Significance
The beneficial interactions between ant-acacias and ants is a textbook example of animal–plant mutualism. In exchange for protection, ant-acacias produce specialized traits (“swollen thorn syndrome”) that provide ants with food and shelter. Although this syndrome is important for plant survival, it does not develop until several weeks after germination. The basis for this apparent paradox is unknown. We show that the appearance of the swollen thorn syndrome is correlated with a change in the expression of genes in the miR156/miR157-SPL vegetative-phase change pathway under a variety of environmental conditions. These results suggest a molecular mechanism for the development of the swollen thorn syndrome and shed light on why syndrome development is age dependent.
Keywords: ant-acacia, age-dependent defenses, vegetative-phase change, miR156, SPL
Abstract
Age-dependent changes in plant defense against herbivores are widespread, but why these changes exist remains a mystery. We explored this question by examining a suite of traits required for the interaction between swollen thorn acacias (genus Vachellia) and ants of the genus Pseudomyrmex. In this system, plants provide ants with refuge and food in the form of swollen stipular spines, protein-lipid–rich “Beltian” bodies, and sugar-secreting extrafloral nectaries—the “swollen thorn syndrome.” We show that this syndrome develops at a predictable time in shoot development and is tightly associated with the temporal decline in the microRNAs miR156 and miR157 and a corresponding increase in their targets—the SPL transcription factors. Growth under reduced light intensity delays both the decline in miR156/157 and the development of the swollen thorn syndrome, supporting the conclusion that these traits are controlled by the miR156-SPL pathway. Production of extrafloral nectaries by Vachellia sp. that do not house ants is also correlated with a decline in miR156/157, suggesting that this syndrome evolved by co-opting a preexisting age-dependent program. Along with genetic evidence from other model systems, these findings support the hypothesis that the age-dependent development of the swollen thorn syndrome is a consequence of genetic regulation rather than a passive developmental pattern arising from developmental constraints on when these traits can develop.
Plants exhibit a wide diversity of morphological, chemical, and behavioral defenses against herbivory. In most plants, these defenses change, appear, or disappear at discrete times in an organism’s life cycle (1–4). Although changes in defense are often thought to be evolved responses to shifts in selection pressure during a plant’s ontogeny, it is possible that these patterns are instead a consequence of developmental constraints on when the traits can develop (5). Evaluating these alternatives is difficult without an understanding of the mechanism controlling the trait of interest.
One hypothesis for age-dependent patterns of defense is that resource limitations lead to trade-offs between growth and defense (1, 6). As plants age, these trade-offs are predicted to shift due to changes in stored reserves, root–shoot ratios, or the development of reproductive structures. This hypothesis predicts that plants produce defenses at a time in development when the cost of allocating resources to these defenses does not prevent the production of other selectively advantageous traits. Implicit in this hypothesis is the assumption that plants could develop the defensive trait at a different time in development if favored by selection.
An alternative possibility is that age dependency arises from factors that constrain the development of defensive traits regardless of selective forces. Such limitations are known as developmental constraints and can arise from multiple sources (7–9). One possibility is that the size of the shoot or root system might limit the types of defenses that can develop at different times in a plant’s growth (5, 10). For example, the stem-derived domatia of many obligate ant-plants develop only once the stem is of a sufficient diameter (11). In this case, the defense trait is “regulated” passively by factors inherent to shoot development, and selection for variation in the timing of trait development would be limited by shoot growth. Another possibility is that the age-dependent development of defense traits is passively regulated as a consequence of constraints imposed by underlying cellular or molecular mechanisms. For example, the molecular mechanism of polar auxin transport determines where leaves are initiated on the shoot apical meristem and is thus responsible for the stable and highly stereotypical patterns of leaf arrangement observed in higher plants (known as phyllotaxy) (12). Phyllotactic patterns that might be selectively advantageous could fail to evolve because they cannot be accommodated by this molecular mechanism (9). Additionally, if defensive traits evolved by co-option of a preexisting genetic regulatory pathway, their age dependence may be a consequence of “inherited” pleiotropic constraints (13–15). Therefore, pathways that control such age-dependent transitions are a prime candidate for investigating the developmental mechanisms of plant defenses (5).
One such pathway—the vegetative-phase change pathway—controls the transition between juvenile and adult phases of shoot development (5, 16). This network of the temporally regulated microRNAs miR156 and miR157 and their transcription factor targets, members of the Squamosa Promoter-binding Protein Like (SPL) gene family, is responsible for age-dependent changes in multiple vegetative traits (17, 18). miR156/157-regulated SPL genes promote adult vegetative traits. Although SPL genes are transcribed in all leaves, high levels of miR156/157 in the first few leaves prevent their translation. As miR156 and miR157 decline in successive leaves, the abundance of SPL proteins increases (17–21). The fact that SPL genes are capable of acting at the earliest stages of shoot development (17, 22–24) means that the traits controlled by these genes could be produced earlier in development simply by a reduction or loss of miR156 and miR157 expression.
We examined the role of this regulatory pathway in a classic model system for the study of the evolution of indirect defenses and symbiotic relationships—the mutualism between Acacia plants (genus Vachellia) and ants of the genus Pseudomyrmex (25, 26). In this system, the plants provide food via nectar secretion from nectaries and food bodies on leaves and shelter in the form of hollow stipular spines at the base of a leaf (the “swollen thorn syndrome”). In exchange for these services, ants protect plants from competitors, herbivores, and pathogens (27, 28).
