Abstract
Objective:
Clozapine, an antipsychotic reserved for management of treatment-resistant schizophrenia, is associated with severe adverse effects, including myocarditis. This study aims to determine the incidence of clozapine-induced myocarditis at a large tertiary hospital compared to what is reported in the literature.
Methods:
Medical records of adult patients admitted to psychiatry units receiving clozapine between January 1, 2010, and July 31, 2016, were retrospectively reviewed. Cases of clozapine-induced myocarditis were defined as having elevated C-reactive protein (CRP) or detectable troponin and at least 1 sign or symptom of myocarditis, in the absence of alternative plausible aetiologies. The primary outcome was incidence of clozapine-induced myocarditis during the study period. Secondary outcomes included rate and description of the management of clozapine-induced myocarditis.
Results:
In total, 316 patients were screened; 10 patients met the case definition for clozapine-induced myocarditis. The incidence of this adverse drug reaction over the study period was 3.16%. Reduced left ventricular ejection fraction was observed in 60% of cases, and electrocardiography changes were noted in 60% of cases. Clozapine was discontinued in all cases. Rechallenge was performed in 2 patients; recurrent CRP elevation resulted in discontinuation in each case. Medications for management of myocarditis were used in 50% of cases. Although 2 patients required transfer to critical care, the in-hospital mortality rate was 0%.
Conclusions:
The incidence of clozapine-induced myocarditis at the study hospital was consistent with the higher range reported in the literature. Further research is necessary to elucidate risk factors, definitive diagnostic criteria, and effective management of clozapine-induced myocarditis.
Keywords: clozapine, myocarditis, adverse drug reaction
Abstract
Objectif:
La clozapine, un antipsychotique réservé à la prise en charge de la schizophrénie réfractaire au traitement, est associée à des effets indésirables graves, dont la myocardite. Cette étude vise à déterminer l’incidence de la myocardite induite par clozapine dans un grand hôpital de soins tertiaires comparativement à ce que déclare la littérature.
Méthodes:
Les dossiers médicaux de patients adultes hospitalisés dans des unités psychiatriques qui recevaient de la clozapine entre le 1er janvier 2010 et le 31 juillet 2016 ont été examinés rétrospectivement. Les cas de myocardite induite par clozapine ont été définis présenter une protéine C-réactive (PCR) élevée ou une troponine détectable et au moins un signe ou symptôme de myocardite, en l’absence d’autres étiologies plausibles. Le résultat principal était l’incidence de la myocardite induite par clozapine durant la période de l’étude. Les résultats secondaires étaient notamment le taux et la description de la prise en charge de la myocardite induite par clozapine.
Résultats:
Trois cent seize patients ont été examinés; 10 patients répondaient à la définition de cas de la myocardite induite par clozapine. L’incidence de cette réaction indésirable au médicament pour la période de l’étude était de 3,16%. Une diminution de la fraction d’éjection ventriculaire gauche a été observée dans 60% des cas, et des changements à l’électrocardiographie ont été notés dans 60% des cas. La clozapine a été interrompue dans tous les cas. Une nouvelle tentative a été menée chez 2 patients; une élévation récurrente de la PCR a entraîné une interruption dans les 2 cas. Les médicaments pour la prise en charge de la myocardite ont été utilisés dans 50% des cas. Bien que 2 patients aient nécessité un transfert aux soins intensifs, le taux de mortalité à l’hôpital était de 0%.
Conclusions:
L’incidence de la myocardite induite par clozapine à l’hôpital de l’étude correspondait au taux élevé déclaré dans la littérature. Il faut plus de recherche afin d’élucider les facteurs de risque, les critères diagnostiques définitifs, et la prise en charge efficace de la myocardite induite par clozapine.
