modifications and, at the same time, to exclude any amnestic effect of the compound on adaptive behaviors. For this reason, we chose to use a long time period for treatment (between 4 and 5 weeks of treatment).
Elevated Plus Maze
The elevated plus maze test was performed with 20 vehicle‐ and 20 MLC901‐treated mice to evaluate their unconditioned anxiety level (Fig. 1C). The test was carried out after 4 weeks of treatment. The device (Bioseb, Vitrolles, France) was made of plastic material and consisted of two open (30 × 5 cm) and two closed (30 × 5 × 15 cm) arms connected to a central area (5 × 5 cm), with the arms of the same type facing each other. The open arms were surrounded by a 1‐mm‐high ledge to prevent the mice from falling. The whole apparatus was elevated to a height of 50 cm above floor level. The illumination was set at 20 lux. At the beginning of the test, mice were individually placed at the center facing an open arm, and their behavior was video recorded for 5 min. The number of entries and the time spent in the open and closed arms were then analyzed using Etholog 2.25 (Ottoni, 2000). The percentage of entries and time spent in the open arms relative to the total entries and time spent in the four arms, which are classical indices of anxiety, were calculated a posteriori. The number of stretched attend postures (SAP; forward elongation of the body followed by its retraction to the initial position) was also noted as an ethoexperimental variable.
Novel Object Recognition Task
In this test, mice are expected to show exploratory preference for a novel object over another one that they had previously explored (Fig. 1C). This preference denotes the capacities of the object memory in each animal. Mice (14 vehicle‐ and 16 MLC901‐treated mice from among those used for the elevated plus maze test) were familiarized with an open field (30 × 30 × 30 cm) for 15 min on the first day of the experiment and for 15 min 2 days later. On the fourth day, for the sample trial mice were placed in the open field, where two different objects called “old” objects had been placed, and they were allowed to explore the objects freely for 10 min. Five hours later, mice were again placed into the open field for the 10‐min‐duration test trial in which one of the objects previously explored had been replaced with a “new” one (the replaced objects across test trials were alternated to avoid a place preference bias). We chose a 5‐hr interval between sample and test trials because it represents a very challenging condition for the object memory of C57BL/6 mice (Sik et al., 2003). The exploratory activity toward each of the objects was recorded during both sample and test trials. The exploratory preference index was calculated for the test trial as (time spent exploring the novel object/total exploration time) × 100.
Actimetry Test
The actimeter (Imetronic) allows the study of the spontaneous activity of mice over a prolonged period (Fig. 1C). Three days after the completion of the novel object recognition task, 10 animals treated with either vehicle or MLC901 for 5 weeks were left in individual cages (20.5 × 11.5 × 16.5 cm) equipped with infrared beams able to detect horizontal and vertical movements. The cages were placed in an enclosure under an inverse 12‐hr light/dark cycle (lights on at 8:00 pm) for 3 days.
Morris Water Maze
The Morris water maze task is currently used to investigate spatial learning and memory, two hippocampus‐dependent cognitive processes (Fig. 1D; Supp. Info. Fig. 1). Briefly, mice had to locate a platform hidden under the water's surface in a circular swimming pool (90 cm in diameter, water at 24°C ± 1°C) by referring to some visual cues arranged all around the experimental room. The test began after a 5‐week treatment. On the day before the start of the learning trials, 10 vehicle‐ and 10 MLC901‐treated mice were familiarized with the experimental conditions (except that cues were hidden). First, they were individually left in the pool without the platform for 1 min. Five minutes later, they were placed onto the platform positioned at the center of the pool for 30 sec. The learning trials were conducted four times per day (intertrial interval, 6 min) for 5 consecutive days. Four different start positions were used (south, west, north, east) each day. The platform was located in the northwest (NW) quadrant. The latency before reaching it was measured. If a mouse did not find the platform within 1 min, it was gently guided to it. After reaching it, mice were left on the platform for 20 sec. Then, they were returned to their cages. Ten days after the last learning trial, animals were subjected to a probe test; they were left in the pool for 1 min after the platform was removed, and the number of crossings of the previous platform location (called “annulus crossings”) and the time spent in the NW quadrant were measured. Two days after the probe test, the platform was moved to the southeast quadrant, and the reversal learning trials began. The reversal learning trials consisted of daily sessions of four trials each for 3 days and were conducted to assess the cognitive flexibility of animals. On the day after the last session of reversal learning, we performed a four‐trial cue test with the platform visible to ensure that MLC901 did not influence motor function, visual–motor abilities, or motivation of mice to escape water. In addition to the latency before reaching the platform, the classical variable recorded in the Morris water maze test, we took into account the strategies used by mice to carry out the task. Our strategy categories, which were inspired by the work of the Kempermann group (Garthe et al., 2009; Garthe and Kempermann, 2013), reached from thigmotaxis (mice swim along the wall of the maze), which is an emotion‐related behavior usually observed during the first exposures, to direct swimming (mice swim straight to the platform), which is the most demanding and efficient spatial strategy (Supp. Info. Fig. 1). These strategies stereotypically emerge in the order presented in Supporting Information Figure 2, in which they are further described. For purposes of clarity, we decided to group together thigmotaxis, random search, and scanning into the same untargeted search strategies category, and directed search, focal search, and direct swimming formed the targeted search strategies category. Chaining had a special status because it could appear either after scanning or after the targeted search strategies as a result of overtraining (Garthe and Kempermann, 2013). Perseverance was scored each time mice searched for the platform where it had been before having been moved for the reversal learning. All trials were video recorded and later analyzed in Anymaze tracking software (Stoelting, Wood Dale, IL).
Figure 2.
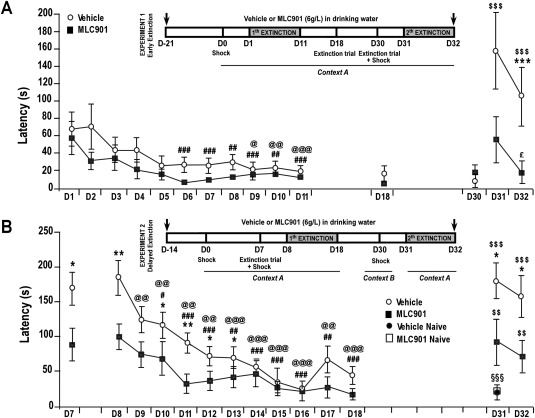
Effect of MLC901 in the passive avoidance test. A: Experiment 1: early extinction; latencies before entering the dark compartment in seconds ± SEM. Because of the requirement of the validity criteria for ANOVA, the experiment 1 data were log10 transformed. @, comparison with D1 latency in the vehicle‐treated group; #, comparison with D1 latency in the MLC901‐treated group; $, comparison with D30 latency in the vehicle‐treated group; *, comparison between vehicle‐treated and MLC901‐treated groups; §, comparison between vehicle‐treated mice and vehicle‐treated naïve mice; £, comparison with D31 latency in the MLC901‐treated group. Single symbol, P < 0.05; double symbols, P < 0.01; triple symbols, P < 0.001. B: Experiment 2: delayed extinction; latencies before entering the dark compartment in seconds ± SEM. @, comparison with D8 latency in the vehicle‐treated mice; #, comparison with D8 latency in the MLC901‐treated group; $, comparison with D18 in both groups; *, comparison between vehicle‐treated and MLC901‐treated groups. Single symbol, P < 0.05; double symbols, P < 0.01; triple symbols, P < 0.001.
