Abstract
Purpose
Cardiovascular (CV) diseases in type 2 diabetes (T2DM) represent an enormous burden with high mortality and morbidity. Sodium-glucose cotransporter 2 (SGLT2) inhibitors have recently emerged as a new antidiabetic class that improves glucose control, as well as body weight and blood pressure with no increased risk of hypoglycemia. The first CV outcome study terminated with empagliflozin, a specific SGLT2 inhibitor, has shown a reduction in CV mortality and in heart failure hospitalization, suggesting a beneficial impact on cardiac function which remains to be demonstrated. This study was designed to examine the chronic effect of empagliflozin on left ventricular (LV) systolic and diastolic functions in a genetic model of T2DM, ob/ob mice.
Methods and Results
Cardiac phenotype was characterized by echocardiography, in vivo hemodynamics, histology, and molecular profiling. Our results demonstrate that empagliflozin significantly lowered HbA1c and slightly reduced body weight compared to vehicle treatment with no obvious changes in insulin levels. Empagliflozin also improved LV maximum pressure and in vivo indices of diastolic function. While systolic function was grossly not affected in both groups at steady state, response to dobutamine stimulation was significantly improved in the empagliflozin-treated group, suggesting amelioration of contractile reserve. This was paralleled by an increase in phospholamban (PLN) phosphorylation and increased SERCA2a/PLN ratio, indicative of enhanced SERCA2a function, further supporting improved cardiac relaxation and diastolic function. In addition, empagliflozin reconciled diabetes-associated increase in MAPKs and dysregulated phosphorylation of IRS1 and Akt, leading to improvement in myocardial insulin sensitivity and glucose utilization.
Conclusion
The data show that chronic treatment with empagliflozin improves diastolic function, preserves calcium handling and growth signaling pathways and attenuates myocardial insulin resistance in ob/ob mice, findings suggestive of a potential clinical utility for empagliflozin in the treatment of diastolic dysfunction.
Keywords: Diastolic dysfunction, Diabetes, ob/ob mice, SGLT2 inhibitor, Empagliflozin, Calcium handling
Introduction
Type 2 diabetes (T2DM), and its frequently associated comorbid risk factors obesity, hypertension, and insulin resistance, represents an enormous burden with high mortality and morbidity [1]. It is one of the most prevalent diseases globally, and it is the fourth or fifth leading cause of death in most developed countries [2]. Hyperglycemia is considered to be the principal cause of diabetes [3]. Glycemic control is therefore essential for effective diabetes management and reducing diabetes-induced complications [4]. However, hyperglycemia is often poorly controlled despite the availability of a number of pharmaco-therapeutic modalities [5–7].
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, a new class of antidiabetic agents, independent of insulin, that promote urinary glucose excretion via inhibition of glucose reabsorption in the kidney, have been regarded to offer a unique opportunity to improve the outcomes of patients with T2DM [8–10]. Indeed, in addition to glucose control, SGLT2 inhibitors are associated with weight loss and blood pressure reductions with no increased risk of hypoglycemia [11].
In phase II trials in patients with T2DM, empagliflozin, a selective SGLT2 inhibitor [12], provided improvements in glycosylated hemoglobin (HbA1c) and other measures of glycemic control when given as monotherapy or add-on to metformin, as well as reductions in body weight and systolic blood pressure [13, 14]. Phase III studies have also reported a good safety profile along with significant improvements in HbA1c, body weight, and blood pressure, with no increased risk of hypoglycemia versus placebo [15–18]. Empagliflozin has also been shown to improve arterial stiffness in type 1 diabetes individuals [19]. Moreover, a CVoutcome study terminated with empagliflozin showed for the first time a spectacular 38% reduction in CV mortality and 35% reduction in heart failure hospitalization [20]. Nonetheless, the effect of empagliflozin on cardiac function has not been examined in metabolically unhealthy states such as diabetes and obesity, particularly when associated with a high risk of heart failure. Therefore, the present study was designed to evaluate the effect of chronic empagliflozin treatment on left ventricular (LV) systolic and diastolic functions in a well-established model of T2DM and obesity, ob/ob mice. We have also explored potential mechanisms involved with a focus on intracellular Ca2+ handling, insulin resistance, as well as activation of stress signaling including extracellular signal-regulated kinase (ERK), JNK, and p38 MAP kinases.
Methods
Animals
Male 10- to 12-week-old leptin-deficient homozygous ob/ob T2DM obese mice (stock #000632) and lean ob/+ mice (n = 10–15/group) were obtained from Jackson Laboratory. Lean control mice were fed regular chow, while obese mice were divided into two groups and fed with either vehicle containing diet or 10 mg/kg/day of empagliflozin mixed in diet for 6 weeks. All mice were kept on a 12-h light-dark cycle and given free access to food and water. Animals were obtained and handled as approved by the Mount Sinai Institutional Animal Care and Use Committee in accordance with the “Principles of Laboratory Animal Care by the National Society for Medical research and the Guide for the Care and Use of Laboratory Animals” (National Institutes of Health Publication No. 86–23, revised 1996).
Echocardiography
Echocardiograms were obtained at baseline and after 6 weeks of treatment. The examinations were performed under sedation with ketamine 80–100 mg/kg injected intraperitoneally. Sedation was optimized by (a) giving the lowest dose of ketamine needed to restrain the animal and prevent motion artifact and (b) maintaining the heart rate as close as possible to a physiological reading of 550 beats/min. Short-axis parasternal two dimensional (2D) views of the LV at the mid-papillary level and long-axis parasternal views of the LV were obtained using a Vivid7 echocardiography apparatus with a 14-MHz linear array probe (i13L probe, General Electric). The analyses were performed offline blinded to treatment group and to study time point using a workstation equipped with the Echopac PC software (GE Vingmed Ultrasound; Horten, Norway). All measurements were averaged over six cardiac cycles. LV M-mode at the mid-papillary level measurements of the size of the LV walls and cavities were obtained by 2D guidance from the short-axis view of the LV. LV internal diameter, LV posterior wall thickness, and LV anterior wall thickness were measured at end diastole. LV mass was derived by cubic method. In addition, LV systolic diameter was measured and LV fractional shortening and LV ejection fraction were calculated.
In order to evaluate LV diastolic function, mitral inflow was obtained using pulsed-wave Doppler from apical fourchamber view. Since we aimed to investigate LV function by ultrasound close to physiological conditions, we only slightly sedated the animals to keep heart rates higher, although we anticipated the complexity of the noninvasive assessment of LV diastolic function in light anesthesia.
Invasive Hemodynamics Measurements
At end-point, global cardiac function was evaluated by obtaining LV pressure-volume (P-V) loops. Mice were anesthetized with inhaled 5% (volume/volume) isoflurane for induction, and subsequently intubated and mechanically ventilated. Isoflurane was lowered to 2–3% (volume/volume) for surgical incisions. The chest was opened through a median sternotomy. A 1.2F mouse PV-4.5 mm elect spacing catheter (Scisense) was inserted into the LVapex through an apical stab performed with a 25-GA needle. Hemodynamic recordings were performed after 5 min of stable heart rate. Anesthesia was maintained at 0.5–1% isoflurane to keep the animal sedated and a stable heart rate at around 400–450 beats/min. Hemodynamics were recorded subsequently through a Scisense P-V Control Unit (FV8968). The intrathoracic inferior vena cava was transiently occluded to vary venous return during the recording to obtain load-independent P-V relationships. Linear fits were obtained for end-systolic pressure-volume relationships (ESPVR) and end-diastolic pressure-volume relationships (EDPVR).
