Introduction
Model organisms are meant to reduce complexity and allow testing of mechanisms. However, for nervous system control of arm and hand movements, testing in rodents poses several challenges. First, much time is spent trying to get rodents to understand what is expected of them, rather than building skill (motor learning), per se. Shaping behavior varies by the skill of the experimenter and how natural the behavior is for the animal. In addition, rodents are able to perform tasks using a wide repertoire of movements in health and compensatory strategies after injury or disease1,2. This makes discrimination of how a task is performed and the level of task performance difficult. Finally, one of the most accurate methods of tracking hand movements in people is through marker based motion tracking3,4. However, using similar technology in rodents is very challenging given the small size of rodents (particularly the paw) and the difficulty of attaching markers on their paws without being gnawed.
Current assays of rodent forelimb movements meet several of these challenges, but they have important limitations. Since most tasks are performed and scored by human experimenters, they are labor-intensive, and the evaluation of kinematics can be subjective and largely qualitative5,6. We argue that these limitations are the biggest motivation for inventing automated devices7–11. By automation, we mean that human tasks are done by a machine or computer, including training or analysis of forelimb movements7.
In this Point of View article, we weigh the use of automated forelimb tasks against traditional manual tasks. We first describe the different types of manual tasks that are currently in use, followed by discussion of available automated tasks. We detail the challenges of adopting automated systems, as well as ways to mitigate these challenges. We conclude by describing some attributes for design of future automated tasks and the validation studies that might lead to their wide scale adoption.
Manual Tasks
We define manual tasks as those that require human experimenters for testing and analysis. This method involves directly observing or video recording rodents reaching, walking, or manipulating. Examples of manual tasks that rely on human experimenters are shown in Fig. 112,13. Much of our current understanding about motor skills comes from studies using these tasks5,14–19. Examples include the single pellet reaching task (SPRT), Irvine, Beatties, and Bresnahan (IBB) task20, Montoya staircase test, vermicelli manipulation task13, and the pasta matrix reaching task21. Among these tasks, the SPRT is both common and representative of reaching, a key forelimb task, so we will use this task to illustrate many of our points.
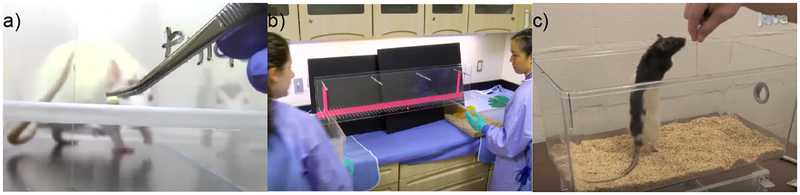
Figure 1.
Manual rodent tasks. A) An experimenter baits the single pellet reaching task. B) Two experimenters run the horizontal ladder task. C) The vermicelli handling task requires an experimenter to actively bait and video record the behavior. Figure B and C are courtesy of JoVE.
We have chosen to specifically focus on SPRT due to three specific advantages it has over other available tasks. First, despite its labor intensive nature, many scientists prefer this task over others because it is highly sensitive to chronic neuronal injuries. Second, it also provides success rate outcomes along with kinematics of movements. Finally, this task tests a forelimb movement that is homologous to human movement22. There are other tasks that are widely used by many laboratories like the Montoya staircase, but it is beyond the scope of this article to compare automated tasks to these all available tasks. To facilitate the discussion on distinctions between Manual and Automated tasks, we have summarized the advantages and disadvantages of both the ‘Automated Tasks’ and ‘Manual Tasks’ in Table 1.
Table 1:
Comparison of Automated and Manual Forelimb Tasks.
| Parameters | Manual | Automated Tasks |
|---|---|---|
| Time Commitment (Training and Testing) | Train: ++ Test: +++ |
Train: + Test: + |
| Time Commitment (Analysis) | Analysis: +++ | Analysis: ++ (initial) / + (subsequent) |
| Objectivity | Low | High |
| Sensitivity | Low | High |
| Kinematics | Qualitative | Quantitative (not always) |
| Repertoire (range of captured mvmts) | Full Range | Full Range |
| Ease of Use | Initial: +++ Ongoing: +++ |
Initial: + Ongoing: ++ |
| Overall Cost (Hardware/Software) | Equipment: $ Labor: $$$ |
Equipment: $$/$$$ Labor: $ |
Note: this analysis is relative and may change over time for both automated and manual methods.
Advantages of manual tasks
There are number of reasons why manual tasks are still very popular today. Many researchers prefer these methods because they have a track record of efficacy and an extensive publication history which allows for comparison against previous studies. Also, these tasks are intuitive for both the rodents performing the task and human experimenter. In addition, they have low start-up costs, are simple to implement, and do not take up much space. For example, in our experience new Single Pellet Reaching Task (SPRT) costs only around US $700 for the clear plastic materials and the video camera, and this can be set up on any lab bench.