Immunohistochemistry
Cell proliferation and survival in the dentate gyrus were evaluated by immunohistochemical staining with Ki67 and 5‐bromo‐2′‐deoxyuridine (BrdU), respectively. Ki67 is a protein specifically expressed during the active phases of the cell cycle, and BrdU is a nucleotide analog incorporated in DNA during replication. Nine days after the beginning of the MLC901 treatment (Fig. 1D), mice used for the Morris water maze test were injected with BrdU (75 mg/kg, i.p., 200 µl every 2 hr for a total of four injections). Three days after the completion of the Morris water maze protocol (i.e., 7 weeks after the BrdU injections), mice were anesthetized with sodium pentobarbital (50 mg/kg) and were intracardially perfused with cold phosphate‐buffered saline (PBS) for 5 min and with 4% paraformaldehyde (PFA) for 3 min. Brains were then removed, placed in 4% PFA, and stored at 4°C overnight. On the next day, brains were serially cut at 40 µm through the hippocampus with a vibratome. The free‐floating sections were placed in an antifreeze solution at −20°C until immunohistochemical processing. The latter was performed on every sixth section of the part of the hippocampus extending from bregma −1.34 mm to bregma −3.26 mm (Heurteaux et al., 2006). Briefly, sections were first incubated with mouse anti‐BrdU antibody (1:8,000; BD Biosciences, San Jose, CA) overnight. After having been washed with PBS, they were incubated for 1 hr with biotinylated goat anti‐mouse antibody (1:100; Vector Laboratories, Burlingame, CA) and then in a biotinylated horseradish peroxidase–avidin solution (ABC kit; Vector Laboratories) according to the manufacturer's recommendations. The peroxidase activity of the immune complexes was revealed by 3,3′‐diaminobenzidine staining (Vector Laboratories). The BrdU‐labeled cells in the subgranular zone of the dentate gyrus were blindly counted under light microscopy at ×400. The protocol used for Ki67 revelation was the same, except that rabbit anti‐Ki67 antibody (1:800; Novus Biologicals, Littleton, CO) was chosen as the primary antibody.
The phenotype of BrdU‐positive cells was determined with fluorescent double labeling. Sheep (1:200; Interchim, Los Angeles, CA) and mouse (1:8,000; BD Biosciences) anti‐BrdU antibodies allowed detection of BrdU‐positive cells. Rabbit antiglial fibrillary acidic protein antibody (1:250; Dako, Carpinteria, CA) and mouse antineuron‐specific nuclear protein (NeuN) antibody (1:250; Millipore, Billerica, MA) were used to label glial cells and mature neurons, respectively. After an overnight incubation with the primary antibodies, sections were incubated with secondary antibodies conjugated with Alexa Fluor 488 and 594 (1:1,000; Molecular Probes, Eugene, OR) for 2 hr. The double‐labeled cells were blindly counted under confocal microscopy at ×40.
Statistical Analysis
ANOVA was preferred for statistical analysis. Two‐way (repeated measures × treatment) ANOVA was conducted for procedures involving either spatial learning (Morris water maze) or behavioral extinction (passive avoidance). Normality and homoscedasticity of data were checked with Shapiro‐Wilk and Levene's tests, respectively. Sphericity was also verified when repeated measures (RM) were performed. When necessary, raw data were log10 transformed to meet the ANOVA validity criteria. Post hoc comparisons were performed with Bonferroni correction. If ANOVA could not be conducted, even after transformation, the nonparametric Mann‐Whitney (for nonpaired comparisons) and Wilcoxon (for paired comparisons) tests were applied. A nonparametric Friedman test, followed by a post hoc Wilcoxon test, was used in cases of repeated measures. Statistical significance was set at P < 0.05. Observed statistical power (1 – β) was provided when calculable and was symbolized by π. Statistical analyses were performed in Statistica 8 (Dell, Aliso Viejo, CA), R 3.0.2 (r-statistics.com), and XLSTAT 2014 (Addinsoft, Paris, France).
RESULTS
Step‐Through Avoidance Test
Preliminary data have shown that MLC901 influences behavioral extinction in the passive avoidance test. Experiments 1 and 2 were designed to characterize such an effect further and to clarify whether it was the result of passive amnesia or of the active extinction process.
The threshold and intensity of the spontaneous nociceptive behaviors induced by electrical footshocks were not influenced by the type of treatment administered (P > 0.05 for all reaction latencies and reaction intensities; data not shown). The latency before entering the dark compartment on the day of conditioning was not significantly different in vehicle‐ and MLC901‐treated mice either in experiment 1 (U = 33.5, P > 0.05) or in experiment 2 (U = 81.5, P > 0.05). MLC901 lowered the spontaneous locomotor activity of mice in the device as measured on the day before conditioning (vehicle‐treated mice 665.1 ± 37.54 [SEM] movements, MLC901‐treated mice 518 ± 37.52 [SEM] movements; U = 19, P < 0.05). The shorter latency before entering the dark compartment observed on the first day of extinction in experiment 1 (first day of extinction = D1) compared with experiment 2 (first day of extinction = D7) was very likely the result of the latent inhibition effect (Dubrovina and Red'kina, 2012); the pre‐exposure of mice to the passive avoidance device before conditioning in experiment 1 weakened the avoidance behavior induced by the shock.
Experiment 1: early extinction
The 11 extinction trials from D1 to D11 induced a progressive and significant decrease of the conditioned avoidance response both in the vehicle‐ and in the MLC901‐treated mice (RM effect: F10,180 = 10.56, P < 0.001, π > 0.99; Fig. 2A). Although neither significant treatment (F1,18 = 3.13, P = 0.09, π = 0.39) nor repeated measures × treatment interaction (F10,180 = 1.42, P > 0.05, π = 0.7) was detected during the extinction training, it must be noted that a floor effect most likely prevented any significant intergroup differences from emerging by D6. Unlike the MLC901‐treated mice that displayed latencies less than 10 sec from D6 to D8, the vehicle‐treated mice took between 22 and 27.5 sec to enter the dark compartment. Moreover, latencies were significantly reduced compared with D1 scores as early as D6 in mice treated with MLC901 (P < 0.001) but only from D9 in the vehicle‐treated mice (P < 0.05). These results clearly suggest that MLC901 promotes behavioral extinction. The latencies displayed by both groups at D18 and D30 were not significantly different from those noted at D11, indicating the lack of spontaneous recovery of the conditioned response. ANOVA revealed significant treatment × repeated measure interaction (F2,36 = 12.82, P < 0.001, π = 0.99) concerning the latencies during the period from D30 to D32. Although reconditioning achieved on D30 resulted in a dramatic increase of latency at D31 in the vehicle‐treated mice (P < 0.001), that was not the case for the MLC901‐treated mice (P > 0.05). The latencies decreased on the following day in both groups, but significance was reached only for the MLC901‐treated mice (P < 0.05) that very quickly entered the dark compartment (13.2 sec). Compared with MLC901‐treated mice, vehicle‐treated mice took ninefold longer to enter the dark compartment (P < 0.001). Two‐way ANOVA, with latencies observed on D1 and D31 as repeated measure, detected a significant treatment × repeated measure interaction (F1,18 = 7.34, P < 0.05, π = 0.73). Although the conditioned response induced by the reconditioning was similar to the D1 response in the MLC901‐treated mice, it was much stronger in the vehicle‐treated group. Altogether, these results suggest that MLC901 promotes behavioral extinction following fear conditioning and strongly weakens the potentiation effect of fear reconditioning.
Experiment 2: delayed extinction
Seven days after the conditioning, the latency before entering the dark compartment was significantly higher in the vehicle‐treated mice than in the MLC901‐treated mice (U = 54, P < 0.05, π = 0.5; Fig. 2B). The latency on D8 was similar to that noted on D7 in both groups (vehicle U = 26, P > 0.05, π = 0.12; MLC901 U = 32, P > 0.05, π = 0.09). In comparison with vehicle treatment, MLC901 led to significantly reduced latencies for most of the first half of the subsequent extinction training (D8 U = 47, P < 0.01, π = 0.83; D10 U = 61.5, P < 0.05, π = 0.33; D11 U = 47.5, P < 0.01, π = 0.78; D12 U = 54.5, P < 0.05, π = 0.53; D13 U = 60.5, P < 0.05, π = 0.13). A nonparametric Friedman test revealed that the avoidance response declined in the course of the extinction trials for both vehicle‐treated (Q = 72.26, P < 0.001) and MLC901‐treated (Q = 74.57, P < 0.001) mice. Compared with the scores on D8, the avoidance time of the dark compartment significantly decreased by D9 in the vehicle‐treated mice (T = 1, P < 0.01, π = 0.92) but only from D10 in mice treated with MLC901 (T = 21.5, P < 0.05, π = 0.67). This small delay probably resulted from the fact that the margin of reduction was reduced in the MLC901‐treated mice because of their mean latency, which was already very low on D8 (97.87 sec for the MLC901‐treated mice vs. 192.33 sec for the vehicle‐treated mice). On D31, the reinstatement procedure led to the resurgence of the conditioned response (D18 vs. D31: vehicle T = 2, P < 0.001, π = 0.99; MLC901 T = 8, P < 0.01, π = 0.83), the magnitude of which was comparable to that observed on D7 and D8 for both groups. As a consequence, the MLC901‐treated mice entered the dark compartment more quickly than the vehicle‐treated mice on D31 (U = 59.5, P < 0.05, π = 0.5) and D32 (U = 60.5, P < 0.05, π = 0.5). The naïve mice, shocked only in context B, on D30 entered the dark compartment much more quickly on D31 than did the conditioned mice, irrespective of the treatment they received (vehicle‐naïve vs. vehicle‐conditioned animals U = 9.5, P < 0.001, π = 0.98; MLC901‐naïve vs. MLC901‐conditioned animals U = 44.5, P = 0.09, π = 0.08; vehicle‐naïve vs. MLC901‐naïve animals U = 40.5, P > 0.05, π = 0.05). These results show that the increase of the conditioned response between D18 and D31 is not attributable to the generalization of the emotional response to the shock received in context B but actually results from reinstatement of the initial conditioning in context A. As a whole, experiment 2 indicates that the extinction training is not required to observe the MLC901‐induced reduction of the conditioned fear response. An amnestic effect of MLC901 is not very likely because the reinstatement procedure unmasked the conditioned response in mice treated with the compound.
