Abstract
Bronchopulmonary dysplasia (BPD) is the most common complication in preterm infants and often complicated by pulmonary hypertension (PH), leading to substantial morbidity and mortality. Sildenafil is often used to treat PH and improve symptoms in this condition, even though evidence of safety and effectiveness is scarce. The aim of this study was to perform a systematic review and meta-analysis about the effectiveness and safety of chronic use of sildenafil in preterm infants with BPD-associated PH. Data sources were PubMed, EMBASE, and Medline. Studies reporting the effectiveness of sildenafil therapy in BPD-associated PH in newborns and infants were included. All-cause mortality, improvement in PH, improvement in respiratory scores, and adverse events were extracted. Five studies were included, yielding a total of 101 patients with 94.2 patient-years of total follow-up. The pooled mortality rate was 29.7%/year (95% confidence interval [CI] = 6.8–52.7). Estimated pulmonary arterial pressure improved > 20% in 69.3% (95% CI = 56.8–81.8) of patients within 1–6 months. Respiratory scores improved in 15.0% (95% CI = 0.0–30.4) of patients within 2–7 days. There were no serious adverse events during sildenafil therapy. This systematic review shows that in the treatment of BPD-associated PH in preterm infants, sildenafil may be associated with improvement in PAP and respiratory scores. However, there is no clear evidence of its effect on mortality rates. Considering BPD as a complex disease with variable expression patterns, these results support the need for a prospective registry and standardized approach.
Keywords: sildenafil, pediatrics, bronchopulmonary dysplasia, pulmonary hypertension
Introduction
Bronchopulmonary dysplasia (BPD) is the most common complication in preterm infants and is associated with adverse short-term and long-term outcomes.1 Twenty-seven percent of infants born at 28 weeks of gestational age will develop BPD and this incidence is still increasing, due the growing number of preterm infants surviving birth.2–5 BPD can be categorized according to severity.1,6 Pulmonary hypertension (PH) is a frequently occurring complication in infants with BPD, with a reported incidence in the range of 18–37%.7–10 This condition is associated with a high mortality rate, in the range of 20–38% within two years.11–13
PH is a symptom of various forms of pulmonary vascular diseases and can be categorized into five groups based on clinical course and underlying disease according to the World Health Organization (WHO) classification.14
Many new drugs have been developed to treat patients with PH in group 1 (pulmonary arterial hypertension [PAH]).15,16 Sildenafil is one of the drugs influencing the nitric oxide-pathway, which, together with the prostacyclin pathway and endothelin receptors, are three main targets for intervention in PAH.15,16 Sildenafil inhibits the enzyme phosphodiesterase-5A (PDE-5A), enhancing the availability of cyclic guanosine monophosphate (cGMP), which leads to vasodilatation and smooth-muscle growth inhibition.17 Chronic treatment with sildenafil has been shown to be effective in PAH and is one of the most important oral drugs for PAH nowadays.18 BPD-associated PH, categorized in group 3 (PH due to lung diseases), is very different in etiology and vascular morphology compared with PAH. Nevertheless, many clinicians nowadays frequently apply treatment strategies developed for group 1 to other forms of PH, for example in the treatment of BPD-associated PH.19 However, studies on the effectiveness of sildenafil in the treatment of BPD-associated PH in preterm infants are scarce, often small in sample size and an overview of reported outcome is lacking. In addition, there has been an official warning from the Food and Drug Administration (FDA) on the use of sildenafil in children aged 1–17 years, especially when using higher doses.20 The aim of this study was to perform a systematic review and meta-analysis of reported effectiveness and safety of chronic use of sildenafil in preterm infants with BPD-associated PH.
Methods
Search strategy and selection of studies
This systematic review was conducted according to the PRISMA statement for systematic reviews.21 On January 14, 2018, the EMBASE, Medline, Ovid, and Cochrane databases were searched by a biomedical information specialist (supplement). The search was limited to studies that were written in English. After extracting duplicates, the remaining publications were reviewed by two independent reviewers (MG and LR) to assess the relevance of each study.