Glucose and Insulin Levels
Ten-hour fasting blood glucose levels were measured in whole blood drawn from the tail vein using the OneTouch Ultra 2 Meter (LifeScan, Inc. New Brunswick NJ). Insulin and HbA1c levels were determined using an Ultra-Sensitive Mouse Insulin ELISA Kit and Mouse Hemoglobin A1c Kit, respectively (Crystal Chem, Inc. Downers Grove, IL).
Tissue Sampling and Histological Assessment of Fibrosis
Mice were sacrificed at the end of the invasive hemodynamic measurements. The hearts were separated and weighed; then, a transverse ring of LV tissue at the mid-papillary level was embedded in Tissue-Tek® O.C.T. Compound for histological analysis, and the rest of the LV tissues were frozen for protein and RNA extraction.
Frozen 8 μm tissue sections of rat LV transverse sections were stained by Masson’s Trichrome (MT) following standard protocols. The extent of fibrosis in myocardial tissue sections was quantified with the ImageJ software as the relative area of positive MTstained area (blue fibrosis) normalized to the total tissue area.
Protein Extraction and Western Blot
Proteins were prepared from rat LV tissue using a lysis buffer containing protease inhibitors (Roche) and phosphatase inhibitor cocktails (Sigma). Cell lysates were separated by SDS-PAGE and transferred onto nitrocellulose membrane. The membranes were blocked with 5% nonfat milk and incubated overnight at 4 °C with the following primary antibodies: SERCA2a (custom made in our lab); phospho-Phospholamban (A010–12 for pSer16; A010–13 for pThr17) and total Phospholamban (A010–140) from Badrilla; Gapdh (GTX627408, GeneTex), Phospho-Akt (9271S), total Akt (9272), phospho-ERK (4377S) phospho-JNK (9252), phospho-p38 (9215S), total ERK (4695), total JNK (9252), total p38 (9212), phospho-Ser307-IRS1 (2381S), IRS1 (2382) Bax (2772S), and Bcl2 (2870P) from Cell Signaling Technology; Glut4 (07–1404) and Glut1 (07–1401) from Millipore; PDK4 (sc-17024) and β-actin (sc-1616R) from Santa Cruz Biotechnology. The membranes were incubated with appropriate secondary antibodies conjugated to horseradish peroxidase (Pierce) and signal intensities were visualized by enhanced chemiluminescence (EMD Millipore). Films from at least three independent experiments were scanned and densities of the immunoreactive bands were evaluated using the NIH Image software. GAPDH and β-actin were used as an internal controls.
RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
After RNA extraction from LV tissue and cDNA synthesis, quantitative real-time PCR was performed with SYBR-green-based detection of double-stranded DNA using mouse specific primers and the ABI-7500 real-time PCR system (Applied Biosystems). For each set of primers, a no template control and a no reverse transcription control were included. Postamplification dissociation curves were performed to verify the presence of a single amplification product in the absence of genomic DNA contamination. Results were expressed as fold change in mRNA expression compared to the control (reference group) using reference genes (ΔΔCt approach, fold change = 2ΔΔCt).
Statistical Analysis
Data were expressed as mean ± SD. For comparisons of quantitative variables within and between groups, we used Wilcoxon signed-rank and Mann-Whitney nonparametric tests, respectively, as appropriate. p < 0.05 was considered statistically significant.
Results
General Features of Animals Following Empagliflozin Treatment
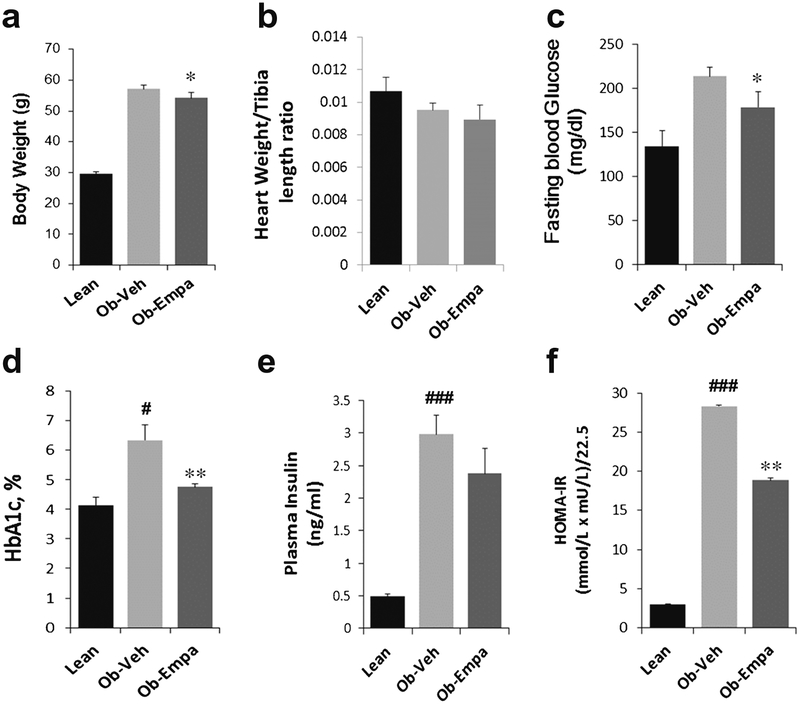
Six weeks treatment with empagliflozin led to a marginal but significant reduction in body weight compared to vehicle treated ob/ob mice (Fig. 1a). Moreover, heart weight/tibia length ratio was not statistically different in ob/ob mice treated with vehicle or empagliflozin (Fig. 1b). Empagliflozin significantly reduced fasting glucose and HbA1c levels in ob/ob mice compared to vehicle-treated group (Fig. 1c, d, respectively). Although there was no statistical effect of empagliflozin on insulin levels with only a trend towards reduction (Fig. 1e), empagliflozin significantly lowered the HOMA-IR index (Fig. 1f), suggesting improved insulin sensitivity.
Fig. 1.
General features of animals: a body weight, b heart weight/tibia length ratio, c fasting blood glucose, d HbA1c, e plasma insulin levels, and f HOMA-IR index of lean, ob/ob + vehicle (Ob-Veh) and ob/ob + empagliflozin (Ob-Empa). The data are expressed as mean ± s.d. (n = 10–15/group). *p = 0.05 and **p < 0.01 vs. Ob-Veh; #p < 0.05 and ###p < 0.001 vs. lean
Effect of Empagliflozin on Cardiac Function
Echocardiographic Analysis of LV
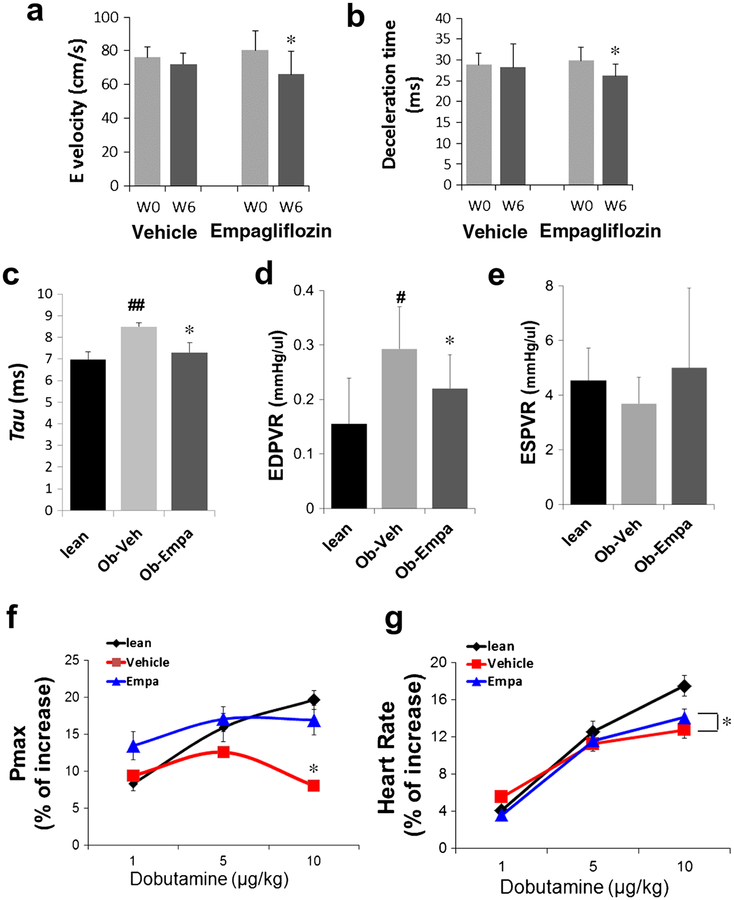
As a first assessment of the effect of empagliflozin on cardiac function we utilized noninvasive transthoracic echocardiography in the empagliflozin-treated ob/ob mice and age-matched vehicle controls. LV function and dimensions were measured at base-line and 6 weeks post-treatment (Table 1). M-mode analysis determined both groups have no significant modification of LV dimensions and morphology between baseline and follow-up. Importantly, LV fractional shortening and LVejection fraction (measures of systolic function) were also not modified during the study in both groups (Table 1). As expected the heart rates were high in both groups at both times points (mean > 550 beats/min; Table 1) and as such, the early (E) and late (A) velocities of mitral inflow are usually fused under these conditions. While peak E wave (Fig. 2a) and E wave deceleration time (DT) (Fig. 2b) were unchanged in vehicle group, these indices linked to LV diastolic function were significantly modified during the study period in empagliflozin group (Fig. 2a, b).
