Abstract
Neonatal herpes simplex virus (nHSV) infections cause devastating morbidity and mortality in infants.Most nHSV cases are associated with primary maternal infection, consistent with the hypothesis that maternal immunity is protective. In humans, we found HSV-specific neutralizing antibodies in newborns of immune mothers, indicating that placentally transferred HSV-specific antibody is protective. Using a murine model, we showed that passive administration of HSV-specific antibody to dams prevented disseminated infection and mortality in pups. Maternal immunization with an HSV-2 replication-defective vaccine candidate, dl5–29, led to transfer of HSV-specific antibodies into neonatal circulation that protected against nHSV neurological disease and death. Furthermore, we observed considerable anxiety-like behavior in adult mice that had been infected with low doses of HSV as neonates, despite a notable lack of signs of infection. This phenotype suggests that nHSV infection can have an unsuspected and permanent impact on behavior. These behavioral sequelae of nHSV were prevented by maternal immunization with dl5–29, demonstrating an unexpected benefit of immunization. These findings also support the general concept that maternal immunization can prevent neurotropic neonatal infections and associated morbidity and mortality.
INTRODUCTION
Neonatal infections are a severe and traumatic manifestation of herpes simplex virus (HSV). Infection in the newborn is due to exposure to HSV-1 or HSV-2 during either parturition or the early postnatal period (1, 2). HSV infection in adults is often asymptomatic, but neonates are particularly susceptible, with about 50% of infected newborns developing disseminated disease or encephalitis (3). Without treatment, mortality is high and surviving infants with central nervous system (CNS) involvement suffer long-term neurodevelopmental disabilities, incurring substantial economic burden (3). Antiviral drugs such as acyclovir and its derivatives are the current standard treatment, but initiation of such therapy requires a high degree of clinical suspicion. Even aggressive acyclovir treatment of neonates with CNS infections leaves an estimated 70% of individuals with neurological sequelae (4–6). Globally, HSV-2 causes more than 70% of neonatal HSV (nHSV), but HSV-1 is the major cause in the Americas, Europe, and the Western Pacific (7). The global incidence of nHSV is estimated to be 1.03 per 10,000 live births (7, 8). However, estimates of nHSV have been reported to be as high as 1 in 3200 live births in the United States, suggesting regional variation (7). It is imperative, therefore, that additional therapies be considered for prevention of HSV infection in this vulnerable population.
HSV-1 and HSV-2 are neurotropic pathogens that infect epithelial tissues and nerve termini, before retrograde spread within the peripheral nervous system, wherein viral latency is established (9). Although primary infection or reactivation of virus can result in visible lesions, they are frequently asymptomatic, which renders diagnoses and preventative measures challenging, especially during the perinatal period (10,11). Most nHSV infections are acquired via mucosal and cutaneous contact during birth. Hence, the virus can be found in lesions on the skin, eye, and mouth, disseminated in visceral organs, and in the CNS (12). CNS disease often presents with non-specific sepsis-like symptoms, leading to delayed antiviral treatment and high risk of mortality and morbidity (13–15).
The risk of vertical transmission of HSV is substantially higher during maternal primary infections (>55%) than during reactivation (<1%) (10, 11). This discrepancy in risk is consistent with the hypothesis that protection is conferred through transfer of maternal antibodies (16, 17), which are absent during primary infection. Immunoglobulin G (IgG) antibodies cross the placenta and supplement the developing fetal and neonatal immune system to protect against a variety of congenital infections (18–22). Whereas vertical transfer of antibody between maternal and fetal circulation is well understood, our recent work demonstrated that maternal antibodies also access neural tissues of the fetus with unexpected efficiency (23). In mice, this maternal IgG is sufficient to prevent neonatal HSV-1 neurological infection (23). Because maternal antibodies appear crucial to the outcome of nHSV, we investigated whether passive antibody treatment and maternal immunization could protect against disseminated infection and associated morbidity in the neonate. Maternal immunization has not been widely considered for nHSV (24), although HSV vaccine candidates, including glycoprotein subunits, attenuated viruses, DNA vaccines, and replication-defective mutant viruses, have been broadly tested in a variety of animal systems (25). These include mouse and guinea pig genital infection, mouse eye models, and nonhuman primates (26–29). To date, none of these candidates have proved successful in clinical trials for prevention of horizontal adult-to-adult transmission (30, 31). The candidate used in this study, HSV-2 dl5–29, is a replication-defective virus (32, 33) and protects mice and guinea pigs against ocular and genital infections respectively (34–36). This promising vaccine candidate was chosen for our study because it is currently in a phase 1 clinical trial (HSV529, NCT02571166).
The purpose of this study was to investigate the ability of maternal immunity to protect neonates against the short-and long-term consequences of HSV infection. This research strongly supports maternal immunization as a strategy to protect neonates against morbidity and mortality from HSV and potentially other neurotropic pathogens.
RESULTS
In humans, maternal HSV-specific antibodies are transferred to offspring and are potently neutralizing
Human IgG is efficiently transferred to fetal circulation via the placenta and protects the fetus and neonate from pathogens (37). This, however, has not been shown directly for HSV. If maternal immunity is involved in the prevention of nHSV, then HSV-specific neutralizing antibodies should be transferred to the fetus. To address this, we assessed HSV-1–and HSV-2–specific IgG from maternal and paired cord, neonatal (1 month), and infant (18 months) sera. Maternal HSV-specific antibodies were abundant in cord samples (Fig. 1A), with HSV-1 gD and gC-specific antibodies found at higher quantities in cord relative to maternal serum. Maternal antibodies were also present in neonates (Fig. 1B) but were absent by 18 months (fig. S1). To examine the biological activity of these transferred antibodies, we ranked and binned cord serum into tertiles based on HSV-specific antibody quantities and assessed neutralizing activities. High responders had antibody quantities that ranked in the top third, and low responder groups had antibody quantities that ranked in the bottom third, among all cord samples. Cord serum that contained more HSV-specific antibodies had higher neutralizing titers (Fig. 1C). These data showed that human cord serum contains HSV-specific antibody of maternal origin and that these antibodies effectively neutralize HSV-1.
Fig. 1. Human maternal HSV-specific antibody is transferred to offspring and is neutralizing.
