Abstract
Background:
Inflammation and vaso-occlusion play key roles in Sickle Cell Disease (SCD) pathophysiology. Lipoxygenase products of the omega-3 fatty acids (O3FAs), docosahexaenoic (DHA) and eicosapentaenoic (EPA) acids, are potent anti-inflammatory mediators modulating pain. O3FAs decrease episodes of vaso-occlusion in SCD.
Methods:
We assessed erythrocyte fatty acid composition in two major cell membrane phospholipids, phosphatidylcholine and phosphatidylethanolamine, in children with SCD HbSS-disease (n=38) and age/race-matched HbAA-controls (n=18). Ratio of pro-inflammatory arachidonic acid (AA) to anti-inflammatory DHA and EPA (FA-Ratio), and its relationship to hs-CRP were evaluated.
Results:
FA-Ratios were increased in both phosphatidylcholine and phosphatidylethanolamine in HbSS compared to controls. Correlations were noted in HbSS subjects between hs-CRP and FA-Ratios (p=0.011). FA-Ratios increased with age (p=0.0007) due to an increase in pro-inflammatory AA with a concomitant decrease in antiinflammatory DHA.
Conclusions:
Findings demonstrate relative deficiencies in HbSS of the anti-inflammatory precursor fatty acids DHA and EPA, which correlates positively with hs-CRP.
Keywords: sickle cell anemia, polyunsaturated fatty acids, omega-3 fatty acids, docosahexaenoic acid, inflammatory biomarkers, C-reactive protein
1. Introduction
Sickle Cell Disease (SCD), the most common Hemoglobinopathy, is caused by a mutant hemoglobin, hemoglobin S (HbS). The most common and debilitating feature of the disease is the painful vaso-occlusive crisis (VOC) characterized by micro-vessel occlusion [1-5]. Evidence from both clinical and transgenic animal models strongly suggest that VOC is a chronic inflammatory pathology [6-10]. Using a transgenic mouse model, Kaul and Hebbel have linked the reperfusion injury paradigm to the VOC event [8]. Later studies by Frenette and his colleagues, and others have focused on activated leucocytes and their released inflammatory mediators as playing a critical role in the pathogenesis of VOC [10-12]. In subjects with SCD, studies have documented elevated levels of inflammatory cytokines [13-15] including interleukin-1ß (IL-1ß) and tumor necrosis factor-α (TNF-α) and lipid mediators including leukotriene B4 (LTB4) [16]. These mediators promote cell-cell adhesion, endothelial activation, up-regulation of endothelial adhesion molecules, activation of transcription factors including nuclear factor (NF) κB, and further leukocyte recruitment [9-12,17]. In addition, these inflammatory mediators sensitize nociceptors acting as agonists for the transient receptor potential (TRP) vanilloid subtype-1 (TRPV1) and ankyrin subtype-1 (TRPA1) which together with other TRP channels play a role in neural and nociceptive pain [18,19].
Polyunsaturated fatty acids (PUFAs) are structural and functional components of cell membranes. These fatty acids are present as acyl chains primarily esterified at the sn-2 position of glycerol in phospholipids (PLPs) including phosphatidylethanolamine (PE) and phosphatidylcholine (PC). PE and PC are the major PLPs of the inner and outer leaflet of the plasma membrane, respectively [20,21]. Based on the position of the terminal double bond, PUFAs are grouped into either omega-3 or omega-6 fatty acids. The omega-3 fatty acids (O3FAs), eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), and the omega-6 fatty acid, arachidonic acid (AA), have been extensively evaluated by many investigators. AA is the precursor for the production of majority of pro-inflammatory lipid modulators [22-24]. This fatty acid can also serve as the substrate for the synthesis of lipoxins, a minor sub-group of eicosanoids with anti-inflammatory and pro-resolving properties [25]. EPA and DHA serve as the precursors for the synthesis of antiinflammatory and pro-resolving lipid mediators [25,26]. These fatty acids have profound effects on multiple biologic and pathologic processes including inflammation and pain [reviewed in 26-29]. In addition, studies have evaluated health benefits of O3FAs in cardiovascular disease, rheumatoid arthritis, diabetes, inflammatory bowel disease, asthma, neurologic disorders, and pain [reviewed in 26,30-34, and references 35,36]. While some studies have documented clinical benefits of O3FAs and their protective effects against inflammation, others have failed to demonstrate any health benefits.
Based on what is known about the pathophysiology of SCD, the demonstrated effects of the O3FAs on leukocytes, endothelium and inflammatory mediators, O3FAs may have great potential, by way of multiple mechanisms, to favorably impact the vaso-occlusive inflammatory and the painful complications of SCD. In fact, limited clinical and nutritional studies in subjects with SCD and sickle cell mouse models have shown that O3FA therapy provided protection against VOC-related pain, lowered NFκB gene expression, and down-regulated adhesion molecule expression [37-41]. While previous studies have shown that the levels of O3FAs are reduced under basal condition in subjects with SCD [42-48], no studies to date have assessed the relationship between the levels of O3FAs and steady state inflammation in SCD. The aim of our study was to assess whether the PUFA levels in SCD correlate with the levels of high sensitivity-C-reactive protein (hs-CRP), the well-characterized biomarker of both acute and chronic inflammatory processes [49-51].
2. Methods and Materials
2.1. Subjects
Individuals with SCD (n=38, age range 1.7 to 20 years) with homozygous hemoglobin S (HbSS) were at baseline steady state health. The individuals with SCD were recruited for the study between 2008 and 2012 when hydroxyurea (HU) therapy had not been established as a definitive and highly recommended therapy for individuals with SCD. These subjects were recruited for the study at St Christopher Hospital Children, Drexel University School of Medicine, Philadelphia, PA. Children receiving HU therapy or chronic transfusion, and subjects with any acute illness, hospitalization, or vaso-occlusive episode within 2 weeks pre- and post- blood sampling were excluded from the study. Age- and race- matched control subjects (n=18) were recruited at St Christopher’s Hospital for Children and Thomas Jefferson University, Philadelphia, PA. Plasma and washed erythrocytes, prepared from blood samples collected in citrate anticoagulant, were stored frozen at −80°C until processed. The study was approved by the Institutional Review Committee for Human Subjects Protection at both study sites. In accordance with the Declaration of Helsinki, blood samples were obtained following informed consent. For minors, subject’s assent was also obtained.