Fifty years ago, Janzen (29) reported that the swollen thorn syndrome is absent early in the development of Vachellia cornigera. However, the generality of this phenomenon and its molecular basis has never been investigated. Here, we show that the swollen thorn syndrome is temporally regulated in multiple species of Vachellia and is correlated with the expression of the miR156-SPL pathway, providing a possible regulatory link. This finding in combination with experimental manipulation of this pathway in other systems supports the idea that age-dependent development is a consequence of developmental constraints from the underlying genetic program and of not passive biophysical or cellular constraints on development.
Results
Development of the Swollen Thorn Syndrome Is Age Dependent.
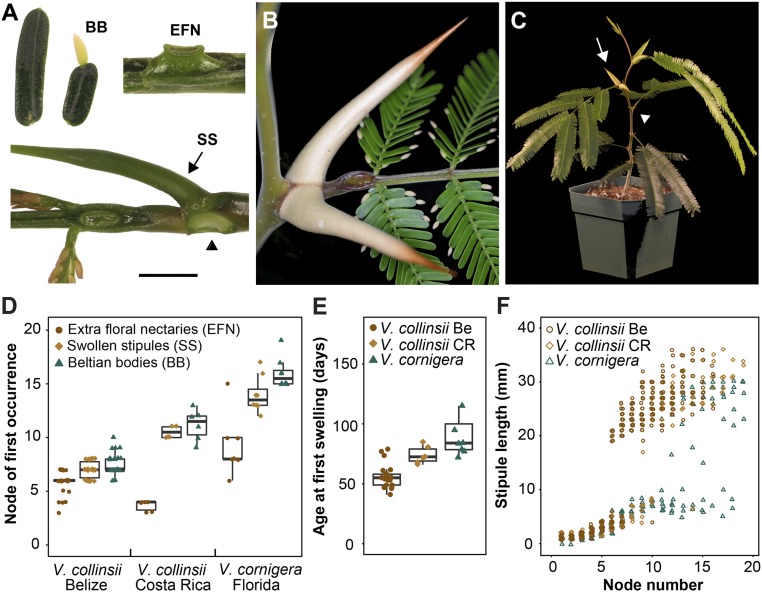
Previous authors have noted that ant-acacias from Central America do not produce “Beltian” bodies (BB), enlarged extrafloral nectaries (EFN), and swollen stipular spines (SS) immediately after germination (Fig. 1 A–C) (29, 30). However, the production of these structures under carefully controlled conditions has never been characterized. Consistent with Janzen’s observations of V. cornigera in the wild, we found that both V. cornigera and V. collinsii plants grown from seed in either a greenhouse or growth chamber began producing these traits between nodes 5 and 15 and did so in sequence (Fig. 1). Extrafloral nectaries appeared first, followed by the swelling and elongation of stipules, and then by Beltian bodies (Fig. 1D). The timing and position of this transition differed between species and accessions, with V. collinsii Belize producing swollen stipules first, followed by V. collinsii Costa Rica and then V. cornigera Florida (Fig. 1 D and E). The development of enlarged stipules was particularly striking in V. collinsii, where it occurred within one node and involved a greater than 5-fold increase in stipule length (Fig. 1 C and F). Both of the V. collinsii accessions that we examined continued to produce swollen stipules at subsequent nodes. However, in V. cornigera, intermediate-sized stipules were occasionally produced during the transition period (Fig. 1F), and many individuals alternated between producing swollen and unswollen stipules as they developed.
Fig. 1.
Ant-acacias have an early period in their life cycle without traits necessary for mutualistic interactions. (A) The traits comprising the swollen thorn syndrome. BB: leaflets with and without a Beltian body growing from their tip. EFN: enlarged extrafloral nectary from leaf 8 of V. collinsii Belize. SS: late-stage swollen stipule primordia (arrow) showing relative position on leaf (arrowhead marks site of the second removed stipule) (Scale bar, 1 cm.) (B) Relative position of mature syndrome traits in V. collinsii Costa Rica. (C) A 2-mo-old V. collinsii Belize seedling showing the early transition from unswollen (arrowhead) to swollen (arrow) stipular spines. (D) Node of first occurrence for each syndrome trait. Boxes outline first and third quartile; center line marks the median. (E) Age of the plant when the leaf primordium bearing the first swollen stipules was between 3 and 5 mm in length. Boxes same as in D. (F) Stipule length as a function of node number.
The Appearance of the Swollen Thorn Syndrome Is Correlated with a Decline in miR156/157.