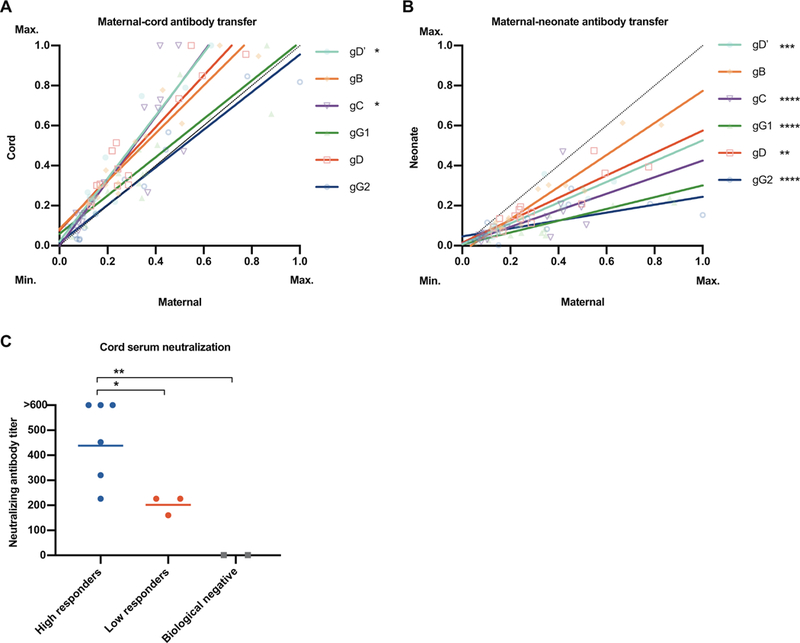
Human maternal and paired cord and neonate sera were probed for HSV-1– and HSV-2 (gG2)–specific antibodies via multiplex. Antibodies found in (A) maternal-cord pairs and (B) maternal-neonate pairs are plotted by glycoprotein binding (maximum binding, 1; minimum binding, 0). Regression lines for each glycoprotein are shown in corresponding colors. The dotted line of identity refers to a 1:1 transfer ratio between maternal and cord or neonate samples. Above the dotted line represents a higher corresponding cord or neonatal value, whereas below represents a higher corresponding maternal value. gD’refers to the ectodomain of HSV-1 gD. (C) Cord samples were binned into tertiles based on mean antibody binding in (A); high responder (above second tertile) and low responder (below first tertile) groups were assayed for neutralizing antibodies against HSV-1 via a serum neutralization assay. Serum from HSV-1 seronegative adults was used as a biological negative control. Statistical significance was determined using one-way analysis of variance (ANOVA) with Dunnett’s multiple comparisons. In (A) and (B), regression lines were compared to the line of identity. In (C), bars represent the mean. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Antibodies in sera and trigeminal ganglia from latently infected animals neutralize HSV-1
We demonstrated previously that passive transfer of antibodies from HSV-1 latently infected mice to naïve adult mice can efficiently access the trigeminal ganglia (TG), the site of latency for HSV-1 (23). Having shown here that HSV-specific antibodies are transferred between infected human mothers and their neonates, we wished to characterize the antibodies found in latently infected mice ≥21 days after infection. Therefore, we analyzed matched sera and TG samples for the presence and quantities of HSV-specific antibodies (Fig. 2, A and B). Sera from latently infected C57BL6 (B6) mice had potent reactivity to HSV-1 glycoproteins gD, gC, and gB and immediate-early protein ICP4 relative to naïve sera. For the latently infected TG extracts, binding activity was significantly higher for gD, gB, and ICP4 relative to naïve controls (P = 0.000078, 0.000028, and 0.005). To test viral neutralization, we assayed pooled sera and TG tissue extracts from latently infected animals in a plaque reduction assay. As expected, sera from latently infected B6 mice had increased HSV neutralizing activity (or plaque reduction) relative to sera from latently infected muMT (mature B cell deficient) and naïve B6 mice (Fig. 2C). Extracts from latently infected B6 TGs also had significantly higher neutralizing activity relative to TG extracts from muMT or naïve B6 mice (P = 0.0068 and 0.0043) (Fig. 2D). Latently infected mice, therefore, have neutralizing antibodies to HSV proteins in serum and TG tissues.
Fig. 2. Antibodies in sera and TGs from latently infected mice neutralize HSV-1.
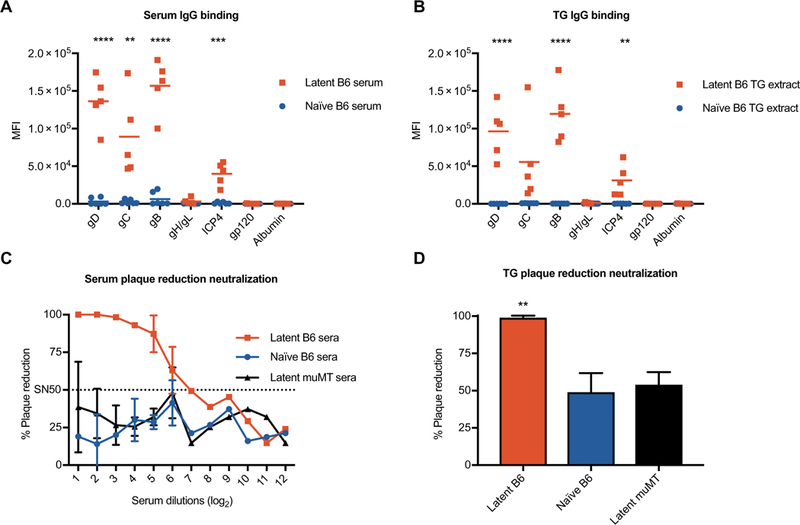
Antibody responses to HSV-1 and control antigens were defined via multiplexed antibody assay for matched (A) sera and (B) TG extract samples. Plaque reduction neutralization assays of (C) sera and (D) diluted TG extracts from latently infected B6 (red), naïve B6 (blue), and latently infected muMT (black) mice. SN50 (dotted line) refers to the serum dilution that neutralized 50% of the virus. Statistical significance for (A) and (B) was determined using an unpaired t test between sera/extracts from latently infected animals and those of naïve animals for each antigen. Statistical significance for (D) was determined by one-way ANOVA with Tukey’s multiple comparisons. MFI, median fluorescence intensity. Error bars indicate SD (A and B). **P < 0.01, ***P < 0.001, and ****P < 0.0001. Serum and TG extracts were pooled from 10 to 18 mice in each group, and data are representative of two independent experiments.
Passive IgG treatment protects neonates from lethal HSV-1 challenge
We showed previously that the offspring of latently infected mice are protected against nHSV (23). We therefore hypothesized that transfer of sera from latently infected dams (immune sera) to pregnant dams could prevent neonatal disease in their offspring after challenge with HSV. To test this, we treated naïve pregnant muMT dams with pooled immune or naïve sera before parturition. muMT mice were used to ensure that any antibody transferred to offspring was from serum treatments. We then challenged pups intranasally with HSV-1 (strain 17) after birth and assessed viral titers in various organs. Pups from dams that received immune sera had significantly reduced (P < 0.0007) viral titers in the brain, TG, and visceral organs relative to pups from naïve sera–treated dams at 3 days after infection (Fig. 3A). Although disseminated disease was reduced in these animals, we found that intranasal infection predisposes the pups to high lung viral titers. However, in survival studies, pups from immune sera–treated females were largely protected, whereas pups from naïve sera–treated mice succumbed to infection by 6 days after infection (Fig. 3B). These results demonstrated that transfer of immune sera can protect offspring in the neonatal mouse model. Essentially identical data were obtained after administration of purified immune IgG (Fig. 3, C and D). Thus, IgG in immune sera was sufficient for protection against viral challenge.
Fig. 3. Maternal passive antibody treatment protects against neonatal herpes.
