ABSTRACT
Long non-coding RNAs (lncRNAs) have been confirmed to be aberrantly expressed and involved in the progression of neuroblastoma. This study aimed to explore the expression profile of lncRNA X-inactive specific transcript (XIST) and its functional involvement in neuroblastoma. In this study, the relative level of XIST in neuroblastoma tissues and cell lines was detected by qPCR, and DKK1 protein expression was determined using western blot. The effect of XIST on cell growth, invasion and migration in vitro and in tumorigenesis of neuroblastoma was assessed. The level of H3K27me3 in DKK1 promoter was analyzed with ChIP-qPCR. Interaction between XIST and EZH2 was verified by RNA immunoprecipitation (RIP) and RNA pull-down assay.
XIST was significantly upregulated in neuroblastoma tissues (n = 30) and cells lines, and it was statistically associated with the age and International Neuroblastoma Staging System (INSS) staging in neuroblastoma patients. Downregulation of XIST suppressed the growth, migration and invasion of neuroblastoma cells. EZH2 inhibited DKK1 expression through inducing H3 histone methylation in its promoter. XIST increased the level of H3K27me3 in DKK1 promoter via interacting with EZH2. Downregulation of XIST increased DKK1 expression to suppress neuroblastoma cell growth, invasion, and migration, which markedly restrained the tumor progression.
In conclusion, XIST downregulated DKK1 by inducing H3 histone methylation via EZH2, thereby facilitating the growth, migration and invasion of neuroblastoma cells and retarding tumor progression.
KEYWORDS: Neuroblastoma, XIST, EZH2, DKK1, histone methylation
Introduction
Neuroblastoma (NB) is the most common solid tumor diagnosed in infants and children up to 5 years old, featured with bad prognosis and high recurrence [1]. Originating from precursor cells of the sympathetic nervous system, neuroblastoma is aggressive and often develops rapidly and leads to high mortality [2]. Our team is always devoting ourselves to illuminating the underlying mechanism of neuroblastoma pathogenesis and have highlighted the tumor suppressor role of Wnt inhibitory factor-1 via regulating Wnt/β-catenin signaling [3]. Recently, Dickkopf Wnt signaling pathway inhibitor 1 (DKK1), a secreted glycoprotein, is highly paid attention for its close association with tumorigenesis [4]. In neuroblastoma cell lines SHEP-21N and SKNAS-NmycER, DKK1 has been proven to be downregulated by MYCN and inhibited neuroblastoma cell proliferation [5]. MYCN amplification occurs in about 20% of primary neuroblastoma cases and is an adverse prognostic marker that is strongly associated with poor outcome [6]. In the other hand, DKK1-induced apoptosis and inhibited the mobility of human neuroblastoma cells [7]. In our previous study, we manifested that DDK1 was lower expressed in neuroblastoma tissues than that in gangliocytoma tissues and normal adrenal tissues [8]. These studies reveal a significant impact of DKK1 on neuroblastoma cell proliferation, mobility, and apoptosis, while its role in neuroblastoma cells with or without MYCN amplification has not been comparatively analyzed.
Increasing evidences of DNA methylation modification of DKK1 have been verified to be implicated in tumor initiation and progression [9,10], implying its potential role in affecting neuroblastoma development via DNA methylation. However, the histone methylation of DKK1 and its association with tumorigenesis have seldom been focused on. Histone methylation is another kind of epigenetic regulation that plays crucial roles in biological and pathological processes [11]. It has been reported that Enhancer of Zeste Homolog 2 (EZH2) mediated repression of DKK1 in hepatic stellate cells, through methylation of Histone H3 trimethyl-lysine 27 (H3K27me3) [12]. EZH2, a histone H3 lysine-27-specific methyltransferase, is involved in controlling gene expression via inducing histone methylation to participate in cancers progression. In neuroblastoma, aberrant upregulation of EZH2 silenced several tumor suppressors, which contributed to the maintenance of the undifferentiated phenotype and poor prognostic status of this tumor [13]. It can be inferred that downregulation of DKK1 in neuroblastoma may result from the histone methylation mediated by EZH2.
Lately, the binding relationship between long non-coding RNA (lncRNA) X-inactive specific transcript (XIST) and EZH2 in osteosarcoma has been proposed [14]. Identified as an oncogene, lncRNA XIST has been reported to be abnormally expressed in diverse cancers and related to tumor development and progression [15]. Moreover, Yao et al. have proclaimed that XIST expression was upregulated in glioma tissues and human glioblastoma stem cells [16]. Therefore, we hypothesized that XIST may participate in the pathogenesis of neuroblastoma, through interacting with EZH2 to downregulate DKK1. Herein, the regulatory effect of XIST on EZH2 and DKK1 histone methylation and its influence on neuroblastoma cell growth, invasion, and migration were explored. This study aimed to provide novel insight into tumorigenesis and solid foundation for developing new therapeutic strategies.
