Abstract
After the identification of the high-affinity glutamate-ureido scaffold, the design of several potent 18F- and 68Ga-labeled tracers has allowed spectacular progress in imaging recurrent prostate cancer by targeting the prostate-specific membrane antigen (PSMA). We evaluated a series of PSMA-targeting probes that are 18F-labeled in a single step for PET imaging of prostate cancer. Methods: We prepared 8 trifluoroborate constructs for prostate cancer imaging, to study the influence of the linker and the trifluoroborate prosthetic on pharmacokinetics and image quality. After 1-step labeling by 19F–18F isotopic exchange, the radiotracers were injected in mice bearing LNCaP xenografts, with or without blocking controls, to assess specific uptake. PET/CT images and biodistribution data were acquired at 1 h after injection and compared with 18F-DCFPyL on the same mouse strain and tumor model. Results: All tracers exhibited nanomolar affinities, were labeled in good radiochemical yields at high molar activities, and exhibited high tumor uptake in LNCaP xenografts with clearance from nontarget organs. Most derivatives with a naphthylalanine linker showed significant gastrointestinal excretion. A radiotracer incorporating this linker with a dual trifluoroborate-glutamate labeling moiety showed high tumor uptake, low background activity, and no liver or gastrointestinal track accumulation. Conclusion: PSMA-targeting probes with trifluoroborate prosthetic groups represent promising candidates for prostate cancer imaging because of facile labeling while affording high tumor uptake values and contrast ratios that are similar to those obtained with 18F-DCFPyL.
Keywords: PSMA, 18F-trifluoroborate, 18F labeling, positron emission tomography, prostate cancer
The prostate-specific membrane antigen (PSMA), a transmembrane metalloenzyme (1), is highly overexpressed in prostate cancer and tumor-associated neovasculature (2). PSMA-targeting constructs have been designed and evaluated as imaging agents for visualizing prostate cancer, most notably by PET (3–6). The diamino acid glutamate-ureido is commonly used for PSMA targeting because of synthetic ease, rapid pharmacokinetics, and high contrast ratios (7). 68Ga-PSMA-11 is currently the most commonly used radioligand for prostate cancer imaging (8,9). The short half-life of 68Ga (68 min) generally restricts distribution to clinics that are close to a 68Ge–68Ga generator, which itself limits daily production to 2–4 clinical doses unless direct production using a more complex solid-target apparatus is implemented (10). In contrast, 18F has several advantages, including a longer half-life (109.8 min); higher spatial resolution than 68Ga due to its short positron range; and on-demand, scalable production of 18F-fluoride ions up to a few hundred gigabecquerels (11).
To this effect, 18F-labeled PSMA-targeting radiotracers such as 18F-DCFPyL (12) and 18F-PSMA-1007 (13) have been introduced in clinical studies. We sought to explore a new 19F–18F isotope exchange reaction on organotrifluoroborate (RBF3−) groups to develop PSMA-targeting radiotracers. With this approach, a precursor is converted into a radiotracer of identical chemical composition. Isotope exchange labeling of RBF3− groups provides good activity yields (15%–60%) and high molar activity values (≥75 GBq/μmol) (14,15). This method has been successfully applied to several 18F-RBF3−–based radiotracers (15–23).
We report the synthesis, radiolabeling, and PET imaging of radiotracers based on the glutamate-ureido-lysine scaffold bearing RBF3− radioprosthetic groups (compounds 1–8, Fig. 1). We measured their binding affinity toward PSMA and LogD7.4 values and then acquired PET images and ex vivo biodistribution data in mice bearing PSMA-expressing LNCaP prostate cancer xenografts. These results were compared with those of 18F-DCFPyL, a clinically emergent 18F-labeled tracer for prostate cancer imaging.
FIGURE 1.
Trifluoroborate probes resembling scaffolds of DCFPhL, DCFPyL, and PSMA-617. In red: AMBF3 prosthetic; in blue: pyrBF3 prosthetic.
MATERIALS AND METHODS
Synthesis of Trifluoroborate Probes and Radiosynthesis
18F-DCFPyL was prepared following literature procedures (24). Precursors for tracers 1–8 were synthesized as described in the supplemental data section (available at http://jnm.snmjournals.org) to give azide-bearing precursors (6,18,21,24–28), which were conjugated to previously reported alkyne-bearing RBF3− (19). After conjugation, the final trifluoroborate conjugate was purified by high-performance liquid chromatography (HPLC), and purity was confirmed by electrospray ionization–mass spectrometry. Representative crude and quality control HPLC traces are provided in the supplemental data section. 18F-1–8 were labeled via previously reported procedures (15,29). Briefly, 30–40 GBq of no-carrier-added 18F-fluoride were trapped on a QMA light cartridge and eluted with 0.9% saline or phosphate-buffered saline (typically 100 μL) directly into a septum-sealed falcon tube containing 80–100 nmol of precursors 1–8 dissolved in 50:50 dimethylformamide:water containing 1 M pyridazinium-HCl buffer (pH 2.5). The reaction was heated to 80°C, and a vacuum was applied to reduce the reaction volume. After 15–20 min, the reaction was quenched by addition of 2 mL of 40 mM ammonium formate or phosphate-buffered saline, and the contents were purified by semipreparative HPLC. Radiochemical purity was confirmed by HPLC analysis using an analytic RP-C18 column with gradients of acetonitrile and water (both containing 0.1% trifluoroacetic acid). Measurements of molar activity values were based on standard curve analysis.
Cell Culture
The LNCaP cell line was obtained from ATCC (LNCaP clone FGC, CRL-1740). The cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL) at 37°C in a MCO-19AIC (Panasonic Healthcare) humidified incubator containing 5% CO2. Cells grown to 80%–90% confluence were then washed with sterile phosphate-buffered saline (1 × phosphate-buffered saline, pH 7.4) and trypsinized. The collected cell number was counted with a Bal Supply 202C laboratory counter.