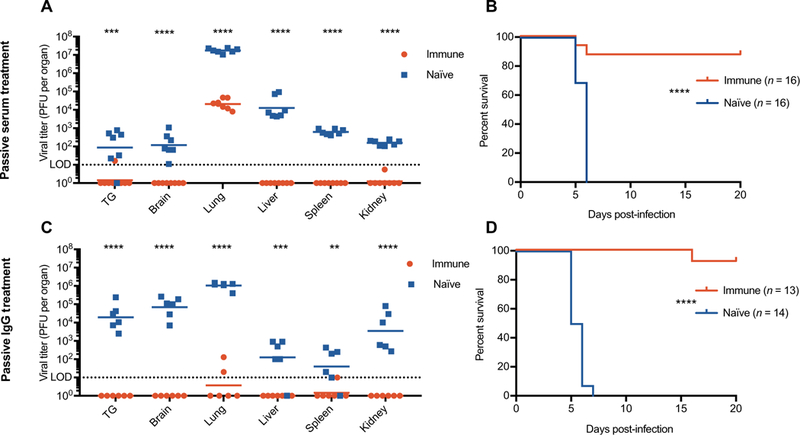
Pooled sera or purified IgG from latently infected (immune) or naïve mice was administered to muMT dams before parturition, and pups were infected intranasally with 105 PFU (plaque-forming units) (A), 104 PFU (B and C), or 103 PFU (D) of HSV-1. Viral titers were determined in pups 3 days after infection. Data are shown as viral titers in individual pups from dams treated with immune sera/IgG (red) or naïve sera/IgG (blue). Statistical significance was determined by multiple t tests (A and C) and log-rank test (B and D). **P < 0.01, ***P < 0.001, and ****P < 0.0001. Data are representative of at least three independent experiments.
Immunization-derived, HSV-specific antibodies are efficiently transferred to the neonate
Having shown that passive vertical transfer of HSV-specific IgG is protective to neonates, we wished to address whether maternal immunization could protect neonates. HSV-2 dl5–29 is a replication-defective virus that lacks the genes for the UL5 helicase-primase subunit and the UL29 (ICP8) DNA binding protein. It has demonstrated promising efficacy in animal models of genital herpes (26, 35) with strong T helper 1 (TH1) cytokine and T cell responses (34) and has been in a phase 1 clinical trial (HSV529, NCT02571166). Maternal subcutaneous immunization with dl5–29 reduced visceral spread of HSV-2 in mouse pups but did not prevent replication at the site of entry, spread to the CNS, or lethal encephalitis (38). Recent work revealed that intramuscular immunization with dl5–29 is more effective than the subcutaneous route (35); thus, our studies tested whether maternal immunization by the intramuscular route could prevent mortality in offspring. To this end, we immunized B6 female mice with mock-infected cell lysate or dl5–29 (Fig. 4A), which should generate sera capable of neutralizing both HSV-2 and HSV-1 (26, 35). To determine whether IgG antibodies resulting from immunization could access the neonatal circulation, we assessed the presence and quantities of HSV-specific antibody in serum of paired dams and pups (Fig. 4, B and C) and observed that immunized dams produce antibodies specific for gD, gC, gB, and ICP4. Likewise, serum from the offspring of the dl5–29–immunized group had significantly increased antibody binding to gD, gC, gB, and ICP4 relative to offspring of mock-immunized dams (P = 0.0005, 0.0102, 0.00001, and 0.00002). We then tested whether IgG antibodies could access the neonatal nervous system. Using immunofluorescence microscopy, we compared the TGs of uninfected pups from dl5–29-and mock-immunized dams (Fig. 4D). The data showed elevated staining for mouse IgG in the TGs from the immunized group (fig. S2). Together, these results indicate that dams mount an antibody response after immunization that can be transferred to the circulation and nervous systems of their offspring.
Fig. 4. Immunization with HSV-2 dl5–29 allows maternal transfer of HSV-specific antibodies.
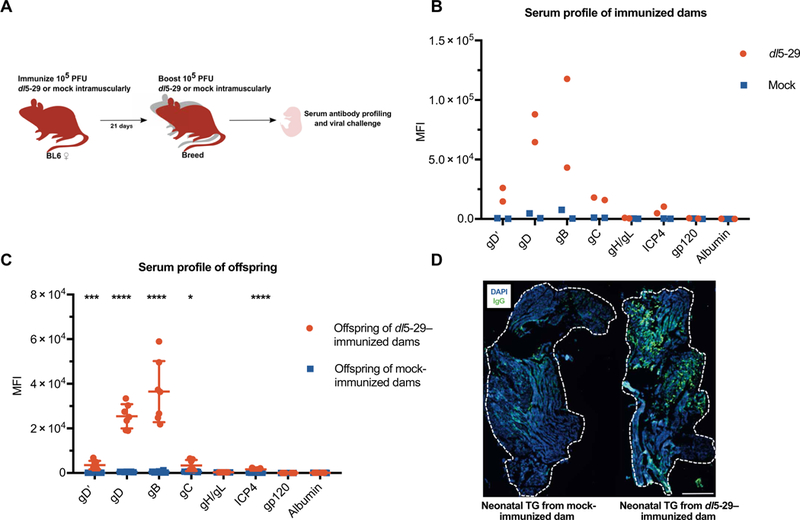
(A) B6 females were vaccinated with HSV-2 dl5–29 virus or mock lysate and boosted and bred 21 days later. i.m., intramuscularly. (B) Sera from immunized dams and (C) their naïve offspring were assessed for HSV-1 specificity. gD’ refers to the ectodomain of HSV-1 gD. (D) Immunofluorescence of TG tissue from naïve neonates of mock-immunized dams (left) and that of dl5–29–immunized dams (right), stained with anti-mouse IgG (green) and 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bar, 500 μm. Statistical significance in (C) was determined by unpaired t test for each antigen. Data are representative of two to three independent experiments *P < 0.05, ***P < 0.001, and **** P <0.0001.
Maternal immunization with dl5–29 protects against neonatal herpes
Having shown that vaccine-derived maternal antibody can be transferred to progeny, we examined whether immunization was sufficient to protect against nHSV in mice. After the experimental immunization plan in Fig. 4A, we challenged pups intranasally with lethal doses of HSV-1. Pups of dl5–29–immunized dams showed significantly decreased viral burden in the CNS (P < 0.000001) and peripheral nervous system (P = 0.0121) and in various visceral organs (P < 0.003) (Fig. 5A). These pups survived the HSV challenge, whereas pups of mock-immunized dams succumbed to infection (Fig. 5B). Similarly, we challenged offspring of immunized dams to a heterologous and low-passage clinical HSV-2 isolate (strain G) and found comparable protection (Fig. 5C). Protection extended to at least four subsequent pregnancies (≥245 days) from these dams, demonstrating that the antibody response was long-lasting and provides protective immunity against nHSV (fig. S3). Humans and mice both transfer immunoglobulins vertically via placental transfer to the fetus, but mice additionally pass mammary antibody secretions to suckling offspring before gut closure. To address this difference, and to separate the relative contributions of placenta-and milk-derived antibodies to protection, we used fostering techniques. Offspring from mock-or dl5–29–immunized dams were removed immediately at birth from their birth dam and fostered by equivalent or reciprocal (mock-or dl5–29–immunized) dams before being challenged with HSV-1 (Fig. 5D). Equivalent survival was observed between litters that received immunization-derived antibodies via the placenta only or milk only. Thus, maternal vaccination with dl5–29 was protective against both HSV-1 and a heterologous HSV-2 strain, and antibody transferred via the placenta was sufficient to completely protect offspring.
Fig. 5. Maternal immunization with dl5–29 protects against neonatal herpes.