The ability to combine multiple manual tasks into one study is another major advantage of using these tasks. Combining several tasks provides performance evaluation over a larger repertoire of movements and ability levels. For example, while the SPRT or Montoya staircase evaluates reaching and grasping skill, the cylinder test measure paw movement during rearing and preference between the paws. Manual tests like the cylinder test and vermicelli handling task that measure paw preference can evaluate differences between paws that are often missed in single forelimb assessments.
Disadvantages of Manual Tasks
The main disadvantages of manual tasks are 1) they are labor-intensive to conduct and to analyze, 2) the analysis is subjective, and 3) often the outcomes are qualitative. The large amount of time needed to train, test, and analyze the data from these tasks means that it takes significant resources. One study estimated that it can take up 47 hours of training time and 141 of testing to collect and score data from a longitudinal experiment with 10 rats6.
Kinematic performance measures produced by manual scoring are often qualitative. For example, scoring of kinematics of the SPRT usually involves assigning values to different aspects of movement based on a categorical scale such as normal, abnormal, or absent. This type of scoring system cannot track movements with high resolution. Precise limb movements are necessary for rodent models because nervous system injury or manipulation often causes more subtle effects than those observed in humans, who rely more on dexterous movement. It should be noted that success rate is the main outcome measure in this task, which is computed in a quantitative manner.
Another major drawback to manual tasks is that they rely on human observers for kinematic evaluation, which can introduce variability. Errors can arise from multiple users scoring data from same subject (low inter-observer reliability) or from one observer measuring performance from multiple subjects or time points (intra-observer reliability). For example, the IBB test used to measure fine motor functions of forelimb and digits after cervical SCI in rodents requires grading forelimb function using a 9 point scale20. In one study, the authors identified at least six possible ways that raters can differ in their scores. There was more than 1 point difference in scoring for novice raters, and expert input on the scoring did not reduce this difference. Such variability lowers the power of studies that use manual assessment methods. However, subjectivity can be mitigated to an extent by adopting good laboratory practices with standardized protocols and properly training staff. Such training lowered the variability of a manual clinical movement test, the Fugl-Meyer23, in people with stroke.
Automated Tasks
Innovations in the behavioral neuroscience community have led to the development of many types of automated tasks to test forelimb function. Several factors drive this innovation. Computing power and operator expertise have improved tremendously in recent decades along with a drop in prices of the devices. In addition, sensors, including cameras and microcontrollers (like Arduinos), are also becoming cheaper and easier to use. Finally, innovations in computer vision and machine learning algorithms have enabled the automated detection of movements. In this section, we point out how automated approach to some currently available manual tests and some newly invented ones may allow more precise measurement of impairments as well as save time and money in the long run.
Advantages of Automated Tasks
All tests of forelimb function require training, testing, and analysis. Automation has attempted to decrease human participation and increase objectivity and quantification of each of these components.
a). Automated training
Training animals involves habituating to the environment, teaching the task, baiting with food rewards, and shaping out unwanted movements during execution of the task. In order to illustrate how automation can help with these complex steps, we will discuss an automated system that was specifically developed for the SPRT. Training in the traditional manual version of SPRT task involves placing animals in the reaching box and encouraging them to reach and grasp the pellets through an aperture. The most common outcome measure is the percentage of pellets successfully retrieved.
Baiting the animals to reach towards pellets in the SPRT task can be done with an automated dispenser. One research group has developed an Automatic Pellet Presenting (APP) system that places food rewards on the left or right of the aperture to the reaching box8. The APP is only initiated when the rodent breaks an infrared beam fixed at the back of the box; this forces the animal to go to the back of cage after each attempt thereby allowing a reset between trials. Automating the baiting of the task enables more trials, and this leads to faster acquisition of the task. It typically takes 3 weeks to train animals to proficiency using a traditional training paradigm of 25-trial session per day. Using the APP, training only took about 3 days. The rats were trained up to 250 trials each day, resulting in a rapid attainment of peak performance. The large number of trials is difficult to achieve and maintain using manual methods.
A similar automated reward presentation has also been implemented in a rodent version of the center-out reaching task that is frequently used in human and primate experiments24,25. However, in the rodent version water droplets are presented in an automated manner to head-fixed mice26. The main advantage of such automatic reward presentation is that animals spontaneously learned to reach and grasp within 3–5 sessions, each lasting around 30 minutes. In addition, the reward position can also be automated to three separate locations: Right, Center or Left, which provides a method to study the effect of target change.
Another benefit of automated training is that it allows standardized training protocols. It is difficult for human users to treat every animal the same when training animals with traditional methods. Differences in training between trainers or between animals can produce variability in task acquisition and performance. Even with standard protocols and experimenter training, the skill and even the sex of the human experimenter can affect the animals27. Training animals with automated equipment and computer protocols is likely to reduce this variability.