Materials and methods
Patient data and tissue samples
Thirty neuroblastoma tissues (NB, n = 30) were obtained from the patients with neuroblastoma and admitted to the First Affiliated Hospital of Zhengzhou University, with the normal adrenal tissues (normal, n = 30) served as control. Documented informed consent was obtained from all the patients before tissue donation. Detailed pathologic and clinical data for all samples were collected, such as age, sex, International Neuroblastoma Staging System (INSS), and MYCN gene amplification. The diagnoses of the samples were all validated by pathologists. Approval to conduct this study was obtained from the ethics committee of the First Affiliated Hospital of Zhengzhou University, and the research was performed in accordance with the Helsinki Declaration.
Quantitative real-time PCR (qPCR)
Total RNA was extracted from the neuroblastoma and adrenal tissue samples using the RNeasy Mini Kit (Qiagen, Germany) according to the manufacturer’s instructions. 2μg of obtained RNA was reversely transcribed into cDNA by using the Taqman MicroRNA Reverse Transcription Kit (Applied Biosystems, USA). The relative level of XIST was detected by qPCR with following conditions: denaturing the DNA at 95°C for 5 min, followed by 40 cycles of amplification: 94°C for 60 s, 58°C for 60 s, 72°C for 60 s for data collection, which was performed on an ABI 7500 thermocycler (Applied Biosystems) using SYBR Premix Ex TaqTM (Perfect Real Time) Kits (TaKaRa, Japan) following the manufacturer’s instructions. GAPDH was used as an internal control. The primer sequences were listed: XIST: F 5ʹ-CTCTCCATTGGGTTCAC-3ʹ, R 5ʹ–GCGGCAGGTCTTAAGAGATGAG-3ʹ; EZH2: F 5ʹ-TGCAGTTGCTTCAGTACCCATAAT-3ʹ, R 5ʹ-ATCCCCGTGTACTTTCCCATCATAAT-3ʹ; DKK1: F 5ʹ-AGTACTGCGCTAGTCCCACC-3ʹ, R 5ʹ-TCCTCAATTTCTCCTCGGAA-3ʹ; GAPDH: F 5ʹ-AGAAGGCTGGGGCTCATTTG-3ʹ, R 5ʹ-AGGGGCCATCCACAGTCTTC-3ʹ.
Western blotting
Neuroblastoma tissues or adrenal tissues were dissolved in the RIPA lysis buffer (Beyotime, China) and centrifuged for protein extraction. After quantified by the BCA protein assay kit (Pierce, USA), protein extracts were loaded onto 10% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, USA). The PVDF membrane was then blocked in the Tris-buffered saline (TBS) containing 5% non-fat milk and 0.1% Tween-20 for 2 h at room temperature and subsequently incubated with primary antibodies (Abcam, UK) against DKK1 andβ-actin at 4°C overnight. The membrane was washed and incubated in HRP-bounded secondary antibody for 2 h at room temperature and the proteins were imaged by Tanon Chemiluminescence Imaging system with an ECL regent (Thermo Fisher, USA). The data were quantified by automated densitometry using Image P-Plus software (Bio-Rad, USA), which was normalized by GAPDH.
Cell culture and cell transfection
Neuroblastoma cells including the cell line without MYCN amplification (SH-SY5Y) and MYCN-amplified cell line SK-N-BE (2) were purchased from the China Center for Type Culture Collection. The Human Embryonic Kidney 293 (HEK293) cells cultured in our laboratory acted as a control. Cells were cultured in RPMI1640 medium (Life Technologies) containing 10% fetal bovine serum (FBS, Life Technologies), 100U/ml penicillin, and 100μg/ml streptomycin; and incubated at 37°C in a humidified atmosphere with 5% CO2. For cell transfection, siRNA-XIST or its negative control (siRNA-control) was provided by GenePharma Co. Ltd (Shanghai, China) and transfected into cells using Lipofectamine2000 (Invitrogen, USA) according to its manufacturer’s specifications. The sequence of siRNA-XIST was listed: 5ʹ-GUAUCCUAUUUGCACGCUAdTdT-3ʹ, as well as the sequence of siRNA-control: 5ʹ-UUCUCCGAACGUGUCACGUdTdT-3ʹ.
Plasmid construction and lentivirus production
Silence of XIST in Neuroblastoma cells was achieved by shRNA-XIST transfection, with sh-control acted as the scrambled control shRNA. Designed shRNA fragments or sh-control fragments were synthesized and constructed into pLKO.1 plasmid (Addgene, USA). Then the generated plasmids containing XIST knockdown fragment or control plasmid together with pMD2.G (Addgene) and psPAX2 (Addgene) plasmids were co-transfected into HEK293T cells using Lipofectamine 2000 reagent (Invitrogen). Seventy-two hours following transfection, cell supernatants containing shRNA-XIST or sh-control lentiviruses were obtained, followed by sieving using 0.45 μm filterable membranes. SK-N-BE (2) and SK-SY5Y cells were infected with harvested shRNA-XIST or sh-control lentivirus supernatants for 2 days and screened with puromycin (Sigma-Aldrich) for at least 7 days, and the shRNA-XIST or sh-control stably transfected SK-N-BE (2) and SK-SY5Y cell line was obtained. The sequence of shRNA-XIST was listed: 5ʹ-GUAUCCUAUUUGCACGCUAdTdT-3ʹ, as well as the sequence of sh-control: 5ʹ-UUCUCCGAACGUGUCACGUdTdT-3ʹ.
