ABSTRACT
The high mortality of epithelial ovarian cancer (EOC) is primarily due to vast intraperitoneal dissemination. 1-calcium phosphate-uracil (1-CP-U) has previously shown the function of inhibiting migration and invasion in multiple tumor cell lines. In this study, we further assessed the possible role of 1-CP-U in suppressing the peritoneal metastasis of EOC cells. First, we demonstrated that 1-CP-U had an inhibitory effect on EOC cells in cell-matrix adhesion, migration and invasion assay in vitro. Within the in vivo model, animals were intraperitoneally inoculated with SKOV3-Luc cells and then 1-CP-U intraperitoneal (i.p.) injection was performed every 5 d for a total of 3 wk. At the 7th d, omenta from each group were analyzed with luciferase activity and bioluminescence imaging assay, which showed a significant reduction of luciferase activity in the omenta from 1-CP-U group. In addition, the rest mice continued treatment and consistent detection of bioluminescence imaging. The data indicated that intraperitoneal metastatic nodules were less-developed in 1-CP-U group. Peritoneal metastatic tumor nodules were detected for immunofluorescent staining, which showed a reduction in FAK and p-FAK staining with 1-CP-U treatment group. Meanwhile, expressions of FAK and its downstream signaling were detected by western blot in tumor tissues and EOC cell lines, which showed significant decreases in the 1-CP-U treatment group. In conclusion, 1-CP-U had a profound inhibitory effect on adhesion, invasion and metastasis of EOC in vitro and suppressed intraperitoneal dissemination and cancer growth in vivo assay, which was associated with inhibition on the FAK pathway.
KEYWORDS: 1-calcium phosphate-uracil, FAK, EOC, adhesion, metastasis
Introduction
Ovarian cancer is the most lethal of the gynecologic cancers, which ranks eighth for both incidence and mortality worldwide for women produced by the International Agency for Research on Cancer [1]. In 2018, 22,240 new cases and 14,070 deaths of ovarian cancer were estimated in the United States [2]. Above age 40, more than 90% are epithelial ovarian cancer (EOC) and the risk increases with age, peaking in the late 70s [3]. Patients may appear asymptomatic in the early stages; thus, 70–75% of them are often diagnosed after cancer has circulated throughout the peritoneal cavity [4,5]. However, effective treatments are limited and little progress has been made in cancer prevention or containment of primary tumors from metastasizing [6]. Therefore, there is a need for new anticancer drugs to prevent the primary tumor from spreading.
EOC expands via direct extension of cancer cells from the primary tumor into the peritoneum-covered surfaces and organs of the peritoneal and pelvic cavity with the peritoneal fluid flow [7]. Subsequently, cancer cells adhere to peritoneal tissues; then, migrate into the sub-mesothelial matrix and finally form secondary lesions [4,8]. The peritoneum is comprised of a single layer of mesothelial cells and its associated underlying extracellular matrix (ECM), which cover the vast surface of the peritoneal and pelvic cavity. Omentum, containing abundant milky spots where the stromal matrix is exposed, is the most common site for EOC metastatic spread [9,10].
Attachment of cancer cells to the sub-mesothelial ECM, mainly mediated by integrins, is the initial stage of peritoneal metastasis [10,11], which subsequently triggers the autophosphorylation of focal adhesion kinase (FAK) [12]. After autophosphorylation at Tyr397, FAK then initiates a cascade of phosphorylation events and new protein–protein interactions to stimulate tumor progression and metastasis through their regulation of cell migration, invasion, epithelial-mesenchymal transition (EMT) and angiogenesis [13]. Numerous studies have shown that increased FAK and p-FAK expression are frequently correlated with malignant or metastatic disease and poor patient prognosis in different tumors [14–17]. Thus, inhibiting FAK has become a hotly pursued approach to treat tumor metastasis in recent years [18].
1-calcium phosphate-uracil (1-CP-U), a novel synthetic pyrimidine derivative, has been documented to demonstrate a variety of different biological activities [19]. Our previous study has documented for the first time the function of 1-CP-U in tumor proliferation, apoptosis and invasion with specific effects against a series of malignant tumors originating in different organs in vitro, including EOC cells [19]. We also observed that 1-CP-U appeared to be more effective at inhibiting cell migration and invasion than inducing apoptosis. In the present study, we further performed a series of experiments employing in vitro and in vivo models to assess the possible role of 1-CP-U in suppressing the peritoneal metastasis of EOC cells. Our results demonstrated that 1-CP-U had an inhibitory effect on the adhesion and invasion of EOC possibly though suppressing FAK signaling pathway. 1-CP-U inhibited the adhesion, migration and invasion of EOC cells in vitro. In addition, 1-CP-U suppressed EOC metastasis into the omentum, as well as reduced intraperitoneal dissemination and cancer growth in vivo assay. Those effects were noted together with a reduction in FAK and its downstream signaling.
Materials and methods
Cell lines and culture
Human ovarian cancer cell line SKOV3, HEY, OVCAR3 and Caov3 were obtained from the American Type Culture Collection (Manassas, VA, USA). They were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL, Carlsbad, CA, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS; HyClone, Logan, UT, USA) and 100 U/ml penicillin and streptomycin, and were cultured at 37°C in a 5% CO2 incubator. The SKOV3 was stably transfected with the lentiviral vectors carrying a luciferase protein sequence (CMV-Luciferase-2A-Puromycin, Clontech) and selected by puromycin (2 μg/ml) for 48 h.
Chemotherapeutic drug and antibodies
1-CP-U, generously provided by Ms. Qizhi Ning, was diluted to the required concentrations in conditional medium and then stored at 4°C according to the reported procedure [19]. Matrigel was purchased from Corning, USA (356234). The antibody to FAK (ab131435) and p-FAK Tyr397 (ab81298) were from Abcam, UK. p-Src Tyr416 antibody (6943) and paxillin antibody (12065) were purchased from Cell Signaling Technology, MA, USA. Anti-β-actin, goat anti-mouse and anti-rabbit secondary antibodies were from Boster Biological Technology (CA, USA).
Cell-matrix adhesion assay
The cell adhesion assay was based on an established method [20]. Briefly, the solution containing Matrigel (3mg/ml) was added to each well of a 96-well tissue culture plate. This was allowed to air-dry, after which the gel was rehydrated. The EOC cells (SKOV3, HEY, OVCAR3 and Caov3) were subjected to 1-CP-U (0.7, 1.0 and 1.4 µmol/l) for 24 h. The control group was incubated with an identical volume of drug-free dilute solution. Then, tumor cells were harvested, resuspended at a concentration of 1 × 105 cells/ml and 200 µl was added to each well. After incubating at 37°C for 1 h, the non-adherent cells were gently washed off. The adherent cells were fixed using a 4% paraformaldehyde solution for 30 min and then stained with 0.5% crystal violet. The number of adherent cells were then counted under a microscope and expressed as the cell number per high power field.
