Key Points
Question
What is the growth rate of atrophic lesions in patients with Stargardt disease?
Findings
In this cohort study, the mean progression of definitely decreased autofluorescence lesions was 0.76 mm2 per year, and the mean progression of the area of total decreased fundus autofluorescence was 0.64 mm2 per year. Rates of progression depended on initial lesion size.
Meaning
The growth rate of atrophic lesions as determined by fundus autofluorescence may be a suitable outcome measure of treatment trials for patients with Stargardt disease.
This cohort study estimates the progression rate of atrophic lesions over a 12-month period in the eyes of individuals with Stargardt disease.
Abstract
Importance
Sensitive outcome measures for disease progression are needed for treatment trials of Stargardt disease.
Objective
To estimate the progression rate of atrophic lesions in the prospective Natural History of the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) study over a 12-month period.
Design, Setting, and Participants
This multicenter prospective cohort study was conducted in an international selection of tertiary referral centers from October 21, 2013, to February 15, 2017. Patients who were affected by Stargardt disease, aged 6 years and older at baseline, and harboring disease-causing variants of the ABCA4 gene were enrolled at 9 centers in the United States, United Kingdom, and continental Europe. Data analysis occurred from November 2016 to January 2017.
Exposures
Autofluorescence images obtained with a standard protocol were sent to a central reading center, and areas of definitely decreased autofluorescence, questionably decreased autofluorescence, and the total combined area of decreased autofluorescence were outlined and quantified. Progression rates were estimated from linear mixed models with time as the independent variable.
Main Outcomes and Measures
Yearly rate of progression, using the growth of atrophic lesions measured by autofluorescence imaging.
Results
A total of 259 study participants (488 eyes; 230 individuals [88.8%] were examined in both eyes) were enrolled (mean [SD] age at first visit, 33.3 [15.1] years; 118 [54.4%] female). Gradable images were available for evaluation for 480 eyes at baseline and 454 eyes after 12 months. At baseline, definitely decreased autofluorescence was present in 306 eyes, and the mean (SD) lesion size was 3.93 (4.37) mm2. The mean total area of decreased autofluorescence at baseline was 4.07 (4.04) mm2. The estimated progression of definitely decreased autofluorescence was 0.76 (95% CI, 0.54-0.97) mm2 per year (P < .001), and the total area of both questionably and definitely decreased autofluorescence was 0.64 (95% CI, 0.50-0.78) mm2 per year (P < .001). Both progression rates depended on initial lesion size.
Conclusions and Relevance
In Stargardt disease, autofluorescence imaging may serve as a monitoring tool and definitely decreased autofluorescence and total area as outcome measures for interventional clinical trials that aim to slow disease progression. Rates of progression depended mainly on initial lesion size.
Introduction
Autosomal-recessive Stargardt macular dystrophy (STGD1; OMIM: 248200) attributable to disease-causing variants in the ABCA4 gene is the most common form of juvenile macular degeneration.1,2 Although there are no approved therapies available, possible treatment approaches, including pharmacotherapy, gene augmentation therapy, and stem cell therapy, are all in early clinical development.2,3 However, the natural history of STGD1 remains poorly characterized, and sensitive, reliable, and clinically relevant outcome measures are needed for clinical trials.4 Hence, the retrospective and prospective multicenter Natural History of the Progression of Atrophy Secondary to Stargardt Disease (ProgStar) studies were launched to characterize the natural history of the disease.5 In both studies, the primary outcome measure is the yearly rate of progression of STGD1 as reflected by the growth or the development of atrophic lesions measured by autofluorescence (AF) imaging.5 There is precedence of this outcome measure in clinical trials and natural history studies in geographic atrophy secondary to age-related macular degeneration (AMD),6,7 especially as regulatory agencies have accepted the growth of atrophy as a primary outcome measure.8 While data on the incidence of atrophic lesions and growth rates of such lesions in the retrospective study were previously reported,9,10 we present herein the data derived from AF imaging over a 12-month period of the prospective ProgStar cohort.
Methods
The prospective ProgStar study is a multicenter, observational cohort study conducted at 9 centers in the United States, the United Kingdom, and continental Europe. The study design with inclusion and exclusion criteria has been described in detail previously,5 and therefore we focus here on key points relevant to AF imaging analysis. Eligible patients had to have at least 2 disease-causing variants in ABCA4; however, patients with 1 variant could be enrolled, as long as they had a clinical phenotype typical for STGD1. Further inclusion criteria were a minimum age at the first visit of 6 years or older, a willingness to undergo examinations every 6 months for a 2-year period, and the ability to undergo and perform all examinations. Patients needed to have a well-demarcated lesion of atrophy measuring at least 300 μm and less than 5 standard disc areas (equal to 12 mm2). The main exclusion criterion was previous or current participation in any interventional study to treat STGD1. The schedule consisted of a baseline visit and 4 planned follow-up visits every 6 months (with a time window for each visit of ±5 weeks). Relevant patient data including age at enrollment, sex, race, smoking history, and vitamin A supplementation (alone or in multivitamins) were collected. In addition, full-field electroretinograms (ERG) obtained according to International Society for Clinical Electrophysiology of Vision standards11 were recorded once at the baseline visit, or results were submitted if examinations were performed within 5 years of the baseline visit or during follow-up. Electroretinograms were evaluated by the local principal investigators in comparison with the locally healthy cohort established at each of the participating centers. Scotopic and/or photopic responses were graded as abnormal when amplitudes were reduced or implicit times were delayed.
This analysis used data from the prospective ProgStar study (ClinicalTrials.gov identifier: NCT01977846), which was approved by the Western institutional review board, local institutional review boards, and the Human Research Protection Office of the United States Army Medical Research and Materiel Command. Written informed consent was obtained from each participant or their representative prior to enrollment in the study.
Image Acquisition
The AF imaging protocol in the prospective ProgStar study resulted from a number of considerations.5 To prevent possible confounding effects on disease progression by serial imaging in this longitudinal study, short-wavelength, reduced-illuminance autofluorescence imaging created by reducing laser power, as proposed by Cideciyan et al,12 was implemented in the study design. The detailed acquisition protocol was previously published.5 The use of this approach compared with conventional AF imaging with respect to grading results was evaluated in a pilot study, and comparable results were revealed.13
Grading of Atrophic Lesions on Fundus Autofluorescence
The grading protocols applied in the retrospective ProgStar studies were used as previously described in the grading of AF images.5,9,13,14,15 Additional details are available in eMethods 1 in the Supplement.
