Significance
The TGFβ family of ligands plays fundamental roles in controlling diverse signaling events throughout multicellular organisms. The family is divided into 3 classes: activin, BMP, and TGFβ, with each signaling through specific combinations of type I and type II receptors. We demonstrate that activin ligands bind receptors similarly to BMP ligands, but they utilize the ligand fingertip to make specificity-determining contacts with the type I receptors. We also show that different molecular mechanisms have emerged for how GDF11 and TGFβ bind the same type I receptor, whereby GDF11 is more dependent on direct receptor contacts. Thus, this study provides insight at the molecular level into how the activin class ligands bind and assemble their type I and type II receptors.
Keywords: GDF11, TGF-β superfamily, activin, ternary signaling complex, Alk5
Abstract
TGFβ family ligands, which include the TGFβs, BMPs, and activins, signal by forming a ternary complex with type I and type II receptors. For TGFβs and BMPs, structures of ternary complexes have revealed differences in receptor assembly. However, structural information for how activins assemble a ternary receptor complex is lacking. We report the structure of an activin class member, GDF11, in complex with the type II receptor ActRIIB and the type I receptor Alk5. The structure reveals that receptor positioning is similar to the BMP class, with no interreceptor contacts; however, the type I receptor interactions are shifted toward the ligand fingertips and away from the dimer interface. Mutational analysis shows that ligand type I specificity is derived from differences in the fingertips of the ligands that interact with an extended loop specific to Alk4 and Alk5. The study also reveals differences for how TGFβ and GDF11 bind to the same type I receptor, Alk5. For GDF11, additional contacts at the fingertip region substitute for the interreceptor interactions that are seen for TGFβ, indicating that Alk5 binding to GDF11 is more dependent on direct contacts. In support, we show that a single residue of Alk5 (Phe84), when mutated, abolishes GDF11 signaling, but has little impact on TGFβ signaling. The structure of GDF11/ActRIIB/Alk5 shows that, across the TGFβ family, different mechanisms regulate type I receptor binding and specificity, providing a molecular explanation for how the activin class accommodates low-affinity type I interactions without the requirement of cooperative receptor interactions.
The transforming growth factor β (TGFβ) family of ligands is composed of more than 30 dimeric growth factors essential in the development and homeostasis of all animals (1–3). Signaling occurs when ligands engage 2 type I and 2 type II serine/threonine kinase receptors, forming a hexameric signaling complex (4, 5). Both the type I and type II receptors consist of a small extracellular ligand-binding domain tethered to an intracellular kinase domain. Upon assembly, type II receptors phosphorylate and activate the type I receptors, which, in turn, phosphorylate intracellular SMAD proteins. Signaling is then transduced into the nucleus, where activated SMADs act as transcriptional factors, enabling signaling outcomes dependent on ligand–receptor combinations. Given the diversity of biological responses and processes controlled by the family, it is somewhat surprising that only 7 type I and 5 type II receptors are found within mammals (1).
Based on sequence homology and receptor utilization, the TGFβ family can be divided into 3 general classes of ligands: activins, BMPs, and TGFβs. From this, 2 general signaling paradigms have emerged—the TGFβ class, which activates SMAD 2/3; and the BMP class, which activates SMAD 1/5/8—with specificity for each paradigm dependent on the activation of different type I receptors. Interestingly, ligands have different affinities for type I receptors, with BMP class ligands binding type I receptors with higher affinity than TGFβ class ligands (5, 6). Structural studies describing ligand–receptor interactions have revealed how TGFβ and BMP ligands adopt different strategies to assemble receptors, accounting for the differences in type I affinity (7–11). The ternary structure of BMP2 revealed independent receptor binding sites, with the type II receptors binding on the convex “knuckle” region of the ligand and the type I receptors binding through extensive contacts in the concave cleft formed by the “wrist helix” at the dimer interface (12). In stark contrast, receptor binding for TGFβ ligands uses a cooperative assembly mechanism that facilitates binding of the type I receptor. Here, the type II receptor is shifted toward the ligand fingertips and comes into direct contact with the type I receptor; thus, binding of the type I receptor is significantly stronger when the ligand is preoccupied with the type II receptor (9, 10). These studies highlight that different mechanisms of ligand–receptor interactions have emerged within the TGFβ family.
The activin class, which includes activin A (ActA), activin B (ActB), Growth and Differentiation Factor 8 (GDF8), and GDF11, shares aspects of both the TGFβ and BMP signaling paradigms. Similar to TGFβ, activin class ligands bind type II receptors with high affinity, including ActRIIA and ActRIIB, and type I receptors with very low affinity, to activating SMAD2/3 (1). However, type II receptor positioning for the activin class is similar to BMP ligands, suggesting that a cooperative mechanism between type I and type II receptors is not used by activin ligands to facilitate binding of the low-affinity type I receptors, as is the case for TGFβ (13, 14). Whereas TGFβ primarily signals use the type I receptor Activin receptor-like kinase 5 (Alk5), the activin class ligands are more promiscuous and can use different combinations of the type I receptors: Alk4, Alk5, and Alk7 (15–17). For example, whereas ActA predominantly signals using Alk4, ActB can signal effectively using both Alk4 and Alk7 (5, 18, 19). GDF8 and GDF11 can both signal using Alk4 and Alk5, with GDF11 extending specificity to Alk7 (17). Although structural studies have revealed how the TGFβ and the BMP ligands assemble the type I and type II receptors, the molecular details for how activin class ligands form a ternary signaling complex and account for type I receptor specificity is unknown. To address this, we sought to structurally characterize a ternary complex of an activin class ligand.
Results and Discussion
Ternary Complex Structure of GDF11/ActRIIB-ECD/Alk5-ECD.
Crystals of an activin class ternary complex were generated by combining GDF11 and the ectodomains (ECDs) of both the high-affinity type II receptor ActRIIB and the low-affinity type I receptor Alk5. Diffraction data were collected to 2.3-Å resolution, and the structure was solved by molecular replacement using the previously determined individual components as search models (data collection and refinement statistics are provided in SI Appendix, Table S1). Within the lattice, the asymmetric unit contains 1 GDF11 monomer bound to 1 ActRIIB-ECD and 1 Alk5-ECD with the full complex being related through crystallographic symmetry. Crystal contacts are exclusively through receptor contacts from symmetry-related complexes (SI Appendix, Fig. S1). The ternary structure shows ActRIIB binding at the knuckle regions of GDF11 and Alk5 binding in the concave clefts formed at the interface of the GDF11 dimer (Fig. 1). As expected, both ActRIIB and Alk5 adopt the conserved 3-finger toxin fold with slight variation in antiparallel β-strand length (SI Appendix, Fig. S2). Electron density was well defined for the following residues: GDF11 (300–407), ActRIIB (26–120), and Alk5 (33–112). Two previously disordered loops within Alk5 were resolved: the loop from β3 to β4 and the loop connecting α-helix1 to β5 (9). All residues at the interfaces between each component were well defined in electron density.
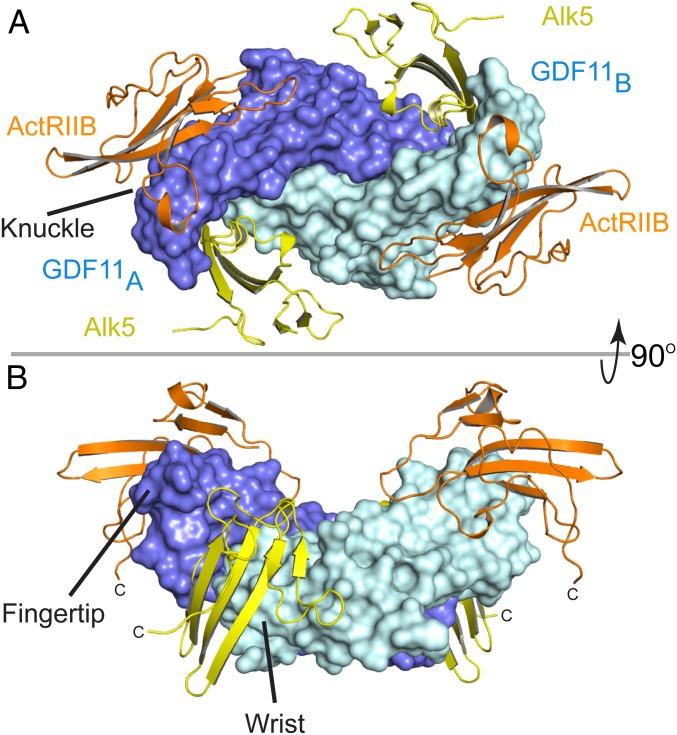
Fig. 1.
