Abstract
New effective therapies are greatly needed for metastatic uveal melanoma, which has a very poor prognosis with a median survival of less than 1 y. The melanocortin 1 receptor (MC1R) is expressed in 94% of uveal melanoma metastases, and a MC1R-specific ligand (MC1RL) with high affinity and selectivity for MC1R was previously developed. Methods: The 225Ac-DOTA-MC1RL conjugate was synthesized in high radiochemical yield and purity and was tested in vitro for biostability and for MC1R-specific cytotoxicity in uveal melanoma cells, and the lanthanum-DOTA-MC1RL analog was tested for binding affinity. Non–tumor-bearing BALB/c mice were tested for maximum tolerated dose and biodistribution. Severe combined immunodeficient mice bearing uveal melanoma tumors or engineered MC1R-positive and -negative tumors were studied for biodistribution and efficacy. Radiation dosimetry was calculated using mouse biodistribution data and blood clearance kinetics from Sprague–Dawley rat data. Results: High biostability, MC1R-specific cytotoxicity, and high binding affinity were observed. Limiting toxicities were not observed at even the highest administered activities. Pharmacokinetics and biodistribution studies revealed rapid blood clearance (<15 min), renal and hepatobillary excretion, MC1R-specific tumor uptake, and minimal retention in other normal tissues. Radiation dosimetry calculations determined pharmacokinetics parameters and absorbed α-emission dosages from 225Ac and its daughters. Efficacy studies demonstrated significantly prolonged survival and decreased metastasis burden after a single administration of 225Ac-DOTA-MC1RL in treated mice relative to controls. Conclusion: These results suggest significant potential for the clinical translation of 225Ac-DOTA-MC1RL as a novel therapy for metastatic uveal melanoma.
Keywords: melanocortin 1 receptor, 225Ac alpha therapy, uveal melanoma, mouse model
Uveal melanoma is the most common primary intraocular malignancy and differs from the more common cutaneous melanoma in terms of risk factors, primary treatment, anatomic spread, molecular changes, and response to systemic therapy (1,2). Patients who develop uveal melanoma metastases, primarily in the liver, have a very poor prognosis with a median survival of about 1 y. Because uveal melanomas have different characteristic mutations from cutaneous melanomas, targeted therapies that have been effective for the latter, such as BRAF, are not indicated for the former (3). Immune checkpoint inhibition therapies that are successful in cutaneous melanoma have had poor efficacy in ocular melanoma, with fewer than 10% of patients responding and with rapid recurrence (3).
The melanocortin 1 receptor (MC1R) is highly expressed in uveal melanoma metastases (4). MC1R is a member of a family of 5 G-protein–coupled melanocortin receptors, 4 of which bind melanocyte-stimulating hormone (MSH) and related ligands (MC1R, 3R, 4R, and 5R) (5). Unlike the other members of this G-protein family, MC1R is not expressed in most normal human tissues (6), lessening concern for therapy-related toxicity. Although expression is found in the brain (7) and normal melanocytes (8), this is not a major concern because conjugates can be designed to not cross the blood–brain barrier and, in the most severe cases of melanocyte loss, the most serious symptom is vitiligo (9). MC1R expression has been reported on activated monocytes, macrophages, and dendritic cells derived from monocytes (10). This is also not a significant concern since the population of activated monocytes and macrophages can be replenished within days and lymphoid dendritic cells, which do not express MC1R, will not be depleted. MC1R is highly polymorphic (11), but the wild-type frequency is about 50% (8) and the most common mutations occur with a frequency of 21.5% in cytoplasmic domains, 19.7% in transmembrane domains, and 0% in the extracellular domain (11). Hence, most patients will have an MC1R isoform that is suitable for ligand binding. An MC1R-specific ligand (MC1RL) and conjugates were previously developed with high specificity (>200 fold) and affinity (0.2–0.4 nM inhibition constant) for MC1R (12,13). A fluorescent-dye conjugate was rapidly internalized by MC1R-expressing tumor cells, does not cross the blood–brain barrier, and is rapidly cleared from circulation (7).
Herein is reported the preclinical development and testing of a novel MC1R-targeted radiopharmaceutical, 225Ac-DOTA-MC1RL, for targeted α-particle therapy (TAT) (14,15) of uveal melanoma. α-particle emissions consist of dicationic helium nuclei (He2+) that have high linear-energy transfer and a short mean free path of only a few cell diameters (<100 μm) in tissue (16). 225Ac is an α-particle–emitting radionuclide that has a 10-d half-life (17), 4 α-emissions in its decay chain, and high (28 MeV) total energy release (18).
MATERIALS AND METHODS
Compound Synthesis and Loading with Lanthanide
MC1RL (13) was synthesized according to a conventional Nα-fluorenylmethyloxycarbonyl (Fmoc) peptide synthesis strategy, except the Fmoc-Lys(Alloc)-OH was coupled to allow orthogonal alloc deprotection of the linker on the ε-amino group of the lysine after the linear peptide synthesis. The alloc group is removed, and Fmoc-aminohexanoic acid linker and tri-t-butyl-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate (DOTA; TCI Chemicals) were coupled sequentially using O-(1H-6-chlorobenzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate activation. The DOTA-MC1RL peptide was cleaved from the resin with a cocktail of trifluoroacetic acid (Chem-Impex International), water, and triisopropylsilane (Sigma-Aldrich) (95:2.5:2.5, v/v), precipitated in cold diethyl ether, pelleted/decanted, and lyophilized. The crude white powder was purified by reverse-phase high-performance liquid chromatography (Agilent) and characterized by both matrix-assisted laser desorption/ionization time-of-flight mass spectroscopy (JEOL USA Inc.) and analytic high-performance liquid chromatography. A scrambled peptide ligand (DOTA–substance P [SP]) was synthesized by changing the order of amino acids (sequence: 4-phenylbutyric acid-Trp-Gly-His-Arg-(d)-Phe-Lys(aminohexanoic acid-DOTA)-CONH2). The europium–diethylenetriaminepentaacetic acid (DTPA)–MC1RL was synthesized as described before (19) except that MC1RL was used as the binding ligand (Supplemental Figs. 1A–1C; supplemental materials are available at http://jnm.snmjournals.org). Competition binding assays were performed as previously described using the europium-DTPA-NDPα-MSH ligand. The europium-DTPA-MC1RL binding affinity was determined using saturation binding assays. To determine MC1RL binding affinity for murine MC1RL, saturation binding assays were performed using the europium-DTPA-MC1RL and B16-F10 murine melanoma cells with high expression of murine MC1R (12).
Cell Culture and Characterization
Uveal melanoma cell lines were acquired (OCM1, OCM3, and OCM8 from June Kan-Mitchel, University of Southern California; OMM1 from Gregorius P. Luyten, University Hospital at Rotterdam; and MEL270, MEL290, and OMM2.3 from Timothy Murray, Bascom Palmer Eye Institute) and grown in RPMI medium, 10% fetal bovine serum, a 100 units/mL concentration of penicillin, a 100 mg/mL concentration of streptomycin, 1% 200 mM l-glutamine, 1% 100 mM sodium pyruvate, 1% minimal essential medium essential vitamin mixture (×100), 1% nonessential amino acid mixture (×100), and 1% 1 M (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) in 5% CO2 at 37°C. A375, A375/MC1R human cutaneous melanoma cells, and Hek293/MC1R cells were obtained and grown as before (12,20). Cells were authenticated per American Type Culture Collection guidelines (21), monitored for original morphology, and tested for Mycoplasma (MycoAlert kit; Lonza), and only passage numbers of less than 25 cells were used. MC1R expression and receptor number were determined as previously described (12,13) except that europium-DTPA-MC1RL was used for saturation binding. Cytotoxicity was determined as described in Supplemental Figure 2.