Studies were considered relevant if the study was an original study reporting mortality after sildenafil therapy for BPD-associated PH in infants. Studies only describing a mixed population including patients with other chronic lung diseases besides BPD, without reporting BPD-specific mortality rates separately, were excluded. Case reports, case series, and conference abstracts were also excluded. In case of overlapping study populations, only the most recent or most complete study was included. When a disagreement occurred between the reviewers, an agreement was negotiated with a third reviewer (BB). The references of included articles were screened for relevant studies.
BPD was defined according to the current National Institutes of Health definition.6,22 According to the guidelines, PH was defined as a mean pulmonary artery blood pressure (mPAP)≥ 25 mmHg at rest, as measured by cardiac catheterization.23,24 Because of the invasiveness of cardiac catheterization and its subsequently less frequent use in pediatric practice, the peak tricuspid regurgitation jet velocity (TRJV) measured by echocardiography was also accepted as an estimate of PH.24,25 The following surrogate parameters using echocardiography were also considered as confirmation of the diagnosis: TRJV ≥ 2.8 m/s; systolic flattening of the bowed interventricular septum; estimated PAP/systolic blood pressure ratio of >0.5 or time to peak velocity to right ventricular ejection time ratio <0.35.26
Data extraction
Data of each study were extracted independently by two reviewers (MG, LR), using Microsoft Office Excel 2010 (Microsoft Corp., Redmond, WA, USA, version 14.0.7177.5000). In case of disagreements between the reviewers, an agreement was negotiated. The following study and baseline patient characteristics were collected: year of publication; study design; the number of included patients; the inclusion period; the duration of the follow-up; the gestational age at birth; co-medication for BPD and PH; the postnatal age at the start of sildenafil treatment; duration of sildenafil treatment; and the initial, maximum, and median sildenafil dose.
The primary outcome parameter was all-cause mortality. Secondary outcome parameters were: improvement in PH; respiratory improvement; and adverse events (AE). Improvement in PH was defined as a decrease of ≥20% in right ventricular (RV) systolic pressure or TRJV gradient, improvement of interventricular septum flattening (≥1°) or reduction of PAP as measured by cardiac catheterization.27 Respiratory improvement was defined as a decrease of ≥20% in requirement of inspired oxygen fraction or a decrease in the respiratory severity score (calculated as mean airway pressure x FiO2).28
Statistical analyses
Baseline patient characteristics were pooled with the use of sample size weighting. In case data were reported in medians, the median was used as an approximation of the mean. If the SD was not available, the range was divided by 4, or the interquartile range by 1.35, as an approximation of the SD. Linearized occurrence rates of outcome measures were pooled with the use of inverse variance weighting according to the number of patient-years of total follow-up, when the definition of outcome parameters was comparable across studies. If the total follow-up duration (in patient-years) was not reported, it was calculated by multiplying the number of patients with the mean follow-up of that study. When the number of studies was large enough (≥3 studies) to estimate the between-study variance (Tau2), a random effects model was used, otherwise a fixed effects model was used. Outcomes were pooled on a linear (non-logarithmic) scale as the Shapiro–Wilk test showed no evidence of skewness among the included studies in any of the outcome measures. A P value of <0.05 was considered statistically significant. Heterogeneity among the studies was analyzed with the Cochran Q statistic and the I2 index. Publication bias was assessed with use of funnel plots. The authors take full responsibility for the integrity of the data.
