Abstract
Stem cell has immense potential in regenerative cellular therapy. Mesenchymal stem cells (MSCs) can become a potential attractive candidate for therapy due to its remarkable ability of self-renewal and differentiation into three lineages, i.e., ectoderm, mesoderm, and endoderm. Stem cell holds tremendous promises in the field of tissue regeneration and transplantation for disease treatments. Globally, medicinal plants are being used for the treatment and prevention of a variety of diseases. Phytochemicals like naringin, icariin, genistein, and resveratrol obtained from plants have been extensively used in traditional medicine for centuries. Certain bioactive compounds from plants increase the rate of tissue regeneration, differentiation, and immunomodulation. Several studies show that bioactive compounds from plants have a specific role (bioactive mediator) in regulating the rate of cell division and differentiation through complex signal pathways like BMP2, Runx2, and Wnt. The use of plant bioactive phytochemicals may also become promising in treating diseases like osteoporosis, neurodegenerative disorders, and other tissue degenerative disorders. Thus, the present review article is aimed at highlighting the roles and consequences of plant extracts on MSCs proliferation and desired lineage differentiations.
1. Background
Stem cells are precursor biological cells that have the ability to self-renew and differentiate into multiple mature cells [1]. Stem cells divide into two major categories, i.e., embryonic stem cells and adult stem cells. Depending upon the differentiation capacity, they can be classified into unipotent, multipotent, pluripotent, or totipotent stem cells. These cells provide the platform to investigate cellular development, maintenance, and differentiation [2]. In 1976, Friedenstein and his coworkers discovered MSCs from mouse bone marrow [3]. MSCs are multipotent stem cells which are nonhematopoietic and possess the ability to differentiate into multilineage cells. The International Society for Cellular Therapy (ISCT) proposes minimal criteria to define human MSC: they are plastic adherent; express CD105, CD73, and CD90; lack expression of CD45, CD34, CD14 or CD11b, CD79a or CD19, and HLA-DR surface molecules; and are capable of differentiating into multilineage cells, i.e., osteoblasts, adipocytes, and chondroblasts in vitro [4]. Human MSCs show morphological subpopulation like rapidly self-renewing cells, spindle-shaped cells, and flattened cells (FC) [5]. Several studies have shown that under standard environmental condition, MSCs can be isolated from different sites including the bone marrow [6, 7], adipose tissue [8, 9], cord and peripheral blood [10, 11], placenta [12], umbilical cord [13, 14], fetal liver [15], fetal lungs [16], dental pulp [17, 18], periodontal ligament [19], trabecular bone [20], compact bone [21], synovial membrane [22], cruciate ligaments [23], amniotic fluid [24, 25], and endometrium [26]. MSCs have been used for several clinical trials for tissue repairing and treating immune-mediated disease including cardiac ischemia, limb ischemia, amyotrophic lateral sclerosis, diabetes, ischemic stroke, osteoarthritis, liver cirrhosis, liver failure, graft versus host disease, Crohn's disease, multiple sclerosis, respiratory distress syndrome, amyloidosis, and rheumatoid arthritis [27–29]. At the National Institute of Health (NIH), USA, several clinical trials are running in different aspects of MSCs used for treatment and regenerative therapy. A total of 945 studies have been found that involve the use of MSCs for different clinical phages among which 264 studies have been completed. Among them, some studies have used dietary supplements including herbal compounds for the trial [30].
From the initial development of human civilization, plants have been used as a medicine for improving growth and development. Medicinal plants are widely acceptable for the treatment of a variety of diseases. The World Health Organization (WHO) declared that the best sources of a variety of drugs are plant derivatives [31]. Globally, around 75% of the population from the developing and developed nations like Britain, Germany, and France use plants and their extracts as a medicine [32]. Out of 150 thousand plants being studied, med-clinically important components have been observed in many of them [33]. Plant derivatives have shown to promote stem cell proliferation and multilineage differentiation. The bioactive compounds obtained from plant extracts could become an alternative, cost effective treatment for bone marrow transplantation and cancer [34]. Most plants have been used both traditionally and therapeutically, but the exact mechanism of action on MSCs of only few plant extracts has been proved. Establishing the differentiation of MSCs into desired lineage-committed progenitors in the presence of a certain plant extract can open a new horizon for regenerative medicine and treatment. Thus, the present review highlights the role of bioactive compounds from plant extracts on MSCs proliferation and differentiation and their use in regenerative therapy and medicine.
2. MSCs Proliferation Potential
MSCs are divided by mitosis but are not capable of unlimited cell division in vitro due to senescence, also called irreversible growth arrest phenomenon first described by Hayflick in the 1960s [35]. Increased expression of senescence-associated β-galactosidase (SA-β-Gal) is responsible for stoppage of further division of MSCs [36]. With the increase in SA-β-Gal gene expression and accumulation of excessive reactive oxygen species (ROS) and progressive shortening of the telomeres or modified telomeric structure [35, 37, 38], morphological and biological changes occur and cell undergoes senescence. Morphologically, MSCs change into enlarged and irregular-shaped cells. Different studies reported that single cell-derived colonies of MSCs can expanded up to 30-50 population doublings in about 10-18 weeks [39–41]. In passages 6 and 12, population doubling time (PDT) is the shortest for umbilical cord-derived stem cell (UC-MSC) compared to bone marrow-derived stem cell (BM-MSC) and adipose tissue-derived stem cell (AT-MSC); also, the proliferation rate is the highest from UC-MSCs [42]. The proliferation and persistence rates of stem cells have been influenced by tissue sources, donor's age, and culture conditions [43]. In addition, older donor's cells (>66 years) have lower proliferative ability than younger ones (about <30 years of age) and pediatric donors have the highest proliferation rate in in vitro standard condition [41, 44]. Meanwhile, the absence of irreversible growth arrest could mean neoplastic transformation of MSCs. Furthermore, the culture system also influences homing and differentiation abilities of stem cells. The three-dimensional culture system has more expansion than the two-dimensional culture system [45]. The study has shown that UC-MSCs exhibit a higher proliferation capacity than BM-MSCs [46] and BM-MSCs have greater proliferation capability compared to muscle-derived stem cells (MD-MSCs) and AT-MSCs [47].
3. MSCs Multilineage Differentiation Potential
According to ISCT criteria, MSCs must be able to differentiate into multilineage cells including osteoblasts, adipocytes, and chondroblasts but it depends upon in vitro conditions as well as the cell source [4]. Depending upon the source, UC-MSCs have high potential to differentiate into osteoblast, chondrocyte, adipocyte, skeletal muscle cells, endothelial cells, cardiomyocyte-like cells, and neuronal cells. BM-MSCs differentiate into osteoblast, chondrocyte, adipocyte, tenocyte, and vascular smooth muscle cells. In addition, periosteum MSCs (P-MSCs), synovial MSCs (S-MSCs), adipose tissue MSCs (AT-MSCs), circulating MSCs (C-MSCs), and tendon-derived MSCs (TD-MSCs) also have potential of multilineage differentiation under in vitro standard condition [47].
3.1. Adipogenic Differentiation
Adipocyte-specific gene expression, which brings the appearance of intracellular lipids, characterizes phenotypic adipocyte. Sequential action of transcription factors C/EBPβ (CCAAT/enhancer binding protein β), C/EBPα (CCAAT/enhancer binding protein α), and PPARγ (peroxisome proliferator-activated receptor γ) is necessary for 3T3-L1 preadipocyte differentiation [48]. Mitochondrial metabolism is important for adipocytic differentiation by increased expression of UCP-1, UCP-2, and UCP-3 mRNA. The increased level of UCP1 is associated with the brown fat phenotype in newly differentiated adipocytes [49]. In addition, fibroblast growth factor-2 (FGF2) and 17-beta estradiol have induced adipocyte characteristics in cell [50, 51]. Studies show that BM-MSCs [52], S-MSCs [53], and UC-MSCs [54] differentiate into adipocytes. In the presence of dexamethasone and insulin supplement in the medium, UC-MSCs differentiate into adipocytes [54].
3.2. Chondrogenic Differentiation
Transforming growth factor-beta (TGF-β) and bone morphogenetic proteins (BMPs) are the most important inducers for chondrogenic differentiation of MSCs [55]. The activation of the Wnt signaling pathway is also involved in chondrogenesis and development of cartilage, and this pathway is activated by glycogen synthase kinase 3 (GSK-3) [56, 57]. Several studies showed that MSCs from different sources differentiated into chondrocytes including BM-MSCs [11, 58], S-MSCs [59], AD-MSCs [60], peripheral blood MSCs (PB-MSCs) [11], and TD-MSCs [61]. Under controlled in vitro condition, supplements such as transforming growth factor-β1, ascorbate-2-phosphate, dexamethasone, and growth and differentiation factor-5 (GDF5) [54, 62, 63] promote chondrogenic differentiation. Formation of shiny cell spheres which express type II collagen in cultures is the evidence for chondrogenic differentiation of MSCs which can be demonstrated by molecular technique and immunohistochemistry.
