Abstract
The reversibility of pulmonary arterial hypertension (PAH) determines the operability of congenital heart disease (CHD) complicating with PAH, but it lacks a method for evaluating the reversibility. The current study aims to investigate the serum survivin level in irreversible PAH rats and to explore its potential as a biomarker for evaluating the reversibility of PAH in CHD patients. Irreversible PAH rats were characterized by prominent obstructive lesions resulting from the intimal formation, which was associated with decreased apoptosis and increased survivin expression, while reversible PAH rats were featured by medial hypertrophy resulting in mild occlusion, with increased apoptosis and unchanged survivin expression. In addition, the serum survivin was significantly increased in irreversible PAH rats when compared to both reversible PAH and control rats, and a positive correlation of serum survivin with survivin expression in the lung was confirmed. Third, the preoperative serum survivin was significantly higher in patients with irreversible CHD-PAH than in these with reversible CHD-PAH, and significant correlations between the serum survivin and BNP, preoperative pulmonary vascular resistance index, and postoperative mean pulmonary arterial pressure were also identified. In conclusion, the increased survivin level is a feature of irreversible PAH and the serum survivin represents a candidate biomarker reflecting the operability of CHD-PAH patients.
Keywords: congenital heart disease, pulmonary arterial hypertension, apoptosis, biomarker, survivin
Introduction
Pulmonary arterial hypertension (PAH) is a common complication of congenital heart diseases (CHD) with left-to-right shunts and a key factor in identifying the feasibility for the closure of the shunts. Patients with reversible PAH will benefit from the closure of the shunts, while the improper closure of the shunts in patients with irreversible PAH will deteriorate the clinical status. It is very important to distinguish the reversible and irreversible PAH in CHD before the surgery. However, there is no widely accepted method to accurately predict the changes in postoperative pulmonary arterial pressure (PAP) in patients with PAH. Invasive right heart catheterization (RHC) has currently been considered the gold standard for the diagnosis of PAH, but the hemodynamic data acquired by this method were not able to accurately predict the progress of PAH. The morphometric analysis of the lung biopsy may be useful in predicting the late outcomes. PAH secondary to CHD might be reversible when histology shows medial hypertrophy, but irreversible when develops neointimal lesions and/or plexiform lesions.1 The high invasiveness of lung biopsy limited its application in clinical practice and lung biopsy is rarely used in the assessment of operability. Less invasive and more efficient biomarkers for predicting the decrease in postoperative PAP are of great value in managing patients with PAH secondary to CHD and emergently needed.
Vascular remodeling resulting from the accumulation of hyperproliferative and apoptosis-resistant pulmonary artery smooth muscle cells (PASMC) and pulmonary artery endothelial cells is an important pathological feature of PAH.2 Apoptosis plays a key role in the progressive vascular remodeling in the development of PAH and failure of endothelial cell apoptosis might result in apoptosis-resistant and hyperproliferative vascular endothelial cells and smooth muscle cells,3 which is an important mechanism of the irreversibility of PAH. Decreased apoptosis based on caspase-3 activity was shown in endothelial cells isolated from the pulmonary arteries of patients with idiopathic PAH; the anti-apoptotic protein BCL-2 overexpressed in the lung from patients with irreversible pulmonary hypertension (PH) but was not from patients with reversible PH.3 PASMCs from severe PAH patients are resistant to apoptosis and express the anti-apoptotic protein survivin.4 An apoptosis-inducing, anti-tumor drug, daunorubicin, has been shown to be able to reduce pulmonary arterial wall thickness through increasing apoptosis and to induce apoptosis in cultured human PASMCs.5 Apoptosis resistance can develop in malignant tumor progression and apoptosis-related markers have been reported to be able to improve the diagnostic accuracy and provide information on the prognosis. Therefore, considering the cancer-like characteristics of pulmonary vascular cells as over-proliferation and anti-apoptosis in PAH, the biomarkers of apoptosis might be of great value in evaluating reversibility in patients with PAH secondary to CHD.6
Survivin, a member of the inhibitor of apoptosis protein (IAP) family, is virtually undetectable in normal adult differentiated tissues,7 but has been found to be highly expressed in most human tumors8–10 and is a valuable prognostic index in several tumors series.11,12 However, the diagnostic and prognostic significance of survivin in irreversible PAH is unclear. Whether the level of survivin in serum is helpful in predicting the decrease of PAP after shunt closure remains to be tested. In the present study, the correlation between the level of survivin and the extent of pulmonary proliferative lesions was explored in an irreversible PAH rat model induced by monocrotaline injection following left pneumonectomy and the potential of survivin as a biomarker for evaluating the reversibility of PAH in CHD-PAH patient was furtherly investigated as well.
Methods
Animal study
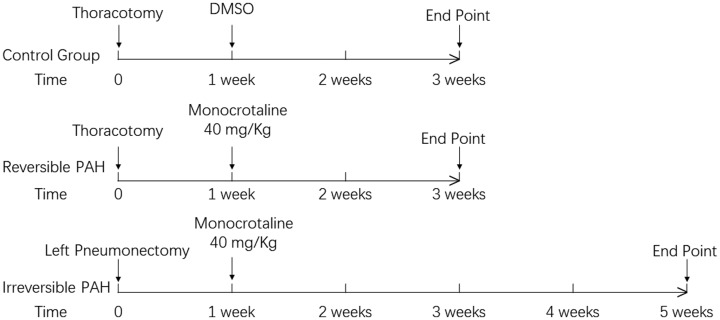
Thirty Sprague-Dawley rats (weighing 300–350 g, from Beijing Weitong Lihua Laboratory Animal Technology Ltd. Co.) were housed in laboratory conditions with free access to food and tap water. They were randomly divided into three groups, each containing 10 rats. Irreversible PAH was induced by subcutaneous injection of monocrotaline at 40 mg/kg (resolved in 0.2 mL DMSO sc, Sigma-Aldrich, USA) one week after left pneumonectomy in rats with the mean weight of 340 g as previously described13 (irreversible PAH group), with intimal formation in pulmonary arteries as its feature in four weeks. Reversible PAH rats underwent low dose of monocrotaline administration (40 mg/kg in 0.2 mL DMSO; reversible PAH group) for two weeks and characterized by medial hypertrophy in pulmonary arteries. Control rats received vehicle (0.2 mL DMSO) and thoracotomy (control group). The time schema for the animal study was shown in Fig 1. This study was approved by the Ethics Committee of Beijing Anzhen Hospital; the methods were in line with the ethical principles.