Structure of GDF11/ActRIIB/Alk5 ternary complex. (A) GDF11/ActRIIB/Alk5 as viewed on the membrane surface. GDF11 has 2 monomers represented in slate (monomer A) and cyan (monomer B). ActRIIB-ECD is represented in orange, with Alk5-ECD in yellow. (B) Ninety-degree upward view of the ternary complex. ActRIIB binds at the convex knuckle region of GDF11 whereas Alk5 binds at the concave interface of GDF11 formed between the fingertip of monomer A and the wrist helix of monomer B GDF11.
Overall, receptor assembly resembles that of the BMP paradigm, with each receptor binding independently and in comparable locations (7, 8). Two ActRIIB molecules bind on the knuckle segment of the GDF11 fingers, similar to that observed for ActA and BMP9 (11, 13). Here, ActRIIB forms a concave surface with a hydrophobic core, anchored by a triad of aromatic residues (Tyr60, Trp78, and Phe101), that buries a total of 720 Å2 per receptor (Fig. 2A and SI Appendix, Fig. S3 A and B). Each ActRIIB interacts exclusively with 1 GDF11 monomer. In contrast, Alk5 contacts both GDF11 monomers by binding in the concave interface formed at the dimer interface. Here, the β4–β5 loop and the α1 helix of Alk5 project into the concave cleft of the GDF11 dimer (SI Appendix, Fig. S3 D and E). This results in 4 main contact points with GDF11, including the prehelix loop, fingertip, N terminus, and wrist helix (Fig. 2A and SI Appendix, Fig. S3A). No major conformational changes (RMSD = 1.76 Å over 216 residues, structural alignment) are observed in GDF11 compared with an unbound state (PDB ID code 5E4G), suggesting the absence of conformational changes upon receptor binding (20). As observed for the BMP and TGFβ ternary complexes, the C-terminal ends of the receptors are oriented in the same direction, consistent for a cell surface signaling complex (Fig. 1B).
Fig. 2.
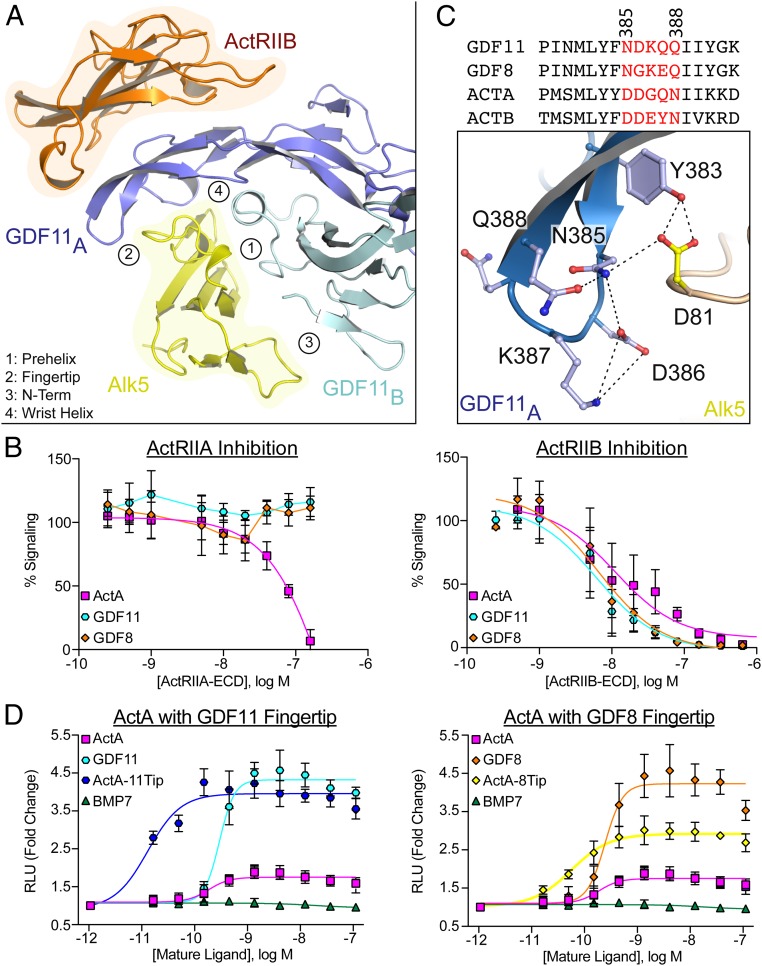
Differences in receptor specificity for the activin class. (A) Ribbon representation of one half of the GDF11/ActRIIB/Alk5 complex with GDF11/Alk5 interfaces numbered and labeled accordingly. (B) Inhibition curves (CAGA-luciferase) for respective ligands following the titration of the ECD of ActRIIA (Left) or ActRIIB (Right). (C) Sequence alignment of activin class ligands with fingertip residues represented in red. Numbers correlate to residues in GDF11. Specific molecular interactions between the GDF11 fingertip and the Alk5 β4–β5 loop highlighting the formation of a hydrogen bonding network with dashed lines. (D) Split β-galactosidase dimerization assay (DiscoverX) specific for ActRIIB/Alk5 assembly. For B and D, each data point represents the mean ± SD of triplicate experiments measuring relative luminescence units (RLU) (B) or β-galactosidase activity (D). For B, 100% signaling represents uninhibited signaling of each respective ligand.
Molecular Determinants of Receptor Specificity for the Activin Class.
Next, we wanted to determine if the ternary complex of GDF11 could provide insight into receptor specificity across the broader activin class. We first compared interactions of GDF11 at the type II interface with previously determined structures, including ActA/ActRIIB (13). Although the position of the type II receptor is consistent with previous structures, GDF11 forms more extensive contacts throughout the interface. This includes a unique salt bridge between Lys334 in GDF11 and Glu94 in ActRIIB that is not present in the ActA/ActRIIB complex (SI Appendix, Fig. S3 B and C) (8, 13). In total, GDF11 buries 12.5% more surface area at the type II interface than ActA. Analysis of the interface stability in jsPISA reveals that the GDF11/ActRIIB interface is more energetically favorable with a ΔG P value of 0.2, compared with a value of 0.4 for both ActA and BMP2 (21). Further comparison of ActRIIA versus ActRIIB revealed potential differences in how GDF11 binds to each type II receptor. For example, ActRIIA would not form the aforementioned salt bridge because Glu94 of ActRIIB is a lysine in ActRIIA (SI Appendix, Fig. S3 B and C). Although both ActRIIA and ActRIIB have been shown to be capable of signaling with the activin class, other studies have suggested a preference of GDF8 for ActRIIB (15). Therefore, we wanted to further investigate if these observed structural variations translate into differences in type II receptor binding.