2.2. Materials
Lipid standards for thin layer chromatography (TLC) and reference fatty acid methyl ester (FAME) standards for gas chromatography (GC) analyses were obtained from Avanti Polar Lipids (Alabaster, AL) and Sigma-Aldrich (St Louis, MO). All chromatographic-grade solvents and reagents were obtained from Fisher Scientific (Wilmington, DE).
2.3. Fatty Acid Analysis
Red cell fatty acids were assayed by capillary GC as previously described [42,52]. In brief, total lipids from erythrocytes (106 cells) were extracted using a mixture of chloroform and methanol as described by Folch et al [53] in the presence of 0.01% butylated hydroxytoluene. Lipid extracts were further subjected to TLC to isolate PE and PC. FAMEs were prepared by transmethylating the isolated PLPs with anhydrous methanolic-HCI (0.5 N) [54], and analyzed using an Agilent 6850 Gas Chromatograph equipped with a polyethyleneglycol capillary column (30m × 250μm × 0.25μm, Agilent INNO-wax) and flame ionization detector. Sample peaks were identified by comparing their retention times with those from authentic FAME standards. Peak areas were integrated using the Agilent ChemStation Software, and quantitated using a FAME standard curve. Recoveries of erythrocyte lipids, monitored using appropriate synthetic PLPs [1,2-dipentadecanoyl-sn-glycero-3-phosphoethanolamine (a synthetic PE), and 1,2-diheptadecanoyl-sn-glycero-3-phosphocholine (a synthetic PC)], were found to be >80%.
2.4. Analysis of hs-CRP
Plasma hs-CRP levels were measured using an ELISA kit (ALPCO Immunoassays, Salem, NH) as previously described [49].
2.5. Analysis of LDH and Hematologic Parameters
Plasma LDH levels were measured using an LDH assay kit (TOX07, Sigma-Aldrich) as previously described [49]. Hematologic parameters (erythrocyte count, hemoglobin, WBC counts, neutrophil counts, and platelet counts) were obtained using a Coulter Counter (Model StK). Reticulocytes were enumerated in blood stained with methylene blue.
2.6. Data analysis
All blood samples were evaluated for red cell membrane lipid markers including fatty acids (AA, EPA and DHA), and the Fatty Acid Ratio (FA-Ratio). FA-Ratio was obtained by dividing AA (in nMol) by DHA plus EPA (in nMols), and was employed as a measure to assess the relative deficiency of O3FAs. Statistical evaluation including unpaired t-test, Mann-Whitney Rank-sum test, Pearson correlation and Spearman rank correlation tests were performed using SigmaPlot (Version 12, Systat Software, San Jose CA).
3. Results
3.1. Hematologic Characteristics of individuals with SCD
Hematologic parameters from individuals with SCD (n=38) enrolled in the study are presented in Table-1. Hematologic indices from SCD were compared to those from the age- and race-matched controls (n=18). Compared to HbAA controls, children with SCD demonstrated decreased levels of hemoglobin and red cell count (p<0.001), and increased levels of reticulocytes, platelets, total white blood cells and neutrophils (p=0.008). Plasma levels of LDH were also increased in children with SCD (p<0.001).
TABLE 1:
Hematological parameters in Children with SCD and Age- and Race- matched HbAA Controls
| Hematological parameters |
HbAA Control (n=18) |
SCD HbSS (n=38) |
p-value |
|---|---|---|---|
| Age (years) | 11.90 ± 5.24 ‡14.17 (8.21, 15.83) |
10.76 ± 5.62 11.54 (5.81, 16.23) |
ns |
|
Erythrocytes (M/μl) |
4.68 ± 0.39 ‡4.67 (4.51, 4.91) |
2.91 ± 0.63 2.90 (2.52, 3.26) |
p<0.001 |
|
Hemoglobin (g/dl) |
*12.21 ± 1.01 12.20 (11.78, 12.83) |
8.81 ± 1.19 8.80 (8.05, 9.70) |
p<0.001 |
|
Reticulocytes (K/μl) |
31 ± 21 ‡29 (11, 46) |
216 ± 85 198 (153, 282) |
p<0.001 |
|
Platelets (K/μl) |
310 ± 110 ‡278 (238, 374) |
426 ± 138 398 (338, 505) |
p=0.001 |
|
WBCs (K/μl) |
*8.01 ± 3.37 6.85 (5.50, 10.30) |
11.07 ± 4.03 10.60 (8.00, 14.40) |
p=0.008 |
|
Neutrophils (K/μl) |
3.30 ± 2.24 ‡2.45 (1.70, 4.60) |
5.08 ± 2.47 4.87 (3.29, 6.17) |
p<0.001 |
|
LDH (mU/ml) |
265 ± 71 ‡252 (223, 294) |
675 ± 217 661 (459, 869) |
p<0.001 |
|
hs-CRP (ng/ml) |
287 ± 339 ‡125 (67, 502) |
3361 ± 3318 1837 (961, 5629) |
p<0.001 |
Results presented are the mean ± SD values (first line), and the median and (1st, and 3rd quartile) levels (second line). Hematologic parameters were compared between groups using * unpaired t-test if the data set passed both normality and equal variance tests. ‡Mann-Whitney Rank Sum test was employed if the data set failed normality and/or equal variance tests. Only partial data was available for comparison from children with SCD for erythrocytes (n=37), hemoglobin (n=37), reticulocytes (n=33), platelets (n=36), WBC (n=35), neutrophils (n=35) and LDH (n=32). Only data from 16 controls was available for reticulocyte comparison. Abbreviations used in the Table: WBCs= white blood cells, LDH= lactate dehydrogenase, hs-CRP= high sensitivity-C-reactive protein
3.2. Composition of red cell membrane AA, EPA and DHA
Composition of erythrocyte membrane AA, EPA and DHA from the PC and PE fractions and the FA-Ratio are shown in Table-2. Consistent with published reports [42-48], DHA levels in subjects with SCD were significantly decreased in both PC (p=0.047) and PE (p=0.042) fractions when compared to those from control children. While EPA levels were similar in the PC fraction, they were significantly decreased in the PE fraction from individuals with SCD (p<0.001). In contrast to DHA and EPA, AA levels were significantly increased in the PC fraction from subjects with SCD (p=0.039). No differences were noted in the PE fraction. The FA-Ratios in children with SCD were significantly increased in both PC (p=0.003) and PE (p=0.035) fractions when compared to those from control children. In the PC fraction, the observed increase in fatty acid ratio was due to a significant decrease in DHA with a concomitant elevation in AA content. In the PE fraction, the abnormal ratio was due to decreases in both DHA and EPA content.