Soft agar assay
SK-N-BE (2) and SH-SY5Y cells proliferation was examined with the Soft agar assay [17]. Briefly, cells (5 × 103) were mixed with 0.05% Nobel agar (Fisher Scientific, USA) in the culture medium. They were seeded onto 6-well plates containing a solidified bottom layer and incubated for 21 days. After the number of cell colonies counted under the microscope, cells were fixed with 100% methanol and stained with 0.5% crystal violet dye.
Determination of cell viability
Cell proliferative capacity of SK-N-BE (2) and SH-SY5Y was determined using a Cell Counting Kit (CCK-8, Beyotime Biotechnology, Shanghai, China). Briefly, cells (5 × 103/well) were seeded into 96-well plates and maintained overnight at 37°C. Then, 10μl of CCK-8 solution was added to the culture medium, followed by incubation in a humidity incubator for 3 h at 37°C. Absorbance at 450nm was measured using a microplate reader (Bio-Rad, USA).
Transwell assay
Transwell inserts that have 6.5-mm polycarbonate filter membranes with pores at a size of 8.0 μm (Corning, USA) were used for the migration assay. Matrigel invasion assay was performed using membranes that pre-coated with Matrigel Basement Member Matrix (BD Science, USA). Homogeneous single-cell suspensions (1 × 105 cells/well) of SK-N-BE (2) and SH-SY5Y were added to the upper chambers and allowed to invade for 24 h at 37°C in a CO2 incubator, with complete medium containing 20% FBS (Gibco, ThermoFisher Scientific, USA) added into the lower compartments. Migrated or invaded cells were stained with 0.1% crystal violet for 10 min at room temperature and the amount of cells was counted under the light microscopy.
QPCR-chromatin immunoprecipitation (ChIP-qPCR)
Chromatin immunoprecipitation was performed to verify the effect of EZH2 on H3K27me3 of the DKK1 promoter region [18]. Briefly, DNA-protein complexes were cross-linked with 1% formaldehyde for 15 min. Immune complexes were formed with either nonspecific IgG or chromatin immunoprecipitation grade antibodies (Abcam) against DKK1, H3K27me3 and EZH2. DNA was eluted and purified from complexes, followed by PCR amplification of the DKK-1 promoter and qPCR detection.
RNA immunoprecipitation (RIP)
Subsequently, binding and interplay between XIST and EZH2 in SH-SY5Y cells were assessed. The Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore) was used for RIP assay. SK-N-BE (2) cells were lysed with RIP buffer and then 100μl cell extract was incubated with RIP buffer containing magnetic beads conjugated with anti-AGO2 antibody (Millipore) or normal mouse IgG as a negative control. RNA-protein complexes were immunoprecipitated with protein A agarose beads and qPCR was performed to detect XIST level.
RNA pull-down assay
RNA pull-down assay was conducted with synthesized XIST as a probe. Performed according to the manufacturers’ protocol, a Magnetic RNA-Protein Pull-Down Kit (Thermo Fisher) was used. With DNA fragment including the whole XIST sequence was synthesized and biotinylated (GenePharma Co., Ltd, China), biotin-labeled RNAs were bound to streptavidin magnetic beads and then to the RNA-binding protein. The RNA-binding protein complexes were eventually washed and eluted for western blot or qPCR analysis.
Neuroblastoma xenograft model
Two-month-old male BALB/c nude mice were used for neuroblastoma xenograft model construct. 1 × 106 tumor cells SH-SY5Y stably transfected with shRNA-control or shRNA-XIST via lentivirus infection were injected subcutaneously into mice (n = 7 per group) in the upper back. The tumor volume was evaluated once a week. One month later, the tumor volume was calculated by the equation V = (ab2)/2, in which V refers to volume, and a represents the length of the major axis, and b represents the length of the minor axis. A Kaplan–Meier plot was used to analyze the survival of mice in shRNA-control (n = 7) and shRNA-XIST group (n = 7). Finally, mice were sacrificed and the tumor tissue was taken for XIST and DKK1 expression analysis.
Statistical analysis
The correlations between XIST expression and neuroblastoma clinicopathological parameters were analyzed by one-way analysis of variance (ANOVA). SPSS 21.0 software (SPSS Inc., USA) was applied for statistical analyses, and all the data were presented as mean ± standard deviation. Differences between two groups were analyzed with Student’s t test, and a value of p< 0.05 was considered significant. Comparison among more than two groups was completed with ANOVA followed by the Newman-Keuls post hoc test.