Fig. 1.
Time schema of animal study. Rats in the control (n = 10) and reversible PAH groups (n = 10) received subcutaneous injection of 0.2 mL DMSO or monocrotaline at 40 mg/kg in 0.2 mL DMSO one week after thoracotomy, respectively. The irreversible PAH rats (n = 10) underwent monocrotaline injection at 40 mg/kg in 0.2 mL DMSO one week after left pneumonectomy. The hemodynamics measurements and tissue harvest were performed three weeks after the beginning of the experiment in the control group and reversible PAH group, and at five weeks in the irreversible PAH group.
Hemodynamics and histopathologic examinations
Both hemodynamics and histopathologic analysis were employed to determine the severity of PAH. Animals were anesthetized with ketamine (60 mg/kg, i.p) and xylazine (3 mg/kg, i.p). A pulmonary artery catheter was inserted from the right carotid vein through right atrium and ventricle for measurement of mean PAP (mPAP) with fluid-filled force transducers. Animals were sacrificed by high-potassium injection after hemodynamics measurement and their lung tissues were harvested for further analysis. The right ventricle (RV) and left ventricle plus septum (LV+S) were also collected and weighed to calculate the ratio of RV/(LV+S), which can act as an indirect indicator of pulmonary artery remodeling. The right upper lobe of the lung was fixed in 10% formalin solutions overnight and then embedded in paraffin. The neointimal formation as the pathological feature of irreversible PAH was identified by elastic Van Gieson stain; vascular occlusion score (VOS) was calculated in 30 vessels from each sample to evaluate the neointimal occlusive lesions following Nishimura's method.14 In addition, quantitative analysis was performed to determine the proportion of vessels suffering from intimal lesions in 30 consecutive vessels in each sample.15 The apoptotic cells were detected by TUNEL method and the percentage of TUNEL positive cells was calculated under a microscope. The expression of survivin in the pulmonary artery was observed by conventional immunohistochemistry using a rabbit anti-rat polyclonal anti-survivin primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA); the percentage of survivin positive immunoreactivity nuclei among 100 cell nuclei in the area of highest immunoreactivity was calculated. All assessments including histological measures and scoring were performed by two authors blinded for grouping to ensure optimal reproducibility.
Western blot analysis
Protein samples were extracted from rat lung tissues. The expression of survivin (SC-10143, 1:1000, clone, 2H5H2, Santa Cruz Biotechnology) was then detected by western blot analysis as described previously,16 with β-actin (Cell Signaling Technology, USA) as an internal control. Immune complexes were visualized with horseradish peroxidase-conjugated secondary antibodies on the ECL Plus system (Amersham Biosciences, USA). Images were scanned, followed by densitometry analysis with Gel-Pro software (Media Cybernetics, USA).
Enzyme-linked immunosorbent assay (ELISA)
Lung tissues were washed with cold saline, minced, and heated in 0.1 M HCl at 100 ℃ for 10 min and then homogenized. After centrifugation at 15,000×g for 30 min, the supernatant was lyophilized and redissolved in 400 μL assay buffer. Serum was also separated according to the instruction of the ELISA kit (PA1-16836, 1:400, Thermo Scientific, USA). Survivin levels in serum and the lung homogenate were determined by ELISA according to the manufacturer's instructions.
Participants
All the participants provided written informed consent from a protocol approved by an institutional review board. Sixty consecutive CHD patients with severe PAH who underwent shunt closure at Beijing Anzhen Hospital were enrolled with their consent between January 2015 and December 2017. All included patients were diagnosed as non-restrictive ventricular septal defects (>1 cm) or atrial septal defects (>2 cm) associated with PAH and were aged >2 years. The pulmonary vascular resistance index (PVRi) calculated from the RHC measurements in the included patients were >4 Woods unit·m2, which suggested that the operability need further evaluation by additional clinical data according to the current guideline.17 The decision regarding operability was based on clinical history, physical examination, and all aspects of non-invasive and invasive evaluation. The characteristics of these patients are shown in Table 1. The included patients were divided into reversible and irreversible groups by whether the mPAP detected via a Swan-Ganz in the pulmonary artery three days after shunt closure decreased to the normal value ( ≤ 25 mmHg).
Table 1.
The clinical features of patients with PAH secondary to CHD.
| Reversible PAH group (n = 38) | Irreversible PAH group (n = 22) | P value | |
|---|---|---|---|
| Age (years) (mean ± SD) | 22.5 ± 8.7 | 29.0 ± 5.7 | <0.05 |
| Sex (M/F) | 17/21 | 9/13 | >0.05 |
| Diagnosis (VSD/ASD) | 17/21 | 14/8 | >0.05 |
| Resting SpO2 (%) (mean ± SD) | 97.6 ± 1.44 | 96.0 ± 1.0 | >0.05 |
| Post-exercise SpO2 (%) (mean ± SD) | 97.1 ± 1.4 | 95.4 ± 1.0 | >0.05 |
| MAP (mmHg) (mean ± SD) | 79.1 ± 3.5 | 80.7 ± 3.3 | >0.05 |
| mPAP (mmHg) (mean ± SD) | 65.8 ± 7.4 | 70.8 ± 6.2 | >0.05 |
| mPAP/MAP (mean ± SD) | 0.83 ± 0.09 | 0.87 ± 0.07 | >0.05 |
| PVRi (Wood unit) (mean ± SD) | 7.6 ± 1.6 | 8.0 ± 2.0 | >0.05 |
| Qp/Qs (mean ± SD) | 1.4 ± 0.2 | 1.3 ± 0.2 | >0.05 |
| AVT (P/N) | 10/28 | 6/16 | >0.05 |
| Targeted therapy (Y/N) | 34/4 | 20/2 | >0.05 |
PAH, pulmonary arterial hypertension; CHD, congenital heart disease; VSD, ventricular septal defect; ASD, atrial septal defect; MAP, mean arterial pressure; mPAP, mean pulmonary arterial pressure; PVRi, pulmonary vascular resistance index; Qp, pulmonary blood flow; Qs, systemic blood flow; AVT, acute vasoreactivity testing; P, positive; N, negative.
Patients with other cardiac anomalies or risk factors for PAH were excluded from the study. In addition, brain natriuretic peptide (BNP) was detected as references at the same time. Serum was collected from peripheral vein blood before surgery according to the ELISA kit instruction and stored at −80 ℃ until measurements. The serum level of survivin (PA1-16836, 1:400, Thermo Scientific, USA) and BNP (MA1-91673, 1:400, Thermo Scientific, USA) were then determined by ELISA according to the manufacturers' instructions.
