Significance
It is believed that the Bcl-2 family protein Bok has a redundant role similar to Bax and Bak in regulating apoptosis. We report that this protein interacts with the key enzyme involved in uridine biosynthesis, uridine monophosphate synthetase, and positively regulates uridine biosynthesis and chemoconversion of 5-fluorouracil (5-FU). Bok-deficient cell lines are resistant to 5-FU. Bok down-regulation is a key feature of cell lines and primary colorectal tumor tissues that are resistant to 5-FU. Our data also show that through its impact on nucleotide metabolism, Bok regulates p53 level and cellular proliferation. Our results have implications for developing Bok as a biomarker for 5-FU resistance and for the development of BOK mimetics for sensitizing 5-FU-resistant cancers.
Keywords: Bok, apoptosis, UMPS, chemoresistance, metabolism
Abstract
BCL-2 family proteins regulate the mitochondrial apoptotic pathway. BOK, a multidomain BCL-2 family protein, is generally believed to be an adaptor protein similar to BAK and BAX, regulating the mitochondrial permeability transition during apoptosis. Here we report that BOK is a positive regulator of a key enzyme involved in uridine biosynthesis; namely, uridine monophosphate synthetase (UMPS). Our data suggest that BOK expression enhances UMPS activity, cell proliferation, and chemosensitivity. Genetic deletion of Bok results in chemoresistance to 5-fluorouracil (5-FU) in different cell lines and in mice. Conversely, cancer cells and primary tissues that acquire resistance to 5-FU down-regulate BOK expression. Furthermore, we also provide evidence for a role for BOK in nucleotide metabolism and cell cycle regulation. Our results have implications in developing BOK as a biomarker for 5-FU resistance and have the potential for the development of BOK-mimetics for sensitizing 5-FU-resistant cancers.
In metazoans, the intrinsic or mitochondrial apoptosis pathway is regulated by the BCL-2 family of proteins. Among the different classes of the BCL-2 family proteins, the BH3-only proteins act as the sentinels of cell death response. These proteins act either directly to promote cell death, by activating the adaptor proteins BAX and BAK at the mitochondrial surface, or indirectly by displacing the inhibitory multidomain anti-apoptotic BCL-2 family proteins from BAX and BAK, allowing the latter to oligomerize and form pores on the mitochondrial membrane, leading to apoptosis (1).
The function and regulation of most mammalian BCL-2 family proteins have been well characterized, with the exception of BOK. BOK was identified in a yeast 2-hybrid screen, using the BCL-2 family member MCL-1 as the bait (2). When ectopically overexpressed, BOK seems to act as a proapoptotic protein, and its expression seemed to be restricted to reproductive tissues such as ovaries (2). Subsequent studies have reported BOK homologs in flies and birds, confirming it as a member of the BCL-2 family protein, based on its conserved BH domains (3). BOK has been reported to have various functions other than apoptosis, such as its role in IP3R stability, as a neuroprotective factor during seizure-induced neuronal injury (4–6), and in autophagy regulation through its effect on MCL-1-BECLIN interaction in human placenta (7). However, deletion of this gene had minimal impact on apoptosis, despite it having a very broad tissue expression pattern. Double-knockout Bok−/−: Bax−/− females displayed a subtle phenotype in oocytes (8), and the triple-knockout Bok−/−: Bax−/−: Bak−/− mice had severe developmental abnormalities compared with the double-knockout mice (9). This led to the conclusion that BOK, with its structural similarity to BAX and BAK, could have overlapping/redundant functions (9).
To get an insight into the cellular function of BOK, we undertook a yeast 2-hybrid screen, using mouse BOK (mBOK) as bait to identify its interaction partners. Screening of a mouse embryonic cDNA library identified the bifunctional enzyme uridine monophosphate synthetase (UMPS) as an interacting partner. In this study, we conduct a detailed characterization of this interaction and its functional consequence. We provide substantive proof for BOK-UMPS interaction significantly increasing UMPS enzyme activity. As a result, BOK regulates uridine metabolism, cell proliferation, and chemoconversion of 5-fluorouracil (5-FU), a widely used drug used in adjuvant chemotherapy for treating various types of cancers. We also report that BOK down-regulation is a key feature in cell lines and patient-derived colorectal cancer (CRC) cell organoids grown in culture, and primary CRC tissue samples that are resistant to 5-FU.