Radiochemical Synthesis and Characterization
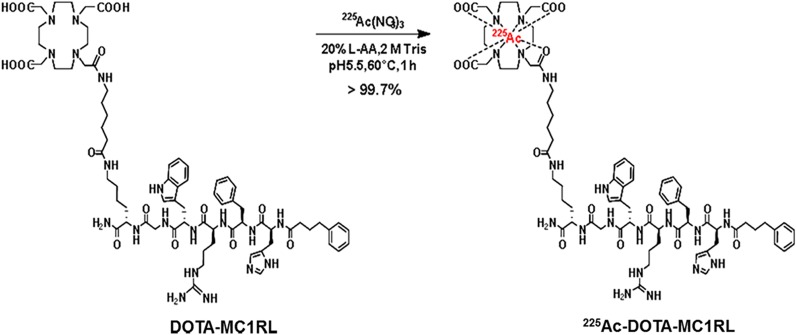
DOTA-MC1RL or DOTA-SP (10 μg/10 μL of water), 225Ac(NO3)3 (3.4 MBq), 90 μL of water, and 10 μL of 20% l-ascorbic acid were added to a 1.5-mL tube followed by pH adjustment to 5.5–6 (1 M Tris buffer;10–12 μL) and incubation at 60°C for 1 h (Fig. 1). Specific activity was calculated using a standard method (22). Radiochemical purity was assessed 24 h after collection by a γ-counter, and in vitro serum stability was determined by adding 50 μL of 225Ac-DOTA-MC1RL (2,072 kBq) to 1 mL of human serum (n = 4), incubated at 37°C for 10 d, and quantified at multiple time points by thin-layer chromatography and γ-counting using established methods (23).
FIGURE 1.
Radiochemical synthesis of 225Ac-DOTA-MC1RL.
Animal Studies
All protocols were approved (University of South Florida Institutional Animal Care and Use Committee protocol IS00000805 and Wake Forest University Health Sciences Institutional Animal Care and Use Committee protocol A11-144). Male and female animals were used. Sprague–Dawley rats, 10–12 wk old and weighing 200–250 g, were purchased with jugular vein catheters installed (Charles River). Nontumor studies used BALB/c mice (10–12 wk old, 18–22 g; Charles River). Severe combined immunodeficient (SCID) mice (6–8 wk old, 15–20 g; Charles River) were used for xenografting cell lines. Tail vein catheters were used for agent administration to mice.
For xenografting, 10 × 106 cells in 80 μL of phosphate-buffered saline and 20 μL of Matrigel (phenol red–free; Corning) were injected subcutaneously into the flank. Tumor volumes were determined by caliper using the following formulas: volume = (length × width2)/2 for A375 and A375/MC1R, and volume = (length × width × height)/2 for MEL270 tumors, which were initially flat with a gradual shift to a rounded shape.
Histology and Immunohistochemistry
Excised tissues were prepared for histology, hematoxylin and eosin staining, MC1R immunohistochemistry staining, and slide scanning as previously described (12). Metastasis burden was determined using images of 3 sections (25%, 50%, and 75%) through each liver and lung. Metastasis area was determined by segmentation using intensity and size threshold classifiers on the triple-red channel (Visiopharm software, version 6.7.0.2590). Total tissue area was determined with an intensity threshold classifier on the immunohistochemistry intensity channel, and the percentage metastasis was calculated.
To quantify MC1R expression in tumors, images from serial hematoxylin and eosin and immunohistochemistry sections were analyzed using Visiopharm, version 2017.7. Each serial section pair (hematoxylin and eosin and immunohistochemistry) was aligned using the tissue align module, and viable tumor was segmented by thresholding the hematoxylin channel. A multithreshold-marker-area analysis was then performed within the viable tumor region on each immunohistochemistry image. Each pixel was categorized as negative, weak, moderate, or strong on the basis of thresholds set by a pathologist, and the percentages of each category were normalized by total area of interest.
Maximum Tolerated Dose (MTD)
The MTD study was performed as previously described (23).
Measurement of Activity
Because α-particles from 225Ac cannot be directly measured in tissue because of the short mean free path (18), 225Ac α-activities were estimated using measurements of related γ-emissions. For the initial MTD study, syringes were prepared with a range of activities as determined by the γ-counter (Wallac 1470 Wizard; Perkin-Elmer). For subsequent studies, a dose calibrator (Atomlab 500; BioDex) was used to prefill syringes with 148 kBq ± 10% (per Appendix E of the BioDex manual) of 225Ac-conjugate activity. Activities were measured for 2 min using dial number 38.2 as recommended by Biodex. Activities of 225Ac, and the 221Fr and 213Bi daughter products (18), were measured by acquiring isomeric γ-spectra (Supplemental Fig. 3) before administration using a 4π well-type wipe-test γ-counter (Atomlab 500). Activities (225Ac) were calculated using factors for γ-ray abundance per α-decay using calibration parameters and correction coefficients from Appendices A and E of the instrument manual. A full energy window (0–800 keV) was used for spectra acquisition that included γ-counts from 225Ac (99.8-keV peak, 1% abundance) and 2 γ-emitting daughters, 221Fr (218.1-keV peak, 11.4% abundance) and 213Bi (440.5-keV peak, 25.9% abundance) (24). The α-activities were determined by fitting each peak with a multigaussian fit and integrating to determine the net number of counts while incorporating the acquisition time. Spectra were acquired at least 24 h after radiosynthesis or tissue rendering, ensuring that 225Ac and daughters were in secular equilibrium (25). Activity remaining in the syringe and catheter after injection was calculated and subtracted to determine net administered activity.
Blood Pharmacokinetics
Sprague–Dawley rats were weighed before injection with radioactivity and injected with 148 kBq (±10%) of 225Ac-DOTA-MC1RL in the syringe. Serial blood draws (45 μL) were taken from 5 min to 24 h after injection. 225Ac α-activity was calculated as described above. Data were fitted using an exponential decay nonlinear regression.
Biodistribution
Non–tumor-bearing BALB/c mice, or SCID mice bearing MEL270 xenografts (160–650 mm3) or A375 and A375/MC1R bilateral xenografts (189–1,680 mm3), were intravenously administered 148 kBq (±10%) of 225Ac α-activity in the syringe. Tissues were rendered and weighed at multiple time-points between 24 h and 3 wk after injection. For each tissue, 225Ac, 221Fr, and 213Bi α-activities were calculated as described above and reported as percentage injected activity per gram (%IA/g).
Radiation Dosimetry
Biodistribution data for the different tissues were fitted using an exponential decay nonlinear regression, and dosimetry calculations were performed for 225Ac, 221Fr, 217At, 213Bi, and 213Po using the generalized internal dosimetry schema of the MIRD Committee for α-particle emitters (26,27). The β− decay branching ratio for 217At to 217Rn is only 0.01%; therefore, it was assumed that all decays of 217At were by α-emission to 213Bi. The branching ratios for decay of 213Bi to 213Po (98%) or 209Tl (2%) were included in the calculation. Because of the relatively low linear-energy transfer and the small dimensions of the target tissues, the β− emissions from 217At, 213Bi, 209Tl, and 209Pb were assumed negligible and were not included in the calculations (28). The following assumptions were made: uniform distribution of activity in the tissue volume; no α-particles escaping from the source tissue due to the short range; and electron and photon contributions that were negligible compared with α-particle energy deposition (28). It was also assumed that α-particles from 221Fr (4.9-min half-life), 217At (32.2-ms half-life), 213Bi (46-min half-life), and 213Po (4.2-μs half-life) were deposited in the same location as 225Ac (10-d half-life) because of the relatively shorter half-lives of these daughter isotopes. Although 217At and 213Po do not have detectable γ-emissions, under the assumption that the decay chain had reached secular equilibrium, the accumulated activity of these 2 daughters would equal that of 221Fr and 213Bi, respectively. The total absorbed α-particle dose was calculated from the summation of doses from 225Ac, 221Fr, 217At, 213Bi, and 213Po.