Results
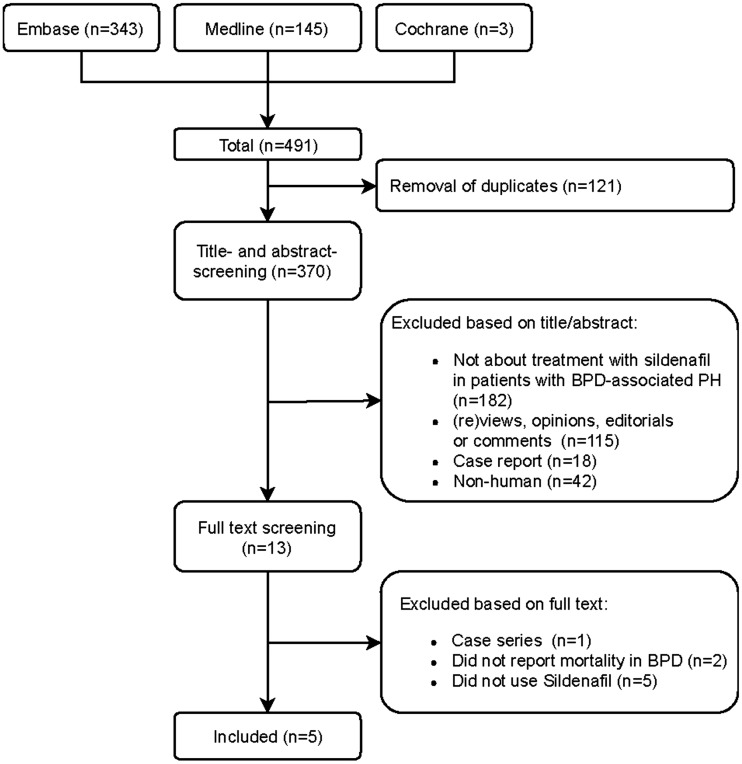
The search identified 370 unique publications of which 13 were included for full-text screening and 5 were included in this systematic review (Fig. 1).29–33 This yielded a total of 101 patients with 94.2 patient-years of total follow-up (pooled mean follow-up 1.0 year) (Table 1). All studies were retrospective in design. Table 1 gives an overview of study and baseline patient characteristics. The pooled mean gestational age at birth was 26.3 ± 2.7 weeks and the postnatal age at start of sildenafil therapy was 21.9 ± 15.9 weeks. Initial sildenafil dose was in the range of 0.3–1.5 mg/kg/day, with a maximum dose of 6.0–8.0 mg/kg/day.
Fig. 1.
Flowchart of study selection, based upon PRISMA statement for systematic reviews.21
Table 1.
Study and patient characteristics.
| Study | Year of publication, Journal | Patients (n) | Male (%) | Gestational age at birth (weeks) | Postnatal age at start of sildenafil (weeks) | Initial PAP (mmHg) | Initial sildenafil dose (mg/kg/day) | Maximum sildenafil dose (mg/kg/day) | Median sildenafil dose (mg/kg/day) |
|---|---|---|---|---|---|---|---|---|---|
| Mourani et al.30 | 2009, Journal of Pediatrics | 18 | 60.9 | 28.0 ± 4.5 | 26.3 ± 22.1 | NR | 1.5 | 8.0 | NR |
| Nyp et al.31 | 2012, Journal of Perinatology | 21 | 54.5 | 27.0 ± 2.5 | 23.9 ± 8.0 | 65.0 ± 15.5 | 0.3–0.5 | 6.0 | NR |
| Tan et al.32 | 2015, European Journal of Pediatrics | 22 | 57.1 | 25.6 ± 1.3 | 24.1 ± 9.8 | 56.5 ± 10.4 | 0.8 | 6.0 | NR |
| Trottier-Boucher et al.33 | 2015, Pediatric Cardiology | 23 | 77.8 | 26.0 ± 2.2 | 15.1 ± 9.0 | NR | 1.0 | 7.3 | 4.4 ± 1.5 |
| Kadmon et al.29 | 2016, Pediatric Pulmonology | 17 | 45.0 | 25.0 ± 2.0 | 20.9 ± 26.1 | 32.0 ± 3.8 (n = 8) | NR | 8.0* | 3.0 |
| Pooled total† | 101 | 59.1 | 26.3 ± 2.7 | 21.9 ± 15.9 |
Results are given in mean (±SD).
Following the FDA warning in August 2012 regarding high-dose sildenafil use in pediatric PH, the dose was reduced to 1 mg/kg/dose in all patients.
Sample size weighting.
PAP, pulmonary arterial pressure; NR, variable not reported.
All included studies followed the guidelines for the diagnosis of BPD. Since not all patients underwent cardiac catheterization to confirm the diagnosis of PH, we also used the surrogate parameters assessed by echocardiography (see “Methods”). Initial mPAPs were in the range of 27–42 mmHg and systolic RV pressures in the range of 38–100 mmHg.29,31,32 One study assessed pulmonary vascular resistance (PVR) by cardiac catheterization in a subset of patients, in the range of 2.3–9.2 WU.29
The majority of patients also received other medication besides sildenafil during follow-up. This included systemic (10.7%) or inhaled (21.4%) steroids, milrinone (11.1%), or other PH-medication such as bosentan (10.3%), and inhaled nitric oxide (iNO) (54.9%) (Table 2).
