Abstract
The long-chain n-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) play a crucial role in health, but previous National Health and Nutrition Examination Survey (NHANES) analyses have shown that EPA and DHA intake in the United States is far below recommendations (~250–500 mg/d EPA+DHA). Less is known about docosapentaenoic acid (DPA), the metabolic intermediate of EPA and DHA; however, evidence suggests DPA may be an important contributor to long-chain n-3 fatty acid intake and impart unique benefits. We used NHANES 2003–2014 data (n = 45,347) to assess DPA intake and plasma concentrations, as well as the relationship between intake and plasma concentrations of EPA, DPA, and DHA. Mean DPA intake was 22.3 ± 0.8 mg/d from 2013–2014, and increased significantly over time (p < 0.001), with the lowest values from 2003–2004 (16.2 ± 1.2 mg/d). DPA intake was higher in adults (20–55 y) and seniors (55+ y) compared to younger individuals. In regression analyses, DPA intake was a significant predictor of plasma EPA (β = 138.5; p < 0.001) and DHA (β = 318.9; p < 0.001). Plasma DPA was predicted by EPA and DHA intake (β = 13.15; p = 0.001 and β = 7.4; p = 0.002), but not dietary DPA (p = 0.3). This indicates that DPA intake is not a good marker of plasma DPA status (or vice versa), and further research is needed to understand factors that affect the interconversion of EPA and DPA. These findings have implications for future long-chain omega-3 fatty acids dietary recommendations.
Keywords: eicosapentaenoic acid, docosahexaenoic acid, omega-3 fatty acids, oily fish, fish oil supplements
Introduction
Long-chain n-3 fatty acids play a crucial biological role in health [1]. Regular intake of fish/seafood (providing ~250–500 mg/d of eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA]) is recommended by the American Heart Association (AHA) [2, 3], Academy of Nutrition and Dietetics [4], and in the 2015–2020 Dietary Guidelines for Americans (DGA)[5] to promote health and reduce the risk of cardiovascular disease (CVD) in the general population. Much less is known about docosapentaenoic acid (DPA), the metabolic intermediate of EPA and DHA. However, existing evidence suggests that DPA may also contribute to the health benefits attributed to EPA and DHA [6–8]. Based on these potential health benefits, it is important to assess the usual DPA intake of the US population compared to habitual EPA and DHA intakes.
The majority of the population in the United States (US) consumes far less than the recommended amount of EPA and DHA [9–12]. For instance, an analysis of National Health and Nutrition Examination Survey (NHANES) data (2003–2008) found that in US adults the mean intake of EPA and DHA from foods was 20 mg/d and 60 mg/d, and 40 mg/d and 70 mg/d when accounting for foods plus supplements [9]. We previously reported that the mean total n-3 fatty acid intake (including EPA, DHA, and EPA-equivalents accounting for potential conversion of alpha-linolenic acid and stearidonic acid) was 170 mg/d (NHANES 2003–2008) [10]. Even when accounting for the potential endogenous, albeit limited, conversion of shorter chain plantbased n-3 fatty acids, over 90% of the study population (n = 24,621) consumed less than the recommended ~500 mg/d [10]. A recent analysis that included the most current NHANES cycles (2003–2014) also found similarly inadequate EPA and DHA intake [13].
Much less is known about DPA consumption. A small number of studies in Australia [14–16], France [17], the UK [18], Japan [19, 20], Norway [21], the Netherlands [22], and Belgium [23] have reported estimated mean DPA intake, with values ranging from 10 – 106 mg/d. These analyses indicate that DPA may contribute an appreciable proportion of total long-chain n-3 fatty acid intake, depending on the population. For instance, in an analysis of the Australian 1995 National Nutrition Survey (n = 13,858) median DPA intake (40 mg/d and 60 mg/d for women and men, respectively) accounted for 29% of the mean total long-chain n-3 fatty acid intake [14, 24]. With regard to the US, DPA intake has been reported as part of What We Eat in America (WWEIA) since NHANES 1999–2000, with 10 mg/d reported for 1999–2000 (n = 8,604) and 20 mg/d for each of the subsequent cycles in men and women 2 years and older [25–32]. However, these reports have been limited to mean intakes rather than deciles, and to our knowledge, no comprehensive analysis of DPA intake over this period has been published, particularly for specific age, sex, and race/ethnicity subgroups. Additionally, little is known about the relationship between dietary DPA intake and plasma concentrations of EPA, DPA, and DHA.
The objective of the current analysis was to provide an updated and comprehensive assessment of DPA intake in the US using data from NHANES 2003–2014, as well as to compare this to EPA and DHA intake using the most recent data. Long-chain n-3 fatty acid intake (EPA, DPA, DHA, EPA+DHA, and EPA+DPA+DHA) from foods was analyzed for the total US population, and by age, sex, and race/ethnicity subgroups. We also evaluated the relationship between self-reported dietary intake and plasma concentrations of each fatty acid.
Methods
Six cycles of the National Health and Nutritional Examination Survey (NHANES; 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012, and 2013–2014) were used for the analysis. NHANES is a cross-sectional survey conducted by the National Center for Health Statistics (NCHS), under the Centers for Disease Control and Prevention, using a complex multistage probability sample that is designed to be representative of the national civilian US population [33]. Sampling weights were adjusted to account for multiple cycles. Males and females aged 1 year or older were included. Adult men with kilocalorie intakes per day <800 or >8000 were excluded. Similarly, adult women with kilocalorie intakes per day <600 or >6000 were excluded. Daily intake of eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA) were calculated for the total US population and the following age groups: infants (1 – 5 years [y]), children (6 – 11 y), adolescents (12 – 19 y), adults (20 – 55 y) and seniors (55+ y). Intake of n-3 fatty acids was reported as mg/d per 1000 kcal in order to account for differences in caloric intake according to age. Each of the age groups were also analyzed by sex. Intakes were also analyzed based on the following race/ethnicity groups: Mexican Americans, Hispanics, Non-Hispanic whites, Non-Hispanic blacks, and Other (including multi-racial). The combined sample included 45,347 individuals.
As part of the “What We Eat in America” (WWEIA) component of the NHANES examination, trained dietary interviewers collected detailed information on all foods and beverages consumed by respondents in the previous 24-hour time period (midnight to midnight). A second dietary recall was administered by telephone 3 to 10 days after the first dietary interview, but not on the same day of the week as the first interview. Average EPA, DPA, and DHA was calculated based on these two dietary interviews. If an individual did not complete the second dietary interview, data from only the first dietary interview was used. Using these averages, EPA + DHA and EPA + DPA + DHA were then calculated. The sum of EPA + DHA was considered missing only if both EPA and DHA were missing. Similarly, the sum EPA + DHA + DPA was considered missing only if values for all three fatty acids were missing.
