Abstract
Case:
A patient presenting with an infected diabetic foot ulcer and Staphylococcus aureus chronic osteomyelitis was studied to validate the clinical importance of bacterial colonization of osteocytic-canalicular networks, as we recently reported in a mouse model. We utilized transmission electron microscopy to describe the deformation of S. aureus, from round cocci to rod-shaped bacteria, in the submicron osteocytic-canalicular networks of amputated bone tissue.
Conclusion:
To our knowledge, this is the first evidence of S. aureus deformation and invasion of the osteocytic-canalicular system in human bone, which supports a new mechanism of persistence in the pathogenesis of chronic osteomyelitis.
It is well established that patients with long-term diabetes, peripheral polyneuropathy, and ischemia are at risk for the development of foot ulcers, which often become infected1. Approximately 25% of patients progress from superficial skin ulcers to bone infections, resulting in a diagnosis of chronic osteomyelitis, with an amputation rate of 10% to 30%2. While these infections are generally polymicrobial, Staphylococcus aureus is frequently identified as the pathogen that is most difficult to eradicate because of the development of methicillin-sensitive S. aureus (MSSA) and methicillin-resistant S. aureus (MRSA) strains. Despite extensive antibiotic treatment and surgical debridement, approximately 20% to 30% of patients have recurrent infections2. Determining how S. aureus remains persistent, despite these rigorous treatment protocols has led researchers to examine chronic osteomyelitis biofilm formation using transmission electron microscopy (TEM). In vitro TEM studies have examined S. aureus invasion infection of embryonic chicken calvarial and human osteoblasts3,4; in vivo studies have confirmed the viability status of bacteria within embryonic chicken and human osteoblasts4,5. To our knowledge, only 1 patient with a TEM study after a surgical case of chronic osteomyelitis has been described in the literature; this case revealed intracellular bacteria within osteocytes6. Collectively, these studies have led researchers to conclude that in vivo S. aureus sequestration and survival within bone cells serves as the most likely reservoir for reinfection.
Recently, de Mesy Bentley et al. published a comprehensive TEM study from an in vivo mouse implant model of S. aureus osteomyelitis, which documented bacterial invasion into the osteocytic-canalicular system7. In contrast to the established morphology of S. aureus, which is known to be cocci 1.0 to 1.5 μm in diameter, this study’s TEM imaging revealed deformed, rod-shaped bacteria occupying and proliferating within the narrow submicron spaces (0.2 to 0.4 μm in diameter) of the canaliculi. To explain these unprecedented observations, we concluded that S. aureus invasion into the osteocytic-canalicular system utilized previously unknown mechanisms, including haptotaxis, durotaxis, and “motility by division,” to deeply invade live cortical bone. Because these novel findings have been documented only in an experimental animal model of osteomyelitis, their clinical relevance was unknown. Thus, we performed TEM on retrieved bone tissue from patients who underwent minor amputations of the distal phalanges because of infected diabetic foot ulcers that were culture-positive for S. aureus. Positive findings from 1 of the 3 cases are presented herein.
The patient was informed that data concerning the case would be submitted for publication, and he provided consent.
Case Report
A 73-year-old man with peripheral neuropathy and Charcot arthropathy presented for evaluation of an open wound on the distal phalanx of the right fourth toe of approximately 1-month duration, likely attributable to poorly fitting shoes. The patient had a history of poorly controlled diabetes since 2003, with hemoglobin A1c (HbA1c) ranging from 7.4% to 13.0%. He was referred to the endocrinology department because of the poorly controlled diabetes. The patient also had a history of infections of the right second and third toes, which required partial amputations in conjunction with oral (Augmentin [amoxicillin clavulanate], cephalexin) and intravenous (ciprofloxacin, cefazoline) antibiotics from 2013 to 2015. Deep wound cultures collected in a sterile environment from previous operations yielded a monomicrobial MSSA infection.
