Abstract
Small bowel diaphragm disease is a rare complication related to non-steroidal anti-inflammatory drug (NSAID) use. It presents with non-specific symptoms such as vomiting, abdominal pain, subacute bowel obstruction and occasionally as an acute abdominal condition. We report a case of diaphragm disease in a 33-year-old female who presented with vomiting, constipation and abdominal pain started 5 days earlier. Physical examination revealed palpated abdominal mass. The patient’s past medical history was remarkable for NSAID use. The patient was managed by surgical resection of involved intestine and diagnosis was confirmed by histological examination. Although there are few published cases of diaphragm disease in the medical literature, we recommend that this disease should be considered as one of the differential diagnoses when assessing patients presenting with non-specific abdominal symptoms with remarkable past medical history of NSAID use.
INTRODUCTION
Non-steroidal anti-inflammatory drugs (NSAIDs) are one of the most prescribed medications by medical healthcare providers worldwide. Recently, the pathological manifestations caused by these drugs have increased [1], including diaphragm disease, which was first described in 1988 by Lang et al. [2]. Debenham [3] was the first to link the long-term of using NSAIDs to enteropathy. What makes diaphragm disease clinically important is that it presents with non-specific symptoms such as anemia, abdominal pain, subacute bowel obstruction and occasionally as an acute abdominal condition. As a result, most cases are diagnosed during laparotomy [4]. The differential diagnosis of diaphragm disease may include Crohn’s disease, lymphoma and gastrointestinal (GI) adenocarcinoma [5]. Remarkable past medical history of prolonged use of NSAIDs can help to narrow the area of differential diagnosis and take diaphragm disease into account [4].
CASE PRESENTATION
A 33-year-old Caucasian female presented to the surgical department complaining of vomiting, constipation and abdominal pain that had started 5 days ago. The patient’s past medical history includes hypertension treated with bisoprolol 2.5 mg and aspirin 81 mg for 2 years. No past surgical history was noted. No history of malignancy in family, and she did not report any constitutional symptoms. The pain was colic and started in the epigastric and periumbilical regions and then radiated to the right lower quadrant. Physical examination revealed poorly localized, non-specific tenderness. The abdomen was soft and not distended. A mass in the right lower quadrant was palpated. No abnormal findings were found on digital rectal examination. The patient’s vital signs were normal. Laboratory studies revealed elevated white blood count 11 200/μl and normal liver and renal function tests.
Abdominal X-ray showed air–fluid levels. On abdominal ultrasonography, kidneys, liver, gall bladder, biliary ducts and spleen all were normal. However, ultrasound revealed peritoneal effusion about 200 ml. CT scan showed dilated small bowel without identifying the underlying cause.
Initially, conservative therapy with nasogastric decompression, intravenous fluids and monitoring was applied. Twelve hours later, symptoms of bowel obstruction persisted, WBC count elevated to 14 000 and peritoneal effusion increased to 400 ml on ultrasonography. Consequently, we performed an exploratory laparotomy, which showed a mass involving the distal ileum and small bowel dilation proximal to the obstruction (Figs. 1 and 2). The terminal ileum was resected with right hemicolectomy and primary ileocolic end-to-side anastomosis was performed using simple interrupted suture (vicryl suture 2-0) (Fig. 3).
Figure 1.
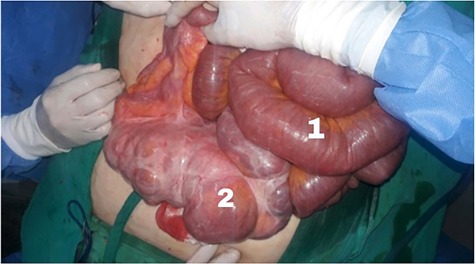
(1) Dilated small intestine proximal to the obstruction and (2) a 20 cm ileal mass.
Figure 2.
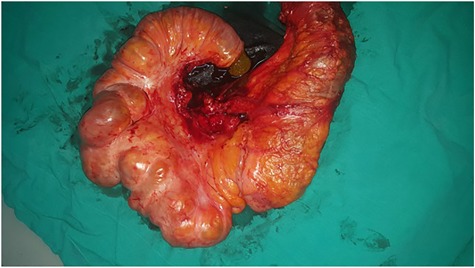
Excisional biopsy of the mass with part of the ascending colon.
