ABSTRACT
The development mechanism of endometriosis remains unknown. Water channel aquaporin-1 (AQP1) enhances water flux across cell membranes, which is highly expressed and associated with cell migration, metastasis and angiogenesis in some human cancers. In this study, the role of the Wnt signaling pathway mediated by AQP1 in endometriosis was investigated, in a bid to provide new therapeutic targets for endometriosis. Microarray expression profiles were screened to acquired differentially expressed genes related to endometriosis. Mouse models with endometriosis were established and grouped. The level of endometriosis was evaluated by measurement of the volume of ectopic region. The expression of AQP1, pathway-related factors (Wnt1 and Wnt4), adhesion molecules (VCAM-1 and ICAM-1), invasive factors (MMP-2, MMP-9, TIMP-1 and TIMP-2), angiogenic factors (VEGF-A, VEGFR1 and VEGFR2) and apoptotic factors (Caspase-3, Caspase-9, Bax and BcL-2) was measured by RT-qPCR and western blot analysis. Furthermore, the role of AQP1 in adhesion, invasion, angiogenesis, and apoptosis of ectopic endometrial cells was determined by transfection of si-AQP1 plasmid. AQP1 was robustly expressed in endometriosis. AQP1 gene silencing alleviated the progression of endometriosis by activating the Wnt signaling pathway in mice with endometriosis. Specifically, silencing of AQP1 gene inhibited ectopic endometrial cell adhesion and invasion abilities, suppressed angiogenesis while promoted apoptosis. Collectively, the present study highlights the role of AQP1 in the regulation of the Wnt signaling pathway in endometriosis mouse models, suggesting that AQP1 could represent a new target aimed at improving the survival of patients with endometriosis.
KEYWORDS: AQP1, Wnt signaling pathway, endometriosis, adhesion, invasion, angiogenesis, apoptosis
Introduction
Endometriosis is defined as the existence of endometrial matrix and glands outside the uterine cavity, mainly but not only in the pelvic cavity [1]. The main symptoms of endometriosis are pelvic pain and infertility [2]. Endometriosis seems to affect all aspects of the female genital system, leading to pregnancy loss or infertility for women [3]. Furthermore, endometriosis is not a cancer disease, but is frequently associated with the pathogenesis of ovarian clear-cell and endometrioid carcinomas [4]. Endometriosis has long diagnostic delays due to various healthcare and socio-economic factors, and the median time intervals between the onset of symptoms and diagnosis are usually 89 months, which suggests endometriosis is a complicated gynecological disease [5]. Treatment for patients with endometriosis consists of analgesics, surgical treatment (including hysterectomy) and hormonal treatment (oral contraceptives, medroxyprogesterone, danazol, and gonadotropin-releasing hormone (GnRH) agonists) [6]. Extensive researches suggest both environmental and genetic factors lead to its risk [7]. Heritable genetic risk such as chromosome alteration has been demonstrated to be strongly involved with endometriosis [8]. In addition, mutations of multiple cancer-associated genes are linked to endometriosis, like oncogene of KRAS and anti-oncogenes of PTEN and TNF [8]. Nevertheless, the pathogenesis of endometriosis is still not well understood since a lack of targeted medical treatment [9].
Aquaporin-1 (AQP1) is a kind of membrane transporter protein, promoting flux of small solutes like water across cell membranes and acting as a water channel that keeps body fluid homeostasis [10]. The aberrant expression of AQP1 has been found in multiple diseases. For instance, in a study, the author suggested that high expression of AQP1 is associated with poorer prognosis in endometrioid carcinoma and mucinous carcinoma [11]. Another study demonstrated that AQP1 delays the development of renal cysts in polycystic kidney disease by inhibiting the Wnt signaling pathway [12]. The Wnt signaling pathway is one of the well-known key cascades that regulate stemness and development, and the function of the Wnt signaling pathway in oncogenesis is presented in many types of tumors [13]. A recent study has revealed that secreted frizzled-related protein 2 (SFRP2) regulates the growth of endometriosis lesions and indicates the borders of endometriosis lesions together with CTNNB1, functioning as a typical stimulant of Wnt/CTNNB1 signaling pathway in endometriosis [9]. Furthermore, the engraftment and survival of desquamated endometrium are critically dependent on angiogenesis, and the Wnt/β-catenin signaling pathway plays a significant role in driving neovascularization [14]. From the above studies, we can speculate that AQP1 gene may affect endometriosis via the Wnt signaling pathway. By exploring the correlation between them, this study is performed to understand the mechanism of endometriosis progression and hence may provide new therapeutic targets.
Materials and methods
Ethics statement
This study protocol was approved by the Experimental Animal Ethics Committee of the First Hospital of Jilin University. The animal experiment was strictly adhered to the principle of using the least number of animals to complete the experiment and to minimize the pain of experimental animals.
Microarray-based analysis
National Center for Biotechnology Information (NCBI) was a public platform storing gene expression datasets, original sequences and records, from which an endometriosis-related dataset GSE25628 was obtained. The data downloaded from the Gene Expression Omnibus (GEO) included 16 endometriosis samples and six normal control samples. The important differentially expressed genes (DEGs) were analyzed by an Empirical Bayes method using the Bioconductor-based “limma” package in the R Programming Language. Finally, the DEGs were annotated by the “‘annotate’” package. p < 0.05 was considered statistically significant.
Protein–protein interaction (PPI) information of the DEGs was obtained from the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (http://www.stringdb.org/). Then, PPI networks were constructed using the Cytoscape software. p < 0.05 was considered statistically significant.
Cell culture, plasmid transfection, and identification
Endometrial stromal cells purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% calf serum, into which the 1: 1 mixed penicillin-streptomycin solution was added till the final concentration was 100 U/mL. Cells were then cultured in an incubator with 5% CO2 at 37°C, detached with 0.25% trypsin, and passaged at a ratio of 1: 3. Then, the cells were inoculated into a six-well plate at the density of 3 × 105 cells/well. When cell confluence reached 70–80%, the cells were employed for subsequent experiments.
The endometrial stromal cells in the logarithmic growth phase were assigned into si-negative control (si-NC) group (cells transfected with fluorescence-labeled nonsense sequence plasmids) and si-AQP1 group (cells transfected with fluorescence-labeled si-AQP1 plasmid). The transfected plasmids and fluorescent labeling of plasmids were all constructed by Shanghai Sangon Biotechnology Co., Ltd. (Shanghai, China). Cells were transfected in strict accordance with the instructions of lipofectamine 2000 (11668–019, Invitrogen, New York, California, USA). After 48 h of transfection, the transfection efficiency of cells was observed using a fluorescence microscope and the fluorescence response of the same cell image under fluorescence and non-fluorescence visual fields was compared. The cells showing green fluorescence under a fluoroscope indicated successful plasmid transfection.