Initially, we tested the ability of the recombinant ECD of both ActRIIA and ActRIIB to inhibit ActA and GDF11 signaling. ActRIIB inhibited ActA and GDF11 signaling with similar IC50 values, whereas ActRIIA inhibited only ActA signaling (Fig. 2B). ActRIIA-ECD failed to inhibit GDF11 and GDF8 signaling (Fig. 2B). Similar to previous studies, mutation of Leu79 in ActRIIB to alanine eliminates ActA binding while maintaining affinity for GDF11; however, the modified receptor fails to inhibit both ActA and GDF11 signaling (SI Appendix, Fig. S4) (22). Although direct binding measurements using surface plasmon resonance were complicated as a result of nonspecific interactions, native PAGE analysis was used to further analyze the interactions, given that binary ligand–receptor complexes are readily visualized. By titrating increasing amounts of receptors, GDF11 more readily forms a complex with ActRIIB compared with ActRIIA, whereas ActA shows little preference for the type II receptors (SI Appendix, Fig. S5A). To further test type II receptor specificity, we purified complexes of ActRIIA and ActRIIB bound to either GDF11 or ActA (SI Appendix, Fig. S5B). Using these complexes, we performed a series of displacement experiments to determine if free receptor could displace ligand-bound receptor. Titration of ActRIIA-ECD failed to displace ActRIIB from a preformed GDF11/ActRIIB complex, whereas ActRIIB could readily displace a GDF11/ActRIIA complex (SI Appendix, Fig. S5C). In contrast with GDF11, ActRIIA readily displaced ActRIIB from the ActA/ActRIIB complex (SI Appendix, Fig. S5C). Last, equimolar addition of both ActRIIA and ActRIIB to GDF11 revealed a clear preference for the formation of GDF11/ActRIIB binary complex, whereas ActA readily formed a complex with both receptors (SI Appendix, Fig. S5D). These results suggest binding differences between activin class ligands and their cognate type II receptors.
The ternary structure also provided insight into type I receptor specificity. For the activin class, it has been shown that different ligands signal through specific type I receptors, sometimes with overlapping interactions (16, 17, 23). For example, ActA signals primarily through Alk4, whereas GDF8 and GDF11 signal through both Alk4 and Alk5. Type I receptor specificity has been partly attributed to the prehelix loop of the ligand, as ActA can be engineered to signal through Alk5 by substitution of the prehelix loop of GDF8 (24). Examination of the GDF11 ternary receptor complex failed to reveal distinct molecular interactions that could account for this observation. However, on the opposite end of the type I interface, we noticed significant interactions of the β4–β5 loop of Alk5 with the fingertips of GDF11. Here, a network of hydrogen bonds throughout the fingertip of GDF11 stabilizes interactions with Alk5, specifically Asp81 (Fig. 2C). Interestingly, across the different type I receptors (Alk4, Alk5, and Alk7), the β4–β5 loop exhibits significant variability in length and composition, which, in Alk5, contains a 5-amino acid extension flanked by proline residues that has been shown to be essential for receptor binding (SI Appendix, Fig. S6) (25). Thus, we hypothesized that the fingertip interactions of activin class ligands contribute to type I specificity.
Through mutagenesis, we generated both an ActA-GDF8 and an ActA-GDF11 fingertip chimera. Here, we replaced the ActA fingertip residues 405-DDGQN-409 with fingertip residues 354-NGKEQ-358 or 386-NDKQQ-390 of GDF8 and GDF11, respectively, with the expectation of conferring Alk5 binding to ActA (Fig. 2C). Purified, recombinant ActA, ActA8Tip, and ActA11Tip were generated and shown to signal in a luciferase reporter assay using HEK 293T cells (SI Appendix, Fig. S7). As HEK cells express multiple activin type I receptors, information about receptor specificity cannot be derived. Thus, to determine if the ligand chimeras can interact with Alk5 specifically, we utilized an enzyme fragment complementation assay to measure assembly of type I and type II receptors (PathHunter; DiscoverX). ActRIIB and Alk5 were fused to inactive fragments of β-galactosidase and measured for the ability to reconstitute β-galactosidase upon addition of ligand, indicating ligand-mediated receptor dimerization. As expected, titration of GDF8 and GDF11 produced a strong signal, whereas ActA showed no ability to reconstitute β-galactosidase in cells expressing ActRIIB and Alk5 (Fig. 2D). Strikingly, upon titration of ActA11Tip or ActA8Tip, we observed a robust activation of the ActRIIB/Alk5 receptor dimerization assay. These results indicate that ActA can be converted into a ligand that can assemble a signaling complex with Alk5 through specific alteration of fingertip residues. These results suggest that type I receptor specificity is derived from the fingertip regions of the ligand, which in turn interact with the β4–β5 loop of the receptor (24).
Although similar effects on Alk5 specificity were observed between the mutants, ActA11Tip and ActA8Tip, there were differences in the potency of Alk5/ActRIIB dimerization whereby GDF11 exhibited a stronger signal than GDF8. This is consistent with previous work in which GDF11 was shown to more potently signal through Alk5 and have a higher apparent binding affinity compared with GDF8, despite only 11 amino acids being different (14, 19). The structure reveals that the majority of the residue differences between GDF8 and GDF11 congregate in the concave, type I interface (SI Appendix, Fig. S8A). Interestingly, a substitution occurs at the fingertips whereby Asp387 in GDF11 is replaced by Gly355 in GDF8, disrupting the hydrogen bonding network of the region (SI Appendix, Fig. S8C). In fact, our previous study showed that replacement of either Val316 of the prehelix or Gly355 of the fingertip in GDF8 with Met or Asp of GDF11, respectively, conferred enhanced signaling activity to GDF8 (19). These results, together with the structure of the ternary complex, indicate that the increase in GDF11 signaling is a result of specific changes in the fingertip and prehelix regions that promote more favorable interactions with Alk5.
Comparison with the TGFβ Paradigm.
The structure of GDF11/ActRIIB/Alk5 allows us to compare the activin receptor assembly to that of the TGFβ class and offers a direct comparison of how the 2 ligands engage the same receptor, Alk5 (Fig. 3A). Although GDF11 and TGFβ both have low affinity for Alk5, the structural comparison highlights significant differences in how they accommodate this low affinity. Whereas TGFβ utilizes a cooperative receptor binding mechanism to enhance type I affinity, GDF11 lacks type II and type I receptor interactions and thus does not use a cooperative mechanism. Instead, GDF11 utilizes the unique interactions at the fingertip to differentially interact with Alk5. These differences alter the placement of Alk5 in the type I receptor binding cleft between GDF11 and TGFβ. Also observed is a significant difference in the configuration of the ligand dimer (Fig. 3B). For TGFβ3, the monomers remain in a linear configuration, whereas, for GDF11, monomer B of the ligand angles upward, moving the N terminus of GDF11 out of the type I binding cleft. The difference in ligand conformation appears necessary for Alk5 to adopt a different position in the type I binding cleft. Superposition of the complexes through monomer A allows one to appreciate the differences in how Alk5 is oriented relative to the ligand. For GDF11, Alk5 positions deeper into the dimer interface, with a shift of 9.2 Å toward monomer B and a shift of 5.8 Å toward the fingertips of monomer A (Fig. 3C). This orientation of Alk5 is restricted with TGFβ3 as a result of steric hindrance from the N-terminal α-helix of the ligand (Fig. 3D). Therefore, GDF11 and TGFβ adopt different molecular interactions and assembly mechanisms to bind the same receptor.
Fig. 3.