Qualitative and Quantitative Parameters
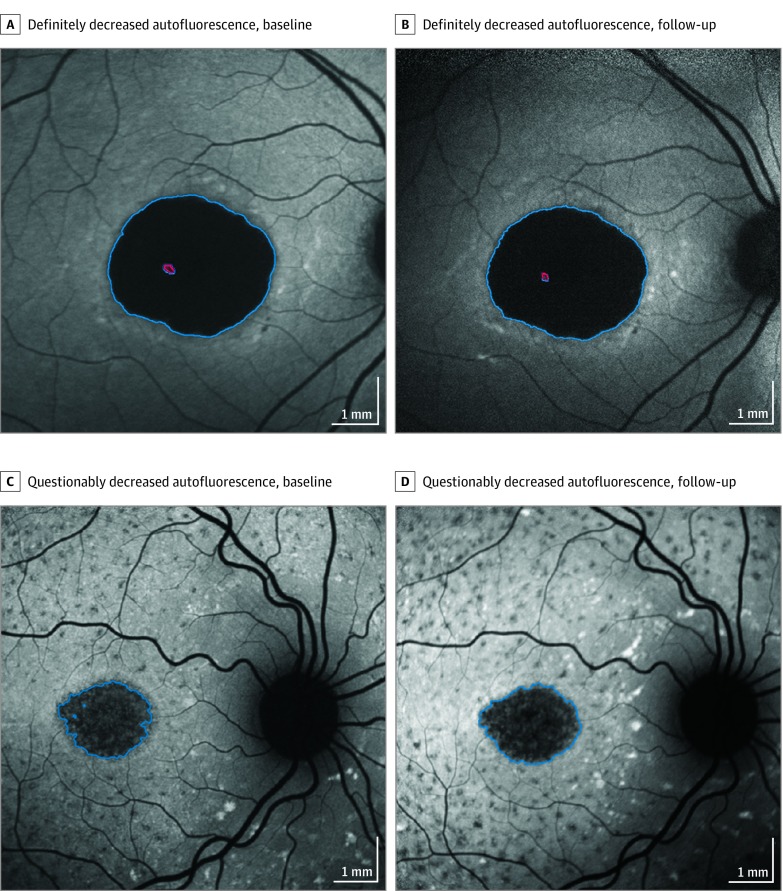
Qualitative parameters (as previously published9,10,13) included background heterogeneity, presence of flecks, increased AF at the lesion edge, and the number of lesions of definitely decreased autofluorescence (DDAF). A semiautomated software tool (RegionFinder [Heidelberg Engineering]) was used for quantitatively grading atrophic lesions on AF images, and 2 distinct types of decreased autofluorescence (DAF) were quantified: the level of darkness of an area of DAF was defined qualitatively as definite or questionable, based on its appearance in comparison with blood vessels or the optic nerve head (a reference point for the 100% level of darkness) and the retinal background seen in the periphery as the opposite reference point (0% blackness; Figure 1). The term definitely decreased autofluorescence was defined for areas in which the level of darkness was at least 90% in reference to the optic nerve head, while regions with levels between 50% and 90% darkness were termed questionably decreased AF (QDAF; Figure 1).
Figure 1. Examples of Progression of Lesions of Definitely Decreased Autofluorescence and Questionably Decreased Autofluorescence.
A, A lesion of definitely decreased autofluorescence (7.87 mm2) at baseline. B, The same eye after 12 months; the lesion is enlarged to 8.48 mm2. C, A lesion of questionably decreased autofluorescence at baseline (2.45 mm2). D, The same eye after 12 months, with the lesion enlarged to 2.90 mm2. Red indicates an excluded area without definitely decreased autofluorescence.
Statistical Methods
Statistical methods are provided in eMethods 2 in the Supplement. Linear models with generalized estimating equations were used to estimate the associations of baseline DAF lesion areas with participant characteristics. Longitudinally, linear mixed models with time as the independent variable were used to estimate the yearly change for each outcome. The models include random effects for the intercept and the slope for time, which take into account the potential correlations between eyes and between repeated measurements of the same eye. The rate of lesion area change associated with each variable was first estimated in an univariate analysis. Multivariate analysis was further run by including variables that were significantly associated with the rate of area change with P values less than .10 in univariate analyses.
Exploratory inspection of lesion growth over time showed a strong dependency on lesion size at the first visit in the retrospective cohort of ProgStar.5 For both DDAF and total DAF lesion area, stratified analyses by lesion size at the first visit were performed for eyes with small lesions (ie, ≤1.90 mm2), eyes with intermediate lesions (>1.9-5.0 mm2), and eyes with large lesions (>5.0 mm2). All analyses were conducted using SAS version 9.3 (SAS Institute), and P values for 2-sided tests are reported, with those less than .05 considered significant.
Results
A total of 489 eyes of 259 participants (including 230 individuals [88.8%] who were examined in both eyes) were enrolled in the prospective ProgStar study between October 21, 2013, and January 30, 2015. Their mean (SD) age at first visit was 33.3 (15.1) years; 118 (54.4%) were female. The eTable in the Supplement further summarizes the patient characteristics and demographics at the baseline visit.
The AF images were gradable for 480 eyes at the baseline visit. (An image was not available for 1 eye, 5 eyes had ungradable images, and 1 AF image for another eye was captured without following the study protocol.) Of these, AF images were gradable for 447 eyes at the 6-month visit (after excluding 3 ungradable images) and 455 eyes (after excluding 4 ungradable images) at the 12-month visit.