3.3. Osteogenic Differentiation
The two important transcription factors that promote osteoblastic differentiation are runt-related transcription factor 2 (Runx2) and osterix (Osx) [64]. Osterix (Osx) also called Sp7 belonging to the Sp transcription factor family is regulated by Runx2 that specifically binds with the Osx promoter region that regulates osteoblast differentiation in vitro and in vivo [65]. The role of Runx2 in osteogenic regulation is by the formation of heterodimer with cotranscription factor core-binding factor beta (Cbf β) and binding to DNA [66, 67]. In addition, the MSC to osteogenic differentiation increases the expression of early-marker alkaline phosphatase gene and late-marker osteopontin gene [24]. Of the different sources of MSCs differentiating into osteoblast-like BM-MSCs [52], S-MSCs, P-MSCs [59], or AT-MSCs [53], in vitro supplements including dexamethasone, β-glycerophosphate, ascorbic acid, and 1,25-dihydroxy-vitamin D3 help in osteogenic differentiation from MSCs [68–71]. The differentiation can be demonstrated by detection of the Runx2 gene by a molecular method and also von Kossa or alizarin red staining methods.
3.4. Tendocytic Differentiation
Tendons are tissues of mesodermal origin. MSCs are also considered promising for tendon repair in cell-based therapy. Expression of the transcription factor Scleraxis (Scx) regulates the tendon formation [72]. Mohawk activation is essential for tendon development and to modulate the expression of Scx and tendon-specific extracellular matrix molecules both in vitro and in vivo [73]. Another cytokine called bone morphogenetic protein-12 (BMP-12) [74] also known as growth factor and differentiation factor [75] is superiorly capable of promoting repair of tendon as well as tendon-like tissue formation from MSCs. Studies showed that BM-MSCs [76] and TD-MSCs [77] can differentiate into tendocyte.
3.5. Neurogenic Differentiation
In a normal state, MSCs express low levels of neural gene markers, such as nestin, Nurr1, enolase 2, glial fibrillary acidic protein (GFAP), and beta-tubulin III [78]. MSCs also differentiate into NSC-like cells under specific culture conditions that are morphologically and phenotypically similar [79]. This indicates that MSCs have the capability to differentiate into nonmesenchymal-origin cells in the presence of stimuli. In the presence of growth factors, NSCs differentiated into the neural phenotypes: astroglia, oligodendroglia, and neurons [80]. Along with this, increased expression of neuronal markers—neuron-specific enolase (NSE), β-tubulin III, neurofilament-M (NF-M), and microtubule-associated protein 2 (MAP2)—has been observed in vitro [81]. Neuronal cells can be derived from BM-MSCs [78, 79], amniotic fluid MSCs (AF-MSCs) [25], and UC-MSCs [80]. Neurons cells can be detected by using histochemical staining for neuronal Nissl bodies.
3.6. Smooth Muscle Differentiation
MSCs differentiation into functional smooth muscle cells (SMCs) requires potential regulators miR-503 and miR-222-5p. Stimulation of transforming growth factor-β1 (TGFβ1) is required for genotypic and phenotypic expression and acts as a strong inducer of myogenic differentiation of MSCs [82]. TGF-β3 also induces MSCs differentiation into SMCs by activating myocardin and myocardin-related transcription factor-A (MRTF-A) [83]. In addition, involvement of sphingosylphosphorylcholine induces contractile SMCs differentiation from human adipose tissue-derived MSCs [84].
4. Effect of Medicinal Plant Extracts on MSCs
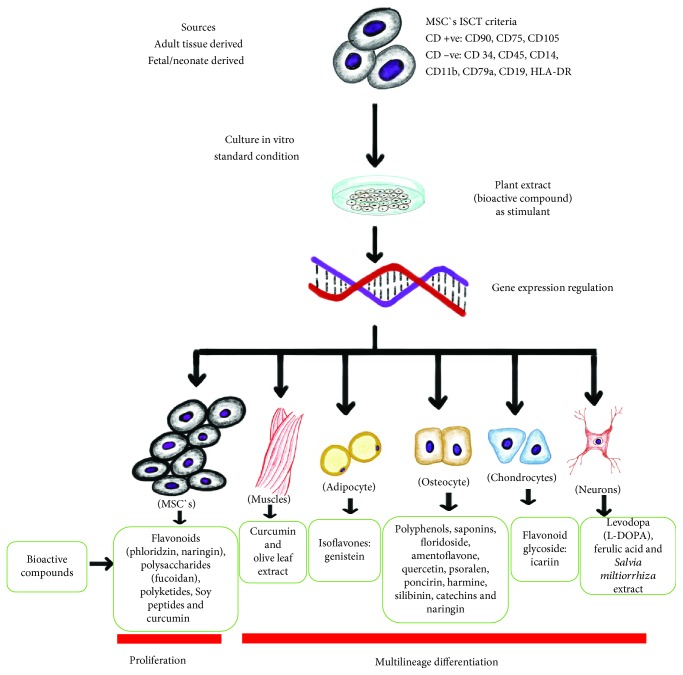
Globally, plants and their products are used for improving health. Plants have been providing endless sources of medicine throughout history. Their method of production, purpose, and method of use vary. The USA has categorized plants into dietary supplements (intended to supplement the diet and usually consist of vitamins and minerals), drugs (over-the-counter drugs), and botanical drugs (complex extracts used for treatment) [85]. Extracts from different parts of a plant (root, bark, flower, leaf, and seed) may be used for different therapeutic purposes. Ayurveda, South-East and Middle-East Asian, and Chinese traditional medicines are the roots for use of natural products in treating diseases. Plant extracts contain bioactive compounds like polyphenols, flavonoids, and many other compounds and chemical substances which play important roles to treat both communicable and noncommunicable diseases [86]. Due to health benefits, phytochemicals from plants generate a lot of interest, demanding further scientific evaluation [87]. According to the National Institutes of Health, USA database, of the 680 clinical trials on MSCs, 27 have used dietary supplements including herbal compounds [86]. Natural compounds isolated from blueberry, green tea, catechin, carnosine, and vitamin D3 have shown to promote the proliferation of stem cell of bone marrow. Dietary fatty acids (oleic acid and linoleic acid) promote the proliferation of haemopoietic stem cells [34]. Under standard in vitro condition, supplementing plant extract may induce increased rates of MSCs proliferation and multilineage differentiation, as shown in Figure 1. Moreover, studies have shown that extracts also increase pluripotent stem cell proliferation and anticancer potency.
Figure 1.
MSCs isolated from different sources derived from adult and fetal tissues. MSCs must be positive for cluster of differentiation CD90, CD75, and CD105 and negative for CD34, CD45, CD14, CD11b, CD79a, CD19, and HLA-DR according to ISCT criteria [4]. Bioactive compound derived from plants regulates MSC gene expression, which may be responsible for the cellular proliferation and multilineage differentiation into osteocyte, muscle cells, nerve cells [86, 88], adipocyte [89], and chondrocyte [90].
5. Proliferation and Differentiation Stimulants
Medicinal plants and herbs have always been valuable in disease treatment. Recently, researchers have investigated and identified those pharmacologically active substances which are responsible for disease prevention and treatment. Recently, medicinal plants have received considerable attention as stimulants for stem cell proliferation in vivo and in vitro [34, 91, 92]. In vitro studies of natural bioactive compounds have suggested that plant-derived substances enhance the adult stem cell proliferation and on the other hand inhibit the proliferation of cancer cells [86]. Several studies have suggested that the proliferation ability of MSCs is influenced by the dose of the stimulant compound, where higher doses of cellular toxicity appear. Using 1-100 μg/ml extract from a citrus increased the human BM-MSCs proliferation and osteogenic differentiation, while using 200 μg/ml concentration decreases BM-MSCs growth [93]. In rat BM-MSCs, naringin 50 μg/ml concentration increased growth of MSCs and a higher concentration at 100 μg/ml suppressed the rate of proliferation [94]. In addition, extracts from brown algae Laminaria japonica (fucoidan) enhance the proliferation of human-derived MSCs when using 0.1–10 μg/ml concentration [95]. Studies have shown MSCs differentiation into osteogenic, neurogenic, and endothelial/vascular progenitor cells in the presence of plant extract supplements. Certain phytochemicals may increase the cellular proliferation and at the same time reduce the time required, as shown in Table 1. The effects of plant extracts on MSCs differentiation and their possible mechanism have been shown in Table 2.
Table 1.