Results
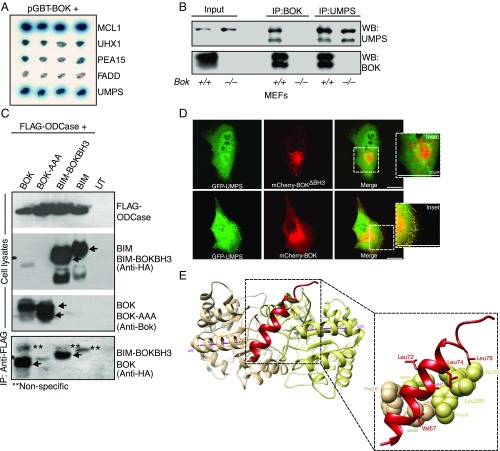
BOK Interacts with UMPS through Its BH3 Domain.
A yeast 2-hybrid screening of a mouse embryonic library yielded a cDNA clone that was identified as mouse UMPS (accession no. NM_009471.3). The specificity of the interaction was confirmed in yeast interaction with various nonspecific baits and with MCL-1 as a positive control (Fig. 1A). This interaction was further confirmed at the endogenous level by immunoprecipitation in mouse embryonic fibroblasts (MEFs). Reciprocal immunoprecipitation of BOK or UMPS confirmed the interaction, whereas Bok−/− cells did not yield any interaction confirming the antibody specificity (Fig. 1B).
Fig. 1.
BOK interacts with UMPS through its BH3 domain. (A) Yeast 2 hybrid assay showing that BOK specifically interacts with UMPS. MCL-1 was used as a positive control, whereas Uhx1, Pea 15, and FADD are used as negative controls. (B) Immunoprecipitation analysis of BOK-UMPS interaction in MEFs at physiological levels of expression. (C) The BH3 domain of BOK mediates the interaction. Mutating the BH3 domain (BOK-AAA = L72A,R73A,L74A) abolishes the interaction (lane 2), and a chimeric BIM mutant with the BOK-BH3 domain could interact with the ODCase domain, whereas BIM by itself does not. (D) Confocal microscopy of MDCK cells expressing GFP-tagged UMPS either with mCherry-tagged BOK-ΔBH3 (Top) or BOK WT (Bottom). (E) Molecular modeling of the BOK-BH3 domain docking on ODCase. Cartoon diagram of BOK BH3 docked to ODCase dimer, viewed down the 2-fold symmetry axis (Top). Protein docking predictions place BOK (red) at the dimer interface between α2b helices (Bottom) from both monomers (monomers are colored tan and khaki, respectively, with α2b helix axis in magenta).
In lower forms of eukaryotes and in prokaryotes, the final 2 steps of de novo uridine biosynthesis are catalyzed by 2 enzymes (i.e., orotate phosphoribosyltransferase [OPRTase], converting orotate to orotidine monophosphate, and orotidine decarboxylase [ODCase], which catalyses the decarboxylation of orotidine monophosphate to uridine monophosphate [UMP]). In mammals, these 2 steps are catalyzed by the bifunctional enzyme UMPS (10). We conducted a deletion experiment in HEK 293T cells and mapped the BOK interaction domain to be the ODCase domain of UMPS. The OPRTase domain did not have any role in this interaction (SI Appendix, Fig. S1 A and B). A previous study had reported that the BH3 domain of BOK was crucial for its function (11), and therefore, we tested whether the BH3 domain was important for UMPS-BOK interaction. Mutation in the conserved BH3 domain of BOK (LRL72-74 to AAA) abolished the interaction (Fig. 1C). This result was further corroborated by replacing the BH3 domain of Bim (an intrinsically unstructured protein [12]) with that of Bok. Although Bim failed to interact with ODCase, replacing the BH3 domain with that of BOK resulted in a strong interaction (Fig. 1C), suggesting the BH3 domain is sufficient in mediating the interaction between BOK and UMPS. We also have confirmed this interaction by confocal microscopy (Fig. 1D). Molecular modeling suggests that the BOK BH3 domain binds ODCase at the dimer interface (Fig. 1E). Conserved hydrophobic residues on BOK are predicted to interact with hydrophobic residues lining the surface of the ODCase dimer interface (Fig. 1E).