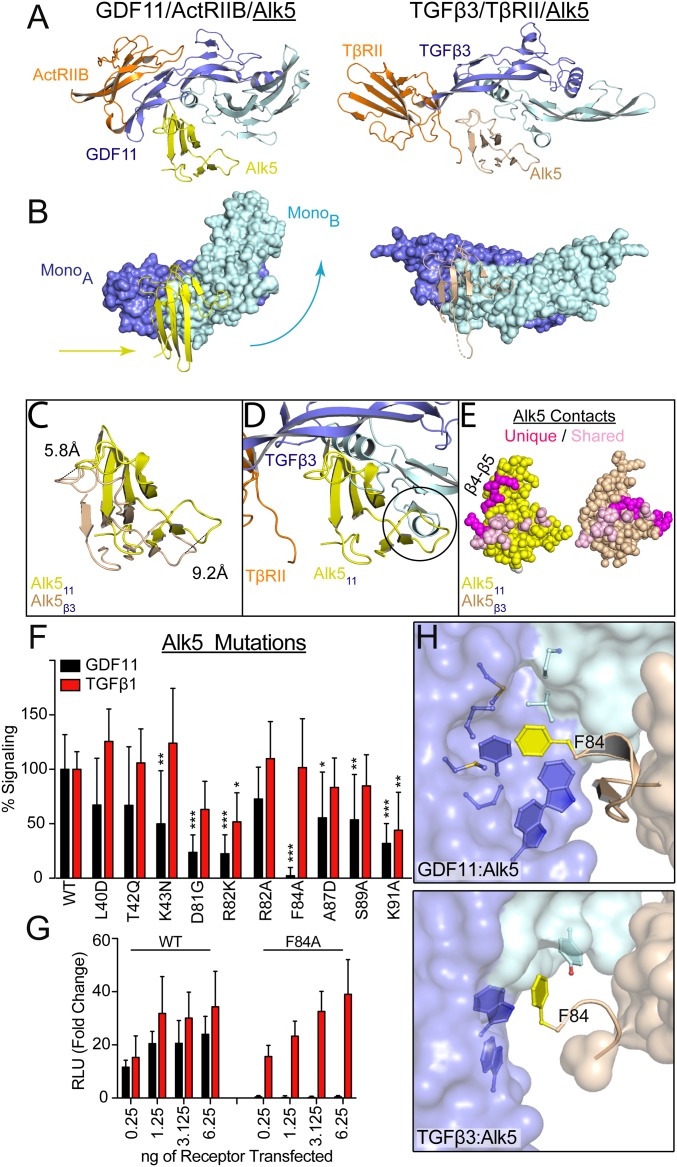
Differential binding of shared type I receptor Alk5 to GDF11 and TGFβ. (A) Ribbon showing 1 type I and 1 type II for GDF11/ActRIIB/Alk5 (PDB ID code 6MAC) and TGFβ3/TβRII/Alk5 (PDB ID code 3KFD), with orientation consistent with alignment of monomer A of the ligand (9). (B) Surface representation of GDF11 (Left) and TGFβ3 (Right) to highlight the relative positional differences of monomer B and Alk5. Arrows indicate the directional shift of GDF11 and Alk5 relative to TGFβ3. (C) Overlay of Alk5 bound to GDF11 (yellow) and TGFβ3 (sand), respectively. Alignment was performed using monomer A. Only the receptor is shown to highlight the shifts in both the β4–β5 loop and the N-terminal region. (D) Overlay of GDF11-bound Alk5 onto TGFβ3 complex using monomer A for superposition. Circle indicates a steric clash with the N terminus of the ligand that would occur if Alk5 were to bind TGFβ3 in a similar position as GDF11. (E) Surface interactions (within 5 Å) of Alk5 with both GDF11 and TFGβ3, with shared residues in pink and unique ligand-interacting residues in magenta. (F and G) Luciferase reporter assay in R1B L17 cells of GDF11 and TGFβ1 signaling following transient transfection of 1.25 ng of Alk5 variants and 2.5 ng (CAGA)12 promoter (F) or titration of Phe84-Ala (F84A) Alk5 transfection (G) (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001, 1-way ANOVA). (H) Differences in how F84 of Alk5 binds to GDF11 (Top) and TGFβ3 (Bottom).
The differences in Alk5 binding results in both unique and common interactions between GDF11 and TGFβ (Fig. 3E). These differences mainly arise from differential interactions with the β4–β5 loop of Alk5. For GDF11, the β4–β5 loop contacts the ligand fingertip, which in turn curls around the receptor. This is in contrast to the flatter extended conformation of the TGFβ3 fingers, which interact with the β4–β5 loop using a combination of the ligand fingers and the N terminus of TβRII (9). Interestingly, the curved fingertip of GDF11 seems to parallel the interreceptor interactions between TβRII and Alk5 (Fig. 2C and SI Appendix, Fig. S9B). Thus, even though GDF11 does not utilize interreceptor interactions to facilitate Alk5 binding, the curvature of the fingertips provides a similar framework. Further molecular differences are observed across the type I receptor–ligand interface. Most apparent is the placement of Arg82 of Alk5, which interacts with the GDF11 fingertips forming a pi stack with family-conserved tryptophan residues. For TGFβ3, Arg82 is shifted away from the ligand and contacts the type II receptor, TβRII (SI Appendix, Fig. S9B) (9). Differences also occur at the C-terminal portion of the β4–β5 loop, where Lys91 of Alk5 caps the wrist helix of GDF11 (Fig. 4D). In TGFβ3, this interaction is missing, and, in fact, residues 90-SKTG-93 of the β4–β5 loop are unresolved in the TGFβ3 ternary structure, implying disorder in this region (SI Appendix, Fig. S9C).
Fig. 4.
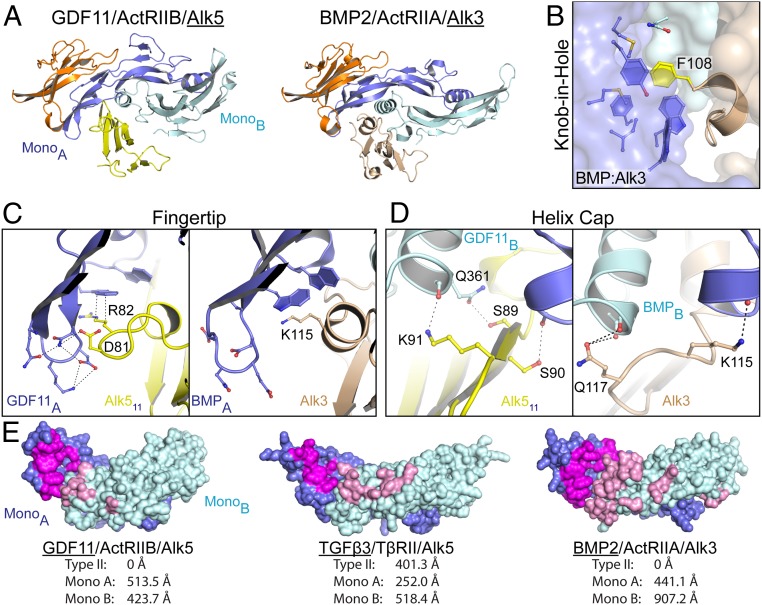
Comparison of type I receptor engagement across the TGFβ family. (A) Ribbon showing 1 type I and 1 type II for GDF11/ActRIIB/Alk5 (PDB ID code 6MAC) and BMP2/ActRIIA/Alk3 (PDB ID code 2GOO), with orientation consistent with alignment of monomer A of the ligand. (B) Knob-in-hole motif observed between F108 of Alk3 and BMP2. (C and D) Comparison of the ligand fingertip-type I interface (C) and the ligand wrist helix cap (D) between GDF11/Alk5 (Left) and BMP2/Alk3 (Right). (E) Ligand surface representation of type I binding interface, with interacting residues on monomer A colored magenta and those on monomer B colored pink. Buried surface area calculations for each of the interfaces are shown as determined by using jsPISA.
Given these molecular differences, we next sought to determine the importance of specific Alk5 residues on GDF11 and TGFβ signaling. For this, we used a cell-based luciferase reporter assay in R1B L17 cells, which lack expression of endogenous Alk5, and tested the signaling activity of transiently expressed wildtype or mutant Alk5. Both wildtype and mutant receptors were expressed to similar levels (SI Appendix, Fig. S10). As expected, both GDF11 and TGFβ1 robustly signal through wildtype Alk5. Conversion of both Asp81-Gly and Arg82-Lys (corresponding residues of Alk4) impacted signaling for the ligands, with a larger effect observed for GDF11. Remarkably, Phe84-Ala completely abolished GDF11 signaling while maintaining interaction with TGFβ1 (Fig. 3F). These observations were confirmed through titration of wildtype and Phe84-Ala Alk5, in which TGFβ1 signaling remains consistent, whereas GDF11 signals through only wildtype Alk5 (Fig. 3G). Comparison of the complexes reveals differences in how Phe84 is positioned relative to GDF11 and TGFβ. In the GDF11 complex, Phe84 forms a “knob-in-hole” interaction with a hydrophobic cleft facilitated by the family-conserved tryptophan residues, whereas, in complex with TGFβ, Phe84 is shifted, forming a tangential interaction with the hydrophobic surface of the ligand (Fig. 3H). This support the conclusion that, even though both GDF11 and TGFβ signal using the same type I receptor, the ligands have adopted different strategies to accommodate low-affinity interactions with Alk5.