Clozapine is an effective antipsychotic agent that is typically reserved for the management of treatment-resistant schizophrenia.1 Compared to other antipsychotics, clozapine may be more effective for patients with persistent suicidal ideation and may be beneficial for those with persistent aggression.1,2 While the association between clozapine and potentially fatal agranulocytosis is well established, it is also associated with severe cardiovascular events, including myocarditis.1
The reported incidence of myocarditis varies in the literature, from 0.06% in the product monograph3 to 3.88% in a more recent cohort study in Australia.4 This inconsistency may be due to factors such as lack of consensus in the diagnosis of myocarditis due to its nonspecific clinical presentation and variable adverse drug reaction reporting mechanisms.5 Furthermore, the reported mortality risk in patients with clozapine-induced myocarditis is also variable, with reports ranging from 10% to 30%.6–8 Myocarditis risk has been reported to be highest in the first month of clozapine therapy, particularly in the third week of therapy.7,9 Delayed reactions after several years of clozapine treatment have also been described.10
The exact mechanism of clozapine-induced myocarditis is not well elucidated. It has been postulated to be an immunoglobulin E (IgE)–mediated hypersensitivity reaction, as up to two-thirds of patients with clozapine-induced myocarditis present with hypereosinophilia.8 In animal models, clozapine treatment has been associated with a hypercatecholaminergic state resulting in an inflammatory response and myocardial inflammation,11 as well as increased myocardial oxidative stress, downregulation of endogenous antioxidants, and apoptosis,12 all of which are proposed to contribute to the pathogenesis of myocarditis.
Several risk factors for clozapine-induced myocarditis have been proposed, including increased age, concomitant medications, and rapid dose titration.4,13 One case-control study found that the risk of myocarditis increased by 26% for each additional 250 mg of clozapine administered in the first 9 days of clozapine titration, concomitant sodium valproate doubled the risk, and that risk of myocarditis increased by 31% with each decade in age.13 In contrast, a small, retrospective cohort study found no association between myocarditis risk and increased age, dose titration, or use of sodium valproate but did find a significant association of clozapine-induced myocarditis with use of selective serotonin reuptake inhibitors.4
Initial presentation of clozapine-induced myocarditis can include nonspecific symptoms, such as fever, lethargy, fatigue, diarrhea, vomiting, and dysuria.6,7 Specific signs and symptoms include tachycardia, basal crepitations, third heart sounds, peripheral edema, elevated jugular venous pressure, and chest pain with nonspecific electrocardiogram (ECG) changes. C-reactive protein (CRP) begins to increase at symptom onset, while troponin increases within 1 to 5 days. Cases of clozapine-induced myocarditis without accompanying symptoms have also been reported.7 Left ventricular impairment by echocardiography is seen in approximately two-thirds of patients, but function recovers rapidly (within 5 days) after withdrawal of clozapine. Endomyocardial biopsy is held as a gold standard for diagnosis of myocarditis, but this procedure is rarely performed due to its clinical risks, including bleeding at the access site, transient conduction defects and arrhythmias, tricuspid regurgitation, pulmonary embolism, and right ventricular perforation.14 Cardiac magnetic resonance imaging (MRI) has been proposed as a noninvasive means to confirm myocarditis.14
The management and outcomes of patients with clozapine-induced myocarditis have not been well studied. After withdrawal of the drug, strategies such as general supportive care and possible treatment with diuretics, angiotensin-converting enzyme (ACE) inhibitors, digoxin, or beta-blockers to alleviate symptoms have been described.5 It is believed that clozapine should be permanently discontinued in patients who have experienced myocarditis, although there are some case reports of successful rechallenge in selected patients and cases where myocarditis has resolved spontaneously without discontinuation of the medication.15–17
St. Paul’s Hospital (SPH) is a large tertiary hospital that serves an inner-city population and has 4 general, inpatient mental health units with 60 beds. Preprinted order sets and a monitoring algorithm for neutropenia/agranulocytosis and myocarditis were implemented in 2015 (see Supplemental Figure S1), adapted from a protocol published by Ronaldson et al.7 based on expert consensus. Anecdotally, the incidence of clozapine-induced myocarditis at SPH was thought to be higher than what is reported in the literature. Therefore, this study aimed to determine the incidence of myocarditis in the inpatient setting at SPH and to describe patient outcomes after clozapine-induced myocarditis. Secondary objectives were to characterize demographics and clinical characteristics of patients affected and to describe the common management approaches of clozapine-induced myocarditis.
Methods
This single-centre, retrospective chart review was approved by the University of British Columbia Clinical Research Ethics Board–Providence Health Care Research Ethics Board. The need for informed consent was waived.