Table 1.
Echocardiography parameters: echocardiography was performed at baseline then 6 weeks following treatment with vehicle or empagliflozin
| Ob-vehicle | Ob-empagliflozin | |||||
|---|---|---|---|---|---|---|
| Baseline | 6 weeks follow-up | p | Baseline | 6 weeks follow-up | p | |
| Weight (g) | 47.7 ±2.1 | 59.1 ±2.8 | 0.004 | 45.2 ± 2.9 | 53.3 ±4.8 | 0.002 |
| Heart rate (beats/min) | 580 ± 42 | 608 ± 35 | 0.82 | 565 ± 39 | 568 ±52 | 1 |
| LV diastolic diameter (mm) | 3.1 ±0.2 | 2.9 ± 0.2 | 0.31 | 2.9 ± 0.2 | 3.0 ± 0.2 | 0.50 |
| LV systolic diameter (mm) | 1.5 ±0.2 | 1.4 ±0.1 | 0.44 | 1.5 ±0.3 | 1.6 ±0.3 | 0.43 |
| LV mass (mg) | 89.7 ± 12.2 | 83.6 ± 13.3 | 0.44 | 79.2 ± 8.9 | 82.4 ± 8.2 | 0.70 |
| LVFS (%) | 51.0 ±6.3 | 52.6 ±4.8 | 0.81 | 48.2 ± 7.5 | 46.1 ± 5.8 | 0.23 |
| LVEF (%) | 87.0 ± 4.5 | 88.6 ±3.2 | 0.63 | 84.7 ± 5.5 | 82.9 ± 5.6 | 0.20 |
| E (cm/s) | 76 ± 6.3 | 72.2 ±6.5 | 0.13 | 80.4 ± 11.6 | 66.1 ± 13.7 | 0.03 |
| DT (ms) | 28.8 ± 2.8 | 28.2 ±5.6 | 0.81 | 29.8 ± 3.2 | 26.2 ± 2.7 | 0.02 |
Data expressed as mean ± standard deviation (n = 10–15/group)
LV left ventricular, LVEF left ventricular ejection fraction, LVFS left ventricular fractional shortening, E mitral inflow peak velocity, DT E wave deceleration time
Fig. 2.
Cardiac function measured by echocardiography and in vivo invasive hemodynamics. a E wave, mitral inflow peak velocity and b E wave deceleration time in ob/ob + vehicle and ob/ob + empagliflozin before (W0) and after (W6) treatment (n = 10/group);*p < 0.05 and *p < 0.05 vs. W0. c Tau and d EDPVR (end-diastolic pressure-volume relationship)—indexes of diastolic function. e ESPVR (end-systolic pressure volume relationship a measure of contractility; no significant effect but a trend to increase) in lean, ob/ob + vehicle (Ob-Veh) and ob/ob + empagliflozin (Ob-Empa) mice (n = 5–10/group); *p < 0.05 vs. Ob-Veh; #p < 0.05 vs. lean. e, f Dobutamine stimulation. Maximum pressure (Pmax, left) and heart rates (HR, right) of lean, ob/ob + vehicle and ob/ob + empagliflozin mice. Dobutamine stress effect is blunted in vehicle but responsive in empagliflozin group, while HR is significantly increased in empagliflozin group (n = 5–10). The % of increase was calculated by normalizing the dobutamine values to the steady state (i.e., nonstimulation) values. *p < 0.05 vs. Ob-Veh
In Vivo Hemodynamics Measurements
To further characterize cardiac function following empagliflozin treatment of ob/ob mice, we conducted a LV pressure-volume (PV) analysis. PVanalysis revealed abnormalities in diastolic parameters including increased relaxation time and tau (Fig. 2c) and increased end-diastolic pressure-volume relationship (EDPVR) in ob/ob compared to lean mice (Fig. 2d). Lusitropic defects are classical indicators of cardiac dysfunction accompanying T2DM [21]. Treatment with empagliflozin significantly improved LV relaxation (Fig. 2c) and compliance (EDPVR) compared to vehicle (Fig. 2d), suggesting improvement of diastolic function, further confirming the echocardiography findings. Again, PV analysis also revealed that systolic function was not affected in ob/ob mice. The end-systolic pressure volume relationship (ESPVR)—a parameter of cardiac contractility—was not changed between the groups, indicating empagliflozin does not affect the inotropic state of the ob/ob mice (Fig. 2e).
In order to further assess the effects of empagliflozin on cardiac function, we administered dobutamine (1–10 μg/kg). Lean mice and empagliflozin-treated ob/ob mice responded immediately with increased maximal LV pressure (Pmax) (Fig. 2f) and heart rates (Fig. 2g); while vehicle-treated ob/ob mice lacked a significant response to dobutamine infusion with minimal changes in Pmax (Fig. 2f).