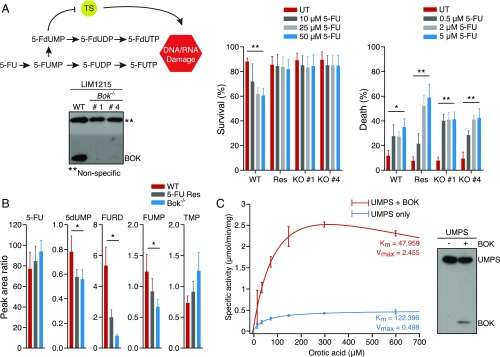
BOK Regulates UMPS Activity and Chemosensitivity.
In cancer cells, UMPS is the enzyme primarily responsible for the conversion of the chemotherapeutic drug 5-FU to its toxic metabolites (13). Therefore, if BOK interaction regulates UMPS activity, that should be manifested in the 5-FU response of Bok−/− cells. In agreement with this, Bok−/− MEFs were resistant to 5-FU compared with wild-type (WT) controls (SI Appendix, Fig. S2A). The 5-FU sensitivity in the WT MEFs could be reversed by the pan-caspase inhibitor Q-VD-OPh, suggesting that the toxicity of 5-FU was mediated by apoptosis (SI Appendix, Fig. S2A). Etoposide induced apoptosis equally in both WT and Bok−/− MEFs, suggesting there was no generalized defect in the apoptotic pathway in Bok−/− MEFs. Human colorectal cancer cell lines with CRISPR/Cas9 deletion of Bok consistently showed resistance to 5-FU (SI Appendix, Fig. S2B). Similarly, treating Bok−/− mice with 5-FU resulted in significantly reduced cell death in the colon epithelium compared with WT mice (SI Appendix, Fig. S2C).
The first step in the conversion of 5-FU to its toxic metabolites (i.e., conversion of 5-FU to 5-FUMP) is catalyzed by OPRTase (14). If the binding of BOK to the ODCase domain had any impact on the UMPS enzyme activity on the whole, it would be reflected in the relative sensitivity of WT and Bok−/− cells to one of the downstream metabolites of 5-FU. Accordingly, treating WT and Bok−/− LIM1215 CRC cells with 5-FdUMP, one of the major metabolites of 5-FU (15), resulted in similar levels of apoptotic cell death in both cell lines. Bok−/− LIM1215 cells were clearly protected against 5-FU, confirming that the conversion of 5-FU to 5-FUMP was the bottleneck in 5-FU resistance seen in Bok−/− cells (Fig. 2A). In agreement with these observations, metabolomic analysis of 5-FU metabolites in WT and Bok−/− LIM1215 cells revealed that there was a generalized reduction in 5-FU metabolites in Bok−/− cells (Fig. 2B). All these results suggested that binding of BOK to UMPS positively regulates its activity. Consistent with this supposition, we performed an in vitro UMPS assay using BOK and UMPS expressed in insect cells, and found that for equivalent amounts of UMPS, presence of BOK increased UMPS activity by 3-fold (Fig. 2C).
Fig. 2.
Conversion of 5-FU to its toxic metabolites is the bottleneck in BOK-dependent apoptosis. (A) In the schematic, conversion of 5-FU to 5-FUMP is the step mediated by UMPS. Accordingly, Bok−/− LIM1215 clones (western blot) are resistant to 5-FU (Left), whereas they are equally sensitive to the 5-FU metabolite 5-FdUMP (Right) compared with the BOK-proficient controls. Cell survival was measured 72 h posttreatment by annexin V/PI staining. (B) Metabolomic analyses of 5-FU (in the medium) and its metabolites (in the lysates) in LIM1215 cells. (C) Enzyme kinetics of UMPS in the presence and in the absence of BOK coexpression in insect cells. The Western blot shows the relative levels of protein in the lysates used in the assay. (B and C) “Res” refers to in vitro developed 5-FU-resistant cells; also see Fig. 3. Error bars ± SEM (n = 3), except in C (±SD, n = 4 for 5-FU and n = 2 for PBS control) and E (±SD, n > 10). *P ≤ 0.05; **P ≤ 0.005.
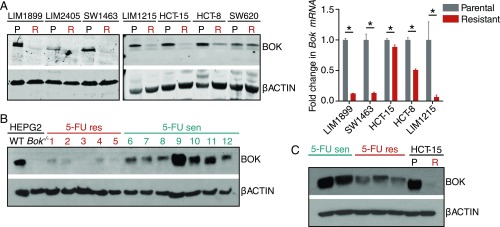
BOK Is a Marker of Chemoresistance.