Statistics
Values are means ± SD. All statistical analyses were performed with SPSS 23 statistical software (IBM, USA). The quantitative data were analyzed with one-way ANOVA followed by Bonferroni post hoc test or Kruskal–Wallis H test. The categorical data were analyzed using the χ2 test. The diagnostic accuracies of serum survivin and BNP levels were assessed by receiver operating characteristic (ROC) curve analysis, respectively. All points of each ROC curve were used as independent variables in the formula “Index = Sensitivity + Specificity – 1.” Then, the maximum value of the index (Youden's index) was used as a criterion for selecting the optimum cut-off point; the corresponding sensitivity and specificity values were the sensitivity and specificity in predicting the reversibility of PAH after the procedure. The correlations between the concentration of survivin and postoperative PAP and BNP level were evaluated with Pearson correlation coefficient. A P value < 0.05 was considered statistically significant.
Results
More severe PAH developed in irreversible PAH rats
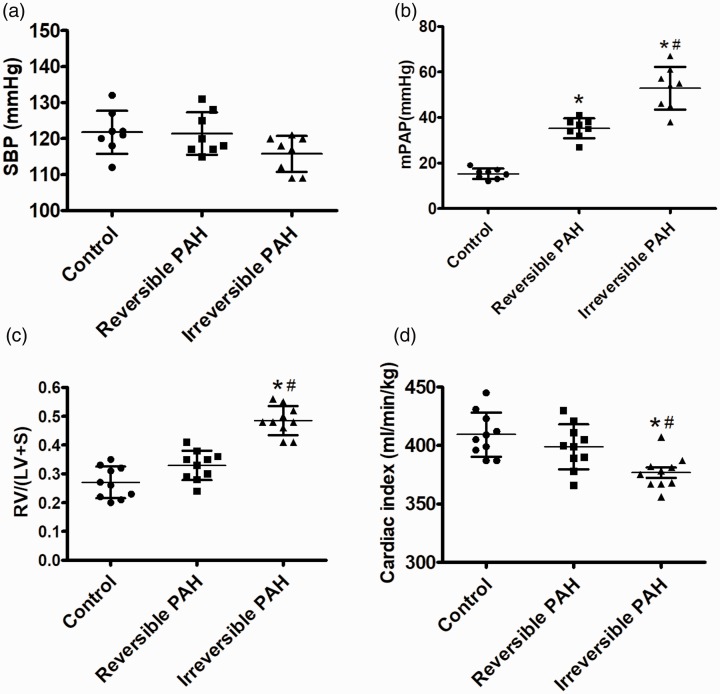
In the present study, the rats in each group have a similar level of systolic blood pressure (control group: 122 ± 6 mmHg; reversible PAH group: 121 ± 6 mmHg; irreversible PAH group: 116 ± 5 mmHg, P > 0.05 among three groups; Fig. 2a). The mPAP was increased in both the reversible PAH rats (35 ± 5 mmHg, P < 0.05) and the irreversible PAH group (53 ± 10 mmHg, P < 0.05) compared to that in the control rats (15 ± 2 mmHg); the difference of mPAP between the reversible PAH and irreversible PAP group was also significant (P < 0.05; Fig. 2b). The increased indexes of right ventricular hypertrophy were found in the irreversible PAH group (0.49 ± 0.05 vs. control group, P < 0.05), but the values of right ventricular hypertrophy index in the reversible PAH and control groups were similar without a difference (0.33 ± 0.05 vs. 0.27 ± 0.06, P > 0.05; Fig. 2c). Cardiac index was normal in both the controls (409 ± 19 mL/min/kg) and reversible PAH rats (399 ± 18 mL/min/kg), but decreased in the irreversible PAH group (377 ± 14 mL/min/kg vs. control or reversible PAH groups, both P < 0.05; Fig. 2d).
Fig. 2.
Increased mPAP and decreased cardiac index in irreversible PAH rats. (a) The systemic arterial pressure measured by catheterizing into the femoral artery were similar among the three groups. (b) The mPAP measured through a pulmonary arterial catheter was increased in rats from both the reversible PAH and irreversible PAH groups when compared to the control group. (c) The right ventricular hypertrophy index indicated by the ratio of RV/(LV+S) were significantly increased in the irreversible PAH group, but changed insignificantly in the reversible PAH group. (d) Cardiac index measured using Fick equation was significantly lower in the irreversible PAH group than in both the control and reversible PAH groups. The differences among the three groups (n = 10 in each group) were analyzed by one-way ANOVA followed by Bonferroni post hoc test. SBP, systemic blood pressure; mPAP, mean pulmonary arterial pressure; RV/(LV+S), right ventricle weight/(left ventricle + septum weight); CI, cardiac index. *P < 0.05 vs. control group, #P < 0.05 vs. reversible PAH group.
Severe occlusive lesions resulted from intimal formation developed in irreversible PAH rats
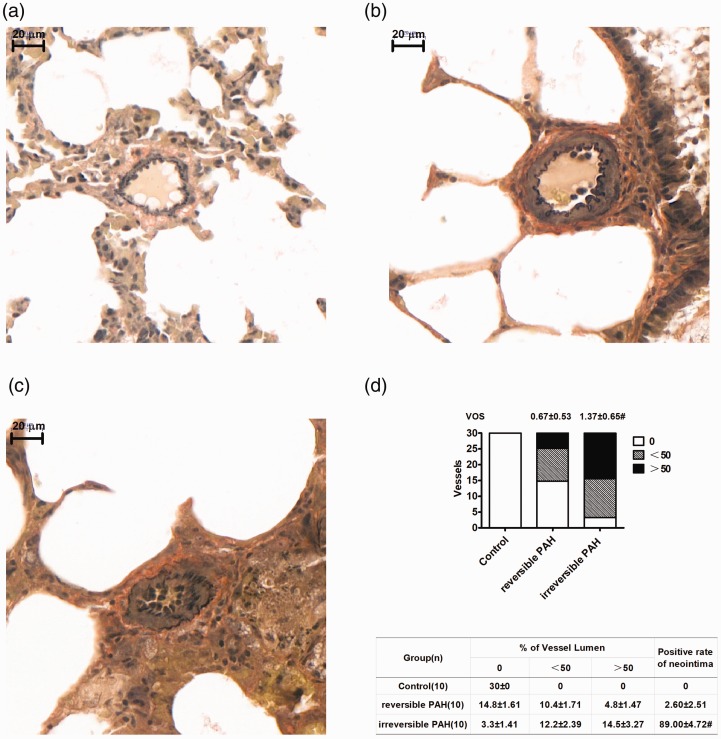
Most of the small pulmonary arteries in the irreversible PAH group rats showed intimal lesions characterized by disappeared intima in elastic Van Gieson's stained lung sections, while the small vessels in reversible PAH rats showed medium hypertrophy with rare intimal formation (Fig. 3a–c and e). In addition, the pulmonary arteries in irreversible PAH rats showed more severe occlusive lesions resulting from intimal formation than that resulted from medium hypertrophy in reversible PAH rats (VOS: reversible PAH group = 0.67 ± 0.53 vs. irreversible PAH group = 1.37 ± 0.65, P < 0.05; Fig. 3d).