Characteristics of Eyes With DDAF
At the baseline visit, a DDAF lesion was present in 306 eyes (62.3%), with a mean (SD) area of 3.93 (4.37) mm2. Flecks were present in 285 eyes and absent in 13 eyes. There was no difference in mean lesion size between the groups. Mean lesion size did also not differ between eyes with unifocal or multifocal lesions. (In eyes with multifocal lesions, this lesion size was the sum of all DDAF lesions.) Eyes with flecks present outside the arcades had significantly larger lesions at baseline (absent, 2.65 [95% CI, 1.91-3.40] mm2; present, 4.81 [95% CI, 3.89-5.73] mm2; P < .001), as did eyes with a heterogenous background (homogenous, 2.58 [95% CI, 1.87-3.29] mm2; heterogenous, 4.97 [95% CI, 4.03-5.92] mm2; P < .001; Table 1). Further baseline characteristics regarding ERG responses (normal, 3.07 [95% CI, 2.40-3.74] mm2; abnormal, 4.75 [95% CI, 3.58-5.92] mm2; P = .02) and nonsignificant differences in contiguity of DDAF lesions, smoking history, and use of vitamin A supplementation of these eyes are summarized in Table 1.
Table 1. Association Between Baseline Characteristics and Areas of Definitely Decreased Autofluorescence and Total Area of the Lesions.
| Characteristic | Definitely Decreased Autofluorescence at First Visit | Definitely and/or Questionably Decreased Autofluorescence, Combined | ||||
|---|---|---|---|---|---|---|
| Patients, No. (%) | Area, Mean (95% CI), mm2 | P Valuea | Patients, No. (%) | Total Area, Mean (95% CI), mm2 | P Valueb | |
| Eyes, No. | 306 | NA | NA | 480 | NA | NA |
| Demographics | ||||||
| Age at baseline, y | ||||||
| <18 | 47 (15.4) | 3.18 (2.22-4.14) | .005 | 83 (17.3) | 4.14 (3.12-5.16) | .003 |
| ≥18-<50 | 194 (63.4) | 3.14 (2.48-3.80) | 318 (66.2) | 3.57 (3.04-4.11) | ||
| ≥50 | 65 (21.2) | 6.72 (4.86-8.59) | 79 (16.5) | 6.72 (5.13-8.31) | ||
| Age at onset of symptoms, yc | ||||||
| <18 | 130 (42.5) | 3.34 (2.58-4.10) | .12 | 204 (42.5) | 3.92 (3.26-4.59) | .36 |
| ≥18 | 153 (50.0) | 4.37 (3.33-5.41) | 241 (50.2) | 4.39 (3.63-5.16) | ||
| Duration of onset of symptoms, y | ||||||
| ≤2 | 15 (5.3) | 1.87 (0.49-3.25) | .13 | 52 (11.6) | 1.80 (1.10-2.50) | <.001 |
| >2-5 | 37 (13.0) | 4.30 (2.19-6.40) | 86 (19.1) | 3.35 (2.23-4.48) | ||
| >5 | 233 (81.7) | 3.94 (3.21-4.68) | 311 (69.3) | 4.75 (4.12-5.38) | ||
| Sex | ||||||
| Male | 136 (55.6) | 3.57 (2.92-4.21) | .30 | 267 (55.6) | 3.84 (3.26-4.41) | .17 |
| Female | 170 (44.4) | 4.20 (3.18-5.21) | 213 (44.4) | 4.51 (3.74-5.29) | ||
| Qualitative autofluorescence grading parameters | ||||||
| Flecks | ||||||
| Absent | 13 (4.3) | 3.65 (2.68-4.61) | .51 | 39 (8.1) | 3.24 (2.37-4.10) | .02 |
| Present | 285 (93.1) | 3.98 (3.32-4.65) | 424 (88.3) | 4.37 (3.84-4.90) | ||
| Questionable/ ungradable | 8 (2.6) | NA | 17 (3.6) | NA | ||
| Flecks beyond the arcadesd | ||||||
| Absent | 124 (40.8) | 2.65 (1.91-3.40) | <.001 | 254 (53.6) | 2.81 (2.32-3.29) | <.001 |
| Present | 179 (58.9) | 4.81 (3.89-5.73) | 219 (46.2) | 5.81 (4.99-6.64) | ||
| Questionable/ ungradable | 1 (0.3) | NA | NA | 1 (0.2) | NA | NA |
| Increased autofluorescence at DDAF lesion edge | ||||||
| Absent | 182 (59.5) | 4.08 (3.37-4.78) | .81 | 250 (52.1) | 4.43 (3.83-5.03) | .41 |
| Present | 82 (26.8) | 3.90 (2.51-5.30) | 159 (33.1) | 4.04 (3.18-4.91) | ||
| Questionable/ ungradable | 42 (13.7) | NA | NA | 71 (14.8) | NA | NA |
| Background | ||||||
| Homogenous | 134 (43.8) | 2.58 (1.87-3.29) | <.001 | 274 (57.1) | 2.71 (2.24-3.18) | <.001 |
| Heterogenous | 172 (56.2) | 4.97 (4.03-5.92) | 206 42.9) | 6.11 (5.27-6.95) | ||
| No. of lesions of definitely decreased autofluorescence | ||||||
| Unifocal | 233 (76.1) | 3.93 (3.29-4.57) | .95 | 233 (48.5) | 5.28 (4.66-5.91) | <.001b |
| Multifocal | 73 (23.9) | 3.90 (2.97-4.83) | 73 (15.2) | 5.28 (4.36-6.20) | ||
| Not present | NA | NA | 174 (36.3) | 2.19 (1.80-2.58) | ||
| Other | ||||||
| Vitamin A supplementation | ||||||
| Yes | 39 (12.7) | 6.10 (3.59-8.62) | .07 | 64 (13.3) | 5.81 (3.99-7.64) | .06 |
| No | 267 (87.3) | 3.61 (2.99-4.22) | 416 (86.7) | 3.96 (3.47-4.46) | ||
| Smoking | ||||||
| Never | 211 (68.9) | 3.49 (2.78-4.19) | .12 | 353 (73.5) | 3.88 (3.33-4.43) | .05 |
| Former | 55 (18.0) | 5.52 (3.77-7.27) | 68 (14.2) | 4.06 (2.67-5.46) | ||
| Current | 40 (13.1) | 4.13 (2.21-6.05) | 59 (12.3) | 6.00 (4.49-7.52) | ||
| Full-field electroretinograme | ||||||
| Normal | 178 (61.8) | 3.07 (2.40-3.74) | .02 | 302 (66.5) | 3.44 (2.92-3.97) | .001 |
| Abnormalf | 110 (38.2) | 4.75 (3.58-5.92) | 152 (33.5) | 5.33 (4.35-6.32) | ||
Abbreviations: DDAF, definitely decreased autofluorescence; NA, not applicable.