Our data indicate that BOK status appears to be a major determinant of 5-FU resistance in these knockout cell lines. To get a perspective on the role of BOK in 5-FU resistance in CRCs, we generated 5-FU-resistant CRC cell lines by iterative treatment with incremental doses of 5-FU in cell culture. We generated 7 such cell lines, and intriguingly, Western blot analysis revealed that a vast majority of these cell lines had lost BOK expression (Fig. 3A). Ectopic expression of BOK restored 5-FU sensitivity in LIM1215 cells to a significant extent, suggesting that 5-FU resistance in these cells is mostly due to the lack of BOK expression (SI Appendix, Fig. S3A). These cells had cross-resistance against oxaliplatin, but were sensitive to topoisomerase I inhibitor irinotecan (SI Appendix, Fig. S3 B and C). Since etoposide and irinotecan are topoisomerase inhibitors (16, 17) and oxaliplatin induces DNA adducts (18), it is conceivable that these 5-FU-resistant cells may have additional mutations affecting DNA repair pathways differentially, independent of BOK status. Consistent with this notion, BOK−/− cells were sensitive to oxaliplatin and had a robust p53 response, determined by a p53-GFP reporter (ref. 19 and SI Appendix, Fig. S3 D and E). The reduction in BOK was also seen in 5-FU-resistant primary human colorectal tumor samples and in samples grown in organoid cultures (Fig. 3 B and C). The 5-FU-sensitive colorectal samples had varying levels of BOK, suggesting that they may be at a transitional stage of developing resistance. The reduction in BOK protein was reflected in the levels of Bok mRNA in 5-FU-resistant cells (Fig. 3A). Promoter methylation is one of the means of silencing BOK expression in nonsmall cell lung carcinoma (20); however, analyzing the BOK promoter by either bisulfite sequencing or high-resolution melting did not reveal any correlation between the promoter methylation status and 5-FU sensitivity/BOK mRNA expression levels (SI Appendix, Fig. S4 A and B), consistent with the report by Carberry et al. (21).
Fig. 3.
BOK is a marker of chemoresistance. (A) Analysis of various 5-FU sensitive (P or parental) and resistant (R) colorectal cancer cell lines (developed in vitro) for BOK protein status by Western blot (Left) and the Bok mRNA status by droplet digital PCR (Right). (B) Analysis of 5-FU-sensitive and 5-FU-resistant primary colorectal cancer tissues for BOK protein levels. WT and Bok−/− HEPG2 cells were used as the antibody control. (C) Analysis of 5-FU-sensitive and 5-FU-resistant colorectal cancer organoids for BOK protein levels. WT and Bok−/− HCT15 cells were used as the antibody control. Error bars ± SEM (n = 3), *P ≤ 0.005.
The 5-FU sensitivity in Bok−/− MEFs and in HeLa cells could be restored by the ectopic expression of WT BOK, but not with the BH3 domain mutant (LRL72-74 to AAA) form of BOK, consistent with the interaction of BOK BH3 domain with UMPS and regulation of its activity (SI Appendix, Fig. S3 F and G). Similar to 5-FU, we also observed cytosine arabinoside (cytarabine) resistance (AraC) in Bok−/− cells. Furthermore, CRISPR/Cas9-mediated deletion of UMPS in MEFs led to resistance to AraC, similar to their resistance to 5-FU (SI Appendix, Fig. S5 A–C), suggesting that AraC resistance could be the result of BOK regulation of UMPS activity. However, analyzing patient-derived, AraC-resistant acute myeloid leukemia samples showed that there was a total down-regulation of BOK in all acute myeloid leukemia samples, irrespective of their AraC resistance status (SI Appendix, Fig. S5D). The reason for this down-regulation is not known, but is consistent with reports that BOK may be acting as a tumor suppressor (20–22). A role for the BOK/UMPS axis in AraC sensitivity could be reconciled in light of the promiscuous nature of OPRTase for its substrate recognition. It could be argued that cytarabine is a surrogate substrate for OPRTase, and that the conversion of cytarabine to cytarabine phosphate is mediated by OPRTase (10, 23).