Table 2.
Medication used concomitantly during sildenafil therapy.
| Exogenous iNO (%) | Milrinone (%) | Bosentan (%) | Systemic steroids (%) | Inhaled steroids (%) | |
|---|---|---|---|---|---|
| Mourani et al.30 | 72.0 | 16.0 | 8.0 | 0.0 | 0.0 |
| Nyp et al.31 | 52.4 | 19.0 | 0.0 | 42.9 | 47.6 |
| Tan et al.32 | 4.5 | NR | 0.0 | 0.0 | 0.0 |
| Trottier-Boucher et al.33 | 91.3 | 0.0 | 17.4 | 0.0 | 34.8 |
| Kadmon et al.29 | NR | NR | 29.4 | NR | NR |
| Pooled total | 54.9 | 11.1 | 10.3 | 10.7 | 21.4 |
NR, variable not reported.
Study outcomes
The pooled mortality rate during a mean follow-up of 1.0 year was 29.7%/year (95% confidence interval [CI] = 6.8–52.7%/year).29–33 The cause of death was specified in fifteen patients from four studies: 12/15 died due to “respiratory failure in BPD-PH”, 2/15 patients due to sepsis, and 1/15 due to neurological injury.29–32. Estimated PAP improved in 69.3% (95% CI = 56.8–81.8%) of patients within 1–6 months.31–33 One study reported changes in PAPs as measured by echocardiography (56.5 ± 10.4 to 34.3 ± 8.3 mmHg).32 Respiratory scores (FiO2 and RSS) improved in 15.0% (95% CI = 0.0–30.4%) of patients within 2–7 days (Table 3).31–33 Pooled respiratory scores and estimates of PAP could not be assessed in 2/5 studies, due to the fact that these outcome parameters were not reported separately for BPD-associated PH patients treated with sildenafil.29,30 All studies assessed possible AEs during sildenafil treatment. There were no serious AEs. AEs were all minor and included transient hypotension (11.8%), hypotension needing temporary cessation (3.0%), and frequent erections (1.0%).29–33 Publication bias could not be assessed due to the limited number of studies.
Table 3.
Pooled estimates of the outcome parameters.
| Study | Mean follow-up duration (months) | Mortality rate (%/year) | Improvement in the estimate of the PAP (%) | Improvement in respiratory scores (%) |
|---|---|---|---|---|
| Mourani et al.30 | 7.9 ± 7.6 | 25.2 | NR | NR |
| Nyp et al.31 | 12.0 ± 2.9 | 19.0 | 70.0 | 14.3*†,‡ |
| Tan et al.32 | 9.0 ± 3.0 | 36.2 | 66.7 | 0.0§† |
| Trottier-Boucher et al.33 | 5.6 ± 2.4 | 74.3 | 71.4 | 34.8§†‡ |
| Kadmon et al.29 | 24.0 ± 15.6 | 2.9 | NR | NR |
| Pooled effect (95% CI) | 11.2 ± 7.5 | 27.6 (12.6–60.4) ** | 69.3 (56.8–81.8) †† | 15.0 (0.0–30.4) ** |
| I2 | 80.6 | 0.0 | 58.2 | |
| X2 P value | <0.001 | 0.9 | 0.05 |
Improvement within 48 h.
Using FiO2 measurements.
Using RSS.
Improvement within 168 h.
Using random effects model.
Fixed effect model.
NR, variable not reported; PAP, pulmonary arterial pressure.
Discussion
This systematic review shows that in the treatment of BPD-associated PH in preterm infants, sildenafil is associated with reduction in PAP and improvements in respiratory score. However, there is no clear evidence of its effect on mortality rates. No serious AEs were reported. The results of this study raise the question: Is chronic use of sildenafil effective as a treatment for BPD-associated PH?