Comparison with the BMP Paradigm.
At first glance, the structure of GDF11/ActRIIB/Alk5 mimics the receptor orientation observed with the BMP2 and BMP9 receptor assemblies, where the type I receptor binds independently from the type II receptor (Fig. 4A) (7, 8, 11). In addition, there are similar molecular features throughout the interface that indicate common mechanisms of binding. Specifically, Alk3 and Alk5 utilize a knob-in-hole strategy whereby a phenylalanine (Phe108-Alk3 and Phe84-Alk5) projects into a hydrophobic pocket in the ligand (Figs. 3H and 4B) (7). Similarly, Arg82 of Alk5 and Lys115 in Alk3 perform a similar role by forming a hydrophobic interaction with the family-conserved tryptophan residues of the ligand (Fig. 4C). Last, both Alk3 and Alk5 utilize the C-terminal end of the β4–β5 loop to cap the wrist helix of the ligand (Fig. 4D) (7).
Despite these similarities, the structure highlights major differences between the GDF11 and BMP complexes in how the type I receptor binds at ligand dimer interface. Here, the BMP complex utilizes a much larger degree of surface area to bind the type I receptor compared with the GDF11 complex. This is consistent with the relative affinities for the type I receptors, in which, in general, BMP ligands bind with higher affinity than activin class ligands. Specifically, interaction of BMP2 with Alk3 buries 1,350 Å2 versus 940 Å2 for the GDF11 Alk5 interface (8). This difference is a result of more substantial interactions between the receptor and monomer B of BMP2, which buries an additional 480 Å2 over the monomer B of GDF11 (Fig. 4E). This increase in surface area is a result of multiple residues within the prehelix loop of BMP2 extending into the palm of Alk3 compared with GDF11, which interacts with Alk5 through only a single residue (Met348). However, in contrast, GDF11 buries slightly more surface area on monomer A as a result of increased interactions between the β4–β5 loop of Alk5 and the fingertips. Interestingly, BMP type I receptors Alk3 and Alk6 have shorter β4–β5 loops, which limits the ability to interact with the fingertip of the ligand (Fig. 4C and SI Appendix, Fig. S6). Thus, fingertip interactions are unique to the GDF11 receptor assembly, further differentiating the activin paradigm of receptor assembly from that of TGFβ and BMP. Although the apparent assembly mechanism appears similar between the BMP and activin class, differences in affinity and surface area utilization lead to diverging receptor assembly mechanisms.
Conclusions.
Studies within the TGFβ family have revealed distinct mechanisms for receptor assembly that are defined by receptor affinities, receptor positioning, and SMAD activation: the TGFβ paradigm, which utilizes cooperative receptor interactions during assembly, and the BMP paradigm, which utilizes a noncooperative lock-and-key receptor binding mode (7, 9, 14). A third class, the activins, has commonalities with both paradigms: receptor affinities similar to that of the TGFβ class and receptor orientation similar to that of the BMP class. However, how the activins engage and interact with their cognate type I receptors to form specific contacts remains uncharacterized. The structure of GDF11/ActRIIB/Alk5 provides insights into the activin class and context for type I and type II interactions across the broader TGFβ family.
Previous biochemical work has suggested that activin class ligands interact differently with the type II receptors ActRIIA and ActRIIB (16, 23). However, for GDF11, both ActRIIA and ActRIIB have been shown to be important for signaling during the patterning of axial vertebrae, implying an ability to signal using either type II receptor (26). Previous crosslinking and binding studies have indicated that GDF8 has a preference for ActRIIB (15, 16). In contrast, studies using the Fc-fusion forms of ActRIIA or ActRIIB, which benefit from avidity, show high-affinity binding and antagonism to both GDF8 and GDF11 (27, 28). From previous structures of ligand–receptor complexes, the general binding interface of the type II receptor for GDF11 was anticipated; however, the structure of ternary GDF11 complex highlights ligand differences within the activin class that account for preferential interactions with type II receptors. Using only the ECD of the type II receptors, we further investigated the preference of GDF11 and ActA for type II receptors. We show that ActRIIB, and not ActRIIA, can inhibit GDF11 signaling in a luciferase reporter assay. Native gel analysis shows a similar preference of GDF11 for ActRIIB over ActRIIA. This preference is not observed with ActA, in which complex formation is similar between ActRIIA and ActRIIB. Interestingly, in the presence of equal quantities of ActRIIA and ActRIIB, GDF11 forms complex with only the latter, suggesting that GDF11 likely prefers ActRIIB when both receptors are present at the cellular surface. This preference is not observed for ActA, in which the ligand can bind to either type II receptor. For GDF11, this might ensure that only 1 type II receptor binds in the ternary complex, whereas ActA has the potential to form a ternary complex that contains 1 ActRIIA and 1 ActRIIB. This implies that cell surface representation of the type II receptors, which are different in several tissues, might be used to modulate ligand sensitivity (22, 29, 30).
Given the similarities in general receptor positioning between GDF11 and BMP ligands, it is interesting to consider why ligands have developed different strategies for binding type I receptors. A major difference in the ligands appears in the flexibility of the dimer interface. For the activin class, multiple ligands have been shown to exhibit significant flexibility of the dimer interface, which disrupts the architecture of the type I binding cleft. This is supported by various structures, including the recent structures of GDF11 and GDF8, in which the ligand is described in an “open” (fully formed type I cleft) or a “closed” state typically associated with a disordered wrist region (13, 19, 20, 24, 31–37). In contrast, BMP ligands are consistently observed in the open ligand state, implying rigidity of the ligand dimer. It is possible that the flexibility of activin class ligands prevents interactions with BMP type I receptors, such as Alk3 and Alk6, which may favor a more rigid type I interface. Given the evidence, it is intriguing to speculate that activin ligand flexibility might be used to accommodate the positioning of type I receptors and that, along with low affinity, might allow certain ligands to be more promiscuous with type I receptors.
Previous studies have implicated the prehelix loop as an element for determining type I receptor specificity (24). For BMP ligands, specific interactions of the prehelix loop are important for type I interactions. For BMP2, single changes in the prehelix loop can render the ligand deficient in receptor binding (e.g., L51P), whereas activin ligands are less dependent on specific residues (6, 38). In line with this, specific differences within the prehelix loop between BMP2 and GDF5 have been shown to directly account for differences in utilization of type I receptors Alk3 and Alk6 (39–41). Although significant variations of the prehelix loop exist across activin class members (SI Appendix, Fig. S6), it is possible that these differences account for differences in ligand flexibility, which might impact how particular ligands accommodate type I receptors.
The study identified that additional elements of type I specificity occur in the fingertip region of the ligand. We show that GDF11 uses the fingertip region to engage the β4–β5 loop of Alk5, forming a network of hydrogen bonds. The variation in fingertip composition observed in both GDF8 and ActA likely weakens this network, therefore destabilizing Alk5 interactions. Structural comparison across the TGFβ family reveals that the interactions between the fingertip and the β4–β5 loop are unique to the activin class (Fig. 4C and SI Appendix, Fig. S9B). Interestingly, activin type I receptors Alk4 and Alk5 uniquely possess an extension in the β4–β5 loop, allowing them to extend interactions into the fingertip interface, indicating a divergence of type I receptors for interactions with activin class ligands. Interestingly, this extension is absent in Alk7 and might provide differential interactions with activin class ligands. Therefore, our study identifies the fingertip as a major determinant of type I specificity for the activin class.
Comparison of Alk5 with the BMP receptor Alk3 revealed a common knob-in-hole motif. Here, a phenylalanine wedges into a hydrophobic pocket created at the dimer interface. Sequence alignment of the type I receptors reveals a phenylalanine or equivalent hydrophobic residue in similar positions in all type I receptors, save for Alk1, which uses a glutamate residue to form hydrogen bonds with the ligand (11). For Alk5, this interaction is not observed in complexes with TGFβ in which the phenylalanine adopts a different sidechain rotamer and does not protrude into a hydrophobic cleft. Remarkably, mutation of Phe84 to alanine abolished GDF11 signaling through Alk5 but did not disrupt TGFβ signaling, indicating that the 2 ligands differently utilize receptor interactions. This indicates that TGFβ is less sensitive than GDF11 to disruptive mutations at the type I ligand interface, presumably because of the additional contacts offered by the type II receptor, TβRII.
