Abstract
Pharmacogenomics aims to associate human genetic variability with differences in drug phenotypes in order to tailor drug treatment to individual patients. The massive amount of genetic data generated from large cohorts of patients with variable drug phenotypes have led to advances in this field. Understanding the application of pharmacogenomics in dermatology could inform clinical practice and provide insight for future research. The Pharmacogenomics Knowledge Base and Clinical Pharmacogenetics Implementation Consortium are among the resources to help clinicians and researchers navigate the many gene-drug associations that have already been discovered. The implementation of clinical pharmacogenomics within health care systems remains an area of ongoing development. This review provides an introduction to the field of pharmacogenomics and to current pharmacogenomics resources using examples of gene-drug associations relevant to the field of dermatology.
Keywords: Pharmacogenomics, pharmacogenetics, personalized medicine, precision medicine, dermatology
Introduction
For decades, clinicians have noted that patients can have very different responses to the same medication.1 While a patient’s response to a medication is often influenced by environmental factors, interest in the effect of genetic variation on drug phenotypes has recently increased.2 This interest has been facilitated by the increased availability of both large-scale genomic data and the tools to analyze this data.2
Pharmacogenomics (used interchangeably with the term pharmacogenetics) studies the link between human genetic variation and drug-related phenotypes, including efficacy and adverse events.2 Variation in genes involved in a drug’s pharmacokinetics and pharmacodynamics, in addition to genes involved in disease and immune system pathways, can all contribute to the pharmacogenomics of drug response.3
While the clinical application of pharmacogenomics is still in its early phases, we have already seen its potential to change practice. Abacavir, an antiretroviral medication used to treat HIV, can cause a dangerous hypersensitivity syndrome; human leukocyte antigen-B*57:01 (HLA-B*57:01) has been associated with a markedly increased risk of this adverse event.4,5 A prospective double-blind randomized controlled trial demonstrated that, by pre-screening for HLA-B*57:01 prior to prescribing abacavir, immunologically confirmed hypersensitivity syndrome could be drastically reduced.5 Cost-effectiveness studies of HLA-B*57:01 testing have demonstrated a cost-effectiveness ratio of $36,700 per quality-adjusted life year when compared to no testing.6 Thus, with proper implementation, pharmacogenomics hopes to improve patient outcomes and save health care dollars through tailored therapy.
Here, we will summarize some of the tools used to discover gene-drug associations, before introducing pharmacogenomics resources for researchers and clinicians, using examples of pharmacogenomics that are applicable to dermatology. Since the ultimate goal of pharmacogenomics research is clinical translation, we will also discuss some of the implementation challenges and the current state of possible solutions.
Discovery in Pharmacogenomics
Clinical observations of patients with different responses to the same drug motivated the first pharmacogenomic studies. Early work in pharmacogenomics noted differences in enzymatic activity between patients with variable drug responses; later, increased understanding of genetics explained the reason for these differences.1 The decreased cost of genotyping and next generation sequencing has allowed these techniques to be readily leveraged in pharmacogenomics research.2
Genotyping arrays interrogate prespecified variants within the genome, usually areas that have been noted to have significant variability between individuals or have clinical significance. Sequencing, on the other hand, determines the DNA sequence of an entire region of interest – a gene, the exome, or the whole genome.7