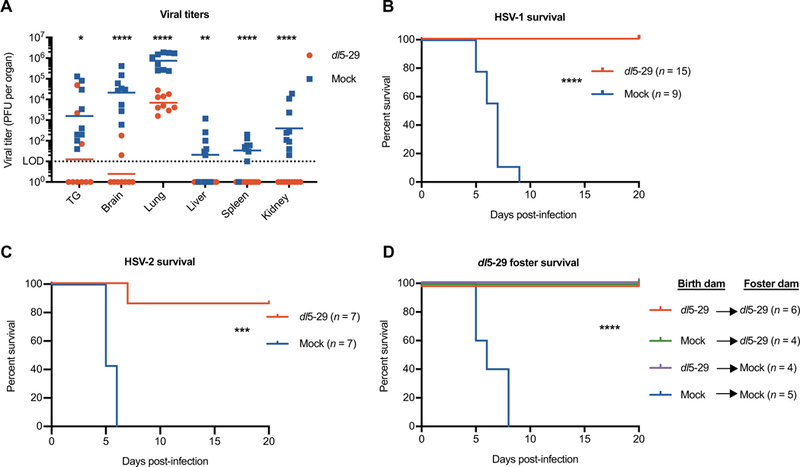
B6 females were immunized and boosted as shown in Fig. 4A. Neonates PI-2 from dams immunized with dl5–29 (red) or from mock-immunized dams (blue) were challenged with HSV. (A) Viral titers in perfused organs from neonates infected with 104 PFU of HSV-1. Survival of neonates challenged with 103 PFU of HSV-1 (B) or HSV-2 (C). (D) Offspring from mock- or dl5–29–immunized dams were removed immediately at birth from their birth dam, fostered by equivalent or reciprocal (mock- or dl5–29-immunized) dams, and challenged P2 with 103 PFU of HSV-1 to assess milk (green) and placental (purple) protective contributions. Statistical significance was determined by multiple t test (A) or log-rank test (B to D). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. Data are representative of at least two independent experiments.
Behavioral morbidity is prevented by vaccination
Even with antiviral treatment, infants that survive nHSV infection of the CNS are left with lifelong debilitating neurological impairments (12,15, 39). To establish a model system for monitoring these neurological sequelae, we used the open field test (OFT), a behavioral assay that measures general ambulatory ability, novel environment exploration, and anxiety in rodents (40, 41). When mice are introduced into a novel environment, they demonstrate exploratory behavior. Thigmotaxis, a tendency to remain close to the periphery of an enclosure, is indicative of anxiety-like behavior in both mice and humans (40, 42). Offspring (P1–2) of latently infected and mock-infected dams were challenged with a low dose (100 PFU) of HSV-1 and monitored until 5 weeks of age. A low dose was used to ensure survival of both cohorts, and at this dose, no differences in mortality, weight, water consumption, or motor function were observed (fig. S4). However, the OFT revealed that offspring of mock-infected dams had an affinity for the periphery and corners of the test arena, thus demonstrating elevated thigmotaxis (Fig. 6A). Offspring of latently infected dams, conversely, spent similar amounts of time exploring the outer and central areas (Fig. 6, B and C). We next assessed whether maternal immunization could similarly prevent this anxiety-like behavior in offspring challenged with HSV. As expected, we observed that offspring of mock-immunized females exhibited increased thigmotaxis (Fig. 6, D to F) regardless of distance traveled in the OFT (fig. S5). Offspring of dl5–29–immunized dams displayed normal thigmotaxis and their behavior was comparable to naïve, age-matched controls (Fig. 6, D to G). These findings suggested that even low dose and sublethal neonatal infection with HSV-1 can result in behavioral morbidity, and this morbidity is preventable by maternal immunity or immunization.
Fig. 6. Neonatal herpes causes anxiogenic behavior, which can be prevented by maternal immunization.
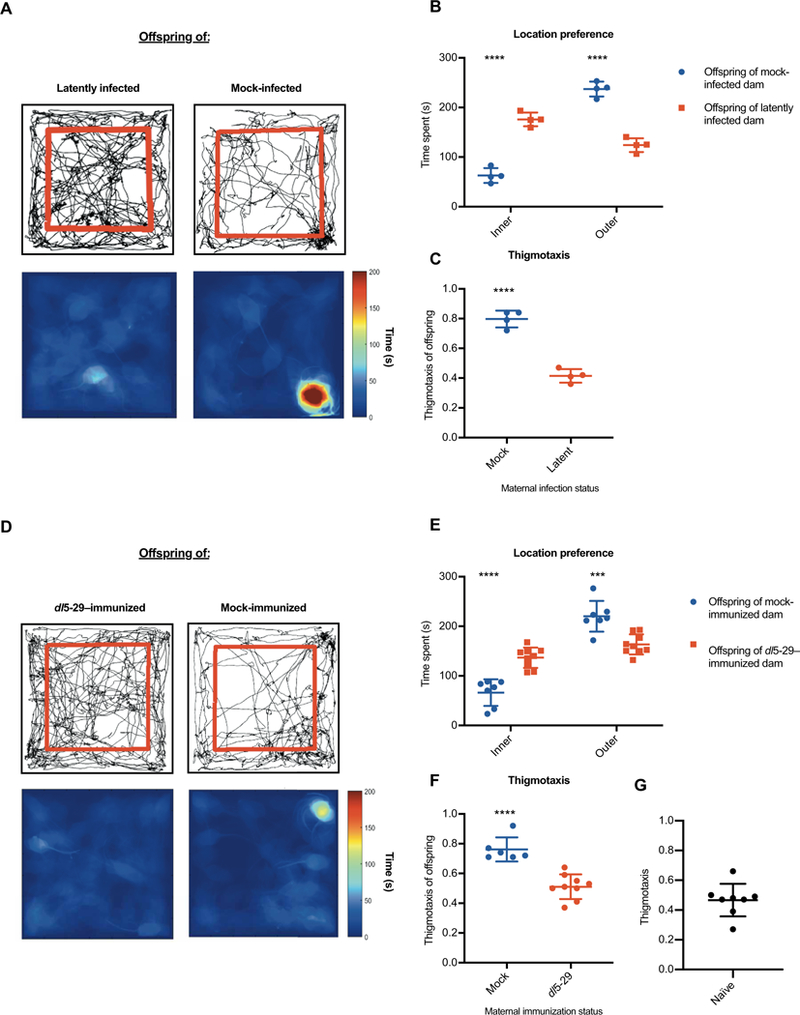
Mice challenged with 100 PFU of HSV-1 as neonates (P1–2) were analyzed in the OFT 5 weeks after infection. (A) Open field behavior in offspring of latently infected and mock-infected dams is shown by movement tracking (top) and heat map (bottom). Location preferences (B) can be quantified as time spent in the inner region (within red lines) and outer periphery or (C) as thigmotaxis, a ratio of time spent in the outer perimeter over total time. Similarly, offspring of dl5–29– and mock-immunized dams (D) were assessed for location preference (E) and thigmotaxis (F). As a control, thigmotaxis of age-matched, untreated, naïve mice was quantified (G). Data are represented as individual animals, and statistical significance was determined by unpaired t test. Error bars represent SD. ***P < 0.001 and ****P < 0.0001. Data are representative of two to three independent experiments.