Figure 3.
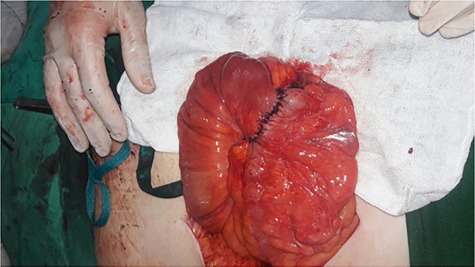
Primary ileocolic anastomosis.
On macroscopic examination, the ileal mass showed multiple mucosal diaphragms dividing the ileum into compartments. The cut surface of the terminal ileum revealed narrowing of the lumen and small polypoid thickness (Fig. 4).
Figure 4.
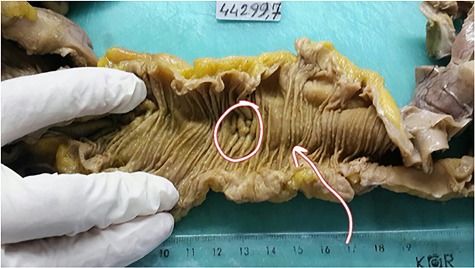
The cut surface of the terminal ileum reveals narrowing of the lumen and small polypoid thickness.
On pathological examination, sections through the diaphragms showed loss of the normal villous pattern suggesting regenerative changes and slightly fibrotic submucosa with muscle fibers running the plane of the muscularis mucosae rather than muscularis propria (Fig. 5). Sections of the polypoid thickness of the terminal ileum revealed marked hyperplasia of the lymphoid tissue in the mucosa and superficial submucosa with many lymphoid follicles containing conspicuous germinal center. Sections of the appendix revealed no pathological changes. Sections of the ascending colon revealed mild mononuclear inflammatory infiltration in the mucosa. Sections of all resected lymph nodes showed reactive changes. These findings are consistent with NSAID-induced diaphragm disease.
Figure 5.
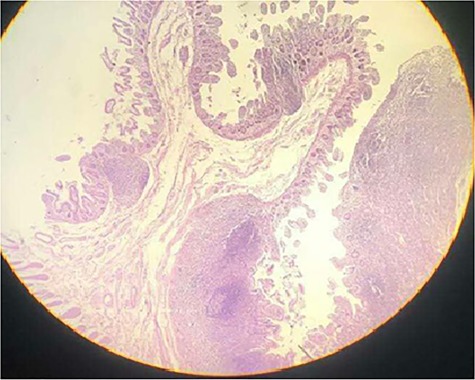
Sections through a diaphragm shows loss of the normal villous pattern suggesting regenerative changes and slightly fibrotic submucosa with muscle fibers running the plane of the muscularis mucosae rather than muscularis propria (hematoxylin and eosin stain) ×40.
The final histological diagnosis was NSAID-induced diaphragm disease and no malignancy was reported.
After surgical management, the patient began to improve and she started oral nutrition 24 h after operation and was discharged home 5 days later.
DISCUSSION
Small bowel diaphragm disease is a rare complication related to NSAID use. It may occur from mucosal and submucosal fibrosis causing a diaphragm or mucosal web, with luminal narrowing and consequently leading to obstruction [4].
The current actual prevalence of diaphragm disease is still unknown [1].
The pathogenesis is also not well understood; nevertheless, it is believed that NSAIDs play an important role in the developing of the disease. NSAIDs inhibit cyclooxygenase 1 (COX-1) and consequently prostaglandins in small intestine result in mucosal injury, fibrosis and mucosal barrier breakdown inducing an inflammatory response leading to circumferential ulceration and diaphragm formation [6].
The term ‘Diaphragm’ describes the rings of collagenous scar tissue, which are positioned like a drawstring across the bowel lumen, inducing the obstruction [7].
Previous studies reported that selective COX-2 inhibitors may be significantly less injurious to GI tract than traditional NSAIDs. This disease may occur in any part of GI tract but mostly located in the small intestine and especially in the ileum [8].
Diaphragm disease presents with non-specific symptoms such as vomiting, abdominal pain, subacute bowel obstruction and occasionally as an acute abdominal condition [9]. In our case, the patient complained of vomiting, constipation and abdominal pain. Additionally, on physical examination, a mass in the right lower quadrant was palpated, which is an interesting finding.