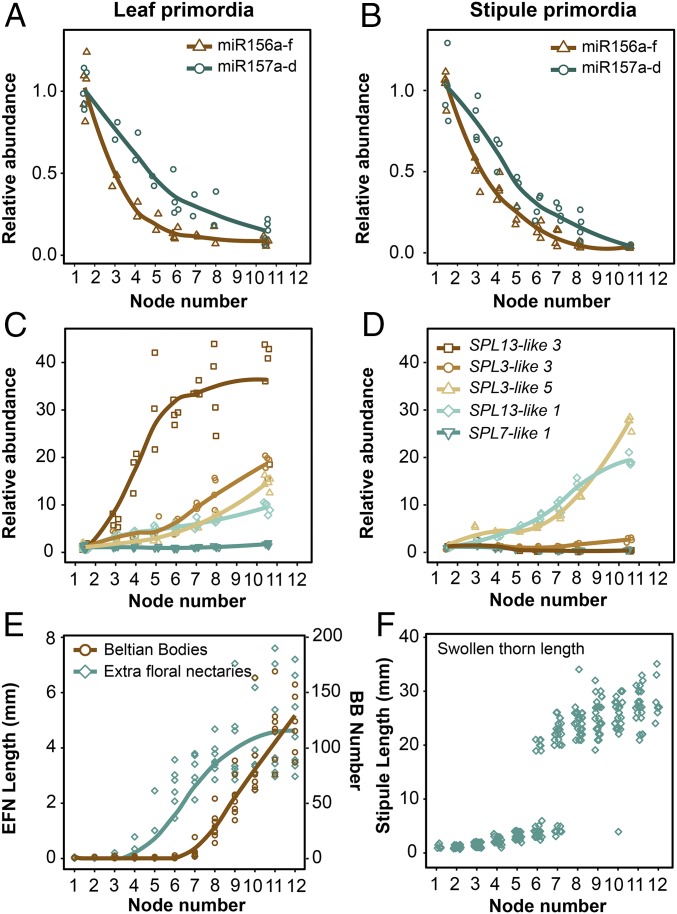
To determine if the development of BB, EFN, and SS might be regulated by the miR156/157 pathway, we used RT-qPCR to measure the abundance of miR156 and miR157 in 1- to 3-mm leaf and stipule primordia from V. collinsii Belize. We found that miR156 levels dropped over 8-fold in leaves and over 19-fold in stipules between nodes 1 and node 8 and remained relatively constant after this point (Fig. 2 A and B). Over the same period, miR157 dropped 3.5- and 6-fold in leaf and stipules, respectively, and continued to decline in later nodes (Fig. 2 A and B). In leaves, the gradual decline in miR156/157 was correlated with a gradual increase in the length of EFN and an increase in the number of BB (Fig. 2E). Stipule swelling occurred at the nodes with the lowest level of miR156 and miR157 (nodes 6 through 8) (Fig. 2F). Conversely, levels of miR156 did not decline, and miR157 dropped by only 2-fold in fully expanded leaves across these same nodes (SI Appendix, Fig. S1 A and B). Thus, miR156/157 display only a major change in gene expression at the developmental stage when the morphological fate of the leaf is being specified.
Fig. 2.
Variation in the abundance of miR156/miR157 and SPL transcripts is correlated with the appearance of the swollen thorn syndrome in V. collinsii Belize. (A, C, and E) Leaf primordia. (B, D, and F) Stipule primordia. Node number represents the position relative to the base of the shoot. (A and B) Relative abundance of the mature miR156a-f and miR157a-d small RNAs in leaf and stipule primordia. Curves represent conditional means using a Loess smoother. (C and D) Relative abundance of miR156/157-targeted (SPL3-like 3, SPL3-like 5, SPL13-like 1, and SPL13-like 3) and untargeted (SPL7-like 1) transcripts in leaf and stipule primordia. Plotting as in A and B. (E and F) The length of EFN and the number of BB on leaves at successive nodes. Plotting as in A and B.
To characterize the molecular basis of this phenomenon in more detail, it was necessary to identify the genes that encode miR156, miR157, and SPL transcription factors in V. collinsii. For this purpose, we sequenced a 450-bp insert genomic library of V. collinsii Belize using Illumina 250PE format (SI Appendix, Supplementary Materials and Methods). This generated 50× of overlapping reads, which were assembled using MaSuRCA (31). The resulting assembly covered 89% of the 518-Mb genome in 122,266 contigs with an NG50 of 6,528 bp (SI Appendix, Table S1). Annotation of this assembly revealed 8 putative MIR156 genes, 5 putative MIR157 genes, and 23 putative SPL genes (Dataset S1).
PCR primers were designed to a unique sequence within the predicted coding region of each SPL gene and to the predicted hairpin region of MIR156 and MIR157 genes. These primers were then used to measure the abundance of these transcripts in leaf and stipule primordia at nodes 1 through 2 and 9 through 12, using semiquantitative RT-PCR (SI Appendix, Supplementary Materials and Methods and Fig. S2 A and B). Twelve of the 13 MIR156/MIR157 genes that we analyzed produced detectable transcripts, and all of these transcripts were less abundant at nodes 9 through 12 than at nodes 1 through 2 in either leaves or stipules, or in both organs. This is consistent with the abundance of the mature miR156 and miR157 transcripts at these nodes. All but one of the 17 SPL transcripts with a predicted miR156/157 target site was more abundant at nodes 9 through 12 than at nodes 1 through 2 in either leaf or stipule primordia. In contrast, SPL transcripts that lacked a predicted miR156/157 target site showed variable patterns of abundance, with the majority being equally abundant at nodes 1 through 2 and nodes 9 through 12 or decreasing in abundance between these positions (SI Appendix, Fig. S2B).