In Vitro Competition Binding Assay
Inhibition constants (Ki) of 1–8 and DCFPyL to PSMA were measured by in vitro competition binding assays using 18F-DCFPyL as the radioligand. LNCaP cells were plated onto a 24-well poly-d-lysine coated plate for 48 h (400,000/well). Growth medium was removed and replaced with 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffered saline (50 mM HEPES, pH 7.5, 0.9% sodium chloride) After 1 h, 18F-DCFPyL (0.1 nM) was added to each well (in triplicate) containing varied concentrations (0.5 mM–0.05 nM) of tested compounds (DCFPyL, 1–8). Nonspecific binding was determined in the presence of 10 μM unlabeled DCFPyL. The assay mixtures were incubated for 1 h at 37°C with gentle agitation followed by 2 washes with cold HEPES buffered saline. A trypsin solution (0.25%, 400 μL) was then added to each well to harvest the cells. Radioactivity was measured by γ-counting, and Ki values were calculated using the “1 site—fit Ki” built-in model in Prism 7 (GraphPad). The dissociation constant value for 18F-DCFPyL, used for Ki determination, was 0.49 nM, as previously measured by saturation assays using LNCaP cells (30).
Distribution Coefficient (LogD7.4) Measurements
LogD7.4 values were measured using the shake flask method. Briefly, an aliquot of 18F-labeled tracer was added to a vial containing 2.5 mL of n-octanol and 2.5 mL of phosphate buffer (0.1 M, pH 7.4). The mixture was vortexed for 2 min and then centrifuged at 3,000g for 10 min. A sample of the n-octanol (0.1 mL) and buffer (0.1 mL) layers was counted using a γ-counter. Values of LogD7.4 were calculated using the following equation: LogD7.4 = log10 [(counts in n-octanol phase)/(counts in buffer phase)].
PET/CT Imaging and Biodistribution Studies
Imaging and biodistribution experiments were performed using NODSCID IL2RγKO male mice. All experiments were conducted according to the guidelines established by the Canadian Council on Animal Care and approved by the Animal Ethics Committee of the University of British Columbia. Mice were anesthetized by inhalation with 2% isoflurane in oxygen and implanted subcutaneously with 1 × 107 LNCaP cells behind the left shoulder. The mice were imaged or used in biodistribution studies once the tumor reached 5–8 mm in diameter (5–6 wk).
PET imaging experiments were conducted using an Inveon preclinical PET/CT scanner (Siemens). Compounds 18F-1,2,3,5,7, and 8 were formulated in 10% ethanol/normal saline, whereas 18F-4 and 6 were formulated in 10% ethanol/phosphate-buffered saline. Each tumor-bearing mouse was injected with 6–8 MBq of 18F-1–8 or 18F-DCFPyL through the tail vein under sedation (2% isoflurane in oxygen). For blocking controls, the mice were coinjected with DCFPyL (0.5 mg). After injection, the mice were allowed to recover and roam freely in their cage. After 50 min, the mice were sedated by 2% isoflurane inhalation and positioned in the scanner. A CT scan was performed first for localization and attenuation correction. This was followed by a 10-min static PET scan. The mice were kept warm by a heating pad during image acquisition. PET images were reconstructed using the IAW software (Siemens), using 2 iterations of the ordered-subset expectation maximization algorithm followed by 18 iterations of the maximum a posteriori algorithm.
For biodistribution and blocking studies, the mice were injected with 1–3 MBq of radiotracer. At 60 min, the mice were anesthetized with 2% isoflurane inhalation and euthanized by CO2 inhalation. Blood was withdrawn by cardiac puncture, and the organs and tissues of interest were collected, weighed, and counted using an automatic γ-counter (PerkinElmer). Uptake values were expressed as the percentage of the injected dose per gram of tissue (%ID/g).
Statistical Analysis
A standard 1-way ANOVA was performed to determine whether statistically significant differences in tumor uptake occurred between radiotracers. Each radiotracer was compared with 18F-DCFPyL using the Dunnett test (a many-to-one t test comparison). This analysis was also performed for kidney and blood activity and for tumor-to-blood and tumor-to-muscle ratios. Reported P values were adjusted for multiple comparisons. The analysis was performed using Prism 8 (GraphPad).
RESULTS
Radiolabeling
Starting with 37 GBq of 18F-fluoride, 1–8 (80–100 nmol) were successfully labeled within 25 min, with activity yields ranging from 4% to 16% (Table 1) at high molar activities (≥56 GBq/μmol). In all cases, the radiochemical purity was at least 99% by analytic HPLC. Compounds bearing the pyridine-trifluoroborate (pyrBF3) prosthetic (4, 6) were labeled in higher yields and molar activities than conjugates bearing the ammoniomethyl-trifluoroborate (AMBF3) prosthetic (3, 5). Although HPLC was used to isolate tracers at greater than or equal to 99% radiochemical purity, HPLC purification can be avoided: 18F-6 was purified on a Sep-Pak C18 cartridge according to reported procedures (29). In that case, the radiochemical purity was at least 95%. In addition, we deliberately labeled 2 at lower molar activity; starting with 37 GBq of no-carrier-added 18F-fluoride and 1 μmol of precursor; 18F-2 was obtained in 34% activity yield at 13.3 GBq/μmol (Table 2).
TABLE 1.