Antitumor Efficacy
Tumor-bearing mice (n = 11/group) were injected with activities of 225Ac-DOTA-MC1RL or 225Ac-DOTA-SP, cold lanthanum-DOTA-MC1RL, or saline solution (0.9%, Cardinal Pharmaceuticals). Surpassing a 2,000 mm3 tumor volume was the experimental endpoint unless clinical endpoints, such as 20% weight loss, tumor ulceration, hunched back, lack of grooming, or lethargy, were observed. Metastasis formation was identified by necropsy.
Statistical Analysis
The t test was used for the MTD study. The following analyses were used for comparison of the efficacy study groups: Kaplan–Meier for time to endpoint, a mixed-model analysis for tumor growth change, a paired Wilcoxon signed-rank test for initial decrease in tumor volume, a Fisher exact test with corrections for multiple testing using the Holm stepdown method for metastasis burden, and a nonparametric Kruskal–Wallis test for immunohistochemistry staining.
RESULTS
Synthesis and Characterization of Parent Compound and Lanthanide Chelates
The unmetallated DOTA-MC1RL was synthesized and, since there are no nonradioactive isotopes of actinium, the analogous lanthanum-DOTA-MC1RL chelate was prepared for use as a nonradioactive control (Supplemental Figs. 4–8) (23,29). Both DOTA-MC1RL and lanthanum-DOTA-MC1RL had high binding affinity for human MC1R, 0.24 ± 0.20 and 0.23 ± 0.18 nM inhibition constants, respectively (Supplemental Fig. 9A). The binding affinity of europium-DTPA-MC1RL to human MC1R was determined to be a 4.4 ± 2.3 nM dissociation constant (Supplemental Fig. 9B). Lower, 1.3 μM, dissociation constant affinity was observed for europium-DTPA-MC1RL binding to murine MC1R (Supplemental Fig. 9C). The scrambled peptide controls, lanthanum-DOTA-SP and europium-DOTA-SP, did not bind (Supplemental Figs. 9D and 9E).
Radiosynthesis and Characterization of 225Ac Radiopharmaceutical
Radiochemical purity of 99.8% and specific activity of 181.3 ± 92.5 kBq/μg and 140.6 ± 55.5 kBq/μg for 225Ac-DOTA-MC1RL and 225Ac-DOTA-SP, respectively, were observed (Supplemental Fig. 10). In vitro serum stability was high, with 90% intact after 10 d (Table 1).
TABLE 1.
In Vitro Serum Stability of 225Ac-DOTA-MC1RL
| % intact |
||
| Day | Thin-layer chromatography scanner | γ-counter |
| 0 | 100 | 100 |
| 2 | 97.3 ± 0.5 | 96.9 ± 0.4 |
| 4 | 95.6 ± 1.1 | 95.1 ± 0.8 |
| 6 | 93.5 ± 0.8 | 93.2 ± 1.3 |
| 8 | 91.4 ± 1.2 | 91.0 ± 0.9 |
| 10 | 90.2 ± 0.7 | 89.9 ± 1.3 |
MC1R Expression on Uveal Melanoma Cell Lines and Xenograft Tumors
MC1R messenger RNA and protein expression were confirmed in a set of uveal melanoma cell lines (Supplemental Fig. 11). Only MEL270, OMM2.3, and OMM1 cells carry the GNAQ or GNA11 mutations found in nearly all uveal melanomas (30). The MEL270 and OMM1 cells formed tumors in immunocompromised mice, and all xenografts had high and uniform MC1R protein expression.
Receptor Number for Tumor Cell Lines
MEL270 cells were selected for the in vivo studies, and it was determined that MEL270 cells have 410,000 receptors per cell (Supplemental Fig. 12), which is a higher level of endogenous expression than that of the engineered A375/MC1R cells, which have 75,000 receptors per cell (12). The parental A375 melanoma cell line has extremely low expression, at 400 ± 93 MC1Rs per cell (31).
In Vitro MC1R-Specific Cytotoxicity
Cytotoxicity assays were performed with the goal of demonstrating target-specific cytotoxicity. Assay conditions were not optimized to demonstrate maximal toxicity. Significantly reduced proliferation (P < 0.0001) was observed in uveal melanoma cells and the engineered A375/MC1R cells treated with 225Ac-DOTA-MC1RL relative to the untargeted 225Ac-DOTA-SP or phosphate-buffered saline controls (Supplemental Fig. 2). All cell lines also had a significant (P < 0.001) response to incubation with 225Ac-DOTA-SP relative to phosphate-buffered saline. However, there was no significant difference in A375 cell proliferation (extremely low MC1R) when treated with either the targeted or the untargeted radiopharmaceutical. These results demonstrate MC1R-specific cytotoxicity. Assay replicates yielded comparable results.
MTD
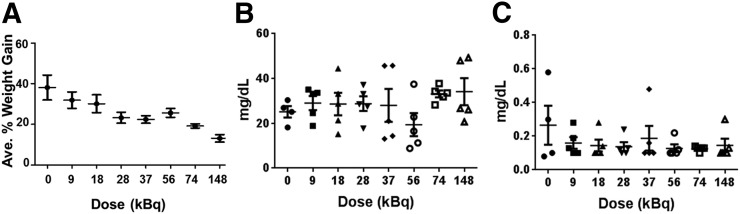
The MTD was evaluated in immune-competent non–tumor-bearing BALB/c mice (n = 5/cohort). Cohorts received a single intravenous injection of 225Ac-DOTA-MC1RL over the range of 0–148 kBq in the syringe. At completion of the study (>11 225Ac half-lives, 118 d after injection), serum and tissues (adipose, bone, cecum, colon, duoden, esophageal, heart, ileum, kidney, liver, lung, lymph nodes, muscle, pancreas, small intestine, spleen, and stomach) were collected for histology and then examined in a masked manner by a veterinary pathologist to assess radiation-induced tissue damage. No remarkable damage was observed in any of the tissues (Supplemental Figs. 13–16). For example, the control kidneys had minimal multifocal interstitial fibrosis and minimal medullary protein in tubules, which were both considered to be incidental findings. The incidental minimal medullary protein was also found in some kidneys from the groups that received treatment activities, but each treatment group also included kidneys that were within normal limits for all types of damage. The cortex of one kidney from the group with the highest administered activity had a focal extracellular cortical hyaline substance that was healing and was considered to be an incidental finding (Supplemental Fig. 14D). Blood urea nitrogen and creatinine, which are important indicators of renal function, were also determined and were not significantly elevated among the groups (Figs. 2B and 2C). All animals had gained weight by the end of the study, albeit less weight was gained by animals at the highest dose level than in the lowest (Fig. 2A).
FIGURE 2.
MTD study for non–tumor-bearing mice: percentage weight gain (A), blood urea nitrogen (B), and blood creatinine (C).