DISCUSSION
Neonates are particularly vulnerable to infection, and at this stage of life, pathogen-specific immunity relies predominantly on maternal- derived immunoglobulins (37, 43). Maternal antibody is passed to offspring via the placenta and ingestion of breast milk (44). For HSV, clinical evidence has suggested that the presence of maternal HSV-specific antibody is crucial for protection against nHSV because mothers with a previous history of HSV rarely transmit the virus to their babies (17, 45–47). Previous research in animal models has also supported the important role of antibody in the prevention of nHSV (48–56). We recently demonstrated the presence of HSV-specific IgG in human fetal neural tissue, suggesting that the protection afforded by IgG may also occur in the nervous system (23). In this study, we show that maternal HSV-specific neutralizing antibodies are transferred via the placenta in humans. Seropositive humans and mice raise a similar profile of HSV glycoprotein-specific antibodies. Administration of these antibodies to pregnant dams can protect against nHSV. Currently, passive IgG therapy [intravenous immunoglobulin (IVIG)] is used to prophylactically protect at-risk neonates from varicella zoster and hepatitis B viruses but requires additional IVIG treatments for subsequent births. This limitation can potentially be resolved via immunization, which can simultaneously protect women, fetuses, and neonates even through multiple pregnancies (43). Our approach shows that maternal immunization with a vaccine candidate, HSV-2 dl5–29, results in a robust antibody response that is transferred to and protects neonates through up to five consecutive pregnancies. This antibody response was cross-reactive to HSV-1 epitopes, providing crucial evidence that an HSV-2 vaccine would likely be sufficient to protect against neonatal HSV-1 and HSV-2 infections. This study has demonstrated that in mice, HSV-specific IgG transferred through either the milk or placenta alone can protect against nHSV. This is a key observation as it supports the notion that human transplacental antibody could also be sufficient for protection from perinatal infections (57).
nHSV is life-threatening, and subsequent complications can lead to long-term neurological sequelae even with aggressive antiviral treatment (58). In this study, we found that neonatal exposure to sublethal doses of HSV can cause behavioral changes in mice that may model the neurological morbidities observed in humans (42). Although only a single behavioral assessment was used here, this observation is important because it provides a system to test the abilities of prophylactic and therapeutic interventions to affect behavioral changes driven by nHSV infection. These behavioral changes could be caused by neuronal loss from viral replication or indirectly by immunopathological changes induced by infection of the CNS. Increasing evidence suggests a role for the immune response in behavior (59, 60), and it will be important to investigate the neurobiological basis of the observed protection with multiple behavioral tests. In particular, infection with pathogens that can cause neurological complications, including Toxoplasma gondii, group B streptococcus, malaria, Zika virus, and dengue virus, induces cognitive behavioral changes in rodent models (61–66). Infection triggers proinflammatory cytokines and microglial activation in the brain and can affect mood, cognition, and behavior (59, 65). Accumulating evidence suggests that prenatal and postnatal immunostimulation influences neurogenesis, cognitive function, and behavior in offspring (67–74). This study raises the potential specter of permanent behavioral changes caused by nHSV in babies born to mothers without preexisting immunity. This may represent another example of how the timely acquisition of immunity to a pathogen can lead to considerable benefits for the offspring. The maternal virome is therefore likely an important determinant of human health.
Prevention of clinical disease is typically the endpoint for the U.S. Food and Drug Administration’s licensure of vaccines. There has been considerable discussion of reduction of viral shedding, seroconversion, or other laboratory results as measures of efficacy of HSV vaccine candidates (25, 35, 75). Our results raise the possibility of measuring other correlates of disease protection provided by HSV vaccines. There is also potential for vaccine efficacy against other medical outcomes, such as behavioral pathologies and age-dependent cognitive decline (76).
We suspect that maternal antibody can access fetal neural tissue through the developing blood-brain and blood-nerve barriers and prevent infection by vertically transmitted pathogens. The TORCH pathogens (Toxoplasma, other, rubella, cytomegalovirus, and HSV) and Zika virus can cause severe neurological sequelae in fetuses and neonates (77–79). Administration of vaccines and passive antibody therapy to women of reproductive age should be explored as approaches to mitigate disease in this at-risk population (20, 79–82). Moreover, highly potent monoclonal antibodies could be engineered to increase their affinity and breadth of pathogen recognition and to enhance their placental transfer. Our work provides strong evidence that the presence of neutralizing antibodies is sufficient to protect against nHSV. Clinical trials of HSV vaccines have exclusively assessed horizontal transmission and reactivation in adults (8, 31). Although vaccination has not been assessed for prevention of vertical transmission, such an approach could prove useful in reducing perinatal and postnatal acquisition of HSV (24). The devastating consequences of nHSV should compel and energize us to further develop such vaccine and therapeutic antibody approaches.
MATERIALS AND METHODS
Study design
The rationale for this study was based on previous findings (23). Overall, we wanted to determine whether maternal vaccination could provide sufficient antibody to offspring and whether this could ultimately protect against neonatal herpes. Sample sizes and time points were determined on the basis of previous studies with the neonatal murine model. Mouse studies were conducted using litters from six to eight different dams per group. One to three litters are represented in each graph, with data points representing individual animals. Endpoints for survival studies were defined as excessive morbidity (hunched, spasms, or paralysis) or more than 10% weight loss. For behavioral studies, B6 mice were transferred to a dedicated behavioral room 1 week before testing and were not handled during that time. Environmental conditions (light cycles, sound, and study time of day) were consistent throughout, and researchers were not present in the room during recorded sessions. All videos were coded and scored with the same parameters using published software (83) that had been manually validated. Experiments were performed in accordance with animal protocols approved by the Center for Comparative Medicine and Research at the Geisel School of Medicine at Dartmouth in compliance with the Institutional Animal Care and Use Committee. Primary data are reported in data file S1.
Subjects
Maternal and child blood were obtained from a birth cohort study of maternal determinants of infant immunity at the Hôpital Saint-Pierre, Brussels, following written informed consent (84). The study was approved by the hospital’s Ethics Committee. Samples analyzed in the present study included cord blood and maternal blood collected at birth from 24 mother-newborn pairs. Additional blood samples were collected at 1 month of age from 14 children and at 18 months of age from another 10 children. Mothers did not present chronic disorders, including autoimmune or inflammatory diseases, and did not receive immunosuppressive therapy before pregnancy. Mean maternal age was 29 years. Mothers were from sub-Saharan Africa (71%), Europe (25%), and South America (4%). Mean gestational age was 39.5 weeks, and 17% were delivered by cesarean section. The biological negative samples for the cord serum neutralization assay were collected from known seronegative adults.
Statistical analysis
All data were analyzed using GraphPad Prism software. For human data (Fig. 1, A and B), a linear regression analysis was completed for binding to bead type and a one-way ANOVA with Dunnett’s multiple comparisons was used to compare the line of identity to the slope of each regression line. For neutralization assays, groups were compared using one-way ANOVA with Tukey’s test for multiple corrections. All viral titer data were log-transformed with undetected samples set as 1 PFU. Geometric mean is shown for all viral titer data. Two-tailed Student’s t test (α = 0.05) was used for comparing behavioral data, viral titers, and samples probed by the same bead type. For Kaplan-Meier plots, log-rank P values are presented.
Supplementary Material
Acknowledgments:
We thank N. Deluca, G. Cohen, R. Eisenberg, F. Gilli, A. Pachner, D. Royce, D. Bucci, J. Weiner, S. Fiering, A. Charron, R. Manivanh, B. North, J. Merbach, P. Canova, A. Wolfert, S. Cerón, and S. Katzenell for materials and/or helpful discussion. We also thank the mothers and infants for their participation in the studies and T. Goetghebuer and V. Olislagers for the clinical management of mothers and infants and for the management of the clinical samples.
Funding: This study was supported by the Munck-Pfefferkorn Education and Research Fund to D.A.L.; P01 AI098681 to D.A.L., D.M.C., and D.M.K.; R01 AI131975 to M.E.A.; R01 AI057552 to D.M.K.; R01 EY09083 to D.A.L.; Fondation Roi Baudouin to A.M.; and T32AI007519 to C.D.P.