Recent experimental research has shown a positive effect of sildenafil in the prevention of PH in neonatal rats with BPD. Administration of sildenafil in this model alleviated the development of lung injury, due to improvement of alveolarization and recovery of the pulmonary angiogenesis.35,36 In addition, sildenafil may enhance RV function, as has been demonstrated in experimental studies.37 These data suggest that preterm infants with BPD at risk for PH may benefit from preventive sildenafil therapy. Our results show that sildenafil appears to be associated with improvements in estimates of PAP and respiratory scores; however, sildenafil showed no effect on mortality rates. Prior publications have reported mortality rates of 20–36% in infants with BPD-associated PH without sildenafil therapy.9,10,12 There may be several reasons for the discrepant results between changes in estimates of PVR and mortality in this systematic review.
Although the studies reported in this systematic review have added important information to the clinicians, this analysis also indicates some weaknesses that should be taken into account. First, it should be noted that various evaluation methods were used to diagnose PH. The formal definition is a mPAP > 25 mmHg. Unfortunately, only one study measured PAPs invasively, the others inferred PAPs from echocardiographic indices as TRJV or septal flattening. Second, clinical effects of a drug, or the lack thereof, should be evaluated in combination with the effective drug dosage. There is no information available regarding the dose-effect relation in the use of sildenafil for treatment of BPD-associated PH. Most institutions gradually increase the dosage to avoid side effects as systemic hypotension. Confusion may arise from the fact that low-dose sildenafil has been shown to improve ventilation-perfusion leading to a reduction on RSS, whereas higher dosages are needed to affect pulmonary vascular remodeling. Indeed, the study performed by Trottier-Boucher et al. reported that improvement in RSS was achieved after 48 h, but echocardiographic-derived reduction in PAP only after 19 days. This dose-dependent and hence time-dependent effect of sildenafil on clinical improvement should be incorporated in prospective registries. Also, careful assessment of potential pulmonary vein stenosis is necessary when evaluating the effect of sildenafil.11
In addition, although PH does increase the risk of mortality in BPD, it is not the only contributor.10 Mortality among infants with BPD is influenced by multiple factors, such as prematurity and associated co-morbidities. One factor relating to outcome not analyzed in any of these studies is the reduction in lung volume associated with prematurity. The changes in the pulmonary vasculature in BPD, categorized in group 3 according to the classification of the WHO, may differ from those in PAH, categorized in group 1. Sildenafil has been tested in children with PAH in a randomized controlled trial in which start of therapy and the follow-up were standardized.38 Unfortunately, the diagnosis of PH, timing of intervention, dosing, and follow-up have not been standardized in infants with BPD. Consequently, there may have been selection bias due to the lack of a standardized approach, leading to an overrepresentation of more severe cases.
The gold standard for evaluation of PH is measurement of PAP by cardiac catheterization.39 However, clinicians hesitate to perform cardiac catheterization in preterm infants with pulmonary disease due to its invasiveness, need for intubation, and reported adverse effects.40 Still, the recommendation is that cardiac catheterization should be performed before the initiation of PAH-targeted therapy, such as sildenafil. A prerequisite for a prospective study or registry is a proper definition and standardized diagnostic approach. Fortunately, many centers can nowadays perform invasive measurements with sedation rather than intubation and general anesthesia. The hemodynamic evaluation should include the assessment of ratio of PA and SA pressure and, if possible, resistances. While echocardiography is widely used, there is no consensus in the literature about the usefulness of echocardiography in the follow-up of PH.34,41,42 The sensitivity of echocardiography to assess PH is suboptimal, due to the requirement of tricuspid valve regurgitation to observe signs of PH. A recent study proposed guidelines for detection of PH in infants with BPD that recommends a staged approach using echocardiography as a screening tool for PH and subsequently cardiac catheterization before initiating treatment with long-term medication to confirm the diagnosis and to assess severity and potential contributing factors.43 The interpretation of the data in this review was hampered by different echocardiographic techniques to assess the PAP in preterm infants with BPD. Only one study assessed PVR using cardiac catheterization at baseline in a subset of patients.29 PVR in this study was in the range of 2.3–9.2 WU, suggesting that not all patients fulfilled the PH criteria. These data call for a prospective registration and standardized diagnostic and therapeutic approach for patients with BPD-associated PH.