TABLE 2:
Red Cell Membrane Phospholipid Fatty Acids in Children with SCD and Age- and Race- matched HbAA Controls
| Phospholipid Fraction |
Fatty Acid | HbAA Control (n=18) |
SCD HbSS (n=38) |
p-value |
|---|---|---|---|---|
| PC Fraction | FA-Ratio | 5.39 ± 2.30 ‡4.99 (3.94, 6.63) |
7.35 ± 2.52 6.88 (5.15, 9.10) |
0.003 |
|
AA (nMol/109 RBCs) |
15.59 ± 3.73 ‡14.06 (13.00, 18.52) |
18.00 ± 3.59 17.29 (15.81, 20.57) |
0.039 | |
|
DHA (nMol/109 RBCs) |
*2.72 ± 0.90 2.33 (1.98, 3.75) |
2.22 ± 0.82 2.19 (1.61, 2.53) |
0.047 | |
|
EPA (nMol/109 RBCs) |
0.53 ± 0.44 ‡0.60 (0.01, 0.69) |
0.48 ± 0.48 0.45 (0.01, 0.76) |
ns | |
| PE Fraction | FA-Ratio |
*6.13 ± 2.16 5.94 (4.21, 7.98) |
7.36 ± 1.94 7.21 (5.91, 8.58) |
0.035 |
|
AA (nMol/109 RBCs) |
33.50 ± 16.89 ‡26.42 (21.30, 50.83) |
27.74 ± 6.51 26.57 (24.82, 30.77) |
ns | |
|
DHA (nMol/109 RBCs) |
4.69 ± 2.25 ‡4.29 (2.87, 5.95) |
3.47 ± 1.30 3.50 (2.25, 4.12) |
0.042 | |
|
EPA (nMol/109 RBCs) |
1.00 ± 0.54 ‡1.13 (0.68, 1.32) |
0.54 ± 0.31 0.58 (0.34, 0.73) |
<0.001 |
Results presented are the mean ± SD values (first line), and the median and (1st, and 3rd quartile) levels (second line). Biomarker levels were compared between groups using *unpaired t-test if the data set passed both normality and equal variance tests. ‡Mann-Whitney Rank Sum test was employed if the data set failed normality and/or equal variance tests. Abbreviations used in the Table: AA= arachidonic acid, DHA= docosahexaenoic acid, EPA= eicosapentaenoic acid, PC= phosphatidylcholine, PE= phosphatidylethanolamine, FA-Ratio= fatty acid ratio (ratio of AA to DHA plus EPA), ns= not significant
3.3. Association of Fatty Acid Ratios with age
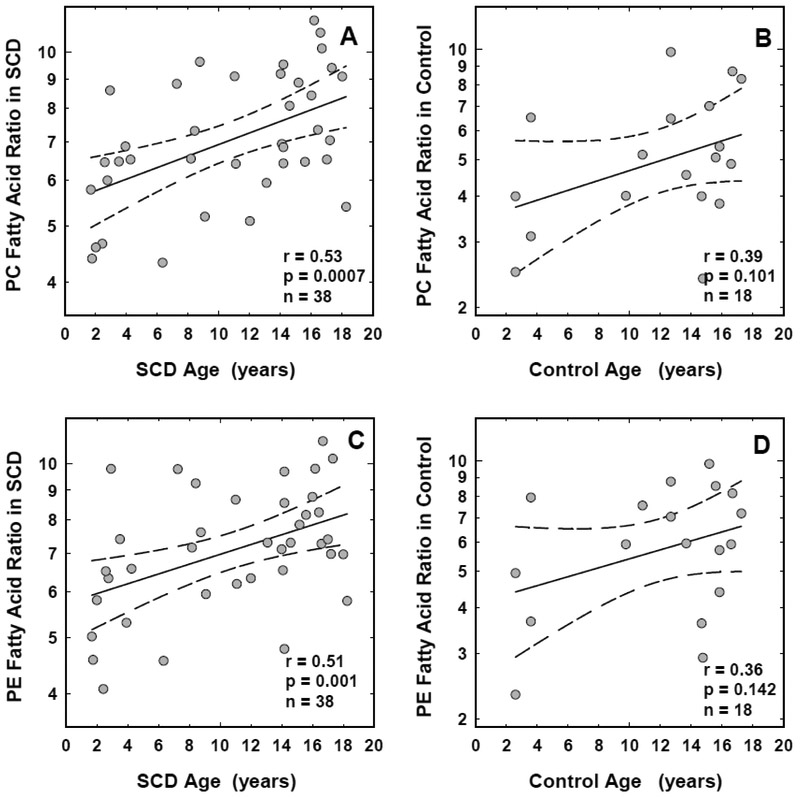
While we have noted significant positive correlations between age and the FA-Ratios from both PC (r=0.53, p=0.0007, n=38) and PE (r=0.51, p=0.001, n=38) fractions from SCD erythrocytes, no significant associations were noted in the control group (r=0.39, p=0.101 with PC and r=0.36, p=0.142 with PE, n=18, Figure-1). Age correlated negatively with levels of DHA in the red cell PC fraction (r=−0.42, p=0.009, n=38) in the subjects with SCD. In additional regression analysis, we assessed the correlation between age and hs-CRP and found no age-related association with hs-CRP in either the SCD (r=0.21, p=0.20, n=38) or the control subjects (r=0.03, p=0.89, n=18).
FIGURE 1: Association between age and fatty acid ratios in red cell phospholipids from children with sickle cell disease and race-matched controls.
Association between age and the fatty acid ratios in red cell PC (panel A) and PE (panel C) fractions from children with sickle cell disease, and the PC (panel B) and PE (panel D) fractions from race-matched controls are shown. The solid and the dotted lines represent the linear regression fit to the data, and the 95% confidence interval curves, respectively. The correlation coefficient (r) and the significance (p) values shown for each correlation were obtained using the Pearson correlation test with logtransformed data. Similar results were observed when the data was analyzed using the Spearman rank correlation test.