Wound-healing migration assay
Wound-healing assay was performed as described previously [21]. The cell motility was measured by a wound-healing assay. Initially, equal numbers of tumor cells were allowed to grow in six-well plates overnight. The next day, the cells were scraped with pipette tips and washed with PBS. The cells were then treated with or without the 1-CP-U (1.0 µmol/l) under starvation. After 24-h incubation, the cells migrated into the wound surface were recorded under an inverted microscope, and the average distance of migrating cells was calculated by photoshop.
Cell invasion assay
1x105 cells were seeded onto the upper part of a Matrigel-coated polycarbonate membrane filter (pore size, 8 µm) in 24-well inserts from Merck Millipore, German. Medium without serum was supplemented into the upper well in the absence or presence of 1.0 µmol/l 1-CP-U and medium containing 10% FBS was supplemented into the lower well. After 24-h incubation at 37°C with 5% CO2, the filters were stained with crystal violet. Five random fields were counted per chamber using an inverted microscope and then quantified.
In vivo studies in a peritoneal metastatic model
All animal experiments were approved by the Institutional Use and Care of Animals Committee and conducted according to the approved animal protocol of the Animal Centre of Tongji University. The nude mice (BALB/cAnSlac-nu/nu) were provided by the Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). In a preliminary experiment, 5–6 wk old female mice were equally distributed into control and experimental groups (five mice each for a total of four groups). Animals were intraperitoneally inoculated with SKOV3-Luc cells (3x106 cells in 200 µl PBS) prior to treatment.and then received an i.p. injection of 100, 200 and 400 mg/kg of 1-CP-U every 5 d for a total of 3-wk treatment after tumor inoculation. When the animals were terminally sacrificed, peritoneal metastatic tumor nodules were recorded and weighed. After the optimal therapeutic dose of i.p. 1-CP-U had been determined, Figure3(d) shows the experimental schedule for the in vivo study of this peritoneal metastatic model. 1 d after tumor inoculation, mice began to receive an i.p. injection of 200mg/kg 1-CP-U every 5 d for a total of 3-wk treatment. Drug-free dilute solution injections for control animals were performed with the same volumes.
Figure 3.
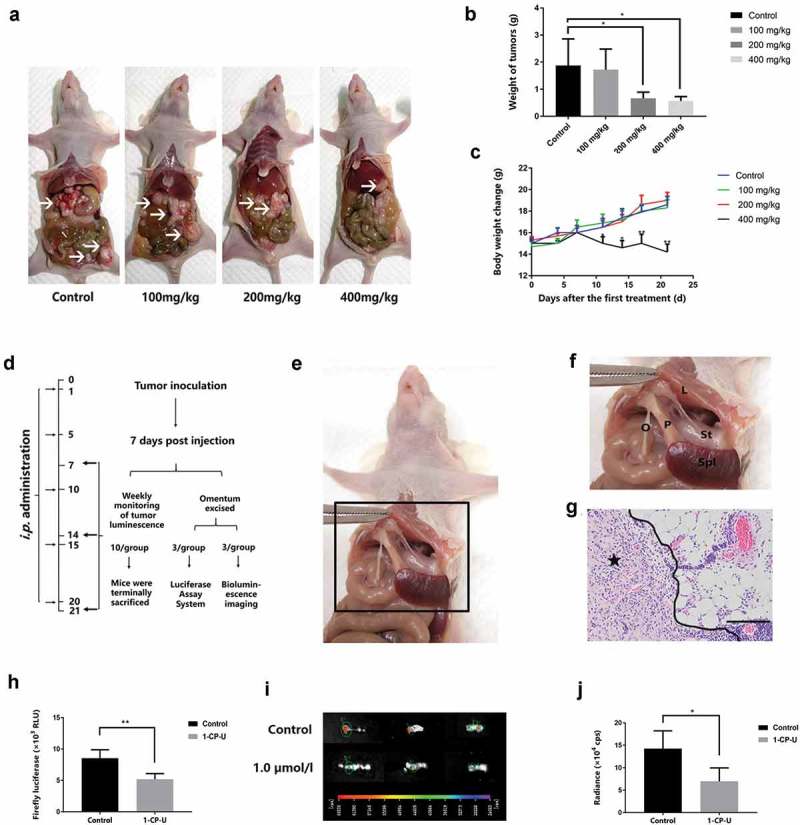
Suppression of EOC adhesion to omentum by 1-CP-U in vivo assays. (a) A preliminary experiment was performed to determine the optimal therapeutic dose of i.p. 1-CP-U. When the animals were terminally sacrificed, peritoneal metastatic tumor nodules were recorded and weighed. Arrows indicate tumors. (b) Mice that received intraperitoneal injection with 200 and 400 mg/kg of 1-CP-U showed a significant reduction in the weight of tumors, while there was no noticeable difference in the reduction of tumor weight between 100 mg/kg of 1-CP-U treated group and control group. (c) Notable weight loss was observed in 400 mg/kg of 1-CP-U group mice throughout the course of treatment. (d) Experimental schedule in a peritoneal metastatic model. (e) The location of murine omentum and surrounding organs. (f) An enlarged view of the area with the omentum (O), spleen (Spl), liver (L), stomach (St) and pancreas (P). (g) H&E staining showed that the SKOV3 cells established metastatic tumors in the omentum. Tumor cells were admixed with immune cells. Star indicates tumors. Scale bars represent 50 µm. (h) After the 1-CP-U i.p. injection for 7 d, omenta were isolated. Luciferase activity was measured using a Luminometer and the Luciferase Assay System. RLU = relative light unit. n = 3 for each group. (i) Bioluminescence images of isolated omenta were captured following 1-wk intraperitoneal administration of 1-CP-U. n = 3 for each group. (j) Fluorescent signals (expressed in counts/s) from the images were quantified using the IndiGo software provided with the in vivo imaging system (Berthold Technologies). cps = counts/second. *P< 0.05; **P< 0.001.
At the 7th d, omenta of three mice from each group were acquired and luciferase activity was analyzed using Luciferase Assay System as described below. At the same time, another three omenta from each group were dissected for bioluminescence images. The rest 10 mice in each group continued treatment and detection of bioluminescence imaging at day 7, 14 and 21. When the animals were terminally sacrificed, peritoneal metastatic tumor nodules were dissected, recorded, weighed and prepared for Haemotoxylin & Eosin (H&E) staining, immunofluorescent analysis and western blot. Body weight change of mice was recorded during the process to analyze the systemic toxicity of 1-CP-U.