Results
XIST expression was increased in neuroblastoma tissues and cell lines
Neuroblastoma tissues (NB, n = 30) and the normal adrenal tissues (normal, n = 30) were taken for clinical analysis. As shown in Figure 1(a), the expression of XIST in neuroblastoma tissues was remarkably higher than that in normal adrenal tissues. Moreover, the XIST expression was significantly correlated with age (p= 0.001), and International Neuroblastoma Staging System (INSS) staging (p= 0.011); while no significant correlation was observed between XIST expression and sex (p= 0.122) or MYCN gene amplification (p= 0.223) (Table 1). Western blot analysis showed that the protein expression of DKK1 was significantly decreased in neuroblastoma tissues (n = 30) (Figure 1(b)). We further compared the expressions of XIST in neuroblastoma cell lines and HEK293 cells. When comparing with HEK293 cells, the expression of XIST was significantly increased in neuroblastoma cells including SK-N-BE (2) and SH-SY5Y, and no significant difference was noted between these two neuroblastoma cell lines (Figure 1(c)); while the DKK1 protein level was reduced in SK-N-BE (2) and SH-SY5Y cells (Figure 1(d)). The results demonstrated that XIST expression was promoted in neuroblastoma tissues and cells, suggesting it may play a role in neuroblastoma oncogenesis and progression.
Figure 1.
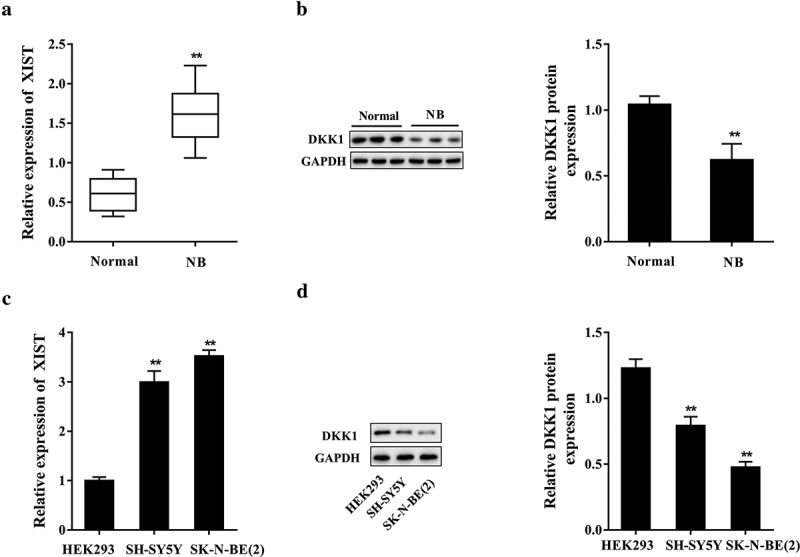
XIST expression was increased in neuroblastoma tissues and cell lines. Neuroblastoma tissues (NB, n = 30) and the normal adrenal tissues (normal, n = 30) were taken for clinical analysis. (a) The expression of XIST in neuroblastoma and adrenal tissues was determined by qPCR. (b) The level of DKK1 protein in neuroblastoma and adrenal tissues was analyzed with western blot and the densitometry was performed (compared to β-actin). **p< 0.05 compared with the normal group. Neuroblastoma cell lines SK-N-BE (2) and SH-SY5Y cells were cultured, with HEK293 cells served as control. (c) The expression of XIST in cells was determined by qPCR. (d) The level of DKK1 protein in cells was examined by western blot and the data were quantified by densitometry (compared to β-actin). **p< 0.05 compared with HEK293 cells.
Table 1.
Correlation between XIST expression and neuroblastoma clinicopathological parameters.
| XIST Expression |
||||
|---|---|---|---|---|
| Variables | No. Patients | Low | High | P |
| Total case | 30 | 18 | 12 | |
| Age (mo) | 0.001* | |||
| ≤18 | 13 | 4 | ||
| >18 | 5 | 8 | ||
| Sex | 0.122 | |||
| Male | 10 | 7 | ||
| Female | 8 | 5 | ||
| INSS staging | 0.011* | |||
| Stage 1 | 2 | 0 | ||
| Stage 2 | 4 | 1 | ||
| Stage 3 | 3 | 2 | ||
| Stage 4 | 6 | 8 | ||
| Stage 4s | 3 | 1 | ||
| MYCN | 0.223 | |||
| Amplified | 7 | 5 | ||
| Non-amplified | 11 | 7 | ||
INSS, International Neuroblastoma Staging System.
*indicates p < 0.05.
Downregulation of XIST-suppressed neuroblastoma cell growth, migration, and invasion
Since the high expression of XIST in neuroblastoma cells has been confirmed, its impact on cell growth, invasion, and migration activities deserves further investigation. Subsequently, SK-N-BE (2) and SH-SY5Y cells were transfected with siRNA-control or siRNA-XIST, respectively, and XIST expression was clearly downregulated after siRNA-XIST transfection (Figure 2(a)). Detection on the number of colonies indicated that inhibition of XIST restrained neuroblastoma cell growth (Figure 2(b)); meanwhile, the cell proliferation, migration, and invasion were also repressed with XIST silence (Figure 2(c–e)). It was clearly proven that downregulation of XIST suppressed the neuroblastoma cells growth, proliferation, migration, and invasion of in vitro.
Figure 2.