Complementary Tests
Elevated plus maze test
The purpose of the plus maze test was to discover the potential effect of MLC901 on unconditioned anxiety (Fig. 3A–D). This was of interest not only in itself but also because such an effect could influence mouse behaviors in the passive avoidance task.
Figure 3.
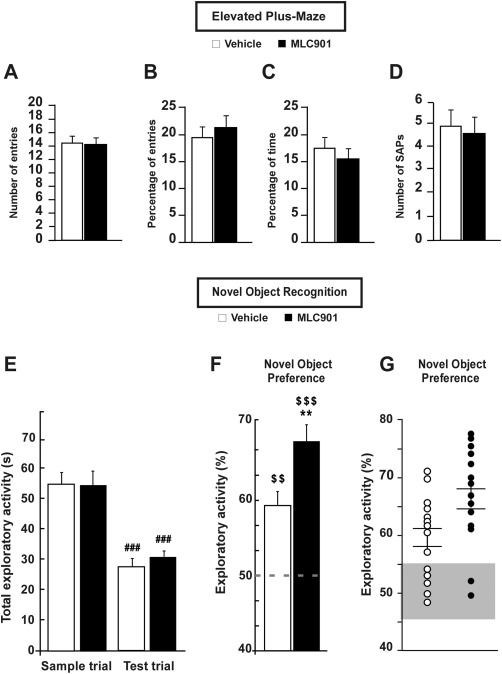
Effect of MLC901 in the elevated plus‐maze and novel object recognition tests. A: Elevated plus maze; number of entries in the closed arms ± SEM. B: Elevated plus maze; entries in the open arms in percentage ± SEM. C: Elevated plus maze; time spent in the open arms in percentage ± SEM. D: Elevated plus maze; number of SAPs achieved ± SEM. E: Novel object recognition; time spent to explore both objects in seconds ± SEM. ### P < 0.001, comparison between the sample and the test trials in both groups. F: Novel object recognition; exploratory activity devoted to the novel object in percentage ± SEM. Dotted line represents the chance level (50% of the exploratory activity). $, comparison with the chance level; *, comparison between the vehicle‐treated and MLC901‐treated groups. Double symbols, P < 0.01; triple symbols, P < 0.001. G: Novel object recognition; individual plots for the exploratory activity devoted to the novel object in percentage ± SEM. Whiskers indicate mean ± the SEM. Gray area marks the 45–55% interval of the total exploratory activity devoted to the novel object.
The locomotor activity of mice in the elevated plus maze, as indicated by the number of closed arms entries, was not modified by MLC901 (U = 183, P > 0.05, π = 0.05; Fig. 3A). Neither the percentage of entries (t = −0.53, P > 0.05, π = 0.08; Fig. 3B) nor the percentage of time spent in the open arms (U = 191.5, P > 0.05, π = 0.09; Fig. 3C) was significantly different in the vehicle‐ vs. the MLC901‐treated mice, nor was the number of SAPs that they achieved (U = 193, P > 0.05, π = 0.08; Fig. 3D). These data indicate that MLC901 treatment probably does not modify the level of unconditioned anxiety in mice.
Novel object recognition test
The objective of this test was to verify that MLC901 exerted some cognitive effect beyond the restricted area of hippocampus‐dependent learning and memory, measured with the passive avoidance test (Fig. 3E–G).
The total exploratory activity of all the mice decreased by half between the sample and the test trials, denoting habituation to the experimental conditions (repeated measure effect F1,28 = 93.58, P < 0.001, π > 0.99; Fig. 3E). The treatment did not significantly change the total time spent in exploring the objects (treatment effect F1,28 = 0.07, P > 0.05, π = 0.06; Fig. 3E) or the drop of exploratory activity between both trials (treatment × repeated measure interaction F1,28 = 0.54, P > 0.05, π = 0.11; Fig. 3E). Although the mice in both groups showed an exploratory preference for the novel object during the test trial (comparison with the chance level: vehicle U = 101.5, P < 0.01, π = 0.99; MLC901 U = 120, P < 0.001, π > 0.99; Fig. 3F), the MLC901‐treated group explored it significantly more than did the vehicle‐treated group (U = 50, P < 0.01, π = 0.72; Fig. 3F). Furthermore, 36% of the vehicle‐treated mice did not show a clear preference for the novel object (between 45% and 55% of the total exploratory activity dedicated to the novel object), whereas that figure was only 12.5% for the MLC901‐treated mice (Fig. 3G). These results suggest that MLC901 strengthens cognitive processes underlying novel object recognition without affecting the exploratory motivation of mice.
Actimetry test
A slight locomotion decrease induced by MLC901 was detected in the passive avoidance test. The actimetry test was conducted to highlight a potential nonspecific effect of the compound on mouse activity (Supp. Info. Fig. 2).
Irrespective of the treatment that the mice received, their horizontal (Supp. Info. Fig. 2A) and vertical (Supp. Info. Fig. 2C) activities followed a regular nycthemeral rhythm, with two peaks during the dark phase and almost no movement during the light phase. Because of a lack of homoscedasticity, ANOVA could not be performed on these variables. Having concluded that hourly comparisons would not be very meaningful, we decided to investigate the changes of the mean nocturnal locomotor and vertical peaks over the 3 days for which the experiment lasted. The peak value was the hourly average between the second and the fifth hours of the dark phase (i.e., between 9:00 am and 12:00 am, inclusive). The nocturnal peak of locomotor activity clearly decreased over the 3 days of the experiment in both groups (repeated measure effect F2,36 = 12.84, P < 0.001, π = 0.99; Supp. Info. Fig. 2B). Furthermore, its punctual intensity (treatment effect F1,18 = 0.61, P > 0.05, π = 0.11; Supp. Info. Fig. 2B) and its decrease over time (treatment × repeated measure interaction F2,36 = 1.58, P > 0.05, π = 0.31; Supp. Info. Fig. 2B) were not significantly influenced by the type of treatment. The decrease in the peak of vertical activity over the experiment duration was even more pronounced than that of the locomotor activity (repeated measure effect F2,36 = 23.36, P < 0.001, π > 0.99; Supp. Info. Fig. 2B). Consistent with what was observed for the latter, neither the rearing behavior peak itself (treatment effect F2,36 = 0.54, P > 0.05, π = 0.11; Supp. Info. Fig. 2D) nor its evolution over time (treatment × repeated measure interaction F2,36 = 0.06, P > 0.05, π = 0.06; Supp. Info. Fig. 2D) was significantly modified by the MLC901 treatment. These results demonstrate that MLC901 fails to alter the mice activity and its nycthemeral structure.
Morris water maze test
The Morris water maze is the gold standard for investigating spatial learning and memory (Figs. 4, 5). The objective was to assess the effect of MLC901 on a hippocampus‐dependent task in which the emotional aspect was less important than in the passive avoidance paradigm.
Figure 4.