Molecular Characterization of Empagliflozin Cardiac Phenotype
SGLT2 Expression and Effect of Empagliflozin on Hypertrophy Markers
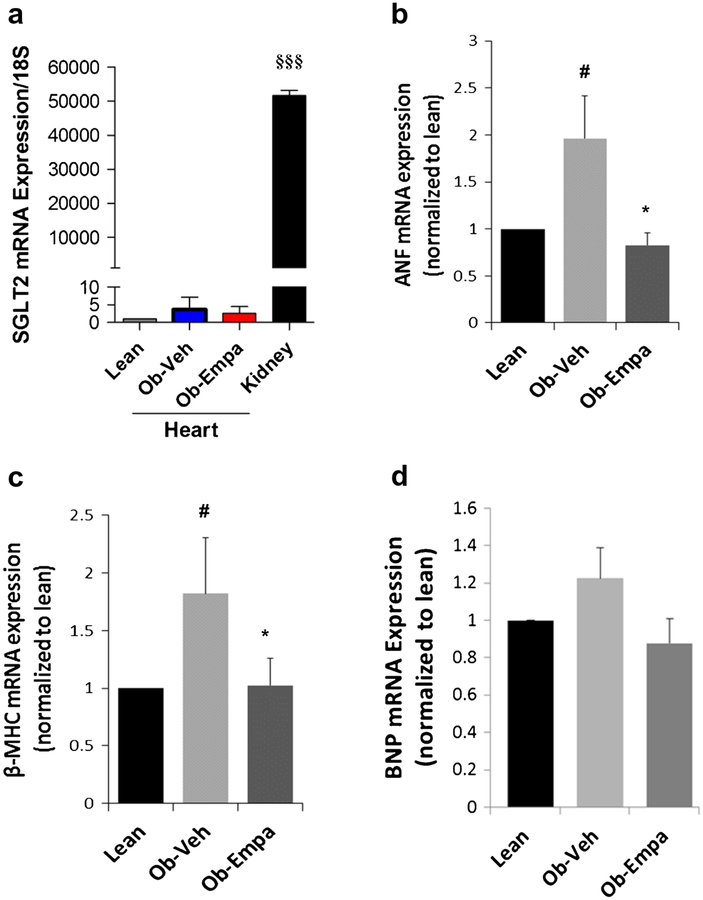
Given that empagliflozin is a selective SGLT2 inhibitor, we first confirmed that SGLT2 is highly specifically expressed in the kidney compared to the heart (Fig. 3a). We then determined whether empagliflozin would have any effects on molecular markers of cardiac hypertrophy, generally presented by diabetic subjects. Analysis of mRNA expression of hypertrophic fetal genes (i.e., atrial natriuretic peptide/factor—Anf—beta-myosin heavy chain—β-Mhc and B-type natriuretic peptide—Bnp) post empagliflozin treatment showed that empagliflozin decreased diabetes-induced expression of Anf (Fig. 3b) and β-Mhc (Fig. 3c) in ob/ob mice compared to vehicle treatment but had no measurable effect on Bnp (Fig. 3d).
Fig. 3.
SGLT2 cardiac expression cardiac hypertrophic markers. mRNA expression of SGLT2 (a), ANF (b), beta-MHC (c), and BNP (d) were evaluated by real-time quantitative PCR in heart samples from lean, ob/ob + vehicle (Ob-Veh) and ob/ob + empagliflozin (Ob-Empa). mRNAvalues were normalized to S18 RNA and plotted as a function of lean values. The data are expressed as mean ± s.d.(n = 5–7). §§§p < 0.0001 vs. Ob-Empa; *p < 0.05 vs. Ob-Veh;#p < 0.05 vs. lean
Effect of Empagliflozin on Calcium Cycling Proteins
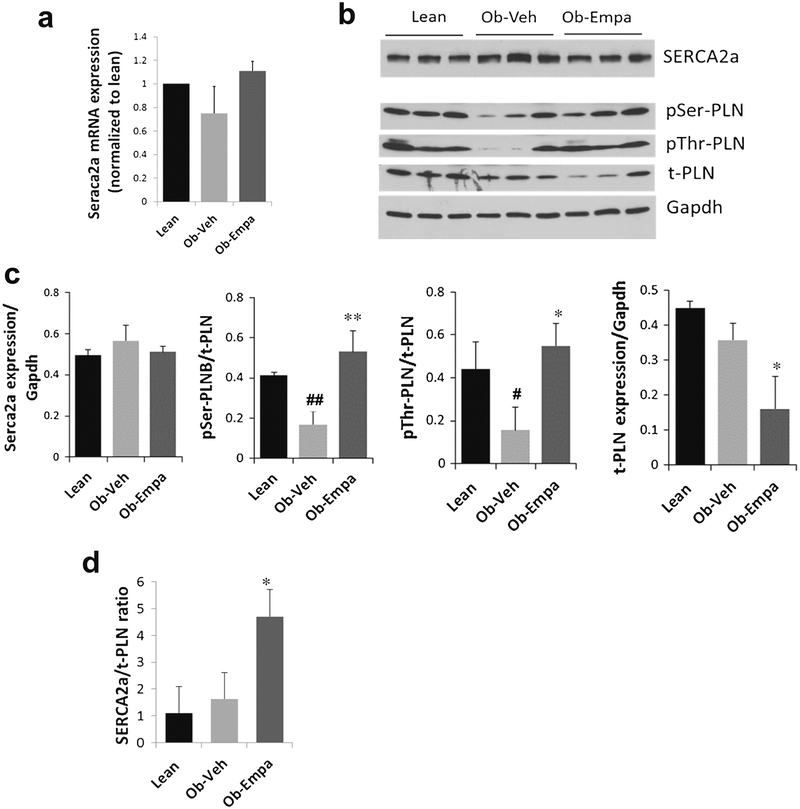
Changes in diastolic function prompted us to examine the expression of calcium handling proteins regulating cardiac contractility. The Ca2+-ATPase (also known as SERCA2a) is the major sarcoplasmic reticulum (SR) enzyme involved in Ca2+ reuptake into the SR whose function is regulated by phospholamban (PLN). Alterations in SERCA2a expression and function lead to diastolic dysfunction and cardiac failure [22]. We performed immunoblotting analyses to assess the protein levels of SERCA2a and PLN as well as the phosphorylation state of PLN. We observed that SERCA2a protein and mRNA expression (Fig. 4a–c) was not significantly changed between the groups, although in the presence of empagliflozin mRNA levels tend to go up (Fig. 4a). However, the phosphorylation state of PLN was significantly changed in the presence of empagliflozin (Fig. 4b, c). PLN is an endogenous small cardiac-specific SR membrane protein that binds to SERCA2a and regulates its function by inhibiting its activity. Increased phosphorylation of PLN both at the serine and threonine sites (Fig. 4c) supports augmented SERCA2a function and subsequently improved diastolic function. Furthermore, the data showed that the expression of PLN was also decreased following empagliflozin treatment (Fig. 4b, c). With a stable SERCA2a expression (Fig. 4c) and a decrease in PLN expression, the SERCA2a/PLN ratio is significantly increased (Fig. 4d), leading to a faster relaxation. Taken together, the data suggest that the changes in SERCA2a activity (i.e., increased PLN phosphorylation and SERCA2a/PLN ratio) are indicative of enhanced SR Ca2+ cycling, which correlates with the improvement in cardiac diastolic performance observed following empagliflozin treatment.
Fig. 4.