Should we design a prospective randomized trial to investigate the effectiveness of sildenafil in the treatment of PH in BPD? Suppose we aim for an absolute reduction in mortality of 15% within one year, assuming an estimated mortality of 25% in the control group, a total of 214 patients with BPD-associated PH would be required to detect this difference with 80% power at a 0.05 significance level with a 1:1 sampling ratio. This might be feasible in a large multicenter study at a considerable cost. Yet, given the complexity of the origin of BPD-associated PH, it is doubtful whether mono-therapy with sildenafil, or any other PAH-targeting therapy, will lead to sufficient clinical effects. Recent experience with treatment of adult patients with PH advocate the start of dual or even triple therapy at time of diagnosis.44 Another complicating issue in pediatric trial design is the definition of a proper endpoint. In this respect, mortality, a frequently reached endpoint in BPD-associated PH may be a relevant endpoint. Secondary endpoints could be quality of life as reflected in RSS scores and oxygen dependency. These practical aspects may further complicate the design of a proper pediatric trial. An alternative strategy may be a prospective cohort including diagnostic criteria and standardized follow-up, observing both short-term and long-term outcomes, based on recent research and guidelines.45–49
Strengths and limitations
This systematic review and meta-analysis is the first to review the effectiveness and safety of sildenafil in infants with BPD-associated PH and encompasses the largest number of patients so far. All included studies were retrospective and observational in design and had no standardization of patient inclusion, treatment, and follow-up. As such, the inherent limitations of meta-analyses of retrospective observational studies should be taken into consideration. The retrospective nature of the studies precludes interpretation on the effects of co-medication. One study reported that in some cases co-medication for PAH was started when the use of sildenafil did not improve PH.30 These data suggest that the effect of sildenafil might has been overestimated. Selection bias could have influenced our results, because case reports, case series, and unpublished data were not included. The limited number of studies precluded assessment of publication bias.
Conclusion
BPD-associated PH is a complex disease with variable expression patterns. This systematic review shows that in the treatment of BPD-associated PH in preterm infants, sildenafil may be associated with improvement in PAP and respiratory scores. However, there is no clear evidence of its effect on mortality rates. These results support the need for a prospective registration and standardized approach, including entry criteria, to allow optimization for the child with BPD-associated PH.
Supplemental Material
Supplemental Material for Sildenafil for bronchopulmonary dysplasia and pulmonary hypertension: a meta-analysis by Marisa van der Graaf, Leonne Arindah Rojer, Willem Arnold Helbing, Irwin Karl Marcel Reiss, Jonathan Richard Gregory Etnel and Beatrijs Bartelds in Pulmonary Circulation
Acknowledgments
The authors thank Wichor Bramer (biomedical information specialist, Erasmus University Medical Center) for his assistance with the literature search.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding
BB acknowledges the support from the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation, Dutch Federation of University Medical Centers, the Netherlands (CVON 2012-08 PHAEDRA). JE is funded by the Dutch Heart Foundation (2013T093).