Effect of plant extract on MSC proliferation.
| Plant | MSC source | Mechanism of action | References |
|---|---|---|---|
| Epimedium pubescens (TCM) | hBMSCs | 20 μg/ml increases significant proliferation | [96] |
| Glycine max var. (vegetable soy peptides) | hAD-MSCs and CB-MSCs | 25% and 20% increase cell proliferation rate, and TGF-β1 plays a crucial role to induce proliferation | [97] |
| Ocimum basilicum | hDP-MSCs and BM-MSC | Induces MSC proliferation and reduces doubling time (DT) at 10 μg/ml concentration | [98] |
| Paullinia cupana (guaraná) | hAD-MSCs | 5 and 10 mg/ml concentrations stimulate proliferation. Increases the catalase (CAT) activity and SOD2, CAT, and GPx gene expression | [99] |
| Glycyrrhiza glabra (licorice root) | hBM-MSC | Increases significant level of proliferation at concentration 10-50 μg/ml | [100] |
| Thymbra spicata var. intricata | h-Dental pulp (DP) and BM-MSCs | Reduces the doubling time (DT) at 10 μg/ml for MSCs and acts as a good proliferation inducer | [101] |
| ZD-I: TCM | Telomerized hMSCs | 0.78–25 μg/ml stimulates the proliferation | [102] |
| Rhizoma drynariae | hBM-MSC | 0–200 μg/ml concentration of the naringin solution enhances the proliferation | [93] |
| Foeniculum vulgare | hBM-MSC | Proliferation activity is seen with a dose of 5 μg/ml | [103] |
| Cissus quadrangularis (Linn.) | Wistar rat BM-MSCs | 300 μg/ml concentration increases the proliferation rate by 2-fold | [91] |
| Apple | h-AD MSCS and CB-MSCs | Proliferation promotes by ERK-dependent cytokine production | [104] |
| Ferula gummosa | hBM-MSCs | 0.5 to 5 μg/ml increases significant cell proliferation | [105] |
| Ginkgo biloba | hBM-MSCs | 25 mg/l increases the cell proliferation by 30% | [106] |
Table 2.
Effect of plant extracts on MSC differentiation.
| Plant extracts | MSC source | Differentiate into | Mechanism of action | References |
|---|---|---|---|---|
| Fructus Ligustri Lucidi (FLL) | — | Osteogenic | Increases ALP activity, expression of osteogenesis-stimulating genes, β-catenin, BMP-2, cyclin D1, MT1MMP (membrane type-1 matrix metalloproteinase), osteoprotegerin, and TBX3 (T-box 3) | [107] |
| Fructus Ligustri Lucidi (FLL) | Rat MSC | Osteogenic | Increases ALP activity, osteoprotegerin- (OPG-) to-receptor activator for nuclear factor-κB ligand (RANKL) mRNA level increase | [108] |
| China Herba epimedii | h BM-MSC | Osteogenic | Increases ALP activity and enhances mRNA expression of BMP-2, Runx2 (runt-related transcription factor 2), and OPN (osteopontin) | [109] |
| Rhizoma drynariae | hBM-MSC | Osteogenic | Increases expression of ALP, collagen I, osteopontin, and osteocalcin genes | [93] |
| Ferula gummosa | hBM-MSC | Osteogenic | Increases alkaline phosphatase activity | [105] |
| TCM: ZD-I | Telomerized hMSCs | Osteogenic | Increases mRNA expression of ALP, Runx2, and osteocalcin | [102] |
| Ginkgo biloba | hBM-MSC | Osteogenic | Increases transcriptional levels of bone morphogenetic protein 4 (BMP4), runt-related transcription factor 2 (Runx2), β-catenin, and cyclin D1 | [106] |
| Berberis aristata | h BM-MSC | Osteogenic | Enhances Runx2, osteocalcin (OCN), and osteopontin (OPN) expression and activation of the canonical Wnt/β-catenin pathway | [110] |
| Mucuna gigantea | hBM-MSC | Neurogenic | Increases expression of mRNA for nestin (a neural precursor marker) and β-tubulin III (an immature neuron marker) | [111] |
| Salvia miltiorrhiza | hUC-WJ MSCs | Neurogenic | Induces expression of nestin, beta-tubulin III, neurofilament (NF), and glial fibrillary acidic protein (GFAP) | [112] |
| Olea europaea leaf | — | Endothelial/vascular genesis | Increases gene expression for vascular endothelial growth factor, platelet-derived growth factor receptor, and vascular endothelial growth factor receptor (VEGFR)-1 | [113] |
| Salvia miltiorrhiza | hMSC | Osteogenic | Increases expression of alkaline phosphatase activity, osteopontin, Runx2, and osterix and promotes osteogenesis by activating the ERK signaling pathway | [114] |
| Angelica sinensis | AD-MSCs | Neurogenic | Increases expression of neuron-specific enolase (a specific marker of neurons) | [115] |
| Epimedium pubescens (TCM) | hM-MSCs | Osteogenic | Increases activity of ALP and the amount of calcified nodules and expression of BMP-2 also increase | [96] |
| Ocimum basilicum | DP-MSCs | Osteogenic | Osteonectin and osteocalcin levels increase | [98] |
| Glycyrrhiza glabra (licorice root) | hBM-MSC | Osteogenic | Osteocalcin, Runx2, BMP2, and ALP gene expression upregulate | [100] |
| Foeniculum vulgare | hBM-MSC | Osteogenic | 17β-Estradiol and ALP activity increase | [103] |
| Thymbra spicata var. intricata | h-DP and BM-MSCs | Osteogenic | Osteocalcin (OCN) (late osteogenic marker) level increases | [101] |
| Cissus quadrangularis (Linn.) | Wistar rat BM-MSCs | Osteogenic | Increases ALP activity | [91] |
5.1. Phytochemical Compounds
5.1.1. Naringin
Naringin (naringenin 7-O-neohesperidose) belongs to the flavonoid group, has an antioxidant effect, is anticancerous, and is used for reducing the cholesterol level. It is also used for the treatment of bone disorders like osteoporosis and osteoarthritis. Naringin has a potential to induce proosteogenic effects which could promote the proliferation of stem cell [116]. In in vitro condition, it has shown to enhance the osteogenic differentiation by increasing the expression of Runx2, OXS, OCN, and Col1 and increase the proliferation by activating the ERK signaling pathway on human BM-MSCs [117]. In rat BMSCs, naringin increases the mRNA levels of osteogenic genes and Notch1 expression [94]. In human amniotic fluid-derived stem cells (hAFSCs), naringin promotes osteogenesis via BMP and Wnt-β-catenin signaling pathways. In addition, it increases the expression of bone morphogenetic protein 4 (BMP4), runt-related transcription factor 2 (Runx2), β-catenin, and cyclin D1 in a dose-dependent manner by 1-100 μg/ml [118]. At 1 μM concentration, it promotes the proliferation and differentiation of human periodontal ligament stem cells (hPDLSCs) both in vitro and in vivo [119]. The proliferation and differentiation are dependent on the dose of naringin in dog-originated BM-MSCs [120]. Rhizoma drynariae is used commonly in the treatment of osteoporosis and bone nonunion in traditional Chinese medicine [93]. The flavanone may become a potential therapeutic candidate to promote the osteogenesis.
5.1.2. Icariin
Icariin (ICA) is the main extract of Herba epimedii which is widely used in traditional Chinese medicine (TCM). Icariin, a natural flavonoid glycoside, possesses anti-inflammatory (through inflammatory cytokines and phosphorylation of p38 and JNK) [121], antiatherosclerosis [122], and anticancer [123] activities and treats type 2 diabetes mellitus [124]. ICA promotes bone formation by stimulating osteogenic differentiation of BMSCs. ICA can promote chondrogenic differentiation by activating the Wnt/β-catenin signaling pathway [90]. In rat BMSCs, proliferation is achieved by activating ERK and p38 MAPK signaling [125]. In Sprague-Dawley (SD) rats, ICA has shown to increase the phosphorylation level of GSK-3β and cyclin D1 protein in BM-MSCs [126]. Icariside II (ICA II) is a kind of metabolite of ICA (loss of the glycosyl moiety at the C-7 position of ICA) [127]. Icariside II (ICS II) is a prenylated active flavonol and has antiosteoporosis, antihypoxia, and anticancer activities. ICS II increases ALP activity and calcium deposition which enhance the osteogenic differentiation of BMSCs at optimal concentration [128] also via enhanced expression of osteogenesis proteins/genes and increases the PI3K/AKT/mTOR/S6K1 signaling pathways [129, 130]. It promotes osteogenesis by upregulating Runx2, ALP, and collagen I and inhibits adipogenesis by downregulating PPARγ, Fabp4, and adipsin gene expression [131].
5.1.3. Genistein
Genistein has structural similarity to human estrogen, so it is also called phytoestrogen. It is one of the most abundant isoflavones in soy. Isoflavones belong to the group of flavonoids, and they act as phytoestrogens, antioxidants, and anticancer agents. Genistein when added to medium (10−7 M and 10−8 M) promotes bone formation and also increases the level of alkaline phosphatase activity and DNA content [132]. Genistein promotes the h-BMSCs (human-BMSCs) to osteogenic differentiation through an ER-dependent mechanism. Also, BMP-dependent SMADs and Runx2 signaling play important roles in the process [133]. In addition, it could stimulate differentiation through the p38 MAPK-Cbfa1 pathway [134]. However, studies have shown that it also induces adipogenic differentiation, promotes triglycerides activity in hBMSC, and suppresses osteogenic potential by upregulating the expression of PPAYγ [89]. An in vitro study shows that genistein stimulates hMSC-induced cellular proliferation and survival of cells and enhances antiapoptotic capacity [135].
5.1.4. Hyaluronic Acid
Hyaluronic acid (HA) as a potential agent for medical use is already documented. HA in combination with BMSCs enhances cartilage regeneration for chondral defects in canines [136]. In addition, an in vivo study done in pigs found that HA with MSCs improves the cartilage healing both histologically and morphologically at 6 and 12 weeks after injection [137]. In humans, HA increases the proliferation which is dose and time dependent. In HA-treated amniotic MSCs, upregulation of the expression of the Wnt/β-catenin pathway has been seen which enhances mRNA expression and protein level of wnt3a, β-catenin, and cyclin D1 [138].