Establishment of mouse models with endometriosis
A total of 70 clean inbred female BALB/c mice (weighing 18–20 g, aged 6–8 weeks) were purchased from the Animal Experimental Center of Southern Medical University (Guangzhou, Guangdong, China). Among these mice, 15 mice were selected into the sham group (the left uteruses of these mice were found by laparotomy, followed by abdominal incision suture without plasmid transfection). The remaining 55 mice were used to establish the mouse model of endometriosis [15]. Mice succeeded in endometriosis modeling exhibited the features similar to those previously reported by Vernon et al. [16]. Mice with endometriosis were randomly classified into three groups with 15 mice in each group: endometriosis group (mice with endometriosis without any treatment), si-NC group (mice with endometriosis injected with endometrial stromal cells carrying si-NC), and si-AQP1 group (mice with endometriosis injected with endometrial stromal cells carrying si-AQP1). On the 1st–12th day after the operation, mice in the endometriosis group were injected via the tail vein with purified water in the morning and intragastrically administered with 0.5 mg/kg estradiol valerate tablets in the afternoon. While mice in the si-NC group and the si-AQP1 group were injected via the tail vein with 0.002 μg endometrial stromal cells carrying si-NC and si-AQP1, respectively, in the morning and intragastrically administered with 0.5 mg/kg estradiol valerate tablets in the afternoon. For the sham group, mice were injected via the tail vein with purified water at 1 mL/100 g. One hour after the last administration of estradiol valerate on the 12th day, the mice in the sham group were intraperitoneally injected with the same amount of normal saline, while the mice in the other groups were intraperitoneally injected with oxytocin at 0.2 mL/mouse to induce a writhing response. The writhing latency and frequency of the mice within 30 min were observed and recorded.
Measurement of ectopic focus volume
After the writhing observation, the mice were anesthetized by intraperitoneal injection of 5% pentobarbital sodium and then euthanatized. The abdominal cavity was opened layer by layer on the aseptic operating table to observe the morphologies of the ectopic foci and measure the volume of the graft in millimeters with a vernier caliper. After that, the ectopic foci tissues were taken out immediately, some of which were immediately fixed with 4% paraformaldehyde solution, conventionally paraffin-embedded, and sliced into serial sections with the thickness of 4 μm. Some tissues were immediately placed in liquid nitrogen and then preserved at −80°C.
Hematoxylin-eosin (HE) staining
The ectopic foci tissues from five mice in each group were fixed with 4% paraformaldehyde solution overnight, routinely dehydrated for 1 min/time, cleared two times using xylene, and finally cooled down on the cold bench of a paraffin embedding machine. The paraffin-embedded sections were deparaffinized into the water, stained with hematoxylin (PT001, Shanghai Bogoo Biotechnology. Co., Ltd., Shanghai, China) at room temperature for 10 min and differentiated with 1% hydrochloric acid alcohol for 30 s. Next, the sections were stained with eosin (0001-H, Beijing XinHuaLvYuan Science and Technology Co., Ltd., Beijing, China) at room temperature for 1 min, dehydrated with gradient alcohol (70%, 80%, 90%, 95% and 100%; each for 1 min), and cleared two times with xylene I and II (GD-RY1215-12, each for 1 min; Shanghai Guduo Biotechnology Co., Ltd., Shanghai, China) respectively. Subsequently, the sections were mounted with neutral balsam in the ventilator cabinet. Finally, a Zeiss fluorescence microscope (Primo Star iLED, Beijing Boris Technology Co., Ltd., Beijing, China) was used to observe the morphological changes of the ectopic foci tissues in each group.
Immunohistochemistry
The ectopic foci tissues of five mice in each group were paraffin-embedded, sliced into sections, and deparaffinized into water. Afterwards, the sections were dehydrated by gradient alcohol and put in methanol containing 3% H2O2 for 20 min, followed by antigen retrieval in a water bath. After being cooled down under tap water, the sections were added with normal goat serum blocking liquid (C-0005, Shanghai Haoran Biological Technology Co., Ltd., Shanghai, China) at room temperature for 20 min, followed by an incubation overnight at 4°C with the following primary antibody, rabbit anti-mouse antibodies to AQP1 (1: 200, ab65837), vascular endothelial growth factor-A (VEGF-A; 1: 250, ab32152), and microvessel density (MVD; sc-376975, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Next, the sections were probed with secondary antibody, goat anti-rabbit to immunoglobulin G (IgG) (1: 1000, ab6785) and then binded with horseradish peroxidase (HRP)-labeled streptavidin protein working solution (0343-10000U, Imunbio, Beijing, China) at 37°C for 20 min. All antibodies were purchased from Abcam Inc. (Cambridge, MA, USA) except for MVD. After that, the sections were developed using diaminobenzidine (DAB; ST033, Whiga Biotechnology Co., Ltd., Guangzhou, Guangdong, China), counterstained with hematoxylin (PT001, Shanghai Bogoo Biotechnology Co., Ltd., Shanghai, China) for 1 min, and treated with 1% ammonia, followed by dehydration using gradient alcohol. After being cleared by xylene and mounted by neutral balsam, the sections were observed under the microscope with the images captured. Five high-power visual fields were randomly picked from each section containing at least 100 cells in each visual field. The cells were negative when the number of positive cells < 10%; positive cells ≥ 10% and < 50% referred to positive, and positive cells > 50% were strongly positive [17].
MVD results were evaluated based on the method of Weidner et al. [18,19]: any cell or cell cluster stained brownish-yellow in endometrial tissues clearly discriminated from the adjacent microvessels and glandular tissues were considered as a new blood vessel; if the structure was not connected, its branches were counted as a blood vessel. Tube-like structure formation was not a necessary condition for judgment. Relatively large lumens with thick muscular layers or lumen with more than eight red blood cells were not counted. Areas with the most microvessels observed under a low-power medium microscope (4 × 10) were counted with three visual fields selected under a medium-power microscope (20 × 10). The average was obtained as MVD. MVD was positive if the average value ≥ MVD threshold, and negative if the average value < MVD threshold [20].