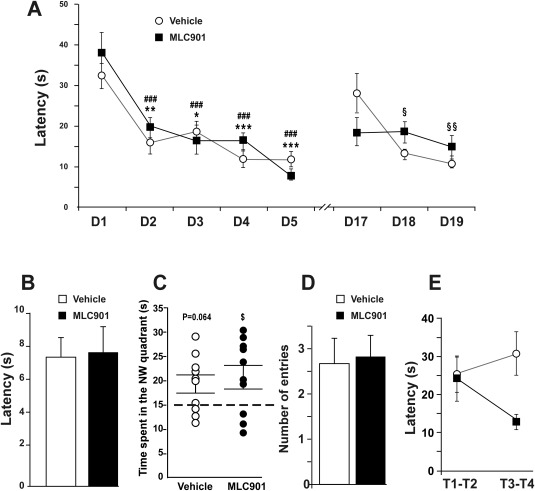
Effect of MLC901 in the Morris water maze test. A: Evolution of the daily average of the latency before reaching the platform over the learning trials (D1–D5) and the relearning trials (D17–D19) in seconds ± SEM. *, comparison with the score performed on the first day of learning (D1) in the vehicle‐treated group; #, comparison with the score performed on the first day of learning (D1) in the MLC901‐treated group; §, comparison with the score performed on the first day of relearning (D17) in the vehicle‐treated group. Single symbol, P < 0.05; double symbols, P < 0.01; triple symbols, P < 0.001. B: Latency before reaching the platform (averaged over four trials) during the cue test in seconds ± SEM. C: Time spent in the NW quadrant during the probe test in seconds ± SEM. Dotted line represents the chance level. $ P < 0.05 compared with the chance level. D: Number of crossings of the previous platform location ± SEM. E: Evolution of the latency before reaching the platform during the first day of the relearning (D17) in seconds ± SEM. T1–T2, average of the two first trial performances; T3–T4, average of the two last trial performances.
Figure 5.
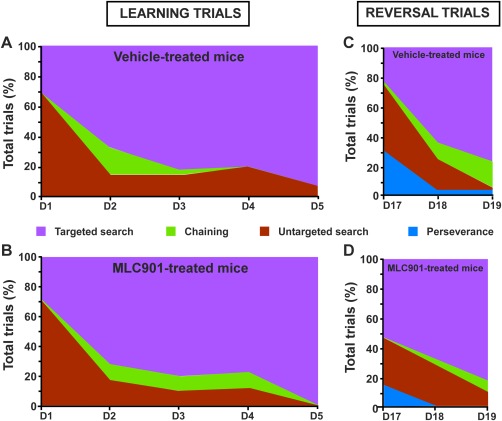
Strategies used in the Morris water maze. A: Learning trial strategies used during the learning trials in the vehicle‐treated mice (A) and B: the MLC901‐treated mice (B). Reversal trial strategies used during the reversal trials by the vehicle‐treated mice (C) and the MLC901‐treated mice (D).
The results obtained in the cue test excluded any specific effect of MLC‐901 on the motivational or motor factors that could influence the latency before reaching the platform (U = 43, P > 0.05, π = 0.05; Fig. 4B). The learning trials resulted in performance increases both in the vehicle‐ and in the MLC901‐treated mice (repeated measure effect F4,72 = 26.69, P < 0.001, π > 0.99; Fig. 4A). The performances per se (treatment effect F1,18 = 0.41, P > 0.05, π = 0.09; Fig. 4A) and their improvement over the learning trials (treatment × repeated measure interaction F4,72 = 1.23, P > 0.05, π = 0.37; Fig. 4A) did not depend on the treatment received. The latency to reach the platform significantly decreased from D2 in both groups compared with their performances on D1 (vehicle‐treated mice P < 0.01 on D1, P < 0.05 on D3, and P < 0.001 on D4 and D5; MLC901‐treated mice P < 0.001 on D2, D3, D4, and D5). In line with the time spent by animals seeking the platform, the strategy progression during the learning trials was highly similar in both groups (Fig. 5). The MLC901‐treated mice exhibited a significant preference for the NW quadrant on the probe test, suggesting that they collectively remembered the area where the platform had been before it was removed (comparison with the chance level U = 47, P < 0.05, π = 0.5; Fig. 4C). This was not the case with the vehicle‐treated mice (comparison with the chance level U = 46, P = 0.064, π = 0.48; Fig. 4C). Nevertheless, the raw data were highly similar for both groups, and the comparison with the chance level almost reached significance for the vehicle‐treated mice (P = 0.064). Furthermore, there were no significant intergroup difference with regard to the time spent in the NW quadrant of the maze (U = 45, P > 0.05, π = 0.07; Fig. 4C). Likewise, the number of annulus crossings was not significantly modified by MLC901 (U = 48.5, P > 0.05, π = 0.05; Fig. 4D). From the comparisons with the chance level, it is difficult to assume that MLC901 improved remembering of the spatial location of the platform. On the contrary, the treatment significantly influenced the performance evolution over the reversal trials (treatment × repeated measure interaction F2,36 = 3.75, P < 0.05, π = 0.75; Fig. 4A). According to the Bonferroni post hoc approach, only the vehicle‐treated mice reached the platform significantly more quickly on the second and third days of the reversal trials compared with the latency noted on the first day (D18 vs. D17 P < 0.05; D19 vs. D17 P < 0.01; Fig. 4A). Nevertheless, the lack of performance improvement in the MLC901‐treated mice during the 3 days of reversal was probably the result of a floor effect caused by their already very low latency on the first day. Although the post hoc analysis did not detect any significant difference between the mean scores of the two groups on that day, we wondered whether the good performances of the MLC901‐treated mice might reflect a relearning facilitation by the compound. Seeking an answer to this question, we decided to investigate further the potential differences between mouse scores on D17 (i.e., the first day of reversal). The two first trial latencies and the two last trial latencies observed on that day were averaged. The two‐way ANOVA performed on the log10‐transformed data showed that evolution of performances between the first and the last trials was significantly influenced by the treatment administered (F1,18 = 4.91, P < 0.05, π = 0.55; Fig. 4E). Although the very conservative Bonferroni test did not reveal any significant difference between the two groups on the first or last trials, the latency exhibited by the MLC901‐treated mice strongly decreased over time (P = 0.08; Fig. 4E) compared with the vehicle‐treated mice. In addition, the analysis of strategies showed that targeted search strategies were used by mice treated with MLC901 in more than 50% of the trials on D17, whereas the vehicle‐treated mice adopted them in only 25% of their trials (Fig. 5). Conversely, mice treated with vehicle were much more persevering than the MLC901‐treated mice on the same day (twice as many trials with persevering behaviors). Although MLC901 does not modify the spatial learning and memory capacities of mice, it seems that it could enhance the cognitive flexibility necessary for quick relearning.
MLC901 Stimulated Neurogenesis
Increased hippocampal neurogenesis with promoted proliferation, neuronal differentiation, and survival of young neurons could contribute to the procognitive effects of MLC901 (Fig. 6). Our objective was to analyze the survival of newborn neurons long term (7 weeks) rather than only up to 3 weeks, as it had been performed in previous studies (Heurteaux et al., 2010; Quintard et al., 2011, 2014).
Figure 6.
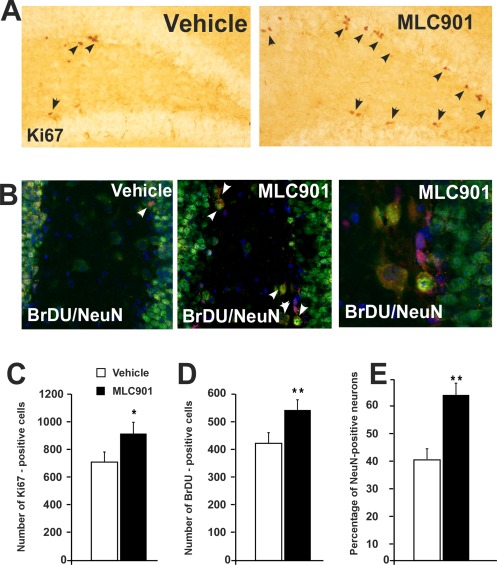
Effect of MLC901 on neurogenesis. A: Representative photographs showing Ki67 in the subgranular zone of the dentate gyrus in vehicle‐ and MLC901‐treated mice (arrowheads). B: Representative photographs showing BrdU/NeuN colocalizations in the subgranular zone of the dentate gyrus in vehicle‐ and MLC901‐treated mice (arrowheads; ×60 left and middle, ×120 right). C,D: Histograms showing the number of Ki67‐ and BrdU‐positive cells counted in the subgranular zone of the dentate gyrus.*P < 0.05, **P < 0.01 comparison between the vehicle‐treated and MLC901‐treated mice. E: Histograms showing the percentage of NeuN‐positive neurons counted in the subgranular zone of the dentate gyrus. **P < 0.01 comparison between the vehicle‐treated and MLC901‐treated mice.