5.1.5. Resveratrol
Resveratrol (RSVL) is a natural type of polyphenolic phytoestrogen. RSVL is mainly found in red grapes, blueberries, peanuts, and other plants [139]. The effect of RSVL on stem cell is well documented. It enhances the hBMSC proliferation and potential to differentiate into osteocyte by activation of extracellular signal-regulated kinase 1/2 (ERK1/2) and p38 mitogen-activated protein kinase (MAPK) signaling through an ER-dependent mechanism [140]. RSVL showed the effect on HMSCs in dose- and time-dependent manners for the self-proliferation and differentiation. 0.1 μM RSVL promotes cell proliferation, but 5 μM or above inhibits cell self-renewal by increasing the senescence rate and cell cycle arrest in S phage. It also helps MSC differentiation into osteogenic cells and suppresses differentiation into the adipogenic lineage [141]. Resveratrol enhances osteogenic differentiation by upregulating HMSC mediated through the SIRT1/FOXO3A. It activates and enhances the proteins SIRT1 and FOXO3A, respectively, in an independent manner. Resveratrol also promotes osteogenesis by upregulating Runx2 gene expression [142].
6. Future Prospective
Recent advancement in science and technology and advanced research on plant extracts is bringing into light their importance in regenerative and therapeutic medicine. As we know very less about the exact site and mechanism of action and side effect of the use of plant extracts, extensive research on humans will help to replace synthetic pharmaceutical drugs to treat diseases. If protocols for proliferation and differentiation of stem cells into desired lineage cells by use of plant extracts can be established, it will help to treat many untreatable diseases like aplastic anemia, leukemia, bone diseases, and cardiovascular diseases. MSCs have promising roles in regenerative therapy due to their broader differentiation potential [4]. From the last few decades, scientist have been aiming to use MSCs for tissue regeneration in bone injury [143], cartilage injury [144], spinal cord injury [145], graft-versus-host disease [146], Crohn's disease [147], and hematopoietic cell recruitment [148]. Though very less side effects of plant extracts on humans have been noted, they may still show adverse drug effects for certain medical condition which are not well known. With better knowledge of the effects of plant extracts, we may also be able to restrict their undesirable use under certain circumstances. The therapeutic doses can also be well established to have desired effects as well as control toxic effects.
Medicinal plants are being widely accepted and increasingly used by the general public for treatment. They are also used as complementary supplements to reduce the side effects produced by Western medicine [149]. The bioactive compounds derived from plants have shown to be potential candidates to activate stem cells for proliferation and differentiation. Currently, recombinant and synthetic cytokines, growth factors, and other proteins are being produced by using bacterial cell, plants cells, and mammalian cells for stem cell growth supplement. These compounds have significant side effects [150–152] and lead to neoplastic cell transformations [153] with high cost, less stability, and limited application and requiring continuous use making them unaffordable for low-income countries. Certain medicinal plants have always been grown and used as cultural values for primary health benefits. With more knowledge on values of commonly available plants in the community, it will help people to preserve and use them for healthy living and preventive and curative medicine and also restrict undesirable use. This will decrease the health care economic burden for primary health care problems. Thus, plant-derived compounds will be proven as promising agents for stem cell therapy for public health with easy availability and affordability and least or no side effects.
7. Conclusion
MSCs along with medicinal plant extracts have a potential hope in stem cell and regenerative therapy. Plant extracts as stimulants significantly affect proliferation and differentiation into multilineage cells. Bioactive compounds from plants precisely regulate the MSCs through different protein pathways. Medicinal plants/herbs produce less toxic effects, are affordable, and can help to increase disease-treating capability using MSC cell therapy for both noninfectious and infectious diseases. With continued research, by using medicinal plant extracts, improved proliferation and differentiation potential of MSCs will be achieved in the near future and development of cost-effective technology for cellular therapy will be possible.
Acknowledgments
We thank Saroj Adhakari and Suroj Maharjan for their support.
Abbreviations
- MSCs:
Mesenchymal stem cells
- ISCT:
International Society for Cellular Therapy
- PDT:
Population doubling time
- UC-MSCs:
Umbilical cord-derived mesenchymal stem cells
- BM-MSCs:
Bone marrow-derived mesenchymal stem cells
- AT-MSCs:
Adipose tissue-derived mesenchymal stem cells
- MD-MSCs:
Muscle-derived mesenchymal stem cells
- P-MSCs:
Periosteum-derived mesenchymal stem cells
- S-MSCs:
Synovial-derived mesenchymal stem cells
- AD-MSCs:
Adipose tissue-derived mesenchymal stem cells
- C-MSCs:
Circulating mesenchymal stem cells
- TD-MSCs:
Tendon-derived mesenchymal stem cells
- Osx:
Osterix
- TCM:
Traditional Chinese Medicine
- SA-β-Gal:
Senescence-associated β-galactosidase
- ALP:
Alkaline phosphatase
- Runx2:
Runt-related transcription factor 2
- TGF-β:
Transforming growth factor-beta
- BMPs:
Bone morphogenetic proteins
- TGF-β:
Transforming growth factor-beta
- SMC:
Smooth muscle cells
- h-UC-WJ:
Human-Umbilical cord Wharton's jelly.
Ethical Approval
Ethical approval was taken from the Nepal Health Research Council, Ramshah Path, Kathmandu, Nepal (registration no. 334/2017).
Conflicts of Interest
There are no conflicts of interest.
Authors' Contributions
Bhuvan Saud and Kanti Shrestha prepared the manuscript. Rajani Malla made critical comments on the manuscript. All the authors finalized and approved the manuscript.
References
- 1.Chagastelles P. C., Nardi N. B. Biology of stem cells: an overview. Kidney International Supplements. 2011;1(3):63–67. doi: 10.1038/kisup.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramalho-Santos M., Willenbring H. On the origin of the term ‘stem cell. Cell Stem Cell. 2007;1(1):35–38. doi: 10.1016/j.stem.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein A. J., Gorskaja J. F., Kulagina N. N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Experimental Hematology. 1976;4(5):267–274. [PubMed] [Google Scholar]
- 4.Dominici M., le Blanc K., Mueller I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 5.Haasters F., Prall W. C., Anz D., et al. Morphological and immunocytochemical characteristics indicate the yield of early progenitors and represent a quality control for human mesenchymal stem cell culturing. Journal of Anatomy. 2009;214(5):759–767. doi: 10.1111/j.1469-7580.2009.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gronthos S., Zannettino A. C., Hay S. J., et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. Journal of Cell Science. 2003;116(9):1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 7.Miao Z., Jin J., Chen L., et al. Isolation of mesenchymal stem cells from human placenta: comparison with human bone marrow mesenchymal stem cells. Cell Biology International. 2006;30(9):681–687. doi: 10.1016/j.cellbi.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Zuk P. A., Zhu M., Ashjian P., et al. Human adipose tissue is a source of multipotent stem cells. Molecular Biology of the Cell. 2002;13(12):4279–4295. doi: 10.1091/mbc.e02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debnath T., Chelluri L. K. Standardization and quality assessment for clinical grade mesenchymal stem cells from human adipose tissue. Hematology, Transfusion and Cell Therapy. 2019;41(1):7–16. doi: 10.1016/j.htct.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tondreau T., Meuleman N., Delforge A., et al. Mesenchymal stem cells derived from CD133-positive cells in mobilized peripheral blood and cord blood: proliferation, Oct4 expression, and plasticity. Stem Cells. 2005;23(8):1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 11.Chong P. P., Selvaratnam L., Abbas A. A., Kamarul T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. Journal of Orthopaedic Research. 2012;30(4):634–642. doi: 10.1002/jor.21556. [DOI] [PubMed] [Google Scholar]
- 12.Fukuchi Y., Nakajima H., Sugiyama D., Hirose I., Kitamura T., Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22(5):649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 13.Sarugaser R., Lickorish D., Baksh D., Hosseini M. M., Davies J. E. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23(2):220–229. doi: 10.1634/stemcells.2004-0166. [DOI] [PubMed] [Google Scholar]