Comparisons between subgroups.
P value indicates testing for any difference among groups.
Unknown for 21 patients, of whom 2 were asymptomatic (with definitely decreased autofluorescence); unknown for 31 patients, of whom 4 were asymptomatic (with respect to the total area of questionably decreased autofluorescence).
The presence of flecks outside the arcades was assessed using information derived from clinical examinations.
Not performed at baseline or not available in 24 eyes.
Not known for 2 eyes.
Characteristics of Eyes With Any Lesion
The mean (SD) area lesion size of QDAF lesions was 4.07(4.04) mm2. Flecks were present in 424 eyes (88.3%) and absent in 39 eyes (8.1%), and eyes with flecks present had larger lesions than eyes without any flecks (absent, 3.24 [95% CI, 2.37-4.10] mm2; present, 4.37 [95% CI, 3.84-4.90] mm2; P = .02); eyes with flecks beyond the arcades also had larger lesions than eyes without flecks (absent, 2.81 [95% CI, 2.32-3.29] mm2; present, 5.81 [95% CI, 4.99-6.64] mm2; P < .001; Table 1). Additional characteristics at baseline in eyes with any lesions of DAF are also summarized in Table 1.
Rates of Lesion Progression
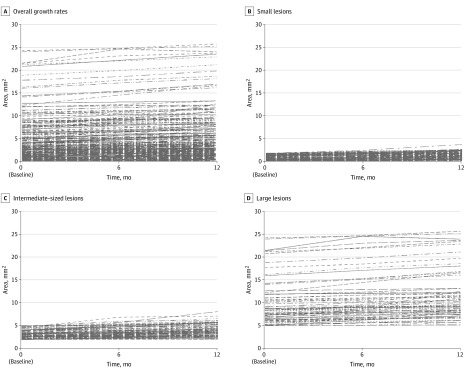
In eyes presenting with DDAF at baseline, the rate of DDAF growth was 0.76 (95% CI, 0.54-0.97) mm2 per year (P < .001). However, growth rates were dependent on initial lesion size (Figure 2): the estimated growth rate in eyes with small lesion sizes (≤1.90 mm2) was 0.40 (95% CI, 0.16-0.64) mm2 per year; it was 0.72 (95% CI, 0.44-0.99) mm2 per year in intermediate-sized DDAF lesions (>1.90-5 mm2) and 1.41 (95% CI, 1.10-1.73) mm2 per year in large lesions (>5.0 mm2; Table 2). The estimated difference in the rate of DDAF growth was 0.31 (95% CI, −0.05 to 0.68) mm2 per year (P = .09) between eyes with smaller lesions and those with intermediate-sized DDAF lesions and 1.01 (95% CI, 0.62-1.40) mm2 per year (P < .001) between eyes with small and large lesions.
Figure 2. Estimated Growth Rates of Lesions of Definitely Decreased Autofluorescence.
Estimated growth rates of lesions of definitely decreased autofluorescence are shown as overall growth rates (A) and subgroups of lesion size at baseline: small lesions (≤1.90 mm2) (B); intermediate-sized lesions (>1.90-5 mm2) (C), and large lesions (>5.0 mm2) (D).
Table 2. Estimates of Yearly Growth Rates for Definitely Decreased Autofluorescence and the Total Area by Baseline Lesion Size and Baseline Characteristics in Univariate Analysis and Multivariate Analysis, After Adjusting for Initial Lesion Size.
| Baseline Characteristic | Definitely Decreased Autofluorescence (95% CI), mm2/y | Definitely and Questionably Decreased Autofluorescence (95% CI), mm2/y | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimated Progression Rate per Univariate Analysis | Estimated Rate Difference in Progression per Univariate Analysis | Estimated Rate Difference in Progression per Multivariate Analysisa | Estimated Progression Rate per Univariate Analysis | Rate Difference per Univariate Analysis | Rate Difference per Multivariate Analysisa | |||||
| Rate | P Value | Rate | P Value | Rate | P Value | Rate | P Value | |||
| Overall size, mm2 | ||||||||||
| ≤1.90 | 0.40 (0.16-0.64) | 0.31 (−0.05 to 0.68) | .09 | 0.18 (−0.20 to 0.57) | .34 | 0.29 (0.10-0.48) | 0.32 (0.05-0.59) | .02 | 0.14 (−0.37 to 0.65) | .53 |
| >1.9-≤5.0 | 0.72 (0.44-0.99) | 0.61 (0.43-0.80) | ||||||||
| >5.0 | 1.41 (1.10-1.73) | 1.01 (0.62-1.40)b | <.001b | 0.78 (0.36-1.20)b | <.001b | 1.17 (0.94-1.39)b | 0.87 (0.58-1.17)b | <.001b | 0.49 (−0.06 to 1.05)b | .08b |
| Age at baseline, y | ||||||||||
| <18 | 0.71 (0.17-1.24) | −0.07 (−0.67 to 0.53) | .82 | NA | NA | 0.55 (0.22-0.88) | 0.01 (−0.36 to 0.38) | .95 | NA | NA |
| 18-50 | 0.64 (0.37-0.91) | 0.56 (0.39-0.74) | ||||||||
| ≥50 | 1.13 (0.67-1.59) | 0.43 (−0.28 to 1.14)c | .24c | NA | NA | 1.02 (0.68-1.36) | 0.47 (−0.01 to 0.94)c | .05c | NA | NA |
| Age at onset of symptoms, y | ||||||||||
| <18 | 0.57 (0.25-0.90) | 0.24 (−0.20 to 0.69) | .28 | NA | NA | 0.68 (0.36-0.99) | 0.18 (−0.25 to 0.61) | .42 | NA | NA |