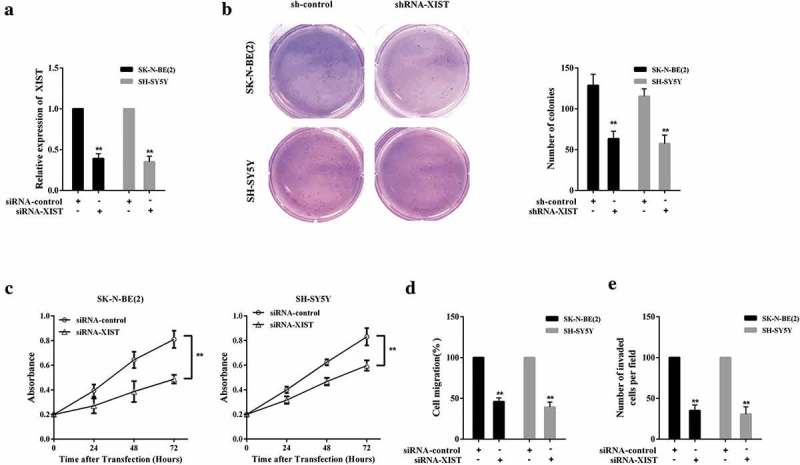
Downregulation of XIST-suppressed cell growth, invasion, and migration in neuroblastoma. SK-N-BE (2) and SH-SY5Y cells were transfected with siRNA-control or siRNA-XIST, respectively. siRNA-control, the negative control of siRNA-XIST. (a) The expression of XIST in SK-N-BE (2) and SH-SY5Y cells was detected by qPCR. (b) Neuroblastoma cells SK-N-BE (2) and SH-SY5Y colony formation of was evaluated by soft agar assay. (c) The CCK-8 assay was conducted to estimate SK-N-BE (2) and SH-SY5Y cells proliferation. (d) Neuroblastoma cells SK-N-BE (2) and SH-SY5Y migration was evaluated by Transwell assay. (e) Transwell assay was performed to assess the invasion of neuroblastoma cells SK-N-BE (2) and SH-SY5Y. **p< 0.05 compared with sh-control or siRNA-control.
EZH2 inhibited DKK1 expression through histone methylation
Regulation mechanism of EZH2 on DKK1 expression was then explored. SK-N-BE (2) and SH-SY5Y cells were separately treated with histone transmethylase inhibitor DZNep (10μM); the result indicated that histone transmethylase inhibitor DZNep induced the expression of DKK1 protein (Figure 3(a)). After EZH2 downregulated by short hairpin RNA (shRNA) in SK-N-BE (2) and SH-SY5Y cells, the DKK1 protein expression was promoted (Figure 3(b)). More importantly, downregulated EZH2 dramatically reduced the H3K27me3 in DKK1 promoter (Figure 3(c)). With the above findings, we manifested that EZH2 inhibited DKK1 expression through inducing H3 histone methylation.
Figure 3.

EZH2 inhibited DKK1 expression through histone methylation. Interaction between EZH2 and DKK1 was evaluated in SK-N-BE (2) and SH-SY5Y cells. (a) After histone transmethylase inhibitor DZNep (10μM) treatment, the DKK1 protein level in SK-N-BE (2) and SH-SY5Y cells was analyzed by western blot, which was quantified by densitometry (compared to β-actin). (b) Interference of EZH2 was achieved via shRNA-EZH2 (with shRNA-NC served as negative control) transfection into SK-N-BE (2) and SH-SY5Y cells, and the expression of DKK1 protein was determined with western blot, and densitometry was performed (compared to β-actin). (c) After EZH2 interference, the level of H3K27me3 in DKK1 promoter in SK-N-BE (2) and SH-SY5Y cells was analyzed with ChIP-qPCR. **p< 0.05 compared with shRNA-NC.
XIST restrained DKK1 expression via histone methylation by binding with EZH2
In SK-N-BE (2) cells, the interaction between XIST and EZH2 was assessed. With RIP assay, the XIST level in EZH2 precipitation complex was determined. Compared with IgG, XIST was abundantly gathered in the EZH2 precipitation complex (Figure 4(a)). Compared with the negative control (NC) group, plenty of EZH2 protein was found in the complex pulled down by XIST (Figure 4(b)). After SK-N-BE (2) and SH-SY5Y cells, respectively, transfected with siRNA-control or siRNA-XIST, the DNA H3K27me3 complex level was decreased, while the DKK1 protein level was upregulated (Figure 4(c,d)). The results manifested that XIST restrained DKK1 expression via H3 histone methylation by binding with EZH2.
Figure 4.

XIST restrained DKK1 expression through binding with EZH2. (a) RNA immunoprecipitation (RIP) was conducted to assess the binding relationship between XIST and EZH2, and the XIST level in EZH2 precipitation complex was determined with qPCR. (b) The interplay between XIST and EZH2 was evaluated with RNA pull-down assay, and the EZH2 protein level in the XIST pull-down complex was analyzed by western blot. (c) For XIST downregulation, siRNA-XIST (with siRNA-control served as negative control) was separately transfected into SK-N-BE (2) and SH-SY5Y cells, and the H3K27me3 level of DKK1 promoter was quantified with ChIP-qPCR. (d) DKK1 protein in SK-N-BE (2) and SH-SY5Y cells was analyzed by western blot and the data were quantified by densitometry (compared to β-actin). **p< 0.05 compared with IgG; **p< 0.05 compared with siRNA-control.