Targeted genotyping and sequencing of candidate genes in cases and controls has led to the discovery of important relationships between genetic variants and drug phenotypes.2 Many times, these candidate genes are selected based on prior knowledge of the drug’s metabolism, mechanism of action, or interaction with the immune system. For example, candidate gene studies were used to discover the association of abacavir hypersensitivity syndrome with HLA-B*57:01.4,5
On a larger scale, genome-wide association studies (GWASs) have been used to discover novel associations by genotyping up to several thousand cases and controls.2,8 These studies do not usually have an a priori hypothesis about what genes or variants may be associated with the drug phenotype of interest.2 GWAS has traditionally used DNA arrays to probe hundreds of thousands to millions of single nucleotide polymorphisms, located across the genome, to discover significant genetic differences in patients with variable drug phenotypes. These phenotypes can be discrete, such as an adverse reaction, or continuous, such as therapeutic drug dose.2 Because of the large number of hypotheses tested, these studies need large sample sizes to be adequately powered.2
More recently, with the falling price of genomic sequencing, there have been pharmacogenomics studies using exome sequencing in lieu of genotyping arrays.9,10
Sequencing has the ability to capture population-specific variants in diverse populations; these variants are not routinely assayed for by genotyping arrays since many genotyping arrays were designed based on prevalent variants in European descent populations.9 Additionally, exome or genome sequencing can also capture rare variants, some of which may be unique to the individual tested.11 However, characterizing the clinical effects of these rare variants can be a challenge since their prevalence is low.8 And though the cost of sequencing has been falling, exome and genome sequencing are still significantly more expensive than using genotyping arrays.7
While GWASs can establish an association between a genetic variant and a drug phenotype, these studies do not establish causation. Additional functional studies looking at changes in gene expression or protein activity are often needed. These functional studies can help determine whether the observed phenotype is likely caused by the variant identified by the GWAS or a neighboring linked variant.8 Furthermore, clinical studies are required to determine which variant-phenotype associations could have clinical impact.
Research and Clinical Resources for Pharmacogenomics
With the rapid increase of discovered gene-drug associations, researchers and clinicians need tools to navigate the massive amount of available data.
The Pharmacogenomics Knowledgebase (PharmGKB) collects, curates and disseminates pharmacogenomic knowledge from multiple sources, including the scientific literature, clinical dosing guidelines and drug labels.12 This knowledge is manually curated by expert scientific curators and can be accessed via the PharmGKB website (www.pharmgkb.org). All PharmGKB data are freely available for use under a Creative Commons license, further details of which can be found at https://www.pharmgkb.org/page/dataUsagePolicy.12
Every gene, drug, variant and phenotype recorded in the PharmGKB has its own page on the website where the pharmacogenomic information linked to that particular entity can be easily accessed. As an example, the page for the drug mycophenolate mofetil (MMF), an immunosuppressive steroid-sparing agent used to treat dermatological diseases such as bullous pemphigoid and dermatomyositis, shows evidence-based pharmacokinetic and pharmacodynamic pathways, which have been produced by PharmGKB curators (Figure).13,14
Figure:
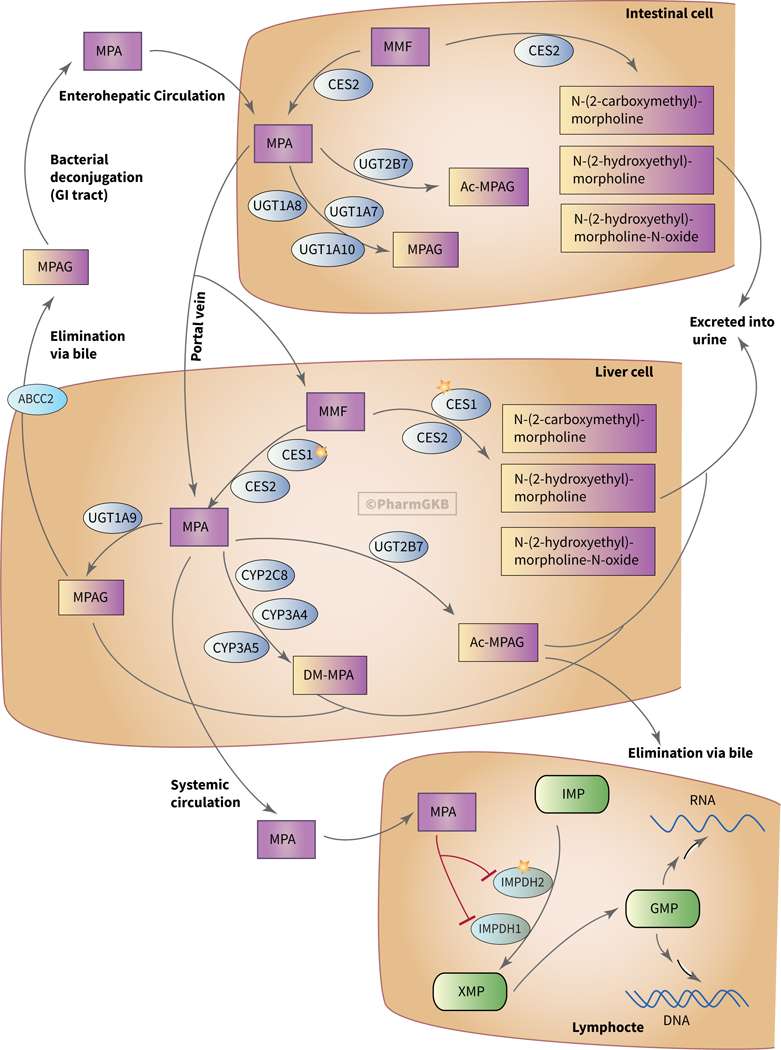