Participants were also asked if they had taken a dietary supplement in the past 30 days, how long they had been taking it, how many days it was taken in the past 30 days, the amount that was taken on those days, and the reason(s) that they were taking it. Label information such as supplement name, manufacturer and/or distributor, serving size, form of serving size, and ingredients were recorded for each supplement reported by participants. For each supplement, the amount of EPA and DHA provided was obtained from the supplement label. When the EPA and DHA content was not specified on the supplement label, the EPA and DHA content was imputed based on the proportion of EPA and DHA in the n-3 fatty acid-containing ingredient (i.e., 18% EPA and 12% DHA per 1 g of fish oil; 8% EPA and 10% DHA per 1 g of cod liver oil; and 8% EPA and 12% DHA per 1 g of salmon oil). Supplements containing fish oil, cod liver oil, salmon oil, krill oil, and DHA-only preparations were included in this analysis.
Fatty acid concentrations were measured in a subset of NHANES 2003–2004 plasma samples (n = 1,845). Plasma samples were collected from adults ≥ 20 years of age following an 8 hour overnight fast. Fatty acid concentrations were quantified using a modified version of the method described by Lagerstedt et al. [34]. In brief, a 100 μL plasma sample was spiked with a 100 μL mixture of 11 internal standards (fatty acids labeled with stable isotopes) to account for recovery. Esterified fatty acids were hydrolyzed from lipids (e.g., triglycerides, phospholipids, and cholesteryl esters) using sequential treatment with acid then base. Following base hydrolysis, samples were re-acidified and total fatty acids were hexane-extracted from the matrix along with internal standards. Extracts were derivatized with pentafluorobenzyl bromide in the presence of triethylamine to form pentafluorobenzyl (PFB) esters and were reconstituted in hexane. PFB-fatty acid derivatives were injected onto a capillary gas chromatographic column to resolve individual cis-fatty acids of interest from other matrix constituents. Analytes were detected using electron capture negative-ion mass spectrometry. For each fatty acid, recovery was estimated and results were adjusted using the most appropriate isotopically-labeled internal standard.
Statistical Analysis
All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC). Descriptive statistics and regression analyses were computed using SURVEYFREQ, SURVEYMEANS, and SURVEYREG, which account for complex survey design and sampling weight. The Rao-Scott chi-square test was used to assess the association between categorical variables. Continuous variables were compared using a regression model (SURVEYREG). For all tests, α was set at 0.05.
Results
The demographic characteristics of the NHANES participants included in the present analysis are provided in Table 1.
Table 1.
Characteristics of analyzed NHANES participants (n = 45,347)1
| n | Mean (SE) | |
|---|---|---|
| Age (y) | 45347 | 37.2 (0.3) |
| Income ($1K per month) | 42415 | 2.9 (0.04) |
| Gender | ||
| Male (%) | 22056 | 48.5 |
| Female (%) | 23291 | 51.5 |
| Race | ||
| Mexican American (%) | 9421 | 9.9 |
| Hispanic (%) | 3627 | 5.0 |
| White (%) | 18118 | 66.5 |
| Black (%) | 10600 | 12.1 |
| Other (%) | 3581 | 6.4 |
| Education | ||
| < High School (%) | 19047 | 32.2 |
| High School/GED (%) | 6748 | 19.5 |
| > High School (%) | 14025 | 48.3 |
| Pregnant | ||
| Yes (%) | 762 | 4.8 |
| No (%) | 8381 | 92.1 |
| Do not know (%) | 287 | 3.1 |
| Age group | ||
| Infant (1–5) (%) | 5495 | 6.9 |
| Child (6–11) (%) | 5550 | 8.3 |
| Adolescent (12–19) (%) | 8186 | 11.5 |
| Adult (20–55) (%) | 15937 | 50.2 |
| Senior (56+) (%) | 10179 | 23.1 |
DPA intake in the US population
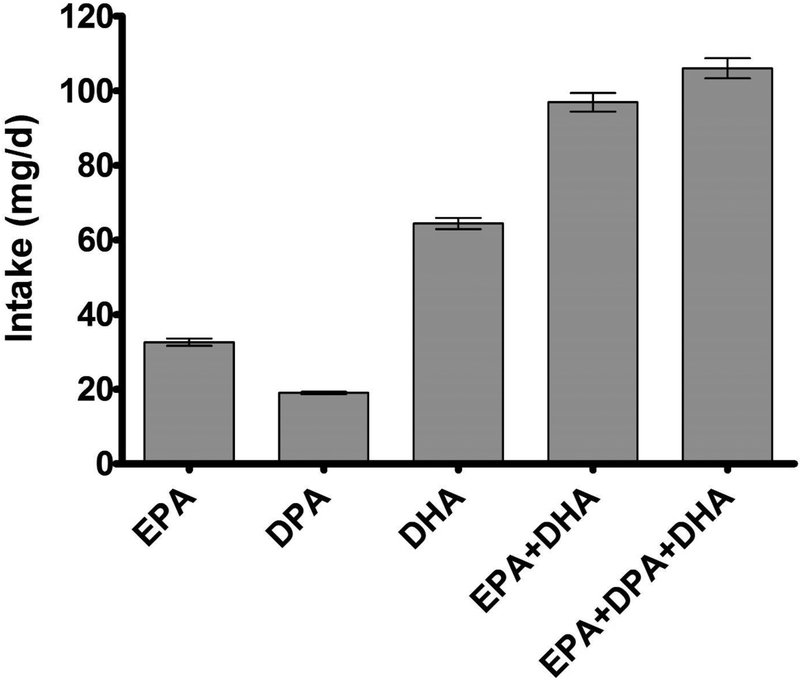
The mean intake of EPA, DPA, DHA), EPA + DHA, and EPA + DPA + DHA is shown in Figure 1. The values for EPA and DHA intake are reproduced from Thompson et al. [13] in order to compare those values to mean DPA intake. Mean DPA intake (19.0 ± 0.3 mg/d) was lower than that of EPA (32.6 ± 1.0 mg/d) and DHA (64.4 ± 1.5 mg/d). Even when accounting for DPA intake, total long-chain n-3 fatty acid intake (EPA+DPA+DHA; 106 ± 2.7 mg/d) was less than half the estimated amount provided by consuming 8 oz/wk of fish/seafood (~250–500 mg/d). Deciles of DPA intake for the total participant population and the subgroups of sex, age, and race/ethnicity are presented in Table 2.
Figure 1.
Mean daily intake eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), docosahexaenoic acid (DHA), EPA + DHA, and EPA + DPA + DHA in the total NHANES population (n = 45,347). Values are means with standards errors represented by vertical bars.
Table 2.