Luciferase assay
About 50–100 mg omenta tissues were dissected and lysed with 500 µL passive lysis buffer (Promega, Madison, Wisconsin, USA). After centrifugation at 12,000 rpm for 10 min at 4°C, the supernatant was collected. Luciferase activity was measured using the Luciferase Assay System (Promega, E1500) according to the manufacturer′s instructions in a luminometer (Molecular Devices, SpectraMax M5).
Luminescence imaging
A nude mouse (∼25 g) was first anesthetized using 100 µl of chloral hydrate (4%). Then, D-luciferin (125 mg/g, Promega) in PBS (pH 7.4) was intraperitoneally injected 10 min prior to bioluminescent imaging in order to provide substrate for the luciferase-expressing cancer cells. The peritoneal tumor engraftment was imaged by the in vivo imaging system (NightOWL LB983; Berthold Technologies, Germany). Fluorescent signals (expressed in counts/s) from the images were calculated and all images were analyzed using the IndiGo software provided with the in vivo imaging system (Berthold Technologies).
Immunofluorescent staining of FAK and p-FAK
Tumor tissues from the in vivo peritoneal metastasis were fixed overnight in 10% formalin and subsequently processed and embedded in paraffin. The thickness of 4 µm sections was air-dried and then fixed in a mixture of 50% acetone and 50% methanol. After rehydrating, sections were incubated for 20 min in 10% goat serum of blocking solution and probed with the specific antibodies to FAK and p-FAK with a 1:100 concentration at 4°C overnight. The sections were first washed with PBS and then incubated with FITC-conjugated secondary antibodies (Sigma-Aldrich, German) for 1 h at 37°C. Then, nuclei were stained with DAPI. The slides were finally visualized under an Olympus BX51 fluorescence microscope at x400 objective magnification.
Immunoblot analyses
For immunoblotting analyses of murine tissues, tumor masses extracted from the in vivo models were prepared, and protein concentrations were determined using Bio-Rad protein assays (Bio-Rad, CA, USA). Then, proteins were separated in 10% SDS-PAGE and transferred onto PVDF membranes. The primary antibody was diluted and the membrane was incubated at 4°C overnight with the following antibodies: FAK (1:1000), p-FAK (1:1000), p-Src (1:1000) and paxillin (1:1000). After membranes were incubated with the secondary antibodies, visual detection was performed using the enhanced chemiluminescence method (Merck Millipore, German).
The SKOV3 and HEY cells were treated with 0.7 and 1.0 µmol/l of 1-CP-U at different incubation time points (6 h and 24 h), while the control group was incubated with an identical volume of drug-free dilute solution at different incubation time points (6 h and 24 h) corresponding to the treatment group. When the protein expressions of FAK, p-FAK, p-Src and paxillin expressions were detected, no significant difference was found between 6-h and 24-h control group. Based on this, we choose the 24-h one as the control group in immunoblot analyses. Whole cellular protein was extracted and protein concentration was determined. Then, the rest of the procedure was similar to that used on tissue protein. Finally, total lane intensities were determined using Image J software (Inc. Germany).
Statistical analysis
The results are expressed as the mean ± standard deviation and statistically compared by one-way analysis of variance test or unpaired Student’s t-test in different experiments. P< 0.05 was considered to indicate a statistically significant difference.
Results
1-CP-U inhibits the adhesion of EOC cells in vitro
In order to address the effect of this compound on adhesion of EOC cells to Matrigel-coated surface, four types of EOC cells (SKOV3, HEY, OVCAR3 and Caov3) were subjected to 1-CP-U (0.7, 1.0 and 1.4 µmol/l) for 24 h, and then the efficiency of their adhesion to Matrigel was examined (Figure 1(a)). The results showed that 1-CP-U significantly inhibited the adhesion of SKOV3, HEY, OVCAR3 and Caov3 cell lines at the concentration of 1.0 µmol/l (48.26 ± 6.47, 53.27 ± 7.04, 47.93 ± 10.02 and 43.98 ± 13.86%, respectively; P < 0.001) and in the 1.4 µmol/l groups (19.90 ± 6.97, 26.63 ± 6.03, 21.76 ± 10.33 and 18.80 ± 14.56%, respectively; P < 0.001), whereas 0.7 µmol/l of 1-CP-U only inhibited adhesion of HEY and OVCAR3 cell lines (67.34 ± 7.04%, P < 0.001; 92.14 ± 9.62%, P = 0.043, respectively) (Figure 1(b)), which indicated that 1-CP-U concentration-dependently suppressed adhesion to Matrigel of EOC cells.
Figure 1.
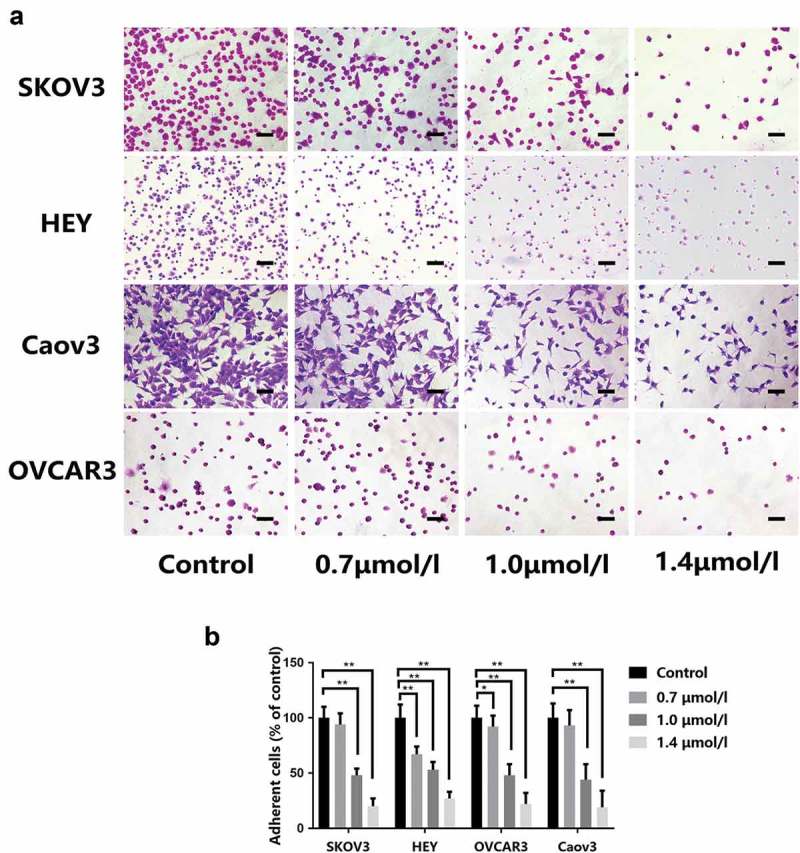
Effect of 1-CP-U on the adhesiveness of ovarian cancer cell to a Matrigel-coated surface. (a) Representative images captured from SKOV3, HEY, OVCAR3 and Caov3 cells which were pre-treated with control, 0.7, 1.0 and 1.4 µmol/l of 1-CP-U (magnification, x 40). Scale bars represent 200 µm. (b) Number of adherent cells were quantified by manual counting. The ratio of the number of adherent cells in the treatment group to that in the corresponding control group was quantified and presented in the bar chart. 1-CP-U concentration-dependently suppressed adhesion to Matrigel and treatment with 1.0 µmol/l of 1-CP-U resulted in >50% inhibitory effect on cell adhesion. Data are presented as the mean ± standard deviation of three separate experiments. *P < 0.05; **P < 0.001.