Pharmacokinetics and Biodistribution
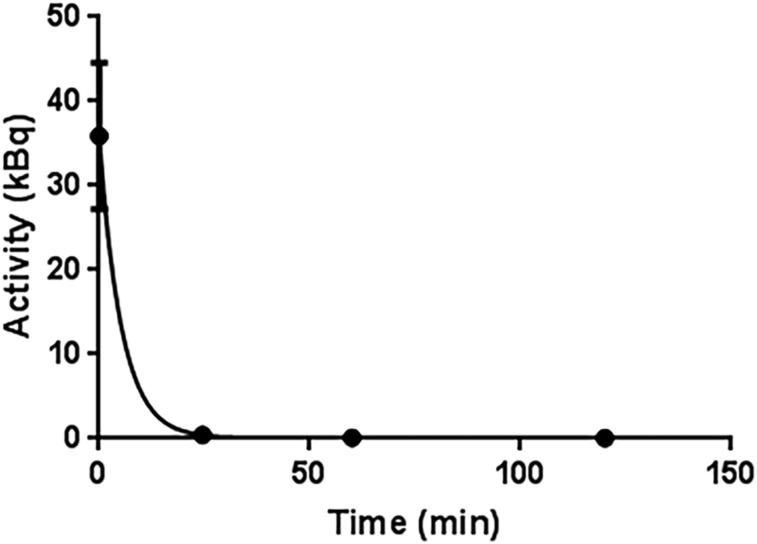
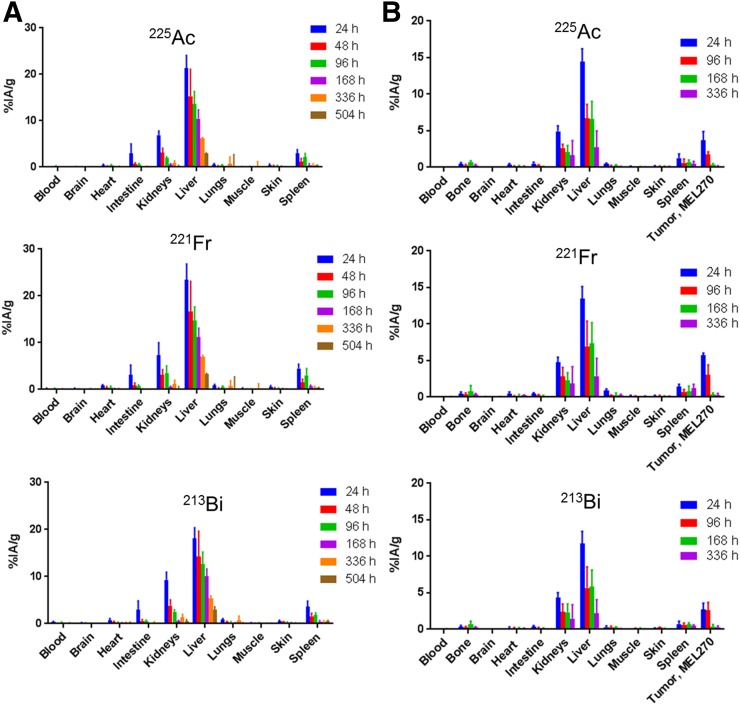
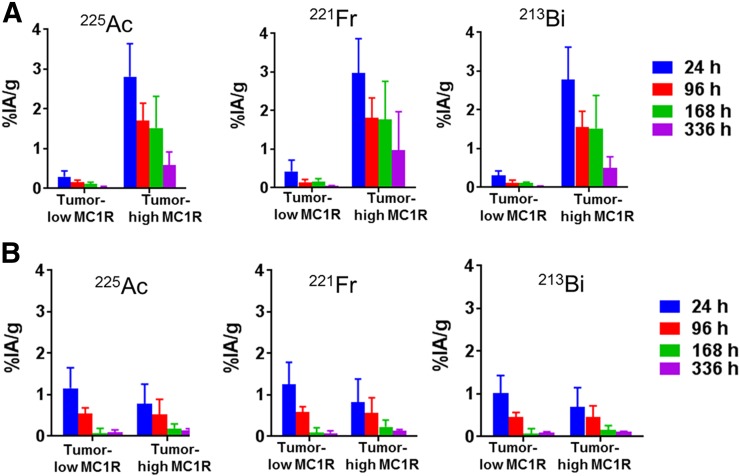
In rats, 225Ac-DOTA-MC1RL rapidly cleared (<15 min after injection) from blood circulation (Fig. 3). After administration to non–tumor-bearing BALB/c mice, 225Ac activities were observed primarily in clearance tissues. At 24 h after injection of 225Ac-DOTA-MC1RL, the liver, kidneys, spleen, and intestine had 21.2 ± 2.8, 6.9 ± 0.9, 2.9 ± 0.8, and 2.9 ± 2.0 %IA/g, whereas negligible activity was observed in the other tissues measured. Activity had largely cleared from the tissues at 1–3 wk (Fig. 4A). For tumor-bearing animals, activity was retained in MC1R-positive tumors, that is, MEL270 (Fig. 4B) and A375/MC1R tumors (Fig. 5A), which had 3.6 ± 1.2 and 2.8 ± 0.8 %IA/g, respectively, compared with the nominal 0.30 ± 0.1 %IA/g in the MC1R-negative A375 tumors at 24 h after injection. The clearance tissues in tumor-bearing animals had lower activities than in non–tumor-bearing mice: for example, 14.4 ± 1.7 %IA/g in the livers of MEL270 tumor-bearing mice at 24 h (Fig. 4B), compared with the 21.2 ± 2.8 %IA/g in the non–tumor-bearing mice (Fig. 4A). The 225Ac-DOTA-SP tumor distribution in the bilateral A357 and A375/MC1R model was also determined, and as expected, uptake was minimal and did not differ between the positive and negative A375 tumors (Fig. 5B). The distribution of 221Fr and 213Bi was also determined (Figs. 4 and 5). However, since 225Ac and daughters are at secular equilibrium by 24 h after injection and the 221Fr and 213Bi atoms present during injection will be mostly decayed, the 221Fr and 213Bi detected in the samples are from decay of the 225Ac taken into the tissues.
FIGURE 3.
Plot of rat blood clearance: exponential decay nonlinear regression line fit of 225Ac α-activity in rat blood over time, after intravenous administration of 225Ac-DOTA-MC1RL (n = 4 rats).
FIGURE 4.
Biodistribution of 225Ac-DOTA-MC1RL: 225Ac, 221Fr, and 213Bi activities in tissues from non–tumor-bearing BALB/c mice (n = 6 per time point) (A) and SCID mice bearing MEL270 human uveal melanoma tumors (n = 5 per time point) (B).
FIGURE 5.
Biodistribution of 225Ac-DOTA-MC1RL (A) and 225Ac-DOTA-SP (B) in bilateral A375 and A375/MC1R tumors (n = 5 per time point).
Radiation Dosimetry
Biodistribution data were fitted (Supplemental Fig. 17), and clearance kinetics, tissue biologic half-life, accumulated activity, and absorbed dose/injected activity (Gy/kBq) were estimated for each radionuclide in each tissue for non–tumor-bearing and MEL270 tumor-bearing mice (Tables 2 and 3). The effective decay half-lives calculated for 225Ac in tissues—for example, 7.2 d in liver—were shorter than the radiodecay half-life of 225Ac (10 d), indicating biologic clearance. The calculated total absorbed dose per injected activity (Gy/kBq) for 225Ac-DOTA-MC1RL was minimal in all tissues except clearance organs and positive tumor. Since the positive tumors shrank rapidly in response to the treatment and the total absorbed doses were extrapolated from data collected over a 2-wk period, the dose values for the tumors are likely subdued relative to the clearance organs, which did not have appreciable cellular toxicity at the administered activities. The total absorbed dose in the liver was generally lower in mice with tumors than in nontumor mice: for example, 0.284 and 0.704 Gy/kBq, respectively.
TABLE 2.