Activity Yield, Molar Activity, Partition Coefficient, and Binding Affinity of 18F-Labeled PSMA Radiotracers
| Tracer | Activity yield* (%, isolated) | Molar activity† (GBq/μmol) | LogD7.4 (n = 3) | Ki (nM) (n = 3) |
| 18F-DCFPyL | 12 ± 3 (n = 7) | 118 ± 37 (n = 7) | −3.12 ± 0.22 | 2.0 ± 0.8 |
| 18F-1 | 7 ± 4 (n = 4) | 70 ± 19 (n = 4) | −3.43 ± 0.35 | 14.4 ± 2.7 |
| 18F-2 | 4 ± 2 (n = 3) | 89 ± 26 (n = 3) | −4.26 ± 0.04 | 11.8 ± 0.9 |
| 18F-3 | 5 ± 1 (n = 3) | 56 ± 15 (n = 3) | −4.01 ± 0.14 | 25.9 ± 5.7 |
| 18F-4 | 16 ± 2 (n = 3) | 148 ± 89 (n = 3) | −3.34 ± 0.02 | 27.6 ± 3.8 |
| 18F-5 | 13 ± 10 (n = 2) | 137 ± 22 (n = 2) | −3.52 ± 0.21 | 1.14 ± 0.26 |
| 18F-6 | 15 ± 2 (n = 6) | 278 ± 73 (n = 6) | −2.28 ± 0.01 | 1.90 ± 0.68 |
| 18F-7 | 10 ± 5 (n = 3) | 92 ± 22 (n = 2) | −3.24 ± 0.03 | 16.5 ± 5.5 |
| 18F-8 | 7 ± 6 (n = 3) | 211 ± 48 (n = 3) | −3.58 ± 0.36 | 0.22 ± 0.01 |
Activity yields are reported at end of synthesis (1 h for DCFPyL, 40 min for 1–8) (with no correction for decay).
Molar activities are reported at time of quality control injection, shortly after end of synthesis.
TABLE 2.
Changes in Activity Yield and Molar Activity with Higher Quantities of Precursor Material
| Tracer | Activity yield (%) | Molar activity (GBq/μmol) |
| 18F-2 from 100 nmol (n = 3) | 4 ± 2 | 89 ± 26 |
| 18F-2 from 1000 nmol (n = 2) | 34 ± 9 | 13.3 ± 0.74 |
| Change | × 8.5 | ÷ 6.7 |
Binding Assays
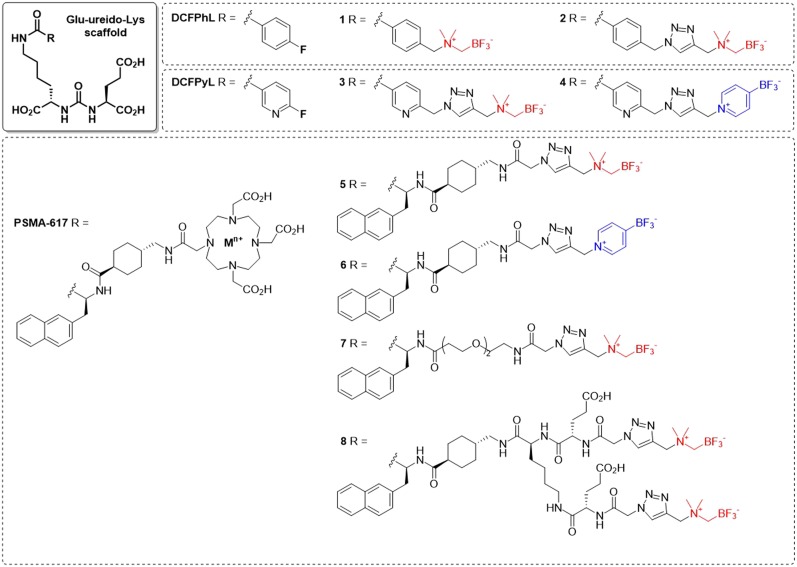
We determined Ki via in vitro competition binding assays using LNCaP cells and 18F-DCFPyL as the radioligand (Fig. 2A). The Ki value for DCFPyL was 2.0 ± 0.8 nM, consistent with the value previously reported by Chen et al. (1.1 ± 0.1 nM) (31). Probes 1–4 and 7 had Ki values in the 10–30 nM range, whereas 5 and 6 had up to 10-fold better affinities, comparable to that measured for DCFPyL. Probe 8 showed excellent binding affinity to PSMA, with a Ki value of 0.22 ± 0.01 nM (Table 1).
FIGURE 2.
(A) Competitive inhibition curves of DCFPyL and 1–8. (B) Values of LogD7.4 for DCFPyL and 1–8 (error bars reflect SD).
LogD7.4
All compounds but 6 had LogD7.4 values similar to 18F-DCFPyL (Figure 2B and Table 1). Using pyrBF3 instead of AMBF3 as the prosthetic group decreased hydrophilicity in 4 and 6 compared with 3 and 5, respectively. Compound 6 was the most lipophilic compound of the series.