The pharmacokinetic and pharmacodynamics pathways for mycophenolate mofetil.14 Genetic variation in these pathways can lead to differential drug phenotypes. An interactive version of this pathway can be found at https://www.pharmgkb.org/pathway/PA165964832. Used with permission of PharmGKB. Abbreviations: CES, carboxylesterases; GMP, guanosine monophosphate; IMP, inosine monophosphate; IMPDH, inosinemonophosphate dehydrogenase; MPA, mycophenolic acid; MMF, mycophenolate mofetil; UGT, UDP glucuronosyl transferase.
Briefly, this pathway shows the pro-drug MMF being absorbed through the intestine after oral intake and being converted to the bioactive compound mycophenolic acid (MPA) by carboxylesterases both in intestinal cells and in liver cells. MPA can then enter the circulation and reach its target - lymphocytes, where it inhibits purine synthesis by blocking the rate-limiting enzyme, inosine monophosphate dehydrogenase. MPA is metabolized by intestinal and liver UDP glucuronosyl transferases, which facilitate glucuronidation and create MPA-7-O-glucuronide (MPAG) and its acyl glucuronide form, Ac-MPAG. MPAG is mainly excreted into the urine. MPAG and Ac-MPAG are also eliminated via bile through the transporter multidrug resistance-associated protein 2, which is encoded by the ABCC2 gene. Additionally, cytochrome P450 (CYP) enzymes in the liver metabolize MPA to 6-O-desmethyl-MPA, which is also excreted via urine.14 If a genetic cause of drug phenotype variability is suspected, but no established drug-gene association exists, PharmGKB’s pathways can provide a starting point for researchers working to identify where genetic variation leads to differential drug response.2
PharmGKB also allows for querying specific gene-drug relationships. For example, searching for the terms dapsone and glucose-6-phosphate dehydrogenase (G6PD) will retrieve any annotations tagged with both dapsone and G6PD.
Pharmacogenomic findings from the scientific literature are captured in the PharmGKB as variant annotations. Variant annotations describe a single finding from a single paper. As a result, papers can be annotated with multiple variant annotations, depending on the content of the paper.
Variant annotations are comprised of a single summary sentence describing the finding. These summary sentences are constructed from a set of standardized terms, allowing multiple variant annotations to be easily compared against each other. PharmGKB curators also tag variant annotations with additional study parameters from the paper, including characteristics of the study population and any statistical analyses that were carried out.12
Once created, variant annotations are used as evidence to produce summaries of the current scientific knowledge regarding a particular variant-drug association. These summaries are the PharmGKB clinical annotations and are assigned a level of evidence by PharmGKB to indicate the strength of evidence supporting the association. PharmGKB curators adjust the assigned evidence level as additional supporting or contradicting data becomes available in the literature. Clinical annotations also include negative results - findings that show no gene-drug association or that contradict the association. As with variant annotations, clinical annotations are tagged with relevant genes, drugs and phenotypes as well as relevant population information.12
Returning to our MMF example, all PharmGKB Clinical Annotations for MMF can also be accessed from the drug page.13,14 For MMF, multiple pharmacogenes are listed; however all have been assigned as Level 3 annotations (i.e. the associations have not yet been independently replicated in the literature) as defined by the PharmGKB curators. For instance, UGT1A8 is listed as a pharmacogene; variation in this gene is associated with increased risk of diarrhea as a side effect in the kidney transplant population; this association is seen only in a single study.15 However, low evidence clinical annotations can provide researchers with a starting point for the design of future studies.