Study Population
Medical records of adult patients receiving clozapine, admitted to general psychiatry wards between January 1, 2010, and July 31, 2016, were identified through the hospital pharmacy database and patients’ charts. Patients under 17 years of age and those who developed acute coronary syndrome during their hospital stay were excluded from the study.
Case Definition
Cases of clozapine-induced myocarditis were defined as having signs or symptoms of 1 or more of the following: lightheadedness with orthostatic hypotension or low resting blood pressure (systolic blood pressure <100 mm Hg), fatigue with decreased exercise tolerance, chest pain/discomfort/pressure, palpitations with increased heart rate (HR >100 bpm or increase more than 30 bpm above baseline), shortness of breath, peripheral edema, fever, and any one of the following: any elevation in high-sensitivity troponin T (14 ng/L or more) or elevation in troponin I (>0.05 mcg/L) or CRP >50 mg/L at any point during treatment with clozapine. This case definition had to be met in the absence of other plausible aetiologies.
Outcomes
The primary outcomes were the incidence of clozapine-induced myocarditis during the study period and rate and description of patient outcomes. Secondary outcomes included rate and description of the management of clozapine-induced myocarditis.
Data Collection
Health records were screened and data were obtained between September 2016 and March 2017. Collected data include demographic information, clozapine dosage (dose, frequency, duration), concomitant medications, history of illicit drug use, signs and symptoms of myocarditis (as defined in case definition above), biopsy results, ECG interpretation and echocardiography, and lab values, including CRP, troponin, N-terminal pro b-type natriuretic peptide (NT-proBNP), and eosinophils; time to discontinuation of clozapine after myocarditis; medications used for treatment; length of stay in hospital; transfer to intensive care unit (ICU); rechallenge with clozapine; and patient mortality.
Statistical Analysis
Simple descriptive statistics were performed to evaluate the demographic data, as well as primary and secondary outcome variables.
Results
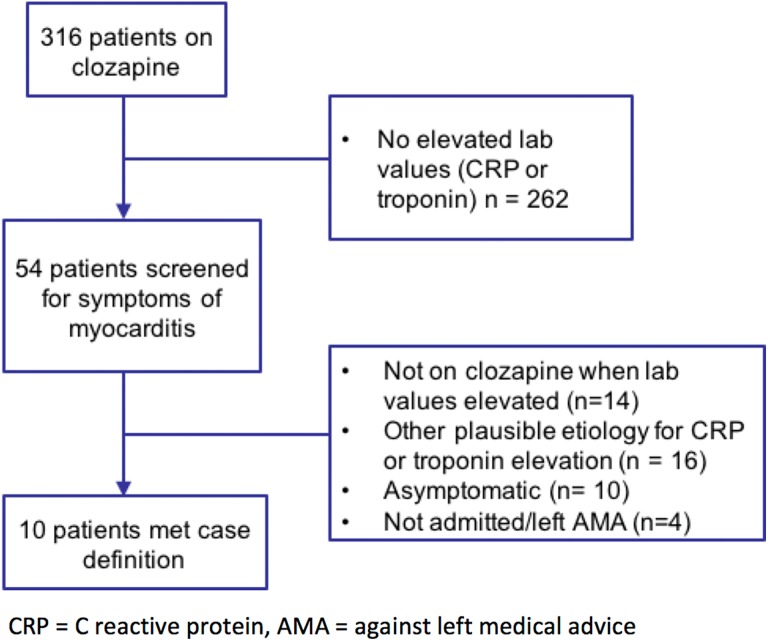
In total, 316 patients met the defined inclusion criteria, as illustrated in Figure 1. Of these, 262 patients did not have elevated CRP or troponin and were assumed not to have myocarditis. Charts of 54 patients with elevated CRP or troponins were screened for symptoms of myocarditis according to the case definition. Thirty-eight patients had elevated CRP, 37 patients had elevated troponin, and 20 patients had both elevated CRP and troponin. Patients were assumed to not have clozapine-induced myocarditis if CRP or troponin elevation did not coincide with when they were on clozapine (n = 14), if there was another plausible aetiology for their signs and symptoms (n = 16), or if they were asymptomatic (n = 4). Four patients who left hospital against medical advice or were not admitted and did not have enough data available to interpret were assumed to not have myocarditis.