PET/CT Imaging and Biodistribution
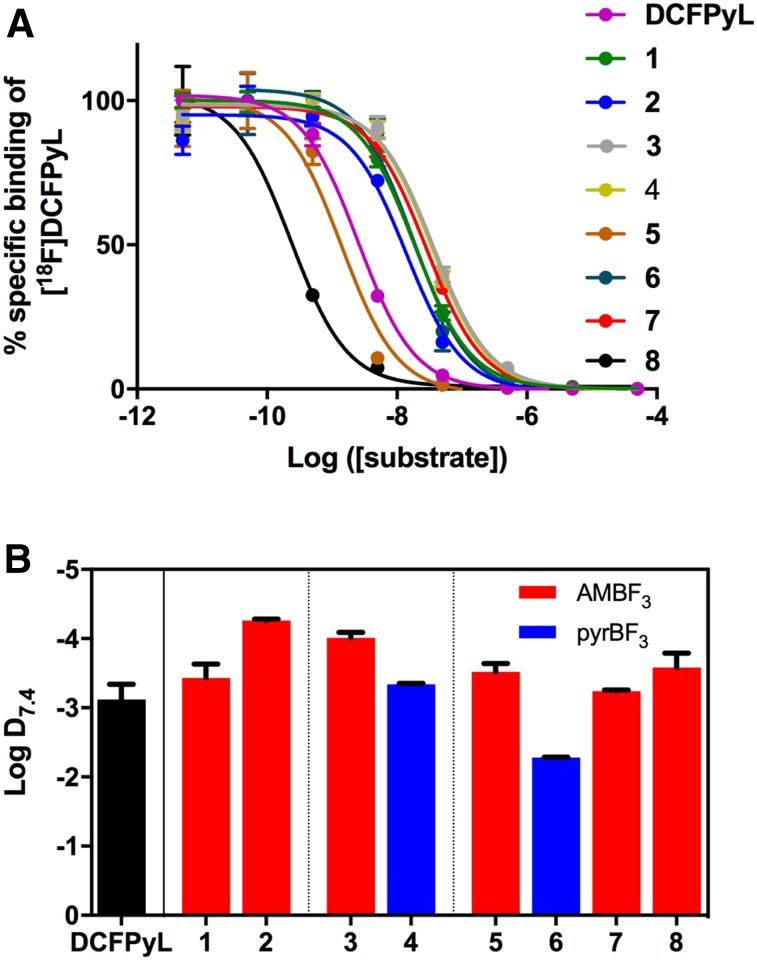
Imaging 18F-DCFPyL confirmed good tumor uptake and fast clearance (31). Similarly, 18F-1–8 showed significant tumor uptake in LNCaP xenografts, which was blocked by coinjection of unlabeled DCFPyL (Fig. 3), thus confirming the specificity of tumor uptake for PSMA. All images also showed high, specific kidney uptake along with urinary excretion. Bone accumulation was negligible for all radiotracers. The blocking agent caused significantly lower tumor and kidney uptake values for all compounds.
FIGURE 3.
PET/CT images (maximum-intensity projections) of LNCaP tumor–bearing mice at 1 h after injection, with and without blocking by coinjection of unlabeled DCFPyL. Arrows locate tumors.
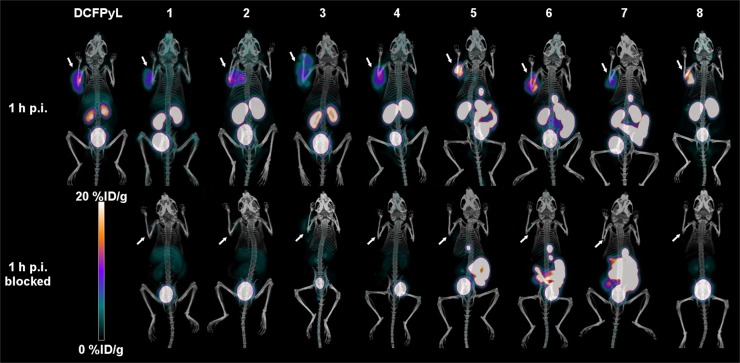
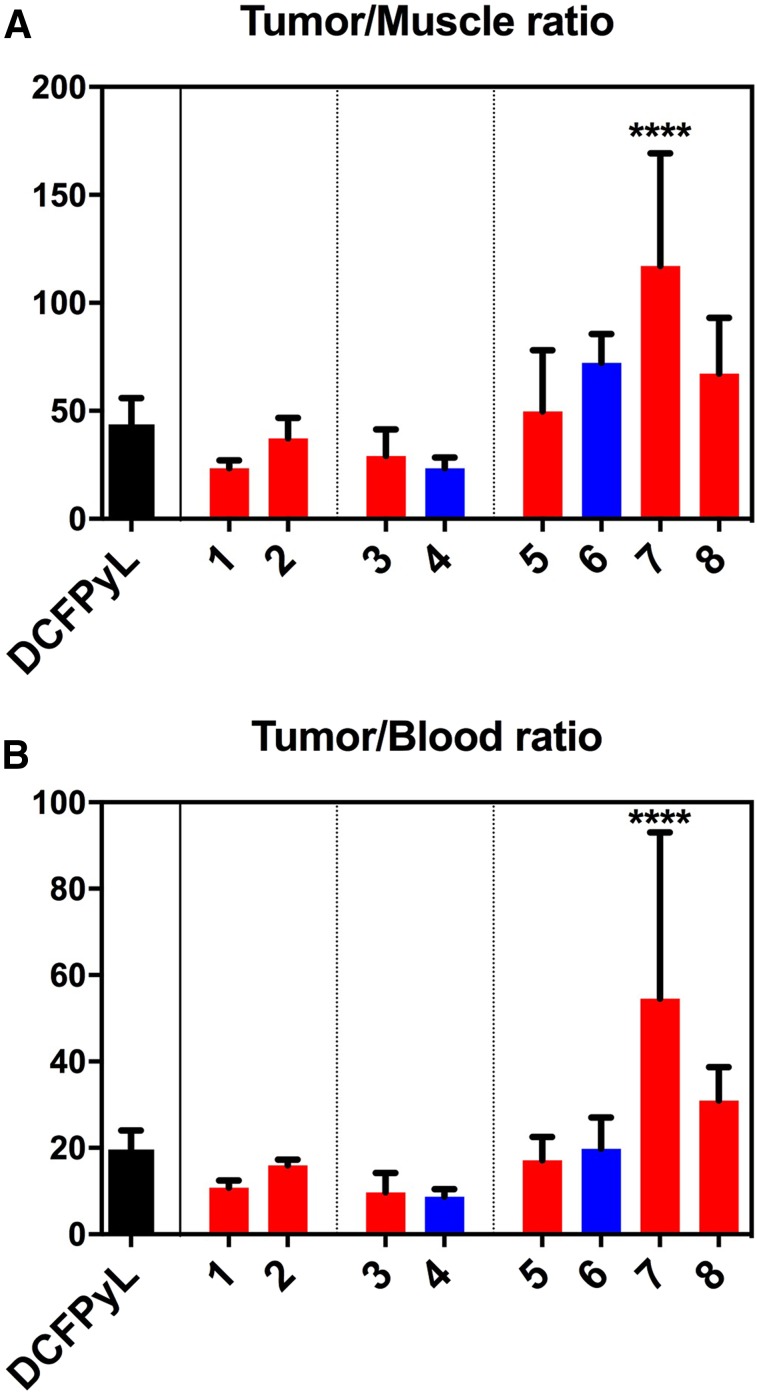
The tracers based on a naphthylalanine-tranexamic acid linker (5 and 6) displayed tumor uptake values of 13.7 ± 5.2 %ID/g and 11.9 ± 2.3 %ID/g, respectively. 18F-1, 2, 3, 4, and 7 had uptake values of 6.0 ± 1.2, 8.3 ± 1.3, 4.4 ± 0.95, 6.3 ± 0.8, and 5.1 ± 1.1 %ID/g, respectively. Compounds 18F-1, 3, 4, and 7 had lower tumor uptake than 18F-DCFPyL (Fig. 4A). Compound 8 had high tumor uptake (16.7 ± 2.7 %ID/g). No statistically significant differences were observed between compounds 2, 5, and 6 and 18F-DCFPyL, whereas compound 8 had higher uptake. Compounds 3 and 7 had lower kidney accumulation (Fig. 4B), whereas compounds 5 and 6 had significantly higher intestinal activity than 18F-DCFPyL (Fig. 4C). The blocking controls showed that intestinal uptake was not receptor-mediated. The tumor-to-blood and tumor-to-muscle ratios were not statistically different from 18F-DCFPyL for any compound except compound 7, which had higher ratios (Fig. 5). Compound 8, with 2 AMBF3-glutamate motifs, had no significant accumulation in the liver, no hepatobiliary excretion, and low background activity (Fig. 6).
FIGURE 4.
Uptake values for tumor (A), kidney (B), and intestine (C) for compounds 1–8 and DCFPyL; in black: DCFPyL; in red: AMBF3 derivatives; in blue: pyrBF3 derivatives (error bars reflect SD values, significance of differences with 18F-DCFPyL indicated at top of bars: **P < 0.01; ****P < 0.0001; ns = not significant). Full data available in Supplemental Data section.