3.4. Association of Fatty Acid Ratios with hs-CRP
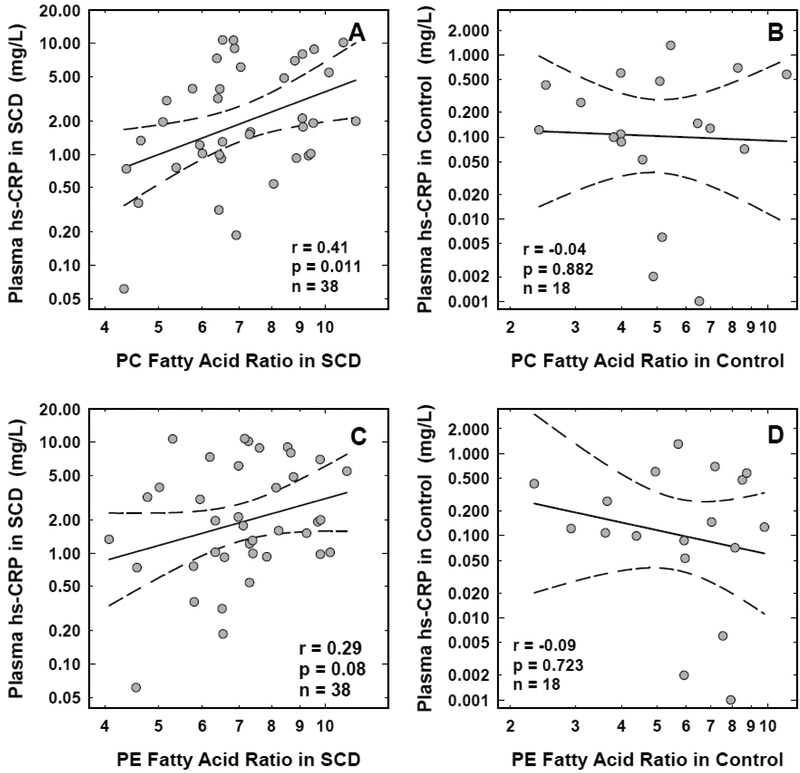
Consistent with previous reports, plasma hs-CRP levels were significantly elevated in children with SCD (p<0.001) when compared to those from the age- and race-matched controls (Table-1). In the SCD group, hs-CRP demonstrated significant positive correlation with the FA-Ratios from the erythrocyte PC fraction (r=0.41, p=0.011, n=38). While a positive relationship was also noted between hs-CRP and the FA-Ratios from the PE fraction, the correlation did not reach statistical significance (r=0.29, p=0.08, n=38). In the control (n=18), hs-CRP did not correlate with the FA-Ratios from either the PC or PE fractions (r=−0.04, p=0.882 with PC and r=−0.09, p=0.723 with PE). The scatter plots of the association between hs-CRP and the FA-Ratios from the erythrocyte PC (panel A) and PE (panel C) fractions from SCD, and the PC (panel B) and PE (panel D) fractions from control erythrocytes are shown in Figure-2.
FIGURE 2: Association between plasma hs-CRP levels and fatty acid ratios in red cell phospholipids from children with sickle cell disease and age- and racematched controls.
Association between the levels of plasma hs-CRP and the fatty acid ratios in red cell PC (panel A) and PE (panel C) fractions from children with sickle cell disease, and the PC (panel B) and PE (panel D) fractions from age- and race-matched controls are shown. The solid and the dotted lines represent the linear regression fit to the data, and the 95% confidence interval curves, respectively. The correlation coefficient (r) and the significance (p) values shown for each correlation were obtained using the Pearson correlation test with log-transformed data. Similar results were observed when the data was analyzed using the Spearman rank correlation test.
4. Discussion
The polyunsaturated fatty acids AA, EPA and DHA have been evaluated by several investigators to assess clinical abnormalities and nutritional status of O3FAs. AA, an omega-6 fatty acid, is the substrate for the synthesis of majority of pro-inflammatory eiocosanoids including prostaglandins, thromboxane and leukotrienes [22-24]. In contrast, DHA and EPA, the major omega-3 fatty acids, are the precursors for the synthesis of potent anti-inflammatory, analgesic and pro-resolving mediators including resolvins, protectins and maresins [25,26]. The balance between omega-3 and omega-6 fatty acids and the relative levels of lipid mediators generated from these fatty acids at the site of inflammation, therefore, may play a role in the pathogenesis of inflammation and its resolution. Studies have shown that O3FA levels in SCD are decreased in multiple lipid fractions from several blood components with marked changes noted in the red cell PC and PE fractions [42-48]. Choline and ethanolamine containing phospholipids are the major inner and outer plasma membrane phospholipids [20,21], respectively. In the present study, we evaluated the levels of AA, DHA and EPA in the red cell PC and PE fractions from children with SCD, and demonstrate that the levels of DHA and EPA are decreased with concomitant increases in AA resulting an increase in the FA-Ratios. Our findings are in agreement with those reported previously in subjects with SCD from Sudan and Nigeria [42-47]. In addition, we demonstrate that the O3FA deficiency in SCD increases with subject’s age and correlates with an increase in plasma levels of the inflammatory bio-marker hs-CRP.
Omega-3 fatty acids modulate many cellular functions including those involved in the pathogenesis of inflammation. Some of the anti-inflammatory effects mediated via EPA and DHA include decreased leukocyte chemotaxis, decreased production of chemo-attractants (such as LTB4), suppressed production of inflammatory cytokines, decreased transcription and expression of adhesion molecules (VCAM-1, ICAM-1 and E-selectin), decreased leukocyte-endothelial interaction, decreased production of inflammatory eicosanoids, and increased production of anti-inflammatory and proresolving mediators, among others [26-29,55-60]. Many of these inflammatory processes have been documented to occur in individuals with SCD [8,11-17]. Since O3FAs can modulate these cellular functions [26-29], they could serve as potential therapeutic agents in the management of VOC pain in SCD. In this regard, it is interesting to note that several clinical studies in SCD have evaluated the beneficial effects of either fish oil (the major source of O3FAs) or O3FA supplements, and have demonstrated a reduction in VOC pain frequency or clinical indicators of pain [37-40]. In addition, studies have also shown that O3FAs reduced oxidant production [61], and the expression of adhesion molecules [41] and NFκB gene in SCD [41]. Since NFκB is a transcription factor which plays a key role in the up-regulation of inflammatory cytokines and adhesion molecules [62,63], this study provides further evidence that O3FA supplementation may have a beneficial effect on the inflammatory processes in SCD.