One more key feature of automated training is that it can be adapted to performance in real-time. Adaptive training protocols can be designed to vary difficulty of the task automatically based on how animals perform. For instance, when training animals on tasks that require them to manipulate a target mounted on a sensor9,28, a computer algorithm can adjust the criterion for task success based on the animals’ performance. The software computes the success rate of the 10 most recent trials and automatically either increases or decreases the difficulty of the task. Adaptive algorithms can be developed to enable training with a failure rate optimized for learning, decreasing training time29. The reward criterion for a task can be adjusted to push animals to their peak performance. From the rehabilitation perspective, varying the difficulty of the task allows optimally challenging subjects to keep them engaged in task for better learning30 and to better match the complexity with skill level of the performer31 than if the task difficulty is the same for all subjects.
b). Automated testing
Adaptive algorithms can also measure task performance more accurately and faster than tests that apply the same task difficulty for each trial32. Although adaptive testing can be done with manual tasks, this procedure can be implemented more easily with automated tasks driven by computer algorithms. In human motor performance, peak performance is usually more informative than performance of a task with a static difficulty. In addition, adaptive testing allows performance measurement across a larger range of ability.
Automated baiting of the SPRT and measurement of the number of pellets left is used to quantify success rate of reaching and grasping. The APP system described above has also been successfully used for assessment8. A pair of infrared LEDs were used in combination with infrared cameras to detect when rats removed a pellet from the tray as well as location of rats between trials (Fig. 2). If the rats failed and the pellet dropped off the trays, a 15 degree ramp allowed dropped pellets to roll toward a camera, which then detected these trials as misses using image analysis. All the information from different sensors were fed to a computer using custom MATLAB software to compute success rate. Thus, automated methods were used to score the success rate of pellet retrieval, obviating the need for hand scoring. The authors report that they spent more than 50% less time per rat (21 min/rat/week vs. 50 mins/rat/week) using the automated setup when compared to manual methods8.
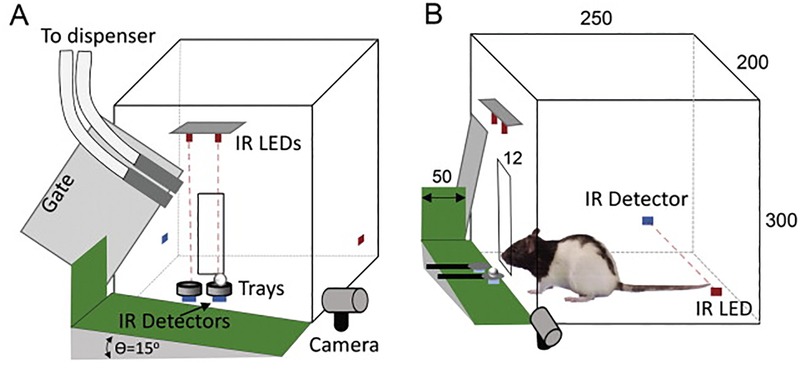
Figure 2:
Automated single pellet reaching task. A) Schematic of rat pellet reaching box is shown with IR LEDs and detectors along with camera to detect fallen pellets. B) Side view of the same reaching box as in (A) is shown with a rat inside the box interacting with pellets. Also shown is another set of IR LEDs and detectors that helps locate animal position inside the box. (Courtesy: Elsevier)
There are a few studies that have attempted to automate the testing of kinematic of reaching as well. Most of these studies use computer vision to track the reaching paw33–35. In one of the studies, the reaching paw of mice was identified by applying green dye to the forepaw and using color contrast analysis to track the reaching movements33. These methods were able to extract the tip of the paw, which allowed measurement of reach trajectory, speed, and smoothness. In a demonstration of the utility of this approach, the authors provide evidence that even though mice spontaneously recovered retrieval of pellets (2 weeks after injury), the kinematics of reach showed sustained deficits, even out to 30 days after injury. It should be noted that authors found deficits in mice using the cylinder test and the foot fault test throughout their testing period. In other words, if the authors were to use the more laborious kinematic analyses of the SPRT test, it is possible that they would have arrived at same conclusions as they did with other manual tasks. However, automated kinematic analysis allows reaching this conclusion quickly and using a single test.
Automated testing has also been implemented in another type of task that require animals to reach, grasp, and manipulate an instrumented device, known as a manipulandum. Thus far we have discussed tasks where animals reach and grasp food rewards directly with forepaws. The main advantage of manipulandum tasks is that they can isolate specific aspects of forelimb movement instead of trying to capture an entire reach and grasp sequence. One example of such task is the knob supination task (Fig. 3) that was designed to specifically measure distal forelimb supination in rodents9,28,36. Supination loss is a relatively specific sign of injury to the descending motor circuits37. In addition to being a sensitive sign of motor circuit impairment, supination loss strongly correlates with loss of hand function38–40. Once animals are trained to supinate, automated testing allows quantifying supination angles without any need for human intervention. Animals are automatically rewarded with food pellets when the knob is turned beyond a user-defined threshold. The angle is used to compute angular velocity and latency to the peak angle among other metrics.