Deciles of DPA intake in the total US population and by sex, age, and race/ethnicity subgroups.
| Mean | Median | Percentiles | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10th | 20th | 30th | 40* | 50th | 60th | 70th | 80th | 90th | |||
| Total population | 19.0 ± 0.3 | 11.6 ± 0.2 | 0 ± 0.1 | 2.6 ± 0.2 | 5.6 ± 0.2 | 8.6 ± 0.2 | 11.6 ± 0.2 | 15.2 ± 0.2 | 19.6 ± 0.3 | 26.4 ± 0.4 | 40.1 ± 0.6 |
| Sex | |||||||||||
| Women | 9.5 ± 0.2 | 9.9 ± 0.2 | 0 ± 0.1 | 2.3 ± 0.2 | 4.9 ± 0.2 | 7.4 ± 0.2 | 9.9 ± 0.2 | 12.9 ± 0.2 | 16.5 ± 0.2 | 21.8 ± 0.4 | 33.5 ± 0.7 |
| Men | 9.6 ± 0.2 | 14.1 ± 0.3 | 0 ± 0.1 | 3.1 ± 0.3 | 6.8 ± 0.3 | 10.3 ± 0.3 | 14.1 ± 0.3 | 18.2 ± 0.3 | 23.2 ± 0.3 | 31.3 ± 0.5 | 46.9 ± 1.1 |
| Race/ethnicity | |||||||||||
| Mexican-American | 9.4 ± 0.3 | 12.2 ± 0.4 | 0 ± 0.2 | 3.6 ± 0.5 | 6.3 ± 0.4 | 9.2 ± 0.4 | 12.2 ± 0.4 | 15.2 ± 0.5 | 19.9 ± 0.6 | 26.7 ± 0.8 | 39.2 ± 1.1 |
| Hispanic | 11.4 ± 0.6 | 13.4 ± 0.5 | 1.0 ± 0.5 | 4.7 ± 0.5 | 7.5 ± 0.5 | 10.5 ± 0.5 | 13.4 ± 0.5 | 17.2 ± 0.5 | 21.7 ± 0.8 | 29.1 ± 1.2 | 45.5 ± 2.5 |
| Caucasian | 8.6 ± 0.2a | 10.6 ± 0.3 | 0 ± 0.1 | 1.9 ± 0.3 | 4.8 ± 0.3 | 7.7 ± 0.3 | 10.6 ± 0.3 | 14.1 ± 0.3 | 18.1 ± 0.3 | 24.2 ± 0.5 | 37.1 ± 0.9 |
| African-American | 11.6 ± 0.4 | 15.3 ± 0.4 | 0.8 ± 0.3 | 5.1 ± 0.3 | 8.4 ± 0.4 | 11.7 ± 0.3 | 15.3 ± 0.4 | 19.7 ± 0.4 | 24.9 ± 0.4 | 33.3 ± 0.6 | 49.4 ± 1.4 |
| Other | 12.9 ± 0.6 | 13.9 ± 0.6 | 0.3 ± 0.1 | 3.7 ± 0.5 | 6.9 ± 0.5 | 10.4 ± 0.6 | 13.9 ± 0.6 | 18.4 ± 0.7 | 24.2 ± 1.1 | 33.3 ± 1.4 | 51.2 ± 2.5 |
| Age groups | |||||||||||
| Infant (1–5 y) | 5.3 ± 0.1 | 4.8 ± 0.2 | 0 ± 0.1 | 0.4 ± 0.1 | 1.8 ± 0.2 | 3.2 ± 0.2 | 4.8 ± 0.2 | 6.7 ± 0.3 | 8.6 ± 0.2 | 11.7 ± 0.4 | 16.9 ± 0.4 |
| Child (6–11 y) | 6.4 ± 0.1 | 8.3 ± 0.3 | 0 ± 0.1 | 1.1 ± 0.3 | 3.6 ± 0.3 | 5.8 ± 0.3 | 8.3 ± 0.3 | 10.7 ± 0.3 | 13.6 ± 0.4 | 17.6 ± 0.4 | 25.7 ± 0.8 |
| Adolescent (12–19 y) | 7.7 ± 0.2 | 10.5 ± 0.3 | 0 ± 0.1 | 2.0 ± 0.3 | 4.9 ± 0.3 | 7.7 ± 0.3 | 10.5 ± 0.3 | 13.6 ± 0.4 | 17.2 ± 0.4 | 22.5 ± 0.5 | 33.3 ± 0.8 |
| Adult (20–55 y) | 10.4 ± 0.2 | 14.1 ± 0.3 | 0 ± 0.1 | 3.9 ± 0.3 | 7.3 ± 0.2 | 10.5 ± 0.3 | 14.1 ± 0.3 | 18.0 ± 0.3 | 22.9 ± 0.3 | 30.9 ± 0.6 | 46.6 ± 1.1 |
| Senior (55+ y) | 11.0 ± 0.3 | 11.8 ± 0.3 | 0 ± 0.1 | 2.9 ± 0.3 | 6.0 ± 0.3 | 8.9 ± 0.3 | 11.8 ± 0.3 | 15.4 ± 0.4 | 19.4 ± 0.4 | 26.4 ± 0.6 | 41.7 ± 1.5 |
Changes in n-3 fatty acid intake over time
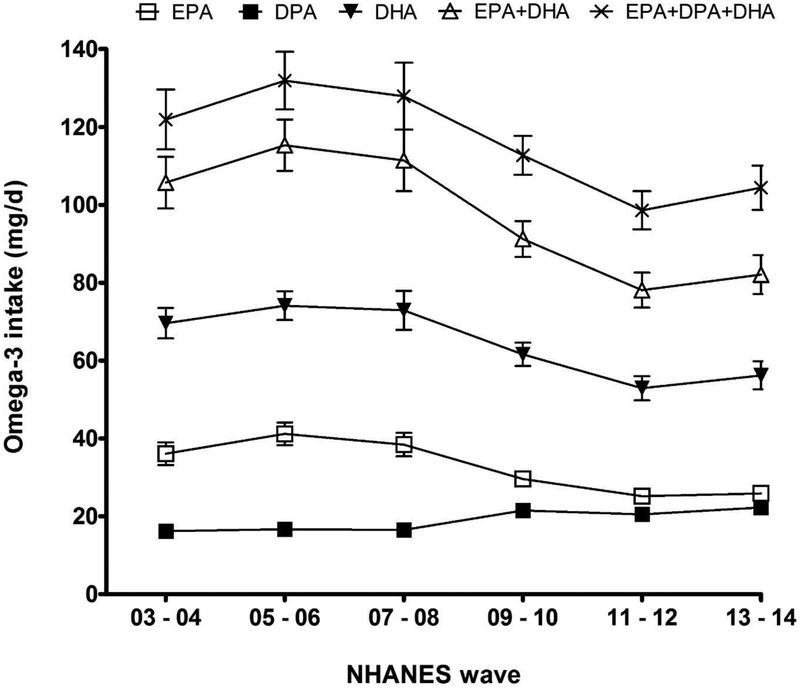
Intake of EPA, DPA, DHA, EPA+DHA, and EPA+DPA+DHA changed significantly over the 11 year period of data collection (main effect of time, p < 0.01; Figure 2). DPA intake increased significantly in later NHANES cycles, with the lowest mean intake observed during the 2003–2004 cycle (16.2 ± 1.2 mg/d). During the 2013–2014 cycle, mean intake increased significantly to 22.3 ± 0.8 mg/d (p < 0.0001). Conversely, EPA intake decreased significantly from 2003–2008 to 2011–2014 (p ≤ 0.04), and mean DHA intake decreased from 2005–2006 to 2011–2014 (p < 0.01). In the most recent NHANES cycle (2013–2014), mean DPA intake was 22.3 ± 0.8 mg/d, whereas mean EPA and DHA intakes were 25.9 ± 1.6 mg/d and 56.2 ± 3.6 mg/d, respectively.