We then performed a more detailed analysis of the expression patterns of four miR156/157-targeted transcripts (SPL3-like 3, SPL3-like 5, SPL13-like 1, and SPL13-like 3) and one nontargeted transcript (SPL7-like 1), using quantitative RT-PCR. SPL7-like 1 was expressed at a constant level in both leaf and stipule primordia across all sampled nodes (Fig. 2 C and D). However, miR156/157-targeted SPL genes had different patterns of abundance in different organs, with changes often mirroring patterns of syndrome development (Fig. 2 C–F). SPL13-like 3 increased nearly 30-fold in leaf primordia between node 1 and node 6, but was expressed at the same level in stipule primordia at different nodes. SPL3-like 3 was expressed in a similar pattern, but increased less dramatically than SPL13-like 3 in leaf primordia. SPL3-like 5 and SPL13-like 1 increased in both leaf and stipule primordia, but increased more in stipules than in leaves (Fig. 2 C and D). Additionally, consistent with miR156/157 abundance in leaves, SPL transcripts were less abundant in fully expanded leaves compared with leaf primordia (SI Appendix, Fig. S1 C–G). These data demonstrate that miR156/157 and their SPL targets are developmentally regulated and that different family members have different temporal expression patterns. One possibility is that different SPL genes regulate different components of the swollen thorn syndrome and that the expression pattern of these functionally distinct genes is temporally coordinated by miR156/157.
Reduced Light Intensity Delays the Appearance of the Swollen Thorn Syndrome and Increases the Abundance of miR156/157.
Genetic analysis of the role of miR156/157 and their targets in the development of the swollen thorn syndrome is hindered by the lack of methods for inactivating gene function in V. collinsii and related species. As an alternative, we explored the observation that in the wild this syndrome develops more slowly in plants growing in shaded conditions (29). To confirm this observation, V. collinsii Belize was grown in a single growth chamber under full illumination or under a cloth-covered shade enclosure that reduced the light intensity by 85%.
Plants grown in low light produced enlarged EFN (Fig. 3A), SS, and BB (SI Appendix, Fig. S3 A and B) significantly later than plants growing in full illumination. To determine if shade affects the development of EFN, SS, and BB by modulating the activity of the miR156/miR157-SPL pathway, we then measured the abundance of miR156 and miR157 in leaf primordia. These miRNAs were expressed in the same temporal pattern in low-light and and full-light plants, but low-light plants had significantly higher levels of both miRNAs than full-light plants (Fig. 3B and SI Appendix, Fig. S3C: ANCOVA, P < 0.001 for both). Consistent with this observation, SPL3-like 3 and SPL3-like 5 were expressed at significantly lower levels in low-light plants than in full-light plants (Fig. 3C and SI Appendix, Fig. S3D: ANCOVA, P < 0.01 and P < 0.001, respectively). Furthermore, the abundance of these transcripts at the first node to produce BB in low-light plants (10.5 on average) was similar to their abundance at the corresponding node in full-light plants (node 6.5 on average) (Fig. 3C and SI Appendix, Fig. S3 A and D). Similarly, although low light did not have a statistically significant effect on the abundance of the SPL13-like 1 or SPL13-like 3 transcripts, their overall levels were lower in low-light plants (SI Appendix, Fig. S3 E and F: ANCOVA, P = 0.98, P = 0.08, respectively). This was not true for SPL7-like 1, which was present at similar levels in both conditions across development (Fig. 3D: ANCOVA, P = 0.29).
Fig. 3.
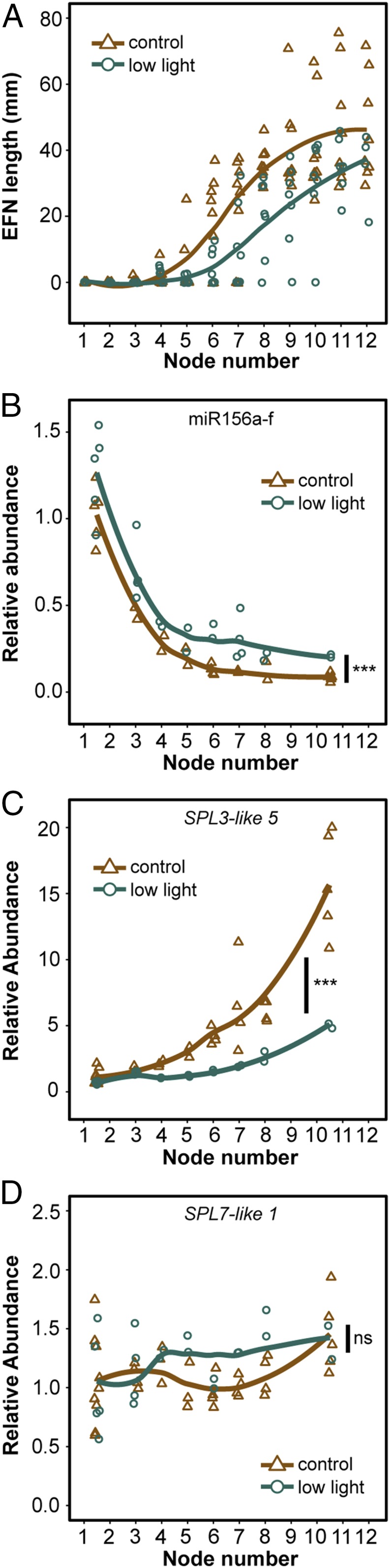
Low-light intensity delays the onset of the swollen thorn syndrome and increases the abundance of miR156/157 in V. collinsii Belize. (A) Length of EFN per node in low light and control conditions. Curves represent conditional means using a Loess smoother. (B) Relative abundance of miR156a-f in leaf primordia. Differences between treatments were tested by ANCOVA. Plotting as in A. (C) Relative abundance of SPL3-like 5, a predicted miR156/157 target. Plotting and statistics as in B. (D) Relative abundance of SPL7-like 1, a gene not targeted by miR156/157. Plotting and statistics as in B. Significance of ANCOVA are indicated: ***P < 0.001; ns: P > 0.05.