References
- 1.Hayes D, Jr, Feola DJ, Murphy BS, et al. Pathogenesis of bronchopulmonary dysplasia. Respiration 2010; 79(5): 425–36. [DOI] [PubMed] [Google Scholar]
- 2.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 2010; 88(1): 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet 2012; 379(9832): 2162–2172. [DOI] [PubMed] [Google Scholar]
- 4.Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol 2014; 100(3): 145–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126(3): 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001; 163(7): 1723–1729. [DOI] [PubMed] [Google Scholar]
- 7.An HS, Bae EJ, Kim GB, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J 2010; 40(3): 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhat R, Salas AA, Foster C, et al. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics 2012; 129(3): e682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DH, Kim HS, Choi CW, et al. Risk factors for pulmonary artery hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Neonatology 2012; 101(1): 40–46. [DOI] [PubMed] [Google Scholar]
- 10.Slaughter JL, Pakrashi T, Jones DE, et al. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol 2011; 31(10): 635–640. [DOI] [PubMed] [Google Scholar]
- 11.Del Cerro MJ, Sabaté Rotés A, Cartõn A, et al. Pulmonary hypertension in bronchopulmonary dysplasia: Clinical findings, cardiovascular anomalies and outcomes. Pediatr Pulmonol 2014; 49(1): 49–59. [DOI] [PubMed] [Google Scholar]
- 12.Khemani E, McElhinney DB, Rhein L, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: clinical features and outcomes in the surfactant era. Pediatrics 2007; 120(6): 1260–1269. [DOI] [PubMed] [Google Scholar]
- 13.Kim GB. Pulmonary hypertension in infants with bronchopulmonary dysplasia. Korean J Pediatr 2010; 53(6): 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin VV, Shah SJ, Souza R, et al. Management of pulmonary arterial hypertension. J Am Coll Cardiol 2015; 65(18): 1976–1997. [DOI] [PubMed] [Google Scholar]
- 15.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004; 351(14): 1425–1436. [DOI] [PubMed] [Google Scholar]
- 16.Lador F, Sekarski N, Beghetti M. Treating pulmonary hypertension in pediatrics. Expert Opin Pharmacother 2015; 16(5): 711–726. [DOI] [PubMed] [Google Scholar]
- 17.Tantini B, Manes A, Fiumana E, et al. Antiproliferative effect of sildenafil on human pulmonary artery smooth muscle cells. Basic Res Cardiol 2005; 100(2): 131–138. [DOI] [PubMed] [Google Scholar]
- 18.Galie N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005; 353(20): 2148–2157. [DOI] [PubMed] [Google Scholar]
- 19.Thompson EJ, Perez K, Hornik CP, et al. Sildenafil exposure in the Neonatal Intensive Care Unit. Am J Perinatol 2019; 36: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FDA. Drug Safety Communication: FDA recommends against use of Revatio (sildenafil) in children with pulmonary hypertension. Washington, DC: FDA, 2012. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm317123.htm.
- 21.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6(7): e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005; 116(6): 1353–1360. [DOI] [PubMed] [Google Scholar]
- 23.Ivy DD, Abman SH, Barst RJ, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 SUPPL.): D117–126. [DOI] [PubMed] [Google Scholar]
- 24.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Hear J 2016; 37(1): 67–119. [DOI] [PubMed] [Google Scholar]
- 25.Bennett D, Marcus R, Stokes M. Incidents and complications during pediatric cardiac catheterization. Paediatr Anaesth 2005; 15(12): 1083–1088. [DOI] [PubMed] [Google Scholar]
- 26.Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010; 23(7): 685–688. [DOI] [PubMed] [Google Scholar]
- 27.Bossone E, D’Andrea A, D’Alto M, et al. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr 2013; 26(1): 1–14. [DOI] [PubMed] [Google Scholar]
- 28.Malkar MB, Gardner WP, Mandy GT, et al. Respiratory severity score on day of life 30 is predictive of mortality and the length of mechanical ventilation in premature infants with protracted ventilation. Pediatr Pulmonol 2015; 50(4): 363–369. [DOI] [PubMed] [Google Scholar]
- 29.Kadmon G, Schiller O, Dagan T, et al. Pulmonary hypertension specific treatment in infants with bronchopulmonary dysplasia. Pediatr Pulmonol 2017; 52: 77–83. [DOI] [PubMed] [Google Scholar]
- 30.Mourani PM, Sontag MK, Ivy DD, et al. Effects of long-term sildenafil treatment for pulmonary hypertension in infants with chronic lung disease. J Pediatr 2009; 154(3): 379–84, 384.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyp M, Sandritter T, Poppinga N, et al. Sildenafil citrate, bronchopulmonary dysplasia and disordered pulmonary gas exchange: Any benefits? J Perinatol 2012; 32(1): 64–69. [DOI] [PubMed] [Google Scholar]