| ≥18 | 0.82 (0.51-1.13) | 0.86 (0.56-1.15) | ||||||||
| Duration of onset of symptoms, y | ||||||||||
| ≤2 | 1.06 (0.11-2.02) | − 0.38 (−1.53 to 0.77) | .51 | NA | NA | 1.01 (0.10-1.93) | −0.16 (−1.27 to 0.95) | .78 | NA | NA |
| >2-5 | 0.68 (0.04-1.33) | 0.85 (0.23-1.47) | ||||||||
| >5 | 0.71 (0.46-0.96) | −0.35 (−1.33 to 0.63)d | .48d | NA | NA | 0.77 (0.53-1.01) | −0.24 (−1.19 to 0.70) | .61 | NA | NA |
| Definitely decreased autofluorescence lesions, No. | ||||||||||
| Unifocal | 0.60 (0.36-0.84) | 0.66 (0.16-1.15) | .01 | 0.62 (0.24-1.00) | .001 | 0.64 (0.41-0.88)e | 0.63 (0.15-1.11) | .01 | 0.55 (0.13 to 0.97) | .01 |
| Multifocal | 1.26 (0.82-1.69) | 1.28 (0.86-1.70)e | ||||||||
| Flecks beyond the arcades | ||||||||||
| Absent | 0.47 (0.14-0.81) | 0.47 (0.04-0.91) | .03 | 0.12 (−0.34 to 0.58) | .61 | 0.42 (0.23-0.61) | 0.49 (0.21-0.78) | <.001 | 0.23 (−0.28 to 0.74) | .38 |
| Present | 0.95 (0.67-1.23) | 0.91 (0.70-1.12) | ||||||||
| Increased autofluorescence at lesion edge | ||||||||||
| Absent | 0.82 (0.55-1.10) | −0.13 (−0.63 to 0.36) | .60 | NA | NA | 0.73 (0.53-0.92) | −0.22 (−0.53 to 0.09) | .17 | NA | NA |
| Present | 0.69 (0.28-1.10) | 0.51 (0.27-0.75) | ||||||||
| Questionablef | 0.59 (0.00-1.17) | −0.23 (−0.88 to 0.41)f | .47f | NA | NA | 0.62 (0.25-0.98) | − 0.11 (−0.53 to 0.30)f | .60f | NA | NA |
| Background heterogeneity | ||||||||||
| Homogenous | 0.45 (0.14-0.77) | 0.56 (0.13-0.99) | .01 | 0.20 (−0.27 to 0.66) | .40 | 0.51 (0.21-0.82) | 0.53 (0.11-0.94) | .01 | 0.16 (−0.36 to 0.69) | .54 |
| Heterogenous | 1.02 (0.73-1.31) | 1.04 (0.76-1.32) | ||||||||
| Vitamin A use | ||||||||||
| No | 0.72 (0.49-0.95) | 0.40 (−0.36 to 1.16) | .31 | NA | NA | 0.62 (0.47-0.77) | 0.13 (−0.34 to 0.60) | .59 | NA | NA |
| Yes | 1.12 (0.40-1.84) | 0.75 (0.31-1.19) | ||||||||
| Smoking | ||||||||||
| Never | 0.65 (0.41-0.92) | 0.37 (−0.20 to 0.94) | .20 | NA | NA | 0.58 (0.42-0.74) | 0.27 (−0.13 to 0.67) | .19 | NA | NA |
| Former | 1.04 (0.53-1.54) | 0.85 (0.48-1.22) | ||||||||
| Currentg | 0.65 (0.02-1.28) | −0.02 (−0.70 to 0.67)g | .96g | NA | NA | 0.63 (0.19-1.08) | 0.05 (−0.42 to 0.53)g | .82g | NA | NA |
| Electroretinograms | ||||||||||
| Normal | 0.62 (0.34-0.90) | 0.33 (−0.12 to 0.79) | .15 | NA | NA | 0.58 (0.40-0.76) | 0.18 (−0.13 to 0.49) | .26 | NA | NA |
| Abnormal | 0.95 (0.59-1.31) | 0.76 (0.50-1.01) | ||||||||
Abbreviation: NA, not applicable.
After adjusting for initial lesion size in variables associated with lesion growth rate in univariate analysis at significance level of less than or equal to .10.
Rate difference between eyes with lesions of 1.90 mm2 or less and those with lesions of more than 5.0 mm2.
Rate difference between eyes of patients younger than 18 years and older than 50 years at baseline visit.
Rate difference between eyes of patients with onset of symptoms less than 2 years ago and those with symptom onset more than 5 years ago.
Growth rates based on contiguity of definitely decreased area within the total area lesion of decreased autofluorescence.
Rate difference between eyes with questionable vs absent increased autofluorescence at the lesion edge.
Rate difference between eyes of those who currently smoke and those who have never smoked.
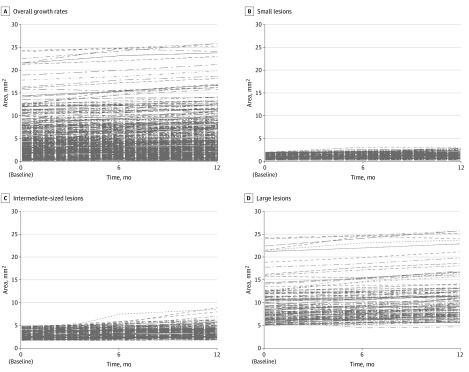
The growth rate of total DAF lesion size overall was 0.64 (95% CI, 0.50-0.78) mm2 per year. However, this varied significantly with initial lesion size (Figure 3): for small lesions, the rate was 0.29 (95% CI, 0.10-0.48) mm2 per year; for intermediate-sized lesions, the rate was 0.61 (95% CI, 0.43-0.80) mm2 per year; and for large lesions, the rate was 1.17 (95% CI, 0.94-1.39) mm2 per year (Table 2).
Figure 3. Estimated Growth Rates of Lesions of Definitely Decreased Autofluorescence Plus Questionably Decreased Autofluorescence Combined.
Overall growth rates (A), with subgroups according to lesion size at baseline: small lesions (≤1.90 mm2) (A); intermediate-sized lesions (>1.90-5 mm2) (B), and large lesions (>5.0 mm2) (C).