Figure 1.
Case identification.
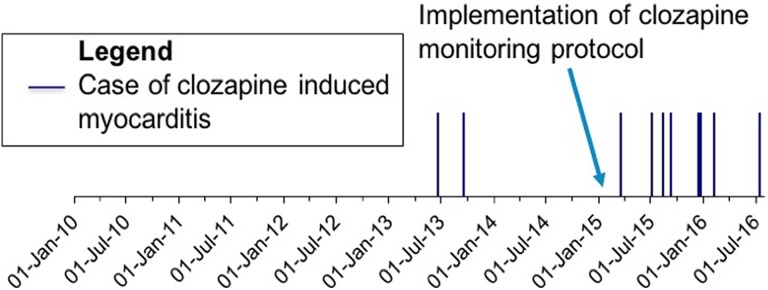
The 10 cases of myocarditis identified represent an incidence of 3.16% over the 6 years and 7 months of the study period. The first case was identified in 2013, with 8 of the 10 cases occurring after implementation of a clozapine monitoring protocol (Figure 2) that formalized myocarditis monitoring practices. Prior to implementation of the monitoring protocol, 237 patients were prescribed clozapine during an inpatient stay at SPH. Following implementation of the monitoring protocol, an additional 79 patients received clozapine as inpatients.
Figure 2.
Timeline of events.
Characteristic features of the identified patients are presented in Table 1. Seven of the 10 patients had a history of illicit drug use, and 5 of these patients had a history of methamphetamine use.
Table 1.
Patient Characteristics.
| Characteristic | Value | ||
|---|---|---|---|
| Age, median (range), y | 48.5 (26-57) | ||
| Sex (male), % | 70 | ||
| BMI, median (range), kg/m2 | 28.5 (19.8-35.7) | ||
| Ethnicity (Caucasian), % | 60 | ||
| History of illicit drug use, n (%) | 7 (70) | ||
| Concomitant Medications | % | Comorbidities | % |
| Other antipsychotic | 80 | Dyslipidemia | 50 |
| Divalproex | 50 | Hepatitis C | 30 |
| Benzodiazepine | 20 | Hypertension | 20 |
| Lithium | 10 | Type 2 DM | 10 |
| Gabapentin | 10 | History of ACS | 10 |
| HIV | 10 | ||
ACS, acute coronary syndrome; BMI, body mass index; DM, diabetes mellitus; HIV, human immunodeficiency virus.
Of the 10 cases, 9 patients were newly initiated on clozapine. One patient had been on clozapine prior to hospital admission at a dose of 450 mg/d. This patient was restarted on a home dose on admission to hospital, which was then titrated up to 550 mg/d over a period of 25 days prior to onset of myocarditis symptoms (Table 2). Valproic acid derivatives have previously been identified as potential risk factors for clozapine-induced myocarditis, and this was found to be a concomitant medication in 50% of cases in our cohort. Selective serotonin reuptake inhibitors (SSRIs), another previously identified potential risk factor, were not used in any of our cases.
Table 2.
Clozapine Dose, Titration, and Symptoms in Patient Cases of Clozapine-Induced Myocarditis.
| Clozapine Dose at Onset of Suspected Myocarditis | 200 mg (100-550 mg) |
|---|---|
| New clozapine starts, median (range): n = 9 | |
| Initial dose, mg | 12.5 (12.5-25) |
| Dose titration, mg/d | 11.31 (5.9-25) |
| Time to onset of suspected myocarditis, days | 16 (10-22) |
| Symptoms Present in Patient Cases | Frequency (%) |
| Fever | 70 |
| Shortness of breath | 70 |
| Lightheadedness with postural BP change or SBP <100 mm Hg | 50 |
| Palpitations with HR above 100 bpm or >30 above baseline | 50 |
| Chest pain, discomfort, or pressure | 40 |
| Fatigue with decreased exercise tolerance | 20 |
| Peripheral edema | 20 |
BP, blood pressure; HR, heart rate; SBP, systolic blood pressure.