FIGURE 5.
Contrast ratios (tumor-to-muscle in A and tumor-to-blood in B) for compounds 1–8 and DCFPyL at 1 h after injection; in black: DCFPyL; in red: AMBF3 derivatives; in blue: pyrBF3 derivatives (error bars reflect SD values, significance of differences with 18F-DCFPyL indicated at top of bars: ****P < 0.0001).
FIGURE 6.
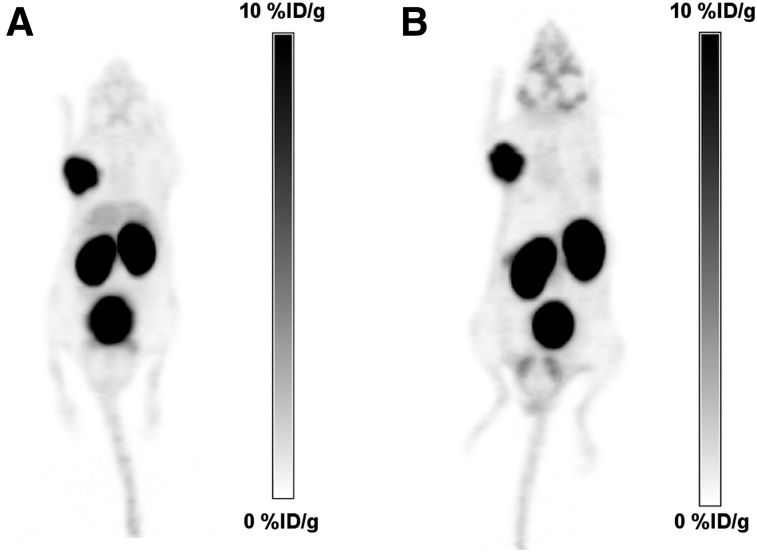
PET/CT images (maximum-intensity projections in black on white to display background activity), comparing 18F-DCFPyL (A) with 18F-8 (B), showing similar image contrast with lower liver accumulation for compound 8. Maximum of scale corresponds to 10 %ID/g for both radiotracers.
DISCUSSION
We designed PSMA-targeting radiotracers that combine the advantages of 1-step aqueous 18F-labeling afforded by 2 RBF3− radioprosthetic groups with certain chemical features found in DCFPyL (or its C-analog DCFPhL) (4) and PSMA-617 (32). Since the 3 carboxylates of Glu-ureido-Lys are needed for binding to PSMA, we introduced modifications at the lysine side chain (31,33), off of which we introduced several well-established linkers along with a suitable RBF3−.
Binding assays confirmed low-nanomolar affinities for compounds 5 and 6, whereas compound 8 had subnanomolar binding affinity. Compounds 1–4 exhibited 10-fold higher affinities than DCFPyL, suggesting that the trifluoroborate prosthetic group may not interact well with the S1 binding pocket in PSMA, which exhibits pronounced affinity for hydrophobic groups (3). Compounds incorporating a naphthylalanine-tranexamic acid motif (5 and 6) exhibited improved binding affinities (Ki = 1.14 nM and 1.90 nM, respectively) similar to those of DCFPyL (Ki = 2.0 nM) and Ga-PSMA-617 (Ki = 2.3 nM) (25). Interestingly, the tranexamic acid linker appears to contribute significantly to affinity, as its replacement by a polyethylene glycol 2 spacer (compound 7) resulted in a higher Ki. The dual glutamate-BF3 motif, introduced to improve the hydrophilicity of the BF3 derivatives with a naphthylalanine-tranexamic acid linker, unexpectedly improved the binding affinity of compound 8, with a Ki approximately an order of magnitude better than DCFPyL.