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) assay
Ectopic foci tissues of five mice in each group were fixed with 4% paraformaldehyde, embedded by paraffin, and sectioned. Following the instructions of TUNEL kit (40302ES20, Shanghai Yeasen Biotechnology Co., Ltd., Shanghai, China), after routine deparaffinizing, the sections were added with 50 μL blocking solution (methanol containing 0.3% H2O2) for 10 min at room temperature, ice-bathed in penetrants for 2 min. Subsequently, the sections were added with 50 μL TUNEL reaction solution in the wet box at 37°C for 1 h, incubated with alkaline phosphatase-labeled antibodies (BAR301, Beijing Bersee Science and Technology Co., Ltd., Beijing, China), then developed with 50 μL DAB (ST033, Whiga Biotechnology Co., Ltd, Guangzhou, Guangdong, China) for 10 min. Afterwards, the sections were counterstained with hematoxylin (Shanghai Fusheng Industrial Co., Ltd., Shanghai, China), dehydrated with gradient ethanol, cleared with xylene, and mounted using balsam. The nuclei of apoptotic cells were stained brown yet those of normal cells were blue. The sections were scanned under a digital pathological section scanner (6,504,523,001, Roche Diagnostics Ltd., Shanghai, China) and observed under a microscope (400 ×). Five visual fields were picked at random in each section to calculate the percentage of the number of apoptotic positive cells by the number of total cells in the ectopic foci tissues in each visual field, and the average was used as the apoptotic index (AI).
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the transfected cells in each group in strict accordance with the instructions of TRIZOL kits (15596–018, Beijing Solarbio science & technology Co., Ltd., Beijing, China), and the concentration of RNA was determined. The primers were synthesized by Takara Biotechnology Ltd. (Dalian, Liaoning, China) (Table 1). Reverse transcription was performed according to the instructions of complementary DNA (cDNA) Reverse Transcription Kits (K1622, Beijing Renata Biotechnology Co., Ltd., Beijing, China). Fluorescence quantitative PCR (ViiA 7, DA AN Gene Co., Ltd of Sun Yat-sen University, Guangzhou, Guangdong, China) was applied for RT-qPCR preformation. Using β-actin as the internal reference, the relative transcription level of target genes was calculated by the relative quantitative method (2−△△CT method) [21].
Table 1.
Primer sequence for reverse transcription quantitative polymerase chain reaction.
| Gene | Sequence (5ʹ – 3ʹ) |
|---|---|
| AQP1 | F: GCCTTGTCTGGGGCAGTAAT |
| R: CTAAGGTGGGGGACTTGCTG | |
| Wnt1 | F: CCCAGGGTTCATAGCGATCC |
| R: TAGGGACCCGAGAGACAAGG | |
| Wnt4 | F: GGACTTTGTTTATGCCTCTGGC |
| R: TGCCTGGAATCTAGCTGCTC | |
| VCAM-1 | F: TGACATCTCCCCTGGATCTC |
| R: CTCCAGTTTCCTTCGCTGAC | |
| ICAM-1 | F: AGGTATCCATCCATCCCACA |
| R: GCCACAGTTCTCAAAGCACA | |
| MMP-2 | F: AGATCTTCTTCTTCAAGGACCGGTT |
| R: GGCTGGTCAGTGGCTTGGGGTA | |
| MMP-9 | F: CGACAGCACCTCCCACTATG |
| R: CCCAACTTATCCAGACTCCT | |
| TIMP-1 | F: CTGGCATCCTCTTGTTGCTA |
| R: AGGGATCTCCAGGTGCACAA | |
| TIMP-2 | F: CATCCAAGTGGGTTCACGCT |
| R: GAAAAGGACCTGGTAGGCCG | |
| VEGF-A | F: GGAGATCCTTCGAGGAGCACTT |
| R: GGCGATTTAGCAGCAGATATAAGAA | |
| Caspase-3 | F: TTAGATTCTGGTATTGAAGC |
| R: GAAATCCTGTCGAGTGGAGCAGG | |
| Caspase-9 | F: AGCTCTTCTTCATCCAGG |
| R: CCCCCAGCCTCATGAAGTT | |
| Bax | F: CGGCGAATTGGAGATGAACTG |
| R: GCAAAGTAGAAGAGGGCAACC | |
| BcL-2 | F: TACCGTCGTGACTTCGCAGA |
| R: GCCAGGCTGAGCAGGGTATT | |
| β-actin | F: AAATCGTGCGTGACATCAAAGA |
| R: CAAGAAGGAAGGCTGGAAAAGA |
Note: F, forward; R, reverse; AQP1, aquaporin-1; VCAM, vascular cell adhesion molecule; ICAM, intercellular adhesion molecule; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinases; VEGF-A, vascular endothelial growth factor-A; Bax, Bcl-2-associated X protein; BcL-2, B-cell lymphoma-2.
Western blot analysis
After 48 h, cells from each group were lysed with protein lysis buffer at 4°C for 30 min and centrifuged at 25764 × g for 20 min at 4°C. The protein concentration of each sample was determined using a bicinchoninic acid (BCA) kit (20201ES76, Shanghai Yeasen Biotechnology Co., Ltd., Shanghai, China). The protein was transferred onto a polyvinylidene fluoride (PVDF) membrane by wet transfer method after separation by polyacrylamide gel electrophoresis. The membrane was blocked in 5% bovine serum albumin (BSA) at room temperature for 1 h and then incubated overnight at 4°C on a shaker with the addition of the primary antibody, rabbit anti-mouse antibodies to AQP1 (ab65837, 1: 1000), Wnt1 (ab15251, 1: 2000), Wnt4 (ab94742, 1: 1000), VCAM-1 (ab134047, 1: 3000), ICAM-1 (ab25375, 1: 1000), MMP-2 (ab37150, 1: 1000), MMP-9 (ab38898, 1: 1000), TIMP-1 (ab38978, 1: 3000), TIMP-2 (ab180630, 1: 1000), VEGF-A (ab51745, 1: 1000), VEGFR1 (ab2350, 1: 500), VEGFR2 (ab11939, 1: 500), cleaved Caspase-3 (ab2302, 1: 500), cleaved Caspase-9 (ab2324, 1: 1000), Caspase-3 (ab4051, 1: 500), Caspase-9 (ab2013, 1: 1000), Bax (ab32503, 1: 5000) and BcL-2 (ab59348, 1: 800). The membrane was washed 3 times with Tris-Buffered Saline and Tween 20 (TBST; 5 min each time), incubated with the addition of HRP-labeled goat anti-rabbit IgG (ab205718, 1: 20000) at room temperature for 1 h. The above antibodies were all obtained from Abcam (Cambridge, MA, USA). The membrane was washed 3 times with TBST (5 min each time), and developed with developing solution. ImageJ 1.48u software (National Institutes of Health, Bethesda, MD, USA) was used for protein quantitative analysis and the relative protein levels were expressed as the gray ratio of each protein to the internal reference glyceraldehyde-3-phosphate dehydrogenase (GAPDH; ab9485, 1: 2500).