In mouse models of SCD, mice treated with an O3FA-enriched diet showed a reduction in vascular activation, inflammatory response and SCD-related end organ damage [64]. DHA supplementation was shown to improve RBC flexibility, decrease irreversibly sickled cells, and reduce cold hypersensitivity [65,66]. In addition, in a most recent study, Matte et al [67] have demonstrated that orally administered 17R-RvD1, a DHA derived SPM, provided protection in SCD mice against hypoxia/reoxygenation-induced systemic/local inflammation, organ damage, and vascular dysfunction in the lung and kidney by modulating multiple biological processes involved in inflammatory and resolution pathology [67]. These findings further highlight the beneficial effects in SCD of DHA, and SPMs derived from this fatty acid.
In this study, we demonstrate that the relative deficiency of DHA and EPA (assayed as the FA-Ratio) increases with age (Figure-1, panels A and C). Clinical complications in subjects with SCD commence in the first year of life, and the disease severity increases with age [68]. While the formation of hemoglobin polymers is the initiating event, multiple pathobiologic processes including inflammation, blood cell adhesion to endothelium, and oxidative stress also play a role in disease pathology [4]. Studies have shown that O3FAs provide protection against these SCD-related pathophysiologic processes [26-29,61,69]. Our results taken together with the documented beneficial effects of O3FAs against inflammation, oxidant stress, and blood cell-endothelial adhesion provide additional complementary evidence to support the initiation of O3FA therapy at an early age in children with SCD.
In our study cohort, we demonstrate that O3FA deficiency is significantly associated with increased levels of plasma hs-CRP. CRP is a well-documented stable plasma biomarker of low-grade, chronic systemic inflammation and it is produced in the liver in response to a host of pro-inflammatory cytokines [50,51]. A previous study from our laboratory has demonstrated that hs-CRP was the most significant correlate of hospitalization for VOC pain in SCD [49]. We have therefore selected hs-CRP as a cardinal inflammatory biomarker to assess the relationship between inflammation and relative O3FA deficiency in children with SCD. We demonstrate that an increased FA-Ratio (a measure of relative O3FA deficiency) in the PC fraction is significantly associated with increased levels of plasma hs-CRP in children with SCD (Figure-2, panel A). While a positive trend was also noted between O3FA deficiency in the PE fraction and hs-CRP, the association did not reach statistical significance (Figure-2, panel C). In contrast to PE, which appears to be relatively stable [70], erythrocyte PC is actively involved in fatty acid turnover, can rapidly incorporate PUFAs [71,72], readily exchange its acyl groups with plasma fatty acids [73], and transfer to other erythrocyte phospholipids including PE [74]. The dynamic nature of red cell PC and the turnover of fatty acids documented in this phospholipid may therefore account for the observed fatty acid abnormalities, and the significant association noted with hs-CRP in SCD. Other studies in patients with coronary artery disease [75] and peripheral artery disease [76] have shown significant inverse correlations between levels of CRP and red cell membrane O3FA content. In a previous study, CRP levels in a cohort of SCD subjects on O3FA supplements were compared to those from an untreated cohort [41]. While the CRP levels were lower in the O3FA-treated group, the changes were not statistically significant probably due to a small sample size (n=18) and a large sample variability, and therefore require confirmation using a larger SCD cohort.
The individuals with SCD were recruited for the study between 2008 and 2012, and all evaluations were made using a naive group of individuals untreated with HU. HU [77,78], and more recently L-glutamine [79,80], are the only FDA approved pharmacologic agents used in the preventive treatment of SCD-related complications. The beneficial effect of HU in SCD is attributed to an increased production of fetal hemoglobin, and a decreased production of leukocytes and reticulocytes [77]. Other pharmacological effects including modulation of erythrocyte adhesion to endothelium, and vaso-dilatory and anti-inflammatory effects through the release of nitric oxide directly from the HU molecule may also contribute to the beneficial effects of HU in this disease entity [81]. Treatment with HU is a standard therapy at the present time for all individuals with HbSS including children and infants as young as 9 months of age [82]. At the time of patient recruitment for our study, parents and caregivers were reluctant to agree to HU therapy for their children due to concern as to the long-term safety of the drug. Thus, the majority of children with SCD were not taking HU at the time of our study. Since HU can also modulate inflammation [81], the blood samples collected at that time of our investigation provided a unique opportunity to assess the relationship between O3FA deficiency and inflammation under baseline steady state condition in the absence of any disease-specific pharmacologic interference. In a cohort of modest sample size, we were able to demonstrate a significant positive correlation between O3FA deficiency in the red cell membrane phospholipid fractions and plasma inflammatory biomarker hs-CRP. Study limitations include a modest sample size and the lack of comparison of plasma fatty acids between healthy controls and individuals with SCD. However, a previous study has shown that O3FA abnormalities similar to those observed in erythrocytes were also noted in plasma [42]. An additional limitation of our study is the lack of repeat measurements to assess either the short-term or long-term intra-individual variability of PUFA levels.
In summary, in children with HbSS we have identified an additional potential cause for the increase in inflammatory tone. This observation is supported by the findings that the membrane levels of EPA and DHA, the precursor fatty acids for the synthesis of anti-inflammatory and pro-resolving protectins, resolvins, and maresins, are decreased in red cells. In contrast, the membrane levels of AA, the precursor fatty acid for the synthesis of majority of pro-inflammatory eicosanoids prostaglandins, leukotriene and thromboxane, is increased, and in addition, the ratio of pro- to anti-inflammatory precursor fatty acids was also increased. We also document that the FA-Ratios in HbSS correlated positively with the inflammatory bio-marker hs-CRP. Our current results taken together with published findings provide evidence that DHA and EPA deficiency plays a component role in the pathogenesis of inflammation in SCD.
HIGHLIGHTS.
Chronic inflammation is one of the hallmarks of sickle cell disease (SCD). We have previously demonstrated that the inflammatory biomarker hs-CRP positively correlated with vaso-occlusive pain in SCD.
In children with SCD, ratios of pro-inflammatory arachidonic acid (AA) to anti-inflammatory docosahexaenoic (DHA) and eicosapentaenoic (EPA) acids are increased in red cell phospholipids due to a relative decrease in omega-3 fatty acids.
A relative deficiency of DHA and EPA correlates positively with hs-CRP in children with SCD.