Fig. 3.
Neointimal formation resulted in vascular obstructive lesions in small pulmonary arteries from irreversible PAH group rats. (a) Normal small pulmonary arteries without occlusion stained by elastic Van Gieson stain in the control group. (b) Medial hypertrophy with mild occlusion (<50%) in the reversible PAH group. (c) Neointimal formation with prominent occlusion (>50%) in the irreversible PAH group. (d) Significant vascular occlusive lesions assessed by vascular occlusion score developed in small pulmonary arteries from irreversible PAH rats (P < 0.05 vs. control group), while the occlusion was mild in the reversible PAH group with an insignificant difference with that in the control group. (e) The neointimal formation developed in most of investigated small pulmonary arteries from the irreversible PAH group, while only a very small amount of investigated small pulmonary arteries from the reversible PAH group developed neointimal formation. VOS, vascular obstruction score. #P < 0.05 vs. reversible PAH group.
Decreased apoptosis associated with an increase of survivin expression in the pulmonary arteries of irreversible PAH rats
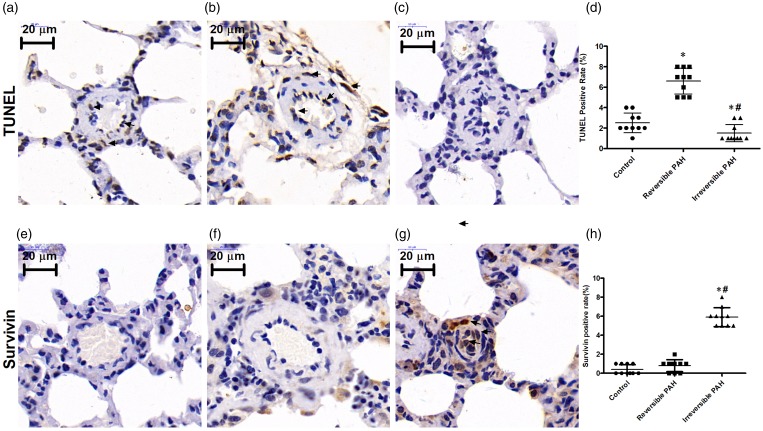
The TUNEL assay indicated that the thickened small pulmonary arteries from reversible PAH rats had increased apoptosis compared to that from the control rats (reversible PAH group: 6.6 ± 1.27% vs. control group: 2.5 ± 0.97%, P < 0.05). However, the small pulmonary arteries with severe proliferative lesions from irreversible PAH rats showed a significant decrease in apoptosis compared to that of reversible PAH rats (irreversible PAH group: 1.5 ± 0.85% vs. reversible PAH group: 6.6 ± 1.27%, P < 0.05), as shown in Fig. 4a–d. Survivin was expressed in the neointima of remodeled small pulmonary arteries in irreversible PAH rats; the number of survivin positive cells was higher than that in reversible PAH rats (irreversible PAH group: 5.9 ± 1.0% vs. reversible PAH group: 0.8 ± 0.6%, P < 0.05; Fig. 4e–h). The small pulmonary arteries from both reversible PAH and control groups showed very low expression of survivin with insignificant difference between them (control group: 0.4 ± 0.33% vs. reversible PAH group: 0.8 ± 0.6%, P > 0.05).
Fig. 4.
Decreased apoptosis associated with increased survivin expression in irreversible PAH rats. (a–c) TUNEL staining in the control group, reversible PAH group, and irreversible PAH group. (d) Apoptosis measured by TUNEL-positive cells increased significantly in the reversible PAH group (P < 0.05 vs. control group) while decreased significantly in the irreversible PAH group. (e–g) Survivin expression measured by immunohistochemical staining in the control group, reversible PAH group, and irreversible PAH group. (h) Survivin expression evaluated by survivin-positive cells was significantly increased in the irreversible PAH group when compared to both the control group and reversible PAH group. The differences among the three groups (n = 10 in each group) were analyzed by one-way ANOVA followed by Bonferroni post hoc test. *P < 0.05 vs. control group, #P < 0.05 vs. reversible PAH group.