For the clinician who wants to incorporate pharmacogenomics into their workflow, clinical dosing guidelines are also annotated on the PharmGKB website. These guidelines come from a number of professional organizations, including the Clinical Pharmacogenetics Implementation Consortium (CPIC), the Royal Dutch Association for the Advancement of Pharmacy - Pharmacogenetics Working Group (DPWG) and the Canadian Pharmacogenomics Network for Drug Safety.12
CPIC produces evidence-based, peer-reviewed prescribing guidelines based on pharmacogenetic test results.16 All guidelines are written using a standardized format and standardized terms.17 It is important to note that CPIC guidelines assume that a patient’s genetic test results are already available; they do not recommend whether or not to order genetic testing. Guidelines produced by CPIC are published in a peer-reviewed journal, have been endorsed by a number of professional societies and are freely available on the CPIC (www.cpicpgx.org) and PharmGKB websites.
Evidence for a CPIC guideline is collected through a systematic review process conducted by a PharmGKB curator. Expert reviewers drawn from CPIC members then assess and score the available evidence before making recommendations for the guideline. Finally, all guidelines are finally reviewed by the CPIC membership before submission to the journal. The process for CPIC guideline creation has been published.18 To date, CPIC has published over 35 different guidelines, covering a range of drugs. Guidelines are also regularly updated to include evidence from recent developments in pharmacogenomic research.
All annotated CPIC guidelines on the PharmGKB website are accompanied by a genotype picker, where users can input specific genotypes and receive the relevant dosing recommendation for that genotype.16 Short video summaries are also available for many CPIC guidelines.
Several gene-drug associations have CPIC guidelines that are applicable to dermatology and are listed in Table 1. In some cases, such as carbamazepine and the HLA-B gene, the adverse event is relevant to dermatological practice. In other cases, such as doxepin and CYP2D6 and CYP2C19, the gene-drug association is important for drug efficacy.
Table 1.
Selected Drugs with CPIC guidelines that are relevant to dermatology
| Drug | Gene(s) | Phenotype | PGx FDA label | Relevance to Dermatology |
PMID |
|---|---|---|---|---|---|
| Abacavir1,2 | HLA-B | Risk of drug hypersensitivity syndrome | Testing required | Adverse reaction presents with cutaneous and systemic symptoms | 22378157 24561393 |
| Allopurinol3,4 | HLA-B | Risk of severe cutaneous adverse reactions – drug hypersensitivity syndrome, Stevens-Johnson syndrome, toxic epidermal necrolysis | None | Adverse reaction presents with cutaneous and systemic symptoms | 23232549 26094938 |
| Azathioprine5,6 | NUDT15 TPMT | Risk of life-threatening myelosuppression | Testing recommended (for TPMT) | Azathioprine is used in immunobullous disorders, eczematous disorders, and photodermatoses.7,8 | 21270794 23422873 |
| Carbamazepine9,10 | HLA-B | Risk of severe cutaneous adverse reactions – drug hypersensitivity syndrome, Stevens-Johnson syndrome, toxic epidermal necrolysis | Testing required | Adverse reaction presents with cutaneous and systemic symptoms | 23695185 |
| Doxepin11,12 | CYP2D6 CYP2C19 | Risk of decreased efficacy or increased toxicity depending on variant effects | Information on actionable PGx, no testing recommendation | Doxepin’s histamine H1 blocking properties are used to treat pruritis.13 | 23486447 27997040 |
| Fluorouracil14,15 | DPYD | Risk of increased toxicity | Information on actionable PGx, no testing recommendation | This guideline is for the systemic form of this drug, but reports exist of this pharmacogene playing a role in topical treatment.16 | 23988873 29152729 |