A relative deficiency of DHA and EPA increases with age in children with SCD
5. Acknowledgments
We would like to thank the staff members of the Division of Hematology, St Christopher’s Hospital for Children, Drexel University, Philadelphia, PA for their efforts at patient recruitment and blood sample collection. This work was supported by grants BTRP U54HL70585 from the NHLBI, and COBRE P20GM109021 from the NIGMS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This work was supported by grants BTRP U54HL70585 from the NHLBI, and COBRE P20GM109021 from the NIGMS, National Institutes of Health, Bethesda MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dampier C, Setty BN, Eggleston B, Brodecki D, O'Neal P, Stuart M, Vaso-occlusion in children with sickle cell disease: clinical characteristics and biologic correlates, J Pediatr Hematol Oncol 26 (2004) 785–790. [PubMed] [Google Scholar]
- 2.Platt OS, Thorington BD, Brambilla DJ, et al. , Pain in sickle cell disease: Rates and risk factors, N Engl J Med 325 (1991) 11–16. [DOI] [PubMed] [Google Scholar]
- 3.Smith WR, Penberthy L. t., Bovbjerg VE, et al. , Daily assessment of pain in adults with sickle cell disease, Ann Intern Med 148 (2008) 94–101. [DOI] [PubMed] [Google Scholar]
- 4.Stuart MJ, Nagel RL, Sickle-cell disease, Lancet 364(9442) (2004) 1343–1360. [DOI] [PubMed] [Google Scholar]
- 5.Piel FB, Steinberg MH, Rees DC, Sickle cell disease, N Engl J Med 376 (2017) 1561–1573. [DOI] [PubMed] [Google Scholar]
- 6.Zhang D, Xu C, Manwani D, Frenette PS, Neutrophils, platelets, and inflammatory pathways at the nexus of sickle cell disease pathophysiology, Blood 127 (2016) 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owusu-Ansah A, Ihunnah CA, Walker AL, Ofori-Acquah SF, Inflammatory targets of therapy in sickle cell disease, Translational Res 167 (2016) 281–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaul DK, Hebbel RP, Hypoxia/reoxygenation causes inflammatory response in transgenic sickle mice but not in normal mice, J Clin Invest 106 (2000) 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.S Platt O, Sickle cell anemia as an inflammatory disease, J Clin Invest 106 (2000) 337–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belcher JD, Bryant CJ, Nguyen J, et al. , Transgenic sickle mice have vascular inflammation, Blood 101 (2003) 3953–3959. [DOI] [PubMed] [Google Scholar]
- 11.Frenette PS, Sickle cell vaso-occlusion: multistep and multicellular paradigm, Curr Opin Hematol 9 (2002) 101–106. [DOI] [PubMed] [Google Scholar]
- 12.Turhan A, Weiss LA, Mohandas N, Coller BS, Frenette PS, Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm, Proc Natl Acad Sci USA 99 (2002) 3047–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowers AS, Reid HL, Greenidge A, Landis C, Reid M, Blood viscosity and the expression of inflammatory and adhesion markers in homozygous sickle cell disease subjects with chronic leg ulcers, PLoS ONE 8 (2013) e68929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keikhaei B, Mohseni AR, Norouzirad R, et al. , Altered levels of pro-inflammatory cytokines in sickle cell disease patients during vaso-occlusive crises and the steady state condition, Eur Cytokine Netw 24 (2013) 45–52. [DOI] [PubMed] [Google Scholar]
- 15.Setty BN, Key NS, Rao AK, et al. , Tissue factor-positive monocytes in children with sickle cell disease: correlation with biomarkers of haemolysis, Br J Haematol 157 (2012) 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Setty BN, Stuart MJ, Eicosanoids in sickle cell disease: potential relevance of neutrophil leukotriene B4 to disease pathophysiology, J Lab Clin Med 139 (2002) 80–89. [DOI] [PubMed] [Google Scholar]
- 17.Hebbel RP, Osarogiagbon R, Kaul D, The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy, Microcirculation 11 (2004) 129–151. [PubMed] [Google Scholar]
- 18.Bautista DM, Pellegrino M, Tsunozaki M, TRPA1: A gatekeeper for inflammation, Annu Rev Physiol 75 (2013) 181–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes` ES, Fernandes MA, Keeble JE, The functions of TRPA1 and TRPV1: moving away from sensory nerves, Br J Pharmacol 166 (2012) 510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Lee M, Fairn GD, Phospholipid subcellular localization and dynamics, J Biol Chem 293 (2018) 6230–6240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Virtanen JA, Cheng KH, Somerharju P, Phospholipid composition of the mammalian red cell membrane can be rationalized by a superlattice model, Proc Natl Acad Sci USA 95 (1998) 4964–4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riccoiotti E, FitzGerald GA, Prostaglandins and inflammation, Arterioscler Thromb Vase Biol 31 (2011) 986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters-Golden M, Henderson WR Jr, Leukotrienes, N Engl J Med 357 (2007) 1841–1854. [DOI] [PubMed] [Google Scholar]
- 24.Innes JK, Calder PC, Omega-6 fatty acids and inflammation, Prostaglandins Leukot Essent Fatty Acids 132 (2018) 41–48. [DOI] [PubMed] [Google Scholar]
- 25.Serhan CN, Discovery of specialized pro-resolving mediators marks the dawn of resolution physiology and pharmacology, Mol Aspects Med 58 (2017) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calder PC, Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance, Biochim Biophys Acta 1851 (2015) 469–484. [DOI] [PubMed] [Google Scholar]
- 27.Calder PC, n-3 fatty acids, inflammation and immunity: new mechanisms to explain old actions, Proc Nutr Soc 72 (2013) 326–336. [DOI] [PubMed] [Google Scholar]
- 28.Ji RR, Xu ZZ, Strichartz G, Serhan CN, Emerging roles of resolvins in the resolution of inflammation and pain, Trends Neurosci 34 (2011) 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li K, Huang T, Zheng J, Wu K, Li D, Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor alpha: a meta-analysis, PLoS ONE 9 (2014) e88103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swanson D, Block R, Mousa SA, Omega-3 fatty acids EPA and DHA: Health benefits throughout life, Adv Nutr 3 (2012) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Superko HR, Superko SM, Nasir K, Agatston A, Garrett BC, Omega-3 fatty acid levels. Clinical significance and controversy, Circulation 128 (2013) 2154–2161. [DOI] [PubMed] [Google Scholar]