Statistical analysis
All data were processed by Statistical Product and Service Solutions (SPSS) 21.0 (IBM Corp. Armonk, NY, USA) and tested by normal distribution and homogeneity of variance. The measurement data conforming to the normal distribution were expressed as mean ± standard deviation yet those not conforming to the normal distribution or homogeneity of variance by interquartile range. Unpaired t-test was used for comparison between two groups with the post hoc test. The skewed distribution data were tested by a non-parametric test, Wilcoxon Signed Rank Test. One-way analysis of variance (ANOVA) was used in comparison among multiple groups. The difference was statistically significant at p < 0.05.
Results
AQP1 gene expression is upregulated in endometriosis
From the GSE25628 dataset downloaded from the GEO database, 2862 DEGs were screened out. Previous studies revealed that AQP1 could act as a soluble regulator of the Wnt signaling pathway, whose dysregulation was associated with AQP1 expression, and AQP1 knockdown promoted the proliferation of lung cancer cells. Moreover, AQP1 has been shown to be associated with various malignancies through the Wnt signaling pathway [22–25]. AQP1 plays a carcinogenic role in many human cancers, but its exact function in human endometriosis is unclear. Therefore, the present study considered AQP1 as a candidate gene and aimed to predict its function and clinical significance in endometriosis. Furthermore, AQP1 ranked higher in the DEGs related to endometriosis. Heatmap of several DEGs in GSE25628 is shown in Figure 1(a). AQP1 gene expression was up-regulated in endometriosis (Figure 1(b)). AQP1 was then predicted to interact with topoisomerase 2-beta (TOP2B) by PPI (Figure 1(c)), which was correlated to the Wnt/β-catenin signaling pathway [12,26,27]. All in all, AQP1 gene is highly expressed in endometriosis and is related to Wnt signaling pathway in silico.
Figure 1.

AQP1 gene expression is elevated in endometriosis. (a), heatmap of the DEGs in GSE25628 dataset, in which the abscissa refers to sample number, ordinate refers to gene name, upper dendrogram represents sample types clustering, and the right color histogram represents gene expression. Red refers to high expression and green refers to low expression. Each box represents the expression of a gene in one sample. Left dendrogram represents the clustering of gene expression. (b), AQP1 gene expression profiling. (c), PPI network, in which blue represents low combined-score and red represents high combined-score. AQP1, aquaporin 1; DEGs, differentially expressed genes; PPI, protein–protein interaction.
Mouse model of endometriosis is successfully established
In order to verify the transfection efficiency and identify whether the model was successfully induced, the fluorescence, graft growth, ectopic foci volume and writhing response of mice in each group were observed. It was found that no green fluorescence was observed in each group under a normal microscope. Under a fluorescence microscope, no fluorescence was observed in the sham group and the endometriosis group, but the si-NC group and the si-AQP1 group showed obvious green fluorescence. The transfection rate in the si-NC group and the si-AQP1 group was 98% and 99%, respectively, indicating that plasmids were successfully transfected (Figure 2(a)).
Figure 2.
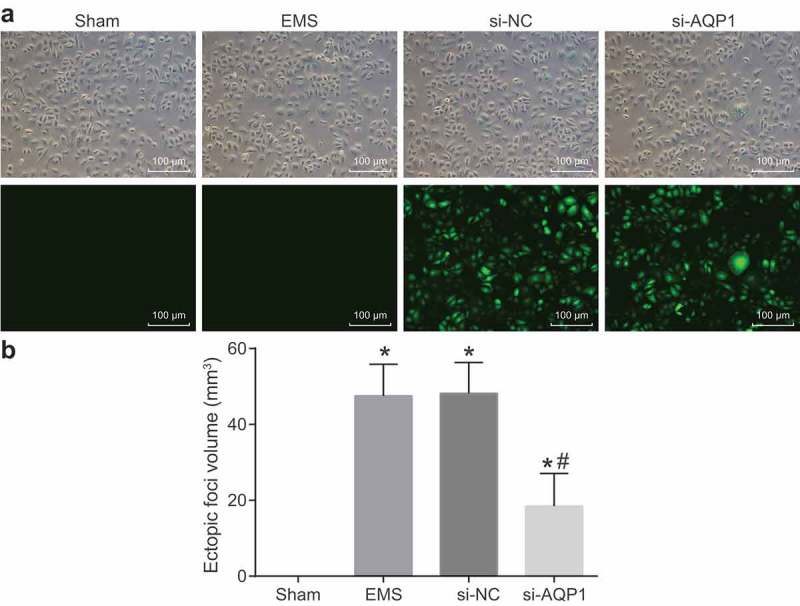
Plasmids are successfully transfected and the mouse models of endometriosis are successfully established. (a), plasmid transfection efficiency under a fluorescence microscope (100 ×); (b), ectopic foci volume of mice in each group; *, p < 0.05 vs. the sham group; #, p < 0.05 vs. the endometriosis and si-NC groups; the ectopic foci volumes were measurement data and were presented as means ± standard deviation and analyzed by one-way ANOVA, n = 15. NC, negative control; ANOVA, analysis of variance; EMS: endometriosis.
The results of graft growth in each group showed that among 55 mice, 45 mice succeeded in modeling with the success rate of 81.81%, seven mice had no graft growth, and three mice died. Except for the sham group, the remaining groups showed graft growth. After opening the abdominal cavity, some grafts were found to be adherent to the surrounding adipose tissues. When the adhesions were separated, it was found that the grafts on the abdominal wall grew well with small vesicles of different sizes containing clear liquid and blood vessels formed on the surface of the grafts.
The ectopic foci volume measurement results (Figure 2(b)) indicated that no ectopic foci appeared in the sham group. In comparison, with the sham group, the ectopic foci volume in the other groups was increased significantly (all p < 0.05). The difference in the endometriosis group and si-NC group was not statistically significant (p > 0.05). When compared with the endometriosis and si-NC groups, the ectopic foci volume in the si-AQP1 group was obviously decreased (both p < 0.05).
The results of writhing response (Table 2) revealed that writhing response occurred in the other groups except for the sham group. Compared with the endometriosis group and the si-NC group, writhing latency was evidently prolonged and writhing frequency was significantly reduced in the si-AQP1 group (all p < 0.05). All the above findings suggest that the mouse model of endometriosis is successfully established.
Table 2.
AQP1 gene silencing suppresses the writhing response of mice with endometriosis.
| Group | n | Writhing latency (s) | Writhing frequency |
|---|---|---|---|
| Sham | 15 | – | – |
| Endometriosis syndrome | 15 | 32.58 ± 9.54* | 21.93 ± 7.91* |
| si-NC | 15 | 33.28 ± 9.63* | 22.73 ± 7.94* |
| si-AQP1 | 15 | 91.46 ± 22.85*# | 8.47 ± 4.76*# |
*, p < 0.05 vs. the sham group; #, p < 0.05 vs. the endometriosis group and the si-NC group; all measurement data were expressed as means ± standard deviation and analyzed using one-way ANOVA; n = 15. AQP1, aquaporin-1; NC, negative control; ANOVA, analysis of variance.