Radiation Dosimetry and Clearance Kinetics Parameters for 225Ac-DOTA-MC1RL in Non–Tumor-Bearing BALB/c Mice
| Parameter | Blood | Brain | Heart | Intestine | Kidney | Liver | Lung | Muscle | Skin | Spleen |
| 225Ac | ||||||||||
| Initial activity/organ (kBq) | ND | 0.0065 | 0.0161 | 1.9113 | 0.7647 | 7.6597 | 0.0512 | 0.0044 | 0.0483 | 0.0426 |
| Effective decay rate constant (h−1) | ND | 0.0070 | 0.0030 | 0.0060 | 0.0060 | 0.0040 | 0.0030 | 0.0030 | 0.0050 | 0.0030 |
| Effective decay half-life (d) | ND | 4.1259 | 9.6270 | 4.8135 | 4.8135 | 7.2203 | 9.6270 | 9.6270 | 5.7762 | 9.6270 |
| Accumulated activity/organ (kBq × h) | ND | 0.7621 | 3.8089 | 260.3419 | 104.1599 | 1484.6038 | 12.1242 | 1.0529 | 7.7927 | 10.0784 |
| Absorbed dose/injected activity (Gy/kBq) | ND | 0.0002 | 0.0023 | 0.0102 | 0.0300 | 0.1485 | 0.0042 | 0.0004 | 0.0024 | 0.0092 |
| 221Fr | ||||||||||
| Initial activity/organ (kBq) | 0.0153 | 0.0222 | 0.0349 | 1.9927 | 1.3795 | 8.4464 | 0.0723 | 0.0211 | 0.0705 | 0.0647 |
| Effective decay rate constant (h−1) | 0.0010 | 0.0030 | 0.0050 | 0.0070 | 0.0080 | 0.0040 | 0.0020 | 0.0030 | 0.0040 | 0.0040 |
| Effective decay half-life (d) | 28.8811 | 9.6270 | 5.7762 | 4.1259 | 3.6101 | 7.2203 | 14.4406 | 9.6270 | 7.2203 | 7.2203 |
| Accumulated activity/organ (kBq × h) | 5.6907 | 5.2622 | 5.6292 | 232.2869 | 139.2517 | 1637.0734 | 21.2642 | 4.9899 | 13.6614 | 12.5317 |
| Absorbed dose/injected activity (Gy/kBq) | 0.0022 | 0.0013 | 0.0037 | 0.0098 | 0.0434 | 0.1770 | 0.0080 | 0.0018 | 0.0045 | 0.0124 |
| 217At | ||||||||||
| Initial activity/organ (kBq) | 0.0153 | 0.0222 | 0.0349 | 1.9927 | 1.3795 | 8.4464 | 0.0723 | 0.0211 | 0.0705 | 0.0647 |
| Effective decay rate constant (h−1) | 0.0010 | 0.0030 | 0.0050 | 0.0070 | 0.0080 | 0.0040 | 0.0020 | 0.0030 | 0.0040 | 0.0040 |
| Effective decay half-life (d) | 28.8811 | 9.6270 | 5.7762 | 4.1259 | 3.6101 | 7.2203 | 14.4406 | 9.6270 | 7.2203 | 7.2203 |
| Accumulated activity/organ (kBq × h) | 5.6907 | 5.2622 | 5.6292 | 232.2869 | 139.2517 | 1637.0734 | 21.2642 | 4.9899 | 13.6614 | 12.5317 |
| Absorbed dose/injected activity (Gy/kBq) | 0.0025 | 0.0014 | 0.0042 | 0.0110 | 0.0486 | 0.1983 | 0.0090 | 0.0021 | 0.0050 | 0.0139 |
| 213Bi | ||||||||||
| Initial activity/organ (kBq) | 0.0236 | 0.0195 | 0.0309 | 1.8886 | 1.0318 | 6.5122 | 0.0717 | 0.0152 | 0.0627 | 0.0511 |
| Effective decay rate constant (h−1) | 0.0010 | 0.0010 | 0.0020 | 0.0050 | 0.0040 | 0.0040 | 0.0020 | 0.0020 | 0.0030 | 0.0030 |
| Effective decay half-life (d) | 28.8811 | 28.881 | 14.4406 | 5.7762 | 7.2203 | 7.2203 | 14.4406 | 14.4406 | 9.6270 | 9.6270 |
| Accumulated activity/organ (kBq × h) | 8.7917 | 7.2573 | 9.0914 | 304.6111 | 199.9839 | 1262.1858 | 21.0948 | 4.4603 | 14.8376 | 12.1064 |
| Absorbed dose/injected activity (Gy/kBq) | 0.0001 | 0.0000 | 0.0001 | 0.0002 | 0.0012 | 0.0025 | 0.0001 | 0.0000 | 0.0001 | 0.0002 |
| 213Po | ||||||||||
| Initial activity/organ (kBq) | 0.0236 | 0.0195 | 0.0309 | 1.8886 | 1.0318 | 6.5122 | 0.0717 | 0.0152 | 0.0627 | 0.0511 |
| Effective decay rate constant (h−1) | 0.0010 | 0.0010 | 0.0020 | 0.0050 | 0.0040 | 0.0040 | 0.0020 | 0.0020 | 0.0030 | 0.0030 |
| Effective decay half-life (d) | 28.8811 | 28.881 | 14.4406 | 5.7762 | 7.2203 | 7.2203 | 14.4406 | 14.4406 | 9.6270 | 9.6270 |
| Accumulated activity/organ (kBq × h) | 8.7917 | 7.2573 | 9.0914 | 304.6111 | 199.9839 | 1262.1858 | 21.0948 | 4.4603 | 14.8376 | 12.1064 |
| Absorbed dose/injected activity (Gy/kBq) | 0.0044 | 0.0023 | 0.0079 | 0.0168 | 0.0811 | 0.1778 | 0.0103 | 0.0021 | 0.0063 | 0.0156 |
| Total absorbed dose/injected activity (Gy/kBq) | 0.0092 | 0.0053 | 0.0183 | 0.0481 | 0.2042 | 0.7042 | 0.0317 | 0.0064 | 0.0182 | 0.0512 |
ND = not detected.
TABLE 3.
Radiation Dosimetry and Clearance Kinetics for 225Ac-DOTA-MC1RL in SCID Mice Bearing MEL270 Tumors
| Parameter | Blood | Bone | Brain | Heart | Intestine | Kidney | Liver | Lung | Muscle | Skin | Spleen | Tumor |
| 225Ac | ||||||||||||
| Initial activity/organ (kBq) | 0.0010 | 0.0172 | 0.0031 | 0.0151 | 0.3026 | 0.6417 | 4.8193 | 0.0265 | 0.0042 | 0.0242 | 0.0228 | 0.0518 |
| Effective decay rate constant (h−1) | 0.0010 | 0.0030 | 0.0020 | 0.0050 | 0.0130 | 0.0050 | 0.0060 | 0.0050 | 0.0020 | 0.0020 | 0.0040 | 0.0080 |
| Effective decay half-life (d) | 28.8811 | 9.6270 | 14.4406 | 5.7762 | 2.2216 | 5.7762 | 4.8135 | 5.7762 | 14.4406 | 14.4406 | 7.2203 | 3.6101 |
| Accumulated activity/organ (kBq × h) | 0.2726 | 3.2443 | 0.6873 | 2.1154 | 16.7417 | 89.9040 | 588.513 | 3.7089 | 0.9236 | 5.3441 | 3.6979 | 4.9055 |
| Absorbed dose/injected activity (Gy/kBq) | 0.0001 | 0.0041 | 0.0003 | 0.0020 | 0.0012 | 0.0273 | 0.0662 | 0.0025 | 0.0007 | 0.0010 | 0.0107 | 0.0045 |
| 221Fr | ||||||||||||
| Initial activity/organ (kBq) | 0.0036 | 0.0185 | 0.0059 | 0.0192 | 0.2655 | 0.6167 | 4.4828 | 0.0495 | 0.0071 | 0.0362 | 0.0257 | 0.0806 |