The goal of Ki67 staining was to verify whether pro‐neurogenesis effect of MLC901 remained present after almost 2 months of treatment. The timing of BrDU administration (see Fig. 1D) was chosen to determine whether newborn neurons survive for at least 7 weeks and reach complete maturity. MLC901‐treated mice exhibited a significantly larger population of Ki67‐labeled cells in the dentate gyrus than the vehicle‐treated mice (U = 21, P < 0.05, π = 0.55), demonstrating that the compound still stimulated cell proliferation in the hippocampus after almost 2 months of administration (Fig. 6A–C). The significant increase in the number of hippocampal BrdU‐positive cells induced by MLC901 (U = 14, P < 0.01, π = 0.5) was in the same proportion as that for the Ki67‐positive cells (Fig. 6d), indicating that the survival rate of the hippocampal newborn cells after 7 weeks was similar in both groups. Furthermore, MLC901 promoted the differentiation of newborn cells into neurons because 63% of the BrdU‐positive cells were also positive for NeuN in MLC901‐treated mice, whereas only 40% of the BrdU‐positive cells were also positive for NeuN in vehicle‐treated mice (U = 1.5, P < 0.01, π = 0.89; Fig. 6B–E).
DISCUSSION
Previous studies have shown that MLC901 could promote both motor and cognitive recovery in rodent models of stroke, cardiac arrest, and TBI (Heurteaux et al., 2010; Quintard et al., 2011, 2014). However, preliminary results strongly suggest that MLC901 could exert particular cognitive effects in control mice (Quintard et al., 2014). The present study analyzes, as accurately as possible, the nature and extent of these effects in “normal” mice, in which no pathology (such as ischemia or TBI) was induced.
The proneurogenesis properties of MLC901 led us to pay particular attention to hippocampal‐dependent cognitive processes. The passive avoidance paradigm partially relies on such processes (Cimadevilla et al., 2007; O'Sullivan et al., 2007; Quirk and Mueller, 2008; Suarez‐Pereira et al., 2014) and has been extensively used to investigate associative learning and memory both in normal and in genetically or pharmacologically manipulated rodents (Kaplan and Moore, 2011; Huang et al., 2013; Webster et al., 2014). Our results show that the vehicle‐ and the MLC901‐treated mice display almost identical latency before entering the formerly punished compartment on the day after the conditioning trial (i.e., D1 of experiment 1; Fig. 2A), indicating that MLC901 does not modify the associative aversive learning capabilities in animals. On the other hand, the results obtained in experiment 1 (Fig. 2A) clearly show that MLC901 promotes the behavioral extinction of the conditioned response across the extinction training. Behavioral extinction is underlain by two nonexclusive processes, the weakening of fear‐conditioning memory on the one hand and extinction on the other. Extinction is an active mechanism that does not, or does not totally, erase fear conditioning memory but allows inhibition of a conditioned response that has become inappropriate (Myers and Davis, 2007; Davis, 2011; Kaplan and Moore, 2011). The latent memory of the conditioned response is evidenced by its spontaneous recovery, which usually happens a few days after termination of the extinction training (Huang et al., 2013). The lack of such a recovery, both in the vehicle‐ and in the MLC901‐treated mice 7 days after the last extinction trial, is probably a result of the fact that the long extinction training applied in this work resulted in a too robust extinction memory. Nevertheless, the reinstatement effect, i.e., the resurgence of the extinguished conditioned response caused by the administration of shock in a context different from the conditioning context, observed on D31 in experiment 2 demonstrated that fear conditioning memory was maintained in spite of the extinction training in both groups (Fig. 2B). We excluded that the latency decrease over extinction training was the result of locomotor hyperactivity because MLC901‐treated mice were found to move less than vehicle‐treated mice in the device before the conditioning trial. As a potential bias, such a locomotion decrease was expected to promote increased latencies of entry into the dark compartment (Fig. 2). In contrast to observations from the passive avoidance device, the data collected in the actimeter did not detect any influence of the compound on locomotor activity per se. Moreover, the number of movements recorded during the first hours spent in this apparatus as well as the number of closed‐arm entries in the elevated plus maze ruled out that MLC901 influenced novelty‐induced changes in exploratory activity. Although the locomotor activity modifications observed on the day before the conditioning in the passive avoidance test remain questionable, it is not very probable that the MLC901 effect on behavioral extinction was caused by specific motor alterations. Beyond motor activity, the level of unconditioned anxiety is also known to influence mouse behavior in the passive avoidance test. The elevated plus maze test, classically used to evaluate anxiety in rodents (Hogg, 1996; Griebel et al., 1997), did not reveal any MLC901‐induced alteration of unconditioned anxiety. Together, these results suggest that the MLC901 effect on behavioral extinction is purely cognitive and is due mainly to the extinction process. This functional profile is of great interest because the manipulation of extinction is a major way of curing those anxiety‐related disorders in which the fear‐conditioning process plays a central role, such as posttraumatic stress disorder (PTSD) and phobias (Boschen et al., 2009; Sehlmeyer et al., 2009; Fitzgerald et al., 2014). To date, several pharmacological enhancers of behavioral extinction have been studied (Delgado et al., 2006; Davis, 2011; Litz et al., 2012; de Kleine et al., 2013; Parsons and Ressler, 2013; Difede et al., 2014). However, their beneficial action was limited, insufficiently proved, or accompanied by disabling side effects (Litz et al., 2012; de Kleine et al., 2013). Thus, MLC901, because of its high degree of safety (Young et al., 2010), could represent an interesting alternative to these treatments. Nevertheless, the present work demonstrates that MLC901 promotes active extinction in “normal” mice. Additional studies on specific disease models are required to identify MLC901 definitively as a potential treatment for PTSD and other anxiety‐related disorders.
Extinction involves intricate neurobiological mechanisms and would particularly depend on the modulation of amygdala activity by projections from the infralimbic cortex (Quirk and Mueller, 2008; Herry et al., 2010; Pape and Pare, 2010). However, functional modifications of the amygdala are observed in PTSD (Yehuda and LeDoux, 2007). For these reasons, further evaluation of MLC901 in behavioral tasks specifically revealing amygdala function, such as cued fear conditioning (Phillips and LeDoux, 1992), is desirable. Complementary studies with tests accurately modeling etiological factors and physiological symptoms of PTSD, such as predator‐based psychosocial stress or predator scent stress (Daskalakis et al., 2013), are also required to confirm the benefits of MLC901 for treating these kinds of anxiety disorders. In addition to the amygdala, the hippocampus is critically involved in PTSD (Yehuda and LeDoux, 2007). In this respect, experiments with functional ablation have suggested that hippocampal neurogenesis actively contributes to contextual extinction of footshock‐induced fear conditioning (Pan et al., 2012). Thus, the large decrease (about 65%) of adult‐born neurons induced by the temporally controlled deletion of extracellular signal‐related kinase 5 in the neurogenic zone of the hippocampus results in the failure of extinction following repeated exposures to the training context without footshocks (Pan et al., 2012).
The stimulation of hippocampal neurogenesis and the promotion of differentiation of newborn cells in neurons by MLC901 have been repeatedly demonstrated (Heurteaux et al., 2010; Quintard et al., 2011, 2014). The present study further shows that the stimulatory effect of MLC901 is persistent because it is still present after almost 2 months of treatment and that newborn neurons survive for at least 7 weeks, i.e., probably reach complete electrophysiological maturity (Ehninger and Kempermann, 2008). The facilitatory action of MLC901 on extinction could thus rely, in part, on its proneurogenesis properties. Additional investigations with different timings of neurogenesis suppression are required to confirm such a hypothesis and to determine the stage of maturity of the newborn neurons that are potentially involved. In addition to neurogenesis, it has previously been shown that cortical BDNF is an important determinant for efficient extinction (Andero and Ressler, 2012; Psotta et al., 2013). Behavioral extinction can occur to some extent without extinction training. This particular phenomenon has been demonstrated to require the increase of hippocampal‐originating BDNF in the prefrontal cortex. The prior infusion of a BDNF‐inactivating antibody into the infralimbic medial prefrontal cortex suppressed the fear extinction induced by hippocampal infusion of BDNF (Peters et al., 2010). Then, MLC901‐induced cortical increase of BDNF (Heurteaux et al., 2010) could contribute to the decrease of the conditioned response observed in MLC901‐treated mice compared with vehicle‐treated mice 7 days after the conditioning trial, whereas no extinction training was administered (experiment 2; Fig. 2B). Reconditioning, which consists of conditioning under conditions identical to those of initial conditioning after a variable delay, is known to potentiate the conditioned response (An et al., 2012). Beyond its stimulatory effect on behavioral extinction itself, MLC901 favors extinction memory over such a potentiation (Fig. 2A). Because relapse and sensitization to stressful events are major concerns in anxiety‐related disorders involving fear conditioning‐related mechanisms (Boschen et al., 2009), the extinction memory‐promoting effect of MLC901 could support a potential therapeutic use in these disorders.