All the RBF3−-bioconjugates were radiolabeled at activity yields greater than 1.85 GBq at molar activity values of at least 56 GBq/μmol. The pyrBF3-modified conjugates showed higher activity yields than those modified with the AMBF3, along with higher molar activities, consistent with a report that compared both prosthetic groups in the context of LLP2A-RBF3− bioconjugates (19), as well as with the established stabilities of various trifluoroborates, as previously reviewed (34). High molar activities were also achieved with compound 8, with a dual glutamate-BF3 motif. Although imaging and biodistribution studies were performed with HPLC-purified tracers to ensure the highest level of purity, a simple Sep-Pak purification of 18F-6 (<5 min) afforded good radiochemical purity (95%) (supplemental data). This demonstrates potential for HPLC-free labeling where speed is preferred (overall synthesis time < 30 min).
Although radiochemical yields were lower for certain compounds, these syntheses have not been optimized. Notably, yields were dramatically improved by increasing the amount of precursor: the lowest yield (for tracer 18F-2) was increased more than 8-fold to 34% when using 10 times more precursor. Consequently, the average molar activity of 18F-2 decreased by a similar factor from 89 to 13.3 GBq/μmol. This demonstrates that yields dramatically increase when high molar activity is not critically needed.
To evaluate 18F-1–8 for PSMA imaging, PET/CT imaging and biodistribution studies were conducted in mice bearing LNCaP tumor xenografts. Previously, Chen et al. and Harada et al. imaged 18F-DCFPyL in different strains of mice bearing different tumor models (31,33), thus complicating a comparison between this work and prior work. Given these discrepancies, we directly compared 18F-1–8 with 18F-DCFPyL using a single mouse strain and the LNCaP xenograft tumor model, because it expresses PSMA endogenously and is commonly used to evaluate PSMA-targeting radiotracers (25,33).
Imaging and biodistribution studies showed that 18F-1–8 and 18F-DCFPyL were all retained in tumors and cleared from nontarget tissues and organs, mainly through the renal pathway for compounds 18F-1–4 and 8, and a combination of renal and hepatobiliary clearance for compounds 18F-5–7 (Fig. 4). Tumor uptake was higher with 18F-8 than with 18F-DCFPyL, a result that might be explained by improved affinity. All compounds showed significant renal uptake, which was blocked by DCFPyL, consistent with the well-documented, high PSMA expression in mouse kidneys (25,31,33,35–38). As with 18F-DCFPyL, images acquired with 18F-1–4 and 8 showed low uptake in nontarget organs, whereas those acquired with 18F-5–7 showed high accumulation in the gallbladder and intestines. Blocking controls showed that this intestinal uptake was not receptor-mediated. Although it is likely that intestinal uptake is due to the hydrophobic naphthylalanine moiety, this was not noted with 68Ga- or 177Lu-labeled PSMA-617 tracers (32). We presume that the DOTA chelator promotes renal clearance.
Because many radiotracers were compared with 18F-DCFPyL, this study did not have statistical power to evaluate small differences between radiotracers. The results confirmed the versatility of RBF3− prosthetic groups for 18F radiolabeling, and potential strategies to direct radiotracers to favor hepatobiliary or renal clearance.
Renal clearance can be a drawback for prostate cancer imaging, as focal retention in the ureters may be confused with small nodal metastases, and because high bladder activity may obscure the detection of primary prostate tumors or recurrences. Conversely, excessive bowel activity may also be detrimental for detection of small lesions in the pelvis and abdomen. High liver activity, as observed in clinical studies with 18F-DCFPyL (12) and 18F-PSMA-1007 (13), might impair detection of liver tumors, notably for detection of hepatocellular carcinomas, for which PSMA imaging may be of value (39).
Other 18F-labeled PSMA binding radiotracers have recently been reported, notably 18F-PSMA-1007 (13,40), among others (41–44). The RBF3− radiotracers presented in this article were not directly compared with these compounds. With an excellent binding affinity, high tumor accumulation, and no liver or gastrointestinal excretion, 18F-8 represents an attractive radiopharmaceutical for clinical translation.
CONCLUSION
We report promising alternatives to current 18F- and 68Ga-labeled PSMA-targeting agents, as the RBF3− prosthetic groups enable a facile, 1-step 18F-labeling in aqueous medium. Labeling times could be further reduced to 30 min with a simple Sep-Pak purification. The 1-step labeling by isotope exchange provided for the simple production of a precursor that is chemically identical to the radiolabeled product, simplifying aspects of both production and labeling. These radiotracers were designed to explore the influence of both the spacer and the trifluoroborate prosthetic group. Compound 8, with a naphthylalanine-tranexamic acid linker and a dual glutamate-BF3 moiety designed to enhance hydrophilicity, showed excellent binding affinity and high tumor uptake without liver accumulation or hepatobiliary clearance.
DISCLOSURE
This work depicts compounds pertaining to patent WO 2017/117687 A1, which entitles certain authors (Hsiou-Ting Kuo, Mathieu Lepage, Jinhe Pan, Zhibo Liu, Aron Roxin, François Bénard, Kuo-Shyan Lin, and David Perrin) to royalties upon licensing. This work was supported by the Michael Smith Foundation for Health Research, the Canadian Cancer Society (grant #704366), and the Canadian Institutes for Health Research (grant #FDN-148465). No other potential conflict of interest relevant to this article was reported.