- 32.Song C, Shieh CH, Wu YS, Kalueff A, Gaikwad S, Su KP, The role of omega-3 polyunsaturated fatty acids eicosapentaenoic and docosahexaenoic acids in the treatment of major depression and Alzheimer's disease: acting separately or synergistically? Prog Lipid Res 62 (2016)41–54. [DOI] [PubMed] [Google Scholar]
- 33.Davidson MH, Omega-3 fatty acids: new insights into the pharmacology and biology of docosahexaenoic acid, docosapentaenoic acid, and eicosapentaenoic acid, Curr Opin Lipidol 24 (2013) 467–474. [DOI] [PubMed] [Google Scholar]
- 34.Shahidi F, Ambigaipalan P, Omega-3 polyunsaturated fatty acids and their health benefits, Ann Rev Food Sci Technol 9 (2018) 345–381. [DOI] [PubMed] [Google Scholar]
- 35.Vesco AT, Lehmann J, Gracious BL, Arnold LE, Young AS, Fristad MA, Omega-3 supplementation for psychotic mania and comorbid anxiety in children, J Child Adolesc Psychopharmacol 25 (2015) 526–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yassine HN, Feng Q, Azizkhanian I, et al. , Association of serum docosahexaenoic acid with cerebral amyloidosis. JAMA Neurol 73 (2016) 1208–1216. [DOI] [PubMed] [Google Scholar]
- 37.Tomer A, Kasey S, Connor WE, Clark S, Harker LA, Eckman JR, Reduction of pain episodes and prothrombotic activity in sickle cell disease by dietary n-3 fatty acids, Thromb Haemost 85 (2001) 966–974. [PubMed] [Google Scholar]
- 38.Okpala I, Ibegbulam O, Duru A, et al. , Pilot study of omega-3 fatty acid supplements in sickle cell disease, APMIS 119 (2011) 442–448. [DOI] [PubMed] [Google Scholar]
- 39.Daak AA, Ghebremeskel K, Hassan Z, et al. , Effect of omega-3 (n-3) fatty acid supplementation in patients with sickle cell anemia: randomized, double-blind, placebo-controlled trial, Am J Clin Nutr 97 (2013) 37–44. [DOI] [PubMed] [Google Scholar]
- 40.Daak AA, Dampier CD, Fuh B, et al. , Double-blind, randomized, multicenter phase 2 study of SC411 in children with sickle cell disease (SCOT trial), Blood Adv 2 (2018) 1969–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daak AA, Elderdery AY, Elbashir LM, et al. , Omega 3 (n-3) fatty acids down-regulate nuclear factor-kappa B (NF-kappaB) gene and blood cell adhesion molecule expression in patients with homozygous sickle cell disease, Blood Cells Mol Dis 55 (2015) 48–55. [DOI] [PubMed] [Google Scholar]
- 42.Ren H, Obike I, Okpala I, Ghebremeskel K, Ugochukwu C, Crawford M, Steady-state haemoglobin level in sickle cell anaemia increases with an increase in erythrocyte membrane n-3 fatty acids, Prostaglandins Leukot Essent Fatty Acids 72 (2005) 415–421. [DOI] [PubMed] [Google Scholar]
- 43.Ren H, Ghebremeskel K, Okpala I, Ugochukwu C, Crawford M, Ibegbulam O, Abnormality of erythrocyte membrane n-3 long-chain fatty acids in sickle cell haemoglobin C (HbSC) disease is not as remarkable as in sickle cell anaemia (HbSS), Prostaglandins Leukot Essent Fatty Acids 74 (2006) 1–6. [DOI] [PubMed] [Google Scholar]
- 44.Ren H, Okpala I, Ghebremeskel K, Ugochukwu CC, Ibegbulam O, Crawford M, Blood mononuclear cells and platelets have abnormal fatty acid composition in homozygous sickle cell disease, Ann Hematol 84 (2005) 578–583. [DOI] [PubMed] [Google Scholar]
- 45.Enomoto TM, Isichei C, VanderJagt DJ, Fry DE, Glew RH, Decreased polyunsaturated fatty acids in sickle cell anaemia, J Trap Pediatr 44 (1998) 28–34. [DOI] [PubMed] [Google Scholar]
- 46.VanderJagt DJ, Trujillo MR, Bode-Thomas F, Huang YS, Chuang LT, Glew RH, Phase angle correlates with n-3 fatty acids and cholesterol in red cells of Nigerian children with sickle cell disease, Lipids Health Dis 2 (2003) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daak AA, Ghebremeskel K, Elbashir MI, Bakhita A, Hassan Z, Crawford MA, Hydroxyurea therapy mobilises arachidonic acid from inner cell membrane aminophospholipids in patients with homozygous sickle cell disease, J Lipids (2011) article ID 718014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Connor WE, Lin DS, Thomas G, Ey F, DeLoughery T, Zhu N, Abnormal phospholipid molecular species of erythrocytes in sickle cell anemia, J Lipid Res 38 (1997) 2516–2528. [PubMed] [Google Scholar]
- 49.Krishnan S, Setty Y, Betal SG, et al. , Increased levels of the inflammatory biomarker C-reactive protein at baseline are associated with childhood sickle cell vasocclusive crises, Br J Haematol 148 (2010) 797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koenig W, Sund M, Frohlich M, et al. , C-Reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) Augsburg Cohort Study, 1984 to 1992, Circulation 99 (1999) 237–242. [DOI] [PubMed] [Google Scholar]
- 51.Verma S, Szmitko PE, Ridker PM, C-reactive protein comes of age, Nat Clin Pract Cardiovasc Med 2 (2005) 29–36. [DOI] [PubMed] [Google Scholar]
- 52.David F, Sandra P, Wylie P, Automated analysis of free fatty acid methyl esters on the HP 6890 Series GC, Application Note 288-334, Hewlett-Packard No (23)5954-3522E (2001). [Google Scholar]
- 53.Folch J, Lees M, Sloane Stanley GH, A simple method for the isolation and purification of total lipides from animal tissues, J Biol Chem 226 (1957) 497–509. [PubMed] [Google Scholar]
- 54.Blau K, Halket J, Handbook of Derivatives for Chromatography (2nd edition). New York: John Wiley & Sons; (1993). [Google Scholar]
- 55.Chen H, Li D, Roberts GJ, Saldeen T, Mehta JL, Eicosapentanoic acid inhibits hypoxia-reoxygenation-induced injury by attenuating upregulation of MMP-1 in adult rat myocytes, Cardiovasc Res 59 (2003) 7–13. [DOI] [PubMed] [Google Scholar]