To characterize the behavioral consequences of chronic MLC901 administration further and also to exclude any amnestic effect, we compared the performances of the vehicle‐ and the MLC901‐treated mice in the Morris water maze and novel object recognition tests. Not only was no impairment noted in the novel object recognition task, but MLC901 seemed even to improve the performances of mice in the challenging version of the test that we used, indicating that the compound could exert procognitive effects on familiarity memory, a hippocampus‐independent process (Dere et al., 2007; Barker and Warburton, 2011; Brown et al., 2012). The Morris water maze test is the gold standard for the assessment of spatial reference memory. For this reason, numerous studies dealing with the effect of hippocampal neurogenesis modification on the rodent performances in this test have been performed (Dupret et al., 2008; Garthe et al., 2009, 2014; Pan et al., 2012). Although most experiments with neurogenesis ablation have supported a role for newborn hippocampal neurons in spatial learning and memory, some negative results have been published. For example, cyclin D2 knockout mice with profound inhibition of hippocampal cell proliferation did not exhibit any impairment affecting the classical variables noted in the Morris water maze (Jaholkowski et al., 2009). Nevertheless, the same mice were unable to adopt spatially precise strategies across training compared with their controls (Garthe et al., 2014). Consistent with these data, age‐related decline of hippocampal neurogenesis has been proved to correlate with diminished efficiency of spatial search strategies (Gil‐Mohapel et al., 2013).
MLC901 changed neither the learning rate (Fig. 4A) nor the spatial memory (Fig. 4C,D) of mice in the Morris water maze. In line with these results, analysis of detailed search strategies used by the mice to find the platform (Supp. Info. Fig. 2) failed to show a positive effect of MLC901 on spatial learning and memory (Fig. 5A,B). In contrast, both latencies (Fig. 4A,E) and search strategies (Fig. 5C,D) used by mice indicated that MLC901 accelerated reversal learning. MLC901‐treated mice were able to find the new location of the platform more quickly by using a more efficient strategy than vehicle‐treated mice. Reversal learning, which is highly dependent on cognitive flexibility, was demonstrated to be impaired by neurogenesis ablation (Garthe et al., 2009; Pan et al., 2012). Therefore, it is possible that the effect of MLC901 on cognitive flexible processing could be partially the result of its stimulatory action on hippocampal neurogenesis. Nevertheless, it has to be kept in mind that the present study shows a potential correlation between behavioral effects and neurogenic properties of MLC901 and not a causal link. In line with our previous studies, the present work demonstrates that a long‐term MLC901 treatment results in an increased number of adult‐born neurons at all stages of maturity, from young, highly plastic neurons to completely mature neurons. In future investigations, temporally controlled inhibition of neurogenesis with genetic, chemical, or X‐ray‐based methods could allow deciphering of the causal contribution of these different newborn neurons to the behavioral effect of MLC901 that we have identified. The administration of BrdU during the learning process could also be more informative for showing a direct role of MLC901‐induced neurogenesis in spatial memory performance.
In conclusion, the present work shows that MLC901 induces various interesting behavioral effects. Its proneurogenesis properties are potentially involved in most of these effects but cannot explain all reported results. From the passive avoidance and Morris water maze tests, it seems that a salient aspect of MLC901 behavioral effects is the promotion of fear extinction and, more generally, of cognitive flexibility, i.e., the cognitive processing underlying the adaptation to environmental changes. As a consequence, MLC901 could be shown to have a potential benefit in the treatment of neuropsychiatric traits such as phobias or PTSD, in which extinction enhancement is a therapeutic option. Moreover, given that cognitive impairments, in particular the loss of cognitive flexibility, and PTSD are common disabling consequences of TBI and stroke (Tate, 1999; Bryant et al., 2010; Edmondson et al., 2013; Kleinman et al., 2013; Williamson et al., 2013; Goldfinger et al., 2014), the results of the present work also provide supplementary evidence for the potential relevance of MLC901 as a treatment for these pathologies. Additional studies investigating more specifically the beneficial effects of MLC901 in specific models of PTSD and anxiety‐related disorders are certainly warranted.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
M.L. is vice‐president for research of Moleac.
REFERENCES
- An B, Hong I, Choi S. 2012. Long‐term neural correlates of reversible fear learning in the lateral amygdala. J Neurosci 32:16845–16856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Ressler KJ. 2012. Fear extinction and BDNF: translating animal models of PTSD to the clinic. Genes Brain Behav 11:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC. 2011. When is the hippocampus involved in recognition memory? J Neurosci 31:10721–10731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavarsad Shahripour R, Shamsaei G, Pakdaman H, Majdinasab N, Nejad EM, Sajedi SA, Norouzi M, Hemmati A, Manouchehri RH, Shiravi A. 2011. The effect of NeuroAiD (MLC601) on cerebral blood flow velocity in subjects’ postbrain infarct in the middle cerebral artery territory. Eur J Intern Med 22:509–513. [DOI] [PubMed] [Google Scholar]
- Boschen MJ, Neumann DL, Waters AM. 2009. Relapse of successfully treated anxiety and fear: theoretical issues and recommendations for clinical practice. Aust N Z J Psychiatry 43:89–100. [DOI] [PubMed] [Google Scholar]
- Brown MW, Barker GR, Aggleton JP, Warburton EC. 2012. What pharmacological interventions indicate concerning the role of the perirhinal cortex in recognition memory. Neuropsychologia 50:3122–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, O'Donnell ML, Creamer M, McFarlane AC, Clark CR, Silove D. 2010. The psychiatric sequelae of traumatic injury. Am J Psychiatry 167:312–320. [DOI] [PubMed] [Google Scholar]
- Caroni P, Donato F, Muller D. 2012. Structural plasticity upon learning: regulation and functions. Nat Rev Neurosci 13:478–490. [DOI] [PubMed] [Google Scholar]
- Chen C, Venketasubramanian N, Gan RN, Lambert C, Picard D, Chan BP, Chan E, Bousser MG, Xuemin S. 2009. Danqi piantang jiaonang (DJ), a traditional Chinese medicine, in poststroke recovery. Stroke 40:859–863. [DOI] [PubMed] [Google Scholar]
- Chen CL, Young SH, Gan HH, Singh R, Lao AY, Baroque AC 2nd, Chang HM, Hiyadan JH, Chua CL, Advincula JM, Muengtaweepongsa S, Chan BP, de Silva HA, Towanabut S, Suwanwela NC, Poungvarin N, Chankrachang S, Wong KS, Eow GB, Navarro JC, Venketasubramanian N, Lee CF, Bousser MG. 2013a. Chinese medicine neuroaid efficacy on stroke recovery: a double‐blind, placebo‐controlled, randomized study. Stroke 44:2093–2100. [DOI] [PubMed] [Google Scholar]
- Chen CL, Venketasubramanian N, Lee CF, Wong KS, Bousser MG. 2013b. Effects of MLC601 on early vascular events in patients after stroke: the CHIMES study. Stroke 44:3580–3583. [DOI] [PubMed] [Google Scholar]
- Y Chen, X Wu, S Yu, X Lin, J Wu, L Li, J Zhao, Y Zhao. 2012. Neuroprotection of tanshinone IIA against cerebral ischemia/reperfusion injury through inhibition of macrophage migration inhibitory factor in rats. PLoS One 7:e40165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadevilla JM, Mendez M, Mendez‐Lopez M, Arias JL. 2007. Unilateral hippocampal blockade reveals that one hippocampus is sufficient for learning a passive avoidance task. J Neurosci Res 85:1138–1142. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Yehuda R, Diamond DM. 2013. Animal models in translational studies of PTSD. Psychoneuroendocrinology 38:1895–1911. [DOI] [PubMed] [Google Scholar]
