Nearly all cancers are diseases of uncontrolled cell growth coupled with dramatic changes in shape and motility. In fact, pathologists have used alterations in cellular and nuclear morphology to identify cancerous tissue for over a hundred years. These fundamental cellular processes—which in cancer also include invasion, dissemination, and secondary site formation—are driven and controlled by the cell’s cytoskeletal network. Embedded in and acting on the actin cytoskeleton, is the force-generating motor protein myosin II, which exists as 3 paralogs (NMIIA, IIB, and IIC) in mammalian, nonmuscle cells. Because of the fundamental nature of these processes, a long-held belief in the cancer field is that components of the cytoskeletal network do not make good targets for anticancer therapy development, despite their altered expression and function in many types of cancer (e.g., refs. 1 and 2). However, Picariello et al. (3) demonstrate that targeting nonmuscle myosin II can be a very effective strategy but must be done in conjunction with targeting additional growth pathways.
Targeting myosin II proteins has always seemed problematic for 2 main reasons. First, the classic textbook view of myosin II has the protein conducting only one major role in the cell: to generate and drive contractility by pulling on antiparallel actin filaments. This simplified view of myosin II in nonmuscle cells puts it at the center of such processes as cell division and motility, implying that because these processes are common across many cell types in the body, targeting myosin II would be toxic to the overall organism.
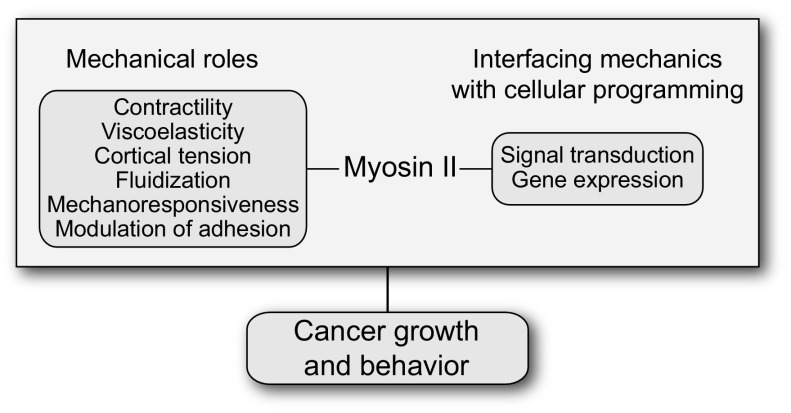
This streamlined view fails to recognize that the nonmuscle myosin IIs, encoded by the 3 paralogs, each have distinct roles in motility, adhesion, and other mechanical processes. More importantly, their contributions to cellular function are multifaceted. In addition to contractility, myosin IIs directly promote or impact cell mechanics, cortical tension and fluidity, elasticity and viscoelasticity, the modulation of cell adhesion both to substrates and to other cells, mechanosensing, and the integration of mechanical inputs with signaling pathways and gene expression (e.g., refs. 4–6) (Fig. 1). The traditionally assigned role also fails to recognize that myosin II is not strictly essential for cell division in many cell types, including a number of mammalian cells (7–9).
Fig. 1.
Myosin II sits at a key node linking the cell’s mechanical and adhesive properties to signal transduction and gene expression, thereby determining cancer cell behavior.
Second, myosin II (specifically the most abundant paralog, NMIIA) recently has been discovered to have tumor-suppressive activity in squamous cell carcinomas (10, 11). In addition to myosin II’s effect on cell growth locally, its tumor-suppressive activity may impact the metastatic potential of cancer cells where loss of myosin II increases survival in response to shear stress, similar to the stresses that would be experienced by the tumor cell in the circulatory system (12). In contrast, another recent study points to further complexity where, in a different tumor type, myosin II is essential for tumor progression (4). The differential requirements for myosin IIs in tumor progression likely depend on 2 key features. First, the type of tumor, including the cell type of origin and the genetic drivers associated with the tumor, plays a role. However, perhaps even more relevant are the specific physical demands being placed on the tumor cell by its microenvironment and neighboring cells. These mechanical demands must be counterbalanced to a significant degree by the nonmuscle myosin IIs in the cancer cell. Yet, the ability of the cell to appropriately respond to the physical challenges depends on the abundance of myosin II, the specific paralogs being expressed, and likely the ratio of those paralogs to each other.