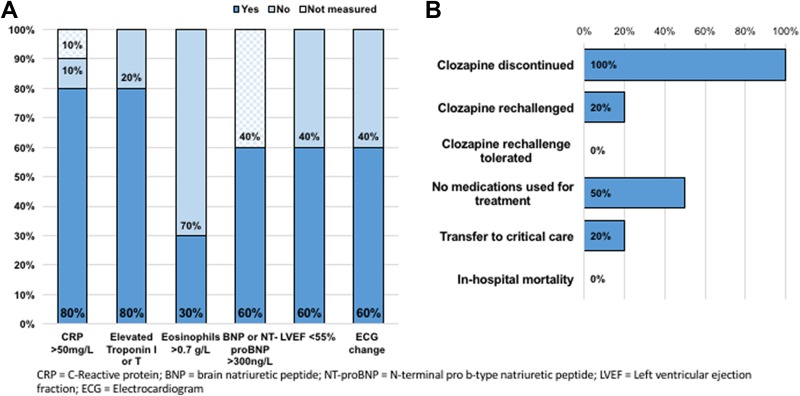
The diagnostic features of the cases are presented in Figure 3a. In the 9 cases where CRP was measured, the mean peak CRP was 145.6 mg/L (interquartile range [IQR], 133.8 to 205.8 mg/L). The method of measuring troponin at our institution changed during the course of our study from troponin I (TnI) to high-sensitivity troponin T (hsTnT). All 10 cases had troponin measured, and elevations were found in 8 of the 10 cases. In the 6 patients with detectable TnI, the median peak elevation was 1.1 mcg/L (IQR, 0.52 to 1.39 mcg/L). Two patients had peak hsTnT of 17 and 18 ng/L respectively. Two patients had elevated CRP in the absence of elevated troponin. Cardiology was consulted in 9 of the 10 cases, in all 9 of these cases, a clinical diagnosis of suspected clozapine-induced myocarditis was made by cardiologists. None of the patients underwent cardiac biopsy to confirm myocarditis. Sixty percent of patients had left ventricular ejection fraction (LVEF) of 55% or lower, and 30% had LVEF less than 40%, as estimated by cardiac MRI or echocardiogram. Cardiac MRI was used as a diagnostic tool in 1 patient case, which revealed inflammation in the myocardium supportive of a clinical diagnosis of myocarditis.
Figure 3.
(a) Diagnostic features. (b) Patient management and outcomes.
Patient management and outcomes are described in Figure 3b. Medications for symptom management were used in 50% of cases, with furosemide being the most commonly used treatment. Discontinuation of clozapine was otherwise sufficient for management of myocarditis in the other 50% of cases. Two patients required transfer to critical care, and there was no inpatient mortality observed.
Two patients were rechallenged with clozapine after experiencing myocarditis. In both cases, after clozapine was reinitiated, CRP increased and clozapine was ultimately discontinued. One of the patients rechallenged at a low dose of 12.5 mg/d had an asymptomatic increase in CRP to a peak of 58.5 mg/L and detectable troponin after 8 days of therapy at this dose. Cardiology was consulted and recommended discontinuation in the context of the patient’s significant cardiac risk factors, including history of acute coronary syndrome and reduced LVEF. The other patient rechallenged developed chest pain, in addition to increased CRP and eosinophilia, which resolved following discontinuation of clozapine.
Discussion
The incidence of myocarditis at SPH is higher than what is reported in the manufacturer’s monograph and within the higher end of the range of what has been described in the literature. The clustering of identified cases after 2015 may reflect the increased awareness and monitoring after the clozapine monitoring protocol was implemented at SPH. The monitoring protocol included daily nursing assessments for signs and symptoms of myocarditis, as well as baseline and weekly monitoring of CRP and troponin for the first 4 weeks of treatment. One can postulate that the true, overall incidence may be higher after implementation of increased monitoring. Conversely, the true incidence may also be lower than what we found due to lack of generally accepted diagnostic criteria and potential for misdiagnosis on the basis of fairly general symptoms and nonspecific diagnostic tests. Two symptoms used in our monitoring protocol and the case definition for myocarditis, orthostasis and tachycardia, are fairly common adverse effects of clozapine, which may make it difficult for providers to interpret if the patient is experiencing a typical side effect rather than myocarditis. To help elucidate this at our institution, a combination of clinical and laboratory findings is used and cardiology consults are recommended in our monitoring protocol.