- Davis M. 2011. NMDA receptors and fear extinction: implications for cognitive behavioral therapy. Dialogues Clin Neurosci 13:463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kleine RA, Rothbaum BO, van Minnen A. 2013. Pharmacological enhancement of exposure‐based treatment in PTSD: a qualitative review. Eur J Psychotraumatol 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. 2006. Extending animal models of fear conditioning to humans. Biol Psychol 73:39–48. [DOI] [PubMed] [Google Scholar]
- Denny CA, Burghardt NS, Schachter DM, Hen R, Drew MR. 2012. 4‐ to 6‐week‐old adult‐born hippocampal neurons influence novelty‐evoked exploration and contextual fear conditioning. Hippocampus 22:1188–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. 2007. The pharmacology, neuroanatomy, and neurogenetics of one‐trial object recognition in rodents. Neurosci Biobehav Rev 31:673–704. [DOI] [PubMed] [Google Scholar]
- Difede J, Cukor J, Wyka K, Olden M, Hoffman H, Lee FS, Altemus M. 2014. D‐cycloserine augmentation of exposure therapy for posttraumatic stress disorder: a pilot randomized clinical trial. Neuropsychopharmacology 39:1052–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovina NI, Red'kina AV. 2012. [Latent inhibition and extinction of passive avoidance in mice C57BL/6J AND DBA/2J]. Ross Fiziol Zh Im I M Sechenova 98:488–496. [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. 2008. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One 3:e1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D, Richardson S, Fausett JK, Falzon L, Howard VJ, Kronish IM. 2013. Prevalence of PTSD in survivors of stroke and transient ischemic attack: a meta‐analytic review. PLoS One 8:e66435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. 2008. Neurogenesis in the adult hippocampus. Cell Tissue Res 331:243–250. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ, Seemann JR, Maren S. 2014. Can fear extinction be enhanced? A review of pharmacological and behavioral findings. Brain Res Bull 105:46–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke TF, Kaplan DR, Cantley LC. 1997. PI3K: downstream AKTion blocks apoptosis. Cell 88:435–437. [DOI] [PubMed] [Google Scholar]
- Gan R, Lambert C, Lianting J, Chan ES, Venketasubramanian N, Chen C, Chan BP, Samama MM, Bousser MG. 2008. Danqi Piantan Jiaonang does not modify hemostasis, hematology, and biochemistry in normal subjects and stroke patients. Cerebrovasc Dis 25:450–456. [DOI] [PubMed] [Google Scholar]
- Garthe A, Kempermann G. 2013. An old test for new neurons: refining the Morris water maze to study the functional relevance of adult hippocampal neurogenesis. Front Neurosci 7:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. 2009. Adult‐generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One 4:e5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthe A, Huang Z, Kaczmarek L, Filipkowski RK, Kempermann G. 2014. Not all water mazes are created equal: cyclin D2 knockout mice with constitutively suppressed adult hippocampal neurogenesis do show specific spatial learning deficits. Genes Brain Behav 13:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil‐Mohapel J, Brocardo PS, Choquette W, Gothard R, Simpson JM, Christie BR. 2013. Hippocampal neurogenesis levels predict WATERMAZE search strategies in the aging brain. PLoS One 8:e75125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinger JZ, Edmondson D, Kronish IM, Fei K, Balakrishnan R, Tuhrim S, Horowitz CR. 2014. Correlates of posttraumatic stress disorder in stroke survivors. J Stroke Cerebrovasc Dis 23:1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T, Trouche S, Massou I, Verret L, Zerwas M, Roullet P, Rampon C. 2010. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience 171:769–778. [DOI] [PubMed] [Google Scholar]
- Griebel G, Rodgers RJ, Perrault G, Sanger DJ. 1997. Risk assessment behaviour: evaluation of utility in the study of 5‐HT‐related drugs in the rat elevated plus‐maze test. Pharmacol Biochem Behav 57:817–827. [DOI] [PubMed] [Google Scholar]
- Harandi AA, Abolfazli R, Hatemian A, Ghragozlee K, Ghaffar‐Pour M, Karimi M, Shahbegi S, Pakdaman H, Tabasi M, Tabatabae AL, Nourian A. 2011. Safety and efficacy of MLC601 in Iranian patients after stroke: a double‐blind, placebo‐controlled clinical trial. Stroke Res Treat 2011:721613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. 2010. Neuronal circuits of fear extinction. Eur J Neurosci 31:599–612. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, Noble F, Blondeau N, Widmann C, Borsotto M, Gobbi G, Vaugeois JM, Debonnel G, Lazdunski M. 2006. Deletion of the background potassium channel TREK‐1 results in a depression‐resistant phenotype. Nat Neurosci 9:1134–1141. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Gandin C, Borsotto M, Widmann C, Brau F, Lhuillier M, Onteniente B, Lazdunski M. 2010. Neuroprotective and neuroproliferative activities of NeuroAiD (MLC601, MLC901), a Chinese medicine, in vitro and in vivo. Neuropharmacology 58:987–1001. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Widmann C, Moha ou Maati H, Quintard H, Gandin C, Borsotto M, Veyssiere J, Onteniente B, Lazdunski M. 2013. NeuroAiD: properties for neuroprotection and neurorepair. Cerebrovasc Dis 35(Suppl 1):1–7. [DOI] [PubMed] [Google Scholar]
- Hogg S. 1996. A review of the validity and variability of the elevated plus‐maze as an animal model of anxiety. Pharmacol Biochem Behav 54:21–30. [DOI] [PubMed] [Google Scholar]
- Huang AC, Shyu BC, Hsiao S, Chen TC, He AB. 2013. Neural substrates of fear conditioning, extinction, and spontaneous recovery in passive avoidance learning: a c‐fos study in rats. Behav Brain Res 237:23–31. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. 2008. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci 11:1153–1161. [DOI] [PubMed] [Google Scholar]
- Jaholkowski P, Kiryk A, Jedynak P, Ben Abdallah NM, Knapska E, Kowalczyk A, Piechal A, Blecharz‐Klin K, Figiel I, Lioudyno V, Widy‐Tyszkiewicz E, Wilczynski GM, Lipp HP, Kaczmarek L, Filipkowski RK. 2009. New hippocampal neurons are not obligatory for memory formation; cyclin D2 knockout mice with no adult brain neurogenesis show learning. Learn Mem 16:439–451. [DOI] [PubMed] [Google Scholar]
- TK Kao, CY Chang, YC Ou, WY Chen, YH Kuan, HC Pan, SL Liao, GZ Li, CJ Chen. 2013. Tetramethylpyrazine reduces cellular inflammatory response following permanent focal cerebral ischemia in rats. Exp Neurol 247:188–201. [DOI] [PubMed] [Google Scholar]
- Kalm M, Karlsson N, Nilsson MK, Blomgren K. 2013. Loss of hippocampal neurogenesis, increased novelty‐induced activity, decreased home cage activity, and impaired reversal learning one year after irradiation of the young mouse brain. Exp Neurol 247:402–409. [DOI] [PubMed] [Google Scholar]
- Kaplan GB, Moore KA. 2011. The use of cognitive enhancers in animal models of fear extinction. Pharmacol Biochem Behav 99:217–228. [DOI] [PubMed] [Google Scholar]
- Koh PO. 2013. Ferulic acid attenuates the injury‐induced decrease of protein phosphatase 2A subunit B in ischemic brain injury. PLoS One 8:e54217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Linden DJ. 2007. Ubiquitous plasticity and memory storage. Neuron 56:582–592. [DOI] [PubMed] [Google Scholar]
- Kleinman JT, DuBois JC, Newhart M, Hillis AE. 2013. Disentangling the neuroanatomical correlates of perseveration from unilateral spatial neglect. Behav Neurol 26:131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong KH, Wee SK, Ng CY, Chua K, Chan KF, Venketasubramanian N, Chen C. 2009. A double‐blind, placebo‐controlled, randomized phase II pilot study to investigate the potential efficacy of the traditional chinese medicine Neuroaid (MLC 601) in enhancing recovery after stroke (TIERS). Cerebrovasc Dis 28:514–521. [DOI] [PubMed] [Google Scholar]