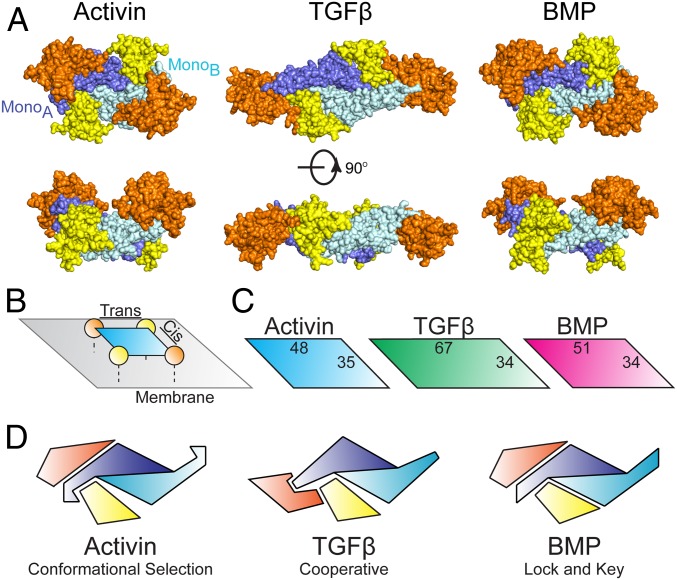
Given that full signaling complexes of each class have been resolved structurally, it is interesting to compare overall receptor positioning across the family (Fig. 5A). At a glance, both BMP and activin ligands position receptors in a similar manner, with TGFβ shifting the type II binding interface toward the fingertip, allowing direct interreceptor interactions to occur with the same-sided (cis-) type I receptor (Fig. 5B). This cis-receptor pair alone has been shown, through mutagenesis, to be sufficient for TGFβ signaling (42). For BMP ligands, 2 type II receptors are required, whereas only 1 type I receptor is sufficient for signaling (43). Whether similar signaling pairs are sufficient for activin class signaling has yet to be determined. However, the GDF11 ternary complex indicates that the overall receptor spacing is similar for the BMP and activin class ligands, even though, for GDF11, the type I receptor interacts more extensively with the ligand fingertips (Fig. 5C).
Fig. 5.
Receptor assembly paradigms of the TGFβ superfamily. (A) Full signaling complex structures from across the TGFβ superfamily: activin (PDB ID code 6MAC), TGFβ (PDB ID code 3KFD), and BMP (PDB ID code 2GOO) (8, 9). Type II and type I receptors are represented in orange and yellow, respectively, with mature ligands in blue. (B) Cartoon representation of the receptor surface complex with cis- and trans-receptor pairs labeled. (C) Distances are calculated from the center of mass for each receptor for cis- and trans-receptor combinations. (D) Schematic representation depicting half the signaling complex for the 3 receptor binding mechanisms of TGFβ family ligands.
In this study, we show that the 3 classes of ligands utilize different molecular strategies to bind type I receptors. TGFβ utilizes a cooperative mechanism between type I and type II receptors, whereas the BMP class uses a high-affinity lock-and-key mechanism (Fig. 5D) (8, 9). The activin class sets itself apart from the TGFβ and BMP classes in which type I receptor specificity is more promiscuous (5, 15–18). This promiscuity is apparent for ActA, which, in addition to activating SMAD2/3 through Alk4, can form a nonsignaling complex with BMP type I receptor Alk2, acting as a competitive inhibitor. However, a single intracellular point mutation of Alk2 switches ActA to an agonist, leading to aberrant ossification and the disease fibrodysplasia ossificans progressiva. Taken together, our structural and specificity data support a third mechanism of ligand–receptor interactions whereby subtle differences in the ligand impact the binding of low-affinity type I receptors, as observed with the fingertip (Fig. 5D). This is consistent with a mechanism whereby the high-affinity type II receptor localizes the ligand on the cell surface, concentrating the ligand to facilitate interactions with low-affinity type I receptors (34). We hypothesize that ligand flexibility is used to maintain low-affinity type I interactions and increase the type I receptor binding profile by facilitating a conformational selection model. The reason the activin class has evolved this mechanism and not simply a different lock-and-key fit remains unclear, but suggests that type I receptor promiscuity might play an important, yet unknown, biological role. Ultimately, additional structural data are needed to compare and contrast receptor specificity within the activin class of TGFβ signaling ligands.
Materials and Methods
ActRIIB, ActRIIA, and Alk5 Expression and Purification.
The extracellular domains of rat ActRIIB (residues 1–120) and human ActRIIA (residues 1–134) were subcloned into the pVL1392 baculovirus vector. ActRIIB was cloned with a C-terminal thrombin cleavage site and a His6 tag, whereas ActRIIA was cloned with C-terminal Flag and His10 tags. Recombinant baculoviruses were generated by using the Baculogold system (ActRIIB; Pharmingen) or the Bac-to-Bac system (ActRIIA; Invitrogen). Virus amplification and protein expression were carried out using standard protocols in SF+ insect cells (Protein Sciences). ActRIIB and ActRIIA were purified from cell supernatants by using Ni Sepharose affinity resin (GE Healthcare) with buffers containing 50 mM Na2HPO4, pH 7.5, 500 mM NaCl, and 20 mM imidazole for loading/washing and 500 mM imidazole for elution. ActRIIB was then digested with thrombin overnight to remove the His6 tag. ActRIIA and ActRIIB were subjected to size exclusion chromatography (SEC) using a HiLoad Superdex S75 16/60 column (GE Healthcare) in 20 mM Hepes, pH 7.5, and 500 mM NaCl. Additionally, human Alk5 (residues 1–101) was expressed in Escherichia coli and was prepared through an oxidative refolding/HPLC purification procedure described previously (44).
Mature ActA, GDF11, GDF8, and TGFβ1 Expression and Purification.
The DNA sequence encoding for human ActA and human GDF11 was cloned into the pAID4T vector containing a UCOE element (Millipore) for increased gene expression. The plasmids were stably transfected in Chinese hamster ovary (CHO) DUKX cells, and pools were generated and used to express ActA and GDF11. Conditioned media (CM) containing ActA was mixed with a proprietary affinity resin made with an ActRIIA-related construct (Acceleron). The pH of the solution was lowered to 3.0 to dissociate the propeptide–ligand complex and raised to 7.5 after a few minutes, and the solution was incubated for 2 h at room temperature. The resin was filtered to remove the conditioned media and then washed with PBS solution and eluted with 0.1 M glycine, pH 3.0. The glycine elution was concentrated over a phenyl hydrophobic interaction column (GE Healthcare) and eluted with 50% acetonitrile/water with 0.1% trifluoroacetic acid (TFA) and then further concentrated by centrifugation in Amicon tubes. The protein was then purified over a preparative reverse-phase C4 column (Vydac) attached to an HPLC with a gradient of water/0.1% TFA and acetonitrile/0.1% TFA. Fractions containing pure protein were combined and lyophilized. The CM containing GDF11 was filtered, concentrated, heated to 75 °C, and cooled to room temperature before protein purification. GDF11 was purified by using a proprietary affinity resin based on an ActRIIB-related construct. The resin was incubated with the prepared CM for 2 h with shaking, followed by a wash with PBS solution and elution with 0.1 M glycine, pH 3.0. Purified material was applied to a preparative C4 reverse-phase column and purified as described with ActA. Pure protein fractions were pooled and dialyzed in 10 mM HCl and then dried with a refrigerated speed vacuum concentrator at −4 °C. For both proteins, purity was assessed by SDS/PAGE analysis. Additionally, mature, human GDF8 was purchased through R&D. Human TGFβ1 was expressed and purified from conditioned medium produced by an overexpressing stably transfected CHO line described previously (45).