- 14.Iwatani S., Yoshida M., Yamana K., et al. Isolation and characterization of human umbilical cord-derived mesenchymal stem cells from preterm and term infants. JoVE. 2019;26(143) doi: 10.3791/58806. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H., Miao Z., He Z., Yang Y., Wang Y., Feng M. The existence of epithelial-to-mesenchymal cells with the ability to support hematopoiesis in human fetal liver. Cell Biology International. 2005;29(3):213–219. doi: 10.1016/j.cellbi.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Zheng C., Yang S., Guo Z., et al. Human multipotent mesenchymal stromal cells from fetal lung expressing pluripotent markers and differentiating into cell types of three germ layers. Cell Transplant. 2009;18(10-11):1093–1109. doi: 10.3727/096368909X12483162197042. [DOI] [PubMed] [Google Scholar]
- 17.Huang G. T. J., Gronthos S., Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. Journal of Dental Research. 2009;88(9):792–806. doi: 10.1177/0022034509340867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledesma-Martínez E., Mendoza-Núñez V. M., Santiago-Osorio E. Mesenchymal stem cells derived from dental pulp: a review. Stem Cells International. 2016;2016:12. doi: 10.1155/2016/4709572.4709572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J. C., Kim J. M., Jung I. H., et al. Isolation and characterization of human periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed PDL tissue: in vitro and in vivo evaluations. Journal of Clinical Periodontology. 2011;38(8):721–731. doi: 10.1111/j.1600-051X.2011.01716.x. [DOI] [PubMed] [Google Scholar]
- 20.Sakaguchi Y., Sekiya I., Yagishita K., Ichinose S., Shinomiya K., Muneta T. Suspended cells from trabecular bone by collagenase digestion become virtually identical to mesenchymal stem cells obtained from marrow aspirates. Blood. 2004;104(9):2728–2735. doi: 10.1182/blood-2003-12-4452. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H., Guo Z. K., Jiang X. X., et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nature Protocols. 2010;5(3):550–560. doi: 10.1038/nprot.2009.238. [DOI] [PubMed] [Google Scholar]
- 22.Hermida-Gómez T., Fuentes-Boquete I., Gimeno-Longas M., et al. Quantification of cells expressing mesenchymal stem cell markers in healthy and osteoarthritic synovial membranes. The Journal of Rheumatology. 2011;38(2):339–349. doi: 10.3899/jrheum.100614. [DOI] [PubMed] [Google Scholar]
- 23.Ho J. H.-C., Ma W.-H., Tseng T.-C., Chen Y.-F., Chen M.-H., Lee O. K.-S. Isolation and characterization of multi-potent stem cells from human orbital fat tissues. Tissue Engineering. Part A. 2011;17(1–2):255–266. doi: 10.1089/ten.tea.2010.0106. [DOI] [PubMed] [Google Scholar]
- 24.Glemžaitė M., Navakauskienė R. Osteogenic differentiation of human amniotic fluid mesenchymal stem cells is determined by epigenetic changes. Stem Cells International. 2016;2016:10. doi: 10.1155/2016/6465307.6465307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng J. X., la X. L., Ma Y., Bi X. J., Wen H. Isolation of human pluripotent mesenchymal stem cells from second-trimester amniotic fluid using two kinds of culture protocol and their differentiation into neuron-like cells. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009;21(12):729–733. [PubMed] [Google Scholar]
- 26.Schwab K. E., Hutchinson P., Gargett C. E. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Human Reproduction. 2008;23(4):934–943. doi: 10.1093/humrep/den051. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Q., Ren H., Han Z. Mesenchymal stem cells: immunomodulatory capability and clinical potential in immune diseases. Journal of Cellular Immunotherapy. 2016;2(1):3–20. doi: 10.1016/j.jocit.2014.12.001. [DOI] [Google Scholar]
- 28.Wang L. T., Ting C. H., Yen M. L., et al. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. Journal of Biomedical Science. 2016;23(1):p. 76. doi: 10.1186/s12929-016-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao F., Chiu S. M., Motan D. A. L., et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell death & disease. 2016;7(1, article e2062) doi: 10.1038/cddis.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US National Library of Medicine. NIH. http://www.clinicaltrials.gov. [DOI] [PubMed]
- 31.Farnsworth N. R., Akerele O., Bingel A. S., Soejarto D. D., Guo Z. Medicinal plants in therapy. Bulletin of the World Health Organization. 1985;63(6):965–981. [PMC free article] [PubMed] [Google Scholar]
- 32.Hasan N. M., Al Sorkhy M. K. Herbs that promote cell proliferation. International Journal of Herbal Medicine. 2014;18(16):18–21. [Google Scholar]
- 33.Saravanan V., Murugan S. S., Rajkumar J. S. I., Krishnan K. R. N., Prdeepa P. In vitro safety assessment of the ethanolic extract of Kalanchoe pinnata on human peripheral lymphocytes. Research Journal of Pharmacy and Technology. 2018;11(6):p. 2595. doi: 10.5958/0974-360X.2018.00480.8. [DOI] [Google Scholar]
- 34.Olatunbosun L. O., Caxton-Martin A., Jimoh O. R., Biliaminu S. A., Ghazal O. K. Proliferative effect of aqueous extracts of parquetina nigrescens on haemopoietic multipotent stem cells in irradiated guinea pigs. Journal of Applied Pharmaceutical Science. 2012;2(6):108–111. doi: 10.7324/japs.2012.2608. [DOI] [Google Scholar]
- 35.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Experimental Cell Research. 1965;37(3):614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 36.Feng G. Mesenchymal stem cells and senescence. Cloning & Transgenesis. 2012;2(1):p. 104. doi: 10.4172/2168-9849.1000104. [DOI] [Google Scholar]
- 37.Estrada J. C., Torres Y., Benguría A., et al. Human mesenchymal stem cell-replicative senescence and oxidative stress are closely linked to aneuploidy. Cell Death & Disease. 2013;4(6):e691–e691. doi: 10.1038/cddis.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Y., Wu Q., Wang Y., Li L., Bu H., Bao J. Senescence of mesenchymal stem cells (review) International Journal of Molecular Medicine. 2017;39(4):775–782. doi: 10.3892/ijmm.2017.2912. [DOI] [PubMed] [Google Scholar]
- 39.Colter D. C., Class R., DiGirolamo C., Prockop D. J. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proceedings of the National Academy of Sciences. 2000;97(7):3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonab M., Alimoghaddam K., Talebian F., Ghaffari S., Ghavamzadeh A., Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biology. 2006;7(1):p. 14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stenderup K., Justesen J., Clausen C., Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33(6):919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Jin H., Bae Y., Kim M., et al. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. International Journal of Molecular Sciences. 2013;14(9):17986–18001. doi: 10.3390/ijms140917986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reyes M., Lund T., Lenvik T., Aguiar D., Koodie L., Verfaillie C. M. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98(9):2615–2625. doi: 10.1182/blood.V98.9.2615. [DOI] [PubMed] [Google Scholar]
- 44.Mareschi K., Ferrero I., Rustichelli D., et al. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. Journal of Cellular Biochemistry. 2006;97(4):744–754. doi: 10.1002/jcb.20681. [DOI] [PubMed] [Google Scholar]
- 45.Sun L.-Y., Hsieh D.-K., Syu W.-S., Li Y.-S., Chiu H.-T., Chiou T.-W. Cell proliferation of human bone marrow mesenchymal stem cells on biodegradable microcarriers enhances in vitro differentiation potential. Cell Proliferation. 2010;43(5):445–456. doi: 10.1111/j.1365-2184.2010.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baksh D., Yao R., Tuan R. S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25(6):1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 47.Via A. G., Frizziero A., Oliva F. Biological properties of mesenchymal stem cells from different sources. Muscles, Ligaments and Tendons Journal. 2012;2(3):154–162. [PMC free article] [PubMed] [Google Scholar]
- 48.Qian S.-W., Li X., Zhang Y. Y., et al. Characterization of adipocyte differentiation from human mesenchymal stem cells in bone marrow. BMC Developmental Biology. 2010;10(1):p. 47. doi: 10.1186/1471-213X-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y., Marsboom G., Toth P. T., Rehman J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS One. 2013;8(10) doi: 10.1371/journal.pone.0077077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kakudo N., Shimotsuma A., Kusumoto K. Fibroblast growth factor-2 stimulates adipogenic differentiation of human adipose-derived stem cells. Biochemical and Biophysical Research Communications. 2007;359(2):239–244. doi: 10.1016/j.bbrc.2007.05.070. [DOI] [PubMed] [Google Scholar]
- 51.Hong L., Colpan A., Peptan I. A., Daw J., George A., Evans C. A. 17-β estradiol enhances osteogenic and adipogenic differentiation of human adipose-derived stromal cells. Tissue Engineering. 2007;13(6):1197–1203. doi: 10.1089/ten.2006.0317. [DOI] [PubMed] [Google Scholar]