AQP1 gene silencing alleviates the progression of endometriosis
The pathological changes of ectopic foci tissues were detected by HE staining and immunohistochemistry. As shown by HE staining results (Figure 3(a)), most glands in the sham group were flat; glandular epithelial cells and interstitial cells invaded into few tissues. There existed insignificant difference between the endometriosis and si-NC groups (p > 0.05). In the endometriosis and si-NC groups, endometrioid glands and matrixes appeared, the glandular epithelial cells were in cylindrical shapes, and glandular epithelial cells interstitial cells were observed to invade the surrounding tissues. In the si-AQP1 group, most glands were atrophied and some tissues were not fully developed. Compared with the endometriosis group and si-NC group, the above histopathological changes in the si-AQP1 group were obviously reduced (p < 0.05).
Figure 3.

AQP1 gene silencing contributes to attenuated endometriosis. (a), pathological changes of ectopic foci tissues of mice in each group by HE staining (400 ×); (b), immunohistochemical staining of AQP1 in ectopic foci tissues of mice in each group (400 ×); (c), positive rate of AQP1 in ectopic foci tissues of mice in each group determined by immunohistochemistry. *, p < 0.05 vs. the sham group; #, p < 0.05 vs. the endometriosis and si-NC groups; all the results above were measurement data; these data were presented as means ± standard deviation and analyzed using one-way ANOVA, n = 5. AQP1, aquaporin-1; HE, hematoxylin-eosin; NC, negative control; ANOVA, analysis of variance; EMS: endometriosis.
Immunohistochemistry results (Figure 3(b and c)) indicated that AQP1 was mainly expressed in cell membrane and cytoplasm. Compared with the sham group, the positive rate of AQP1 in the other groups was increased greatly (all p < 0.05). The difference in the endometriosis group and si-NC group was not statistically significant (p > 0.05). The positive rate of AQP1 in the si-AQP1 group was remarkably decreased versus the endometriosis and si-NC groups (both p < 0.05). It is suggested that silencing of AQP1 could inhibit the pathological process of endometriosis.
AQP1 gene silencing activates the Wnt signaling pathway in endometriosis
In order to detect the interaction between AQP1 gene and the Wnt signaling pathway in endometriosis, RT-qPCR (Figure 4(a)) and western blot analysis (Figure 4(b and c)) were applied to, respectively, measure the mRNA and protein expression of AQP1, Wnt1, and Wnt4. The results revealed that in comparison with the sham group, the mRNA and protein expression of AQP1 was higher while that of Wnt1 and Wnt4 was lower in the si-NC, endometriosis, si-AQP1 groups (all p < 0.05). The expression of AQP1, Wnt1 and Wnt4 did not differ between the endometriosis and si-NC groups (all p > 0.05). In contrast to the endometriosis and si-NC groups, the si-AQP1 group exhibited low mRNA and protein expression of AQP1 but high those of Wnt1 and Wnt4 (all p < 0.05). These findings provide evidence that AQP1 gene silencing facilitates the activation of the Wnt signaling pathway in ectopic foci tissues in mice with endometriosis.
Figure 4.

The Wnt signaling pathway is activated with the silencing of AQP1 gene. (a), mRNA expression of AQP1, Wnt1 and Wnt4 in ectopic foci tissues of mice in each group determined by RT-qPCR; (b), protein bands in western blot analysis of AQP1, Wnt1, Wnt4, and β-actin in ectopic foci tissues of mice in each group; (c), quantification of protein expression of AQP1, Wnt1 and Wnt4 in ectopic foci tissues of mice in each group by western blot analysis; *, p < 0.05 vs. the sham group; #, p < 0.05 vs. the endometriosis and si-NC groups; the above results were all measurement data; these data were expressed as means ± standard deviation and analyzed using one-way ANOVA, n = 15. AQP1, aquaporin-1; RT-qPCR, reverse transcription quantitative polymerase chain reaction; NC, negative control; ANOVA, analysis of variance; EMS: endometriosis.
AQP1 gene silencing inhibits adhesion and invasion abilities of ectopic endometrial cells
In order to find out the effects of APQ1 on adhesion and invasion abilities of ectopic endometrial cells, RT-qPCR and western blot analysis were used to, respectively, examine the mRNA and protein expression of adhesion molecules (VCAM-1 and ICAM-1) and invasion-related factors (MMP-2, MMP-9, TIMP-1, and TIMP-2) in endometrial cells of each group. The results (Figure 5(a–c)) showed that compared with the sham group, the mRNA and protein expression of VCAM-1, ICAM-1, MMP-2, and MMP-9 was markedly increased while that of TIMP-1 and TIMP-2 was obviously decreased in the other groups (all p < 0.05). The difference in adhesion and invasion abilities of ectopic endometrial cells in the endometriosis group and si-NC group was not statistically significant (p > 0.05). Relative to the endometriosis group and si-NC group, the si-AQP1 group displayed downregulation of VCAM-1, ICAM-1, MMP-2, and MMP-9 but upregulated that of TIMP-1 and TIMP-2 (all p < 0.05). Based on these results, the conclusion could be made that silencing of AQP1 suppresses ectopic endometrial cell adhesion and invasion in mice with endometriosis.
Figure 5.

Depleted AQP1 leads to repressed ectopic endometrial cell adhesion and invasion in mice with endometriosis. (a), mRNA expression of adhesion molecules (VCAM-1 and ICAM-1) and invasion-related factors (MMP-2, MMP-9, TIMP-1, and TIMP-2) in each group by RT-qPCR; (b) protein expression of adhesion molecules (VCAM-1 and ICAM-1), invasion-related factors (MMP-2, MMP-9, TIMP-1, and TIMP-2), and internal control (β-actin) in each group by western blot analysis; (c), quantification of protein expression of adhesion molecules (VCAM-1 and ICAM-1) and invasion-related factors (MMP-2, MMP-9, TIMP-1, and TIMP-2) in each group by western blot analysis; *, p < 0.05 vs. the sham group; #, p < 0.05 vs. the endometriosis and si-NC groups; the above results were all measurement data; these data were expressed as means ± standard deviation and analyzed using one-way ANOVA; the experiment was repeated three times; n = 15. AQP1, aquaporin-1; VCAM, vascular cell adhesion molecule; ICAM, intercellular adhesion molecule; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase; RT-qPCR, reverse transcription quantitative polymerase chain reaction; NC, negative control; ANOVA, analysis of variance; EMS: endometriosis.