| Effective decay rate constant (h−1) | 0.0017 | 0.0030 | 0.0010 | 0.0030 | 0.0090 | 0.0040 | 0.0060 | 0.0040 | 0.0020 | 0.0020 | 0.0010 | 0.0070 |
| Effective decay half-life (d) | 16.9889 | 9.6270 | 28.8811 | 9.6270 | 3.2090 | 7.2203 | 4.8135 | 7.2203 | 14.4406 | 14.4406 | 28.881 | 4.1259 |
| Accumulated activity/organ (kBq × h) | 0.8370 | 3.4960 | 1.5396 | 3.6259 | 22.3311 | 99.8475 | 547.425 | 8.0162 | 1.5699 | 8.0166 | 6.7303 | 8.6407 |
| Absorbed dose/injected activity (Gy/kBq) | 0.0002 | 0.0047 | 0.0007 | 0.0037 | 0.0017 | 0.0328 | 0.0666 | 0.0058 | 0.0014 | 0.0017 | 0.0211 | 0.0086 |
| 217At | ||||||||||||
| Initial activity/organ (kBq) | 0.0036 | 0.0185 | 0.0059 | 0.0192 | 0.2655 | 0.6167 | 4.4828 | 0.0495 | 0.0071 | 0.0362 | 0.0257 | 0.0806 |
| Effective decay rate constant (h−1) | 0.0017 | 0.0030 | 0.0010 | 0.0030 | 0.0090 | 0.0040 | 0.0060 | 0.0040 | 0.0020 | 0.0020 | 0.0010 | 0.0070 |
| Effective decay half-life (d) | 16.9889 | 9.6270 | 28.8811 | 9.6270 | 3.2090 | 7.2203 | 4.8135 | 7.2203 | 14.4406 | 14.4406 | 28.881 | 4.1259 |
| Accumulated activity/organ (kBq × h) | 0.8370 | 3.4960 | 1.5396 | 3.6259 | 22.3311 | 99.8475 | 547.425 | 8.0162 | 1.5699 | 8.0166 | 6.7303 | 8.6407 |
| Absorbed dose/injected activity (Gy/kBq) | 0.0003 | 0.0053 | 0.0007 | 0.0041 | 0.0019 | 0.0367 | 0.0746 | 0.0065 | 0.0015 | 0.0019 | 0.0236 | 0.0097 |
| 213Bi | ||||||||||||
| Initial activity/organ (kBq) | 0.0016 | 0.0133 | 0.0027 | 0.0082 | 0.2160 | 0.5664 | 3.9089 | 0.0175 | 0.0017 | 0.0240 | 0.0131 | 0.0381 |
| Effective decay rate constant (h−1) | 0.0013 | 0.0020 | 0.0007 | 0.0040 | 0.0100 | 0.0040 | 0.0060 | 0.0060 | 0.0020 | 0.0050 | 0.0030 | 0.0060 |
| Effective decay half-life (d) | 22.2163 | 14.4406 | 41.2588 | 7.2203 | 2.8881 | 7.2203 | 4.8135 | 4.8135 | 14.4406 | 5.7762 | 9.6270 | 4.8135 |
| Accumulated activity/organ (kBq × h) | 0.4096 | 2.9358 | 0.7531 | 1.3342 | 16.2384 | 91.7024 | 477.346 | 2.1354 | 0.3859 | 3.3617 | 2.4736 | 4.6565 |
| Absorbed dose/injected activity (Gy/kBq) | 2.20E-06 | 7.39E-05 | 5.96E-06 | 2.51E-05 | 2.32E-05 | 5.61E-04 | 1.08E-03 | 2.88E-05 | 6.19E-06 | 1.32E-05 | 1.44E-04 | 8.68E-05 |
| 213Po | ||||||||||||
| Initial activity/organ (kBq) | 0.0016 | 0.0133 | 0.0027 | 0.0082 | 0.2160 | 0.5664 | 3.9089 | 0.0175 | 0.0017 | 0.0240 | 0.0131 | 0.0381 |
| Effective decay rate constant (h−1) | 0.0013 | 0.0020 | 0.0007 | 0.0040 | 0.0100 | 0.0040 | 0.0060 | 0.0060 | 0.0020 | 0.0050 | 0.0030 | 0.0060 |
| Effective decay half-life (d) | 22.2163 | 14.4406 | 41.2588 | 7.2203 | 2.8881 | 7.2203 | 4.8135 | 4.8135 | 14.4406 | 5.7762 | 9.6270 | 4.8135 |
| Accumulated activity/organ (kBq × h) | 0.4096 | 2.9358 | 0.7531 | 1.3342 | 16.2384 | 91.7024 | 477.346 | 2.1354 | 0.3859 | 3.3617 | 2.4736 | 4.6565 |
| Absorbed dose/injected activity (Gy/kBq) | 0.0002 | 0.0052 | 0.0004 | 0.0018 | 0.0016 | 0.0392 | 0.0756 | 0.0020 | 0.0004 | 0.0009 | 0.0101 | 0.0061 |
| Total absorbed dose/injected activity (Gy/kBq) | 0.0007 | 0.0193 | 0.0021 | 0.0115 | 0.0065 | 0.1366 | 0.2842 | 0.0168 | 0.0040 | 0.0056 | 0.0656 | 0.0290 |
Antitumor Efficacy
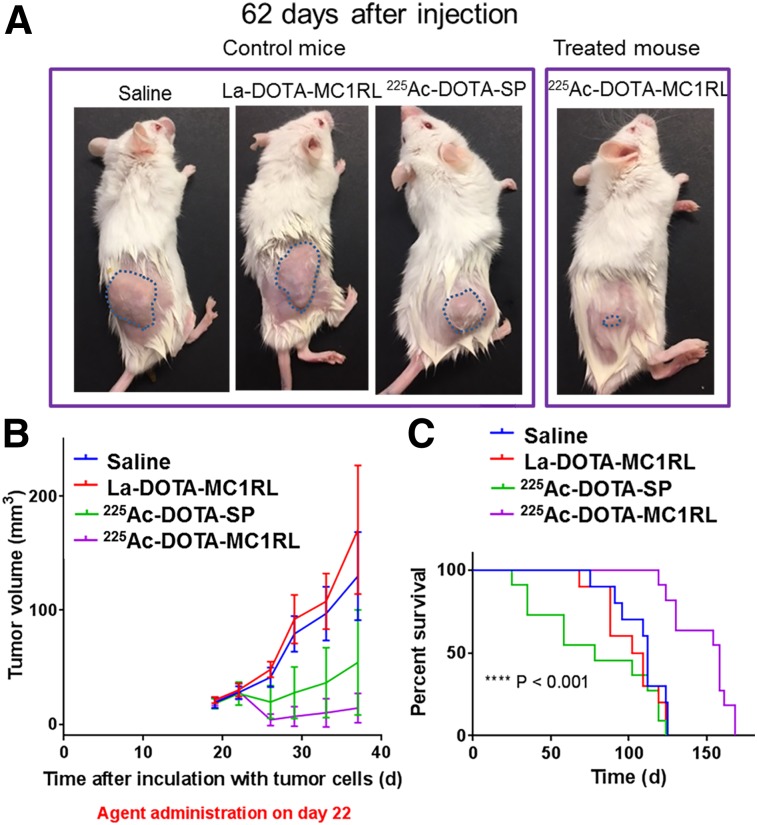
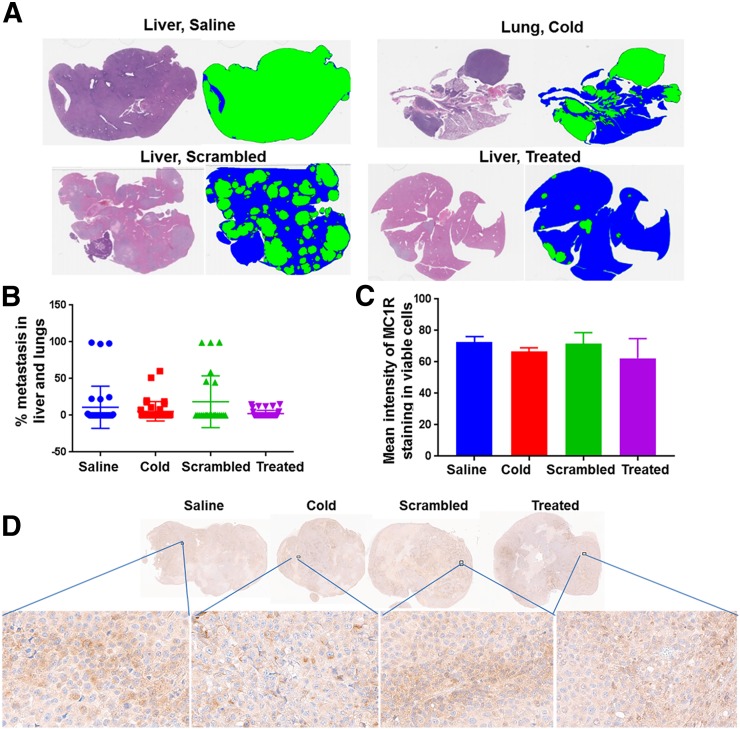
SCID mice bearing MEL270 tumors (124 ± 36 mm3 pretreatment tumor volumes) were injected with a single administration of 225Ac-DOTA-MC1RL (92.5 ± 9.3 kBq), 225Ac-DOTA-SP (99.9 ± 9.9 kBq), lanthanum-DOTA-MC1RL (1 pmol/mouse), or saline. Representative images show much smaller tumors in treated mice than in controls (Fig. 6A), and tumor volumes decreased immediately after treatment relative to controls (P = 0.001) before eventual regrowth (Fig. 6B). Treated mice had a significantly delayed time to experimental or clinical endpoint (P < 0.001), with a median survival of 148 d, compared with the median survival of control groups (79–108 d), and differences among the controls were not significant (Fig. 6C). In this study, some animals were euthanized because of reaching clinical endpoints instead of the experimental endpoint (Supplemental Table 1). Some of the animals that reached clinical endpoints had metastases in the liver or lungs, and metastasis burden was significantly lower in the 225Ac-DOTA-MC1RL treated group than in the controls (P = 0.024) (Figs. 7A and 7B). Mice bearing A375/MC1R tumors (240 ± 110 mm3 pretreatment volume) were also injected with either sterile saline, lanthanum-DOTA-MC1RL, 107.3 ± 11.1 kBq of 225Ac-DOTA-SP, or 59.2 ± 5.9 kBq of 225Ac-DOTA-MC1RL, and significant decreases in tumor volume (P = 0.005) and tumor growth delay (P < 0.0001) were observed. Some tumors that disappeared did not recur, and those mice lived their natural life span (Supplemental Figs. 18A–18C). After tumors reached an endpoint, MC1R staining was quantified, and the level of MC1R expression was not significantly different in treated tumors that responded by shrinking before regrowth relative to control tumors (P = 0.60 for MEL270 and P = 0.82 for A375/MC1R) (Figs. 7C and 7D; Supplemental Fig. 18D).