ActA Propeptide and ActA Fingertip Mutant Expression and Purification.
ActA constructs were untagged and cloned downstream of mouse ActA propeptide (with native signal sequence) by isothermal Gibson assembly of synthetic gene blocks (Integrated DNA Technologies) into an expression vector driven by CMV promoter (46). For purification, propeptide complexes were secreted from CHO-K1 cells generated by Regeneron Protein Expression Sciences Group using proprietary EESYR technology. Noncovalently bound mature ActA dimer in complex with ActA propeptide was purified to homogeneity by heparin chromatography, followed by SEC at 4 °C, and the same purification protocol was used for both wild type Activin A and fingertip mutant complexes. Specifically, CHO-K1 conditioned media was applied without modification to 2 tandem HiTrap Heparin columns (GE Healthcare/Pharmacia) that were equilibrated in 20 mM Tris⋅HCl, pH 7.4, 0.15 M NaCl. Subsequent to sample loading, heparin columns were washed with 15 column volumes of 20 mM Tris⋅HCl, 0.15 M NaCl, pH 7.4, and then 15 column volumes of 20 mM Tris⋅HCl, 0.3 M NaCl, pH 7.4. Bound Activin A propeptide complexes were eluted with a linear NaCl gradient from 0.3 to 1.0 M. Pooled fractions were concentrated and applied to a Sephadex S200 HiPrep column (GE Healthcare/Pharmacia) equilibrated with 20 mM Tris⋅HCl, pH 7.4, and 0.3 M NaCl.
Crystallization and Structure Determination.
Complex formation between recombinant GDF11 and ActRIIB was performed by mixing the 2 proteins at a 2.1:1 molar ratio, followed by incubation at room temperature for 1 h and SEC in 20 mM Hepes, pH 7.5, and 500 mM NaCl. A complex peak was observed and contained both GDF11 and ActRIIB based on SDS/PAGE analysis. The same process was utilized for each binary complex formed for the native PAGE analysis featured in SI Appendix, Fig. S5. To establish appropriate mixing ratios of GDF11/ActRIIB binary complex and Alk5 for crystallization, a titration of Alk5 was performed through native PAGE analysis and optimized to 1:2.1 to minimize the amount of free binary or Alk5. The complex was concentrated to 8 mg/mL and set up by using the hanging drop method in crystallization trials. The final ternary complex crystal was grown in mother liquor containing 0.1 M sodium thiocyanate, 0.1 M sodium acetate, and 16% polyethylene glycol 8000 at pH 6 and 20 °C. Mother liquor supplemented with 25% ethylene glycol was used as a cryoprotectant for crystal freezing. X-ray diffraction data were collected at GM-CA beamline 23ID-B of the Advanced Photon Source at Argonne National Laboratory. Data were indexed and integrated with DIALS and subsequently scaled and merged through AIMLESS (47, 48). Placement of the ternary complex in the crystal lattice was determined through molecular replacement using Phaser and the search models ActRIIB (PDB ID code 1NYS), GDF11 (PDB ID code 5E4G), and Alk5 (PDB ID code 3KFD) (9, 13, 20, 49). Final refinement and model building were performed with Phenix.Refine, PDB-REDO, and Coot (SI Appendix, Table S1) (50–52). Final coordinates are available in the PDB under ID code 6MAC. All native PAGE analysis was carried out as described previously (53). To determine the composition from native gels, particular bands were excised and electro-eluted followed by analysis on SDS/PAGE.
Luciferase Reporter Assays.
Assays using the HEK-293-(CAGA)12 luciferase reporter cells (initially derived from RRID: CVCL_0045) were performed as previously described (19, 24). Briefly, cells were plated in 96-well format (3 × 104 cells per well) and grown for 24 h. For the inhibition comparison assays featured in Fig. 2, the growth media was removed and replaced with serum-free media containing 0.1% BSA and a 2-fold serial titration series of ActRIIA, ActRIIB, or ActRIIB:L79A ECD (0.25 to 160 nM) with constant ligand (ActA, GDF11, GDF8) concentration (0.62 nM). Cells were lysed, and luminescence was measured 18 h after treatment by using a Synergy H1 Hybrid plate reader (BioTek). For assays utilizing the R1B L17 cell line (RRID: CVCL_0596), cells were seeded into the 96-well format (3 × 104 cells per well) and grown for 24 h. The cells were then cotransfected with a total of 100 ng DNA containing the (CAGA)12 luciferase reporter construct and wildtype or mutant receptor containing plasmids (pRK5 rat Alk5 S278T [ST] variant) using Mirus LT-1 transfection reagent. The ST variant is not inhibited by SB-431542, reducing background signaling from low-level basal type I expression (54). Empty pRK5 vector was added to normalize the total DNA concentration. Following overnight transfection, media was exchanged to serum-free media containing 0.1% BSA, 0.62 nM (or titration of) mature GDF11 or TGFβ1, and 10 uM SB-431542. Cells were lysed, and luminescence was measured 18 h after ligand treatment.
PathHunter Dimerization Assays.
The PathHunter eXpress ActRIIB/Alk5 dimerization assays (93-1046E3) were developed by DiscoverX. In this assay, human ActRIIB (residues 1–164) and human Alk5 (residues 1–158) are C-terminally truncated to facilitate fusion to fragments of the β-galactosidase (β-gal) enzyme. Specifically, ActRIIB is fused to a small fragment of β-gal called the enzyme donor, PK1; Alk5 is fused to a larger fragment called the enzyme acceptor, EA; and both receptors are coexpressed within a human osteosarcoma cell line (U2OS). During receptor assembly with a cognate ligand, the fragments come together and form active β-galactosidase enzyme that hydrolyzes substrate to generate a chemiluminescent signal. Cells from the U2OS clones were plated in a 96-well format (1 × 104 cells per well) and grown for 24 h in Eagle’s minimum essential medium supplemented with 2% FBS and 1% penicillin/streptomycin. Media was then exchanged to serum-free media containing 0.1% BSA and a titration of ligand (∼1 pM–80 nM). Following treatment for 16 h, samples were treated with PathHunter FLASH substrate and detection reagents (DiscoverX) followed by chemiluminescence measurement according to the manufacturer’s protocol.
Supplementary Material
Acknowledgments
The authors thank Drs. Michael Becker and Qingping Xu and the staff of the 23-ID Beamline at the Advanced Photon Source, Argonne National Laboratory, for assistance in X-ray data collection.
Footnotes
Conflict of interest statement: T.B.T. is a consultant for Acceleron Pharma and Scientific Founder for Eclode. R.C. and R.K. are current employees of Acceleron Pharma with ownership interest in the company. A.N.E. and V.J.I. are current employees of Regeneron Pharmaceuticals with ownership interest in the company. The other authors report no competing interests.
This article is a PNAS Direct Submission.
Data deposition: The ternary structure of GDF11 bound to ActRIIB-ECD and Alk5-ECD described in this paper has been deposited to the Protein Data Bank, http://www.rcsb.org/ (accession code 6MAC).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1906253116/-/DCSupplemental.