Expression of calcium cycling proteins. a mRNA expression of Serca2a. b representative western blots of SERCA2a and phospholamban (phospho-Serine-PLB—pSer-PLB, phospho-threonine PLB—pThr-PLB, and total t-PLB). c Densitometric quantification of corresponding cross-reactive bans in hearts samples from lean, ob/ob + vehicle (Ob-Veh) and ob/ob + empagliflozin (Ob-Empa). d SERCA2a/PLN ratio. mRNA values were normalized to S18 RNA and plotted as a function of lean values. Gapdh is used as a loading control. The data are expressed as mean ± s.d. *p < 0.05 and **p = 0.01 vs. Ob-Veh; #p < 0.05 and ##p = 0.01 vs. lean
Effect of Empagliflozin on Myocardial Insulin Responsiveness and MAPK Signaling
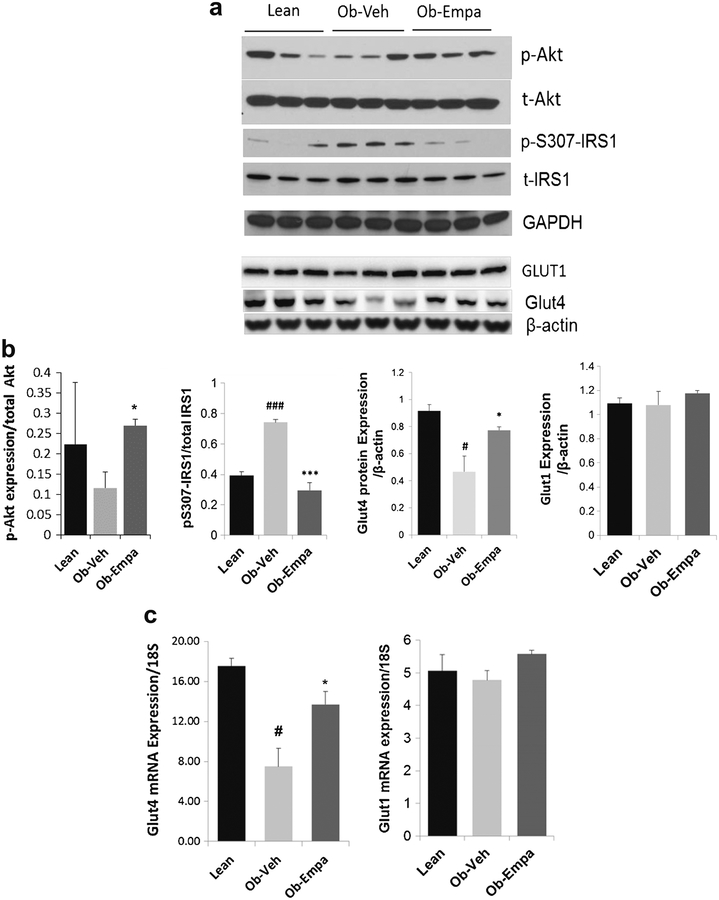
Cardiac insulin resistance and disruption of insulin signaling is a hallmark of T2DM hearts [23]. Our observation that empagliflozin significantly lowered the HOMA-IR index (Fig. 1f) is of particular interest as it may indicate improvement in insulin sensitivity. The effects of insulin, for the most part, are dependent on the activation of PI3-kinase and subsequent phosphorylation and activation of the serine-threonine kinase, Akt, indicating that this intracellular regulatory molecule is a major determinant of this pathway [24, 25]. We examined the activity (phosphorylation) and expression of Akt and observed that diabetes-induced reduction in Akt phosphorylation in ob/ob mice was reversed by empagliflozin treatment compared to vehicle-treated animals (Fig. 5a,b), although empagliflozin itself did not exert any effect on the expression of total Akt. Serine phosphorylation of IRS1 is known to be an indicator of insulin resistance. Compared to vehicle-treated mice, cardiac samples from empagliflozin-treated obese mice displayed decreased serine phosphorylation of IRS1 (pS307-IRS1) (Fig. 5a, b), suggesting that empagliflozin may act as an insulin sensitizer. These findings were paralleled by an increase in glucose transporter 4 (Glut4) protein (Fig. 5a, b) and mRNA (Fig. 5c) cardiac expression but not Glut1, conceivably indicating that attenuation of IRS1 serine phosphorylation activates Akt leading to increased Glut4 signaling and improvement in glucose utilization. Myocardial glucose uptake into cardiomyocytes is facilitated by sarcolemmal glucose transporters Glut1 and Glut4. Glut1 represents basal cardiac glucose uptake while Glut4, the dominant transporter in the adult heart, requires insulin for its activation and is thought to be mediated through the PI3K/Akt axis. These observations suggest that empagliflozin’s effect is associated with amelioration of myocardial insulin sensitivity and potentially with enhanced Glut-mediated glucose uptake in ob/ob hearts which is consistent with the overall improvement of insulin responsiveness illustrated by the decrease in the HOMA-IR index following empagliflozin treatment.
Fig. 5.
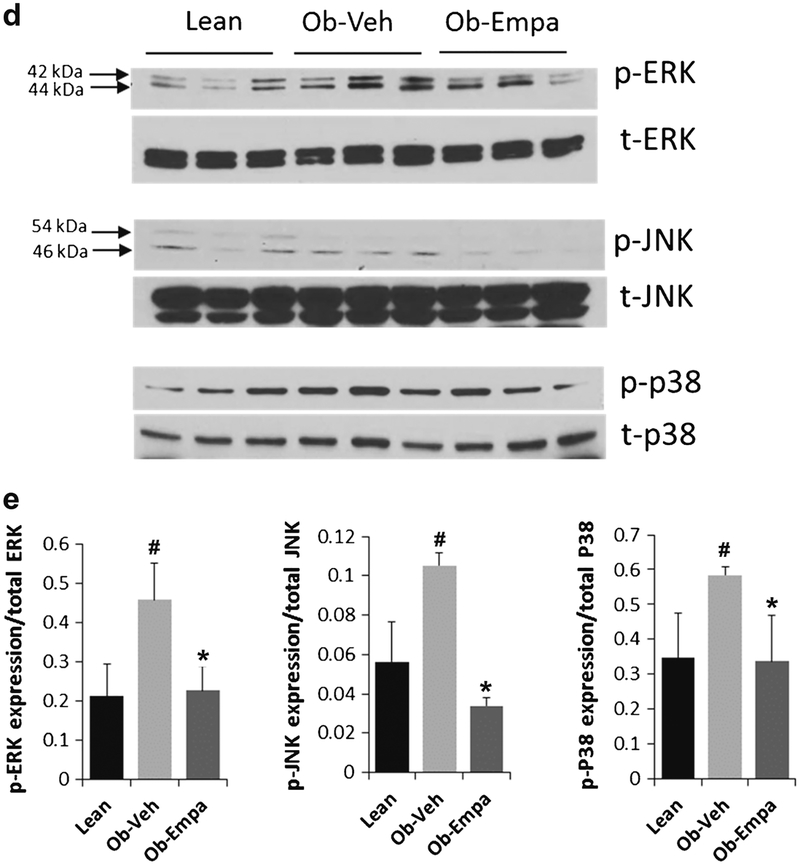
Akt and IRS1 phosphorylation, Glut4 expression, and MAPKs activation. Western blot analyses of a Akt phosphorylation (p-Akt) and total Akt (t-Akt), serine phosphorylation of IRS1 (p-S307-IRS1) and total IRS1 (t-IRS1), Glut1 and Glut4 protein expression and d ERK, JNK, and p38 phosphorylation (p-ERK, p-JNK, p-p38) and total (t-ERK, t-JNK, t-p38) in hearts samples from lean, ob/ob + vehicle (Ob-Veh), and ob/ob + empagliflozin (Ob-Empa). b and e Respective densitometric measurements of phospho-immunoreactive bands normalized to total protein values for each protein are shown. Glut1 and Glut4 are normalized to β-actin. c mRNA expression of Glut4 and Glut1 in hearts samples from lean, ob/ob + vehicle (Ob-Veh) and ob/ob + empagliflozin (Ob-Empa). GAPDH and β-actin were used as loading control. The data are expressed as mean ± s.d. *p < 0.05, **p < 0.01 and ***p < 0.01 vs. Ob-Veh; #p < 0.05 and ###p < 0.001 vs. lean
Given that mitogenic stress pathways such as MAP kinases play an essential role in diabetes/obesity-induced heart dysfunction [26] and are also regulated by insulin signaling, we sought to determine whether diabetes can activate MAPkinase pathways (namely, the extracellular signal-regulated protein kinase 1 and 2 (ERK1/2), c-Jun NH2-terminal kinase (JNK), and p38) in ob/ob hearts and whether empagliflozin can modulate these pathways. The ERKs, JNKs and p38 MAPKs have all been implicated in the development of cardiac remodeling. Data shown in Fig. 5 demonstrated that diabetes led to increased cardiac activity of all the three MAP kinases (Fig. 5d, e). Interestingly, empagliflozin greatly reduced the phosphorylation of ERK1/2, JNK-46 kDa, and p38 to almost lean control levels without affecting the pan expression of these molecules (Fig. 5d, e), further indicating improvement in insulin action and attenuation of cardiac remodeling.