- 56.Marcheselli VL, Hong S, Lukiw WJ, et al. , Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression, J Biol Chem 278 (2003) 43807–43817. [DOI] [PubMed] [Google Scholar]
- 57.McGuinness J, Neilan TG, Sharkasi A, Bouchier-Hayes D, Redmond JM, Myocardial protection using an omega-3 fatty acid infusion: quantification and mechanism of action, J Thorac Cardiovasc Surg 132 (2006) 72–79. [DOI] [PubMed] [Google Scholar]
- 58.Mayer K, Merfels M, Muhly-Reinholz M, et al. , Omega-3 fatty acids suppress monocyte adhesion to human endothelial cells: role of endothelial PAF generation, Am J Physiol Heart Circ Physiol 283 (2002) H811–818. [DOI] [PubMed] [Google Scholar]
- 59.Saku N, Kobayashi J, Kitamura S, Eicosapentaenoic acid modulates arachidonic acid metabolism in rat alveolar macrophages activated by silica, Prostaglandins Leukot Essent Fatty Acids 61 (1999) 51–54. [DOI] [PubMed] [Google Scholar]
- 60.Taccone-Gallucci M, Manca-di-Villahermosa S, Battistini L, Stuffier RG, Tedesco M, Maccarrone M, N-3 PUFAs reduce oxidative stress in ESRD patients on maintenance HD by inhibiting 5- lipoxygenase activity, Kidney Int 69 (2006) 1450–1454. [DOI] [PubMed] [Google Scholar]
- 61.Daak AA, Ghebremeskel K, Mariniello K, Attallah B, Clough P, Elbashir MI, Docosahexaenoic and eicosapentaenoic acid supplementation does not exacerbate oxidative stress or intravascular haemolysis in homozygous sickle cell patients, Prostaglandins Leukot Essent Fatty Acids 89 (2013) 305–311. [DOI] [PubMed] [Google Scholar]
- 62.Hoesel B, Schmid JA, The complexity of NF-kappaB signaling in inflammation and cancer, Mol Cancer 12 (2013) 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Min JK, Kim YM, Kim SW, et al. , TNF-related activation-induced cytokine enhances leukocyte adhesiveness: induction of ICAM-1 and VCAM-1 via TNF receptor-associated factor and protein kinase C-dependent NF-kappaB activation in endothelial cells, J Immunol 175 (2005) 531–540. [DOI] [PubMed] [Google Scholar]
- 64.Kalish BT, Matte A, Andolfo I, et al. , Dietary omega-3 fatty acids protect against vasculopathy in a transgenic mouse model of sickle cell disease, Haematologica 100 (2015) 870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wandersee NJ, Hanson MS, Maciaszek JL, et al. , Dietary supplementation with docosahexanoic acid (DHA) improves RBC flexibility and reduces cold hypersensitivity in mice with sickle cell disease, Blood 120 (2012) 2116. [Google Scholar]
- 66.Wandersee NJ, Maciaszek JL, Giger KM, et al. , Dietary supplementation with docosahexanoic acid (DHA) increases red blood cell membrane flexibility in mice with sickle cell disease, Blood Cells Mol Dis 54 (2015) 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mattè A, Recchiuti A, Federti E, et al. , Resolution of sickle cell disease-associated inflammation and tissue damage with 17R-resolvin D1, Blood 133 (2019) 252–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang WC, Ware RE, Miller ST, et al. , Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG), Lancet 377 (2011) 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Caterina R, Madonna R, Massaro M, Effects of omega-3 fatty acids on cytokines and adhesion molecules, Curr Atherosclerosis Reports 6 (2004) 485–491. [DOI] [PubMed] [Google Scholar]
- 70.Reed CF, Murphy M, Roberts G. Phospholipid exchange between plasma and erythrocytes in man and the dog, J Clin Invest 47 (1968) 749–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Setty BNY, Chen D, Stuart MJ, Sickle red blood cells stimulate endothelial cell production of eicosanoids and diacyglycerol, J Lab Clin Med 128 (1996) 313–321. [DOI] [PubMed] [Google Scholar]
- 72.Oliveira MM, Vaughan M, Incorporation of fatty acids into phospholipids of erythrocyte membranes, J Lipid Res 5 (1964) 156–162. [PubMed] [Google Scholar]
- 73.Dise CA, Goodman DBP, Rasmussen H, Selective stimulation of erythrocyte membrane phospholipid fatty acid turnover associated with decreased cell volume, J Biol Chem 255 (1980) 5201–5207. [PubMed] [Google Scholar]
- 74.Shohet SB, Release of phospholipid fatty acid from human erythrocytes, J Clin Invest 40 (1970) 1668–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Farzaneh-Far R, Harris WS, Garg S, Na B, Whooley MA, Inverse association of erythrocyte n-3 fatty acid levels with inflammatory biomarkers in patients with stable coronary artery disease: The Heart and Soul Study, Atherosclerosis 205 (2009) 538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grenon SM, Conte MS, Nosova E, et al. , Association between n-3 polyunsaturated fatty acid content of red blood cells and inflammatory biomarkers in patients with peripheral artery disease, J Vase Surg 58 (2013) 1283–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanter J, Kruse-Jarres R, Management of sickle cell disease from childhood through adulthood, Blood Reviews 27 (2013) 279–287. [DOI] [PubMed] [Google Scholar]
- 78.Strouse JJ, Heeney MM, Hydroxyurea for the treatment of sickle cell disease: efficacy, barriers, toxicity, and management in children, Pediatr Blood Cancer 59 (2012) 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.No authors listed. FDA Briefing Document, Oncologic Drugs Advisory Committee Meeting, NDA 208587, L-Glutamine, Applicant: Emmaus Medical, Inc. U S Food and Drug Administration; https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM559734.pdf (2017) [Google Scholar]
- 80.DeBaun M, A new therapy for sickle cell disease: FDA approves oral L-glutamine for prevention of acute vaso-occlusive events in children and adults, Hematologist 15(6) (2018). [Google Scholar]
- 81.Ware RE, How I use hydroxyurea to treat young patients with sickle cell anemia, Blood 115 (2010) 5300–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quinn CT, Sickle cell disease in childhood: from newborn screening through transition to adult medical care, Pediatr Clin N Am 60 (2013) 1363–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]