FIGURE 6.
Efficacy study in mice bearing MEL270 tumors: representative images of tumors (outlined) (A); initial tumor growth volumes (B); and Kaplan–Meier plots (C).
FIGURE 7.
Metastasis study in MEL270 uveal melanoma mouse model and MC1R expression in tumors reaching endpoints from each treatment group: representative hematoxylin and eosin staining and corresponding threshold segmentations of sections containing liver and lung metastases (cold = lanthanum-DOTA-MC1RL; scrambled = untargeted; treated = 225Ac-DOTA-MC1RL; blue = normal tissue; green = metastasis) (A); quantified metastasis burden (B); graph (C) and sections (D) for MC1R immunohistochemistry staining of MEL270 tumors after reaching endpoints.
DISCUSSION
We have developed and evaluated a novel MC1R-targeted radiopharmaceutical, 225Ac-DOTA-MC1RL, for TAT of metastatic uveal melanoma. The choice of using a peptide-targeting ligand is reinforced by the recent preclinical and clinical successes of TAT radiopeptides (14,15,32–34). Another group has also reported the development of a peptide-based TAT, 212Pb-CCMSH, that is targeted to melanocortin receptors for treatment of melanoma (35). However, 212Pb-CCMSH was associated with renal toxicity. This is likely due to the use of an α-MSH derivative–targeting ligand, as α-MSH has specificity for multiple melanocortin receptor isoforms, including MC5R, which is expressed in the human kidney and lungs (6). The MC1RL-targeting moiety used in the current work has specificity for the MC1R isoform (13), greatly reducing the potential for renal toxicity. Another advantage of 225Ac-DOTA-MC1RL over the 212Pb-TAT agent is that 225Ac has greater cell-killing potential through generation of 4 α-particle emissions per radionuclide, compared with the single α-emission of 212Pb, in their relative decay chains (35,36).
225Ac-DOTA-MC1RL has high affinity for MC1R, high radiochemistry yield and purity, high biostability, and MC1R-specific cytotoxicity in vitro. In vivo studies demonstrated low toxicity, rapid blood clearance, and uptake into MC1R-positive tumors and clearance organs. Biodistribution studies demonstrated that 225Ac remains in the compartments where 225Ac-DOTA-MC1RL was initially distributed, that is, tumors and clearance organs, and the corresponding clearance kinetics parameters and radiation dose delivered by all α-particle–emitting radioisotopes in the decay chain were calculated. Considering the 10-d half-life of 225Ac, most of the administered 225Ac-DOTA-MC1RL will have either been taken into tumor cells (7) or cleared from the blood before decay. Hence, 225Ac-DOTA-MC1RL likely functions as an in vivo α-particle generator, concentrating α-emissions in the target tumor tissues, with limited translocation of daughter isotopes (32). This is consistent with the recent observations of efficacy with low toxicity observed for an 225Ac-PSMA–targeting small-molecule conjugate (37).
In vivo efficacy studies demonstrated significant tumor and metastasis growth delay and prolonged survival in human uveal and cutaneous melanoma xenograft models in mice after a single treatment of 225Ac-DOTA-MC1RL, including some cures. Tumors that shrank and regrew after treatment had the same MC1R expression levels as controls, suggesting that multiple treatment regimens would increase efficacy.
CONCLUSION
We have developed and evaluated a novel MC1R-targeted radiopharmaceutical for TAT of metastatic uveal melanoma. In vivo studies demonstrated low toxicity, rapid blood clearance, uptake into MC1R-positive tumors and clearance organs, significant tumor and metastasis growth delay, and prolonged survival in human uveal melanoma xenograft models in mice after a single treatment of 225Ac-DOTA-MC1RL. This novel radiopharmaceutical has strong potential to benefit patients with metastatic uveal melanoma, which has had no significant improvement in treatment in the last 20 y.
DISCLOSURE
Funding was provided by a Miles for Moffitt Milestone Award (principal investigator, David Morse), the Moffitt Imaging and Technology Center of Excellence, an NIH/NCI–Moffitt Skin Cancer SPORE (P50CA168536-03) Career Enhancement Program award (principal investigator, David Morse), an NIH/NCI SBIR Phase 1 Contract to Modulation Therapeutics, Inc. (principal investigator, Narges Tafreshi), and a Melanoma Research Alliance Team Science Award (principal investigator, David Morse). This work was supported by the Analytic Microscopy, Bioinformatics and Biostatistics, Molecular Genomics, Proteomics, Small Animal Imaging Laboratory, and Tissue Core Facilities at the H. Lee Moffitt Cancer Center and Research Institute, an NCI-designated Comprehensive Cancer Center (P30-CA076292). David Morse, Thaddeus Wadas, Mark McLaughlin, HyunJoo Kil, and Narges Tafreshi are coinventors on a pending patent application. The pending patent has been licensed to Modulation Therapeutics Inc., and Mark McLaughlin is a cofounder of that company. No other potential conflict of interest relevant to this article was reported.
Supplementary Material
Acknowledgments
Animal studies were conducted in the Moffitt Barrier Vivarium that is administered by the University of South Florida Comparative Medicine Department. The 225Ac isotope used in this research was supplied by the U.S. Department of Energy Office of Science by the Isotope Program in the Office of Nuclear Physics.