1-CP-U inhibits ovarian cancer cell migration and invasioninvitro.
It has been shown in our previous study [19] that 1-CP-U markedly suppressed the migration and invasion in multiple types of cell lines including EOC cells. In this research, we repeated metastasis-related assays which found that 1.0 µmol/l of 1-CP-U significantly decreased the migration (Figure 2(a)) and invasion (Figure 2(b)) potential of SKOV3 and HEY cells. The ratio of the migration distance in the treatment group to that in the corresponding control group was 37.20% and 47.03% for SKOV3 and HEY cells, respectively (P < 0.001) (Figure 2(c)). The number of SKOV3 passing through the Matrigel in the 1.0 µmol/l group was 62.29% of the negative control group (P = 0.023), while the number for HEY was 56.30% of control group (P < 0.001) (Figure 2(d)). Of note, 1-CP-U markedly inhibited SKOV3 and HEY migration and invasion at the level of 1.0 µmol/l.
Figure 2.
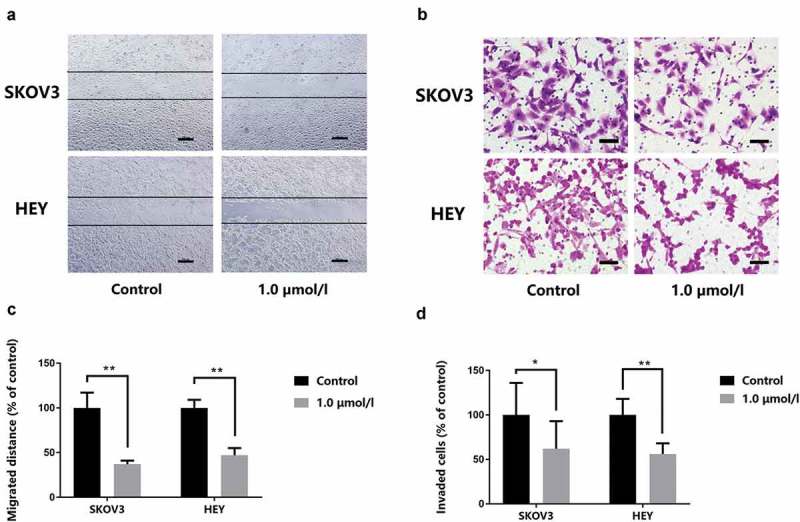
1-CP-U inhibits migration and invasion of tumor cells. (a) Wound-healing assay. Tumor cells were scratched with a pipette tip and then treated with 1.0 µmol/l 1-CP-U. After 24-h incubation, the cells migrated into the wound surface were recorded under an inverted microscope (magnification, x 40). Scale bars represent 200 µm. (b) Cell invasion assay. The cells in the lower surface of the chamber were captured under the microscope (magnification, x 200). Scale bars represent 50 µm. (c) The ratio of the number of migratory distance in the treatment group to that in the corresponding control group was quantified and presented in the bar chart. (d) Invaded SKOV3 and HEY cells were quantified by counting and densitometric analysis of invasive cells as in (b) was shown. *P < 0.05; **P < 0.001.
1-CP-U inhibits the EOC adhesion to omenta in vivo assays
To determine the optimal therapeutic dose of i.p. 1-CP-U, we first performed a preliminary experiment, in which 5–6 wk old female mice were equally distributed into control and experimental groups (five mice each for a total of four groups), and then received an i.p. injection of 100, 200 and 400 mg/kg of 1-CP-U every 5 d for a total of 3-wk treatment after tumor inoculation. When the animals were terminally sacrificed, peritoneal metastatic tumor nodules were recorded and weighed (Figure 3(a)). There was no noticeable difference in the reduction of tumor weight between 100 mg/kg of 1-CP-U treated group and control group (1.72 ± 0.67 g vs. 1.88 ± 0.98 g; P = 0.978), whereas the mice injected with 200 and 400 mg/kg of 1-CP-U showed a significant reduction in weight of tumors (0.66 ± 0.23 g vs. 1.88 ± 0.98 g, P = 0.036; 0.57 ± 0.16 vs. 1.88 ± 0.98 g, P= 0.023) (Figure 3(b)). However, notable weight loss was observed in 400 mg/kg of 1-CP-U group mice throughout the course of treatment, which indicates the systemic toxicity of 1-CP-U at the high concentration of 400 mg/kg (Figure 3(c)). Therefore, we chose i.p. injection of 200 mg/kg 1-CP-U in this procedure, which not only reduced intraperitoneal dissemination but also had no noticeable difference in the body weight change. Figure 3(d) shows the experimental schedule of the suppressive effect of 1-CP-U on EOC adhesion to omentum in vivo. 5–6 wk old female mice were equally distributed into control and experimental groups of 16 mice each for a total of 32 mice. It was at first accurately quantified by luciferase assays using SKOV3-Luc cells. After 7 d treatment of 1-CP-U injection i.p. into mice (200mg/kg), the omentum was excised by cutting at its base. Figure 3(e) shows the view of area with murine omentum and surrounding organs. The omentum was located in the peritoneal cavity between the stomach, pancreas and the spleen. Although analogous to the human omentum in composition and tissue architecture, the mouse omentum consisted of a single ribbon of fat attached to the pancreas (Figure 3(f)). Sections of isolated omenta were subjected to H&E staining, which confirmed the colonization of tumor cells on the omentum (Figure 3(g)). The luciferase activity in the omentum of 1-CP-U group was lower than that in the control group (5.18 × 103 vs. 8.53 × 103 RLU; P < 0.001) (Figure 3(h)). Subsequently, the suppression of EOC cells adhesion to the omentum was further examined in the bioluminescence imaging system. Omentum was excised 10 min after the D-luciferin injections, and bioluminescence image was captured (Figure 3(i)). The luciferase activity in the omentum treated with 1-CP-U was significantly lower than that of control group (6.97 × 104 vs. 14.24 × 104 cps; P = 0.007) (Figure 3(j)). These studies indicated that treatment with 1.0 µmol/l of 1-CP-U significantly decreased the ability of SKOV3-Luc cells to colonize mouse omentum in vivo as compared to control group.