The serum level of survivin was correlated to its expression in the whole lung from rats
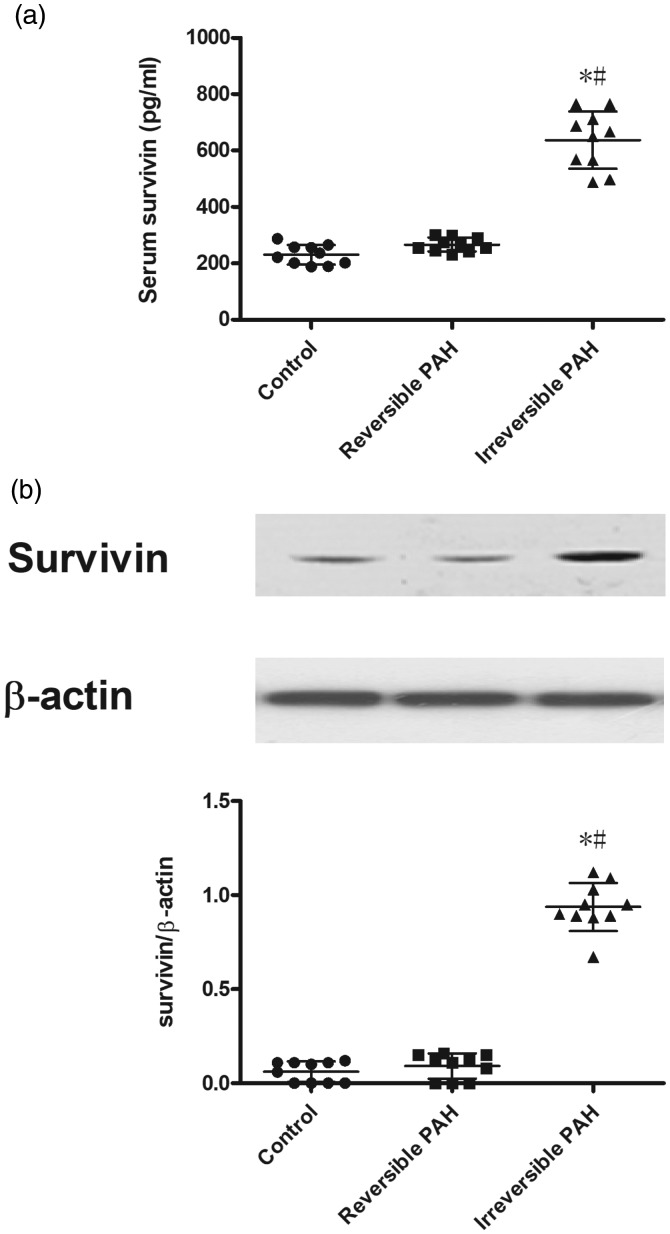
The serum concentration of survivin in irreversible PAH rats was much higher than that in both the control and reversible PAH groups (control group: 230 ± 35 pg/mL vs. irreversible PAH group: 637 ± 102 pg/mL, P < 0.05; reversible PAH group: 267 ± 24 pg/mL vs. 637 ± 102 pg/mL, P < 0.05; Fig. 5a); the difference in serum survivin between the control and reversible PAH groups was insignificant (control group: 230 ± 35 pg/mL vs. reversible PAH group: 267 ± 24 pg/mL, P > 0.05). The protein level of survivin was extremely low in the whole lung homogenate of the control and reversible PAH groups but was significantly upregulated in the irreversible PAH rats (Fig. 5b). Furthermore, the concentration of serum survivin was positively correlated with the expression of total survivin in the whole lung homogenate in all rats (r = 0.759, P < 0.05).
Fig. 5.
Increased serum level of survivin and overexpression of survivin in lung from irreversible PAH rats. (a) Serum level of survivin from peripheral vein in the irreversible PAH group was significantly higher than that in both control and reversible PAH rats. (b) Representative immunoblots and densitometry demonstrating increased protein expression of survivin in lung from irreversible PAH rats. The differences among the three groups (n = 10 in each group) were analyzed by one-way ANOVA followed by Bonferroni post hoc test. *P < 0.05 vs. control group, #P < 0.05 vs. reversible PAH group.
Patients' characteristics
The clinical and hemodynamic characteristics of recruited patients are shown in Table 1. Of the 60 included CHD-PAH patients, 38 were assigned to the reversible PAH group because their mPAP became normal after shunt closure, while the other 22 patients were classified to the irreversible PAH group due to the high postoperative mPAP. The PVRi in all included CHD-PAH patients was >4 Wood unit·m2, which failed to establish whether PAH would be reversible after shunt closure in these borderline patients. Therefore, the operability was further evaluated based on clinical and hemodynamic criteria. All CHD-PAH patients had large intracardiac shunts (atrial septal defect or ventricular septal defect), systolic murmur, normal resting saturations (>95%) without drop during exercise, enlarged left ventricle, and normal cardiac function (defined as normal CI). The pulmonary arteriography showed increased pulmonary blood flow and no sign of rarefaction of the pulmonary arterial tree. The positive acute vasodilator testing with oxygen was detected in 28 patients in the reversible PAH group and 16 in the irreversible PAH group. All included patients received diuretics and digoxin for at least one month before shunt closure despite no clinical evidence of right ventricular failure and in World Health Organization (WHO) functional class (FC) I or II. All the clinical parameters before operation except age in reversible PAH and irreversible PAH group were similar without significant difference between them. The patients in the control group did not show any signs of PAH; thus, RHC was not performed in these patients.
Circulating survivin level and its relationship with pulmonary hemodynamics
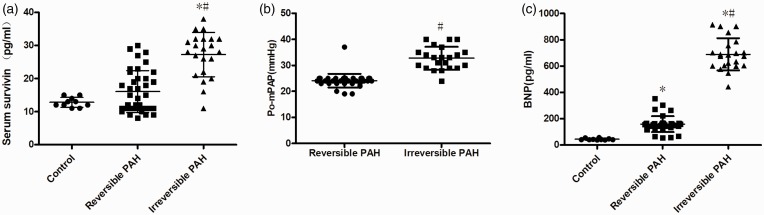
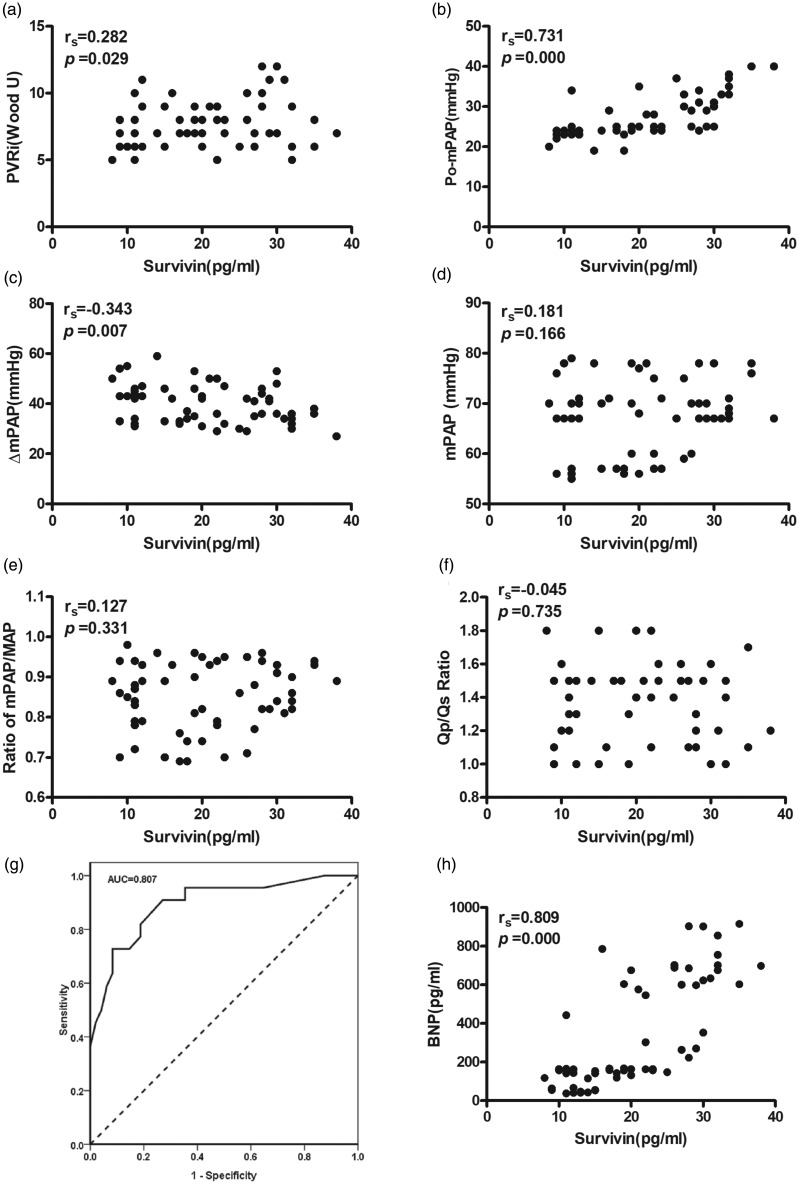
As shown in Fig. 6a, the preoperative serum level of survivin was significantly higher in the irreversible PAH group compared to that in both the reversible PAH and control groups (both P < 0.05), while the serum survivin level in the reversible PAH and control groups had no significant difference (P > 0.05). The mPAP on day 3 after the operation was significantly higher in the irreversible PAH group than that in the reversible PAH group (irreversible PAH group: 33.0 ± 4.0 vs. reversible PAH group: 23.7 ± 1.5, P < 0.05; Fig. 6b). Furthermore, serum survivin level was found to be correlated to the level of preoperative PVRi (r = 0.282, P = 0.029; Fig. 7a), postoperative mPAP (r = 0.731, P = 0.000; Fig. 7b) and ΔmPAP (the decrease of mPAP from before surgery to after surgery: r = –0.343, P = 0.000), respectively. However, no significant correlation was observed between serum survivin and preoperative mPAP, mPAP/MAP, or Qp/Qs (Fig. 7d–f). The diagnostic accuracy of survivin (cut-off at 27.5 pg/mL) determined by the ROC analysis demonstrated a sensitivity of 89.50% and a specificity of 68.2% with an area under the curve (AUC) of 0.807 in predicting the reversibility of PAH. The ROC curve is shown in Fig. 7g.