Regular monitoring for potential clozapine-induced neutropenia is mandatory; patients on clozapine must be enrolled in a registry program before the drug can be dispensed.3 In contrast, monitoring for potential myocarditis is not regularly performed by clozapine prescribers.18 Given the incidence of myocarditis found in our study, further development and implementation of clozapine monitoring protocols to include myocarditis monitoring is an important undertaking.
This study had several limitations. Due to the retrospective nature of the study, cases of myocarditis may have been missed if CRP or troponins were not tested or signs and symptoms were not documented in the chart. Only inpatient data were available, and thus clozapine-induced myocarditis that occurred after patients were discharged from hospital would not be captured unless they presented at SPH during the study period. Last, short of a cardiac biopsy, there is no clear consensus on how to diagnose clozapine-induced myocarditis, and due to the invasive nature of this procedure, none of the patients in our study with presumptive myocarditis had this test performed.
Due to the small number of cases, we were unable to run a logistic regression to look at risk factor association. Further studies, with a larger sample size, are necessary to clarify and validate potential risk factors.
Although clozapine-induced myocarditis has been postulated to be a type I hypersensitivity reaction, only 30% of patient cases had elevated eosinophils. This may reflect the contribution of other mechanisms in the pathogenesis of this adverse event. BNP or NT-proBNP was elevated in all patients who had these lab tests performed and may represent an important marker for recognizing this potentially fatal adverse effect.
At least mild decreases in LVEF were detected in 60% of patients, but none of the patients had a baseline echocardiogram for comparison. Impairment of LVEF has also been observed in asymptomatic patients on low doses of clozapine and in patients chronically treated with the drug.19 Some centres have instituted baseline echocardiograms for all patients newly initiated on clozapine.7 This may aid in the diagnosis of clozapine-induced myocarditis and cardiomyopathy, but at the expense of increased cost and utilization of health care resources.
Management of all patient cases involved discontinuation of clozapine. There have been published cases where myocarditis has resolved despite continuation of clozapine.15,16 As there are no tests available to differentiate infectious versus drug-induced myocarditis, in some of these cases, clozapine discontinuation may not have been necessary due to another plausible aetiology for the condition. Determining which patients require immediate cessation of the drug rather than continuation with careful monitoring is an ongoing clinical dilemma.
Rechallenge with clozapine was unsuccessful in the 2 patients in whom it was attempted. Working to develop an optimal method of rechallenge is an important avenue of further research, as clozapine may otherwise be the most effective drug to manage the patient’s treatment-resistant schizophrenia.
Conclusion
In this study, the incidence of clozapine-induced myocarditis was 3.16% over the 6-year and 7-month study period, comparable with the higher end of the range described in the literature. This finding suggests that cardiac monitoring protocols are warranted given the frequency of this adverse effect and risk of fatal outcome. Notably, withdrawal of clozapine was sufficient for management in a majority of cases. Further research, with a larger sample size, is necessary to elucidate risk factors, to determine definitive diagnostic criteria, and to optimize strategies for appropriate rechallenge.