Figure 2.
Change in n-3 fatty acid intake by NHANES data collection cycle, from 2003 to 2014. Values are means with standards errors represented by vertical bars.
DPA intake by age, sex, and race/ethnicity
DPA intake was significantly higher in men compared to women when intake was reported as mg/d (mean = 22.1 ± 0.4 mg/d vs. 16.1 ± 0.4 mg/d; p < 0.001), but this relationship was no longer present when DPA intake was adjusted for kcal intake. When interpreted as mg/d of fatty acid consumed per 1000 kcal, there was no significant difference between men and women for either DPA intake (p = 0.9) or total intake of EPA+DPA+DHA (p = 0.1). However, DPA intake was significantly different according to race/ethnicity and age (Table 3). With regard to ethnicities, mean DPA intake was highest in the “Other” category (including Asian and multiracial; 24.5 ± 1.4 mg/d), followed by African Americans (24.2 ± 0.7 mg/d). Regardless of sex, higher DPA intake was observed in adults (20–55 y) and seniors (55+ y) when compared to younger age groups, even when total caloric intake was accounted for.
Table 3.
| DPA | EPA+DPA+DHA | |
|---|---|---|
| Sex | ||
| Female | 9.5 ± 0.2 | 60.3 ± 1.8 |
| Male | 9.6 ± 0.2 | 57.4 ± 1.5 |
| Race/ethnicity3 | ||
| Mexican-American | 9.4 ± 0.3a | 53.1 ± 2.2a |
| Hispanic | 11.4 ± 0.6a | 67.9 ± 5.3a |
| Caucasian | 8.6 ± 0.2a | 53.5 ± 1.4a |
| African-American | 11.6 ± 0.4b | 70.5 ± 3.0b |
| Other | 12.9 ± 0.6b | 95.1 ± 6.5b |
| Age group3 | ||
| Infant (1–5 y) | 5.3 ± 0.1a | 28.4 ± 1.1a |
| Child (6–11 y) | 6.4 ± 0.1a,b | 32.3 ± 1.5a,b |
| Adolescent (12–19 y) | 7.7 ± 0.2b | 37.4 ± 1.5b |
| Adult (20–55 y) | 10.4 ± 0.2c | 63.7 ± 1.7c |
| Senior (55+ y) | 11.0 ± 0.3c | 77.6 ± 2.8c |
Different letters within the column indicate a significant difference between groups.
Reported as mg of fatty acid intake per 1000 kcal per day to adjust for differences in caloric needs among age groups.
p<0.001 for main effect of categorical variable on intake of DPA and EPA+DHA+DPA.
Dietary intake and plasma fatty acid concentrations
Mean plasma concentration of EPA, DPA, and DHA was 50.7 ± 1.7 μmol/L, 44.2 ± 0.7 μmol/L, and 138.5 ± 4.0 μmol/L, respectively. Regression analyses demonstrated that plasma concentrations of EPA and DHA were predicted by the dietary intake of those fatty acids (Table 4; p < 0.001). In contrast, DPA intake was a significant predictor of plasma EPA (β = 139; p < 0.001) and DHA (β = 318.93; p < 0.001), but not plasma DPA (p = 0.3). Plasma DPA was predicted only by EPA and DHA intake (β = 13.15; p = 0.001 and β = 7.4; p = 0.002). There was no significant relationship between the dietary intake of EPA, DPA, or DHA and the plasma concentration of arachidonic acid (p ≥ 0.2). The plasma concentrations of all three n-3 fatty acids (EPA, DPA, and DHA) were significantly correlated with one another (p < 0.0001; data not shown).
Table 4.
Relationship between plasma fatty acid concentrations and self-reported dietary intake
| β Coefficient (SE) | R-Square | P-value | |
|---|---|---|---|
| Plasma EPA (μmol/L) | |||
| Dietary EPA (mg/d) | 114 ± 26 | 8.9% | <0.001 |
| Dietary DPA (mg/d) | 139 ± 63 | 2.3% | <0.001 |
| Dietary DHA (mg/d) | 60 ± 19 | 6.8% | <0.001 |
| Plasma DPA (μmol/L) | |||
| Dietary EPA (mg/d) | 13 ± 5 | 0.7% | 0.001 |
| Dietary DPA (mg/d) | 9 ± 8 | 0.1% | 0.3 |
| Dietary DHA (mg/d) | 7 ± 4 | 0.6% | 0.002 |
| Plasma DHA (μmol/L) | |||
| Dietary EPA (mg/d) | 212 ± 31 | 8.6% | <0.001 |
| Dietary DPA (mg/d) | 319 ± 104 | 3.4% | <0.001 |
| Dietary DHA (mg/d) | 122 ± 27 | 7.6% | <0.001 |
| Plasma arachidonic acid (μmol/L) | |||
| Dietary EPA (mg/d) | 76 ± 54 | 0.1% | 0.2 |
| Dietary DPA (mg/d) | 66 ± 101 | 0.1% | 0.6 |
| Dietary DHA (mg/d) | 36 ± 24 | 0.1% | 0.3 |
Discussion
This analysis of NHANES 2003–2014 data demonstrates that the average DPA intake in the US is very low, as is that of EPA and DHA. Even when accounting for DPA intake, the total daily long-chain n-3 fatty acid intake (defined as EPA + DPA + DHA) of the majority of the US population is well below the ~250–500 mg/d amount estimated to be provided by the amount of fish/seafood consumption recommended by the AHA [2] and the 2015–2020 DGA [5]. Similar to previous findings for EPA and DHA in this population [13], DPA intake was lower in women and younger age groups (< 19 y), even when adjusting for differences in caloric needs between age groups. This pattern of DPA intake was also found in Norway [21] and similar trends have been reported for EPA and DHA [9, 10, 13]. These differences may have implications for dietary recommendations and should be further explored. Notably, DPA intake significantly increased over time while EPA and DHA intake significantly declined. Consistent with existing evidence, strong correlations were observed between dietary intake of EPA and DHA, and corresponding plasma concentrations of these fatty acids. However, DPA plasma concentrations were correlated only with EPA and DHA intake, not DPA intake.