Fig. 6.
Increased serum survivin level in patients with residue PAH. (a) The level of survivin in patients with irreversible PAH (n = 22) was significantly higher than that in both control (n = 10) and reversible PAH group patients (n = 38). The differences among the three groups (n = 10 in each group) were analyzed by one-way ANOVA followed by Bonferroni post hoc test. (b) The patients in the irreversible PAH group (n = 22) had increased postoperative mPAP than that in the reversible PAH group (n = 38). The difference between the two groups was tested with independent samples t-test. (c) The level of BNP in patients with irreversible PAH (n = 22) was significantly higher than that in both control (n = 10) and reversible PAH group patients (n = 38). The serum BNP in the reversible PAH group was significantly higher than that in the control group. The differences among the three groups (n = 10 in each group) were analyzed by one-way ANOVA followed by Bonferroni post hoc test. *P < 0.05 vs. control group, #P < 0.05 vs. reversible PAH group. Po-mPAP, postoperative mean pulmonary arterial pressure; BNP, brain natriuretic peptide.
Fig. 7.
Correlations of serum survivin with pulmonary hemodynamics and ROC curve for serum survivin. The serum survivin level was found to be correlated to the level of preoperative PVRi (a), postoperative mPAP (b), and ΔmPAP (c); negative correlation of serum survivin with mPAP (d), mPAP/MAP (e), and Qp/Qs (f). The correlations between the concentration of survivin and pulmonary hemodynamics were evaluated with Pearson's correlation coefficient, respectively. (g) ROC analysis for predicting the reversibility of PAH after shunt closure in patient with PAH secondary to CHD. (h) A positive correlation of serum survivin with BNP was observed. PVRi, pulmonary vascular resistance index; mPAP, mean pulmonary arterial pressure; ΔmPAP, the difference of mPAP between before surgery and after surgery; MAP, mean arterial pressure; Qp/Qs, pulmonary-to-systemic blood flow ratio; BNP, brain natriuretic peptide.
The correlation between serum survivin and BNP levels
Serum BNP level was significantly increased in both reversible PAH patients (vs. control group, P < 0.05; Fig. 6c) and irreversible PAH group patients (vs. control group, P < 0.05; Fig. 6c). Moreover, the BNP level in the irreversible PAH group was significantly higher than that in the reversible PAH group (P < 0.05; Fig. 6c). A significant correlation between the levels of survivin and BNP in serum was observed (rs = 0.809, P = 0.000; Fig. 7h).
Discussion
As a progressive disease of the pulmonary vasculature, PAH is an important determinant of morbidity and mortality in CHD patients; irreversible PAH is difficult to identify based on preoperative testing. The present study found that, in an irreversible PAH rat model, the overexpression of a marker for apoptosis-resistance, survivin, paralleled with the histological lesions of small pulmonary arteries, and the serum level of survivin was also correspondingly increased. Furthermore, a positive relationship between serum level of survivin from peripheral veins and postoperative PAP was identified in PAH patients secondary to CHD. The results indicated that the serum level of survivin might be helpful to assess the operability in patients with CHD complicating with severe PAH.
Evidence of the reversal of pulmonary remodeling in PAH patients is limited and CHD-related PAH is the only form in which the reverse remodeling effects have been demonstrated after surgical treatment.18 Vascular remodeling refers to the structural changes that lead to hypertrophy and/or luminal occlusion, which will become irreversible in the absence of timely surgical intervention and is the principal determinant of the feasibility of surgical treatment. The pulmonary arteries presenting mainly neointimal proliferation and more serious lesions indicated that the PAH had developed to the irreversible stage.1 In the present study, the emergence of the neointimal lesion in small pulmonary arteries was considered as standard for irreversibility; the rats underwent monocrotaline administration following left pneumonectomy developed marked obstructive pulmonary vascular change (intimal lesions), which was an animal model of irreversible PAH.