1-CP-U reduces intraperitoneal dissemination and inhibits the tumor growth in vivo assay
3x106 SKOV3-Luc cells were resuspended in 200μl PBS and injected into the abdomen of 5-6 wk old female Athymic Nude mice. Real-time tracking of tumor metastasis and growth were monitored by bioluminescent imaging and analyzed by detection of overall luciferase activity at day 7, 14 and 21 (Figure 4(a)). As for luciferase activity detection (Figure 4(b)), the two groups had no difference during the first 7 d. 1-CP-U appeared to exhibit a little inhibition effect after 2 wk although this is not statistically significant (14.47×104 vs. 18.61 × 104 cps; P = 0.111). Moreover, 1-CP-U had a marked decrease to luciferase activity which achieved statistical significance (18.90×104 vs. 35.26 × 104 cps; P = 0.023) at the 3rd wk. Those data indicated that intraperitoneal metastatic nodules were less-developed in 1-CP-U group, and it took 3 wk to reach a significant inhibitory effect compared with the control group. Murine peritoneal metastatic locations of involved organs could be visually observed by the luminescence imaging under dissection exposure (Figure 4(c)). To improve visualization, the metastatic tumor nodules and involved organs were dissected. The control group of mice had multiple locations of metastatic nodules on the liver, pancreas, colon, mesentery or even spleen, whereas the metastatic nodules of treatment group were mainly on the colon and pancreas (Figure 4(d)), and histological analysis was performed by H&E stain (Figure 4(e)). Furthermore, mice injected with 1-CP-U showed a significant reduction in the weight of tumors (0.84 ± 0.52 g vs. 1.73±0.82 g; P = 0.033) (Figure 4(f)). During the treatment, there was no noticeable difference in the body weight change between 1-CP-U treated and control mice (Figure 4(g)). In addition, no histological abnormalities were observed in the major organs such as the heart, liver, kidney, pancreas and colon of the 1-CP-U -treated mice, indicating no sign of systemic toxicity (Figure 4(h)).
Figure 4.
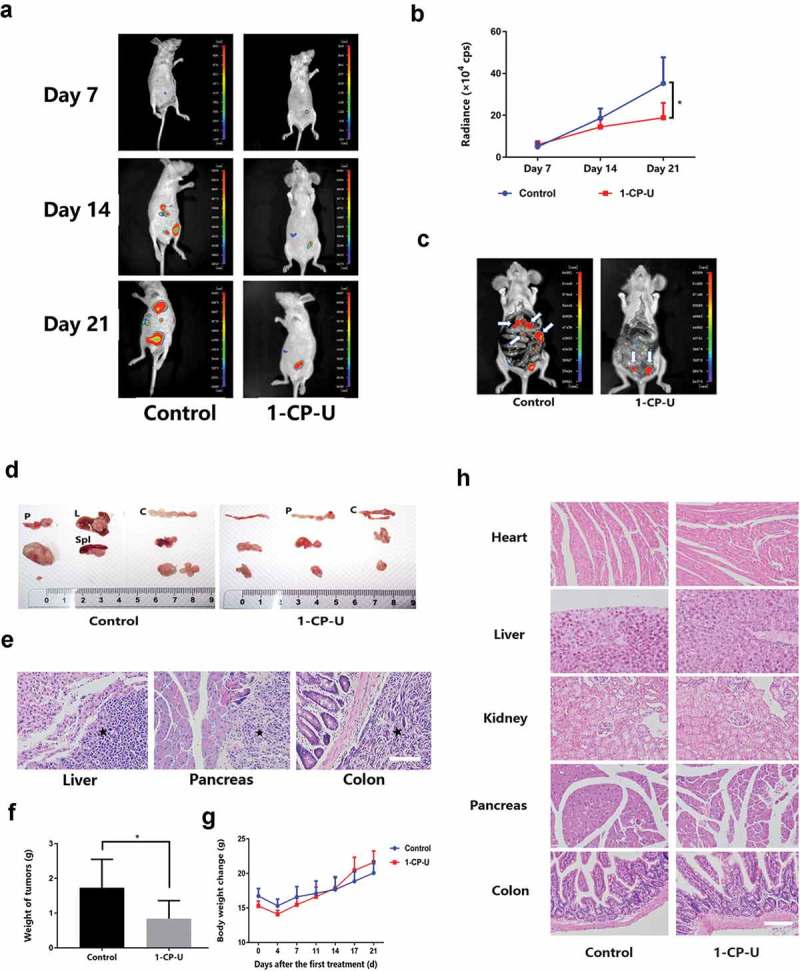
1-CP-U inhibited EOC intraperitoneal metastasis and tumor growth. (a) Intraperitoneal metastasis was monitored by bioluminescent imaging and tumor growth was analyzed by detection of overall luciferase activity at day 7, 14 and 21. n = 10 for each group. (b) The graph shows the luciferase activity after injection which demonstrated 1-CP-U exhibited a significant effect on growth inhibition after 3 wk. (c) In comparison with 1-CP-U treatment group, more sporadic intraperitoneal metastatic colonizations to different organs were detected in control group. Arrows indicate tumors. (d) Representative views of the metastases in the peritoneal cavity are shown. Tumors were located on the spleen (Spl), liver (L), colon (C) and pancreas (P). (e) The metastatic colonizations were identified by H&E staining. Stars indicate tumors. (f) Mice that received intraperitoneal injection of 1-CP-U showed a significant reduction in the weight of tumors. (g) Notable weight loss was not observed in treatment group mice throughout the course of treatment, and the body weight increase in both groups showed no significance. (H) Vital organs were harvested to be stained with H&E stain, there was no noticeable difference in the pictures of heart, liver, kidney, pancreas and colon. Scale bars represent 50 µm. cps = counts/second. *P < 0.05.
1-CP-U suppressed the metastasis potential of EOC cells by inhibiting FAK
After 1-CP-U i.p. treatment for 3 wk, animals were terminally sacrificed, peritoneal metastatic tumor nodules were dissected and prepared for immunofluorescent staining. Tumor cells stained strongly for total FAK in control group, whereas tumors from mice which received 1-CP-U showed a marked reduction in FAK staining (Figure 5(a)). Similar to total FAK, a reduction in p-FAK staining was noted in tumors from mice that received treatment.
Figure 5.