In February 2016, the patient presented with a new infected open wound at the tip of the right fourth hammer toe. The wound showed purulent drainage and direct communication with the distal phalanx. He was placed on a 2-week course of oral cephalexin. Two weeks after the completion of the antibiotic treatment, the right fourth toe showed worsening signs of infection (increased erythema and purulence), which prompted us to proceed with surgical intervention. He had palpable pulses of the dorsalis pedis and posterior tibial arteries, and sensation was diminished throughout the right foot. Presurgical laboratory values revealed an HbA1c of 6.3% (normal, 4% to 6%), an erythrocyte sedimentation rate of 13 mm/hr (normal, 0 to 20 mm/hr), a white blood-cell count of 10 × 103/μL (normal, 4.2 to 9.1 × 103/μL) with a normal differential, and a C-reactive protein level of 45 mg/L (normal, 0 to 10 mg/L). We made a clinical diagnosis of osteomyelitis of the distal phalanx of the right fourth toe based on the medical and surgical history, active purulent drainage, and a positive “probe-to-bone” test. Radiographs of the right foot demonstrated general osteopenia and clawing of the fourth toe (Fig. 1-A), with osteolysis at the tip of the distal phalanx (Fig. 1-B). The distal portion of the phalanx was amputated, and the wound culture specimen was MSSA positive.
Fig. 1.
Preoperative radiographs. Fig. 1-A Anteroposterior view of the right foot and the infected fourth toe tip (arrow) with clawing. Fig. 1-B Lateral view of the right foot and the infected fourth toe. Note the osteolysis at the distal phalanx of the fourth toe (arrow).
Light Microscopy and TEM Methods
The tissue of the amputated distal phalanx of the fourth toe was divided and fixed for paraffin embedding in 10% formalin, and for TEM fixation using 2.5% glutaraldehyde and 4.0% paraformaldehyde in a 0.1M-sodium cacodylate buffer for 24 hours. Both specimens were decalcified in 14% ethylenediaminetetraacetic acid (EDTA).
The decalcified bone destined for TEM imaging was postfixed in buffered 2.0% osmium tetroxide, dehydrated in a graded series of ethanol solutions, infiltrated, and subsequently embedded into EPON-Araldite epoxy resin for polymerization at 60°C for 48 hours. To search for areas within the embedded bone tissue that were infected with S. aureus, light microscopy was used and 1-μm sections stained with toluidine blue were examined. Once areas of dark-blue stained bacteria were identified, the block face was thin-sectioned using an ultramicrotome and a diamond knife to produce thin sections that were 70 nm thick for mounting onto formvar/carbon-coated nickel slot grids. The uranyl acetate and lead citrate-stained grids were examined under a Hitachi 7650 transmission electron microscope. This process was repeated every 5 μm throughout the thickness of the block of tissue to facilitate the finding of osteocytes and/or canaliculi occupied by bacteria.
To classify the bacterial infection in the paraffin-embedded bone tissue, we performed a Gram stain on paraffin sections that confirmed gram-positive bacteria.
Treatment
Initially, the patient was treated by the wound care clinic and underwent a 14-day course of oral cephalexin and local wound care. However, over the course of the month, the wound continued to worsen, at which point he was referred to the orthopaedic clinic and deemed a candidate for amputation. Deep wound cultures collected during the operation again demonstrated growth of MSSA. Following amputation, he was placed on amoxicillin/clavulanic acid for 5 days. Additionally, we recommended that he wear custom diabetic orthotics once he was allowed to wear his normal shoes. The wound healed without complications and without requiring additional antibiotic therapy. The patient was followed over a period of 16 months, and there was no recurrence of infection in the remnant of the affected fourth toe.
Results
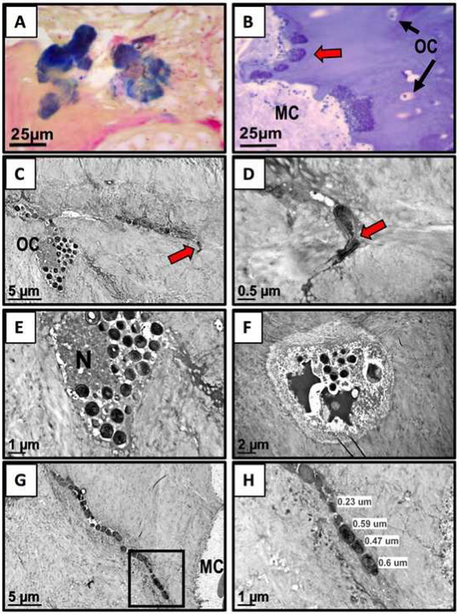
Gram staining of paraffin-embedded bone sections confirmed Gram-positive bacteria scalloping the bone tissue adjacent to the bone marrow (Fig. 2-A). Epoxy-embedded bone tissue surveyed by light microscopy using 1-μm epoxy sections stained with toluidine blue (the stain osmicated the bacteria blue) revealed areas of dark-blue stained bacterial biofilm also scalloping the inner margin of the bone next to the marrow space (Fig. 2-B). Additional thin sectioning of these areas that we examined by TEM revealed bacterial invasion of an osteocyte’s lacunar space and into its submicron canaliculus (Fig. 2-C, red arrow). A remarkable finding at the end of this canaliculus was 2 deformed submicron-sized bacteria displaying evidence of septal plane binary fission (Fig. 2-D, red arrow). A higher magnification of the osteocyte displayed in Figure 2-C (Fig. 2-E) and a neighboring osteocyte (Fig. 2-F) display apoptosis of their nuclei, as well as document bacterial invasion into their lacunar spaces. Additional scrutiny of the same epoxy-embedded block of bone and additional thin sections also revealed deformed rod-shaped bacteria within a submicron canaliculus near the marrow cavity (Fig. 2-G). The higher magnification of the boxed area in Figure 2-G displays the submicron measurement of these rod-shaped bacteria (Fig. 2-H).