Footnotes
Competing interests: D.M.K. is a coinventor on a patent (7,223,411) on the use of dl5–29 as a herpes vaccine, and the technology has been licensed to Sanofi Pasteur by Harvard University. D.M.K. is a member of the AAV Scientific Advisory Board of Applied Genetic Technologies Corporation. A.M. has received consultancy fees from GSK Vaccines. The other authors declare no competing interests.
Data and materials availability: All data associated with this study are present in the paper or the Supplementary Materials.
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/11/487/eaau6039/DCl
REFERENCES AND NOTES
- 1.James SH, Sheffield JS, Kimberlin DW, Mother-to-child transmission of herpes simplex virus. J. Pediatric Infect. Dis. Soc. 3, S19–S23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leas BF, Umscheid CA, Neonatal herpes simplex virus type 1 infection and Jewish ritual circumcision with oral suction: A systematic review. J. Pediatric Infect. Dis. Soc. 4, 126–131 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corey L, Wald A, Maternal and neonatal herpes simplex virus infections. N. Engl. J. Med. 361, 1376–1385 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGrath N, Anderson N, Croxson M, Powell K, Herpes simplex encephalitis treated with acyclovir: Diagnosis and long term outcome. J. Neuro/. Neurosurg. Psychiatry 63, 321–326 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James SH, Kimberlin DW, Whitley RJ, Antiviral therapy for herpesvirus central nervous system infections: Neonatal herpes simplex virus infection, herpes simplex encephalitis, and congenital cytomegalovirus infection. Antivir. Res. 83, 207–213 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris JB, Holmes AP, Neonatal herpes simplex viral infections and acyclovir: An update. J. Pediatr. Pharmacol. Ther. 22, 88–93 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Looker KJ, Magaret AS, May MT, Turner KME, Vickerman P, Newman LM, Gottlieb SL, First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob. Health 5, e300–e309 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottlieb SL, Giersing BK, Hickling J, Jones R, Deal C, Kaslow DC; HSV Vaccine Expert Consultation Group, Meeting report: Initial World Health Organization consultation on herpes simplex virus (HSV) vaccine preferred product characteristics, March 2017. Vaccine, 10.1016/j.vaccine.2017.10.084 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Fields BN, Knipe DM, Howley PM, Everiss KD, Kung HJ, FundamentalVirology (Lippincott-Raven, 1996). [Google Scholar]
- 10.Whitley RJ, Nahmias AJ, Visintine AM, Fleming CL, Alford CA, The natural history of herpes simplex virus infection of mother and newborn. Pediatrics 66, 489–494 (1980). [PubMed] [Google Scholar]
- 11.Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L, Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA 289, 203–209 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Kimberlin DW, Neonatal herpes simplex infection. Clin. Microbiol. Rev. 17, 1–13 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson B, Kimberlin DW, Forgie SE, Delayed recurrence of herpes simplex virus infection in the central nervous system after neonatal infection and completion of six months of suppressive therapy. J. Pediatric Infect. Dis. Soc. 6, e177–e179 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Shah SS, Aronson PL, Mohamad Z, Lorch SA, Delayed acyclovir therapy and death among neonates with herpes simplex virus infection. Pediatrics 128, 1153–1160 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malm G, Forsgren M, el Azazi M, Persson A, A follow-up study of children with neonatal herpes simplex virus infections with particular regard to late nervous disturbances. Acta Paediatr. 80, 226–234 (1991). [DOI] [PubMed] [Google Scholar]
- 16.Yeager AS, Arvin AM, Urbani LJ, Kemp JA, Relationship of antibody to outcome in neonatal herpes simplex virus infections. Infect. Immun. 29, 532–538 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitley RJ, Neonatal herpes simplex virus infections: Is there a role for immunoglobulin in disease prevention and therapy? Pediatr. Infect. Dis. J. 13, 432–439 (1994). [PubMed] [Google Scholar]
- 18.Caboré RN, Maertens K, Dobly A, Leuridan E, Van Damme P, Huygen K, Influence of maternal vaccination against diphtheria, tetanus, and pertussis on the avidity of infant antibody responses to a pertussis containing vaccine in Belgium. Virulence 8, 1245–1254 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y, Mahmood I, Zhong L, Zhang P, Struble EB, Passive immunoprophylaxis for the protection of the mother and her baby: Insights from in vivo models of antibody transport. J. Immunol. Res. 2017, 7373196 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson CS, Cruz DV, Tran D, Bialas KM, Stamper L, Wu H, Gilbert M, Blair R, Alvarez X, Itell H, Chen M, Deshpande A, Chiuppesi F, Wussow F, Diamond DJ, Vandergrift N, Walter MR, Barry PA, Cohen-Wolkowiez M, Koelle K, Kaur A, Permar SR, Preexisting antibodies can protect against congenital cytomegalovirus infection in monkeys. JCI Insight 2, e94002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niewiesk S, Maternal antibodies: Clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front. Immunol. 5, 446 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zinkernagel RM, Maternal antibodies, childhood infections, and autoimmune diseases. N. Engl. J. Med. 345, 1331–1335 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, Patel CD, Manivanh R, North B, Backes IM, Posner DA, Gilli F, Pachner AR, Nguyen LN, Leib DA, Maternal antiviral immunoglobulin accumulates in neural tissue of neonates to prevent HSV neurological disease. MBio 8, e00678–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Y, Leib D, Preventing neonatal herpes infections through maternal immunization. Future Virol. 12, 709–711 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dropulic LK, Cohen JI, The challenge of developing a herpes simplex virus 2 vaccine. Expert Rev. Vaccines 11, 1429–1440 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernard M-C, Barban V, Pradezynski F, de Montfort A, Ryall R, Caillet C, Londono-Hayes P, Immunogenicity, protective efficacy, and non-replicative status of the HSV-2 vaccine candidate HSV529 in mice and guinea pigs. PLOS ONE 10, e0121518 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awasthi S, Hook LM, Shaw CE, Pahar B, Stagray JA, Liu D, Veazey RS, Friedman HM, An HSV-2 trivalent vaccine is immunogenic in rhesus macaques and highly efficacious in guinea pigs. PLOS Pathog. 13, e1006141 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanfield BA, Pahar B, Chouljenko VN, Veazey R, Kousoulas KG, Vaccination of rhesus macaques with the live-attenuated HSV-1 vaccine VC2 stimulates the proliferation of mucosal T cells and germinal center responses resulting in sustained production of highly neutralizing antibodies. Vaccine 35, 536–543 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Royer DJ, Gurung HR, Jinkins JK, Geltz JJ, Wu JL, Halford WP, Carr DJJ, A highly efficacious herpes simplex virus 1 vaccine blocks viral pathogenesis and prevents corneal immunopathology via humoral immunity. J. Virol. 90, 5514–5529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RL, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, hulte JM, Deal CD, Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 366, 34–43 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnston C, Gottlieb SL, Wald A, Status of vaccine research and development of vaccines for herpes simplex virus. Vaccine 34, 2948–2952 (2016). [DOI] [PubMed] [Google Scholar]