Effect of Empagliflozin on Apoptosis and Cardiac Fibrosis
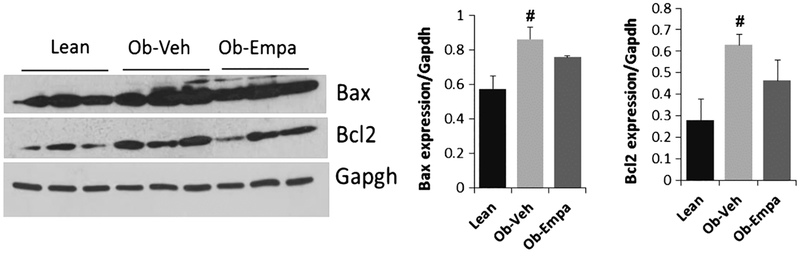
Since cardiac fibrosis and apoptosis are often times associated with cardiac dysfunction, we examined the presence of apoptosis by analyzing the expression of the antiapoptotic molecule Bcl2 and the pro-apoptotic protein Bax. Western blot analysis shows no difference in the expression of bcl2 or Bax between the groups (Fig. 6), indicating that empagliflozin has no effect on the expression of apoptotic proteins.
Fig. 6.
Myocardial apoptosis. Representative Western blot analysis and densitometric quantification of Bcl2 and Bax expression in hearts samples from lean, ob/ob + vehicle (Ob-Veh) and ob/ob + empagliflozin (Ob-Empa). Data normalized to Gapdh expression. The data are expressed as mean ± s.d. #p < 0.05 vs. lean
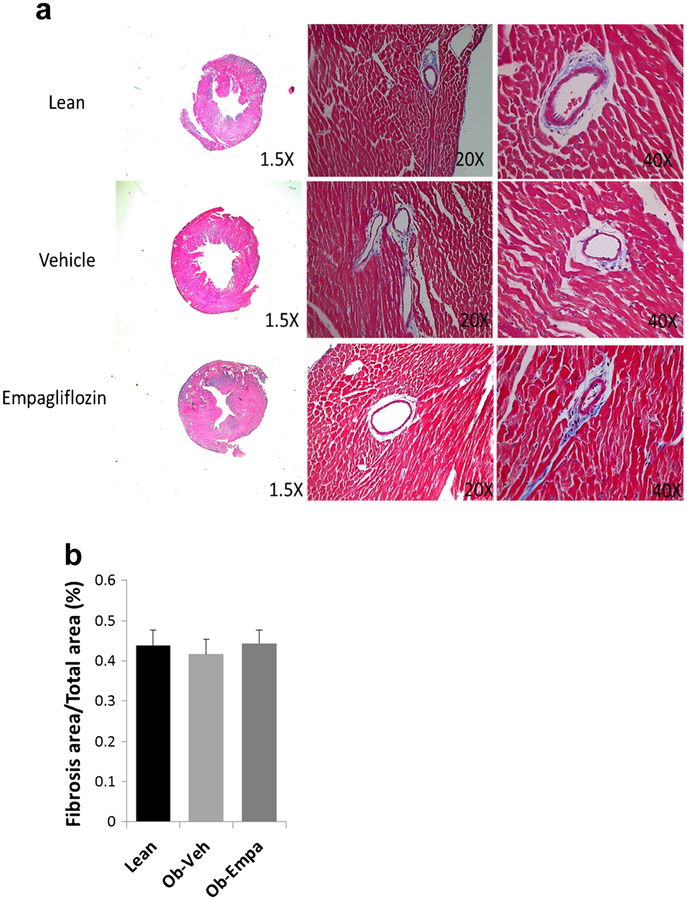
We also determined if empagliflozin alters myocardial fibrosis. Histological examination by Masson’s Trichrome staining of heart sections from lean, and ob/ob treated with vehicle or empagliflozin shows only minor patchy fibrosis (Fig. 7a) not significantly different in the three groups (Fig. 7b).
Fig. 7.
Cardiac fibrosis by histology. a Representative 1.5×, 20×, and 40× micrographs of Trichrome-stained LV sections and b fibrosis quantification from lean, ob/ob + vehicle (Vehicle) and ob/ob + empagliflozin (Empagliflozin) (n = 5)
Discussion
Previous studies have demonstrated that LV diastolic dysfunction and ventricular hypertrophy, frequently presented by diabetic patients, can be improved by tight glycemic control [27], indicating that hyperglycemia is a major risk factor in diabetes-induced cardiac dysfunction. In the present study, we show that chronic treatment with empagliflozin, a new class of antidiabetic agents that inhibit the SGLT2 and lower blood glucose, ameliorates indices of diastolic LV dysfunction in T2DM obese mice likely by lowering hyperglycemia and modulating Ca2+ handling apparatus and growth signaling pathways. These experimental data indicate the potential interest of empagiflozin in heart failure patients. Empagliflozin could be of particular interest in patients with heart failure with preserved ejection fraction in which no treatment has yet shown to reduce morbidity or mortality [28].
Empagliflozin-induced amelioration in diastolic function in our study was documented by significant modification of noninvasive indices linked to diastolic function (peak E wave and DT), increased relaxation time and tau, increased end diastolic pressure-volume relationship (EDPVR, an index of LV compliance), and increased SERCA2a/PLN ratio, leading to enhanced diastolic relaxation velocity. These changes were also associated with reversal of diabetes-blunted mechanical responses to dobutamine assessed by maximal LV pressure. At the molecular level, diabetes-induced cardiac dysfunction is characterized by an increase in PLN expression, a decrease in PLN phosphorylation and often times a decrease in SERCA2a activity or expression. Experimental evidence from transgenic animal models and gene transfer experiments demonstrate that a decrease in the SERCA2a/PLN ratio, such as PLN overexpression, slows cardiac relaxation and adversely affects the frequency response of cardiac myocytes [29]. In contrast, reduction of PLN through antisense suppression or genetic ablation in animal models increased sarcoplasmic reticulum Ca2+ cycling and improved contractility [30]. Our results show that improving Ca2+ cycling by decreasing PLN inhibition to SERCA2a (through empagliflozin-induced PLN phosphorylation) enhances SERCA2a activity and contributes to restoration of diastolic function in diabetic mice. Interestingly, these findings on the cardioprotective action of empagliflozin are consistent with recent clinical trials results that showed for the first time an antidiabetic drug decreasing cardiovascular events. Empagliflozin treatment was accompanied by significantly lower rates of death from cardiovascular causes (38%), hospitalization for heart failure (35%), and death from any cause (32%) [20, 31]. Cardiac protection is further demonstrated in our study by empagliflozin-induced activation of Akt, which extends our knowledge of the relationship between glucose control and Akt in modulating myocardial remodeling and further addresses an involvement of Akt signaling in empagliflozin-produced beneficial effect. This is consistent with reports demonstrating improved cardiac function with Akt [25, 32]. These findings also align with improvement in insulin resistance, commonly associated with cardiomyopathy under diabetic conditions.
Another important aspect of our findings is the regulation of the mitogenic stress MAP kinase pathways. The ERKs, JNKs, and p38 MAPKs have all been implicated in the development of cardiac hypertrophy. We report the sustained activation of the three MAPK signaling pathways in ob/ob hearts which were mitigated by empagliflozin. Since MAPK action is regulated by insulin signaling it is conceivable to suggest that amelioration of insulin sensitivity in ob/ob hearts is responsible for the observed mitigation of the MAPK signaling. It is therefore likely that some of the pleotropic effects of empagliflozin may be ascribed to shunting insulin signaling away from the mitogenic pathway and towards the metabolic pathway. Another explanation to this effect may involve empagliflozin regulation of myocardial calcium homeostasis noted earlier. In this respect, it is likely that a decrease in intracellular Ca2+, as a result of improved SERCA2a activity and calcium cycling, modulates MAPK actions and cardiac hypertrophy since calcium is an essential cofactor in the activation of several hypertrophic signaling pathways including MAPKs.