The DDAF and total DAF growth rates were estimated for different subgroups. There were no significant differences in estimated progression rate based on demographic features, including age at baseline, age at onset of symptoms, duration of disease at baseline, smoking status, or vitamin A supplementation (Table 2). There was also no significant difference between subcohorts with normal vs abnormal full-field ERG responses (Table 2). However, the rates of DDAF and total DAF growth were significantly faster in eyes with flecks beyond the arcades compared with eyes without flecks beyond the arcades (DDAF, 0.47 [95% CI, 0.04-0.91] mm2/y; P = .03; total DAF, 0.49 [95% CI, 0.21-0.78] mm2/y; P < .001) and eyes with heterogenous backgrounds at baseline compared with eyes with homogenous backgrounds (DDAF, 0.56 [95% CI, 0.13-0.99] mm2/y; P = .01; DAF, 0.53 [95% CI, 0.11-0.94] mm2/y; P = .01). In eyes with multifocal DDAF lesions (whether alone or in combination with QDAF) at baseline, those with multifocal lesions had faster growth rates compared with eyes with a unifocal DDAF lesion (alone: unifocal DDAF, 0.60 [95% CI, 0.36-0.84] mm2/y; multifocal DDAF, 1.26 [95% CI, 0.82-1.69] mm2/y; P = .01; combined with QDAF: unifocal DAF, 0.64 [95% CI, 0.41-0.88] mm2; multifocal DAF, 1.28 [95% CI, 0.86-1.70] mm2; P = .01; Table 2).
After applying multivariate models and adjusting for initial lesion size, the difference in growth rates were no longer significant between eyes for absent or present flecks outside the arcades (estimated differences in rate progression: DDAF, 0.09 [95% CI, −0.36 to 0.55; P = .69]; DAF, 0.12 [95% CI, −0.26 to 0.50]; P = .62) and between eyes with homogenous and heterogeneous background (estimated differences in rate progression: DDAF, 0.17 [95% CI, −0.28 to 0.63]; P = .46; DAF, 0.19 [95% CI, −0.20 to 0.59]; P = .95). The difference in DDAF growth rates remained significant between eyes with a unifocal lesion of DDAF vs eyes with multifocal lesions of DDAF (estimated rate difference: DDAF, 0.61 [95% CI, 0.23-0.98]; P = .001) mm2/y; DAF, 0.54 [95% CI, 0.12-0.96] mm2/y; P = .01).
Incidence of DDAF Lesion From Baseline to 12 Months’ Visit
There were 174 eyes without any DDAF lesions at baseline, of which 161 (of 163) eyes had gradable images at month 12. At month 12, 43 of 161 eyes (26.7%) had a new DDAF lesion observed, with a mean (SD) lesion size of 1.02 (1.70) mm2.
Discussion
ProgStar is, to our knowledge, the first international, multicenter study to evaluate the natural history of STGD1 prospectively and was designed as a hybrid study with a retrospective review of medical records and/or a prospective follow-up of patients.5 In this article, we report on ProgStar’s primary outcome measure: namely, the prospective growth rate as determined by AF. Because the lesion growth rate is also characterized by loss of photoreceptors, it is considered a surrogate end point for vision loss.8 The estimation of growth rates in STGD1 using AF was first proposed by Chen et al,16 who observed a mean (SD) rate of 0.94 (0.87) mm2 per year, and then in multiple reports originating from single centers that suggested a wide range of growth rates, from 0.45 mm2 per year (in 67 patients [range, 0.06-4.37 mm2/y for different subgroups])14 to 1.58 mm2 per year (range, 0.13-5.27 mm2/y).17 Of note, all these studies only assessed lesions corresponding to DDAF lesions. The grading protocols of ProgStar also included the grading of QDAF lesions in addition to DDAF lesions, especially as these may represent early stages of disease and may still be rescuable by gene augmentation therapy or pharmacotherapy, for example.2,3
Additionally, short-wavelength, reduced-illuminance autofluorescence imaging was applied in a multicenter setting for the first time. Accumulation of N-retinylidene-N-retinyethanolamine and lipofuscin are believed to be cytotoxic, resulting in dysfunction and cell death of the retinal pigment epithelium (RPE) and photoreceptors. One mechanism is the mediation through blue light–induced damage to RPE cells by photooxidative damage,13,18,19 and an increased rate of lipofuscin accumulation and/or its toxicity could be triggered by the high-intensity and short-wavelength excitation light used in conventional AF imaging.12 A recent comprehensive simulation of photooxidative stress in the RPE in vivo suggested that lipofuscin granules have a 3-fold higher oxygen uptake and light absorption in patients with STGD1 than age-matched control individuals, with the RPE being at increased risk of photooxidative stress, which is amplified by exposure during short-wavelength autofluorescence imaging.20 Furthermore, ophthalmic imaging equipment operates safely below retinal light-damage thresholds; however, those thresholds are mainly based on unaffected retinas, while diseased retinas may have lower light-damage thresholds.12,20
One of the key findings of the analysis of the retrospective medical records review in 215 patients was that growth rates were strongly dependent on initial lesion size and taking into account initial size when reporting progression rates (in mm2 per year) is important.10 As previously reported, 145 of 259 patients enrolled in the prospective study had already been followed up retrospectively. Hence, mean (SD) lesion size at baseline in the retrospective study (DDAF, 2.2 [2.7] mm2; DAF, 2.6 [2.8] mm2) was smaller than in the prospective study (DDAF, 3.92 [4.38] mm2; DAF, 4.09 [4.04] mm2). This may be the reason that the estimated mean progression (DDAF, 0.67 [95% CI, 0.44-0.90] mm2/y; DAF, 0.62 [95% CI, 0.47-0.76] mm2/ year) was greater in the prospective than in the retrospective study (DDAF, 0.51 [95% CI, 0.42-0.61] mm2/y; DAF, 0.35 [95% CI, 0.28-0.43] mm2/ year). One possible explanation could be that a larger initial lesion would have a larger surface area for further growth and thus had a larger rate of growth.