Our findings about habitual DPA intake in the US may have important implications given emerging evidence regarding the bioactive role and potential health effects of DPA [6, 8]. In previous observational studies, plasma DPA has been inversely associated with total mortality [35], nonfatal myocardial infarction [36], and incident CVD in some ethnic groups [37]. Lower serum concentrations of DPA and DPA + DHA have also been associated with greater risk of myocardial infarction [38] and acute coronary events [39], respectively. Furthermore, inverse associations have been found for DPA and intermediate CVD risk factors, such as the inflammatory marker C-reactive protein [7, 40, 41]. Pre-clinical evidence suggests that DPA supplementation may have beneficial effects on triglycerides similar to those of EPA and DHA [6, 42]. Additionally, an inverse association between red blood cell (RBC) DPA and triglyceride concentrations has been documented [7, 43]. Seal oil—a relatively rich source of DPA—has also been shown to reduce triglycerides in some populations [44]. Clinical DPA supplementation studies are needed to clarify these potential biological effects, but existing evidence suggests that the very low DPA intake (19 ± 0.3 mg/d) found in our analysis may have important implications for health.
Relationships between dietary intake and plasma concentrations of EPA, DPA, and DHA may provide insights into the metabolism of DPA and its potential health effects. In this analysis, plasma EPA and DHA concentrations were both significantly predicted by the dietary intake of these fatty acids, which is consistent with the strong correlation between dietary intake and plasma concentrations found for EPA and DHA in prior studies [45, 46]. Conversely, plasma DPA was significantly related to the dietary intake of EPA and DHA, but not DPA. Similar results were found in the Nurses’ Health Study [47] and a study of men in Japan [19]. However, in two additional studies, dietary and plasma DPA levels were found to have a significant correlation in female participants [19, 48], which may suggest a potential sex-related difference in these associations. Because previous evidence has shown that DPA is metabolically active [6, 8], the lack of association between dietary and plasma DPA may indicate that DPA is metabolized to other compounds following consumption. For instance, DPA can be metabolized into a distinct family of specialized pro-resolving mediators [49–52], which could deplete plasma DPA concentrations. DPA may also serve as a biologic pool for EPA, as DPA supplementation has been shown to increase EPA concentrations in both cell-based [53] and clinical studies [54]. In the one clinical DPA supplementation study that has been conducted, DPA supplementation (8 g/d for 7 days) significantly increased the proportion of both DPA and EPA in plasma phospholipids and triglyceride fractions [54]; however, it should be noted that this is a much larger amount of DPA than is consumed by the general population and may result in different blood concentrations than typical dietary intake levels. Additionally, supplementation with EPA (and EPA+DHA) increases RBC and plasma DPA concentrations [44, 55–59], indicating potential inter-convertibility between EPA and DPA. It has been suggested that plasma EPA is a more readily available source of n-3 fatty acids than 22-carbon fatty acids, which that may be preferentially stored in specific tissue compartments [36, 56, 60]. If plasma EPA that is expended on cellular functions can be replenished with DPA, the health implications of this warrant further study. Additional DPA supplementation studies are needed to assess the potential relationship between dietary intake and blood concentrations, as well as aid in the interpretation and clinical significance of any such correlations.
Compared to previous WWEIA reports of DPA intake, our results provide a more comprehensive assessment of DPA intake over a 12-year period, and analysis of changes in intake patterns over time. In NHANES 1999–2000, mean DPA intake was 10 ± 0.1 mg/d [25], whereas we found that the average daily intake from 2003–2014 was 19 ± 0.3 mg/d. Within the 2003–2014 time period, DPA intake was significantly higher during 2009–2014 compared to 2003–2008 (p ≤ 0.02). This is particularly notable given that EPA and DHA intakes significantly decreased over the aforementioned time periods. This may be due in part to the different food sources of EPA/DHA versus DPA. For instance, although fish/fish oil is the primary source of EPA and DHA, it provides relatively little DPA (~2–5% by weight [61]). DPA is found in greater concentrations amounts in red meat (e.g., beef and lamb) [24]. In Australia, Howe et al. found that while the primary food sources of EPA and DHA were fish/seafood products, the primary contributor to DPA intake was meat, poultry, and game (at 73%) [14]. We did not assess changes in the intake of these food categories, but it is possible that the changes in EPA, DPA, and DHA intake found in our analysis may reflect changes in food consumption patterns.
Analyses of DPA intake in other countries demonstrate distinct variations in intake and provide valuable comparisons for our findings regarding US intake. Similarly low DPA intakes were found in the Netherlands (10 mg/d) [22], Belgium (25.3 mg/d) [23], the UK (37.1 mg/d) [18], Canada (40 mg/d for men and 30 mg/d for women) [48] and one study in Japan (10 mg/d) [20]. However, it should be noted that much higher n-3 fatty acid intakes are typically found in Japanese populations, which is consistent with the highest DPA intake (106 mg/d for men and 85 mg/d for women) reported by Kuriki et al. [19]. Higher intakes were also reported in France (75 mg/d for men and 56 mg/d for women) [17], Norway (70 mg/d) [21], and Australia (71 mg/d) [14]. In Japan, France, and Norway, this may be due to a higher intake of oily fish (and thus higher EPA and DHA, as well). Higher DPA intake in Australia has been attributed to the consumption of meat, poultry, and game—which accounted for 73% of DPA intake and 43% of total long-chain n-3 fatty acid intake [14]. Although meat consumption is higher in the US than Australia, the higher DPA intake in Australia is likely due in part to the predominance of grassfed beef, which contains a greater proportion of DPA [14, 62] compared to grain-fed beef in the US. However, red meat may provide an alternative means of increasing DPA intake in the US given the consistently low intake of oily fish by the majority of the US population [9]. Numerous barriers may prevent individuals from following recommendations to regularly consume oily fish, including: personal preferences (e.g., ethical or environmental concerns, aversion to eating fish), unfamiliarity with seafood preparation and cooking methods, cost and/or availability in the local food environment, food allergies, a vegetarian or vegan dietary pattern, concerns about depleting fish stocks, and a perceived risk of pollutants. Red meat (e.g., beef and lamb) is the richest terrestrial source of DPA and is consumed more frequently than oily fish in the US. This may offer a potential means of increasing DPA intake in the US, but it should be noted that DPA-rich grass-fed beef [62, 63] may not be widely available and/or is more costly in the US. The potential benefits of increasing DPA intake via the consumption of red meat should be weighed with the negative impacts of consuming higher-than-recommended quantities. Current recommendations outlined by the US Department of Agriculture (USDA) Dietary Guidelines for Americans advise that red meat consumption should not exceed 26 oz-equivalents/wk and that saturated fat intake remain below 10% of total daily caloric intake [5].