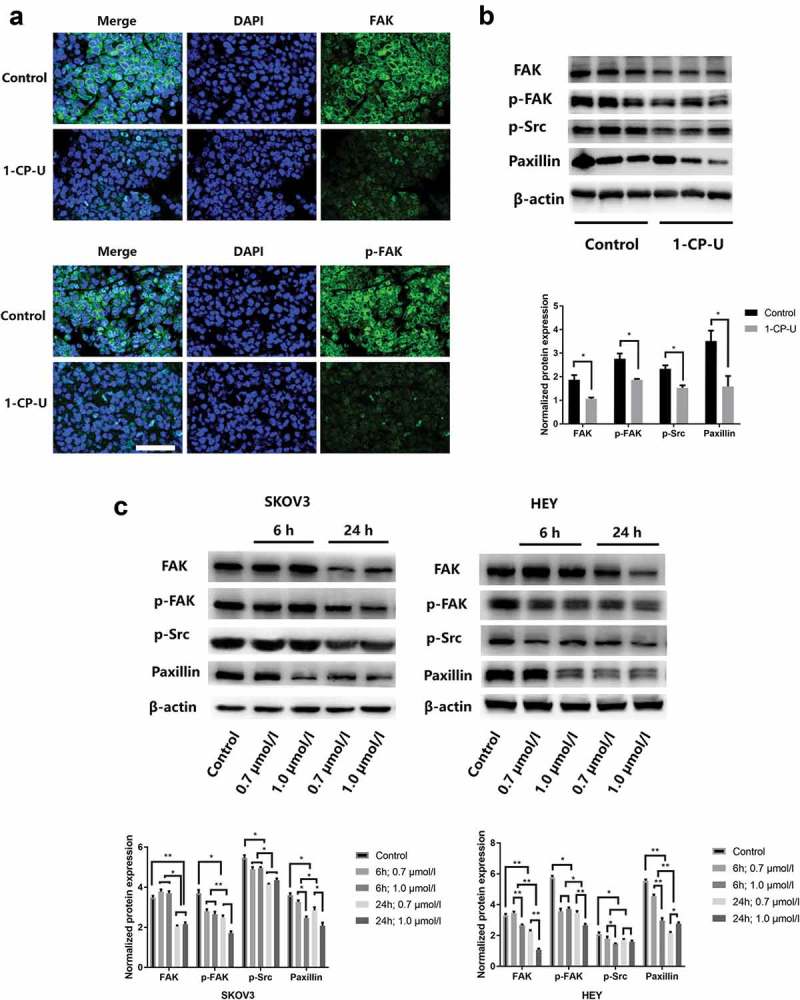
1-CP-U downregulates FAK expression and activation in EOC cells. (a) At the end of 3-wk treatment with i.p. 1-CP-U, peritoneal metastatic tumor nodules were dissected and prepared for Immunofluorescent staining. The staining pattern of FAK and p-FAK in tissue sections was examined, which showed positive staining mainly in the cytoplasm. Nuclei were stained with DAPI (blue). There was a marked reduction in FAK and p-FAK in the treatment group in comparison with the control group. Scale bars represent 25 µm. (b) FAK, p-FAK, p-Src and paxillin expressions from tumor tissues were examined, and the band intensity values showed that all significantly decreased in the 1-CP-U treatment group. (C) SKOV3 and HEY cells were treated with 1-CP-U for 24 h at different concentrations (0.7 and 1.0 µmol/l). With the increasing concentration and treatment time of 1-CP-U, expressions of FAK, p-FAK, p-Src and paxillin were significantly decreased by western blotting analysis.
We further evaluated the effect of 1-CP-U on FAK and its downstream signaling in murine tumor tissues by western blot. For quantification of immunoblotted membranes, the band intensity values for each protein are detected (mean ± SD, n = 3). With the treatment of 1-CP-U i.p., the protein expressions of FAK, p-FAK, p-Src and paxillin expressions were all significantly decreased (Figure 5(b)).
Subsequently, SKOV3 and HEY cells were treated with 0.7 and 1.0 µmol/l of 1-CP-U for indicated time. As shown in Figure 5(c), 1-CP-U suppressed the FAK, p-FAK, p-Src and paxillin expressions in a time- and dose-dependent manner, which implied 1-CP-U inhibited the metastasis potential of EOC cells by inhibiting FAK signaling pathway.
Discussion
1-calcium phosphate-uracil (1-CP-U) is a synthetic uracil derivative, which is a pyrimidine-containing compound and has been suggested to demonstrate a wide range of highly selective functions [19]. Our previous studies have documented a broad spectrum of anti-cancer activities of 1-CP-U against a number of different human tumor cell lines [19]. We also observed that 1-CP-U appeared to be more effective at inhibiting cell migration and invasion than inducing apoptosis [19]. In the present study, we demonstrated that 1-CP-U had an inhibitory effect on the adhesion of SKOV3, HEY, OVCAR3 and Caov3 cells. Moreover, 1-CP-U inhibited the migration and invasion of SKOV3 and HEY cells in vitro. Then, the luciferase activity and bioluminescence imaging assay were performed to find that 1-CP-U suppressed EOC metastasis into the omentum in vivo assay. Meanwhile, 1-CP-U reduced intraperitoneal dissemination and cancer growth of SKOV3-Luc cells by real-time tracking of tumor metastasis and growth monitored by bioluminescent imaging. Furthermore, mice injected with 1-CP-U showed a significant reduction in the weight of tumors, whereas there was no noticeable difference in the body weight change between 1-CP-U treated and control mice, and no histological abnormalities were observed in the major organs such as the heart, liver, kidney, pancreas, colon, and small intestine of the 1-CP-U -treated mice. Finally, expression of FAK and its downstream signaling were detected in tumor tissues and EOC cell lines, which showed significant decreases in the 1-CP-U treatment group.