- 52.Charbord P., Livne E., Gross G., et al. Human bone marrow mesenchymal stem cells: a systematic reappraisal via the genostem experience. Stem Cell Reviews and Reports. 2011;7(1):32–42. doi: 10.1007/s12015-010-9125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakaguchi Y., Sekiya I., Yagishita K., Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis and Rheumatism. 2005;52(8):2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 54.Hou T., Xu J., Wu X., et al. Umbilical cord Wharton’s jelly: a new potential cell source of mesenchymal stromal cells for bone tissue engineering. Tissue Engineering. Part A. 2009;15(9):2325–2334. doi: 10.1089/ten.tea.2008.0402. [DOI] [PubMed] [Google Scholar]
- 55.Indrawattana N., Chen G., Tadokoro M., et al. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochemical and Biophysical Research Communications. 2004;320(3):914–919. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 56.Chun J.-S., Oh H.-H., Yang S.-Y., Park M.-Y. Wnt signaling in cartilage development and degeneration. BMB Reports. 2011;41(7):485–494. doi: 10.5483/bmbrep.2008.41.7.485. [DOI] [PubMed] [Google Scholar]
- 57.MacDonald B. T., Tamai K., He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Developmental Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnstone B., Hering T. M., Caplan A. I., Goldberg V. M., Yoo J. U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Experimental Cell Research. 1998;238(1):265–272. doi: 10.1006/excr.1997.3858. [DOI] [PubMed] [Google Scholar]
- 59.Yoshimura H., Muneta T., Nimura A., Yokoyama A., Koga H., Sekiya I. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell and Tissue Research. 2007;327(3):449–462. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 60.Danisovic L., Varga I., Polak S., Ulicna M., Böhmer D., Vojtassak J. Comparison of in vitro chondrogenic potential of human mesenchymal stem cells derived from bone marrow and adipose tissue. General Physiology and Biophysics. 2009;28(1):56–62. doi: 10.4149/gpb_2009_01_56. [DOI] [PubMed] [Google Scholar]
- 61.Tan Q., Lui P. P. Y., Rui Y. F., Wong Y. M. Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Engineering Part A. 2012;18(7-8):840–851. doi: 10.1089/ten.tea.2011.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Awad H. A., Halvorsen Y. D. C., Gimble J. M., Guilak F. Effects of transforming growth factor β1 and dexamethasone on the growth and chondrogenic differentiation of adipose-derived stromal cells. Tissue Engineering. 2003;9(6):1301–1312. doi: 10.1089/10763270360728215. [DOI] [PubMed] [Google Scholar]
- 63.Feng G., Wan Y., Balian G., Laurencin C. T., Li X. Adenovirus-mediated expression of growth and differentiation factor-5 promotes chondrogenesis of adipose stem cells. Growth Factors. 2009;26(3):132–142. doi: 10.1080/08977190802105917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Augello A., De Bari C. The regulation of differentiation in mesenchymal stem cells. Human Gene Therapy. 2010;21(10):1226–1238. doi: 10.1089/hum.2010.173. [DOI] [PubMed] [Google Scholar]
- 65.Nishio Y., Dong Y., Paris M., O'Keefe R. J., Schwarz E. M., Drissi H. Runx2-mediated regulation of the zinc finger osterix/Sp7 gene. Gene. 2006;372:62–70. doi: 10.1016/j.gene.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 66.Kobayashi H., Gao Y. H., Ueta C., Yamaguchi A., Komori T. Multilineage differentiation of Cbfa1-deficient calvarial cells in vitro. Biochemical and Biophysical Research Communications. 2000;273(2):630–636. doi: 10.1006/bbrc.2000.2981. [DOI] [PubMed] [Google Scholar]
- 67.Yoshida C. A., Furuichi T., Fujita T., et al. Core-binding factor β interacts with Runx2 and is required for skeletal development. Nature Genetics. 2002;32(4):633–638. doi: 10.1038/ng1015. [DOI] [PubMed] [Google Scholar]
- 68.Kern S., Eichler H., Stoeve J., Klüter H., Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 69.Zuk P. A., Zhu M., Mizuno H., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Engineering. 2001;7(2):211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 70.Halvorsen Y.-D. C., Franklin D., Bond A. L., et al. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Engineering. 2001;7(6):729–741. doi: 10.1089/107632701753337681. [DOI] [PubMed] [Google Scholar]
- 71.Malladi P., Xu Y., Yang G. P., Longaker M. T. Functions of vitamin D, retinoic acid, and dexamethasone in mouse adipose-derived mesenchymal cells. Tissue Engineering. 2006;12(7):2031–2040. doi: 10.1089/ten.2006.12.ft-115. [DOI] [PubMed] [Google Scholar]
- 72.Alberton P., Popov C., Prägert M., et al. Conversion of human bone marrow-derived mesenchymal stem cells into tendon progenitor cells by ectopic expression of scleraxis. Stem Cells and Development. 2012;21(6):846–858. doi: 10.1089/scd.2011.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu H., Zhang C., Zhu S., et al. Mohawk promotes the tenogenesis of mesenchymal stem cells through activation of the TGFβ signaling pathway. Stem Cells. 2015;33(2):443–455. doi: 10.1002/stem.1866. [DOI] [PubMed] [Google Scholar]
- 74.Inada M., Katagiri T., Akiyama S., et al. Bone morphogenetic protein-12 and -13 inhibit terminal differentiation of myoblasts, but do not induce their differentiation into osteoblasts. Biochemical and Biophysical Research Communications. 1996;222(2):317–322. doi: 10.1006/bbrc.1996.0742. [DOI] [PubMed] [Google Scholar]
- 75.Docheva D., Müller S. A., Majewski M., Evans C. H. Biologics for tendon repair. Advanced Drug Delivery Reviews. 2015;84:222–239. doi: 10.1016/j.addr.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Violini S., Ramelli P., Pisani L. F., Gorni C., Mariani P. Horse bone marrow mesenchymal stem cells express embryo stem cell markers and show the ability for tenogenic differentiation by in vitro exposure to BMP-12. BMC Cell Biology. 2009;10(1) doi: 10.1186/1471-2121-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rui Y. F., Lui P. P. Y., Lee Y. W., Chan K. M. Higher BMP receptor expression and BMP-2-induced osteogenic differentiation in tendon-derived stem cells compared with bone-marrow-derived mesenchymal stem cells. International Orthopaedics. 2012;36(5):1099–1107. doi: 10.1007/s00264-011-1417-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tondreau T., Lagneaux L., Dejeneffe M., et al. Bone marrow-derived mesenchymal stem cells already express specific neural proteins before any differentiation. Differentiation. 2004;72(7):319–326. doi: 10.1111/j.1432-0436.2004.07207003.x. [DOI] [PubMed] [Google Scholar]
- 79.Ma K., Fox L., Shi G., et al. Generation of neural stem cell-like cells from bone marrow-derived human mesenchymal stem cells. Neurological Research. 2013;33(10):1083–1093. doi: 10.1179/1743132811Y.0000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hermann A. Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. Journal of Cell Science. 2004;117(19):4411–4422. doi: 10.1242/jcs.01307. [DOI] [PubMed] [Google Scholar]
- 81.Tao H., Rao R., Ma D. D. F. Cytokine-induced stable neuronal differentiation of human bone marrow mesenchymal stem cells in a serum/feeder cell-free condition. Development, Growth and Differentiation. 2005;47(6):423–433. doi: 10.1111/j.1440-169X.2005.00810.x. [DOI] [PubMed] [Google Scholar]
- 82.Gu W., Hong X., le Bras A., et al. Smooth muscle cells differentiated from mesenchymal stem cells are regulated by microRNAs and suitable for vascular tissue grafts. Journal of Biological Chemistry. 2018;293(21):8089–8102. doi: 10.1074/jbc.RA118.001739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang N., Ma L., Zhou Z., et al. Regeneration of smooth muscle cells from bone marrow-use of mesenchymal stem cells for tissue engineering and cellular therapeutics. 2009 3rd International Conference on Bioinformatics and Biomedical Engineering; 2009; Beijing, China. [DOI] [Google Scholar]
- 84.Jeon E. S., Park W. S., Lee M. J., Kim Y. M., Han J., Kim J. H. A rho kinase/myocardin-related transcription factor-a-dependent mechanism underlies the sphingosylphosphorylcholine-induced differentiation of mesenchymal stem cells into contractile smooth muscle cells. Circulation Research. 2008;103(6):635–642. doi: 10.1161/CIRCRESAHA.108.180885. [DOI] [PubMed] [Google Scholar]
- 85.Schmidt B., Ribnicky D. M., Poulev A., Logendra S., Cefalu W. T., Raskin I. A natural history of botanical therapeutics. Metabolism. 2008;57(7, Supplement 1):S3–S9. doi: 10.1016/j.metabol.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kornicka K., Kocherova I., Marycz K. The effects of chosen plant extracts and compounds on mesenchymal stem cells—a bridge between molecular nutrition and regenerative medicine- concise review. Phytotherapy Research. 2017;31(7):947–958. doi: 10.1002/ptr.5812. [DOI] [PubMed] [Google Scholar]