| Oxcarbaxepine10 | HLA-B | Risk of severe cutaneous adverse reactions – Stevens-Johnson syndrome, toxic epidermal necrolysis | Testing recommended | Adverse reaction presents with cutaneous and systemic symptoms | 29392710 |
| Phenytoin17 | CYP2C9 HLA-B | CYP2C9 – Risk of decreased efficacy HLA-B - Risk of severe cutaneous adverse reactions – drug hypersensitivity syndrome, Stevens-Johnson syndrome, toxic epidermal necrolysis | Information on actionable PGx, no testing recommendation | Adverse reaction presents with cutaneous and systemic symptoms | 25099164 |
| Tacrolimus18 | CYP3A5 | Risk of decreased efficacy or increased toxicity depending on variant effects | None | Systemic tacrolimus has been used in inflammatory and autoimmune dermatological disease.19 | 25801146 |
| Voriconazole20 | CYP2C19 | Risk of decreased efficacy or increased toxicity depending on variant effects | Information on actionable PGx, no testing recommendation | Broad range of anti-fungal activity | 27981572 |
Abbreviations: CPIC, Clinical Pharmacogenetics Implementation Consortium; CYP, Cytochrome p450; FDA, Food and Drug Administration; PGx, pharmacogenomic(s); PMID, PubMed ID; TPMT, Thiopurine methyltransferase.
At the time of writing, over 500 drug labels with pharmacogenomic information from the US Food and Drug Administration, European Medicines Agency, Health Canada (Santé Canada) and Japan’s Pharmaceutical and Medical Device Agency have been curated by PharmGKB. Every annotated drug label is assigned a pharmacogenomics level by a PharmGKB curator, which indicates the kind of pharmacogenomic information contained in the label. This pharmacogenomics level can range from “Informative Pharmacogenomics”, where a label discusses the role of certain genes or proteins in the metabolism or pharmacodynamics of a drug, to “Testing Required”, where a label specifically states that some form of clinical testing must be undertaken before prescribing the drug. For the drug-gene combinations in Table 1, Food and Drug Administration labeling information has also been included.
The Future of Clinical Pharmacogenomics
While there has been a marked increase in pharmacogenomics research, the use of pharmacogenomics is in its clinical infancy. The aforementioned pharmacogenomics clinical guidelines and drug labels by global regulatory agencies serve as the framework upon which clinical implementation can be developed. Additional work is currently underway to facilitate this translation.
Many of the studies referenced by the pharmacogenomics clinical guidelines are not randomized controlled trials, the gold standard of evidenced-based medicine. In early 2017, the Ubiquitous Pharmacogenomics project was launched in Europe.19 This randomized controlled trial will evaluate the primary endpoint of adverse drug reactions in a patient group that has preemptive pharmacogenomics testing for 42 drugs based on DPWG guidelines versus standard of care.19 Secondary endpoints include health care expenditure and incidence of drug discontinuation due to adverse events or lack of efficacy.19 This trial, which aims to recruit 8,100 patients across 7 European countries (Greece, Slovenia, Spain, the United Kingdom, the Netherlands, Austria, and Italy), will provide additional information on the utility of preemptive pharmacogenetics in practice.19
Physicians have to juggle a massive amount of information in a limited amount of time during every clinical encounter; any implementation of clinical pharmacogenomics cannot interrupt the clinical workflow.2 Decision support tools integrated into the electronic health record (EHR) have been suggested as a possible way to implement clinical pharmacogenomics.2,8 Given the diversity of EHR systems, creating decision support tools will be one of the challenges for clinical pharmacogenomics. Physicians, pharmacists, and researchers have begun to address some of these challenges.