Over the years, several randomized clinical trials have assessed the different infusion strategies of chemotherapy (CHT) in advanced ovarian cancer management. However, because of treatment heterogeneity, the results regarding survival rates are mostly conflicting. Recently, a meta-analysis based only on randomized clinical trials evaluated the efficacy and toxicity of intravenous chemotherapy (i.v.-CHT) and intraperitoneal chemotherapy (i.p.-CHT) and identified differences in outcomes [22]. They found that i.p.-CHT allowed an improvement of both overall survival (OS) (HR 0.79, 95% CI 0.67–0.92) and progression-free survival (PFS) (HR 0.88, 95% CI 0.80–0.98) over i.v.-CHT in advanced OC management. With regard to toxicities, i.v. plus i.p. chemotherapy is more toxic than i.v. alone. However, that is quite unusual for patients receiving both i.p. and i.v. to complete all cycles of chemotherapy, and toxicity is not enough to abandon the IP route of administration because of the increased survival outcomes. Therefore, it is crucial to find a balance between right effective doses and tolerable toxicity. Effective and safe delivery of intraperitoneal chemotherapy might represent the right choice and should be offered as a treatment option. In our study, 1-CP-U i.p. injection was performed every 5 d for a total of 3 wk. And we first assessed the suppression of EOC adhesion to the omentum and then in the real-time tracking of tumor metastasis and growth monitored by bioluminescent imaging, 1-CP-U was found reduce intraperitoneal dissemination and cancer growth of SKOV3-Luc cells. Therefore, we conclude that 1-CP-U i.p. injection suppressed intraperitoneal dissemination and cancer growth in vivo assay. More importantly, no difference in body weight changes or histological abnormalities was observed, indicating no systemic toxicity. 1-CP-U i.p. injection might be a potential choice for treating EOC patients and prevent peritoneal metastasis. As the i.v. infusion of chemotherapy is also an important way in EOC treatment, further researches should be designed to compare the efficacy and toxicity between1-CP-U i.p. and i.v. infusion, and identify the appropriate infusion strategy and schedule of 1-CP-U.
Although ovarian carcinoma cells have the potential to metastasize throughout the peritoneal cavity, it is not entirely random that the primary tumors metastasize to distributed organs. Rather than the bilateral fallopian tubes and ovaries, the most common secondary sites for distant metastasis are the omentum and the peritoneum [23]. The human omentum is a peritoneal fold composed of a single layer of mesothelial cells and its associated underlying ECM. Mesothelial cells protect against, rather than mediate, cancer cell attachment. Instead, the sub-mesothelial ECM is the preferred substrate for ovarian cancer cell attachment [24] and migration [11]. In addition to the similarity of composition and function in omentum from human [25], the omentum from mice offers accessible distinguishment and sampling, thus it is used to detect the early stage metastasis of EOC in vivo. Giving the depth and associated light scattering of bioluminescent signals originating from the omentum, which makes it challenging to employ optical imaging modalities, omentum tissues were directly dissected at the 7th d to perform luciferase activity and luminescence imaging. Therefore, we use murine omentum excised after D-luciferin injection to assess the suppression of EOC adhesion to the omentum in luciferase activity assay and bioluminescence imaging system. The luciferase activities in the omentum treated with 1-CP-U from both assays were significantly lower than those of control group. These studies show that treatment with 1-CP-U significantly decreased the ability of SKOV3-Luc cells to colonize mouse omentum in vivo as compared to control group.
In the ovarian cancer study, FAK overexpression in primary tumor biopsy material was correlated with metastasis to lymph nodes and distant organs, as well as with reduced survival times [15,26]. Phosphorylation of FAK at Tyr397 was found in invasive tumor tissues, linked to poor clinical outcome, but not detected in the normal ovarian epithelium [27], which indicates that FAK pathway might be a useful therapeutic target [28,29]. Metastasizing cancer cells have two main options for attachment to the: the surface mesothelial cells or the exposed ECM [30]. A variety of different adhesion molecules can mediate the attachment of cancer cells to peritoneum, among which the integrins, αβ heterodimeric transmembrane proteins, play a vital role [31]. Blocking β1 integrin inhibits EOC cell attachment and migration on ECM substrate relevant to the peritoneum [32]. EOC cell lines with an aggressive phenotype have an elevated expression of the α2 and β1 collagen-binding integrin subunits as compared to cells with lower invasive capacity [33]. A group of integrins recognizes and binds to the Arg-Gly-Asp (RGD) motif present on the ECM proteins which further activate specific intracellular kinases, such as FAK [34], inducing FAK autophosphorylation at FAK Tyr397 which exposes an SH2 domain-binding site for Src [35]. Src recruitment results in Src-dependent phosphorylation of FAK at FAK Tyr576 and FAK Tyr577 leading to maximal FAK activation [36]. Paxillin is a substrate for the FAK–Src complex that functions as an adaptor molecule for various signaling and structural proteins in adhesion [37]. Previous researches showed that paxillin localizes in dynamic adhesions, which forms and rapidly disassembles at the base of protrusions [38]. Moreover, Paxillin can recruit other molecules to adhesions and regulate the organization of the actin cytoskeleton [39]. In addition, FAK-mediated signaling events induce the expression of matrix metalloproteinases (MMPs). Once MMPs are secreted, they mediate the breakdown of surrounding extracellular-matrix substrates and promote cell invasion [40]. To determine whether 1-CP-U affects FAK signaling, peritoneal metastatic tumor nodules were detected for immunofluorescent staining, which showed a reduction in FAK and p-FAK staining with 1-CP-U treatment group. Then, the protein expressions of FAK, p-FAK, p-Src and paxillin in murine tumor tissues were tested by western blot and demonstrated significantly decrease. Moreover, different concentrations of 1-CP-U were used to treat SKOV3 and HEY cells at the indicated time point. The results showed expressions of FAK, p-FAK, p-Src and paxillin were significantly decreased with the increasing concentration. So we inferred that 1-CP-U might inhibit EOC adhesion and invasion through suppressing FAK signaling pathway.
In conclusion, 1-CP-U had a profound inhibitory effect on adhesion, invasion and metastasis of EOC in vitro and suppressed intraperitoneal dissemination and cancer growth in vivo assay, which was associated with inhibition on the FAK pathway. These data indicate that 1-CP-U may be a potential choice for treating EOC patients and prevent peritoneal metastasis.
Funding Statement
This work was supported by the National Natural Science Foundation of China [81602283];National Natural Science Foundation of China [81602278];National Natural Science Foundation of China [81772762]; National Natural Science Foundation of China [81372305]; Program for Young Excellent Talents in Tongji University.