Previous studies from single centers reported slower progression rates for eyes with a homogenous background, although these were not stratified for initial lesion size.14,17 A report on the progression of geographic atrophy secondary to AMD showed that the mean change in lesion size from baseline to month 12 was significantly larger in patients who had eyes with multifocal atrophic spots compared with those with unifocal spots,7 in keeping with these results on patients with STGD1. There may be shared pathways of both diseases, such as photooxidative stress caused by RPE bisretinoid accumulation and possible links to light exposure in both STGD1 and AMD.21 However, STGD1 and AMD clearly differ in atrophy progression, which was recently found to be significantly faster in AMD compared with late-onset STGD1 (ie, cases in which patients are at least 45 years old at self-reported symptom onset).22
A previous study included electrophysiological assessment and suggested faster progression in patients with abnormal ERGs at baseline; however, the study cohort was very small and did not include genotypic confirmation.17 We could not detect a significant difference in progression rates between eyes with normal and abnormal responses in full-field ERG, although there was a significant difference in lesion size at baseline; however, there was a numerically higher but nonsignificant faster progression rates in eyes with abnormal ERGs. One possible explanation for this difference with previous work is that ProgStar has some bias, based on its inclusion criteria5: to be able to track lesion growth by AF, the maximum lesion size was set to be less than 12 mm2, which inevitably lead to the exclusion of eyes with more severe types of STGD1 both genotypically and phenotypically.
This investigation did not analyze genotypes and rates of progression in part because ABCA4 is a large, complex gene with approximately 1000 disease-causing variants currently reported. Future studies appear warranted regarding a proper genotype-phenotype correlation on progression rates.
The use of vitamin A has potential detrimental effects in patients with disease-causing ABCA4 mutations, at least based on results derived from animal research.23,24 However, 37 of 250 patients (14.3%) took vitamin A in addition to their usual nutrition at baseline; this was surprising, because literally all study investigators advised patients not to take vitamin A supplements. Although we could see a trend toward a faster progression rate in DDAF lesions, this was not significant over a 12-month period.
Limitations
One limitation is the recording of vitamin A intake because a general nutrition diary that would document entire vitamin A intake was beyond the limits of this natural history study. This might be of relevance for upcoming treatment trials for compounds interacting with vitamin A metabolism, however.2,3,25
Smoking has been associated with a myriad of negative ocular effects.26 Recently, subclinical nicotine induced foveal changes in the absence of clinically apparent foveal toxicity in healthy young people who smoked.27 In the limited number of individuals who formerly or currently smoke in this cohort, we did not detect a significant difference in growth rate, at least over the 12-month period. Future studies might potentially explore the possible detrimental outcomes of smoking further.
One essential difference between the retrospective and the prospective study has been the mean observational period. Whereas in the retrospective study, it was 3.9 (1.6) years (range, 0.7-12.1 years), we report herein a follow-up period of 12 months. However, 43 of 161 eyes (26.7%) without a lesion at baseline developed at least 1 DDAF lesion over these 12 months. In the retrospective study, the median time to develop DDAF lesions was 4.9 (95% CI, 4.3-5.6) years, and by 3.0 years, 24% had developed such a lesion.9 This difference in incidence of DDAF lesions can be attributed to the different observational period and the difference in inclusion criteria.
This report provides data on structural changes over a 1-year period, and we cannot determine at this time whether the structural changes correlate with functional changes, such as best-corrected visual acuity and microperimetry-derived retinal light sensitivity.28,29,30,31,32 Furthermore, the outcome of genetic variants on disease progression will be explored over a 2-year period.
Conclusions
In conclusion, AF is a suitable tool to track disease progression in STGD1, especially of DDAF lesions. It may be used in interventional trials that aim to slow down disease progression.
eMethods 1. Grading of atrophic lesions on fundus autofluorescence
eMethods 2. Statistical methods
eReferences.
eTable. Demographic characteristics at baseline visit of patients in the prospective ProgStar study
eAppendix. ProgStar study team
References
- 1.Allikmets R, Singh N, Sun H, et al. . A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15(3):236-246. doi: 10.1038/ng0397-236 [DOI] [PubMed] [Google Scholar]
- 2.Tanna P, Strauss RW, Fujinami K, Michaelides M. Stargardt disease: clinical features, molecular genetics, animal models and therapeutic options. Br J Ophthalmol. 2017;101(1):25-30. doi: 10.1136/bjophthalmol-2016-308823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scholl HP, Strauss RW, Singh MS, et al. . Emerging therapies for inherited retinal degeneration. Sci Transl Med. 2016;8(368):368rv6. doi: 10.1126/scitranslmed.aaf2838 [DOI] [PubMed] [Google Scholar]
- 4.Thompson DA, Ali RR, Banin E, et al. ; Monaciano Consortium . Advancing therapeutic strategies for inherited retinal degeneration: recommendations from the Monaciano Symposium. Invest Ophthalmol Vis Sci. 2015;56(2):918-931. doi: 10.1167/iovs.14-16049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strauss RW, Ho A, Muñoz B, et al. ; Progression of Stargardt Disease Study Group . The natural history of the progression of atrophy secondary to Stargardt disease (ProgStar) studies: design and baseline characteristics, ProgStar report No. 1. Ophthalmology. 2016;123(4):817-828. doi: 10.1016/j.ophtha.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 6.Schmitz-Valckenberg S, Brinkmann CK, Alten F, et al. . Semiautomated image processing method for identification and quantification of geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(10):7640-7646. doi: 10.1167/iovs.11-7457 [DOI] [PubMed] [Google Scholar]
- 7.Schmitz-Valckenberg S, Sahel JA, Danis R, et al. . Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression study). Ophthalmology. 2016;123(2):361-368. doi: 10.1016/j.ophtha.2015.09.036 [DOI] [PubMed] [Google Scholar]