As a member of the inhibitors of apoptosis, survivin has been reported to be involved in the pathogenesis of PAH;4 however, the relationship of survivin to the irreversibility in PAH was rarely reported. The role of survivin in an irreversible PAH model will be more valuable in predicting the prognosis of this disease, especially in evaluating the reversibility of PAH in CHD patients. Intimal lesions were believed to be irreversible occlusive pulmonary proliferative lesions in serial biopsy studies in patients with PAH secondary to CHD.18 In the current experiment, neointimal formation, a human PAH-like irreversible obstructive vasculopathy, developed in small pulmonary arteries from rats receiving monocrotaline injection after left pneumectomy. Decreased TUNEL-positive cells, increased survivin-positive cells, and overexpression of survivin protein in the lung were also observed in the small pulmonary arteries from irreversible PAH rats. Although the relationship between survivin level and “cancer-like” pulmonary vasculopathy has not been reported in PAH, the expression of this anti-apoptotic protein has shown significant correlation with a histopathological grade in tumors. Winther et al. found that the expression of survivin measured by immunohistochemical staining was significantly associated with a histopathological grade in meningiomas.19 In a comprehensive analysis on the effect of survivin on the prognostic and clinicopathological significance in patients with renal cell carcinoma, Xie et al. found that high survivin expression was significantly associated with the TNM stage, pathological T stage, and tumor size.20 The current experiment showed that the survivin increased in pulmonary arteries suffering from neointimal lesions in irreversible PAH rats but remained unchanged in pulmonary arteries showing medial hypertrophy from reversible PAH rats, even if the mPAP was elevated significantly. These results indicated that the overexpression of survivin may be correlated with the extent of pulmonary pathological lesions, as in tumors.
Besides being overexpressed in small pulmonary arteries, the serum level of survivin was increased prominently in the irreversible PAH model compared to the reversible PAH model; a positive correlation between the serum level of survivin and the expression of survivin in the lung was further observed, which suggested that the serum level of survivin might be helpful in predicting the extent of pulmonary lesions. In previous studies, survivin has been reported as a promising prognostic biomarker for cancers because of its high expression in malignancies but absence in normal adult tissues.21–23 In the present irreversible PAH rats, a higher level of survivin was found not only in the lung tissues but also in the serum compared with that in both reversible PAH and control rats; however, the role of survivin in the prediction of prognosis of PAH needs to be further investigated in patients.
In the current PAH population, most of the preoperative results including sex, diagnosis, PAP, PVR, Op/Qs, SpO2, and positive rate of the acute vascular test were similar among patients with reversible or irreversible PAH. Consistent with previous studies,24 none of the clinical and hemodynamic criteria could clearly identify patients with reversible or irreversible PAH in the present study, which indicated the difficulty of evaluating the operability in these patients. Preoperatively, both increased pulmonary blood flow and subsequently sustained vasoconstriction and proliferative vascular remodeling contributed to PAH in patients with moderate-to-large defects, while, postoperatively, residual PAH mainly resulted from the obstructive vascular remodeling. In addition, increased PVR and high postoperative PAP are mainly due to pulmonary vascular remodeling,25 while the high pulmonary flow is the main reason for high preoperative PAP. The serum level of survivin in the irreversible PAH patients was significantly higher than that in reversible PAH patients and positively correlated with PVR and postoperative mPAP, but did not correlate with preoperative mPAP, mPAP/MAP, and Qp/Qs. These results implied that, as in cancers,26,27 increased survivin level might result from the abnormal proliferation of pulmonary vascular cells and provided the support of survivin as a biomarker of pulmonary proliferative vasculopathy in PAH secondary to CHD.
Circulating biomarkers have been considered as potentially non-invasive, objective, and repeatable parameters for the prognosis evaluation. Although some biomarkers have been shown to correlate with the reversibility of PAH secondary to CHD, there is still no widely accepted specific marker for the prediction of reversibility. Smadja et al.24 showed that the circulating endothelial cell count in peripheral blood could help to predict the reversibility of PAH associated with CHD. A higher level of BNP, N-terminal-pro-fragment BNP, asymmetric dimethylarginine, and vascular endothelial growth factor were found in patients with PAH secondary to CHD when compared with healthy controls.28 Recently, Huang et al.29 reported that upregulated caveolin-1, filamin A, and cathepsin D and downregulated glutathione S-transferase mu1 in the lung biopsy may be potential new biomarkers for the reversibility of CHD-PAH. These biomarkers are related to the mechanisms involved in the pathophysiology of PAH including endothelial dysfunction (circulating endothelial cells, asymmetric dimethylarginine, caveolin-1), increased myocardial stress (BNP, NT-proBNP), and cell proliferation (vascular endothelial growth factor, filamin A, cathepsin D, glutathione S-transferase mu1), but the apoptosis-related biomarker has not yet been reported in evaluating reversibility. As an important factor involved in the regulation of apoptosis, survivin showed the potential of a biomarker for predicting reversibility of PAH secondary to CHD from the results of our present study. Among these biomarkers, BNP and NT-proBNP remain the only biomarkers that are widely used in the routine practice of PH centers as well as in clinical trials,17 while the role of BNP and NT-proBNP in evaluating the operability of patients with CHD and PAH was still uncertain. In the present study, a positive correlation between survivin and BNP was also identified in the included patients with CHD complicated with PAH. BNP/NT-proBNP could be considered as “late” markers of disease, since they signify high ventricular wall stress and ischemia, respectively. A “normal” level of these markers would not, therefore, exclude the presence of early disease, and the survivin that represented the extent of pulmonary remodeling will change earlier and be more sensitive.4
There are several limitations to this study. First, the data of lung biopsy from patients with CHD-PAH were lacking in the present study. The pathological change can reflect the extent of PAH more exactly, but the non-uniformity of vascular lesions in the lung likewise impacts the results. The PAP used in the current study is also a widely accepted diagnostic indicator for PAH. Second, the value of survivin in predicting the irreversibility of PAH secondary to CHD was derived from limited cases in the present study; its utility in the clinic should be consistently demonstrated in further, larger, prospective studies.
In conclusion, the experiments in the rats showed that survivin expression was closely correlated with the extent of obstructive pulmonary vasculopathy in irreversible pulmonary remodeling; the serum survivin was paralleled with the survivin expression in lung, which provided evidence that the serum survivin could be a biomarker for the reversibility of pulmonary remodeling. In addition, a positive correlation between the serum level of survivin and the reversibility of PAH secondary to CHD was found in patients receiving repairs of cardiac defects. The consistent results from animal experiments and the clinical study suggested that the serum survivin level in peripheral blood might be valuable in evaluating the operability of CHD complicated with PAH, although its clinical value should be confirmed by studies with a longer follow-up and a larger number of patients.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the National Natural Science Foundation of China (81600383), Beijing Natural Science Foundation (7164252), and Beijing Municipal Administration of Hospitals (QML20170601).