AQP1 gene silencing inhibits angiogenesis of ectopic endometrial cells
After the experiment established the findings proving that the silencing of AQP1 gene could inhibit the adhesion and invasion abilities of ectopic endometrial cells, the focus of the experiment then shifted to how AQP1 gene silencing affects angiogenesis of ectopic endometrial cells. As suggested by the results of immunohistochemistry (Figure 6(a–c)), the expression of VEGF-A protein was mainly located in the cytoplasm, and positive rate of VEGF-A and MVD in the si-NC, endometriosis, si-AQP1 groups were significantly elevated (all p < 0.05) compared with the sham group. The difference in positive rate of VEGF and MVD between the endometriosis group and the si-NC group was not statistically significant (both p > 0.05). The si-AQP1 group presented reduced positive rate of VEGF-A and MVD in contrast to the endometriosis group and the si-NC group (all p < 0.05).
Figure 6.

Angiogenesis in ectopic endometrial cells in mice with endometriosis is inhibited with the silencing of AQP1. (a), immunohistochemical staining of VEGF-A in ectopic foci tissues of mice in each group (400 ×); (b), positive rate of VEGF-A in ectopic foci tissues of mice in each group; (c), MVD in ectopic foci tissues of mice in each group by immunohistochemistry; (d), mRNA expression of VEGF in ectopic foci tissues of mice in each group by RT-qPCR; (e), protein expression of VEGF-A, VEGFR1 and VEGFR2 in ectopic foci tissues of mice in each group; (f), quantification of protein expression of VEGF-A, VEGFR1 and VEGFR2 in ectopic foci tissues of mice in each group by western blot analysis; *, p < 0.05 vs. the sham group; #, p < 0.05 vs. the endometriosis and si-NC groups; the above results were all measurement data; these data were expressed as means ± standard deviation and analyzed using one-way ANOVA; the experiment was repeated three times; n = 15. AQP1, aquaporin-1; VEGF-A, vascular endothelial growth factor-A; VEGFR, vascular endothelial growth factor receptor; MVD, microvessel density; RT-qPCR, reverse transcription quantitative polymerase chain reaction; NC, negative control; ANOVA, analysis of variance; EMS: endometriosis.
The results of RT-qPCR (Figure 6(d)) and western blot analysis (Figure 6(a–f)), indicated that the mRNA expression of VEGF and the protein expression of VEGF-A, VEGFR1 and VEGFR2 in the si-NC, endometriosis, si-AQP1 groups were significantly upregulated compared with the sham group (all p < 0.05). The endometriosis group and the si-NC group showed no significantly different in mRNA expression of VEGF and the protein expression of VEGF-A, VEGFR1 and VEGFR2 (p > 0.05). When compared with the endometriosis group and the si-NC group, the mRNA expression of VEGF and the protein expression of VEGF-A, VEGFR1, and VEGFR2 in the si-AQP1 group was obviously downregulated (p < 0.05). These results reveal that AQP1 gene silencing represses angiogenesis in ectopic endometrial cells in mice with endometriosis.
AQP1 gene silencing promotes apoptosis of ectopic endometrial cells
With the aim to explore the role of AQP1 in apoptosis of ectopic endometrial cells, the mRNA expression of apoptosis-related factors (Caspase-3, Caspase-9, Bax, and BcL-2) was measured using RT-qPCR, the results (Figure 7(a)) of which indicated that compared with the sham group, mRNA expression of Caspase-3, Caspase-9 and Bax was profoundly reduced while that of BcL-2 was dramatically elevated in the si-NC, endometriosis, si-AQP1 groups (all p < 0.05). The difference in the mRNA expression of apoptosis-related factors (Caspase-3, Caspase-9, Bax, and BcL-2) in the endometriosis group and the si-NC group was not statistically significant (all p > 0.05). When in comparison with the endometriosis group and the si-NC group, the si-AQP1 group had elevations in the mRNA expression of Caspase-3, Caspase-9, and Bax but a decline in that of BcL-2 (all p < 0.05). Meanwhile, western blot analysis was conducted to determine the protein expression of apoptosis-related factors (Figure 7(b and c)), which revealed significant enhancements in cleaved Caspase-3, cleaved Caspase-9 and Bax protein expression, significant elevation of BcL-2 (all p < 0.05), while insignificant change in the protein expression of Caspase-3 and Caspase-9 in all group except for the sham group. In comparison, with the endometriosis and si-NC groups, the si-AQP1 group displayed elevations in the protein expression of cleaved Caspase-3, cleaved Caspase-9 and Bax, a decline in that of BcL-2 (all p < 0.05), whereas no significant change in the protein expression of Caspase-3 and Caspase-9 was observed.
Figure 7.

AQP1 gene silencing contributes to enhanced ectopic endometrial cell apoptosis in mice with endometriosis. (a), mRNA expression of Caspase-3, Caspase-9, Bax, and BcL-2 in each group by RT-qPCR; (b), protein expression of Caspase-3, cleaved Caspase-3, Caspase-9, cleaved Caspase-9, Bax, and BcL-2 and internal control (β-actin); (c), quantification of protein expression of Caspase-3, cleaved Caspase-3, Caspase-9, cleaved Caspase-9, Bax, and BcL-2 in each group by western blot analysis; (d), images of apoptosis of ectopic endometrial cells in each group (400 ×) by TUNEL assay; (e), AI of ectopic endometrial cells in each group by TUNEL assay; *, p < 0.05 vs. the sham group; #, p < 0.05 vs. the endometriosis and si-NC groups; the above results were all measurement data; these data were expressed as means ± standard deviation and analyzed using one-way ANOVA; the experiment was repeated three times; n = 15. AQP1, aquaporin 1; Bax, Bcl-2-associated X protein; BcL-2, B-cell lymphoma-2; TUNEL, terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling; AI, apoptotic index; NC, negative control; ANOVA, analysis of variance; EMS: endometriosis.
The results of TUNEL assay (Figure 7(d and e)), which was conducted to determine the AI, revealed that compared with the sham group, AI in the si-NC, endometriosis, and si-AQP1 groups showed a downward trend (all p < 0.05). There was no obvious difference between the endometriosis group and the si-NC group concerning the AI (p > 0.05). Moreover, the si-AQP1 group showed higher AI than the endometriosis group and the si-NC group (p < 0.05). It is demonstrated that the silencing of AQP1 gene accelerates apoptosis of ectopic endometrial cells in mice with endometriosis.
Discussion
Endometriosis is a gynecological disorder, featuring in the existence and growth of functional endometrial-like tissues outside of uterine, influencing women of childbearing age with dysmenorrhea, dyspareunia, chronic pelvic pain and infertility [28]. AQP1, a water channel protein, may play a key role in maintaining maternal-fetal fluid homeostasis at the late duration of pregnancy [29]. So, this study is aimed at investigating the mechanism of AQP1 gene-mediated Wnt signaling pathway in endometriosis and thus finding new therapeutic targets for endometriosis. The results in our study suggested that the silencing of AQP1 activated the Wnt signaling pathway, which subsequently suppressed adhesion, invasion, and angiogenesis while accelerated apoptosis of ectopic endometrial cells in mice with endometriosis.