References
- 1.Hinck A. P., Mueller T. D., Springer T. A., Structural biology and evolution of the TGF- b family. Cold Spring Harb. Perspect. Biol. 8, a022103 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinck A. P., Structural studies of the TGF-betas and their receptors–Insights into evolution of the TGF-beta superfamily. FEBS Lett. 586, 1860–1870 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Weiss A., Attisano L., The TGFbeta superfamily signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 2, 47–63 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Attisano L., et al. , Identification of human activin and TGFb type I receptors that form heteromeric kinase complexes with type II receptors. Cell 75, 671–680 (1993). [DOI] [PubMed] [Google Scholar]
- 5.Wrana J. L., Attisano L., Wieser R., Ventura F., Massague J., Mechanism of activation of the TGF-β receptor. Nature 370, 341–347 (1994). [DOI] [PubMed] [Google Scholar]
- 6.Keller S., Nickel J., Zhang J.-L., Sebald W., Mueller T. D., Molecular recognition of BMP-2 and BMP receptor IA. Nat. Struct. Mol. Biol. 11, 481–488 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Weber D., et al. , A silent H-bond can be mutationally activated for high-affinity interaction of BMP-2 and activin type IIB receptor. BMC Struct. Biol. 7, 6 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allendorph G. P., Vale W. W., Choe S., Structure of the ternary signaling complex of a TGF-beta superfamily member. Proc. Natl. Acad. Sci. U.S.A. 103, 7643–7648 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groppe J., et al. , Cooperative assembly of TGF-β superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol. Cell 29, 157–168 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Radaev S., et al. , Ternary complex of transforming growth factor-β1 reveals isoform-specific ligand recognition and receptor recruitment in the superfamily. J. Biol. Chem. 285, 14806–14814 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townson S. A., et al. , Specificity and structure of a high affinity activin receptor-like kinase 1 (ALK1) signaling complex. J. Biol. Chem. 287, 27313–27325 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenwald J., et al. , The BMP7/ActRII extracellular domain complex provides new insights into the cooperative nature of receptor assembly. Mol. Cell 11, 605–617 (2003). [DOI] [PubMed] [Google Scholar]
- 13.Thompson T. B., Woodruff T. K., Jardetzky T. S., Structures of an ActRIIB:activin A complex reveal a novel binding mode for TGF-beta ligand:receptor interactions. EMBO J. 22, 1555–1566 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadin D., Mueller T. D., Structural insights into BMP receptors: Specificity, activation and inhibition. Cytokine Growth Factor Rev. 27, 13–34 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Lee S.-J., McPherron A. C., Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. U.S.A. 98, 9306–9311 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rebbapragada A., Benchabane H., Wrana J. L., Celeste A. J., Attisano L., Myostatin signals through a transforming growth factor beta-like signaling pathway to block adipogenesis. Mol. Cell. Biol. 23, 7230–7242 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersson O., Reissmann E., Ibáñez C. F., Growth differentiation factor 11 signals through the transforming growth factor-beta receptor ALK5 to regionalize the anterior-posterior axis. EMBO Rep. 7, 831–837 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsuchida K., Nakatani M., Uezumi A., Murakami T., Cui X., Signal transduction pathway through activin receptors as a therapeutic target of musculoskeletal diseases and cancer. Endocr. J. 55, 11–21 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Walker R. G., et al. , Structural basis for potency differences between GDF8 and GDF11. BMC Biol. 15, 19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padyana A. K., et al. , Crystal structure of human GDF11. Acta Crystallogr. F Struct. Biol. Commun. 72, 160–164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krissinel E., Stock-based detection of protein oligomeric states in jsPISA. Nucleic Acids Res. 43, W314–W319 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sako D., et al. , Characterization of the ligand binding functionality of the extracellular domain of activin receptor type IIB. J. Biol. Chem. 285, 21037–21048 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee S.-J. J., Regulation of muscle mass by myostatin. Annu. Rev. Cell Dev. Biol. 20, 61–86 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Cash J. N., Rejon C. A., McPherron A. C., Bernard D. J., Thompson T. B., The structure of myostatin:follistatin 288: Insights into receptor utilization and heparin binding. EMBO J. 28, 2662–2676 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuniga J. E., et al. , The TβR-I pre-helix extension is structurally ordered in the unbound form and its flanking prolines are essential for binding. J. Mol. Biol. 412, 601–618 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul Oh S., et al. , Activin type IIA and IIB receptors mediate Gdf11 signaling in axial vertebral patterning. Genes Dev. 16, 2749–2754 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aykul S., Martinez-Hackert E., Transforming growth factor-β family ligands can function as antagonists by competing for type II receptor binding. J. Biol. Chem. 291, 10792–10804 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dussiot M., et al. , An activin receptor IIA ligand trap corrects ineffective erythropoiesis in β-thalassemia. Nat. Med. 20, 398–407 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goh B. C., et al. , Activin receptor type 2A (ACVR2A) functions directly in osteoblasts as a negative regulator of bone mass. J. Biol. Chem. 292, 13809–13822 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olsen O. E., et al. , Activin A inhibits BMP-signaling by binding ACVR2A and ACVR2B. Cell Commun. Signal. 13, 1–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cash J. N., et al. , Structure of myostatin·follistatin-like 3: N-terminal domains of follistatin-type molecules exhibit alternate modes of binding. J. Biol. Chem. 287, 1043–1053 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrington A. E., et al. , Structural basis for the inhibition of activin signalling by follistatin. EMBO J. 25, 1035–1045 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon B. H., et al. , An activin A/BMP2 chimera, AB204, displays bone-healing properties superior to those of BMP2. J. Bone Miner. Res. 29, 1950–1959 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenwald J., et al. , A flexible activin explains the membrane-dependent cooperative assembly of TGF-β family receptors. Mol. Cell 15, 485–489 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Stamler R., et al. , The structure of FSTL3.activin A complex: Differential binding of N-terminal domains influences follistatin-type antagonist specificity. J. Biol. Chem. 283, 32831–32838 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang X., Fischer G., Hyvönen M., Structure and activation of pro-activin A. Nat. Commun. 7, 12052 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cotton T. R., et al. , Structure of the human myostatin precursor and determinants of growth factor latency. EMBO J. 37, 367–383 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heinecke K., et al. , Receptor oligomerization and beyond: A case study in bone morphogenetic proteins. BMC Biol. 7, 59 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klammert U., et al. , GDF-5 can act as a context-dependent BMP-2 antagonist. BMC Biol. 13, 1–18 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nickel J., Kotzsch A., Sebald W., Mueller T. D., A single residue of GDF-5 defines binding specificity to BMP receptor IB. J. Mol. Biol. 349, 933–947 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Seemann P., et al. , Activating and deactivating mutations in the receptor interaction site of GDF5 cause symphalangism or brachydactyly type A2. J. Clin. Invest. 115, 2373–2381 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang T., et al. , TGF-β signalling is mediated by two autonomously functioning TβRI:TβRII pairs. EMBO J. 30, 1263–1276 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knaus P., Sebald W., Cooperativity of binding epitopes and receptor chains in the BMP/TGFβ superfamily. Biol. Chem. 382, 1189–1195 (2001). [DOI] [PubMed] [Google Scholar]
- 44.Zúñiga J. E., et al. , Assembly of TβRI:TβRII:TGFβ ternary complex in vitro with receptor extracellular domains is cooperative and isoform-dependent. J. Mol. Biol. 354, 1052–1068 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Zou Z., Sun P. D., Overexpression of human transforming growth factor-β1 using a recombinant CHO cell expression system. Protein Expr. Purif. 37, 265–272 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Gibson D. G., et al. , Supporting online material for creation of a bacterial cell controlled by a chemically synthesized genome. Science 329, 1–6 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Evans P. R., Murshudov G. N., How good are my data and what is the resolution? Acta Crystallogr. Sect. D. Biol. Crystallogr. 69, 1204–1214 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clabbers M. T. B., Gruene T., Parkhurst J. M., Abrahams J. P., Waterman D. G., Electron diffraction data processing with DIALS. Acta Crystallogr. Sect. D. Struct. Biol. 74, 506–518 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mccoy A. J., et al. , Phaser crystallographic software. J. Appl. Cryst. 40, 658–674 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Afonine P. V., Ralf W., Headd J. J., Thomas C., Towards automated crystallographic structure refinement with phenix refine. Acta Cryst. D68, 352–367 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joosten R. P., Long F., Murshudov G. N., Perrakis A., The PDB_REDO server for macromolecular structure model optimization. IUCrJ 1, 213–220 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emsley P., Lohkamp B., Scott W. G., Cowtan K., Features and development of Coot. Acta Crystallogr D. Biol Crystallogr. 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Walker R. G., et al. , Molecular characterization of latent GDF8 reveals mechanisms of activation. Proc. Natl. Acad. Sci. U.S.A. 115, E866–E875 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inman G. J., SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 62, 65–74 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.