Taken together, our results provide a key assessment of US DPA intake relative to that of EPA and DHA, along with potential insights regarding the relationship between dietary intake and plasma concentrations of EPA, DPA, and DHA. Similar to EPA and DHA intake, the general US population consumes very little DPA. However, DPA intake significantly increased during the 2009–2014 period, whereas EPA and DHA intake significantly decreased. DPA intake differed significantly according to age and race/ethnicity in this study population. Additional analyses are needed to establish whether specific population subgroups may be more likely to have low intakes. Clinical supplementation studies are needed, but there is increasing evidence to suggest that DPA is a bioactive n-3 fatty acid with both independent and shared effects with EPA and DHA on health outcomes. In conjunction with previous findings, the relationships between dietary intake and plasma concentrations of EPA, DPA, and DHA in our analysis provide insight into the metabolism of DPA and potential inter-conversion of these fatty acids. Additional research is needed to further characterize DPA intake in the US and clarify potential implications for health outcomes.
Strengths and Limitations
This assessment was conducted using a large, representative sample of the US population and provides the first comprehensive assessment of DPA intake in the US.
Furthermore, we analyzed changes in n-3 consumption patterns over time using 12 years of NHANES data. Age, sex, and race/ethnicity subgroups were also analyzed, but patterns of intake in these subgroups may vary in different populations/countries. The 24-hour dietary recall method is considered sufficient for accurately measuring mean dietary intake on the population level as it produces less systematic error and is less likely to alter eating behavior (compared to a Food Frequency Questionnaire), is less burdensome, relies only on short-term memory, and can overcome random error associated with day-to-day fluctuations in intake if days of the week are evenly represented in the data [64]. However, 24-hour recalls are not a reliable indicator of an individual’s habitual dietary intake (may miss days of fish consumption) and may be prone to self-reporting bias (e.g., underreporting intake). Additionally, Howe et al. found substantial underreporting of the DPA content of foods in Australia prior to using an updated fatty acid composition database [14, 16]. Thus, the minimal DPA content in current USDA food composition tables may reflect similarly imprecise fatty acid data or true differences in the n-3 fatty acid content of foods, such as grass-fed versus grain-fed beef [14]. However, if DPA intake is being underreported due to imprecise fatty acid databases, it is unlikely that the magnitude of this difference would substantially alter our primary finding that DPA intake (and total long-chain n-3 fatty acid intake) in the US remains far below recommended values. NHANES does not differentiate between the n-3 and n-6 forms of DPA; however, n-6 DPA content is much lower in most tissues [6] and likely would not alter our findings if the DPA values reported in WWEIA include both the n-3 and n-6 forms. Plasma fatty acid concentrations were only available from one NHANES cycle (2003–2004) and additional analyses are needed to confirm the relationships between dietary intake and plasma fatty acid concentrations observed in this sample.
Abbreviations:
- AHA
American Heart Association
- CVD
cardiovascular disease
- DHA
docosahexaenoic acid
- DPA
docosapentaenoic acid
- EPA
eicosapentaenoic acid
- DGA
Dietary Guidelines for Americans
- NHANES
National Health and Nutrition Examination Survey
- WWEIA
What We Eat In America
Footnotes
Conflict of Interest
The contents are solely the responsibility of the authors. All authors take responsibility for the manuscript’s final content. All of the authors have no conflicts of interest to declare.
References
- 1.Calder PC (2015) Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta 1851: 469–484 [DOI] [PubMed] [Google Scholar]
- 2.Kris-Etherton PM, Harris WS, and Appel LJ (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106: 2747–2757 [DOI] [PubMed] [Google Scholar]
- 3.Kris-Etherton PM, Harris WS, Appel LJ, and Committee AN (2003) Omega-3 fatty acids and cardiovascular disease new recommendations from the American Heart Association. Arteriosclerosis, thrombosis, and vascular biology 23: 151–152 [DOI] [PubMed] [Google Scholar]
- 4.Vannice G, and Rasmussen H (2014) Position of the academy of nutrition and dietetics: dietary fatty acids for healthy adults. J Acad Nutr Diet 114: 136–153 [DOI] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services and U.S. Department of Agriculture, 2015–2020 Dietary Guidelines for Americans, 2015.
- 6.Kaur G, Cameron-Smith D, Garg M, and Sinclair AJ (2011) Docosapentaenoic acid (22:5n-3): a review of its biological effects. Prog Lipid Res 50: 28–34 [DOI] [PubMed] [Google Scholar]
- 7.Skulas-Ray A, Flock M, Richter C, Harris W, West S, and Kris-Etherton P (2015) Red Blood Cell Docosapentaenoic Acid (DPA n-3) is Inversely Associated with Triglycerides and C-reactive Protein (CRP) in Healthy Adults and Dose-Dependently Increases Following n-3 Fatty Acid Supplementation. Nutrients 7: 6390–6404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaur G, Guo XF, and Sinclair AJ (2016) Short update on docosapentaenoic acid: a bioactive longchain n-3 fatty acid. Curr Opin Clin Nutr Metab Care 19: 88–91 [DOI] [PubMed] [Google Scholar]
- 9.Papanikolaou Y, Brooks J, Reider C, and Fulgoni VL 3rd (2014) U.S. adults are not meeting recommended levels for fish and omega-3 fatty acid intake: results of an analysis using observational data from NHANES 2003–2008. Nutr J 13: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richter CK, Bowen KJ, Mozaffarian D, Kris-Etherton PM, and Skulas-Ray AC (2017) Total LongChain n-3 Fatty Acid Intake and Food Sources in the United States Compared to Recommended Intakes: NHANES 2003–2008. Lipids [DOI] [PubMed] [Google Scholar]
- 11.U.S. Department of Agriculture, Agricultural Research Service, Nutrient Intakes from Food and Beverages: Mean Amounts Consumed per Individual, by Gender and Age, in What We Eat in America, NHANES 2011–20122014.