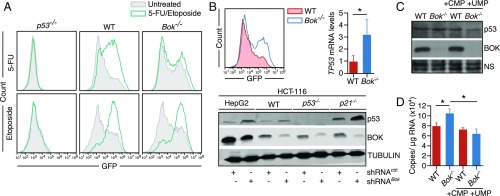
5-FU-Mediated p53 Induction and Genotoxic Stress Are BOK-Dependent.
The tumor suppressor p53 is a critical determinant of sensitivity to the 5-FU metabolite 5-FdU (24). 5-FdU is a thymidylate kinase inhibitor that leads to DNA damage and p53 activation (24). We therefore determined the p53 response to 5-FU in WT and Bok−/− MEFs, using a GFP reporter fused with p53 responsive elements from the Bbc3 (Puma) gene (19). Treating WT cells with 5-FU resulted in a robust induction of the reporter, while Bok−/− MEFs showed no induction, suggesting that 5-FU-mediated p53 induction was BOK-dependent (Fig. 4A). Control experiments showed that the p53 pathway was intact in both cell lines, as demonstrated by GFP reporter induction on treatment with etoposide (Fig. 4A). Furthermore, 5-FU-mediated DNA damage (as measured by phosphorylated gamma histone 2 or H2A.X) could be enhanced in both WT and Bok−/− HCT116 cells by the ectopic expression of WT BOK, but not with the BH3 domain mutant (SI Appendix, Fig. S6). Intriguingly, the basal level p53-GFP was significantly higher in Bok−/− MEFs (Fig. 4B), which was corroborated in Bok−/− liver tissues by mRNA analyses and in BOK-depleted cell lines by Western blot analyses (Fig. 4B). Consistent with the data that nucleotide deficiency is a known inducer of genome instability and p53 response (25), treating BOK-deficient cell lines with nucleotides reversed this phenotype including p21 induction (Fig. 4 C and D), further corroborating the role of the BOK-UMPS axis in regulating nucleotide biosynthesis.
Fig. 4.
5-FU-mediated p53 induction and genotoxic stress is BOK-dependent. (A) MEFs of various genotypes (as indicated) expressing a p53-GFP reporter were treated with either 5-FU (50 µg/mL) or etoposide (1 µg/mL), and the readout (GFP induction) was measured 24 h later by FACS analysis. (B) Basal levels of p53 were measured by the p53 reporter assay in MEFs (Left), RNA analysis in mouse liver samples (Right), and Western blot analysis (Bottom) in WT and their Bok−/− counterparts. (C) The increase in the basal level of p53 in Bok−/− HCT116 cells could be reversed by culturing the cells in UMP and CMP (1 mM). Nonspecific band (NS) is used as loading control. (D) The p53 transcriptional target p21 induction could be reversed by culturing the cells in UMP and CMP (1 mM). Error bars ± SD, n = 3 (except for D, where error bars ± SEM, n = 3). *P ≤ 0.005.
Role of BOK in Cellular Proliferation.
Reduced cell proliferation in Bok−/− cells has previously been reported (11). Defects in nucleotide metabolism could lead to a decrease in cell proliferation (26, 27); therefore, we tested whether this proliferation defect could be reversed by nucleotide supplementation. As a control cell line, we also used Umps−/− MEF cells in which the last 2 steps of the uridine biosynthetic pathway is blocked by the genetic ablation. These cells can only survive with UMP supplementation in the medium. The proliferation defect observed in Bok−/− cells could be partially reversed by the addition of UMP (SI Appendix, Fig. S7 A and B). We also tested this in the ura3 strain of Saccharomyces cerevisiae (ODCase deficient), in which the auxotrophy was complemented by mouse UMPS. Coexpression of BOK in this strain increased cell proliferation significantly compared with BIM (SI Appendix, Fig. S7C). This is consistent with the previous report that the proliferation defect in Bok−/− MEFs could be rescued by the ectopic expression of WT BOK, but not with the BH3 domain mutant of BOK (11). Finally, we compared the liver regeneration capacity of WT and Bok−/− animals (since hepatocytes express high levels of BOK [28] and hepatocytes rely on de novo synthesis of pyrimidine nucleotides [29]) after tetrachloride-induced liver injury (30). The extent of liver damage as assessed by AST/ALT ratio at day 2 after the injection was not significantly different between the WT and Bok−/− mice (SI Appendix, Fig. S8), yet liver regeneration as observed by histology was significantly impaired in Bok−/− mice, with liver sections showing significant patches of hepatocyte loss consistent with proliferation defect (SI Appendix, Fig. S8).