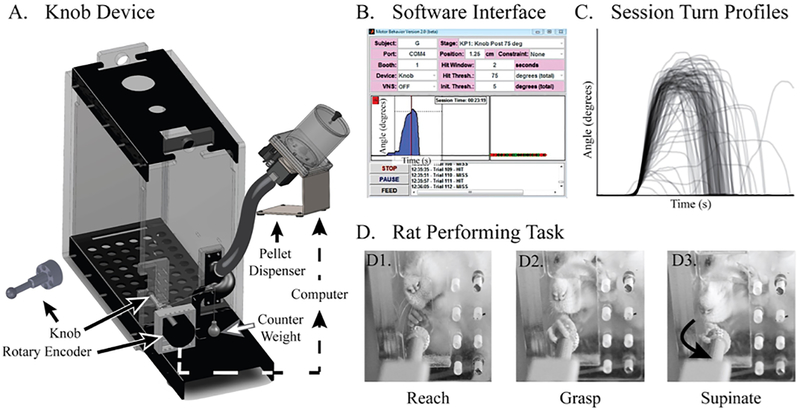
Figure 3:
The knob supination task. (A) The knob supination device. (B) The software user interface. (C) Turn profile from a single 30-minute session. (D) A rat performing a task. (D1) A rat reaches toward the knob, (D2) grasps it with a precision grip and, (D3) turns it in supination. (Courtesy: Sage Publications).
This task showed significant and lasting deficits in rats after unilateral injury to the corticospinal tract9, the most important pathway for voluntary movement, while the SPRT did not1. The isometric pull41 and the lever42 tasks are some other examples of manipulandum tasks designed to quantify other specific forelimb movements: the pull task measures the grip strength and the lever task is used to quantify pressing movement that must be repeated quickly respectively. Besides the specificity offered by these types of tasks, another benefit of this type of task is that they can to be very sensitive. This is due to the sensors that detect forelimb movements which can keep track of subtle changes with a high sampling rate. For example, the knob task can measure angle changes as small as 1/4 of a degree, and position is sampled at 100Hz9,28,36. The ability to measure movements at such high resolution is a theoretical advantage at this time due to lack of head-to-head comparisons with manual assessment of movement kinetics.
Another innovation in automated systems is integration of the devices into the home cage. These allow training and testing of rodents in the same cage in which they are housed. An advantage with home cage based tasks is that animals can engage continuously in the task, allowing quicker habituation and learning. Objective assessments can also be done continuously, which provides a much richer data set about motor learning. All of this can be done without human intervention or movement to a testing apparatus. Such conditions limit human interaction and enable standardized protocols within and between laboratories.
The SPRT has been modified into a home cage system. One such iteration, shown in Fig. 4, consists of three main parts: an automated pellet presenting (APP) system, a barrier mechanism to limit access to the pellet presenting platform based on location of the rat; and sensors that detect the location of the rats. This system allows for automation at three levels: tracking of animals undergoing training; placing pellets in front of the animals; and the barrier mechanism, which helps reset individual trials by ensuring animals move to the back of the cage between reaches. The authors report that the home cage system was very efficient at training and testing animals; home cage trained rats were proficient at task by 5–6 days compared to more than 11 days with a manual approach43.
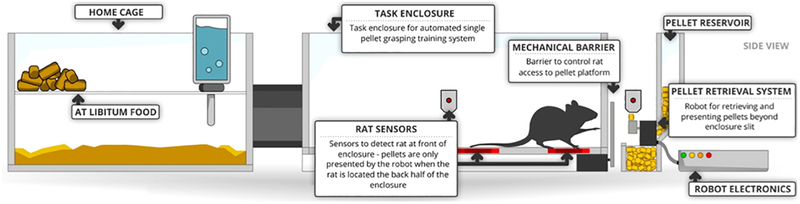
Figure 4:
Home cage based system. Automated training, testing and analysis of forelimb reaching task is achieved by connecting home cage to task enclosure that is coupled with automated pellet presenting (APP) robot. (Courtesy: Elsevier)
Joystick tasks have also been implemented into home cage systems. Joystick tasks are useful because they can track forelimb movements with multiple degrees of freedom, and they can also measure movements with high precision10,44,45. A joystick is part of a home cage system called the automated rodent training system (ARTS)10. The software controlling the system can control multiple home cages simultaneously and independently, using protocols specified by the user. While designed to control a joystick, the authors designed the software to be flexible to run different types of behavior tasks, by writing custom scripts. The capability of automated training and the flexibility to add new behavior tasks makes this system highly modular. However, the authors have yet to add new tasks to take advantage of this modular system, and this should be prioritized in future iterations of the task.
c). Automated analysis
Automation can also help in making data analysis step more robust and efficient. The analyzing data from manual behavior tasks typically involves scoring video recordings after they have been recorded and making judgements about the quality of performance. Many of these steps can be implemented with automated methods. There have been attempts to develop automated analysis using computer vision for reaching and sensor data for tasks involving a manipulandum as well.