- 32.Tan K, Krishnamurthy MB, O’Heney JL, et al. Sildenafil therapy in bronchopulmonary dysplasia-associated pulmonary hypertension: a retrospective study of efficacy and safety. Eur J Pediatr 2015; 174(8): 1109–1115. [DOI] [PubMed] [Google Scholar]
- 33.Trottier-Boucher MN, Lapointe A, Malo J, et al. Sildenafil for the treatment of pulmonary arterial hypertension in infants with bronchopulmonary dysplasia. Pediatr Cardiol 2015; 36(6): 1255–1260. [DOI] [PubMed] [Google Scholar]
- 34.Mourani PM, Sontag MK, Younoszai A, et al. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics 2008; 121(2): 317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Visser YP, Walther FJ, Laghmani EH, et al. Sildenafil attenuates pulmonary inflammation and fibrin deposition, mortality and right ventricular hypertrophy in neonatal hyperoxic lung injury. Respir Res 2009; 10 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park HS, Park JW, Kim HJ, et al. Sildenafil alleviates bronchopulmonary dysplasia in neonatal rats by activating the hypoxia-inducible factor signaling pathway. Am J Respir Cell Mol Biol 2013; 48(1): 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borgdorff MA, Bartelds B, Dickinson MG, et al. Sildenafil treatment in established right ventricular dysfunction improves diastolic function and attenuates interstitial fibrosis independent from afterload. Am J Physiol Heart Circ Physiol 2014; 307(3): H361–369. [DOI] [PubMed] [Google Scholar]
- 38.Barst RJ, Beghetti M, Pulido T, et al. STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive pediatric pulmonary arterial hypertension. Circulation 2014; 129(19): 1914–1923. [DOI] [PubMed] [Google Scholar]
- 39.Abman SH, Ivy DD, Archer SL, et al. Executive Summary of the American Heart Association and American Thoracic Society Joint Guidelines for Pediatric Pulmonary Hypertension. Am J Respir Crit Care Med 2016; 194(7): 898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beghetti M, Schulze-Neick I, Berger RM, et al. Haemodynamic characterisation and heart catheterisation complications in children with pulmonary hypertension: Insights from the Global TOPP Registry (tracking outcomes and practice in paediatric pulmonary hypertension). Int J Cardiol 2016; 203: 325–330. [DOI] [PubMed] [Google Scholar]
- 41.Carlton EF, Sontag MK, Younoszai A, et al. Reliability of echocardiographic indicators of pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. J Pediatr 2017; 186: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossor T, Greenough A. Advances in paediatric pulmonary vascular disease associated with bronchopulmonary dysplasia. Expert Rev Respir Med 2015; 9(1): 35–43. [DOI] [PubMed] [Google Scholar]
- 43.Abman SH, Collaco JM, Shepherd EG, et al. Interdisciplinary care of children with severe bronchopulmonary dysplasia. J Pediatr 2017; 181: 12–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van de Veerdonk MC, Huis in ‘t Veld AE, Markus JT, et al. Upfront combination therapy reduces right ventricular volumes in pulmonary arterial hypertension. Eur Respir J 2017; 49(6). [DOI] [PubMed]
- 45.Okumura K, Humpl T, Dragulescu A, et al. Longitudinal assessment of right ventricular myocardial strain in relation to transplant-free survival in children with idiopathic pulmonary hypertension. J Am Soc Echocardiogr 2014; 27(12): 1344–1351. [DOI] [PubMed] [Google Scholar]
- 46.Lammers AE, Adatia I, Cerro MJ, et al. Functional classification of pulmonary hypertension in children: Report from the PVRI pediatric taskforce, Panama 2011. Pulm Circ 2011; 1(2): 280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fike CD, Aschner JL. Looking beyond PPHN: the unmet challenge of chronic progressive pulmonary hypertension in the newborn. Pulm Circ 2013; 3(3): 454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Di Maria MV, Younoszai AK, Mertens L, et al. RV stroke work in children with pulmonary arterial hypertension: estimation based on invasive haemodynamic assessment and correlation with outcomes. Heart 2014; 100(17): 1342–1347. [DOI] [PubMed] [Google Scholar]
- 49.Di Maria MV, Burkett DA, Younoszai AK, et al. Echocardiographic estimation of right ventricular stroke work in children with pulmonary arterial hypertension: comparison with invasive measurements. J Am Soc Echocardiogr 2015; 28(11): 1350–1357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Sildenafil for bronchopulmonary dysplasia and pulmonary hypertension: a meta-analysis by Marisa van der Graaf, Leonne Arindah Rojer, Willem Arnold Helbing, Irwin Karl Marcel Reiss, Jonathan Richard Gregory Etnel and Beatrijs Bartelds in Pulmonary Circulation