Discussion
Since the discovery of BCL-2 function in apoptosis (31), the role of BCL-2 family members in regulating this process is well established. Understanding the dynamics of interaction between various BCL-2 family members and its impact on the mitochondrial apoptotic pathway has led to the development of novel cancer therapeutics (32). However, in recent years, some of the family members have been reported to possess nonapoptotic/noncell death roles as well. These include regulation of mitochondrial morphology (33), regulation of ATP synthesis (34), regulation of calcium homeostasis in the endoplasmic reticulum (35), and regulation of glucose and lipid metabolism (36). Understanding the structural basis of the interaction between the proapoptotic BCL-2 family protein BAD and glucokinase led to the development of BAD mimetics that have the potential as new-generation glucokinase activators for treating type 2 diabetes (37). In the present study, we provide a very compelling argument for a role for BOK in regulating uridine metabolism and 5-FU resistance (SI Appendix, Fig. S9).
Since its discovery in 1957 by Charles Heidelberger, 5-FU has been one of the most commonly used drugs in adjuvant therapies. (It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system [38]). 5-FU is a widely used chemotherapeutic agent that inhibits cancer cell growth and initiates apoptosis by targeting thymidylate synthase, and by direct incorporation of 5-FU metabolites into DNA and RNA. 5-FU-based chemotherapy improves overall and disease-free survival of patients with colorectal, breast, and aero-digestive cancers (39). The combination of 5-FU with other anticancer drugs such as irinotecan, Tomudex, and oxaliplatin has improved response rates for advanced CRC from 40% to 50% (40). Despite these improvements, there are <12% of patients with advanced CRC who have received systemic 5-FU chemotherapy who are still alive after 2 y (41). De novo and acquired chemoresistance is the major obstacle for the success of 5-FU-based chemotherapy. Although thymidylate synthase protein overexpression is a major 5-FU resistance-inducing factor (42), high thymidylate synthase expression does not account for all nonresponding tumors in patients with CRC treated with 5-FU (41). 5-FU sensitivity is also influenced by expression levels of dihydropyrimidine dehydrogenase, the genetic status of p53, and DNA mismatch-repair genes (40). The experimental and clinical data about the predictive value of these factors are still quite controversial. In addition, the precise molecular mechanisms of 5-FU chemoresistance in patients with cancer are still largely unknown. In the present study, we provide a significant amount of data arguing for the role of BOK in regulating 5-FU resistance and how it could be affecting p53-mediated apoptosis (Fig. 4). Our findings are consistent with a previous study by Carberry et al. (21) that reported a global down-regulation of BOK protein levels in CRC tissues, most of which received 5-FU-based chemotherapy. This study also found that higher BOK levels correlate with poor prognosis, which may appear to be counterintuitive. However, considering the role of BOK in nucleotide metabolism, one would expect the tumors with low/no BOK levels to be impaired in proliferation, as previously reported (11). This is likely to put selection pressure on these tumors to restore BOK expression with additional mutations in the apoptotic pathway, particularly the p53-mediated apoptotic response (SI Appendix, Fig. S9). Therefore, understanding the structural basis of the interaction between UMPS and BOK may aid in the development of small molecule “BOK mimetics” in activating 5-FU sensitivity in cancers. Furthermore, profiling cancer tissues for BOK levels may help in developing BOK as a diagnostic marker for stratifying cancers for 5-FU sensitivity. Regulation of uridine metabolism is the most significant phenotype observed thus far in Bok−/− cells. The phenotype observed in the triple-knockout Bok−/−: Bax−/−: Bak−/− mice (9) should be seen in the context of the present findings. BOK ablation could lead to nucleotide deficiency and up-regulation of p53 and the cell cycle regulator p21, as previously reported (11).