- 12.Zhang Z, Fulgoni VL, Kris-Etherton PM, and Mitmesser SH (2018) Dietary Intakes of EPA and DHA Omega-3 Fatty Acids among US Childbearing-Age and Pregnant Women: An Analysis of NHANES 2001–2014. Nutrients 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson M, Hein N, Hanson C, Smith LM, Anderson-Berry A, Richter CK, Stessy Bisselou K, Kusi Appiah A, Kris-Etherton P, Skulas-Ray AC, and Nordgren TM (2019) Omega-3 Fatty Acid Intake by Age, Gender, and Pregnancy Status in the United States: National Health and Nutrition Examination Survey 2003(–)2014. Nutrients 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howe P, Meyer B, Record S, and Baghurst K (2006) Dietary intake of long-chain omega-3 polyunsaturated fatty acids: contribution of meat sources. Nutrition 22: 47–53 [DOI] [PubMed] [Google Scholar]
- 15.Meyer BJ (2016) Australians are not Meeting the Recommended Intakes for Omega-3 Long Chain Polyunsaturated Fatty Acids: Results of an Analysis from the 2011–2012 National Nutrition and Physical Activity Survey. Nutrients 8: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer BJ, Mann NJ, Lewis JL, Milligan GC, Sinclair AJ, and Howe PR (2003) Dietary intakes and food sources of omega-6 and omega-3 polyunsaturated fatty acids. Lipids 38: 391–398 [DOI] [PubMed] [Google Scholar]
- 17.Astorg P, Arnault N, Czernichow S, Noisette N, Galan P, and Hercberg S (2004) Dietary intakes and food sources of n-6 and n-3 PUFA in French adult men and women. Lipids 39: 527–535 [DOI] [PubMed] [Google Scholar]
- 18.Givens D, and Gibbs R (2006) Very long chain n-3 polyunsaturated fatty acids in the food chain in the UK and the potential of animal-derived foods to increase intake. Nutrition Bulletin 31: 104–110 [Google Scholar]
- 19.Kuriki K, Nagaya T, Tokudome Y, Imaeda N, Fujiwara N, Sato J, Goto C, Ikeda M, Maki S, Tajima K, and Tokudome S (2003) Plasma concentrations of (n-3) highly unsaturated fatty acids are good biomarkers of relative dietary fatty acid intakes: a cross-sectional study. The Journal of nutrition 133: 3643–3650 [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi M, Sasaki S, Kawabata T, Hasegawa K, and Tsugane S (2003) Validity of a selfadministered food frequency questionnaire used in the 5-year follow-up survey of the JPHC Study Cohort I to assess fatty acid intake: comparison with dietary records and serum phospholipid level. J Epidemiol 13: S64–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johansson LR, Solvoll K, Bjorneboe GE, and Drevon CA (1998) Intake of very-long-chain n-3 fatty acids related to social status and lifestyle. European journal of clinical nutrition 52: 716–721 [DOI] [PubMed] [Google Scholar]
- 22.Otto SJ, van Houwelingen AC, Badart-Smook A, and Hornstra G (2001) Changes in the maternal essential fatty acid profile during early pregnancy and the relation of the profile to diet. The American Journal of Clinical Nutrition 73: 302–307 [DOI] [PubMed] [Google Scholar]
- 23.Sioen IA, Pynaert I, Matthys C, De Backer G, Van Camp J, and De Henauw S (2006) Dietary intakes and food sources of fatty acids for Belgian women, focused on n-6 and n-3 polyunsaturated fatty acids. Lipids 41: 415–422 [DOI] [PubMed] [Google Scholar]
- 24.Howe P, Buckley J, and Meyer B (2007) Long-chain omega-3 fatty acids in red meat. Nutrition & Dietetics 64: S135–S139 [Google Scholar]
- 25.Ervin RB, Wright JD, Wang CY, and Kennedy-Stephenson J (2004) Dietary intake of fats and fatty acids for the United States population: 1999–2000. Adv Data: 1–6 [PubMed] [Google Scholar]
- 26.U.S. Department of Agriculture, Agricultural Research Service, Nutrient Intakes from Food: Mean Amounts Consumed per Individual, One Day, 2001–2002, 2002. [Google Scholar]
- 27.U.S. Department of Agriculture, Agricultural Research Service, Nutrient Intakes from Food: Mean Amounts Consumed per Individual, One day, 2003–2004, 2004. [Google Scholar]
- 28.U.S. Department of Agriculture, Agricultural Research Service, Nutrient Intakes from Food: Mean Amounts Consumed per Individual, by Gender and Age, in the United States, 2005–2006, in What We Eat in America, NHANES 2005–2006 2006. [Google Scholar]
- 29.U.S. Department of Agriculture, Agricultural Research Service, Nutrient Intakes from Food: Mean Amounts Consumed per Individual, by Gender and Age, in the United States, 2007–2008, in What We Eat in America, NHANES 2007–2008 2008. [Google Scholar]
- 30.U.S. Department of Agriculture, Agricultural Research Service, Nutrient Intakes from Food: Mean Amounts Consumed per Individual, by Gender and Age, in the United States, 2009–2010, in What We Eat in America, NHANES 2009–2010 2010. [Google Scholar]
- 31.U.S. Department of Agriculture, Agricultural Research Service, Nutrient Intakes from Food: Mean Amounts Consumed per Individual, by Gender and Age, in the United States, 2011–2012, in What We Eat in America, NHANES 2011–2012 2012. [Google Scholar]
- 32.U.S. Department of Agriculture, Agricultural Research Service, Nutrient Intakes from Food: Mean Amounts Consumed per Individual, by Gender and Age, in the United States, 2013–2014, in What We Eat in America, NHANES 2013–2014 2014. [Google Scholar]
- 33.Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, and Dostal J (2013) National health and nutrition examination survey: plan and operations, 1999–2010. Vital Health Stat 1: 1–37 [PubMed] [Google Scholar]
- 34.Lagerstedt SA, Hinrichs DR, Batt SM, Magera MJ, Rinaldo P, and McConnell JP (2001) Quantitative determination of plasma c8–c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol Genet Metab 73: 38–45 [DOI] [PubMed] [Google Scholar]
- 35.Mozaffarian D, Lemaitre RN, King IB, Song X, Huang H, Sacks FM, Rimm EB, Wang M, and Siscovick DS (2013) Plasma phospholipid long-chain omega-3 fatty acids and total and causespecific mortality in older adults: a cohort study. Annals of internal medicine 158: 515–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Q, Ma J, Campos H, Rexrode KM, Albert CM, Mozaffarian D, and Hu FB (2008) Blood concentrations of individual long-chain n-3 fatty acids and risk of nonfatal myocardial infarction. The American Journal of Clinical Nutrition 88: 216–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Oliveira Otto MC, Wu JH, Baylin A, Vaidya D, Rich SS, Tsai MY, Jacobs DR Jr., and Mozaffarian D (2013) Circulating and dietary omega-3 and omega-6 polyunsaturated fatty acids and incidence of CVD in the Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2: e000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oda E, Hatada K, Katoh K, Kodama M, Nakamura Y, and Aizawa Y (2005) A case-control pilot study on n-3 polyunsaturated fatty acid as a negative risk factor for myocardial infarction. Int Heart J 46: 583–591 [DOI] [PubMed] [Google Scholar]