Preoperative diagnosis of diaphragm disease is challenging as it manifests with non-specific symptoms, and radiological findings are usually indefinite. CT scan may reveal small bowel obstruction; otherwise, it is unable to identify the thin diaphragms [4].
Consequently, diagnosis of diaphragm disease is most often made through exploratory laparotomy followed by histopathological examination of resected bowel [4].
Recent techniques such as capsule endoscopy may play a role in the diagnosis. Diaphragm disease diagnosis with capsule endoscopy was first reported by Yousfi et al. [10].
In our case, the CT scan revealed signs of bowel obstruction but common CT findings, such as symmetric ileal strictures and focal bowel wall thickening, were not identified.
Hence, the patient underwent an exploratory laparotomy and diagnosis was confirmed by histopathological examination of the excisional biopsy.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
AUTHOR CONTRIBUTIONS
A.K.D. and A.A.-M. contributed to the conception and design of the study. A.K.D., A.A.-M., A.A.N. and A.K. contributed to the analysis and interpretation of the data. A.A.-M., Y.K.D., A.A.N. and A.K. helped in drafting the article. A.K.D. and A.A.-H. participated in critical revision of the article for important intellectual content. All authors read and approved the final version of the manuscript.
ETHICAL APPROVAL
An informed consent was obtained from the patient.
ACKNOWLEDGEMENTS
We thank Lina Gabro, PhD, consulting pathologist, and Lama Kadoura and Nebras Kabawa, general surgery residents at Aleppo University Hospital, for their support.
REFERENCES
- 1. Pilgrim S, Velchuru V, Waters G, Tsiamis A, Lal R. Diaphragm disease and small bowel enteropathy due to nonsteroidal anti-inflammatory drugs: a surgical perspective. Colorectal Dis 2011;13:463–6. [DOI] [PubMed] [Google Scholar]
- 2. Lang J, Price AB, Levi AJ, Burke M, Gumpel JM, Bjarnason I. Diaphragm disease: pathology of disease of the small intestine induced by non-steroidal anti-inflammatory drugs. J Clin Pathol 1988;41:516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Debenham GP. Ulcer of the cecum during oxyphenbutazone (tandearil) therapy. Can Med Assoc J 1966;94:1182–4. [PMC free article] [PubMed] [Google Scholar]
- 4. Wang Y, Sun G, Cai F, Yang Y. Clinical features, diagnosis, and treatment, strategies of gastrointestinal diaphragm disease associated with nonsteroidal anti-inflammatory drugs. Gastroenterol Res Pract 2016;1:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sarantitis I, Gerrard AD, Teasdale R, Pettit S. Small bowel diaphragm disease mimicking malignancy. BMJ Case Rep 2015; doi: 10.1136/bcr-2015-210174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Matsui H, Shimokawa O, Kaneko T, Nagano Y, Rai K, Hyodo I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J Clin Biochem Nutr 2011;48:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maiden L, Thjodleifsson B, Seigal A, Bjarnason II, Scott D, Birgisson S, et al.. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: a cross-sectional capsule enteroscopy study. Clin Gastroenterol Hepatol 2007;5:1040–5. [DOI] [PubMed] [Google Scholar]
- 8. Ishihara M, Ohmiya N, Nakamura M, Funasaka K, Miyahara R, Ohno E, et al.. Risk factors of symptomatic NSAID-induced small intestinal injury and diaphragm disease. Aliment Pharmacol Ther 2014;40:538–47. [DOI] [PubMed] [Google Scholar]
- 9. Shumaker DA, Bladen K, Katon RM. NSAID-induced small bowel diaphragms and strictures diagnosed with intraoperative enteroscopy. Can J Gastroenterol 2001;15:619–23. [DOI] [PubMed] [Google Scholar]
- 10. Yousfi MM, De Petris G, Leighton JA, Sharma VK, Pockaj BA, Jaroszewski DE, et al.. Diaphragm disease after use of nonsteroidal anti-inflammatory agents: first report of diagnosis with capsule endoscopy. J Clin Gastroenterol 2004;38:686–91. [DOI] [PubMed] [Google Scholar]