One of the most significant findings in our study was that AQP1 expression was upregulated in endometriosis, and the silencing of AQP1 inhibited the pathological process of endometriosis. AQP1 regulates osmotic pressure between cytoplasm and tissue fluid and is demonstrated to be involved in proliferation of many types of tumors. For example, in accordance with the results in this study, increased expression of AQP1 is found in patients with osteosarcoma, and AQP1 knockdown can suppress cell adhesion and invasion in vitro as well as inhibit tumor growth in vivo [30]. Furthermore, lymph node metastasis is found in bladder uroepithelial cell carcinoma patients with evident high expression of AQP1 [31]. In addition, our study also found out that silencing AQP1 promoted the activation of the Wnt signaling pathway in ectopic foci tissues of mice with endometriosis, which was reflected by the increased mRNA and protein expression level of Wnt1 and Wnt4. Biochemical analysis in a study revealed that AQP1 is a new determinant factor in renal cyst development and may be involved in suppressing the Wnt signaling pathway via an AQP1-macromolecular signaling complex [12]. Therefore, this previous study may provide evidence for our result that high expressed AQP1 was involved in tumor growth and knockdown of AQP1 could be associated with the Wnt signaling pathway activation.
Besides, our study also revealed that silencing AQP1 gene restrained adhesion and invasion abilities of ectopic endometrial cells in mice with endometriosis, which showed downregulation of ICAM-1, ICAM-1, MMP-2 and MMP-9 while upregulation of TIMP-1 and TIMP-2. The results from a research suggested that down-regulation of AQP1 can efficiently repress the capabilities of cell adhesion, invasion and proliferation and by targeting focal adhesion genes and the transforming growth factor-β (TGF-β) signaling pathway [30]. Moreover, the data from another study also indicated that silenced AQP1 greatly contributed to the decreased migration and invasion capabilities of AQP1-siRNA cells, and particularly, AQP1 knockdown by siRNA inhibited the expression of MMP-2 and MMP-9 which played promotion marker in tumor progression [32]. Similarly, the results from a current study demonstrated that downregulated AQP1 using siRNA greatly restrained cell migration, invasion and viability and boosted apoptosis of ovarian carcinoma cells [22]. Hence, AQP1 gene regulates tumor cell growth by modulating cellular physiology.
Finally, our study also suggested that the silencing of AQP1 suppressed angiogenesis of ectopic endometrial cells, which was proved by the notably reduced expression of VEGF and MVD. In accordance with our finding, researchers observed a decline in neovascularization in AQP1-deficient mice in comparison with wild type mice using the matrigel plug angiogenesis assay, regardless of VEGF addition [33]. Considering cell apoptosis, in this present study, AQP1 silencing promoted apoptosis of ectopic endometrial cells, which was determined by the elevated expression of apoptosis-related factors (Caspase-3, Caspase-9, and Bax) as well as the reduced expression of BcL-2. A previous study demonstrated that, in line with DNA fragmentation analysis, depleted AQP1‐dependent angiogenesis induced cell apoptosis in tumors [34]. In another study, the author confirmed that the AQP1 inhibitor-bacopaside II inhibited the viability, migration and tube formation of endothelial cells, and enhanced apoptosis [35]. Furthermore, it was found that the knockdown of AQP1 expression in chondrocytes from osteoarthritic rats using siRNA reduced the activity and expression of caspase-3, which indicated that AQP1 conduced to chondrocyte apoptosis and osteoarthritis development [36]. Thereby, AQP1 could regulate angiogenesis and apoptosis of cell in multiple diseases.
Taken together, this study demonstrates that the silence of AQP1 activates the Wnt signaling pathway and thus inhibits the progression of endometriosis (Figure 8). Therefore, we believe that AQP1 gene can provide a reference for diagnostic and therapeutic targets of endometriosis. Certainly, in order to ensure reliability, much more experimental subjects or methods will need to be used for further verification of the results in the future.
Figure 8.
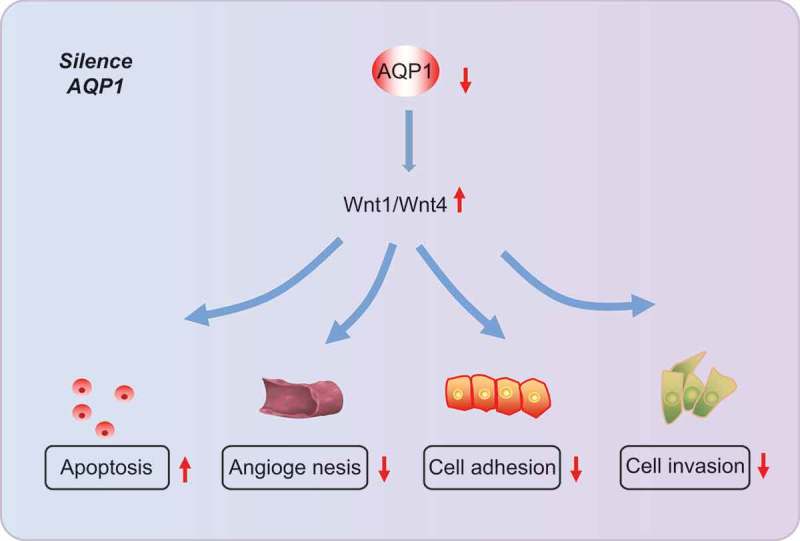
Regulatory mechanism of AQP1 in endometriosis. Silencing AQP1 activates the Wnt signaling pathway to inhibit adhesion, invasion, angiogenesis, and apoptosis of ectopic endometrial cells in mice with endometriosis. AQP1, aquaporin 1.