Supplemental Material
Supplemental Material, Clozapine_monitoring_protocol for Incidence and Management of Clozapine-Induced Myocarditis in a Large Tertiary Hospital: Incidence et prise en charge de la myocardite induite par clozapine dans un grand höpital de soins tertiaires by Julia M. Higgins, Cindy San, Gillian Lagnado, Doson Chua and Tamara Mihic in The Canadian Journal of Psychiatry
Footnotes
Data Access: The data sets generated during the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Canadian Psychiatric Association. Clinical practice guidelines: treatment of schizophrenia. Can J Psychiatry. 2005;50(13, Suppl 1):7S–57S. [PubMed] [Google Scholar]
- 2. Meltzer HY, Alphs L, Green AI. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82–91. [DOI] [PubMed] [Google Scholar]
- 3. Clozaril [clozapine tablets]. Drug Product Database [Internet]. Ottawa (ON): Health Canada; 2016. [cited 7 July 2016]. Available from: http://webprod5.hc-sc.gc.ca/dpd-bdpp/info.do?code=11422&lang=eng. [Google Scholar]
- 4. Youssef D, Narayanan P, Gill N. Incidence and risk factors for clozapine-induced myocarditis and cardiomyopathy at a regional mental health service in Australia. Australas Psychiatry. 2015;24(2):176–180. [DOI] [PubMed] [Google Scholar]
- 5. Reinders J, Parsonage W, Lange D, et al. Clozapine-related myocarditis and cardiomyopathy in an Australian metropolitan psychiatric service. Aust N Z J Psychiatry. 2004;38(11-12):915–922. [DOI] [PubMed] [Google Scholar]
- 6. Haas S, Hill R, Krum H, et al. Clozapine-associated myocarditis. Drug Saf. 2007;30(1):47–57. [DOI] [PubMed] [Google Scholar]
- 7. Ronaldson K, Fitzgerald P, Taylor AJ, et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45(6):458–465. [DOI] [PubMed] [Google Scholar]
- 8. Barry A, Windram J, Graham M. Clozapine-associated myocarditis: case report and literature review. Can J Hosp Pharm. 2015;68(5):427–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ronaldson K, Taylor A, Fitzgerald P, et al. Diagnostic characteristics of clozapine-induced myocarditis identified by an analysis of 38 cases and 47 controls. J Clin Psychiatry. 2010;71(8):976–981. [DOI] [PubMed] [Google Scholar]
- 10. Lang U, Willbring M, von Golitschek R, et al. Clozapine-induced myocarditis after long-term treatment: case presentation and clinical perspectives. J Psychopharmacol. 2008;22(5):576–580. [DOI] [PubMed] [Google Scholar]
- 11. Wang J, Min J, Hampton T, et al. Clozapine-induced myocarditis: role of catecholamines in a murine model. Eur J Pharmacol. 2008;592(1-3):123–127. [DOI] [PubMed] [Google Scholar]
- 12. Abdel-Wahab B, Metwally M. Clozapine-induced cardiotoxicity: role of oxidative stress, tumour necrosis factor alpha and NF-κβ. Cardiovasc Toxicol. 2014;15(4):355–365. [DOI] [PubMed] [Google Scholar]
- 13. Ronaldson K, Fitzgerald P, Taylor A, et al. Rapid clozapine dose titration and concomitant sodium valproate increase the risk of myocarditis with clozapine: a case-control study. Schizophr Res. 2012;141(2-3):173–178. [DOI] [PubMed] [Google Scholar]
- 14. Curto M, Girardi N, Lionetto L, et al. Systematic review of clozapine cardiotoxicity. Curr Psychiatry Rep. 2016;18(7):68. [DOI] [PubMed] [Google Scholar]
- 15. Manu P, Sarpal D, Muir O, et al. When can patients with potentially life-threatening adverse effects be rechallenged with clozapine? A systematic review of the published literature. Schizophr Res. 2012;134(2-3):180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang J, Mihic T, Fennemore M. A case of spontaneous resolution of myocarditis in a schizophrenic patient treated with clozapine without treatment discontinuation. Asian J Psychiatry. 2018;35:24–25. [DOI] [PubMed] [Google Scholar]
- 17. Ronaldson K, Fitzgerald P, Taylor A, et al. Continuation of clozapine following mild myocarditis. Aust N Z J Psychiatry. 2012;46(9):910–911. [DOI] [PubMed] [Google Scholar]
- 18. Goldsmith D, Cotes RO. An unmet need: a clozapine-induced myocarditis screening protocol. Primary Care Compan CNS Disord. 2017;19(4):16102083. [DOI] [PubMed] [Google Scholar]
- 19. Chow V, Yeoh T, Ng A, et al. Asymptomatic left ventricular dysfunction with long-term clozapine treatment for schizophrenia: a multicentre cross-sectional cohort study. Open Heart. 2014;1(1):e000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Clozapine_monitoring_protocol for Incidence and Management of Clozapine-Induced Myocarditis in a Large Tertiary Hospital: Incidence et prise en charge de la myocardite induite par clozapine dans un grand höpital de soins tertiaires by Julia M. Higgins, Cindy San, Gillian Lagnado, Doson Chua and Tamara Mihic in The Canadian Journal of Psychiatry