References
- 1.Van d Feen DE, Bartelds B, De Boer RA, et al. Pulmonary arterial hypertension in congenital heart disease: translational opportunities to study the reversibility of pulmonary vascular disease. Eur Heart J 2017; 38(26): 2034–2040. [DOI] [PubMed] [Google Scholar]
- 2.Vaillancourt M, Ruffenach G, Meloche J, et al. Adaptation and remodelling of the pulmonary circulation in pulmonary hypertension. Can J Cardiol 2015; 31(4): 407–415. [DOI] [PubMed] [Google Scholar]
- 3.Jurasz P, Courtman D, Babaie S, et al. Role of apoptosis in pulmonary hypertension: from experimental models to clinical trials. Pharmacol Ther 2010; 126(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 4.McMurtry MS, Archer SL, Altieri DC, et al. Gene therapy targeting survivin selectively induces pulmonary vascular apoptosis and reverses pulmonary arterial hypertension. J Clin Invest 2005; 115(6): 1479–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki YJ, Ibrahim YF, Shults NV. Apoptosis-based therapy to treat pulmonary arterial hypertension. J Rare Dis Res Treat 2016; 1(2): 17–24. [PMC free article] [PubMed] [Google Scholar]
- 6.Dressen K, Hermann N, Manekeller S, et al. Apoptosis-related biomarkers in patients with gastrointestinal cancer. Int J Clin Pharmacol Ther 2015; 53(12): 1062–1064. [DOI] [PubMed] [Google Scholar]
- 7.Sah NK, Khan Z, Khan GJ, et al. Structural, functional and therapeutic biology of survivin. Cancer Lett 2006; 244(2): 164–171. [DOI] [PubMed] [Google Scholar]
- 8.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 2008; 8(1): 61–70. [DOI] [PubMed] [Google Scholar]
- 9.Pennati M, Folini M, Zaffaroni N. Targeting survivin in cancer therapy: fulfilled promises and open questions. Carcinogenesis 2007; 28(6): 1133–1139. [DOI] [PubMed] [Google Scholar]
- 10.Guha M, Altieri DC. Survivin as a global target of intrinsic tumor suppression networks. Cell Cycle 2009; 8(17): 2708–2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato J, Kuwabara Y, Mitani M, et al. Expression of survivin in esophageal cancer: correlation with the prognosis and response to chemotherapy. Int J Cancer 2001; 95(2): 92–95. [DOI] [PubMed] [Google Scholar]
- 12.Monzó M, Rosell R, Felip E, et al. A novel anti-apoptosis gene: Re-expression of survivin messenger RNA as a prognosis marker in non-small-cell lung cancers. J Clin Oncol 1999; 17(7): 2100–2104. [DOI] [PubMed] [Google Scholar]
- 13.Okada K, Tanaka Y, Bernstein M, et al. Pulmonary hemodynamics modify the rat pulmonary artery response to injury. A neointimal model of pulmonary hypertension. Am J Pathol 1997; 151(4): 1019–1025. [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimura T, Vaszar LT, Faul JL, et al. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation 2003; 108(13): 1640–1645. [DOI] [PubMed] [Google Scholar]
- 15.Oka M, Homma N, Taraseviciene-Stewart L, et al. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circ Res 2007; 100(6): 923–929. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Liu B, Fan Z, et al. Targeted inhibition of survivin with YM155 promotes apoptosis of hypoxic human pulmonary arterial smooth muscle cells via the upregulation of voltage-dependent K+ channels. Mol Med Rep 2016; 13(4): 3415–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37(1): 67–119. [DOI] [PubMed] [Google Scholar]
- 18.Wagenvoort C A, Wagenvoort N, DraulansNoë Y. Reversibility of plexogenic pulmonary arteriopathy following banding of the pulmonary artery. J Thorac Cardiovasc Surg 1984; 87(6): 876–886. [PubMed] [Google Scholar]
- 19.Winther TL, Torp SH. The anti-apoptotic protein survivin can improve the prognostication of meningioma patients. PLoS One 2017; 12(9): e0185217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie YP, Ma X, Gu L, et al. Prognostic and clinicopathological significance of survivin expression in renal cell carcinoma: a systematic review and meta-analysis. Sci Rep 2016; 6: 29794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Teng Z, Wang Y, et al. Prognostic significance of survivin expression in osteosarcoma patients: a meta-analysis. Med Sci Monit 2015; 21: 2877–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv S, Dai C, Liu Y, et al. The impact of survivin on prognosis and clinicopathology of glioma patients: a systematic meta-analysis. Mol Neurobiol 2015; 51(3): 1462–1467. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Wang J, Sui X, et al. Prognostic and clinicopathological value of survivin in diffuse large B-cell lymphoma: a meta-analysis. Medicine (Baltimore) 2015; 94(36): e1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smadja DM, Gaussem P, Mauge L, et al. Circulating endothelial cells: a new candidate biomarker of irreversible pulmonary hypertension secondary to congenital heart disease. Circulation 2009; 119(3): 374–381. [DOI] [PubMed] [Google Scholar]
- 25.Frank DB, Hanna BD. Pulmonary arterial hypertension associated with congenital heart disease and Eisenmenger syndrome: current practice in pediatrics. Minerva Pediatr 2015; 67(2): 169–185. [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Wang G, Wei J, et al. Survivin protein expression positively correlated with proliferative activity of cancer cells in bladder cancer. Indian J Med Sci 2005; 59(6): 235–242. [PubMed] [Google Scholar]
- 27.Adamkov M, Drahošová S, Chylíková J, et al. Survivin in breast lesions: immunohistochemical analysis of 196 cases. Pol J Pathol 2017; 68(4): 297–305. [DOI] [PubMed] [Google Scholar]
- 28.Giannakoulas G, Mouratoglou SA, Gatzoulis MA, et al. Blood biomarkers and their potential role in pulmonary arterial hypertension associated with congenital heart disease. a systematic review. Int J Cardiol 2014; 174(3): 618–623. [DOI] [PubMed] [Google Scholar]
- 29.Huang L, Li L, Hu E, et al. Potential biomarkers and targets in reversibility of pulmonary arterial hypertension secondary to congenital heart disease: an explorative study. Pulm Circ 2018; 8(2): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]