Fig. 2.
Figs. 2-A through 2-H TEM evidence of bacterial deformation and invasion of the submicron osteocytic-canalicular system. MC = marrow cavity, OC = osteocyte, and N = nucleus. Fig. 2-A Light microscopy image demonstrating positive Gram- stained bacteria in a section from the paraffin-embedded bone tissue of the fourth toe (×60). Fig. 2-B From the TEM epoxy-embedded portion of bone, a 1-μm epoxy section stained with toluidine blue reveals dark-blue bacteria scalloping the live bone tissue (red arrow) (note the OCs and black arrows adjacent to the MC) (×60). Fig. 2-C TEM image of a dead OC and its canaliculus (red arrow), colonized by deformed rod-shaped bacteria (×6,000). Fig. 2-D Higher magnification of the area adjacent to the red arrow in Figure 2-C reveals the septal plane of the dividing bacteria within the submicron space of the canaliculus (×30,000). Note the asymmetric plane of the 2 daughter cells. Fig. 2-E High magnification of the osteocyte in Figure 2-C displaying condensed nuclear debris and round bacteria occupying its lacunar space (×15,000). Fig. 2-F Another dead osteocyte’s lacuna containing remnants of condensed chromatin bodies and round bacteria (×8,000). Fig. 2-G A TEM image from thin sections deeper into the bone tissue reveals rod-shaped bacteria within a canaliculus (×6,000). Fig. 2-H Higher-magnification TEM image of the boxed area in Figure 2-G measuring submicron-sized deformed and elongated bacteria (×15,000).
Discussion
Although it has been established that the majority of chronic osteomyelitis cases involve S. aureus infections (monomicrobial or polymicrobial) and that recurrence is common despite aggressive surgical debridement and antibiotic therapy8, the unique mechanism that makes these bacteria such a challenging pathogen in bone remains unknown. The recent discovery that S. aureus is able to deform and invade the submicron canaliculi of live cortical bone7 offers a novel explanation because these bacteria colonize the “red zone” beyond the margins of aggressive debridement and partial amputation. Moreover, the biodistribution of standard-of-care antibiotic therapies may not achieve the minimal inhibitory concentration in infected osteocytic-canalicular networks. Thus, given their clinical importance, we aimed to confirm these experimental findings by performing TEM studies on S. aureus-infected human bone. To our knowledge, we report the first TEM evidence of S. aureus colonization of osteocytic-canalicular networks in a patient with recurrent osteomyelitis. While this case report needs to be substantiated by larger prospective studies, it should be noted that we specifically chose to investigate amputated toe tissue because the costs (money, time, labor) associated with formal TEM interrogation of larger bones (e.g., the tibia and the femur) are prohibitive.
Finally, this identification of a reservoir of bacteria invading deep within bone is most likely why chronic osteomyelitis infections recur. Indeed, reports of S. aureus reactivation 50 to 75 years after the initial infection indicate that a mechanism of slowed growth does occur, and most likely is due to the slow growth of deformed submicron-sized bacteria in the osteocytic-canalicular system9-11. Additional studies of monomicrobially and polymicrobially infected diabetic bone tissue could elucidate whether other species of bacteria invade canaliculi, or whether a heterogeneous bacterial infection modulates the persistence of S. aureus.
Acknowledgments
Disclosure: This work was funded by AOTrauma (Davos, Switzerland) and the National Institutes of Health, grant numbers P30 AR069655 and P50AR054041. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors also checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work.