REFERENCES
- 1.Chattopadhyay C, Kim DW, Gombos DS, et al. Uveal melanoma: from diagnosis to treatment and the science in between. Cancer. 2016;122:2299–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krantz BA, Dave N, Komatsubara KM, Marr BP, Carvajal RD. Uveal melanoma: epidemiology, etiology, and treatment of primary disease. Clin Ophthalmol. 2017;11:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Komatsubara KM, Carvajal RD. Immunotherapy for the treatment of uveal melanoma: current status and emerging therapies. Curr Oncol Rep. 2017;19:45. [DOI] [PubMed] [Google Scholar]
- 4.López MN, Pereda C, Ramirez M, et al. Melanocortin 1 receptor is expressed by uveal malignant melanoma and can be considered a new target for diagnosis and immunotherapy. Invest Ophthalmol Vis Sci. 2007;48:1219–1227. [DOI] [PubMed] [Google Scholar]
- 5.Yang Y. Structure, function and regulation of the melanocortin receptors. Eur J Pharmacol. 2011;660:125–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chhajlani V. Distribution of cDNA for melanocortin receptor subtypes in human tissues. Biochem Mol Biol Int. 1996;38:73–80. [PubMed] [Google Scholar]
- 7.Tafreshi NK, Silva A, Estrella VC, et al. In vivo and in silico pharmacokinetics and biodistribution of a melanocortin receptor 1 targeted agent in preclinical models of melanoma. Mol Pharm. 2013;10:3175–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ainger SA, Jagirdar K, Lee KJ, Soyer HP, Sturm RA. Skin pigmentation genetics for the clinic. Dermatology. 2017;233:1–15. [DOI] [PubMed] [Google Scholar]
- 9.Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386:74–84. [DOI] [PubMed] [Google Scholar]
- 10.Salazar-Onfray F, Lopez M, Lundqvist A, et al. Tissue distribution and differential expression of melanocortin 1 receptor, a malignant melanoma marker. Br J Cancer. 2002;87:414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tagliabue E, Fargnoli MC, Gandini S, et al. MC1R gene variants and non-melanoma skin cancer: a pooled-analysis from the M-SKIP project. Br J Cancer. 2015;113:354–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tafreshi NK, Huang X, Moberg VE, et al. Synthesis and characterization of a melanoma-targeted fluorescence imaging probe by conjugation of a melanocortin 1 receptor (MC1R) specific ligand. Bioconjug Chem. 2012;23:2451–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barkey NM, Tafreshi NK, Josan JS, et al. Development of melanoma-targeted polymer micelles by conjugation of a melanocortin 1 receptor (MC1R) specific ligand. J Med Chem. 2011;54:8078–8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgenstern A, Apostolidis C, Kratochwil C, Sathekge M, Krolicki L, Bruchertseifer F. An overview of targeted alpha therapy with 225actinium and 213bismuth. Curr Radiopharm. 2018;11:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makvandi M, Dupis E, Engle JW, et al. Alpha-emitters and targeted alpha therapy in oncology: from basic science to clinical investigations. Target Oncol. 2018;13:189–203. [DOI] [PubMed] [Google Scholar]
- 16.Baidoo KE, Yong K, Brechbiel MW. Molecular pathways: targeted alpha-particle radiation therapy. Clin Cancer Res. 2013;19:530–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deblonde GJ, Abergel RJ. Active actinium. Nat Chem. 2016;8:1084. [DOI] [PubMed] [Google Scholar]
- 18.Ma D, McDevitt MR, Finn RD, Scheinberg DA. Breakthrough of 225Ac and its radionuclide daughters from an 225Ac/213Bi generator: development of new methods, quantitative characterization, and implications for clinical use. Appl Radiat Isot. 2001;55:667–678. [DOI] [PubMed] [Google Scholar]
- 19.Josan JS, De Silva CR, Yoo B, et al. Fluorescent and lanthanide labeling for ligand screens, assays, and imaging. Methods Mol Biol. 2011;716:89–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Handl HL, Vagner J, Yamamura HI, Hruby VJ, Gillies RJ. Lanthanide-based time-resolved fluorescence of in cyto ligand-receptor interactions. Anal Biochem. 2004;330:242–250. [DOI] [PubMed] [Google Scholar]
- 21.Reid Y, Storts D, Riss T, Minor L. Authentication of human cell lines by STR DNA profiling analysis. In: Sittampalam GS, Coussens NP, Brimacombe K, et al. Assay Guidance Manual. Bethesda, MD: Eli Lily & Company and the National Center for Advancing Translational Science; 2004:1–21. [Google Scholar]
- 22.Bonardi ML, de Goeij JJM. How do we ascertain specific activities in no-carrier-added radionuclide preparations? J Radioanal Nucl Chem. 2005;263:87–92. [Google Scholar]
- 23.Pandya DN, Hantgan R, Budzevich MM, et al. Preliminary therapy evaluation of 225Ac-DOTA-c(RGDyK) demonstrates that Cerenkov radiation derived from 225Ac daughter decay can be detected by optical imaging for in vivo tumor visualization. Theranostics. 2016;6:698–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Apostolidis C, Molinet R, Rasmussen G, Morgenstern A. Production of Ac-225 from Th-229 for targeted alpha therapy. Anal Chem. 2005;77:6288–6291. [DOI] [PubMed] [Google Scholar]
- 25.Robertson AKH, Ramogida CF, Rodriguez-Rodriguez C, et al. Multi-isotope SPECT imaging of the 225Ac decay chain: feasibility studies. Phys Med Biol. 2017;62:4406–4420. [DOI] [PubMed] [Google Scholar]
- 26.Song H, Hobbs RF, Vajravelu R, et al. Radioimmunotherapy of breast cancer metastases with alpha-particle emitter 225Ac: comparing efficacy with 213Bi and 90Y. Cancer Res. 2009;69:8941–8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bolch WE, Eckerman KF, Sgouros G, Thomas SR. MIRD pamphlet no. 21: a generalized schema for radiopharmaceutical dosimetry–standardization of nomenclature. J Nucl Med. 2009;50:477–484. [DOI] [PubMed] [Google Scholar]
- 28.Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted alpha therapy of mCRPC with 225actinium-PSMA-617: dosimetry estimate and empirical dose finding. J Nucl Med. 2017;58:1624–1631. [DOI] [PubMed] [Google Scholar]
- 29.Thiele NA, Wilson JJ. Actinium-225 for targeted alpha therapy: coordination chemistry and current chelation approaches. Cancer Biother Radiopharm. 2018;33:336–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griewank KG, Yu X, Khalili J, et al. Genetic and molecular characterization of uveal melanoma cell lines. Pigment Cell Melanoma Res. 2012;25:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng Z, Xiong Z, Subbarayan M, Chen X, Gambhir SS. 64Cu-labeled alpha-melanocyte-stimulating hormone analog for microPET imaging of melanocortin 1 receptor expression. Bioconjug Chem. 2007;18:765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miederer M, Henriksen G, Alke A, et al. Preclinical evaluation of the alpha-particle generator nuclide 225Ac for somatostatin receptor radiotherapy of neuroendocrine tumors. Clin Cancer Res. 2008;14:3555–3561. [DOI] [PubMed] [Google Scholar]
- 33.Nayak TK, Norenberg JP, Anderson TL, Prossnitz ER, Stabin MG, Atcher RW. Somatostatin-receptor-targeted alpha-emitting 213Bi is therapeutically more effective than beta(−)-emitting 177Lu in human pancreatic adenocarcinoma cells. Nucl Med Biol. 2007;34:185–193. [DOI] [PubMed] [Google Scholar]
- 34.Drecoll E, Gaertner FC, Miederer M, et al. Treatment of peritoneal carcinomatosis by targeted delivery of the radio-labeled tumor homing peptide bi-DTPA-[F3]2 into the nucleus of tumor cells. PLoS One. 2009;4:e5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miao Y, Hylarides M, Fisher DR, et al. Melanoma therapy via peptide-targeted α-radiation. Clin Cancer Res. 2005;11:5616–5621. [DOI] [PubMed] [Google Scholar]
- 36.McDevitt MR, Ma D, Lai LT, et al. Tumor therapy with targeted atomic nanogenerators. Science. 2001;294:1537–1540. [DOI] [PubMed] [Google Scholar]
- 37.Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with 225Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med. 2018;59:795–802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.