Some of the best examples of automated analysis come from automated kinematic data analysis in the SPRT. To quantify of the reaching kinematics, a few groups use computer vision to extract kinematics33–35,46–48. In one such approach, motion tracking was performed by computer vision through machine learning algorithms46. Instead of relying on models of rodents reaching and grasping manipulandum, this method uses a data set from an advanced human pose estimation algorithm called DeeperCut. This tracking system can accurately predict the location of different components of the forelimb, including individual digits. Remarkably, this system reaches a level of accuracy in tracking these body parts that is similar to humans, and using only a small number (141) of labeled frames. There are several advantages of using this particular machine learning approach. First, it does not require building a model of the reaching limb in order to accurately track the position. Also, the approach does not require that markers be placed on the limb, which is less invasive for the animals and easier for the experimenter. In addition, only a small amount of data was needed to train the system accurately, making this method easy to adopt.
A different model-based approach that does require a model was adopted by another group to study reaching34. In this study, video recordings were used to generate a model of the rat paw, an approach adopted by others as well33–35. Rats are video recorded performing single pellet reaching from the front and the side, and the images synchronized and combined34. These video images are then compared to a 3D model of the paw to estimate the pose. This analysis enables correlation of forelimb impairments with specific aspect of reaching. This analysis allows the determination of movements about multiple joints meaningful to patients, like multi-joint coordination. This would not be possible without a highly quantitative automated method. Despite advanced computer vision based approach, this group uses data from only one animal and such, this approach has not been widely adopted perhaps owing to complex image processing and automation requirements.
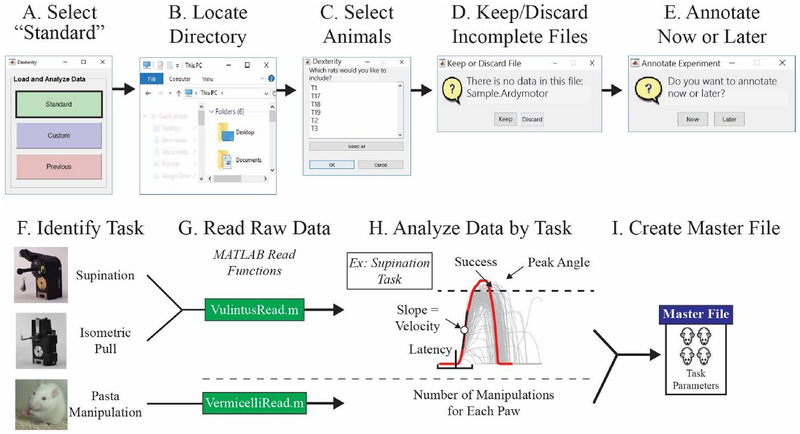
One of the repercussions of collecting data with highly quantitative tools is the large amount of data generated. Analysis methods are being developed to translate voluminous data into sensible outcomes. Dexterity49 is one example of such software, developed in conjunction with the knob supination task. Dexterity is MATLAB-based software that is capable of handling large data sets and quickly visualize or prepare the data sets for more complex analysis. This is made possible by a Graphical User Interface as shown in Fig. 5 (A-E) which is designed to be used by even those investigators without advanced MATLAB or computer programming skills. Another main advantage of Dexterity is that even though it was developed for the knob supination task, it can analyze many different types of data, both automated and manual. For example, it has been used to analyze the automated pull task as well as manual tasks like pasta manipulation, as shown in Fig. 5 (F).
Figure 5.
Dexterity software. (A) A user can select between three options: “Standard” “Custom,” and “Previous.” If the user selects “Standard”, the user will (B) load in their data, and Dexterity will prompt the user to provide the directory where files are located and (C) select subjects. (D) It will then step through each file and prompt the user to keep or discard files with incomplete or no data. (E) If the user chooses, they can annotate their experiment now or later. (F) Dexterity identifies which task it is analyzing, (G) utilizes the appropriate file reading MATLAB function, (H) analyzes that task, and then (I) creates a Master File with all of the processed data organized by subject.