Footnotes
Investigation performed at the Department of Orthopaedics and Center for Musculoskeletal Research, University of Rochester Medical Center, Rochester, New York
The authors thank Gayle Schneider of the Electron Microscope Shared Resource Laboratory for her technical assistance in processing of the bone into epoxy resin and producing the 1-μm and serial thin sections of human bone tissue.
Contributor Information
Karen L. de Mesy Bentley, Center for Musculoskeletal Research, University of Rochester School of Medicine and Dentistry, Rochester, New York; Departments of Pathology and Laboratory Medicine, University of Rochester School of Medicine and Dentistry, Rochester, New York; Department of Orthopaedics, University of Rochester School of Medicine and Dentistry, Rochester, New York.
Ashlee MacDonald, Center for Musculoskeletal Research, University of Rochester School of Medicine and Dentistry, Rochester, New York; Department of Orthopaedics, University of Rochester School of Medicine and Dentistry, Rochester, New York.
Edward M. Schwarz, Center for Musculoskeletal Research, University of Rochester School of Medicine and Dentistry, Rochester, New York; Departments of Pathology and Laboratory Medicine, University of Rochester School of Medicine and Dentistry, Rochester, New York; Department of Orthopaedics, University of Rochester School of Medicine and Dentistry, Rochester, New York.
Irvin Oh, Center for Musculoskeletal Research, University of Rochester School of Medicine and Dentistry, Rochester, New York; Department of Orthopaedics, University of Rochester School of Medicine and Dentistry, Rochester, New York.
References
- 1.Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons NB. Burden of diabetic foot ulcers for Medicare and private insurers. Diabetes Care. 2014;37(3):651–8. Epub 2013 Nov 1. [DOI] [PubMed] [Google Scholar]
- 2.Lipsky BA. Medical treatment of diabetic foot infections. Clin Infect Dis. 2004. August 1;39(Suppl 2):S104–14. [DOI] [PubMed] [Google Scholar]
- 3.Hudson MC, Ramp WK, Nicholson NC, Williams AS, Nousiainen MT. Internalization of Staphylococcus aureus by cultured osteoblasts. Microb Pathog. 1995. December;19(6):409–19. [DOI] [PubMed] [Google Scholar]
- 4.Ellington JK, Harris M, Webb L, Smith B, Smith T, Tan K, Hudson M. Intracellular Staphylococcus aureus. A mechanism for the indolence of osteomyelitis. J Bone Joint Surg Br. 2003. August;85(6):918–21. [PubMed] [Google Scholar]
- 5.Reilly SS, Hudson MC, Kellam JF, Ramp WK. In vivo internalization of Staphylococcus aureus by embryonic chick osteoblasts. Bone. 2000. January;26(1):63–70. [DOI] [PubMed] [Google Scholar]
- 6.Bosse MJ, Gruber HE, Ramp WK. Internalization of bacteria by osteoblasts in a patient with recurrent, long-term osteomyelitis. A case report. J Bone Joint Surg Am. 2005. June;87(6): 1343–7. [DOI] [PubMed] [Google Scholar]
- 7.de Mesy Bentley KL, Trombetta R, Nishitani K, Bello-Irizarry SN, Ninomiya M, Zhang L, Chung HL, McGrath JL, Daiss JL, Awad HA, Kates SL, Schwarz EM. Evidence of Staphylococcus aureus deformation, proliferation, and migration in canaliculi of live cortical bone in murine models of osteomyelitis. J Bone Miner Res. 2017. May;32(5):985–90. Epub 2017 Jan 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byren I, Bejon P, Atkins BL, Angus B, Masters S, McLardy-Smith P, Gundle R, Berendt A. One hundred and twelve infected arthroplasties treated with ‘DAIR’ (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother. 2009. June;63(6): 1264–71. Epub 2009 Mar 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libraty DH, Patkar C, Torres B. Staphylococcus aureus reactivation osteomyelitis after 75 years. N Engl J Med. 2012. February 2;366(5):481–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donati L, Quadri P, Reiner M. Reactivation of osteomyelitis caused by Staphylococcus aureus after 50 years. J Am Geriatr Soc. 1999. August;47(8):1035–7. [DOI] [PubMed] [Google Scholar]
- 11.Al-Maiyah M, Hemmady MV, Shoaib A, Morgan-Jones RL. Recurrence of chronic osteomyelitis in a regenerated fibula after 65 years. Orthopedics. 2007. May;30(5):403–4. [DOI] [PubMed] [Google Scholar]