The effect of empagliflozin is selectively on SGLT2 which is highly specifically expressed in the kidney, as we determined in this study and previously reported by others [33], so the natural question is how empagliflozin exerts its effect on the heart when its primary biological target action is else where, and what are the mechanisms underlying its cardioprotection? Although the exact mechanism(s) is far from clear, it is conceivable to speculate that reduction in glucose load and all the ensuing hyperglycemia-associated events, and reduction in the severity of renal dysfunction—although not examined in this study—likely contributed to the improvement observed on treatment with empagliflozin. This is quite possible as hyperglycemia and renal failure negatively influence cardiac function and mediates cardiac tissue injury through multiple pathways such as generation of reactive oxygen species and increases in advanced glycation end products, which have been shown to directly alter Ca2+ handling proteins function [34]. Therefore, given the fact that empagliflozin is known to improve glycemic indices, the observed pleiotropic effects of empagliflozin on cardiac phenotype and the associated molecular effects may represent secondary manifestations resulting from the improvement in the systemic hyperglycemic condition. Moreover, the sodium loss related to the diuresis effect of the empagliflozin reduces the LV load and probably contributes to the observed improvement of LV function [35]. The possibility that empagliflozin might have also evoked beneficial effects directly on cardiomyocytes and on other metabolic tissues and organs which collectively contributed to the overall improvement observed is far from certain, as no evidence of tangible SGLT2 expression was observed in the heart, precluding a direct action of empagliflozin on myocytes and suggesting an indirect mechanism.
Although hyperglycemia is still considered the principal cause of diabetes and its cardiovascular complications [34, 36], recent evidence indicates that diabetes involves more than abnormal glucose homeostasis. While glycemic control in T2DM patients has proven successful to some extent, recent large-scale clinical trials of the effect of lowering glucose on cardiovascular events and mortality did not fare well, again highlighting the complexity of the outcomes and suggesting that controlling hyperglycemia alone may not be an adequate therapy for diabetes and its complications [37–39]. This is true given the fact that many patients with T2DM have comorbidities such as obesity, hypertension, and dyslipidemia which are also believed to confer increased risk of cardiovascular diseases in this population. Therefore, it is conceivable to suggest that reduced excess body weight, as have been reported for empagliflozin, and lipotoxicity provide additional benefits to the observed cardiac effects of empagliflozin. However, the recent success demonstrated by the empagliflozin clinical trials in terms of reducing cardiovascular events indicates that this type of antidiabetic class may act differently and offers a novel strategy to overcome diabetes-associated cardiac contractile impairment [40]. For instance, we have conceivably proposed that increased circulating levels of β-hydroxybuturate during treatment with empagliflozin may offer cardioprotection in a mechanism that favors oxidation of β-hydroxybuturate over fatty acids leading to a better and efficient energy utilization and restoration of diastolic function [41]. Additional studies are needed to test this hypothesis of potential shift in energetics and to further gain mechanistic insights into empagliflozin-elicited cardiac protection would be instrumental in further improving its action. In addition, the assessment of temporal effects of empagliflozin and testing its long-term biological action desirably in different animal models of diabetic and diastolic heart failure is highly warranted. Furthermore, despite low expression of SGLT2 in the heart, performing studies on isolated cardiomyocytes to directly evaluate empagliflozin effects on cardiomyocyte mechanics and physiology would be interesting and informative.
Limitations
Several limitations to our study need to be acknowledged. The numbers of mice used in the study were relatively small and the treatment duration was limited to 6 weeks. These facts could explain that no significant modification of LV mass was observed during the course of the study which was relatively short. While we demonstrate an improvement of hypertrophic markers at the molecular levels (i.e., molecular remodeling), structural changes (i.e., morphological remodeling) may require longer periods of treatment given the fact that the in vivo physiological settings are more complex. Moreover, it was reported that ob/ob mice exhibited only a mild fibrosis and LV morphological remodeling; LV diastolic dysfunction is the main LVabnormality observed in this model. In our study, the mice were carefully phenotyped and the improvement of LV diastolic function in the treated group was documented by ultrasound, invasive hemodynamics, and molecular analyses. However, the clinical relevance of our findings deserves further investigation. It would be interesting to evaluate the potential effects of this drug in patients with early-stage heart failure with preserved ejection fraction, when the structural changes in the heart and vasculature are still reversible rather than in later stages which are associated with advanced myocardial structural remodeling and fibrosis [42, 43]. In this study, blood pressure was not monitored; however, previous studies have reported a mild but meaningful reduction in blood pressure in patients treated with empagliflozin [44]. The potential contribution of lowering blood pressure drug effect on the observed improvement of LV diastolic function needs further investigations. Nevertheless, the findings of this study in animal models align well with recent clinical trial outcomes and further suggest a potential clinical utility for empagliflozin in the treatment of diastolic dysfunction as there is currently no approved drug to specifically target diastolic heart failure despite the high incidence of this condition.
Conclusion
The present study shows that treatment with empagliflozin led to improvement in indices of diastolic cardiac dysfunction in a diabetic and obese rodent model with severe hyperglycemia. Our findings suggest that reduction in the glucose load with resultant attenuation of myocardial insulin resistance on one hand, and an improvement in diabetes-related abnormalities in calcium handling and MAPkinase signaling pathways on the other have contributed to the protective effects of empagliflozin on LV diastolic function which is impaired early in diabetic cardiomyopathy.
Acknowledgements
The authors thank Shihong Zhang for the technical assistance. This work was supported in part by a grant from the National Institutes of Health R01HL097357 (DL), by an unrestricted research grant from Boehringer Ingelheim (Agr-6547) (DL), and by a grant from the French Federation of Cardiology (NH).
Conflict of Interest Some of the results in this paper have been published previously in an abstract at the American Diabetes Association 76th Scientific Sessions Boston, in June 2015. DL received unrestricted funding for an investigator initiated proposal from Boerhinger Ingelheim to perform this study. EM is an employee of Boehringer Ingelheim. Dr. Komajda has performed consulting/advisory activities for Servier, Bristol-Myers Squibb, AstraZeneca, Menarini, Novartis, MSD, and Sanofi-Aventis. All other authors declare no interests.
Footnotes
Animal Ethical Approval Animals were obtained and handled as approved by the Mount Sinai Institutional Animal Care and Use Committee in accordance with the “Principles of Laboratory Animal Care by the National Society for Medical research and the Guide for the Care and Use of Laboratory Animals” (National Institutes of Health Publication No. 86–23, revised 1996).
Research Involving Human Participants Not applicable.
Informed Consent Not applicable.