Disadvantages of Automated Tests
The most significant downside of adopting automated tasks is the loss of human observation during behavior training and analysis. The role of observation is particularly important for behavior because unexpected changes are often found. For example, many improvements in function in human50 and animal models1 after injury are related to trunk adjustments and automated tasks are specifically designed only to measure paw or wrist movements. These changes are likely to be picked up by a skilled observer, but may go unrecognized with automated methods.
High initial set-up cost is another challenge faced by groups adopting automated tasks. The equipment and material required to set up automated systems can be costly compared to manual methods. For example, the knob supination devices are currently priced at $3,500 per device. Given the high-throughput design of these tasks, it’s not unusual to use several of these devices in a laboratory. Although the long-term savings in personnel more than offset high equipment cost, the initial investment is considerable. The amount of time required to train the animals to proficiency can become a potential downside when using automated devices as well, particularly manipulandum tasks. The main motivation for studying skilled forelimb movements in rodent models is to better understand movement and its neural control. Therefore, it is not surprising that we model human centric behavior in the rodents like reach and grasp. Training the rodents to perform unnatural movements like reaching to grasp a knob can be challenging and time-consuming. Training rodents with automated methods can compound this difficulty, since the food reward is not the target of reach. In addition, a human trainer can provide helpful motivation or novelty to a task.
Another downside of adopting automated tasks is the need for dedicated technical and financial resources to maintain and debug such system. Automated systems are often built with sophisticated hardware and software, and malfunction of these components is unavoidable. In order to maintain uninterrupted operation in labs, there has to be either a technical expert available on-site to deal with such events or pay fees to companies that manufacture these devices and software.
Future Promise
Automated tasks of the future must build on the current advantages of the systems. While current automated devices measure specific movements with high fidelity, the forelimb has enormous behavioral flexibility, and measurement of even a complicated reach and grasp movement tests only a small portion of the large repertoire. Future automated tasks should incorporate interchangeable modules that can test multiple aspects of forelimb movements. Another advantage in some of the available automated systems is that they allow training and testing in the home cage. The next generation of automated tasks must strive towards systems that can be incorporated into standard cages easily and affordably. Home cage based systems should also track multiple animals simultaneously to enable social housing. Modular systems should also be compatible with enriched or more natural environments. Finally, future systems should enable testing of natural rodent behaviors—eating, grooming, and exploration—to determine how these behaviors are controlled in health and disease.
Another important priority is to limit the disadvantages in current automated tasks. Most automated tasks use custom-written complex software that can be difficult to adopt and to modify. Similarly, current tasks often rely on custom built robotic platforms and machinery. Reliance on customized software and hardware necessitates ongoing support of the makers, or local expertise to troubleshoot and maintain the complex automated devices. This adds ongoing expense to the high startup costs. Use of simple user interfaces, such as voice activation and touch screens, could make the operation simple and fast. In addition, with sensors now being developed for mass markets, using these components and the control software can simplify devices and make them more affordable. Designing the automated tasks of the future will require using the power of sophisticated technologies like computer vision and modular robots, but with the methods that are easy to implement and to scale.
Finally, future automated tasks should use artificial intelligence to continuously improve the systems. One of the current advantages of using automated tasks is the use of computer algorithms that adapt to performance. These algorithms need to be optimized empirically and also need to be updated according to the need of the experiment. For example, the algorithm to optimize training will be different than the algorithm to measure performance. The algorithms may also differ by the type of task, species or strain of rodent, and type of intervention. An intelligent system can also partially address the lack of human observation during automated tasks. Specifically, behavior that is observed to be outside the norms of the system could trigger an alert about forelimb performance or compensatory movements of the body, for example. We imagine that such integrated system will utilize computer vision to detect and alert users at desired time intervals for feedback. We also envision a smart automated system that learns from such human feedback when problems arise and can suggest steps to solve such problems based on previous feedback. Further, intelligent systems could compare studies in multiple labs to ensure that data is generated in the same way. Having such automated method to validate data will also encourage wider adoption of these tasks. These systems may, therefore, deepen our understanding of forelimb function through methods that ensure high rigor and reproducibility.
Acknowledgements
We gratefully acknowledge the contribution by Andrew Sloan, PhD, who provided helpful discussions and valuable input on an early draft of the manuscript.
Footnotes
Declaration of Conflicting Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. JBC served as a consultant to Vulintus, Inc. on NINDS SBIR Grant R44NS086344, which helped develop the knob supination task. JBC owns no shares and has no financial interest in Vulintus, Inc. Authors AS and SDB declare no conflict of interests.