In the United States, the Translational Pharmacogenetics Program (TPP) of the National Institutes of Health Pharmacogenomics Research Network has documented clinical pharmacogenomics implementation (based on CPIC guidelines) across 8 health systems - Harvard University, Mayo Clinic, Ohio State University, St. Jude Children’s Research Hospital, University of Chicago, University of Florida, University of Maryland, and Vanderbilt University.20 Each health system created their own system of implementation of both reactive and preemptive pharmacogenomics testing using their native EHR. With reactive testing, the physician orders the pharmacogenomics test when they are about to prescribe a drug with a pharmacogenomics guideline. With preemptive testing, patients have genetic testing using a pharmacogenomics panel prior to any clinical decisions; the data are then available in the EHR. The implemented systems were used across multiple specialties and in both the inpatient and outpatient settings. This process revealed that a broadly translatable pharmacogenomics implementation system is difficult to create. Each health system designed a different framework for implementation; the heterogeneous solutions were due to a lack of standards for representing genomic results in the EHR and differences in workflows between sites.20 Promisingly, as of 2017, about 100,000 pharmacogenomic tests had been ordered in the TPP network of health systems and nearly 1 out of 4 tests had a potentially actionable result.20 In Europe, the Ubiquitous Pharmacogenomics project plans to implement a pharmacogenomics decision support tool in a standardized way across heterogeneous EHR systems that use multiple languages.19 To this end, the goal is to use a web-based content management system as a central knowledge base that can than interface with the various EHR systems.19 Such a central database would address some of the issues encountered in the TPP, where each individual health care system represented genomic and phenotypic results in a different manner due to a lack of standards.19,20 These real world system implementations highlight the informatics challenges but also provide a foundation for further improvement and standardization.
Conclusion
The ready availability and decreasing cost of genotyping and sequencing technology has led to an explosion in the number of studies examining the links between human genetic variability and drug phenotypes. Tools such as PharmGKB can be used to quickly query the vast number of gene-drug associations that have been established and provide foundational knowledge for clinical practice and the design of further research.
The hope is that, as genotyping and genomic sequencing become more readily available in the clinic, dermatologists will be able to use that genetic information to make informed decisions using clinical guidelines, such as those provided by CPIC or DPWG. However, clinical implementation will require surmounting informatics challenges, such as integrating pharmacogenomics data in the daily workflow. Several health systems are currently working on optimizing the implementation of pharmacogenomics data in the clinic through the use of decision support tools integrated with the EHR. The ultimate goal of pharmacogenomics is to provide an evidence-based manner to tailor drug therapy in order to have better efficacy and decrease adverse events.
Acknowledgments
Grant support: This work was supported by NIH/NIGMS R24 GM61374.
References
- 1.Meyer UA Pharmacogenetics - five decades of therapeutic lessons from genetic diversity. Nat Rev Genet 5, 669–76 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Karczewski KJ, Daneshjou R & Altman RB Chapter 7: Pharmacogenomics. PLoS Comput Biol 8, e1002817 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roden DM et al. Opportunities and Challenges in Cardiovascular Pharmacogenomics: From Discovery to Implementation. Circ Res 122, 1176–1190 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallal S et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet 359, 727–32 (2002). [DOI] [PubMed] [Google Scholar]
- 5.Mallal S et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med 358, 568–79 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Schackman BR et al. The cost-effectiveness of HLA-B*5701 genetic screening to guide initial antiretroviral therapy for HIV. AIDS 22, 2025–33 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ji Y, Si Y, McMillin GA & Lyon E Clinical pharmacogenomics testing in the era of next generation sequencing: challenges and opportunities for precision medicine. Expert Rev Mol Diagn 18, 411–421 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Lavertu A et al. Pharmacogenomics and big genomic data: from lab to clinic and back again. Hum Mol Genet 27, R72–R78 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daneshjou R et al. Genetic variant in folate homeostasis is associated with lower warfarin dose in African Americans. Blood 124, 2298–305 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott SA et al. Exome sequencing of extreme clopidogrel response phenotypes identifies B4GALT2 as a determinant of on-treatment platelet reactivity. Clin Pharmacol Ther 100, 287–94 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright GEB, Carleton B, Hayden MR & Ross CJD The global spectrum of protein-coding