- 32.Costa XJ, Jones CA, Knipe DM, Immunization against genital herpes with a vaccine virus that has defects in productive and latent infection. Proc. Natl. Acad. Sci. U.S.A. 96, 6994–6998 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa X, Kramer MF, Zhu J, Brockman MA, Knipe DM, Construction, phenotypic analysis, and immunogenicity of a UL5/UL29 double deletion mutant of herpes simplex virus 2. J. Virol. 74, 7963–7971 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoshino Y, Pesnicak L, Dowdell KC, Lacayo J, Dudek T, Knipe DM, Straus SE, Cohen JI, Comparison of immunogenicity and protective efficacy of genital herpes vaccine candidates herpes simplex virus 2 dl5–29 and dl5–29-41L in mice and guinea pigs. Vaccine 26, 4034–4040 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz FM, Knipe DM, Protection from genital herpes disease, seroconversion and latent infection in a non-lethal murine genital infection model by immunization with an HSV-2 replication-defective mutant virus. Virology 488, 61–67 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Lint AL, Torres-Lopez E, Knipe DM, Immunization with a replication-defective herpes simplex virus 2 mutant reduces herpes simplex virus 1 infection and prevents ocular disease. Virology 368, 227–231 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fouda GG, Martinez DR, Swamy GK, Permar SR, The impact of IgG transplacental transfer on early life immunity. Immunohorizons 2, 14–25 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans IAC, Jones CA, Maternal immunization with a herpes simplex virus type 2 replication-defective virus reduces visceral dissemination but not lethal encephalitis in newborn mice after oral challenge. J. Infect. Dis. 185, 1550–1560 (2002). [DOI] [PubMed] [Google Scholar]
- 39.Kimberlin DW, Why neonatal herpes matters. Lancet Glob. Health 5, e234–e235 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Simon P, Dupuis R, Costentin J, Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav. Brain Res. 61, 59–64 (1994). [DOI] [PubMed] [Google Scholar]
- 41.Seibenhener ML, Wooten MC, Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp, e52434 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walz N, Mühlberger A, Pauli P, A human open field test reveals thigmotaxis related to agoraphobic fear. Biol. Psychiatry 80, 390–397 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Vermillion MS, Klein SL, Pregnancy and infection: Using disease pathogenesis to inform vaccine strategy. Npj Vaccines 3, 6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hurley WL, Theil PK, Perspectives on immunoglobulins in colostrum and milk. Nutrients 3, 442–474 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullender WM, Yasukawa LL, Schwartz M, Pereira L, Hensleigh PA, Prober CG, Arvin AM, Type-specific antibodies to herpes simplex virus type 2 (HSV-2) glycoprotein G in pregnant women, infants exposed to maternal HSV-2 infection at delivery, and infants with neonatal herpes. J. Infect. Dis. 157, 164–165 (1988). [DOI] [PubMed] [Google Scholar]
- 46.Kohl S, West MS, Prober CG, Sullender WM, Loo LS, Arvin AM, Neonatal antibody-dependent cellular cytotoxic antibody levels are associated with the clinical presentation of neonatal herpes simplex virus infection. J. Infect. Dis. 160, 770–776 (1989). [DOI] [PubMed] [Google Scholar]
- 47.Prober CG, llender WM, Yasukawa LL, Au DS, Yeager AS, Arvin AM, Low risk of herpes simplex virus infections in neonates exposed to the virus at the time of vaginal delivery to mothers with recurrent genital herpes simplex virus infections. N. Engl. J. Med. 316, 240–244 (1987). [DOI] [PubMed] [Google Scholar]
- 48.Baron S, Worthington MG, Williams J, Gaines JW, Postexposure serum prophylaxis of neonatal herpes simplex virus infection of mice. Nature 261, 505–506 (1976). [DOI] [PubMed] [Google Scholar]
- 49.Hayashi Y, Wada T, Mori R, Protection of newborn mice against herpes simplex virus infection by prenatal and postnatal transmission of antibody. J. Gen. Virol. 64, 1007–1012 (1983). [DOI] [PubMed] [Google Scholar]
- 50.Kino Y, Eto T, Ohtomo N, Hayashi Y, Yamamoto M, Mori R, Passive immunization of mice with monoclonal antibodies to glycoprotein gB of herpes simplex virus. Microbiol. Immunol. 29, 143–149 (1985). [DOI] [PubMed] [Google Scholar]
- 51.Yamamoto M, Hayashi Y, Tang LL, Mori R, Effects of combined use of acyclovir and antibody in athymic nude mice inoculated intracutaneously with herpes simplex virus. Antivir. Res. 5, 83–91 (1985). [DOI] [PubMed] [Google Scholar]
- 52.Kino Y, Hayashi Y, Hayashida I, Mori R, Dissemination of herpes simplex virus in nude mice after intracutaneous inoculation and effect of antibody on the course of infection. J. Gen. Virol. 63, 475–479 (1982). [DOI] [PubMed] [Google Scholar]
- 53.Bravo F, Bourne N, Harrison CJ, Mani C, Stanberry LR, Myers MG, Bernstein DI, Effect of antibody alone and combined with acyclovir on neonatal herpes simplex virus infection in guinea pigs. J. Infect. Dis. 173, 1–6 (1996). [DOI] [PubMed] [Google Scholar]
- 54.Yorty JL, Bonneau RH, Transplacental transfer and subsequent neonate utilization of herpes simplex virus-specific immunity are resilient to acute maternal stress. J. Virol. 77, 6613–6619 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohl S, Loo LS, The relative role of transplacental and milk immune transfer in protection against lethal neonatal herpes simplex virus infection in mice. J. Infect. Dis. 149, 38–42 (1984). [DOI] [PubMed] [Google Scholar]
- 56.Kohl S, Role of antibody-dependent cellular cytotoxicity in neonatal infection with herpes simplex virus. Rev. Infect. Dis. 13, S950–S952 (1991). [DOI] [PubMed] [Google Scholar]
- 57.Qi Z, Zhao H, Zhang Q, Bi Y, Ren L, Zhang X, Yang H, Yang X, Wang Q, Li C, Zhou J, Xin Y, Yang Y, Yang H, Du Z, Tan Y, Han Y, Song Y, Zhou L, Zhang P, Cui Y, Yan Y, Zhou D, Yang R, Wang X, Acquisition of maternal antibodies both from the placenta and by lactation protects mouse offspring from Yersiniapestis challenge. Clin. Vaccine Immunol. 19, 1746–1750 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krehbiel K, Singh V, Disseminated neonatal herpes simplex virus infection with Escherichia coli coinfection. J. Forensic Sci. 63, 935–938 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW, From inflammation to sickness and depression: When the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dantzer R, Cytokine-induced sickness behaviour: A neuroimmune response to activation of innate immunity. Eur. J. Pharmacol. 500, 399–411 (2004). [DOI] [PubMed] [Google Scholar]
- 61.de Miranda AS, Rodrigues DH, Amaral DCG, de Lima Campos RD, Cisalpino D, Vilela MC, Lacerda-Queiroz N, de Souza KPR, Vago JP, Campos MA, Kroon EG, da D Glória de Souza MM Teixeira AL Teixeira MA Rachid, Dengue-3 encephalitis promotes anxiety-like behavior in mice. Behav. Brain Res. 230, 237–242 (2012). [DOI] [PubMed] [Google Scholar]