- 87.Thangapazham R. L., Sharad S., Maheshwari R. K. Phytochemicals in wound healing. Advances in Wound Care. 2016;5(5):230–241. doi: 10.1089/wound.2013.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Udalamaththa V. L., Jayasinghe C. D., Udagama P. V. Potential role of herbal remedies in stem cell therapy: proliferation and differentiation of human mesenchymal stromal cells. Stem Cell Research & Therapy. 2016;7(1):p. 110. doi: 10.1186/s13287-016-0366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang L. Y., Xue H. G., Chen J. Y., Chai W., Ni M. Genistein induces adipogenic differentiation in human bone marrow mesenchymal stem cells and suppresses their osteogenic potential by upregulating PPARγ. Experimental and Therapeutic Medicine. 2016;11(5):1853–1858. doi: 10.3892/etm.2016.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang J. Y., Yin C. C., Wu C. C., Geng S. G., Yin M. Icaritin promotes chondrogenic differentiation of BMSCs by Wnt/β-catenin signaling pathway. Zhongguo Zhong Yao Za Zhi. 2016;41(4):694–699. doi: 10.4268/cjcmm20160425. [DOI] [PubMed] [Google Scholar]
- 91.Potu B. K., Bhat K. M. R., Rao M. S., et al. Petroleum ether extract of Cissus quadrangularis (Linn.) enhances bone marrow mesenchymal stem cell proliferation and facilitates osteoblastogenesis. Clinics. 2009;64(10):993–998. doi: 10.1590/S1807-59322009001000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gao L. N., An Y., Lei M., et al. The effect of the coumarin-like derivative osthole on the osteogenic properties of human periodontal ligament and jaw bone marrow mesenchymal stem cell sheets. Biomaterials. 2013;34(38):9937–9951. doi: 10.1016/j.biomaterials.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 93.Peng-Zhang, Dai K.-r., Yan S.-g., et al. Effects of naringin on the proliferation and osteogenic differentiation of human bone mesenchymal stem cell. European Journal of Pharmacology. 2009;607(1-3):1–5. doi: 10.1016/j.ejphar.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 94.Yu G., Zheng G. Z., Chang B., et al. Naringin stimulates osteogenic differentiation of rat bone marrow stromal cells via activation of the notch signaling pathway. Stem Cells International. 2016;2016:8. doi: 10.1155/2016/7130653.7130653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim B. S., Kang H. J., Park J. Y., Lee J. Fucoidan promotes osteoblast differentiation via JNK- and ERK-dependent BMP2-Smad 1/5/8 signaling in human mesenchymal stem cells. Experimental & Molecular Medicine. 2015;47(1):p. e128. doi: 10.1038/emm.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin X.-x., Chen Z.-q., Liu Z.-j., Ma Q.-j., Dang G.-t. Icariine stimulates proliferation and differentiation of human osteoblasts by increasing production of bone morphogenetic protein 2. Chinese Medical Journal. 2007;120(3):204–210. doi: 10.1097/00029330-200702010-00006. [DOI] [PubMed] [Google Scholar]
- 97.Lee J., Roh K. B., Kim S. C., Lee J., Park D. Soy peptide-induced stem cell proliferation: involvement of ERK and TGF-β1. The Journal of Nutritional Biochemistry. 2012;23(10):1341–1351. doi: 10.1016/j.jnutbio.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 98.Mendi A. H., Gökçınar Yağcı B., Sarac N., Kızıloğlu M., Yılmaz D., Uçkan D. Effect of Ocimum basilicum on mesenchymal stem cell proliferation and differentiation: does the effect change according to niches? International Journal of Secondary Metabolite. 2017;4(3):1–10. doi: 10.21448/ijsm.356244. [DOI] [Google Scholar]
- 99.Machado A. K., Cadoná F. C., Azzolin V. F., et al. Guaraná (Paullinia cupana) improves the proliferation and oxidative metabolism of senescent adipocyte stem cells derived from human lipoaspirates. Food Research International. 2015;67:426–433. doi: 10.1016/j.foodres.2014.11.056. [DOI] [Google Scholar]
- 100.Azizsoltani A., Piri K., Behzad S., et al. Ethyl acetate extract of licorice root (Glycyrrhiza glabra) enhances proliferation and osteogenic differentiation of human bone marrow mesenchymal stem cells. Iranian Journal of Pharmaceutical Research: IJPR. 2018;17(3):1057–1067. [PMC free article] [PubMed] [Google Scholar]
- 101.Mendi A., Yağcı B. G., Kızıloğlu M., et al. Thymbra spicata var. intricata induces mesenchymal stem cell proliferation and osteogenic differentiation. Brazilian Archives of Biology and Technology. 2017;60 doi: 10.1590/1678-4324-2017160391. [DOI] [Google Scholar]
- 102.Chen M., Feng W., Cao H., et al. A traditional Chinese medicine formula extracts stimulate proliferation and inhibit mineralization of human mesenchymal stem cells in vitro. Journal of Ethnopharmacology. 2009;125(1):75–82. doi: 10.1016/j.jep.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 103.Mahmoudi Z., Soleimani M., Saidi A., Khamisipour G., Azizsoltani A. Effects of Foeniculum vulgare ethanol extract on osteogenesis in human mecenchymal stem cells. Avicenna Journal of Phytomedicine. 2013;3(2):135–142. [PMC free article] [PubMed] [Google Scholar]
- 104.Lee J., Shin M. S., Kim M. O., et al. Apple ethanol extract promotes proliferation of human adult stem cells, which involves the regenerative potential of stem cells. Nutrition Research. 2016;36(9):925–936. doi: 10.1016/j.nutres.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 105.Mahmoudi Z., Soleimani M., Saidi A., Iranshahi M., Azizsoltanli A. Effect of Ferula gummosa ethanolic extract on osteogenesis in human mesenchymal stem cells. Journal of Medicinal Plants. 2013;2(46):50–59. [Google Scholar]
- 106.Gu Q., Chen C., Zhang Z., et al. Ginkgo biloba extract promotes osteogenic differentiation of human bone marrow mesenchymal stem cells in a pathway involving Wnt/β-catenin signaling. Pharmacol. Res. 2015;97:70–78. doi: 10.1016/j.phrs.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 107.Li G., Zhang X. A., Zhang J. F., et al. Ethanol extract of Fructus Ligustri Lucidi promotes osteogenesis of mesenchymal stem cells. Phytotherapy Research. 2009;24(4):571–576. doi: 10.1002/ptr.2987. [DOI] [PubMed] [Google Scholar]
- 108.Ko C., Siu W., Lau C., Lau C. B., Fung K., Leung P. Osteoprotective effects of Fructus Ligustri Lucidi aqueous extract in aged ovariectomized rats. Chinese Medicine. 2010;5(1):p. 39. doi: 10.1186/1749-8546-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang J. F., Li G., Meng C. L., et al. Total flavonoids of Herba Epimedii improves osteogenesis and inhibits osteoclastogenesis of human mesenchymal stem cells. Phytomedicine. 2009;16(6-7):521–529. doi: 10.1016/j.phymed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 110.Tao K., Xiao D., Weng J., Xiong A., Kang B., Zeng H. Berberine promotes bone marrow-derived mesenchymal stem cells osteogenic differentiation via canonical Wnt/β-catenin signaling pathway. Toxicology Letters. 2016;240(1):68–80. doi: 10.1016/j.toxlet.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 111.Kongros B., Bunyaratvej A., Viyoch J., Sila-asna M. The effects of seed extract of Mucuna gigantea on the expression of neural markers in mesenchymal stem cells. Journal of Medicinal Plants Research. 2012;6(7):1297–1303. doi: 10.5897/JMPR11.1406. [DOI] [Google Scholar]
- 112.Ma L., Feng X. Y., Cui B. L., et al. Human umbilical cord Wharton’s jelly-derived mesenchymal stem cells differentiation into nerve-like cells. Chinese Medical Journal. 2005;118(23):1987–1993. [PubMed] [Google Scholar]
- 113.Gomez J. M., Mora R. M., Diaz A. C., De Castro M. D., inventors; SANIDAD Y RESIDENCIAS 21 SA, QUESPER R&D SL, Servicio Andaluz de Salud . Use of olive leaf extracts in a pharmaceutical composition for inducing angiogenesis and vasculogenesis. US Patent 13/320,180; 2012. [Google Scholar]
- 114.Xu D., Xu L., Zhou C., et al. Salvianolic acid B promotes osteogenesis of human mesenchymal stem cells through activating ERK signaling pathway. International Journal of Biochemistry & Cell Biology. 2014;51:1–9. doi: 10.1016/j.biocel.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 115.Wang Q., Zhou L., Guo Y., Liu G., Cheng J., Yu H. Differentiation of human adipose-derived stem cells into neuron-like cells by Radix Angelicae sinensis. Neural Regeneration Research. 2013;8(35):3353–3358. doi: 10.3969/j.issn.1673-5374.2013.35.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lavrador P., Gaspar V. M., Mano J. F. Bioinspired bone therapies using naringin: applications and advances. Drug Discovery Today. 2018;23(6):1293–1304. doi: 10.1016/j.drudis.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 117.Wang H., Li C., Li J., et al. Naringin enhances osteogenic differentiation through the activation of erk signaling in human bone marrow mesenchymal stem cells. Iranian Journal of Basic Medical Sciences. 2017;20(4):408–414. doi: 10.22038/IJBMS.2017.8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu M., Li Y., Yang S. T. Effects of naringin on the proliferation and osteogenic differentiation of human amniotic fluid-derived stem cells. Journal of Tissue Engineering and Regenerative Medicine. 2017;11(1):276–284. doi: 10.1002/term.1911. [DOI] [PubMed] [Google Scholar]