References
- 1.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riva E, Andreoni G, Bianchi R, Latini R, Luvara G, Jeremic G, Traquandi C, Tuccinardi L. Changes in diastolic function and collagen content in normotensive and hypertensive rats with long-term streptozotocin-induced diabetes. Pharmacol Res. 1998;37:233–40. [DOI] [PubMed] [Google Scholar]
- 3.Singleton JR, Smith AG, Russell JW, Feldman EL. Microvascular complications of impaired glucose tolerance. Diabetes. 2003;52: 2867–73. [DOI] [PubMed] [Google Scholar]
- 4.Hemmingsen B, Lund SS, Gluud C, Vaag A, Almdal TP, Hemmingsen C, Wetterslev J. Targeting intensive glycaemic control versus targeting conventional glycaemic control for type 2 diabetes mellitus. The Cochrane Database of Systematic Reviews. 2013;11:Cd008143. [DOI] [PubMed] [Google Scholar]
- 5.Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, Marinopoulos SS, Puhan MA, Ranasinghe P, Block L, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154:602–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esposito K, Chiodini P, Bellastella G, Maiorino MI, Giugliano D. Proportion of patients at HbA1c target <7% with eight classes of antidiabetic drugs in type 2 diabetes: systematic review of 218 randomized controlled trials with 78 945 patients. Diabetes Obes Metab. 2012;14:228–33. [DOI] [PubMed] [Google Scholar]
- 7.Schernthaner G, Barnett AH, Betteridge DJ, Carmena R, Ceriello A, Charbonnel B, Hanefeld M, Lehmann R, Malecki MT, Nesto R, et al. Is the ADA/EASD algorithm for the management of type 2 diabetes (January 2009) based on evidence or opinion? A critical analysis. Diabetologia. 2010;53:1258–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodiumglucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011;32:515–31. [DOI] [PubMed] [Google Scholar]
- 9.Tahrani AA, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. The Lancet. Diabetes & Endocrinology. 2013;1: 140–51. [DOI] [PubMed] [Google Scholar]
- 10.Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. Journal of the American Society of Nephrology: JASN. 2011;22:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raskin P Sodium-glucose cotransporter inhibition: therapeutic potential for the treatment of type 2 diabetes mellitus. Diabetes Metab Res Rev. 2013;29:347–56. [DOI] [PubMed] [Google Scholar]
- 12.Grempler R, Thomas L, Eckhardt M, Himmelsbach F, Sauer A, Sharp DE, Bakker RA, Mark M, Klein T, Eickelmann P. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14:83–90. [DOI] [PubMed] [Google Scholar]
- 13.Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:721–8. [DOI] [PubMed] [Google Scholar]
- 14.Haring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Broedl UC, Woerle HJ. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37:1650–9. [DOI] [PubMed] [Google Scholar]
- 15.Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double-blind, placebocontrolled trial. The Lancet. Diabetes & Endocrinology. 2014;2: 369–84. [DOI] [PubMed] [Google Scholar]
- 16.Haring HU, Merker L, Seewaldt-Becker E, Weimer M, Meinicke T, Woerle HJ, Broedl UC. Empagliflozin as add-on to metformin plus sulfonylurea in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2013;36:3396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ, Broedl UC. Empagliflozin improves glycaemic and weight control as add-on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24-week, randomized, placebo-controlled trial. Diabetes Obes Metab. 2014;16:147–58. [DOI] [PubMed] [Google Scholar]
- 18.Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, Broedl UC. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet. Diabetes & Endocrinology. 2013;1:208–19. [DOI] [PubMed] [Google Scholar]
- 19.Cherney DZ, Perkins BA, Soleymanlou N, Har R, Fagan N, Johansen OE, Woerle HJ, von Eynatten M, Broedl UC. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol. 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 21.Schannwell CM, Schneppenheim M, Perings S, Plehn G, Strauer BE. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology. 2002;98:33–9. [DOI] [PubMed] [Google Scholar]
- 22.Kawase Y, Hajjar RJ. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases. Nature Clinical Practice Cardiovascular Medicine. 2008;5:554–65. [DOI] [PubMed] [Google Scholar]
- 23.Abel ED. Myocardial insulin resistance and cardiac complications of diabetes. Current drug targets. Immune, Endocrine and Metabolic Disorders. 2005;5:219–26. [DOI] [PubMed] [Google Scholar]
- 24.Matsui T, Li L, del Monte F, Fukui Y, Franke TF, Hajjar RJ, Rosenzweig A. Adenoviral gene transfer of activated phosphatidylinositol 3′-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation. 1999;100:2373–9. [DOI] [PubMed] [Google Scholar]
- 25.Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, et al. Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res. 2004;94:884–91. [DOI] [PubMed] [Google Scholar]
- 26.Liang L, Jiang J, Frank SJ. Insulin receptor substrate-1-mediated enhancement of growth hormone-induced mitogen-activated protein kinase activation. Endocrinology. 2000;141:3328–36. [DOI] [PubMed] [Google Scholar]
- 27.Grandi AM, Piantanida E, Franzetti I, Bernasconi M, Maresca A, Marnini P, Guasti L, Venco A. Effect of glycemic control on left ventricular diastolic function in type 1 diabetes mellitus. Am J Cardiol. 2006;97:71–6. [DOI] [PubMed] [Google Scholar]
- 28.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37: 2129–200. [DOI] [PubMed] [Google Scholar]
- 29.Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW 2nd, Walsh RA, Kranias EG. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J Clin Invest. 1996;97:533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.del Monte F, Harding SE, Dec GW, Gwathmey JK, Hajjar RJ. Targeting phospholamban by gene transfer in human heart failure. Circulation. 2002;105:904–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zinman B, Inzucchi SE, Lachin JM, Wanner C, Ferrari R, Fitchett D, Bluhmki E, Hantel S, Kempthorne-Rawson J, Newman J, et al. Rationale, design, and baseline characteristics of a randomized, placebo-controlled cardiovascular outcome trial of empagliflozin (EMPA-REG OUTCOME). Cardiovasc Diabetol. 2014;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui T, Tao J, del Monte F, Lee KH, Li L, Picard M, Force TL, Franke TF, Hajjar RJ, Rosenzweig A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation. 2001;104:330–5. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Williams S, Ho S, Loraine H, Hagan D, Whaley JM, Feder JN. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Therapy: Research, Treatment and Education of Diabetes and Related Disorders. 2010;1:57–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. 2006;98:596–605. [DOI] [PubMed] [Google Scholar]
- 35.Sattar N, McLaren J, Kristensen SL, Preiss D, McMurray JJ. SGLT2 inhibition and cardiovascular events: why did EMPA-REG outcomes surprise and what were the likely mechanisms? Diabetologia. 2016;59:1333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dobrin JS, Lebeche D. Diabetic cardiomyopathy: signaling defects and therapeutic approaches. Expert Rev Cardiovasc Ther. 2010;8: 373–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. [DOI] [PubMed] [Google Scholar]
- 39.Terry T, Raravikar K, Chokrungvaranon N, Reaven PD. Does aggressive glycemic control benefit macrovascular and microvascular disease in type 2 diabetes? Insights from ACCORD, ADVANCE, and VADT. Current Cardiology Reports. 2012;14:79–88. [DOI] [PubMed] [Google Scholar]
- 40.de Leeuw AE, de Boer RA. Sodium-glucose cotransporter 2 inhibition: cardioprotection by treating diabetes-a translational viewpoint explaining its potential salutary effects. European Heart Journal Cardiovascular Pharmacotherapy. 2016;2:244–55. [DOI] [PubMed] [Google Scholar]
- 41.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPAREG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39:1108–14. [DOI] [PubMed] [Google Scholar]
- 42.Borlaug BA. Exercise haemodynamics and outcome in patients with dyspnoea. Eur Heart J. 2014;35:3085–7. [DOI] [PubMed] [Google Scholar]
- 43.Hammoudi N, Laveau F, Helft G, Cozic N, Barthelemy O, Ceccaldi A, Petroni T, Berman E, Komajda M, Michel PL, et al. Low level exercise echocardiography helps diagnose early stage heart failure with preserved ejection fraction: a study of echocardiography versus catheterization. Clin Res Cardiol. 2017;106:192–201. [DOI] [PubMed] [Google Scholar]
- 44.Imprialos KP, Sarafidis PA, Karagiannis AI. Sodium-glucose cotransporter-2 inhibitors and blood pressure decrease: a valuable effect of a novel antidiabetic class? J Hypertens. 2015;33:2185–97. [DOI] [PubMed] [Google Scholar]