Downegulation of XIST-suppressed cell growth, invasion and migration in neuroblastoma via increasing DKK1
To clarify whether DKK1 played a role in XIST modulating neuroblastoma cell growth, invasion, and migration, SK-N-BE (2) and SH-SY5Y cells were allocated into 4 groups: siRNA-control, siRNA-XIST, siRNA-XIST+NC, and siRNA-XIST+siRNA-DKK1. It was displayed that siRNA-XIST blocked neuroblastoma cell growth, but it was reversed after siRNA-DKK1 transfection (Figure 5(a)). Similarly, siRNA-XIST-suppressed cell proliferation, migration and invasion, which were reversed with siRNA-DKK1 transfected (Figure 5(b-d)). It could be confirmed that downregulation of XIST-suppressed cell growth, proliferation, migration and invasion in neuroblastoma via increasing DKK1, which was achieved by binding with EZH2.
Figure 5.

Downregulation of XIST-suppressed cell growth, invasion, and migration in neuroblastoma via increasing DKK1. SK-N-BE (2) and SH-SY5Y cells were allocated into four groups: siRNA-control, siRNA-XIST, siRNA-XIST+NC, and siRNA-XIST+siRNA-DKK1. (a) The colony formation of Neuroblastoma cells SK-N-BE (2) and SH-SY5Y was determined with soft agar assay. (b) The CCK-8 assay was carried out to estimate SK-N-BE (2) and SH-SY5Y cells proliferation. (c) Neuroblastoma cells SK-N-BE (2) and SH-SY5Y migration was evaluated by Transwell assay. (d) Transwell assay was performed to assess the invasion of neuroblastoma cells SK-N-BE (2) and SH-SY5Y. **p< 0.05 compared with siRNA-control. ##p< 0.05 compared with siRNA-XIST+NC.
Inhibition of XIST-controlled neuroblastoma growth in the mouse model
For in vivo function verification, SH-SY5Y cells stably transfected with shRNA-control (n = 7) or shRNA-XIST (n = 7) were subcutaneously injected into mice. As illustrated in Figure 6(a), the survival rate of mice was markedly elevated with XIST interfered (shRNA-XIST, n = 7) when compared with the shRNA-control group (n = 7); and the tumor volume in shRNA-XIST group (n = 7) was notably smaller than that in its control group (shRNA-control, n = 7). Downregulation of XIST through shRNA-XIST transfection was confirmed (Figure 6(b)). In addition, sh-XIST significantly increased DKK1 protein level (Figure 6(c)). These results demonstrated that inhibition of XIST-controlled neuroblastoma growth in the mouse model.
Figure 6.

Inhibition of XIST-controlled neuroblastoma growth in the mouse model. SH-SY5Y cells stably transfected with shRNA-control (n = 7) or shRNA-XIST (n = 7) were subcutaneously injected into mice. (a) A Kaplan–Meier plot was used to analyze the survival of mice in the two groups, and the tumor volume was estimated. (b) The expression of XIST in neuroblastoma tissues was quantified by qPCR. (c) The level of DKK1 protein in neuroblastoma tissues was analyzed with western blot and the densitometry was conducted (compared to β-actin). **p< 0.05 compared with shRNA-control.
Discussion
With a series of experiments including clinical analysis, mechanism exploration in vitro and functional validation in vivo, we elucidate an epigenetic regulatory role of lncRNA XIST in neuroblastoma. It can be concluded that XIST regulates DKK1 expression via histone methylation through the methyltransferase activity of EZH2, and thus influencing cell growth, invasion, and migration in neuroblastoma. This study initially uncovered the carcinogenesis of XIST in neuroblastoma, and further emphasized the well-known roles of EZH2 and DKK1 in this disorder. Above all, the interaction between EZH2 and DKK1 in neuroblastoma was confirmed for the first time, revealing a significant regulatory pathway in neuroblastoma and offering a novel perspective for developing effective therapies.
Although the neuroblastoma has been widely studied in oncogenesis, cell growth, proliferation, invasion, and migration [19,20], the underlying molecular mechanisms contributing to neuroblastoma pathogenesis, recurrence, and metastasis are still far from understood. LncRNAs have emerged as a new kind of non-coding transcripts that exhibit a pivotal influence on neuroblastoma pathogenesis [21]. For instance, lncRNA HOXD-AS1 controlled retinoid acid-induced cell differentiation in SH-SY5Y cell line and targeted the protein-coding genes involved in angiogenesis and inflammation, the hallmarks of cancer metastasis [22]. In a recent report, NBAT-1 was identified to impair cell growth and metastasis, thereby blocking neuroblastoma progression [23]. The present study unraveled a high expression of XIST in neuroblastoma and its positive effect on cell growth, invasion, and migration, justifying its inherent impression to promote cancer development and determine cell fate.