The difference in the responsiveness of SPL3-like and SPL13-like may be due to differences in the mechanism by which they are repressed by miR156/157. In Arabidopsis, SPL13 transcripts respond very little to changes in miR156/157 because these miRNAs regulate SPL13 primarily at a translational level (20, 21), and this may be true for SPL13-like 1 and SPL13-like 3 as well. The expression pattern of SPL genes is also regulated at a transcriptional level, and our data do not disentangle this level of regulation from the effect of miR156/157. Nevertheless, the correlated effect of low light intensity on the development of SS, BB, and EFN and on the expression of genes in the miR156/157-SPL pathway support the hypothesis that the swollen thorn syndrome is regulated by this pathway.
The Swollen Thorn Syndrome Likely Evolved by Co-Opting a Preexisting Regulatory Pathway.
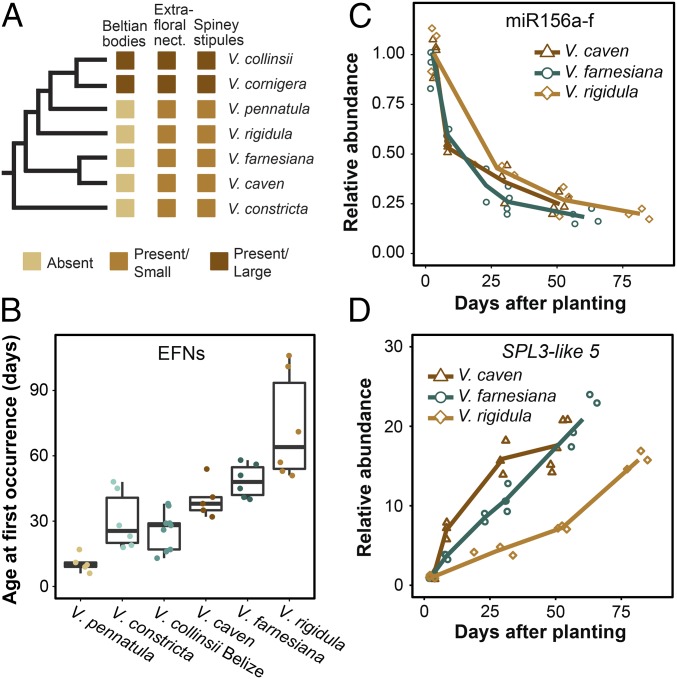
The production of BB is a derived trait within Vachellia (32, 33). However, all species in this genus produce stipular spines and extrafloral nectaries (Fig. 4A), although both of these structures remain relatively small in species that do not establish a relationship with ants. We reasoned that the swollen thorn syndrome may have evolved by co-opting a pathway that controls the development of these preexisting traits. This hypothesis was supported by our observation that EFN are not initially produced in Vachellia species that are closely related to V. collinsii (Fig. 4A) but which do not produce enlarged SS and BB. Indeed, three of these species—V. caven, V. farnesiana, and V. rigidula—produced EFN even later than V. collinsii Belize (Fig. 4B).
Fig. 4.
Components of the swollen thorn syndrome are temporally regulated in non–ant-acacias (genus Vachellia). (A) Phylogenetic distribution of syndrome traits for species used in this study. Non–ant-acacias: V. pennatula, V. rigidula, V. farnesiana, V. caven, and V. constricta. (B) Timing of EFN first occurrence in Vachellia species. Boxes outline first and third quartile; center line marks the median. (C) Abundance of miR156a-f in three non–ant-acacia species. Lines are plotted through the means of samples grouped by similar age. (D) Abundance of SPL3-like 5 in three non–ant-acacia species. Plotting as in C.
To determine if the development of EFN in these species is correlated with the expression of genes in the miR156/157-SPL pathway, we measured the abundance of miR156, miR157, and SPL3-like 5 in shoot apices of V. caven, V. farnesiana, and V. rigidula at four to five different times after planting. The samples collected at the final time point were taken from plants in which at least one prior leaf had had an EFN. The primers used to measure SPL3-like 5 were identical to those used for V. collinsii, and sequencing of the resulting products revealed that the same gene was amplified in all three species. In every species, miR156 and miR157 declined over fourfold and the expression of SPL3-like 5 increased 15- to 20-fold during the period preceding the production of the first EFN (Fig. 4 C and D and SI Appendix, Fig. S4A). In contrast, abundance of SPL7-like 1 showed no consistent pattern between species (SI Appendix, Fig. S4B). These results are consistent with the possibility that the miR156/157-SPL pathway coordinates the timing of vegetative development in many Vachellia species and that this preexisting regulatory network was co-opted during the evolution of the swollen thorn syndrome.