pharmacogenomic diversity. Pharmacogenomics J 18, 187–195 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whirl-Carrillo M et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther 92, 414–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu V & Mackool BT Mycophenolate in dermatology. J Dermatolog Treat 14, 203–11 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Lamba V et al. PharmGKB summary: mycophenolic acid pathway. Pharmacogenet Genomics 24, 73–9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woillard JB et al. Risk of diarrhoea in a long-term cohort of renal transplant patients given mycophenolate mofetil: the significant role of the UGT1A8 2 variant allele. Br J Clin Pharmacol 69, 675–83 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Relling MV & Klein TE CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther 89, 464–7 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caudle KE et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC). Genet Med 19, 215–223 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caudle KE et al. Incorporation of pharmacogenomics into routine clinical practice: the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline development process. Curr Drug Metab 15, 209–17 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blagec K et al. Implementing pharmacogenomics decision support across seven European countries: The Ubiquitous Pharmacogenomics (U-PGx) project. J Am Med Inform Assoc 25, 893–898 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luzum JA et al. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: Outcomes and Metrics of Pharmacogenetic Implementations Across Diverse Healthcare Systems. Clin Pharmacol Ther 102, 502–510 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin MA et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for HLA-B Genotype and Abacavir Dosing: 2014 update. Clin Pharmacol Ther 95, 499–500 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin MA et al. Clinical pharmacogenetics implementation consortium guidelines for HLA-B genotype and abacavir dosing. Clin Pharmacol Ther 91, 734–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hershfield MS et al. Clinical Pharmacogenetics Implementation Consortium guidelines for human leukocyte antigen-B genotype and allopurinol dosing. Clin Pharmacol Ther 93, 153–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito Y et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for human leukocyte antigen B (HLA-B) genotype and allopurinol dosing: 2015 update. Clin Pharmacol Ther 99, 36–7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Relling MV et al. Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther 89, 387–91 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Relling MV et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin Pharmacol Ther 93, 324–5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patel AA, Swerlick RA & McCall CO Azathioprine in dermatology: the past, the present, and the future. J Am Acad Dermatol 55, 369–89 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Schram ME et al. Off-label use of azathioprine in dermatology: a systematic review. Arch Dermatol 147, 474–88 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Leckband SG et al. Clinical Pharmacogenetics Implementation Consortium guidelines for HLA-B genotype and carbamazepine dosing. Clin Pharmacol Ther 94, 324–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hicks JK et al. Clinical Pharmacogenetics Implementation Consortium guideline for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants. Clin Pharmacol Ther 93, 402–8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hicks JK et al. Clinical pharmacogenetics implementation consortium guideline (CPIC) for CYP2D6 and CYP2C19 genotypes and dosing of tricyclic antidepressants: 2016 update. Clin Pharmacol Ther 102, 37–44 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta MA & Guptat AK The use of antidepressant drugs in dermatology. J Eur Acad Dermatol Venereol 15, 512–8 (2001). [DOI] [PubMed] [Google Scholar]
- 33.Caudle KE et al. Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clin Pharmacol Ther 94, 640–5 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amstutz U et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for Dihydropyrimidine Dehydrogenase Genotype and Fluoropyrimidine Dosing: 2017 Update. Clin Pharmacol Ther 103, 210–216 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson MR et al. Life-threatening toxicity in a dihydropyrimidine dehydrogenase-deficient patient after treatment with topical 5-fluorouracil. Clin Cancer Res 5, 2006–11 (1999). [PubMed] [Google Scholar]
- 36.Caudle KE et al. Clinical pharmacogenetics implementation consortium guidelines for CYP2C9 and HLA-B genotypes and phenytoin dosing. Clin Pharmacol Ther 96, 542–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birdwell KA et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin Pharmacol Ther 98, 19–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madan V & Griffiths CE Systemic ciclosporin and tacrolimus in dermatology. Dermatol Ther 20, 239–50 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Moriyama B et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP2C19 and Voriconazole Therapy. Clin Pharmacol Ther 102, 45–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]