- Liss B, Roeper J. 2001. Molecular physiology of neuronal K‐ATP channels [review]. Mol Membr Biol 18:117–127. [PubMed] [Google Scholar]
- Litz BT, Salters‐Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, Hofmann SG. 2012. A randomized placebo‐controlled trial of D‐cycloserine and exposure therapy for posttraumatic stress disorder. J Psychiatr Res 46:1184–1190. [DOI] [PubMed] [Google Scholar]
- Moha Ou Maati H, Borsotto M, Chatelain F, Widmann C, Lazdunski M, Heurteaux C. 2012. Activation of ATP‐sensitive potassium channels as an element of the neuroprotective effects of the traditional Chinese medicine MLC901 against oxygen glucose deprivation. Neuropharmacology 63:692–700. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. 2007. Mechanisms of fear extinction. Mol Psychiatry 12:120–150. [DOI] [PubMed] [Google Scholar]
- KN Nam, KP Kim, KH Cho, WS Jung, JM Park, SY Cho, SK Park, TH Park, YS Kim, EH Lee. 2013. Prevention of inflammation‐mediated neurotoxicity by butylidenephthalide and its role in microglial activation. Cell Biochem Funct 31:707–712. [DOI] [PubMed] [Google Scholar]
- Navarro JC, Chen CL, Lagamayo PD, Geslani MB, Eow GB, Poungvarin N, de Silva A, Wong LK, Venketasubramanian N. 2013. CHIMES‐I: sub‐group analyzes of the effects of NeuroAiD according to baseline brain imaging characteristics among patients randomized in the CHIMES study. Int J Stroke 8:491–494. [DOI] [PubMed] [Google Scholar]
- Navarro JC, Gan HH, Lao AY, Baroque AC 2nd, Hiyadan JH, Chua CL, San Jose MC, Advincula JM, Lee CF, Bousser MG, Chen CL. 2014. Baseline characteristics and treatment response of patients from the Philippines in the CHIMES study. Int J Stroke 9(Suppl A100):102–105. [DOI] [PubMed] [Google Scholar]
- O'Sullivan NC, McGettigan PA, Sheridan GK, Pickering M, Conboy L, O'Connor JJ, Moynagh PN, Higgins DG, Regan CM, Murphy KJ. 2007. Temporal change in gene expression in the rat dentate gyrus following passive avoidance learning. J Neurochem 101:1085–1098. [DOI] [PubMed] [Google Scholar]
- Ottoni EB. 2000. EthoLog 2.2: a tool for the transcription and timing of behavior observation sessions. Behav Res Methods Instrum Comput 32:446–449. [DOI] [PubMed] [Google Scholar]
- Pan YW, Chan GC, Kuo CT, Storm DR, Xia Z. 2012. Inhibition of adult neurogenesis by inducible and targeted deletion of ERK5 mitogen‐activated protein kinase specifically in adult neurogenic regions impairs contextual fear extinction and remote fear memory. J Neurosci 32:6444–6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YW, Storm DR, Xia Z. 2013. Role of adult neurogenesis in hippocampus‐dependent memory, contextual fear extinction and remote contextual memory: new insights from ERK5 MAP kinase. Neurobiol Learn Mem 105:81–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. 2010. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev 90:419–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Ressler KJ. 2013. Implications of memory modulation for posttraumatic stress and fear disorders. Nat Neurosci 16:146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Dieppa‐Perea LM, Melendez LM, Quirk GJ. 2010. Induction of fear extinction with hippocampal‐infralimbic BDNF. Science 328:1288–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. 1992. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci 106:274–285. [DOI] [PubMed] [Google Scholar]
- Psotta L, Lessmann V, Endres T. 2013. Impaired fear extinction learning in adult heterozygous BDNF knockout mice. Neurobiol Learn Mem 103:34–38. [DOI] [PubMed] [Google Scholar]
- Quintard H, Borsotto M, Veyssiere J, Gandin C, Labbal F, Widmann C, Lazdunski M, Heurteaux C. 2011. MLC901, a traditional Chinese medicine, protects the brain against global ischemia. Neuropharmacology 61:622–631. [DOI] [PubMed] [Google Scholar]
- Quintard H, Lorivel T, Gandin C, Lazdunski M, Heurteaux C. 2014. MLC901, a traditional Chinese medicine, induces neuroprotective and neuroregenerative benefits after traumatic brain injury in rats. Neuroscience 277:72–86. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. 2008. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology 33:56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. 2004. Radiation‐induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol 188:316–330. [DOI] [PubMed] [Google Scholar]
- Schulz‐Klaus B, Lessmann V, Endres T. 2013. BDNF‐dependent consolidation of fear memories in the perirhinal cortex. Front Behav Neurosci 7:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. 2009. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One 4:e5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui FJ, Venketasubramanian N, Chan ES, Chen C. 2013. Efficacy and safety of MLC601 (NeuroAiDTM), a traditional Chinese medicine, in poststroke recovery: a systematic review. Cerebrovasc Dis 35(Suppl 1):8–17. [DOI] [PubMed] [Google Scholar]
- Sik A, van Nieuwehuyzen P, Prickaerts J, Blokland A. 2003. Performance of different mouse strains in an object recognition task. Behav Brain Res 147:49–54. [DOI] [PubMed] [Google Scholar]
- Siow CH. 2008. Neuroaid in stroke recovery. Eur Neurol 60:264–266. [DOI] [PubMed] [Google Scholar]
- Suarez‐Pereira I, Canals S, Carrion AM. 2014. Adult newborn neurons are involved in learning acquisition and long‐term memory formation: the distinct demands on temporal neurogenesis of different cognitive tasks. Hippocampus 25:51–61. [DOI] [PubMed] [Google Scholar]
- Q Tang, R Han, H Xiao, J Shen, Q Luo, J Li. 2012. Neuroprotective effects of tanshinone IIA and/or tetramethylpyrazine in cerebral ischemic injury in vivo and in vitro Brain Res 1488:81–91. [DOI] [PubMed] [Google Scholar]
- Tate RL. 1999. Executive dysfunction and characterological changes after traumatic brain injury: two sides of the same coin? Cortex 35:39–55. [DOI] [PubMed] [Google Scholar]
- Tsai MC, Chang CP, Peng SW, Jhuang KS, Fang YH, Lin MT, Tsao TC. 2014. Therapeutic efficacy of Neuro AiD (MLC 601), a traditional Chinese medicine, in experimental traumatic brain injury. J Neuroimmune Pharmacol 10:45–54. [DOI] [PubMed] [Google Scholar]
- Venketasubramanian N, Chen CL, Gan RN, Chan BP, Chang HM, Tan SB, Picard D, Navarro JC, Baroque AC 2nd, Poungvarin N, Donnan GA, Bousser MG. 2009. A double‐blind, placebo‐controlled, randomized, multicenter study to investigate Chinese medicine NeuroAiD efficacy on stroke recovery (CHIMES Study). Int J Stroke 4:54–60. [DOI] [PubMed] [Google Scholar]
- Webster SJ, Bachstetter AD, Nelson PT, Schmitt FA, Van Eldik LJ. 2014. Using mice to model Alzheimer's dementia: an overview of the clinical disease and the preclinical behavioral changes in 10 mouse models. Front Genet 5:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson JB, Heilman KM, Porges EC, Lamb DG, Porges SW. 2013. A possible mechanism for PTSD symptoms in patients with traumatic brain injury: central autonomic network disruption. Front Neuroeng 6:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, LeDoux J. 2007. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron 56:19–32. [DOI] [PubMed] [Google Scholar]
- Young SH, Zhao Y, Koh A, Singh R, Chan BP, Chang HM, Venketasubramanian N, Chen C. 2010. Safety profile of MLC601 (NeuroAiD) in acute ischemic stroke patients: a Singaporean substudy of the Chinese medicine neuroaid efficacy on stroke recovery study. Cerebrovasc Dis 30:1–6. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ, Fields RD, Johansen‐Berg H. 2012. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat Neurosci 15:528–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- P Zhuang, Y Zhang, G Cui, Y Bian, M Zhang, J Zhang, Y Liu, X Yang, AO Isaiah, Y Lin, Y Jiang. 2012. Direct stimulation of adult neural stem/progenitor cells in vitro and neurogenesis in vivo by salvianolic acid B. PLoS One 7:e35636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