Materials and Methods
For organoid cultures, rectal and peritoneal cancer tissues were taken from patients undergoing surgery at the Peter MacCallum Cancer Centre with patient informed consent and approval by the Peter MacCallum Cancer Centre Human Ethics committee (ethics #14/185 and #15/76, respectively). These patients were receiving Folfox (folinic acid/5-FU/oxaliplatin) adjuvant therapy. 5-FU resistance/sensitivity was tested immediately after growing the organoids in culture. The protocol for organoid culture was reported previously (43). Details of other methods, including cell culture conditions, yeast 2-hybrid screen, protein and RNA analyses, CRISPR editing, methylation analyses, apoptosis and cell proliferation assays, fluorescence microscopy, animal experiments, patient samples, p53 transcriptional assay, quantitative metabolomics, computer modeling of the structures, and statistical analyses are presented in SI Appendix, Materials and Methods.
Supplementary Material
Acknowledgments
We thank Julian Grusovin (Commonwealth Scientific and Industrial Research Organization), Dr. Grant Dewson (Walter and Eliza Hall Institute for Medical Research), and Prof. Mike Ryan (Monash University) for help and reagents and Prof. Jim Goding for reviewing the manuscript. This project was funded by La Trobe University Research Focus Area (H.P.) and the Swiss National Science Foundation (#31003A_173006 to T.K.). R.S. and Z.C. are supported by La Trobe University postgraduate scholarships. S.N. is supported by the Graduate School of Cellular and Biomedical Sciences of the University of Bern.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.W.A. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1904523116/-/DCSupplemental.
References
- 1.Doerflinger M., Glab J. A., Puthalakath H., BH3-only proteins: A 20-year stock-take. FEBS J. 282, 1006–1016 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Hsu S. Y., Kaipia A., McGee E., Lomeli M., Hsueh A. J., Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc. Natl. Acad. Sci. U.S.A. 94, 12401–12406 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H., Holzgreve W., De Geyter C., Evolutionarily conserved Bok proteins in the Bcl-2 family. FEBS Lett. 480, 311–313 (2000). [DOI] [PubMed] [Google Scholar]
- 4.D’Orsi B., et al. , Bok is not pro-apoptotic but suppresses poly ADP-ribose polymerase-dependent cell death pathways and protects against excitotoxic and seizure-induced neuronal injury. J. Neurosci. 36, 4564–4578 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulman J. J., et al. , The stability and expression level of Bok are governed by binding to inositol 1,4,5-trisphosphate receptors. J. Biol. Chem. 291, 11820–11828 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soleymanlou N., et al. , A novel Mtd splice isoform is responsible for trophoblast cell death in pre-eclampsia. Cell Death Differ. 12, 441–452 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Kalkat M., et al. , Placental autophagy regulation by the BOK-MCL1 rheostat. Autophagy 9, 2140–2153 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ke F., et al. , Consequences of the combined loss of BOK and BAK or BOK and BAX. Cell Death Dis. 4, e650 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ke F. F. S., et al. , Embryogenesis and adult life in the absence of intrinsic apoptosis effectors BAX, BAK, and BOK. Cell 173, 1217–1230.e7 (2018). [DOI] [PubMed] [Google Scholar]
- 10.McReynolds M. R., Wang W., Holleran L. M., Hanna-Rose W., Uridine monophosphate synthetase enables eukaryotic de novo NAD+ biosynthesis from quinolinic acid. J. Biol. Chem. 292, 11147–11153 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabachini T., et al. , BOK promotes chemical-induced hepatocarcinogenesis in mice. Cell Death Differ. 25, 706–718 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinds M. G., et al. , Bim, Bad and Bmf: Intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets. Cell Death Differ. 14, 128–136 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Griffith M., et al. , Novel mRNA isoforms and mutations of uridine monophosphate synthetase and 5-fluorouracil resistance in colorectal cancer. Pharmacogenomics J. 13, 148–158 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Houghton J. A., Houghton P. J., Elucidation of pathways of 5-fluorouracil metabolism in xenografts of human colorectal adenocarcinoma. Eur. J. Cancer Clin. Oncol. 19, 807–815 (1983). [DOI] [PubMed] [Google Scholar]
- 15.Fukushima M., Nomura H., Murakami Y., Shirasaka T., Aiba K., [Estimation of pathways of 5-fluorouracil anabolism in human cancer cells in vitro and in vivo]. Gan To Kagaku Ryoho 23, 721–731 (1996). [PubMed] [Google Scholar]