Supplementary Material
Acknowledgments
We thank Nadine Colpo and Navjit Hundal-Jabal for their help with the animal studies.
REFERENCES
- 1.Carter RE, Feldman AR, Coyle JT. Prostate-specific membrane antigen is a hydrolase with substrate and pharmacologic characteristics of a neuropeptidase. Proc Natl Acad Sci USA. 1996;93:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 3.Byun Y, Mease RC, Lupold SE, Pomper MG. Recent development of diagnostic and therapeutic agents targeting glutamate carboxypeptidase II (GCPII). In: Supporan CT, Winum J-Y, eds. Drug Design of Zinc-Enzyme Inhibitors. Hoboken, NJ: John Wiley & Sons, Inc.; 2009:881–910. [Google Scholar]
- 4.Chen Y, Foss CA, Byun Y, et al. Radiohalogenated prostate-specific membrane antigen (PSMA)-based ureas as imaging agents for prostate cancer. J Med Chem. 2008;51:7933–7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maurer T, Eiber M, Schwaiger M, Gschwend JE. Current use of PSMA-PET in prostate cancer management. Nat Rev Urol. 2016;13:226–235. [DOI] [PubMed] [Google Scholar]
- 6.Maresca KP, Hillier SM, Femia FJ, et al. A series of halogenated heterodimeric inhibitors of prostate specific membrane antigen (PSMA) as radiolabeled probes for targeting prostate cancer. J Med Chem. 2009;52:347–357. [DOI] [PubMed] [Google Scholar]
- 7.Kiess AP, Banerjee SR, Mease RC, et al. Prostate-specific membrane antigen as a target for cancer imaging and therapy. Q J Nucl Med Mol Imaging. 2015;59:241–268. [PMC free article] [PubMed] [Google Scholar]
- 8.Lütje S, Heskamp S, Cornelissen AS, et al. PSMA ligands for radionuclide imaging and therapy of prostate cancer: clinical status. Theranostics. 2015;5:1388–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calais J, Fendler WP, Herrmann K, Eiber M, Ceci F. Comparison of 68Ga-PSMA-11 and 18F-fluciclovine PET/CT in a case series of 10 patients with prostate cancer recurrence. J Nucl Med. 2018;59:789–794. [DOI] [PubMed] [Google Scholar]
- 10.Lin M, Waligorski GJ, Lepera CG. Production of curie quantities of Ga-68 with a medical cyclotron via the Zn-68 (p,n)Ga-68 reaction. Appl Radiat Isot. 2018;133:1–3. [DOI] [PubMed] [Google Scholar]
- 11.Eberl S, Eriksson T, Svedberg O, et al. High beam current operation of a PETtrace (TM) cyclotron for F-18(−) production. Appl Radiat Isot. 2012;70:922–930. [DOI] [PubMed] [Google Scholar]
- 12.Szabo Z, Mena E, Rowe SP, et al. Initial evaluation of [18F]DCFPyL for prostate-specific membrane antigen (PSMA)-targeted PET imaging of prostate cancer. Mol Imaging Biol. 2015;17:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giesel FL, Hadaschik B, Cardinale J, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44:678–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Li Y, Lozada J, Lin K-S, Schaffer P, Perrin DM. Rapid, one-step, high yielding 18F-labeling of an aryltrifluoroborate bioconjugate by isotope exchange at very high specific activity. J Labelled Comp Radiopharm. 2012;14:491–497. [Google Scholar]
- 15.Liu Z, Pourghiasian M, Radtke MA, et al. An organotrifluoroborate for broadly applicable one-step F-18-labeling. Angew Chem Int Ed Engl. 2014;53:11876–11880. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Jenni S, Zhang CC, et al. Synthesis and evaluation of F-18-trifluoroborate derivatives of triphenylphosphonium for myocardial perfusion imaging. Bioorg Med Chem Lett. 2016;26:1675–1679. [DOI] [PubMed] [Google Scholar]
- 17.Lau J, Liu ZB, Lin KS, et al. Trimeric radiofluorinated sulfonamide derivatives to achieve in vivo selectivity for carbonic anhydrase IX-targeted PET imaging. J Nucl Med. 2015;56:1434–1440. [DOI] [PubMed] [Google Scholar]
- 18.Liu ZB, Pourghiasian M, Benard F, Pan JH, Lin KS, Perrin DM. Preclinical evaluation of a high-affinity F-18-trifluoroborate octreotate derivative for somatostatin receptor imaging. J Nucl Med. 2014;55:1499–1505. [DOI] [PubMed] [Google Scholar]
- 19.Roxin Á, Zhang C, Huh S, et al. Preliminary evaluation of 18F-labeled LLP2A-trifluoroborate conjugates as VLA-4 (α4β1 integrin) specific radiotracers for PET imaging of melanoma. Nucl Med Biol. 2018;61:11–20. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Zhang Z, Lin K-S, et al. Melanoma imaging using 18F-labeled alpha-melanocyte-stimulating hormone derivatives with positron emission tomography. Mol Pharm. 2018;15:2116–2122. [DOI] [PubMed] [Google Scholar]
- 21.Pourghiasian M, Liu ZB, Pan JH, et al. F-18-AmBF3-MJ9: a novel radiofluorinated bombesin derivative for prostate cancer imaging. Bioorg Med Chem. 2015;23:1500–1506. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Amouroux G, Zhang ZX, et al. F-18-trifluoroborate derivatives of Des-Arg(10) kallidin for imaging bradykinin B1 receptor expression with positron emission tomography. Mol Pharm. 2015;12:974–982. [DOI] [PubMed] [Google Scholar]
- 23.Liu Z, Radtke MA, Wong MQ, Lin K-S, Yapp DT, Perrin DM. Dual mode fluorescent 18F-PET tracers: efficient modular synthesis of rhodamine-[cRGD]2-[18F]-organotrifluoroborate, rapid, and high yielding one-step 18F-labeling at high specific activity, and correlated in vivo PET imaging and ex vivo fluorescence. Bioconjug Chem. 2014;25:1951–1962. [DOI] [PubMed] [Google Scholar]