Acknowledgments
We would like to acknowledge the helpful comments on this paper received from our reviewers.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Vercellini P, Vigano P, Somigliana E, et al. Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol. 2014;10(5):261–275. [DOI] [PubMed] [Google Scholar]
- [2].Bulletti C, Coccia ME, Battistoni S, et al. Endometriosis and infertility. J Assist Reprod Genet. 2010;27(8):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mishra VV, Gaddagi RA, Aggarwal R, et al. Prevalence; characteristics and management of endometriosis amongst infertile women: a one year retrospective study. J Clin Diagn Res. 2015;9(6):QC01–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Staal AH, van der Zanden M, Nap AW.. Diagnostic delay of endometriosis in the Netherlands. Gynecol Obstet Invest. 2016;81(4):321–324. [DOI] [PubMed] [Google Scholar]
- [6].Kvaskoff M, Mu F, Terry KL, et al. Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update. 2015;21(4):500–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Borghese B, Zondervan KT, Abrao MS, et al. Recent insights on the genetics and epigenetics of endometriosis. Clin Genet. 2017;91(2):254–264. [DOI] [PubMed] [Google Scholar]
- [8].Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Heinosalo T, Gabriel M, Kallio L, et al. Secreted frizzled-related protein 2 (SFRP2) expression promotes lesion proliferation via canonical WNT signaling and indicates lesion borders in extraovarian endometriosis. Hum Reprod. 2018;33(5):817–831. [DOI] [PubMed] [Google Scholar]
- [10].Pei JV, Kourghi M, De Ieso ML, et al. Differential inhibition of water and ion channel activities of mammalian aquaporin-1 by two structurally related bacopaside compounds derived from the medicinal plant bacopa monnieri. Mol Pharmacol. 2016;90(4):496–507. [DOI] [PubMed] [Google Scholar]
- [11].Sato K, Miyamoto M, Takano M, et al. Different prognostic implications of aquaporin-1 and aquaporin-5 expression among different histological types of ovarian carcinoma. Pathol Oncol Res. 2018. [DOI] [PubMed] [Google Scholar]
- [12].Wang W, Li F, Sun Y, et al. Aquaporin-1 retards renal cyst development in polycystic kidney disease by inhibition of Wnt signaling. Faseb J. 2015;29(4):1551–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36(11):1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhang L, Xiong W, Xiong Y, et al. 17 beta-estradiol promotes vascular endothelial growth factor expression via the Wnt/beta-catenin pathway during the pathogenesis of endometriosis. Mol Hum Reprod. 2016;22(7):526–535. [DOI] [PubMed] [Google Scholar]
- [15].Ma Y, He YL. Study of an antiangiogenesis gene therapy with endostatin on endometriosis in the nude mouse model. Clin Exp Obstet Gynecol. 2014;41(3):328–334. [PubMed] [Google Scholar]
- [16].Greaves E, Horne AW, Jerina H, et al. EP2 receptor antagonism reduces peripheral and central hyperalgesia in a preclinical mouse model of endometriosis. Sci Rep. 2017;7:44169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ren XU, Wang Y, Xu G, et al. Effect of rapamycin on endometriosis in mice. Exp Ther Med. 2016;12(1):101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147(1):9–19. [PMC free article] [PubMed] [Google Scholar]
- [19].Jiang HQ, Li YL, Zou J. Effect of recombinant human endostatin on endometriosis in mice. Chin Med J (Engl). 2007;120(14):1241–1246. [PubMed] [Google Scholar]
- [20].Sundov Z, Tomic S, Alfirevic S, et al. Prognostic value of MVD, LVD and vascular invasion in lymph node-negative colon cancer. Hepatogastroenterology. 2013;60(123):432–438. [DOI] [PubMed] [Google Scholar]
- [21].Ayuk SM, Abrahamse H, Houreld NN. The role of photobiomodulation on gene expression of cell adhesion molecules in diabetic wounded fibroblasts in vitro. J Photochem Photobiol B. 2016;161:368–374. [DOI] [PubMed] [Google Scholar]
- [22].Wang Y, Fan Y, Zheng C, et al. Knockdown of AQP1 inhibits growth and invasion of human ovarian cancer cells. Mol Med Rep. 2017;16(4):5499–5504. [DOI] [PubMed] [Google Scholar]
- [23].Wang Z, Pradhan-Bhatt S, Farach-Carson MC, et al. Artificial induction of native aquaporin-1 expression in human salivary cells. J Dent Res. 2017;96(4):444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Park JY, Yoon G. Overexpression of aquaporin-1 is a prognostic factor for biochemical recurrence in prostate adenocarcinoma. Pathol Oncol Res. 2017;23(1):189–196. [DOI] [PubMed] [Google Scholar]
- [25].Crisp RL, Maltaneri RE, Vittori DC, et al. Red blood cell aquaporin-1 expression is decreased in hereditary spherocytosis. Ann Hematol. 2016;95(10):1595–1601. [DOI] [PubMed] [Google Scholar]
- [26].Grenier JK, Foureman PA, Sloma EA, et al. RNA-seq transcriptome analysis of formalin fixed, paraffin-embedded canine meningioma. PLoS One. 2017;12(10):e0187150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hsu PJ, Wu FJ, Kudo M, et al. A naturally occurring Lgr4 splice variant encodes a soluble antagonist useful for demonstrating the gonadal roles of Lgr4 in mammals. PLoS One. 2014;9(9):e106804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tosti C, Pinzauti S, Santulli P, et al. Pathogenetic mechanisms of deep infiltrating endometriosis. Reprod Sci. 2015;22(9):1053–1059. [DOI] [PubMed] [Google Scholar]
- [29].Luo H, Xie A, Hua Y, et al. Aquaporin 1 gene deletion affects the amniotic fluid volume and composition as well as the expression of other aquaporin water channels in placenta and fetal membranes. Clin Chim Acta. 2018;482:161–165. [DOI] [PubMed] [Google Scholar]
- [30].Wu Z, Li S, Liu J, et al. RNAi-mediated silencing of AQP1 expression inhibited the proliferation, invasion and tumorigenesis of osteosarcoma cells. Cancer Biol Ther. 2015;16(9):1332–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu J, Zhang WY, Ding DG. Expression of aquaporin 1 in bladder uroepithelial cell carcinoma and its relevance to recurrence. Asian Pac J Cancer Prev. 2015;16(9):3973–3976. [DOI] [PubMed] [Google Scholar]
- [32].Wei X, Dong J. Aquaporin 1 promotes the proliferation and migration of lung cancer cell in vitro. Oncol Rep. 2015;34(3):1440–1448. [DOI] [PubMed] [Google Scholar]
- [33].Pulford E, McEvoy J, Hocking A, et al. The effect of aquaporin 1-inhibition on vasculogenic mimicry in malignant mesothelioma. Int J Mol Sci. 2017;18(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Simone L, Gargano CD, Pisani F, et al. Aquaporin-1 inhibition reduces metastatic formation in a mouse model of melanoma. J Cell Mol Med. 2018;22(2):904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Palethorpe HM, Tomita Y, Smith E, et al. The aquaporin 1 inhibitor bacopaside II reduces endothelial cell migration and tubulogenesis and induces apoptosis. Int J Mol Sci. 2018;19(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gao H, Gui J, Wang L, et al. Aquaporin 1 contributes to chondrocyte apoptosis in a rat model of osteoarthritis. Int J Mol Med. 2016;38(6):1752–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]