- 62.de Miranda AS, Lacerda-Queiroz N, de Carvalo Vilela M, Rodrigues DH, Rachid MA, Quevedo J, Teixeira AL, Anxiety-like behavior and proinflammatory cytokine levels in the brain of C57BL/6 mice infected with Plasmodium berghei (strain ANKA). Neurosci. Lett. 491, 202–206 (2011). [DOI] [PubMed] [Google Scholar]
- 63.Evans AK, Strassmann PS, Lee I-P, Sapolsky RM, Patterns of Toxoplasma gondii cyst distribution in the forebrain associate with individual variation in predator odor avoidance and anxiety-related behavior in male Long–Evans rats. Brain Behav. Immun. 37, 122–133 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guha SK, Tillu R, Sood A, Patgaonkar M, Nanavaty IN, Sengupta A, Sharma S, Vaidya VA, Pathak S, Single episode of mild murine malaria induces neuroinflammation, alters microglial profile, impairs adult neurogenesis, and causes deficits in social and anxiety-like behavior. Brain Behav. Immun. 42, 123–137 (2014). [DOI] [PubMed] [Google Scholar]
- 65.drade EB, Magalhães A, Puga A, Costa M, Bravo J, Portugal CC, Ribeiro A, Correia-Neves M, Faustino A, Firon A, Trieu-Cuot P, Summavielle T, Ferreira P , A mouse model reproducing the pathophysiology of neonatal group B streptococcal infection. Nat. Commun. 9, 3138 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Oliveira Souza IN, Frost PS, França JV, Nascimento-Viana JB, Neris RLS, Freitas L, Pinheiro DJLL, Nogueira CO, Neves G, Chimelli L, De Felice FG, Cavalheiro ÉA, Ferreira ST, Assunção-Miranda I, Figueiredo CP, Da Poian AT, Clarke JR, Acute and chronic neurological consequences of early-life Zika virus infection in mice. Sci. Transl.Med 10, eaar2749 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Arsenault D, St-Amour I, Cisbani G, Rousseau L-S, Cicchetti F, The different effects of LPS and poly I: C prenatal immune challenges on the behavior, development and inflammatory responses in pregnant mice and their offspring. Brain Behav. Immun. 38, 77–90 (2014). [DOI] [PubMed] [Google Scholar]
- 68.Walker FR, March J, Hodgson DM, Endotoxin exposure in early life alters the development of anxiety-like behaviour in the Fischer 344 rat. Behav. Brain Res. 154, 63–69 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Bilbo SD, Levkoff LH, Mahoney JH, Watkins LR, Rudy JW, Maier SF, Neonatal infection induces memory impairments following an immune challenge in adulthood. Behav. Neurosci. 119, 293–301 (2005). [DOI] [PubMed] [Google Scholar]
- 70.Spencer SJ, Heida JG, Pittman QJ, Early life immune challenge—Effects on behavioural indices of adult rat fear and anxiety. Behav. Brain Res. 164, 231–238 (2005). [DOI] [PubMed] [Google Scholar]
- 71.French SS, Chester EM, Demas GE, Maternal immune activation affects litter success, size and neuroendocrine responses related to behavior in adult offspring. Physiol. Behav. 119, 175–184 (2013). [DOI] [PubMed] [Google Scholar]
- 72.Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, lyo M, Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: A neurodevelopmental animal model of schizophrenia. Biol. Psychiatry 59, 546–554 (2006). [DOI] [PubMed] [Google Scholar]
- 73.Li Y, Dugyala SR, Ptacek TS, Gilmore JH, Frohlich F, Maternal immune activation alters adult behavior, gut microbiome and juvenile brain oscillations in ferrets. eNeuro 5, ENEUR0.0313–18.2018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dabbah-Assadi F, Alon D, Golani I, Doron R, Kremer I, Beloosesky R, Shamir A, The influence of immune activation at early vs late gestation on fetal NRG1-ErbB4 expression and behavior in juvenile and adult mice offspring. Brain Behav. Immun, 10.1016/j.bbi.2019.02.002 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Knipe DM, Corey L, Cohen JI, Deal CD, Summary and recommendations from a National Institute of Allergy and Infectious Diseases (NIAID) workshop on “Next Generation Herpes Simplex Virus Vaccines”. Vaccine 32, 1561–1562 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen VC, Wu S-I, Huang K-Y, Yang Y-H, Kuo T-Y, Liang H-Y, Huang K-L, Gossop M, Herpes zoster and dementia: A nationwide population-based cohort study. J. Clin. Psychiatry 79, 16m11312 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Arora N, Sadovsky Y, Dermody TS, Coyne CB, Microbial vertical transmission during human pregnancy. Cell Host Microbe 21, 561–567 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coyne CB, Lazear HM, Zika virus—Reigniting the TORCH. Nat. Rev.Microbiol. 14, 707–715 (2016). [DOI] [PubMed] [Google Scholar]
- 79.Muller WJ, Treatment of perinatal viral infections to improve neurologic outcomes. Pediatr. Res. 81, 162–169 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Sapparapu G, Fernandez E, Kose N, Cao B, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, Davidson E, Mysorekar IU, Fremont DH, Doranz BJ, Diamond MS, Crowe JE, Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature 540, 443–447 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, 0. Levy, Protecting the newborn and young infant from infectious diseases: Lessons from immune ontogeny. Immunity 46, 350–363 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Marchant A, Sadarangani M, Garand M, Dauby N, Verhasselt V, Pereira L, Bjornson G, Jones CE, Halperin SA, Edwards KM, Heath P, Openshaw PJ, Scheifele DW, Kollmann TR, Maternal immunisation: Collaborating with mother nature. Lancet Infect. Dis. 17, e197–e208 (2017). [DOI] [PubMed] [Google Scholar]
- 83.Patel TP, Gullotti DM, Hernandez P, O’Brien TW, Capehart BP, Morrison B III, Bass C, Eberwine JE, Abel T, Meaney DF, An open-source toolbox for automated phenotyping of mice in behavioral tasks. Front. Behav. Neurosci 8, 349 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Goetghebuer T, Smolen KK, Adler C, Das J, McBride T, Smits G, Lecomte S, Haelterman E, Barlow P, Piedra PA, van der Klis F, Kollmann TR, Lauffenburger DA, Alter G, Levy J, Marchant A, Initiation of anti-retroviral therapy before pregnancy reduces the risk of infection-related hospitalization in HIV-exposed uninfected infants born in a high-income country. Clin. Infect. Dis. 68, 1193–1203 (2018). [DOI] [PubMed] [Google Scholar]
- 85.Brown SM, Ritchie DA, Subak-Sharpe JH, Genetic studies with herpes simplex virus type 1. The isolation of temperature-sensitive mutants, their arrangement into complementation groups and recombination analysis leading to a linkage map. J. Gen. Virol. 18, 329–346 (1973). [DOI] [PubMed] [Google Scholar]
- 86.Ejercito PM, Kieff ED, Roizman B, Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 2, 357–364 (1968). [DOI] [PubMed] [Google Scholar]
- 87.Manivanh R, Mehrbach J, Knipe DM, Leib DA, Role of herpes simplex virus 1 γ34.5 in the regulation of irf3 signaling. J. Virol. 91, e01156–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rader KA, Ackland-Berglund CE, Miller JK, Pepose JS, Leib DA, In vivo characterization of site-directed mutations in the promoter of the herpes simplex virus type 1 latency-associated transcripts. J. Gen. Virol. 74, 1859–1869 (1993). [DOI] [PubMed] [Google Scholar]
- 89.Brown EP, Licht AF, Dugast A-S, Choi I, Bailey-Kellogg C, Alter G, Ackerman ME, High-throughput, multiplexed IgG subclassing of antigen-specific antibodies from clinical samples. J. Immunol.Methods 386, 117–123 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