Given these complexities, can we even target nonmuscle myosin II in cancer, and if so, how? Picariello et al. (3) begin to identify a potential strategy for myosin II targeting in glioblastoma, one of the deadliest human cancers. Previously, Rosenfeld and coworkers (13, 14) found that inhibiting myosin II blocked the invasiveness of glioblastoma cells. However, in this study (3), deleting NMIIA in the context of a pten-deletion glioblastoma mouse model blocked tumor invasiveness just as expected, but paradoxically with a decrease in mouse survival. Upon further scrutiny, the tumors did not display disrupted growth as the simple model for myosin II contractility might have predicted. Instead, the tumors grew faster and with no increase in chromosomal instability—i.e., no increased failure of cell division. On the other hand, double deletion of NMIIA and NMIIB did have the predicted reduction in tumor growth due to cytokinetic failure.
If NMIIA deletion is not impacting genomic instability, how can its impact on increasing tumor size be explained? Picariello et al. discovered that the loss of NMIIA on soft substrates led to activation of a YAP–RHAMM–ERK pathway, a progrowth signaling pathway, while on stiff substrates, NFκB and downstream stem cell factors (Sox2 and Nanog) become activated. The activation of these pathways was especially sensitive to changes in the mechanical landscape. This is consistent with the notion that the mechanoresponsiveness of myosin IIs allows the cells to respond to and integrate substrate stiffness with growth pathways (ERK pathway on soft substrates, NFκB on stiff substrates). When the authors inhibited the growth pathway in combination with inhibiting NMIIA, tumor progression was significantly inhibited and mouse survival significantly improved. Thus, hitting both growth and myosin II pathways simultaneously may ultimately prove to be a powerful antiglioblastoma treatment strategy and, as the authors demonstrate, appears to apply to breast cancer cells too.
Targeting multiple pathways at once has been a nearly universal paradigm for aggressive disease treatment for a long time. HIV was perhaps the trendsetter in this regard, finally reaping the full benefit with the development of Gilead’s Atripla, the first single pill to include 3 drugs, each targeting distinct pathways essential for HIV replication. Cancer therapies also routinely include multiple strategies. For diseases as complex and deadly as glioblastoma, a multiple pathway targeting strategy is no doubt essential for successfully managing, and hopefully eventually eliminating, this and other aggressive cancers.
Footnotes
The authors declare no conflict of interest.
See companion article on page 15550.
References
- 1.Cross S. E., Jin Y. S., Rao J., Gimzewski J. K., Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol. 2, 780–783 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Surcel A., et al. , Harnessing the adaptive potential of mechanoresponsive proteins to overwhelm pancreatic cancer dissemination and invasion. bioRxiv, 10.1101/190553 (2017). [DOI] [Google Scholar]
- 3.Picariello H. S., et al. , Myosin IIA suppresses glioblastoma development in a mechanically sensitive manner. Proc. Natl. Acad. Sci. U.S.A. 116, 15550–15559 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halder D., et al. , Nonmuscle myosin IIA and IIB differentially modulate migration and alter gene expression in primary mouse tumorigenic cells. Mol. Biol. Cell 30, 1463–1476 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai H., et al. , Yes-associated protein impacts adherens junction assembly through regulating actin cytoskeleton organization. Am. J. Physiol. Gastrointest. Liver Physiol. 311, G396–G411 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiffhauer E. S., Robinson D. N., Mechanochemical signaling directs cell-shape change. Biophys. J. 112, 207–214 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Lozanne A., Spudich J. A., Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science 236, 1086–1091 (1987). [DOI] [PubMed] [Google Scholar]
- 8.Kanada M., Nagasaki A., Uyeda T. Q., Adhesion-dependent and contractile ring-independent equatorial furrowing during cytokinesis in mammalian cells. Mol. Biol. Cell 16, 3865–3872 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poirier C. C., Ng W. P., Robinson D. N., Iglesias P. A., Deconvolution of the cellular force-generating subsystems that govern cytokinesis furrow ingression. PLOS Comput. Biol. 8, e1002467 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schramek D., et al. , Direct in vivo RNAi screen unveils myosin IIa as a tumor suppressor of squamous cell carcinomas. Science 343, 309–313 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conti M. A., et al. , Conditional deletion of nonmuscle myosin II-A in mouse tongue epithelium results in squamous cell carcinoma. Sci. Rep. 5, 14068 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xin Y., et al. , Mechanics and actomyosin-dependent survival/chemoresistance of suspended tumor cells in shear flow. Biophys. J. 116, 1803–1814 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beadle C., et al. , The role of myosin II in glioma invasion of the brain. Mol. Biol. Cell 19, 3357–3368 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivkovic S., et al. , Direct inhibition of myosin II effectively blocks glioma invasion in the presence of multiple motogens. Mol. Biol. Cell 23, 533–542 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]