- 16.Kamer I., et al. , Proapoptotic BID is an ATM effector in the DNA-damage response. Cell 122, 593–603 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Lee B., et al. , A novel mechanism of irinotecan targeting MDM2 and Bcl-xL. Biochem. Biophys. Res. Commun. 514, 518–523 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Ouzon-Shubeita H., Baker M., Koag M. C., Lee S., Structural basis for the bypass of the major oxaliplatin-DNA adducts by human DNA polymerase η. Biochem. J. 476, 747–758 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jabbour A. M., et al. , Myeloid progenitor cells lacking p53 exhibit delayed up-regulation of Puma and prolonged survival after cytokine deprivation. Blood 115, 344–352 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moravcikova E., et al. , BOK displays cell death-independent tumor suppressor activity in non-small-cell lung carcinoma. Int. J. Cancer 141, 2050–2061 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carberry S., et al. , The BAX/BAK-like protein BOK is a prognostic marker in colorectal cancer. Cell Death Dis. 9, 125 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chu J., et al. , B-cell lymphoma 2 ovarian killer suppresses testicular cancer cell malignant behavior, but plays a role in platinum resistance. Anticancer Drugs 29, 839–846 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Schramm V. L., Grubmeyer C., Phosphoribosyltransferase mechanisms and roles in nucleic acid metabolism. Prog. Nucleic Acid Res. Mol. Biol. 78, 261–304 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Yan Y., Qing Y., Pink J. J., Gerson S. L., Loss of uracil DNA glycosylase selectively resensitizes p53-mutant and -deficient cells to 5-FdU. Mol. Cancer Res. 16, 212–221 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Bester A. C., et al. , Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell 145, 435–446 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chi C., et al. , Nucleotide levels regulate germline proliferation through modulating GLP-1/Notch signaling in C. elegans. Genes Dev. 30, 307–320 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernández-Justel D., et al. , A nucleotide-dependent conformational switch controls the polymerization of human IMP dehydrogenases to modulate their catalytic activity. J. Mol. Biol. 431, 956–969 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Echeverry N., et al. , Intracellular localization of the BCL-2 family member BOK and functional implications. Cell Death Differ. 20, 785–799 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le T. T., et al. , Disruption of uridine homeostasis links liver pyrimidine metabolism to lipid accumulation. J. Lipid Res. 54, 1044–1057 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhathal P. S., Rose N. R., Mackay I. R., Whittingham S., Strain differences in mice in carbon tetrachloride-induced liver injury. Br. J. Exp. Pathol. 64, 524–533 (1983). [PMC free article] [PubMed] [Google Scholar]
- 31.Vaux D. L., Cory S., Adams J. M., Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature 335, 440–442 (1988). [DOI] [PubMed] [Google Scholar]
- 32.Merino D., et al. , BH3-Mimetic drugs: Blazing the trail for new cancer medicines. Cancer Cell 34, 879–891 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Frank S., et al. , The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 1, 515–525 (2001). [DOI] [PubMed] [Google Scholar]
- 34.Alavian K. N., et al. , Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat. Cell Biol. 13, 1224–1233 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berridge M. J., Lipp P., Bootman M. D., The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Danial N. N., et al. , BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature 424, 952–956 (2003). [DOI] [PubMed] [Google Scholar]
- 37.Szlyk B., et al. , A phospho-BAD BH3 helix activates glucokinase by a mechanism distinct from that of allosteric activators. Nat. Struct. Mol. Biol. 21, 36–42 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gustavsson B., et al. , A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin. Colorectal Cancer 14, 1–10 (2015). [DOI] [PubMed] [Google Scholar]
- 39.García M. A., et al. , The chemotherapeutic drug 5-fluorouracil promotes PKR-mediated apoptosis in a p53-independent manner in colon and breast cancer cells. PLoS One 6, e23887 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Longley D. B., Harkin D. P., Johnston P. G., 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 3, 330–338 (2003). [DOI] [PubMed] [Google Scholar]
- 41.Sobrero A., et al. , New directions in the treatment of colorectal cancer: A look to the future. Eur. J. Cancer 36, 559–566 (2000). [DOI] [PubMed] [Google Scholar]
- 42.Wang W., Cassidy J., O’Brien V., Ryan K. M., Collie-Duguid E., Mechanistic and predictive profiling of 5-Fluorouracil resistance in human cancer cells. Cancer Res. 64, 8167–8176 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Ramsay R. G., Abud H. E., Exploiting induced senescence in intestinal organoids to drive enteroendocrine cell expansion. Stem Cell Investig. 4, 36 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.