Discussion
Changes in plant defense during ontogeny are widespread (1–4), yet why this developmental pattern exists remains largely unknown. The developmental timing of defense traits may be a consequence of selection, the result of developmental constraints that limit the types of phenotypes seen by selection (although these constraints could themselves be the result of selection) or a combination of both (5). To evaluate these alternatives, it is necessary to understand both how selection acts in a system and the developmental mechanisms that control the traits in question. To date, most work has focused on the former question, and very little is known about the latter (5, 34).
We found that V. cornigera and V. collinsii plants grown from seed in a controlled environment in the absence of ants begin producing SS, EFN, and BB in a stereotypical sequence at least 1 mo after germination. This, and our evidence that the production of SS, EFN, and BB is tightly correlated with changes in the expression of miR156/157 and their SPL targets, strongly suggest that this syndrome is regulated by the vegetative-phase change pathway. Our observation that EFN in species closely related to V. collinsii and V. cornigera develop at approximately the same age as the swollen thorn syndrome in these ant-acacias, and that the appearance of EFN is correlated with a change in miR156 and its targets, further suggest that the swollen thorn syndrome is a modification of a developmental pathway that exists in many, if not all, species in this genus.
Given its value to the plant, it is interesting to consider why the swollen thorn syndrome is temporally regulated. One leading hypothesis for the temporal development of plant defenses posits that such patterns are mediated by developmental constraints (5, 35). This hypothesis predicts that physical or genetic barriers intrinsically limit the development of defense traits. With regard to the swollen thorn syndrome, if there were physical limits on development, this would mean that plants should be unable to produce syndrome traits immediately after germination. However, genetic analyses of the miR156/157-SPL pathway in Arabidopsis do not support this hypothesis. In Arabidopsis, reducing the abundance of miR156/157 transforms the earliest juvenile leaves into adult leaves (23). A more natural example comes from the genus Acacia, where the adult leaf type, known as a phyllode, can be observed as early as leaf one or two in some species, and has been shown to be tightly correlated with the miR156/157-SPL pathway (19). In the future, genetic manipulation of this pathway in V. collinsii could conclusively rule out physical constraints if loss of miR156/157 genes resulted in transgenic plants producing syndrome traits at the earliest nodes.
In addition to passive constraints arising from biophysical limits on development, co-option of the miR156-SPL pathway for the control of syndrome development may itself be constrained by pleiotropy. Given that SPL genes are important regulators of floral development and the adult vegetative phase (36, 37), it is possible that precocious expression of these genes may be constrained by a requirement for the juvenile phase or the cost of flowering too early. This constraint could be overcome through gene duplication and subfunctionalization of the SPL gene family (13–15), which would allow family members to acquire functions specific to the swollen thorn syndrome. However, it is unlikely that the timing of SPL gene expression in Vachellia is constrained by a role for these genes in floral induction because the swollen thorn syndrome appears within a few months, whereas Vachellia species flower years later. Together, these observations suggest that a juvenile phase lacking the swollen thorn syndrome is advantageous. It will be important to determine if age-dependent defenses in other systems are regulated by the miR156/SPL pathway and how the functions of SPL genes in vegetative and reproductive development have evolved to maximize plant fitness.
If the timing of the swollen thorn syndrome is driven by natural selection, we believe trade-offs in resource allocation between whole plant growth and the production of structures associated with this syndrome is a likely cause (1, 5, 38). However, the situation is probably more complicated than this, given that V. cornigera seedlings do not host ants in their first year of growth, even though they begin to produce the swollen thorn syndrome within 1 to 2 mo (29). This disconnect may mean that ant foundresses require a critical mass of domatia and/or resources before they colonize a tree or that other temporally regulated factors, such as volatile organic compounds (VOCs), are required for selection. Foundress queens select a host using VOCs as cues of quality (39). These compounds are produced by fully expanded leaves, and our results indicate that miR156/157 decline more slowly in fully expanded leaves than they do in leaf and stipule primordia, possibly explaining the delay in colonization.
The evidence that the swollen thorn syndrome is regulated by the miR156/157-SPL pathway opens the door to more detailed questions about the mechanism of this phenomenon. For example, nectar secretion from EFN in New World Vachellia species has been shown to depend on jasmonic acid (JA) (32). This is interesting because previous studies in rice (40) and maize (41) indicate that jasmonic acid promotes the juvenile phase, possibly via regulation of miR156/157. However, the JA response is downstream of miR156 and SPL9 in Arabidopsis (16). Either jasmonic acid has the opposite function in New World Vachellia species (i.e., promotes the adult phase) or its effect on nectar secretion reflects an organ-specific function of this hormone. It will also be important to explore the functional significance of the observation that different MIR156/157 and SPL genes have different expression patterns in leaves and stipules. Which of these many genes are required for the development of these structures, and, if so, are they functionally distinct? This latter question will require the development of methods for manipulating gene expression in Vachellia, but it is reasonable to expect that these will become available in the near future.
Materials and Methods
Detailed information about the methods used to determine species identity, genome sequencing and annotation, and RNA abundance is provided in SI Appendix, Supplementary Materials and Methods.
Plant Material and Growth Conditions.
V. cornigera seed were purchased from a vendor in Florida (https://www.etsy.com/shop/MrNature), and V. collinsii Belize seed were purchased from a vendor in Belize (http://www.especies-seeds.com). Seeds of V. collinsii Costa Rica were collected by D. Janzen (University of Pennsylvania) in Costa Rica. V. caven (xDL-89–0115D), V. constricta (xDL-90-0431), V. farnesiana (xDL-90-0341), V. pennatula (xDL-96-0002), and V. rigidula (xDL-92-0153D) were obtained from the Desert Legume Program at the University of Arizona. Plants were grown in a Conviron chamber maintained at 24 °C with 16 h light/8 h dark and 190 to 220 μmol⋅m−2⋅s−1 provided by warm white fluorescent lights.