- 39.Rissanen T, Voutilainen S, Nyyssonen K, Lakka TA, and Salonen JT (2000) Fish oil-derived fatty acids, docosahexaenoic acid and docosapentaenoic acid, and the risk of acute coronary events: the Kuopio ischaemic heart disease risk factor study. Circulation 102: 2677–2679 [DOI] [PubMed] [Google Scholar]
- 40.Reinders I, Virtanen JK, Brouwer IA, and Tuomainen TP (2012) Association of serum n-3 polyunsaturated fatty acids with C-reactive protein in men. Eur J Clin Nutr 66: 736–741 [DOI] [PubMed] [Google Scholar]
- 41.Micallef MA, Munro IA, and Garg ML (2009) An inverse relationship between plasma n-3 fatty acids and C-reactive protein in healthy individuals. Eur J Clin Nutr 63: 1154–1156 [DOI] [PubMed] [Google Scholar]
- 42.Kaur G, Begg DP, Barr D, Garg M, Cameron-Smith D, and Sinclair AJ (2010) Short-term docosapentaenoic acid (22:5 n-3) supplementation increases tissue docosapentaenoic acid, DHA and EPA concentrations in rats. Br J Nutr 103: 32–37 [DOI] [PubMed] [Google Scholar]
- 43.Dai XW, Chen YM, Zeng FF, Sun LL, Chen CG, and Su YX (2016) Association between n-3 polyunsaturated fatty acids in erythrocytes and metabolic syndrome in Chinese men and women. Eur J Nutr 55: 981–989 [DOI] [PubMed] [Google Scholar]
- 44.Meyer BJ, Lane AE, and Mann NJ (2009) Comparison of seal oil to tuna oil on plasma lipid levels and blood pressure in hypertriglyceridaemic subjects. Lipids 44: 827–835 [DOI] [PubMed] [Google Scholar]
- 45.Baylin A, and Campos H (2006) The use of fatty acid biomarkers to reflect dietary intake. Current opinion in lipidology 17: 22–27 [DOI] [PubMed] [Google Scholar]
- 46.Harris WS (2008) The omega-3 index as a risk factor for coronary heart disease. The American Journal of Clinical Nutrition 87: 1997S–2002S [DOI] [PubMed] [Google Scholar]
- 47.Sun Q, Ma J, Campos H, Hankinson SE, and Hu FB (2007) Comparison between plasma and erythrocyte fatty acid content as biomarkers of fatty acid intake in US women. The American Journal of Clinical Nutrition 86: 74–81 [DOI] [PubMed] [Google Scholar]
- 48.Garneau V, Rudkowska I, Paradis AM, Godin G, Julien P, Perusse L, and Vohl MC (2012) Omega-3 fatty acids status in human subjects estimated using a food frequency questionnaire and plasma phospholipids levels. Nutr J 11: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dalli J, Colas RA, and Serhan CN (2013) Novel n-3 immunoresolvents: structures and actions. Scientific reports 3: 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weylandt KH (2016) Docosapentaenoic acid derived metabolites and mediators - The new world of lipid mediator medicine in a nutshell. Eur J Pharmacol 785: 108–115 [DOI] [PubMed] [Google Scholar]
- 51.Vik A, Dalli J, and Hansen TV (2017) Recent advances in the chemistry and biology of antiinflammatory and specialized pro-resolving mediators biosynthesized from n-3 docosapentaenoic acid. Bioorg Med Chem Lett 27: 2259–2266 [DOI] [PubMed] [Google Scholar]
- 52.Markworth JF, Kaur G, Miller EG, Larsen AE, Sinclair AJ, Maddipati KR, and Cameron-Smith D (2016) Divergent shifts in lipid mediator profile following supplementation with n-3 docosapentaenoic acid and eicosapentaenoic acid. Faseb J 30: 3714–3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benistant C, Achard F, Ben Slama S, and Lagarde M (1996) Docosapentaenoic acid (22:5,n-3): metabolism and effect on prostacyclin production in endothelial cells. Prostaglandins Leukot Essent Fatty Acids 55: 287–92 [DOI] [PubMed] [Google Scholar]
- 54.Miller E, Kaur G, Larsen A, Loh SP, Linderborg K, Weisinger HS, Turchini GM, Cameron-Smith D, and Sinclair AJ (2013) A short-term n-3 DPA supplementation study in humans. Eur J Nutr 52: 895–904 [DOI] [PubMed] [Google Scholar]
- 55.Cao J, Schwichtenberg KA, Hanson NQ, and Tsai MY (2006) Incorporation and clearance of omega-3 fatty acids in erythrocyte membranes and plasma phospholipids. Clin Chem 52: 2265–2272 [DOI] [PubMed] [Google Scholar]
- 56.Katan MB, Deslypere JP, van Birgelen AP, Penders M, and Zegwaard M (1997) Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 38: 2012–2022 [PubMed] [Google Scholar]
- 57.Mori TA, Burke V, Puddey IB, Watts GF, O’Neal DN, Best JD, and Beilin LJ (2000) Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. The American Journal of Clinical Nutrition 71: 1085–1094 [DOI] [PubMed] [Google Scholar]
- 58.von Schacky C, and Weber PC (1985) Metabolism and effects on platelet function of the purified eicosapentaenoic and docosahexaenoic acids in humans. J Clin Invest 76: 2446–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krul ES, Lemke SL, Mukherjea R, Taylor ML, Goldstein DA, Su H, Liu P, Lawless A, Harris WS, and Maki KC (2012) Effects of duration of treatment and dosage of eicosapentaenoic acid and stearidonic acid on red blood cell eicosapentaenoic acid content. Prostaglandins Leukot Essent Fatty Acids 86: 51–59 [DOI] [PubMed] [Google Scholar]
- 60.Brown AJ, Pang E, and Roberts DC (1991) Persistent changes in the fatty acid composition of erythrocyte membranes after moderate intake of n-3 polyunsaturated fatty acids: study design implications. The American Journal of Clinical Nutrition 54: 668–673 [DOI] [PubMed] [Google Scholar]
- 61.Byelashov OA, Sinclair AJ, and Kaur G (2015) Dietary sources, current intakes, and nutritional role of omega-3 docosapentaenoic acid. Lipid Technol 27: 79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mann N, Ponnampalam E, Yep Y, and Sinclair A (2003) Feeding regimes affect fatty acid composition in Australian beef cattle. Asia Pacific journal of clinical nutrition 12 [Google Scholar]
- 63.Droulez V, Williams P, Levy G, Stobaus T, and Sinclair A (2006) Composition of Australian red meat 2002. 2 Fatty acid profile. [Google Scholar]
- 64.Ahluwalia N, Dwyer J, Terry A, Moshfegh A, and Johnson C (2016) Update on NHANES Dietary Data: Focus on Collection, Release, Analytical Considerations, and Uses to Inform Public Policy. Adv Nutr 7: 121–134 [DOI] [PMC free article] [PubMed] [Google Scholar]