Bibliography
- 1.Whishaw IQ. Loss of the innate cortical engram for action patterns used in skilled reaching and the development of behavioral compensation following motor cortex lesions in the rat. Neuropharmacology. 2000;39:788–805. [DOI] [PubMed] [Google Scholar]
- 2.Whishaw IQ, Coles BL. Varieties of paw and digit movement during spontaneous food handling in rats: postures, bimanual coordination, preferences, and the effect of forelimb cortex lesions. Behav. Brain Res 1996;77:135–148. [DOI] [PubMed] [Google Scholar]
- 3.Metcalf CD, Notley SV, Chappell PH, Burridge JH, Yule VT. Validation and application of a computational model for wrist and hand movements using surface markers. IEEE Trans Biomed Eng 2008;55:1199–1210. [DOI] [PubMed] [Google Scholar]
- 4.Summers JJ, Kagerer FA, Garry MI, Hiraga CY, Loftus A, Cauraugh JH. Bilateral and unilateral movement training on upper limb function in chronic stroke patients: A TMS study. J. Neurol. Sci 2007;252:76–82. [DOI] [PubMed] [Google Scholar]
- 5.Kleim JA, Boychuk JA, Adkins DL. Rat models of upper extremity impairment in stroke. ILAR J 2007;48:374–384. [DOI] [PubMed] [Google Scholar]
- 6.Sloan AM, Fink MK, Rodriguez AJ, et al. A Within-Animal Comparison of Skilled Forelimb Assessments in Rats. PLoS ONE 2015;10:e0141254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaefer AT, Claridge-Chang A. The surveillance state of behavioral automation. Curr. Opin. Neurobiol 2012;22:170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong CC, Ramanathan DS, Gulati T, Won SJ, Ganguly K. An automated behavioral box to assess forelimb function in rats. J. Neurosci. Methods 2015;246:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sindhurakar A, Butensky SD, Meyers E, et al. An automated test of rat forelimb supination quantifies motor function loss and recovery after corticospinal injury. Neurorehabil. Neural Repair 2017;31:122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poddar R, Kawai R, Ölveczky BP. A fully automated high-throughput training system for rodents. PLoS ONE. 2013;8:e83171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyers EC, Solorzano BR, James J, et al. Vagus nerve stimulation enhances stable plasticity and generalization of stroke recovery. Stroke. 2018;49:710–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dona KR, Goss-Varley M, Shoffstall AJ, Capadona JR. A novel single animal motor function tracking system using simple, readily available software. J. Vis. Exp 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tennant KA, Asay AL, Allred RP, Ozburn AR, Kleim JA, Jones TA. The vermicelli and capellini handling tests: simple quantitative measures of dexterous forepaw function in rats and mice. J. Vis. Exp 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu T, Yu X, Perlik AJ, et al. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones TA, Schallert T. Use-dependent growth of pyramidal neurons after neocortical damage. J. Neurosci 1994;14:2140–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J. Neurosci 1996;16:4529–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J. Neurophysiol 1998;80:3321–3325. [DOI] [PubMed] [Google Scholar]
- 18.Buitrago MM, Ringer T, Schulz JB, Dichgans J, Luft AR. Characterization of motor skill and instrumental learning time scales in a skilled reaching task in rat. Behav. Brain Res 2004;155:249–256. [DOI] [PubMed] [Google Scholar]
- 19.Khazipov R, Sirota A, Leinekugel X, Holmes GL, Ben-Ari Y, Buzsáki G. Early motor activity drives spindle bursts in the developing somatosensory cortex. Nature. 2004;432:758–761. [DOI] [PubMed] [Google Scholar]
- 20.Irvine K-A, Ferguson AR, Mitchell KD, et al. The irvine, beatties, and bresnahan (IBB) forelimb recovery scale: an assessment of reliability and validity. Front. Neurol 2014;5:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballermann M, Metz GA, McKenna JE, Klassen F, Whishaw IQ. The pasta matrix reaching task: a simple test for measuring skilled reaching distance, direction, and dexterity in rats. J. Neurosci. Methods 2001;106:39–45. [DOI] [PubMed] [Google Scholar]
- 22.Sacrey L-AR, Alaverdashvili M, Whishaw IQ. Similar hand shaping in reaching-for-food (skilled reaching) in rats and humans provides evidence of homology in release, collection, and manipulation movements. Behav. Brain Res 2009;204:153–161. [DOI] [PubMed] [Google Scholar]
- 23.See J, Dodakian L, Chou C, et al. A Standardized Approach to the Fugl-Meyer Assessment and Its Implications for Clinical Trials. Neurorehabil. Neural Repair 2013. [DOI] [PubMed] [Google Scholar]
- 24.Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J. Neurosci 1982;2:1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarz C, Hentschke H, Butovas S, et al. The head-fixed behaving rat--procedures and pitfalls. Somatosens Mot Res 2010;27:131–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galiñanes GL, Bonardi C, Huber D. Directional Reaching for Water as a Cortex-Dependent Behavioral Framework for Mice. Cell Rep. 2018;22:2767–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. [DOI] [PubMed] [Google Scholar]