RNA abundance was measured in leaf and stipule primordia of V. collinsii Belize when the leaves bearing these structures were 1 to 3 mm in size. Five to 50 samples were pooled, depending on node and tissue type. RNA abundance in V. caven, V. farnesiana, and V. rigidula was measured in whole apices with leaf primordia less than 3 mm in size.
Genome Sequencing and Identification of MIR156/157 and SPL Genes.
A single V. collinsii Belize plant was sequenced on a HisEq. 2500 using 250PE format of a 450-bp insert library. Reads were merged using FLASH v1.2.11 (42) and assembled using MaSuRCA (31). MIR156/157 and SPL containing scaffolds were identified using BLAST (43, 44) against a database of 6 plant species. MIR156/157 genes were confirmed by the presence of hairpin structures using RNAfold (45), and SPL genes were annotated using the MAKER pipeline (46).
qPCR Analysis of Small RNA and mRNA Abundance.
The abundance of SPL transcripts was normalized to ACT2, and miR156/miR157 abundance was normalized to miR159 and miR168 using the 2−ΔΔCt method (47).
Quantification and Statistical Analysis.
Statistical tests were conducted in R (48). The validity of parametric tests was determined by analyzing the residuals from fitted linear models. Response variables were log-transformed to meet parametric assumptions when necessary. Smoothed conditional means where calculated with a Loess smoother using ggplot2 (49).
Data and Software Availability.
All sequence data have been deposited in GenBank (https://www.ncbi.nlm.nih.gov). The genome assembly and sequencing reads are associated with BioProject PRJNA470667. Gene sequences used for phylogenetic analysis have the following accession numbers: KX959482–KX959487 and MH324479–MH324488. The 3′RACE products of SPL transcripts have the following accessions: MH404175–MH404183.
Supplementary Material
Acknowledgments
We thank Dan Janzen (University of Pennsylvania) and Matthew Johnson and Kenneth Coppola (University of Arizona’s Desert Legume Program) for donating seeds used in this study. This study was funded by the National Institutes of Health Grant GM51893 (to R.S.P.) and Grant T32GM008216 (to A.R.L.) and by the Katherine Esau Postdoctoral Fellowship (to A.R.L.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in GenBank, https://www.ncbi.nlm.nih.gov (accession nos. PRJNA470667, KX959482–KX959487, MH324479–MH324488, and MH404175–MH404183).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1900644116/-/DCSupplemental.
References
- 1.Boege K., Marquis R. J., Facing herbivory as you grow up: The ontogeny of resistance in plants. Trends Ecol. Evol. (Amst.) 20, 441–448 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Barton K. E., Koricheva J., The ontogeny of plant defense and herbivory: Characterizing general patterns using meta-analysis. Am. Nat. 175, 481–493 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Massad T. J., Ontogenetic differences of herbivory on woody and herbaceous plants: A meta-analysis demonstrating unique effects of herbivory on the young and the old, the slow and the fast. Oecologia 172, 1–10 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Quintero C., Barton K. E., Boege K., The ontogeny of plant indirect defenses. Perspect. Plant Ecol. Syst. 15, 245–254 (2013). [Google Scholar]
- 5.Barton K. E., Boege K., Future directions in the ontogeny of plant defence: Understanding the evolutionary causes and consequences. Ecol. Lett. 20, 403–411 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Herms D. A., Mattson W. J., The dilemma of plants: To grow or defend. Q. Rev. Biol. 67, 283–335 (1992). [Google Scholar]
- 7.Alberch P., “Developmental constraints in evolutionary processes” in Evolution and Development, Bonner J. T., Ed. (Springer, 1982), pp. 313–332. [Google Scholar]
- 8.Smith J. M., et al. , Developmental constraints and evolution: A perspective from the mountain lake conference of development and evolution. Q. Rev. Biol. 60, 265–287 (1985). [Google Scholar]
- 9.Amundson R., Two concepts of constraint: Adaptationism and the challenge from developmental biology. Philos. Sci. 61, 556–578 (1994). [Google Scholar]
- 10.Evans G. C., The Quantitative Analysis of Plant Growth (University of California Press, Berkley, CA, 1972). [Google Scholar]
- 11.Brouat C., McKey D., Leaf-stem allometry, hollow stems, and the evolution of caulinary domatia in myrmecophytes. New Phytol. 151, 391–406 (2001). [Google Scholar]
- 12.Reinhardt D., et al. , Regulation of phyllotaxis by polar auxin transport. Nature 426, 255–260 (2003). [DOI] [PubMed] [Google Scholar]
- 13.True J. R., Carroll S. B., Gene co-option in physiological and morphological evolution. Annu. Rev. Cell Dev. Biol. 18, 53–80 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Monteiro A., Podlaha O., Wings, horns, and butterfly eyespots: How do complex traits evolve? PLoS Biol. 7, e37 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wessinger C. A., Hileman L. C., Accessibility, constraint, and repetition in adaptive floral evolution. Dev. Biol. 419, 175–183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao Y. B., et al. , Jasmonate response decay and defense metabolite accumulation contributes to age-regulated dynamics of plant insect resistance. Nat. Commun. 