- 24.Bouvet V, Wuest M, Jans H-S, et al. Automated synthesis of [18F]DCFPyL via direct radiofluorination and validation in preclinical prostate cancer models. EJNMMI Res. 2016;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benešová M, Schafer M, Bauder-Wust U, et al. Preclinical evaluation of a tailor-made DOTA-conjugated PSMA inhibitor with optimized linker moiety for imaging and endoradiotherapy of prostate cancer. J Nucl Med. 2015;56:914–920. [DOI] [PubMed] [Google Scholar]
- 26.Horiuchi T, Chiba J, Uoto K, Soga T. Discovery of novel thieno[2,3-d]pyrimidin-4-yl hydrazone-based inhibitors of Cyclin D1-CDK4: synthesis, biological evaluation, and structure-activity relationships. Bioorg Med Chem Lett. 2009;19:305–308. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee S, van der Donk WA. Mechanistic studies on the substrate-tolerant lanthipeptide synthetase ProcM. J Am Chem Soc. 2014;136:10450–10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Z, Fahrni CJ. A fluorogenic probe for the copper(I)-catalyzed azide-alkyne ligation reaction: modulation of the fluorescence emission via (3)(n,pi*)-(1)(pi,pi*) inversion. J Am Chem Soc. 2004;126:8862–8863. [DOI] [PubMed] [Google Scholar]
- 29.Liu Z, Lin KS, Benard F, et al. One-step F-18 labeling of biomolecules using organotrifluoroborates. Nat Protoc. 2015;10:1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuo HT, Pan J, Zhang Z, et al. Effects of linker modification on tumor-to-kidney contrast of 68Ga-labeled PSMA-targeted imaging probes. Mol Pharm. 2018;15:3502–3511. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Pullambhatla M, Foss CA, et al. 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid, [18F]DCFPyL, a PSMA-based PET imaging agent for prostate cancer. Clin Cancer Res. 2011;17:7645–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kratochwil C, Giesel FL, Eder M, et al. [177Lu]lutetium-labelled PSMA ligand-induced remission in a patient with metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2015;42:987–988. [DOI] [PubMed] [Google Scholar]
- 33.Harada N, Kimura H, Onoe S, et al. Synthesis and biologic evaluation of novel F-18-labeled probes targeting prostate-specific membrane antigen for PET of prostate cancer. J Nucl Med. 2016;57:1978–1984. [DOI] [PubMed] [Google Scholar]
- 34.Perrin DM. F-18-organotrifluoroborates as radioprosthetic groups for PET imaging: from design principles to preclinical applications. Acc Chem Res. 2016;49:1333–1343. [DOI] [PubMed] [Google Scholar]
- 35.Weineisen M, Schottelius M, Simecek J, et al. Ga-68- and Lu-177-labeled PSMA I&T: optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J Nucl Med. 2015;56:1169–1176. [DOI] [PubMed] [Google Scholar]
- 36.Gregor PD, Wolchok JD, Turaga V, et al. Induction of autoantibodies to syngeneic prostate-specific membrane antigen by xenogeneic vaccination. Int J Cancer. 2005;116:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmittgen TD, Zakrajsek BA, Hill RE, et al. Expression pattern of mouse homolog of prostate-specific membrane antigen (FOLHI) in the transgenic adenocarcinoma of the mouse prostate model. Prostate. 2003;55:308–316. [DOI] [PubMed] [Google Scholar]
- 38.Yang D, Holt GE, Velders MP, Kwon ED, Kast WM. Murine six-transmembrane epithelial antigen of the prostate, prostate stem cell antigen, and prostate-specific membrane antigen: prostate-specific cell-surface antigens highly expressed in prostate cancer of transgenic adenocarcinoma mouse prostate mice. Cancer Res. 2001;61:5857–5860. [PubMed] [Google Scholar]
- 39.Kesler M, Levine C, Hershkovitz D, et al. 68Ga-PSMA is a novel PET-CT tracer for imaging of hepatocellular carcinoma: a prospective pilot study. J Nucl Med. July 12, 2018. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40.Cardinale J, Schafer M, Benesova M, et al. Preclinical evaluation of 18F-PSMA-1007, a new prostate-specific membrane antigen ligand for prostate cancer imaging. J Nucl Med. 2017;58:425–431. [DOI] [PubMed] [Google Scholar]
- 41.Rowe SP, Gage KL, Faraj SF, et al. 18F-DCFBC PET/CT for PSMA-based detection and characterization of primary prostate cancer. J Nucl Med. 2015;56:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malik N, Baur B, Winter G, Reske SN, Beer AJ, Solbach C. Radiofluorination of PSMA-HBED via Al18F(2+) chelation and biological evaluations in vitro. Mol Imaging Biol. 2015;17:777–785. [DOI] [PubMed] [Google Scholar]
- 43.Behr SC, Aggarwal R, Van Brocklin HF, et al. First-in-human phase I study of CTT1057, a novel 18F labeled imaging agent with phosphoramidate core targeting prostate specific membrane antigen in prostate cancer. J Nucl Med. November 21, 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zlatopolskiy BD, Endepols H, Krapf P, et al. Discovery of 18F-JK-PSMA-7, a novel PET-probe for the detection of small PSMA positive lesions. J Nucl Med. November 2, 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.