- 119.Yin L., Cheng W., Qin Z., et al. Effects of naringin on proliferation and osteogenic differentiation of human periodontal ligament stem cells in vitro and in vivo. Stem Cells International. 2015;2015:9. doi: 10.1155/2015/758706.758706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang X. B., Liu T. L., Zhang X., Tan R. J., Ge H. L., Huang Y. L. Effect of Naringin on proliferation and osteogenic differentiation of bone marrow stromal cells in vitro. Shanghai Kou Qiang Yi Xue. 2014;23(3):280–284. [PubMed] [Google Scholar]
- 121.Lai X., Ye Y., Sun C., et al. Icaritin exhibits anti-inflammatory effects in the mouse peritoneal macrophages and peritonitis model. International Immunopharmacology. 2013;16(1):41–49. doi: 10.1016/j.intimp.2013.03.025. [DOI] [PubMed] [Google Scholar]
- 122.Hu Y., Liu K., Yan M., Zhang Y., Wang Y., Ren L. Effects and mechanisms of icariin on atherosclerosis. International Journal of Clinical and Experimental Medicine. 2015;8(3):3585–3589. [PMC free article] [PubMed] [Google Scholar]
- 123.Li J., Jiang K., Zhao F. Icariin regulates the proliferation and apoptosis of human ovarian cancer cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncology Reports. 2015;33(6):2829–2836. doi: 10.3892/or.2015.3891. [DOI] [PubMed] [Google Scholar]
- 124.Han Y., Jung H. W., Park Y. K. Effects of Icariin on insulin resistance via the activation of AMPK pathway in C2C12 mouse muscle cells. European Journal of Pharmacology. 2015;758:60–63. doi: 10.1016/j.ejphar.2015.03.059. [DOI] [PubMed] [Google Scholar]
- 125.Qin S., Zhou W., Liu S., Chen P., Wu H. Icariin stimulates the proliferation of rat bone mesenchymal stem cells via ERK and p38 MAPK signaling. International Journal of Clinical and Experimental Medicine. 2015;8(5):7125–7133. [PMC free article] [PubMed] [Google Scholar]
- 126.Fu S., Yang L., Hong H., Zhang R. Wnt/β-catenin signaling is involved in the icariin induced proliferation of bone marrow mesenchymal stem cells. Journal of Traditional Chinese Medicine. 2016;36(3):360–368. doi: 10.1016/s0254-6272(16)30050-4. [DOI] [PubMed] [Google Scholar]
- 127.Xia Q., Xu D., Huang Z., et al. Preparation of icariside II from icariin by enzymatic hydrolysis method. Fitoterapia. 2010;81(5):437–442. doi: 10.1016/j.fitote.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 128.Luo G., Gu F., Zhang Y., Liu T., Guo P., Huang Y. Icariside II promotes osteogenic differentiation of bone marrow stromal cells in beagle canine. International Journal of Clinical and Experimental Pathology. 2015;8(5):4367–4377. [PMC free article] [PubMed] [Google Scholar]
- 129.Hu C., Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. Journal of Cellular and Molecular Medicine. 2018;22(3):1428–1442. doi: 10.1111/jcmm.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guangming Luo Y. H., Xu B. Icariside II promotes the osteogenic differentiation of canine bone marrow mesenchymal stem cells via the PI3K/AKT/mTOR/S6K1 signaling pathways. American Journal of Translational Research. 2017;9(5):2077–2087. [PMC free article] [PubMed] [Google Scholar]
- 131.Huang J.-m., Bao Y., Xiang W., et al. Icariin regulates the bidirectional differentiation of bone marrow mesenchymal stem cells through canonical Wnt signaling pathway. Evidence-Based Complementary and Alternative Medicine. 2017;2017:12. doi: 10.1155/2017/8085325.8085325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Okumura N., Yoshikawa T., Iida J., Nonomura A., Takakura Y. Osteogenic effect of genistein on in vitro bone formation by human bone marrow cell culture - for development of advanced bio-artificial bone. Key Engineering Materials. 2005;284-286:667–670. doi: 10.4028/www.scientific.net/kem.284-286.667. [DOI] [Google Scholar]
- 133.Dai J., Li Y., Zhou H., Chen J., Chen M., Xiao Z. Genistein promotion of osteogenic differentiation through BMP2/SMAD5/RUNX2 signaling. International Journal of Biological Sciences. 2013;9(10):1089–1098. doi: 10.7150/ijbs.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liao Q., Xiao Z., Qin Y., Zhou H. Genistein stimulates osteoblastic differentiation via p38 MAPK-Cbfa1 pathway in bone marrow culture. Acta Pharmacologica Sinica. 2007;28(10):1597–1602. doi: 10.1111/j.1745-7254.2007.00632.x. [DOI] [PubMed] [Google Scholar]
- 135.Lee S. Therapeutic effect of genistein-stimulated human mesenchymal stem cells in myocardial infarction. Journal of Transplantation & Stem Cell Biology. 2014;1(1):p. 7. doi: 10.13188/2374-9326.1000004. [DOI] [Google Scholar]
- 136.Li L., Duan X., Fan Z., et al. Mesenchymal stem cells in combination with hyaluronic acid for articular cartilage defects. Scientific Reports. 2018;8(1) doi: 10.1038/s41598-018-27737-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee K. B. L., Hui J. H. P., Song I. C., Ardany L., Lee E. H. Injectable mesenchymal stem cell therapy for large cartilage defects-a porcine model. Stem Cells. 2007;25(11):2964–2971. doi: 10.1634/stemcells.2006-0311. [DOI] [PubMed] [Google Scholar]
- 138.Liu R.-M., Sun R.-G., Zhang L.-T., et al. Hyaluronic acid enhances proliferation of human amniotic mesenchymal stem cells through activation of Wnt/β-catenin signaling pathway. Experimental Cell Research. 2016;345(2):218–229. doi: 10.1016/j.yexcr.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 139.Burns J., Yokota T., Ashihara H., Lean M. E. J., Crozier A. Plant foods and herbal sources of resveratrol. Journal of Agricultural and Food Chemistry. 2002;50(11):3337–3340. doi: 10.1021/jf0112973. [DOI] [PubMed] [Google Scholar]
- 140.Dai Z., Li Y., Quarles L. D., et al. Resveratrol enhances proliferation and osteoblastic differentiation in human mesenchymal stem cells via ER-dependent ERK1/2 activation. Phytomedicine. 2007;14(12):806–814. doi: 10.1016/j.phymed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 141.Peltz L., Gomez J., Marquez M., et al. Resveratrol exerts dosage and duration dependent effect on human mesenchymal stem cell development. PLoS One. 2012;7(5, article e37162) doi: 10.1371/journal.pone.0037162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tseng P. C., Hou S. M., Chen R. J., et al. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. Journal of Bone and Mineral Research. 2011;26(10):2552–2563. doi: 10.1002/jbmr.460. [DOI] [PubMed] [Google Scholar]
- 143.Griffin M., Iqbal S. A., Bayat A. Exploring the application of mesenchymal stem cells in bone repair and regeneration. The Journal of Bone and Joint Surgery. British volume. 2011;93-B(4):427–434. doi: 10.1302/0301-620X.93B4.25249. [DOI] [PubMed] [Google Scholar]
- 144.Jo C. H., Lee Y. G., Shin W. H., et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32(5):1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 145.Uccelli A. Adult stem cells for spinal cord injury: what types and how do they work? Cytotherapy. 2008;10(6):541–542. doi: 10.1080/14653240802451172. [DOI] [PubMed] [Google Scholar]
- 146.Ozaki K. Novel strategy for GVHD treatment: possible use of mesenchymal stem cells and interleukin-21. Rinshō Ketsueki. 2012;53(5):483–486. [PubMed] [Google Scholar]
- 147.Uccelli A., Mancardi G., Chiesa S. Is there a role for mesenchymal stem cells in autoimmune diseases? Autoimmunity. 2009;41(8):592–595. doi: 10.1080/08916930802200166. [DOI] [PubMed] [Google Scholar]
- 148.Delorme B., Chateauvieux S., Charbord P. The concept of mesenchymal stem cells. Regenerative Medicine. 2006;1(4):497–509. doi: 10.2217/17460751.1.4.497. [DOI] [PubMed] [Google Scholar]
- 149.Wong H.-L., Siu W. S., Shum W. T., Gao S., Leung P. C., Ko C. H. Application of Chinese herbal medicines to revitalize adult stem cells for tissue regeneration. Chinese Journal of Integrative Medicine. 2012;18(12):903–908. doi: 10.1007/s11655-012-1293-3. [DOI] [PubMed] [Google Scholar]
- 150.Ali T. F., Hasan T. Phlorotannin-incorporated mesenchymal stem cells and their promising role in osteogenesis imperfecta. Journal of Medical Hypotheses and Ideas. 2012;6(2):85–89. doi: 10.1016/j.jmhi.2012.09.002. [DOI] [Google Scholar]
- 151.Marion N. W., Mao J. J. Mesenchymal stem cells and tissue engineering. Methods in Enzymology. 2006;420(6):339–361. doi: 10.1016/S0076-6879(06)20016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim B.-S., Kim Y. C., Zadeh H., et al. Effects of the dichloromethane fraction of Dipsaci Radix on the osteoblastic differentiation of human alveolar bone marrow-derived mesenchymal stem cells. Biosci. Biotechnol. Biochem. 2014;75(1):13–19. doi: 10.1271/bbb.100379. [DOI] [PubMed] [Google Scholar]
- 153.Raghavan R. N., Vignesh G., Kumar B. S., Selvaraj R., Dare B. J. Phytochemicals: do they hold the future in stem cell differentiation? International Journal of Research in Ayurveda & Pharmacy. 2015;6(3):379–381. doi: 10.7897/2277-4343.06374. [DOI] [Google Scholar]