8, 13925 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu G., Poethig R. S., Temporal regulation of shoot development in Arabidopsis thaliana by miR156 and its target SPL3. Development 133, 3539–3547 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chuck G., Cigan A. M., Saeteurn K., Hake S., The heterochronic maize mutant Corngrass1 results from overexpression of a tandem microRNA. Nat. Genet. 39, 544–549 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Wang J. W., et al. , miRNA control of vegetative phase change in trees. PLoS Genet. 7, e1002012 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu M., et al. , Developmental functions of miR156-regulated SQUAMOSA PROMOTER BINDING PROTEIN-LIKE (SPL) genes in Arabidopsis thaliana. PLoS Genet. 12, e1006263 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He J., et al. , Threshold-dependent repression of SPL gene expression by miR156/miR157 controls vegetative phase change in Arabidopsis thaliana. PLoS Genet. 14, e1007337 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franco-Zorrilla J. M., et al. , Target mimicry provides a new mechanism for regulation of microRNA activity. Nat. Genet. 39, 1033–1037 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Wu G., et al. , The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell 138, 750–759 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J. W., Czech B., Weigel D., miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138, 738–749 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Belt T., The Naturalist in Nicaragua (J. M. Dent & Sons, London, ed. 1, 1874). [Google Scholar]
- 26.Wheeler W. M., Studies of neotropical ant-plants and their ants. Bull. Mus. Comp. Zool. Harvard 90, 3–262 (1942). [Google Scholar]
- 27.Janzen D. H., Coevolution of mutualism between ants and acacias in central America. Evolution 20, 249–275 (1966). [DOI] [PubMed] [Google Scholar]
- 28.González-Teuber M., Kaltenpoth M., Boland W., Mutualistic ants as an indirect defence against leaf pathogens. New Phytol. 202, 640–650 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Janzen D. H., Interaction of the bull’s-horn acacia (Acacia cornigera L.) with an ant inhabitant (Pseudomyrmex ferruginea F. Smith) in eastern Mexico. Univ. Kans. Sci. Bull. 47, 315–558 (1967). [Google Scholar]
- 30.Hocking B., Insect associations with the swollen thorn acacias. Trans. R. Entomol. Soc. London 122, 211–255 (1970). [Google Scholar]
- 31.Zimin A. V., et al. , The MaSuRCA genome assembler. Bioinformatics 29, 2669–2677 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heil M., et al. , Evolutionary change from induced to constitutive expression of an indirect plant resistance. Nature 430, 205–208 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Gómez-Acevedo S., Rico-Arce L., Delgado-Salinas A., Magallón S., Eguiarte L. E., Neotropical mutualism between Acacia and Pseudomyrmex: Phylogeny and divergence times. Mol. Phylogenet. Evol. 56, 393–408 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Mayer V. E., Frederickson M. E., McKey D., Blatrix R., Current issues in the evolutionary ecology of ant-plant symbioses. New Phytol. 202, 749–764 (2014). [DOI] [PubMed] [Google Scholar]
- 35.Clark L. L., Burns K. C., The ontogeny of leaf spines: Progressive versus retrogressive heteroblasty in two New Zealand plant species. N. Z. J. Bot. 53, 15–23 (2015). [Google Scholar]
- 36.Huijser P., Schmid M., The control of developmental phase transitions in plants. Development 138, 4117–4129 (2011). [DOI] [PubMed] [Google Scholar]
- 37.Jorgensen S. A., Preston J. C., Differential SPL gene expression patterns reveal candidate genes underlying flowering time and architectural differences in Mimulus and Arabidopsis. Mol. Phylogenet. Evol. 73, 129–139 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Heil M., McKey D., Protective ant-plant interactions as model systems in ecological and evolutionary research. Annu. Rev. Ecol. Evol. Syst. 34, 425–453 (2003). [Google Scholar]
- 39.Razo-Belman R., Molina-Torres J., Martinez O., Heil M., Plant-ants use resistance-related plant odours to assess host quality before colony founding. J. Ecol. 106, 379–390 (2017). [Google Scholar]
- 40.Hibara K., et al. , Jasmonate regulates juvenile-to-adult phase transition in rice. Development 143, 3407–3416 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Beydler B., Osadchuk K., Cheng C. L., Manak J. R., Irish E. E., The juvenile phase of maize sees upregulation of stress-response genes and is extended by exogenous jasmonic acid. Plant Physiol. 171, 2648–2658 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magoč T., Salzberg S. L., FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J., Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990). [DOI] [PubMed] [Google Scholar]
- 44.Camacho C., et al. , BLAST+: Architecture and applications. BMC Bioinformatics 10, 421 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lorenz R., et al. , ViennaRNA Package 2.0. Algorithms Mol. Biol. 6, 26 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cantarel B. L., et al. , MAKER: An easy-to-use annotation pipeline designed for emerging model organism genomes. Genome Res. 18, 188–196 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livak K. J., Schmittgen T. D., Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- 48.R Core Team , R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna). https://www.R-project.org/. (Accessed 1 May 2017).
- 49.Wickham H., ggplot2: Elegant Graphics for Data Analysis (Springer, New York, ed. 1, 2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.