In addition, as an X-linked lncRNA that functionally regulates the formation of the inactive X chromosome, the epigenetic regulation mediated by XIST cannot be ignored, through accumulating on the entire length of the chromosome in cis and inducing heterochromatin formation [24]. In most cases, epigenetic activation or silencing of gene expression induced by XIST is generally achieved by posttranslational histone modifications and DNA methylation, in which is the H3K27me3 modification [25,26]. EZH2, a histone H3 lysine-27-specific methyltransferase, is involved in controlling gene expression via inducing histone methylation, participating in various physiological and pathological processes [12]. Activation or high EZH2 is a prognotic indicator of cancer aggressiveness and development [27,28]. Likewise, EZH2 is aberrantly upregulated in neuroblastoma cells and it mediates epigenetic silencing of neuroblastoma suppressor genes, such as CASZ1, CLU, RUNX3, and NGFR, contributing to the oncogenesis [13]. Right here, we highlighted a methylation modification of XIST on DKK1 by enriching EZH2 into target gene promoter, strengthening the understanding of the epigenetic machinery in the development of neuroblastoma.
As a typical inhibitor of the wnt/beta-catenin, DKK1 plays critical roles in tumor pathogenesis [29]. In diverse cancers, DKK1 modulates tumor angiogenesis [30], mediates cancer metastasis [4], regulates chemo-resistance [31], and shows the potential as a serological biomarker for the diagnosis and prognosis of cancer [32]. In neuroblastoma, DKK1 is reported to be downregulated by MYCN, whose amplification and overexpression are poor prognostic factors of neuroblastoma [5]. Furthermore, DKK1 is released by neuroblastoma cells and is able to affect the balance between osteoblastogenesis and osteoclastogenesis, thus favoring the onset of osteolytic metastases [33]. In the current study, we further manifest that upregulation of DKK1 helps to suppress cell growth, invasion, and migration, underscoring its inhibitory effect on neuroblastoma occurrence and metastases.
Taken together, our findings illustrated that XIST expression is correlated with neuroblastoma development, and it epigenetically regulates DKK1 expression through histone methylation via EZH2. This study represents the huge prospect of lncRNA and epigenetic modification in early diagnosis, treatment, and prognostic evaluation of malignancies. In addition to histone methylation, the role of DNA methylation of DKK1 in neuroblastoma and the relationship between them deserve further research in the future.
Funding Statement
This study was supported by the National Natural Science Foundation of China [No. 81502187].
Disclosure statement
The authors declare no conflict of interest.
References
- [1].Chen X, Pan M, Han L, et al. miR-338-3p suppresses neuroblastoma proliferation, invasion and migration through targeting PREX2a. FEBS Lett. 2013;587(22):3729–3737. [DOI] [PubMed] [Google Scholar]
- [2].Pandey GK, Mitra S, Subhash S, et al The risk-associated long noncoding RNA NBAT-1 controls neuroblastoma progression by regulating cell proliferation and neuronal differentiation. Cancer Cell. 2014;26(5):722–737. [DOI] [PubMed] [Google Scholar]
- [3].Zhang J, Zhou B, Liu Y, et al. Wnt inhibitory factor-1 functions as a tumor suppressor through modulating Wnt/beta-catenin signaling in neuroblastoma. Cancer Lett. 2014;348(1–2):12–19. [DOI] [PubMed] [Google Scholar]
- [4].Hu P, Chu J, Wu Y, et al NBAT1 suppresses breast cancer metastasis by regulating DKK1 via PRC2. Oncotarget. 2015;6(32):32410–32425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Koppen A, Ait-Aissa R, Hopman S, et al. Dickkopf-1 is down-regulated by MYCN and inhibits neuroblastoma cell proliferation. Cancer Lett. 2007;256(2):218–228. [DOI] [PubMed] [Google Scholar]
- [6].Ke XX, Zhang D, Zhao H, et al. Phox2B correlates with MYCN and is a prognostic marker for neuroblastoma development. Oncol Lett. 2015;9(6):2507–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang KP, Bai Y, Wang J, et al. Morphine protects SH-SY5Y human neuroblastoma cells against Dickkopf1-induced apoptosis. Mol Med Rep. 2015;11(2):1174–1180. [DOI] [PubMed] [Google Scholar]
- [8].Zhang J, Liu Q-L, Gao J-Y, et al. Expression and clinical significance of DKK-1 in neuroblastoma tissues. J Clin Pediatr Surg. 2015;14(5):388–390. [Google Scholar]
- [9].Liang L, He H, Lv R, et al. Preliminary mechanism on the methylation modification of Dkk-1 and Dkk-3 in hepatocellular carcinoma. Tumour Biol. 2015;36(2):1245–1250. [DOI] [PubMed] [Google Scholar]
- [10].Rawson JB, Sun Z, Dicks E, et al Vitamin D intake is negatively associated with promoter methylation of the Wnt antagonist gene DKK1 in a large group of colorectal cancer patients. Nutr Cancer. 2012;64(7):919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hattori N, Ushijima T.. Compendium of aberrant DNA methylation and histone modifications in cancer. Biochem Biophys Res Commun. 2014;455(1–2):3–9. [DOI] [PubMed] [Google Scholar]