Acknowledgments
1-CP-U was generously provided by Ms. Qizhi Ning, who developed this agent. This study was supported by the National Natural Science Foundation of China under Grant (number 81602283, 81602278, 81372305 and 81772762); and Program for Young Excellent Talents in Tongji University.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- [2].Smith RA, Andrews KS, Brooks D, et al. Cancer screening in the United States, 2018: A review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin. 2018;68(4):297–316. [DOI] [PubMed] [Google Scholar]
- [3].Webb PM, Jordan SJ.. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. [DOI] [PubMed] [Google Scholar]
- [4].Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177(3):1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Marsden DE, Friedlander M, Hacker NF. Current management of epithelial ovarian carcinoma: a review. Semin Surg Oncol. 2000;19(1):11–19. [DOI] [PubMed] [Google Scholar]
- [6].Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2(8):563–572. [DOI] [PubMed] [Google Scholar]
- [7].Hudson LG, Zeineldin R, Stack MS. Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin Exp Metastasis. 2008;25(6):643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kenny HA, Dogan S, Zillhardt M, et al. Organotypic models of metastasis: A three-dimensional culture mimicking the human peritoneum and omentum for the study of the early steps of ovarian cancer metastasis. Cancer Treat Res. 2009;149:335–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Khan SM, Funk HM, Thiolloy S, et al. In vitro metastatic colonization of human ovarian cancer cells to the omentum. Clin Exp Metastasis. 2010;27(3):185–196. [DOI] [PubMed] [Google Scholar]
- [10].Steinkamp MP, Winner KK, Davies S, et al. Ovarian tumor attachment, invasion, and vascularization reflect unique microenvironments in the peritoneum: insights from xenograft and mathematical models. Front Oncol. 2013;3:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Burleson KM, Hansen LK, Skubitz AP. Ovarian carcinoma spheroids disaggregate on type I collagen and invade live human mesothelial cell monolayers. Clin Exp Metastasis. 2004;21(8):685–697. [DOI] [PubMed] [Google Scholar]
- [12].Bogenrieder T, Herlyn M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene. 2003;22(42):6524–6536. [DOI] [PubMed] [Google Scholar]
- [13].Zhao J, Guan JL. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009;28(1–2):35–49. [DOI] [PubMed] [Google Scholar]
- [14].Recher C, Ysebaert L, Beyne-Rauzy O, et al. Expression of focal adhesion kinase in acute myeloid leukemia is associated with enhanced blast migration, increased cellularity, and poor prognosis. Cancer Res. 2004;64(9):3191–3197. [DOI] [PubMed] [Google Scholar]
- [15].Zeng XQ, Li N, Ma -L-L, et al. Prognostic value of Focal Adhesion Kinase (FAK) in human solid carcinomas: a meta-analysis. PLoS One. 2016;11(9):e0162666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Frame MC, Serrels A. FAK to the rescue: activated stroma promotes a “safe haven” for BRAF-mutant melanoma cells by inducing FAK signaling. Cancer Cell. 2015;27(4):429–431. [DOI] [PubMed] [Google Scholar]
- [17].Shibue T, Weinberg RA. Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci U S A. 2009;106(25):10290–10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jiang WG, Sanders AJ, Katoh M, et al. Tissue invasion and metastasis: molecular, biological and clinical perspectives. Semin Cancer Biol. 2015;35 Suppl(Suppl):S244–s275. [DOI] [PubMed] [Google Scholar]
- [19].Peng J, Chen X, Hu Q, et al. 1calcium phosphateuracil, a synthesized pyrimidine derivative agent, has antiproliferative, proapoptotic and antiinvasion effects on multiple tumor cell lines. Mol Med Rep. 2014;10(5):2271–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jiang WG, Ye L, Ji K, et al. Antitumour effects of Yangzheng Xiaoji in human osteosarcoma: the pivotal role of focal adhesion kinase signalling. Oncol Rep. 2013;30(3):1405–1413. [DOI] [PubMed] [Google Scholar]
- [21].Wu S, Du R, Gao C, et al. The role of XBP1s in the metastasis and prognosis of hepatocellular carcinoma. Biochem Biophys Res Commun. 2018;500(3):530–537. [DOI] [PubMed] [Google Scholar]
- [22].Marchetti C, De Felice F, Perniola G, et al. Role of intraperitoneal chemotherapy in ovarian cancer in the platinum-taxane-based era: A meta-analysis. Crit Rev Oncol Hematol. 2019;136:64–69. [DOI] [PubMed] [Google Scholar]
- [23].Sehouli J, Senyuva F, Fotopoulou C, et al. Intra-abdominal tumor dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer. J Surg Oncol. 2009;99(7):424–427. [DOI] [PubMed] [Google Scholar]
- [24].Moser TL, Pizzo SV, Bafetti LM, et al. Evidence for preferential adhesion of ovarian epithelial carcinoma cells to type I collagen mediated by the alpha2beta1 integrin. Int J Cancer. 1996;67(5):695–701. [DOI] [PubMed] [Google Scholar]
- [25].Wilkosz S, Ireland G, Khwaja N, et al. A comparative study of the structure of human and murine greater omentum. Anat Embryol (Berl). 2005;209(3):251–261. [DOI] [PubMed] [Google Scholar]
- [26].Sood AK, Coffin JE, Schneider GB, et al. Biological significance of focal adhesion kinase in ovarian cancer: role in migration and invasion. Am J Pathol. 2004;165(4):1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Grisaru-Granovsky S, Salah Z, Maoz M, et al. Differential expression of protease activated receptor 1 (Par1) and pY397FAK in benign and malignant human ovarian tissue samples. Int J Cancer. 2005;113(3):372–378. [DOI] [PubMed] [Google Scholar]
- [28].Xu B, Lefringhouse J, Liu Z, et al. Inhibition of the integrin/FAK signaling axis and c-Myc synergistically disrupts ovarian cancer malignancy. Oncogenesis. 2017;6(1):e295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Golubovskaya VM. Targeting FAK in human cancer: from finding to first clinical trials. Front Biosci (Landmark Ed). 2014;19:687–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sodek KL, Murphy KJ, Brown TJ, et al. Cell-cell and cell-matrix dynamics in intraperitoneal cancer metastasis. Cancer Metastasis Rev. 2012;31(1–2):397–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Barczyk M, Carracedo S, Gullberg D. Integrins. Cell Tissue Res. 2010;339(1):269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Burleson KM, Casey RC, Skubitz KM, et al. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol. 2004;93(1):170–181. [DOI] [PubMed] [Google Scholar]
- [33].Sodek KL, Evangelou AI, Ignatchenko A, et al. Identification of pathways associated with invasive behavior by ovarian cancer cells using multidimensional protein identification technology (MudPIT). Mol Biosyst. 2008;4(7):762–773. [DOI] [PubMed] [Google Scholar]
- [34].Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. [DOI] [PubMed] [Google Scholar]
- [35].Schaller MD, Hildebrand JD, Shannon JD, et al. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14(3):1680–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995;15(2):954–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000;2(12):E231–E236. [DOI] [PubMed] [Google Scholar]
- [38].Laukaitis CM, Webb DJ, Donais K, et al. Differential dynamics of alpha 5 integrin, paxillin, and alpha-actinin during formation and disassembly of adhesions in migrating cells. J Cell Biol. 2001;153(7):1427–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Webb DJ, Donais K, Whitmore LA, et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6(2):154–161. [DOI] [PubMed] [Google Scholar]
- [40].McLean GW, Carragher NO, Avizienyte E, et al. The role of focal-adhesion kinase in cancer - a new therapeutic opportunity. Nat Rev Cancer. 2005;5(7):505–515. [DOI] [PubMed] [Google Scholar]