- 8.Csaky KG, Richman EA, Ferris FL III. Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2008;49(2):479-489. doi: 10.1167/iovs.07-1132 [DOI] [PubMed] [Google Scholar]
- 9.Strauss RW, Muñoz B, Ho A, et al. ; ProgStar Study Group . Incidence of atrophic lesions in Stargardt disease in the progression of atrophy secondary to Stargardt disease (ProgStar) study: report No. 5. JAMA Ophthalmol. 2017;135(7):687-695. doi: 10.1001/jamaophthalmol.2017.1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strauss RW, Muñoz B, Ho A, et al. ; ProgStar Study Group . Progression of Stargardt disease as determined by fundus autofluorescence in the Retrospective Progression of Stargardt Disease Study (ProgStar Report No. 9). JAMA Ophthalmol. 2017;135(11):1232-1241. doi: 10.1001/jamaophthalmol.2017.4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCulloch DL, Marmor MF, Brigell MG, et al. . ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015;130(1):1-12. doi: 10.1007/s10633-014-9473-7 [DOI] [PubMed] [Google Scholar]
- 12.Cideciyan AV, Swider M, Aleman TS, et al. . Reduced-illuminance autofluorescence imaging in ABCA4-associated retinal degenerations. J Opt Soc Am A Opt Image Sci Vis. 2007;24(5):1457-1467. doi: 10.1364/JOSAA.24.001457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strauss RW, Muñoz B, Jha A, et al. . Comparison of short-wavelength reduced-illuminance and conventional autofluorescence imaging in Stargardt macular dystrophy. Am J Ophthalmol. 2016;168:269-278. doi: 10.1016/j.ajo.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujinami K, Lois N, Mukherjee R, et al. . A longitudinal study of Stargardt disease: quantitative assessment of fundus autofluorescence, progression, and genotype correlations. Invest Ophthalmol Vis Sci. 2013;54(13):8181-8190. doi: 10.1167/iovs.13-12104 [DOI] [PubMed] [Google Scholar]
- 15.Kuehlewein L, Hariri AH, Ho A, et al. . Comparison of manual and semiautomated fundus autofluorescence analysis of macular atrophy in Stargardt disease phenotype. Retina. 2016;36(6):1216-1221. doi: 10.1097/IAE.0000000000000870 [DOI] [PubMed] [Google Scholar]
- 16.Chen B, Tosha C, Gorin MB, Nusinowitz S. Analysis of autofluorescent retinal images and measurement of atrophic lesion growth in Stargardt disease. Exp Eye Res. 2010;91(2):143-152. doi: 10.1016/j.exer.2010.03.021 [DOI] [PubMed] [Google Scholar]
- 17.McBain VA, Townend J, Lois N. Progression of retinal pigment epithelial atrophy in stargardt disease. Am J Ophthalmol. 2012;154(1):146-154. doi: 10.1016/j.ajo.2012.01.019 [DOI] [PubMed] [Google Scholar]
- 18.Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000;41(7):1981-1989. [PubMed] [Google Scholar]
- 19.Boulton M, Dontsov A, Jarvis-Evans J, Ostrovsky M, Svistunenko D. Lipofuscin is a photoinducible free radical generator. J Photochem Photobiol B. 1993;19(3):201-204. doi: 10.1016/1011-1344(93)87085-2 [DOI] [PubMed] [Google Scholar]
- 20.Teussink MM, Lambertus S, de Mul FF, et al. . Lipofuscin-associated photo-oxidative stress during fundus autofluorescence imaging. PLoS One. 2017;12(2):e0172635. doi: 10.1371/journal.pone.0172635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sears AE, Bernstein PS, Cideciyan AV, et al. . Towards treatment of Stargardt disease: workshop organized and sponsored by the Foundation Fighting Blindness. Transl Vis Sci Technol. 2017;6(5):6. doi: 10.1167/tvst.6.5.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindner M, Lambertus S, Mauschitz MM, et al. ; Foveal sparing Atrophy Study Team (FAST) . Differential disease progression in atrophic age-related macular degeneration and late-onset Stargardt disease. Invest Ophthalmol Vis Sci. 2017;58(2):1001-1007. doi: 10.1167/iovs.16-20980 [DOI] [PubMed] [Google Scholar]
- 23.Sparrow JR, Gregory-Roberts E, Yamamoto K, et al. . The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012;31(2):121-135. doi: 10.1016/j.preteyeres.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radu RA, Yuan Q, Hu J, et al. . Accelerated accumulation of lipofuscin pigments in the RPE of a mouse model for ABCA4-mediated retinal dystrophies following vitamin A supplementation. Invest Ophthalmol Vis Sci. 2008;49(9):3821-3829. doi: 10.1167/iovs.07-1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charbel Issa P, Barnard AR, Herrmann P, Washington I, MacLaren RE. Rescue of the Stargardt phenotype in Abca4 knockout mice through inhibition of vitamin A dimerization. Proc Natl Acad Sci U S A. 2015;112(27):8415-8420. doi: 10.1073/pnas.1506960112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galor A, Lee DJ. Effects of smoking on ocular health. Curr Opin Ophthalmol. 2011;22(6):477-482. doi: 10.1097/ICU.0b013e32834bbe7a [DOI] [PubMed] [Google Scholar]
- 27.El-Shazly AA, Farweez YA, Elzankalony YA, Elewa LS, Farweez BA. Effect of smoking on macular function and structure in active smokers versus passive smokers. Retina. 2018;38(5):1031-1040. [DOI] [PubMed] [Google Scholar]
- 28.Strauss RW, Kong X, Bittencourt MG, et al. ; for the SMART Study Group . Scotopic Microperimetric Assessment of Rod Function in Stargardt Disease (SMART) study: design and baseline characteristics (report No. 1). Ophthalmic Res. 2019;61(1):36-43. doi: 10.1159/000488711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ervin AM, Strauss RW, Ahmed MI, et al. ; ProgStar Study Group . A workshop on measuring the progression of atrophy secondary to stargardt disease in the ProgStar studies: findings and lessons learned. Transl Vis Sci Technol. 2019;8(2):16. doi: 10.1167/tvst.8.2.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong X, Fujinami K, Strauss RW, et al. ; ProgStar Study Group . Visual acuity change over 24 months and its association with foveal phenotype and genotype in individuals with Stargardt disease: ProgStar study report No. 10. JAMA Ophthalmol. 2018;136(8):920-928. doi: 10.1001/jamaophthalmol.2018.2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong X, Strauss RW, Cideciyan AV, et al. ; ProgStar Study Group . Visual acuity change over 12 months in the prospective progression of atrophy secondary to Stargardt disease (ProgStar) study: ProgStar report No. 6. Ophthalmology. 2017;124(11):1640-1651. doi: 10.1016/j.ophtha.2017.04.026 [DOI] [PubMed] [Google Scholar]
- 32.Schönbach EM, Strauss RW, Kong X, et al. ; ProgStar Study Group . Longitudinal changes of fixation location and stability within 12 months in Stargardt disease: ProgStar report No. 12. Am J Ophthalmol. 2018;193:54-61. doi: 10.1016/j.ajo.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Grading of atrophic lesions on fundus autofluorescence
eMethods 2. Statistical methods
eReferences.
eTable. Demographic characteristics at baseline visit of patients in the prospective ProgStar study
eAppendix. ProgStar study team