- 28.Butensky SD, Bethea T, Santos J, et al. The Knob Supination Task: A Semi-automated Method for Assessing Forelimb Function in Rats. J. Vis. Exp 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu HG, Miyamoto YR, Gonzalez Castro LN, Ölveczky BP, Smith MA. Temporal structure of motor variability is dynamically regulated and predicts motor learning ability. Nat. Neurosci 2014;17:312–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guadagnoli MA, Lee TD. Challenge point: a framework for conceptualizing the effects of various practice conditions in motor learning. J Mot Behav. 2004;36:212–224. [DOI] [PubMed] [Google Scholar]
- 31.Lin G-H, Huang Y-J, Lee S-C, Huang S-L, Hsieh C-L. Development of a computerized adaptive testing system of the functional assessment of stroke. Arch. Phys. Med. Rehabil 2018;99:676–683. [DOI] [PubMed] [Google Scholar]
- 32.Wainer H Computerized adaptive testing: A primer. Routledge; 2000. [Google Scholar]
- 33.Lai S, Panarese A, Spalletti C, et al. Quantitative kinematic characterization of reaching impairments in mice after a stroke. Neurorehabil. Neural Repair 2015;29:382–392. [DOI] [PubMed] [Google Scholar]
- 34.Palmér T, Tamtè M, Halje P, Enqvist O, Petersson P. A system for automated tracking of motor components in neurophysiological research. J. Neurosci. Methods 2012;205:334–344. [DOI] [PubMed] [Google Scholar]
- 35.Azim E, Jiang J, Alstermark B, Jessell TM. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature. 2014;508:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyers E, Sindhurakar A, Choi R, et al. The supination assessment task: An automated method for quantifying forelimb rotational function in rats. J. Neurosci. Methods 2016;266:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemon RN. Descending pathways in motor control. Annu. Rev. Neurosci 2008;31:195–218. [DOI] [PubMed] [Google Scholar]
- 38.Braendvik SM, Elvrum A-KG, Vereijken B, Roeleveld K. Relationship between neuromuscular body functions and upper extremity activity in children with cerebral palsy. Dev. Med. Child Neurol 2010;52:e29–34. [DOI] [PubMed] [Google Scholar]
- 39.Klotz MCM, Kost L, Braatz F, et al. Motion capture of the upper extremity during activities of daily living in patients with spastic hemiplegic cerebral palsy. Gait Posture. 2013;38:148–152. [DOI] [PubMed] [Google Scholar]
- 40.Mackey AH, Walt SE, Stott NS. Deficits in upper-limb task performance in children with hemiplegic cerebral palsy as defined by 3-dimensional kinematics. Arch. Phys. Med. Rehabil 2006;87:207–215. [DOI] [PubMed] [Google Scholar]
- 41.Hays SA, Khodaparast N, Sloan AM, et al. The isometric pull task: a novel automated method for quantifying forelimb force generation in rats. J. Neurosci. Methods 2013;212:329–337. [DOI] [PubMed] [Google Scholar]
- 42.Hays SA, Khodaparast N, Sloan AM, et al. The bradykinesia assessment task: an automated method to measure forelimb speed in rodents. J. Neurosci. Methods 2013;214:52–61. [DOI] [PubMed] [Google Scholar]
- 43.Fenrich KK, May Z, Hurd C, et al. Improved single pellet grasping using automated ad libitum full-time training robot. Behav. Brain Res 2015;281:137–148. [DOI] [PubMed] [Google Scholar]
- 44.Washburn DA, Rulon MJ, Gulledge JP. A new breed of computer users: rats control a cursor via joystick manipulation. Behav. Res. Methods. Instrum. Comput 2004;36:173–179. [DOI] [PubMed] [Google Scholar]
- 45.Slutzky MW, Jordan LR, Bauman MJ, Miller LE. A new rodent behavioral paradigm for studying forelimb movement. J. Neurosci. Methods 2010;192:228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathis A, Mamidanna P, Cury KM, et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci 2018;21:1281–1289. [DOI] [PubMed] [Google Scholar]
- 47.Ellens DJ, Gaidica M, Toader A, et al. An automated rat single pellet reaching system with high-speed video capture. J. Neurosci. Methods 2016;271:119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nica I, Deprez M, Nuttin B, Aerts J-M. Automated assessment of endpoint and kinematic features of skilled reaching in rats. Front. Behav. Neurosci 2017;11:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Butensky SD, Sloan AP, Meyers E, Carmel JB. Dexterity: A MATLAB-based analysis software suite for processing and visualizing data from tasks that measure arm or forelimb function. J. Neurosci. Methods 2017;286:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levin MF, Michaelsen SM, Cirstea CM, Roby-Brami A. Use of the trunk for reaching targets placed within and beyond the reach in adult hemiparesis. Exp. Brain Res 2002;143:171–180. [DOI] [PubMed] [Google Scholar]