- [12].Han Li C, Chen Y.. Targeting EZH2 for cancer therapy: progress and perspective. Curr Protein Pept Sci. 2015;16(6):559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang C, Liu Z, Woo CW, et al EZH2 Mediates epigenetic silencing of neuroblastoma suppressor genes CASZ1, CLU, RUNX3, and NGFR. Cancer Res. 2012;72(1):315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu T, Jiang W, Fan L, et al. Upregulation of long noncoding RNA Xist promotes proliferation of osteosarcoma by epigenetic silencing of P21. Oncotarget. 2017;8(60):101406–101417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chen DL, Ju HQ, Lu YX, et al Long non-coding RNA XIST regulates gastric cancer progression by acting as a molecular sponge of miR-101 to modulate EZH2 expression. J Exp Clin Cancer Res. 2016;35(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yao Y, Ma J, Xue Y, et al Knockdown of long non-coding RNA XIST exerts tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152. Cancer Lett. 2015;359(1):75–86. [DOI] [PubMed] [Google Scholar]
- [17].Zhang H, Pu J, Qi T, et al. MicroRNA-145 inhibits the growth, invasion, metastasis and angiogenesis of neuroblastoma cells through targeting hypoxia-inducible factor 2 alpha. Oncogene. 2014;33(3):387–397. [DOI] [PubMed] [Google Scholar]
- [18].Hussain M, Rao M, Humphries AE, et al. Tobacco smoke induces polycomb-mediated repression of Dickkopf-1 in lung cancer cells. Cancer Res. 2009;69(8):3570–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cao P, Feng F, Dong G, et al. Estrogen receptor alpha enhances the transcriptional activity of ETS-1 and promotes the proliferation, migration and invasion of neuroblastoma cell in a ligand dependent manner. BMC Cancer. 2015;15:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lu F, Kishida S, Mu P, et al. NeuroD1 promotes neuroblastoma cell growth by inducing the expression of ALK. Cancer Sci. 2015;106(4):390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pandey GK, Kanduri C. Long noncoding RNAs and neuroblastoma. Oncotarget. 2015;6(21):18265–18275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yarmishyn AA, Batagov AO, Tan JZ, et al. HOXD-AS1 is a novel lncRNA encoded in HOXD cluster and a marker of neuroblastoma progression revealed via integrative analysis of noncoding transcriptome. BMC Genomics. 2014;15(Suppl 9):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yang C, Wang G, Yang J, et al. Long noncoding RNA NBAT1 negatively modulates growth and metastasis of osteosarcoma cells through suppression of miR-21. Am J Cancer Res. 2017;7(10):2009–2019. [PMC free article] [PubMed] [Google Scholar]
- [24].Hasegawa Y, Brockdorff N, Kawano S, et al. The matrix protein hnRNP U is required for chromosomal localization of Xist RNA. Dev Cell. 2010;19(3):469–476. [DOI] [PubMed] [Google Scholar]
- [25].Gendrel AV, Heard E. Noncoding RNAs and epigenetic mechanisms during X-chromosome inactivation. Annu Rev Cell Dev Biol. 2014;30:561–580. [DOI] [PubMed] [Google Scholar]
- [26].Yue M, Charles Richard JL, Yamada N, et al. Quick fluorescent in situ hybridization protocol for Xist RNA combined with immunofluorescence of histone modification in X-chromosome inactivation. J Vis Exp. 2014;93:e52053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee SR, Roh YG, Kim SK, et al Activation of EZH2 and SUZ12 regulated by E2F1 predicts the disease progression and aggressive characteristics of bladder cancer. Clin Cancer Res. 2015;21(23):5391–5403. [DOI] [PubMed] [Google Scholar]
- [28].Bae WK, Yoo KH, Lee JS, et al. The methyltransferase EZH2 is not required for mammary cancer development, although high EZH2 and low H3K27me3 correlate with poor prognosis of ER-positive breast cancers. Mol Carcinog. 2015;54(10):1172–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mariz K, Ingolf JB, Daniel H, et al. The Wnt inhibitor dickkopf-1: a link between breast cancer and bone metastases. Clin Exp Metastasis. 2015;32(8):857–866. [DOI] [PubMed] [Google Scholar]
- [30].Park H, Jung HY, Choi HJ, et al. Distinct roles of DKK1 and DKK2 in tumor angiogenesis. Angiogenesis. 2014;17(1):221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jia X, Li N, Peng C, et al miR-493 mediated DKK1 down-regulation confers proliferation, invasion and chemo-resistance in gastric cancer cells. Oncotarget. 2016;7(6):7044–7054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Han SX, Zhou X, Sui X, et al Serum dickkopf-1 is a novel serological biomarker for the diagnosis and prognosis of pancreatic cancer. Oncotarget. 2015;6(23):19907–19917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Granchi D, Corrias MV, Garaventa A, et al. Neuroblastoma and bone metastases: clinical significance and prognostic value of Dickkopf 1 plasma levels. Bone. 2011;48(1):152–159. [DOI] [PubMed] [Google Scholar]


