Abstract
Tumor cells can vary epigenetically during ionizing irradiation (IR) treatment. These epigenetic variegations can influence IR response and shape tumor aggressiveness. However, epigenetic disturbance of histones after IR, implicating in IR responsiveness, has been elusive. Here, we investigate whether altered histone modification after IR can influence radiation responsiveness. The oncogenic CXCL12 mRNA and protein were more highly expressed in residual cancer cells from a hepatoma heterotopic murine tumor microenvironment and coculture of human hepatoma Huh7 and normal IMR90 cells after radiation. H3K4 methylation was also enriched and H3K9 methylation was decreased at its promoter region. Accordingly, invasiveness and the subpopulation of aggressive CD133+/CD24− cells increased after IR. Histone demethylase inhibitor IOX1 attenuated CXCL12 expression and the malignant subpopulation, suggesting that responses to IR can be partially mediated via histone modifications. Taken together, radiation-induced histone alterations at the CXCL12 promoter in hepatoma cells are linked to CXCL12 upregulation and increased aggressiveness in the tumor microenvironment.
Keywords: CXCL12, histone modification, malignancy, radiation, tumor microenvironment
INTRODUCTION
Radiation is one of the principal methods of cancer treatment, but its effectiveness is often restricted by tumor resistance and aggressiveness. Resistance and aggressiveness can be intrinsic to the tumor before radiation treatment or acquired during treatment. A defective DNA damage checkpoint is a common intrinsic factor related to radiation resistance which allows the cell to bypass DNA repair to continue growing and proliferating (Bao et al., 2006). Acquired resistance and aggressiveness can develop after receiving radiation treatment in tumors that were initially sensitive but adapt to become resistant and aggressive via mutations arising during treatment or various other responses such as altered expression of the therapeutic target and activation of alternative compensatory pathways. Moreover, tumors can have a wide spectrum of genetic and epigenetic heterogeneity, so radiation resistance and aggressiveness can arise through radiation-induced selection of small resistant subpopulations that become intensified. Tumors have many adaptive responses to the continuously changing tumor microenvironment during radiation treatment. Most use quick and transient epigenetic mechanisms, but genetic processes can allow tumors to stably respond to radiation and survive.
As our understanding of molecular signatures and genotypes of radiation resistant tumors has advanced, the mechanisms of resistance to radiation learned from earlier studies help predict responsiveness to radiation treatment and elucidate mechanisms of resistance to combinational radiation treatment with molecular targeted agents such as PARP inhibitors (Guillot et al., 2014). Nevertheless, tumors are often adaptable, and the inactivation of death pathways and the activation of survival pathways can lead to aggressiveness. Importantly, epigenetic changes and the influence of the local tumor microenvironment can contribute to malignancy. More recently, treatment failure was attributed to either a small subpopulation of malignant tumor cells or the presence of cancer stem cells (CSCs) and their increased population (Hong et al., 2016; Rich, 2007).
The epigenetic approach in combination with genomic and proteomic techniques is more widely applied to identification of novel genes and signaling pathways that are involved in determining the responsiveness of tumors to a specific treatment. Moreover, epigenetic processes in tumors are critical mechanisms that regulate aspects of cancer cell biology including responsiveness. Epigenetic mechanisms regulate gene expression primarily through DNA methylation, histone modifications such as acetylation, methylation, phosphorylation, and ubiquitination, and miRNA. DNA hypo- and hyper-methylation at promoter regions can lead to transcriptional induction and repression, respectively. Similarly, histone modifications can regulate gene transcription. For example, active promoters are marked with increased trimethylation of H3 at lysine 4 (H3K4me3) or acetylated H3 at lysine 9 (H3K9ac), and inactive promoters are marked with increased trimethylated H3 at lysine 27 (H3K27me3) or lysine 9 (H3K9me3) (Choi and Lee, 2013).
Earlier epigenetic studies largely focusing on DNA methylation of tumors resulted in a substantial increase in our ability to identify genes that are important for the responsiveness of tumors to certain treatment settings and aggressiveness. Thus, there have been limited studies of the influence of ionizing radiation on histone modifications at particular genes and gene expression. To overcome malignancy and increase the effectiveness of radiation treatment, we analyzed histone modifications at the promoters of specific genes linked to responsiveness and malignancy, focusing on differential expression before and after ionizing radiation. For example, the CXCL12 (stromal cell-derived factor-1, SDF-1)-CXC receptor 4/7 (CXCR4/7) axis, which plays a biologically relevant role in tumor progression, angiogenesis, metastasis, proliferation, and survival (Kryczek et al., 2007; Teicher and Fricker, 2010), is highly expressed in multiple cell types including hematopoietic stem cells, lymphocytes, endothelial and epithelial cells, and cancer cells (Kryczek et al., 2007).
Here, we found upregulation of CXCL12 and altered histone modifications at its promoter regions in response to ionizing radiation in a hepatoma heterotopic murine tumor microenvironment and human xenograft and coculture systems. Our results reveal a close relationship between the histone methylation profile at the promoter and expression of the CXCL12 gene. Notably, we indicate that the induced level of CXCL12 may contribute to acquired invasiveness and stemness. Collectively, our results show that CXCL12 upregulation mediated by histone modification dynamics at the promoter in the residual hepatoma cells after radiation treatment may be at least partially responsible for the acquired adaptation to radiation and aggressiveness during treatment in tumor cells. Thus, epigenetic modulation can be a promising therapeutic option to refine radiation treatment.
MATERIALS AND METHODS
A murine in vivo heterotopic tumor model and radiation administration
A spontaneous murine hepatocarcinoma (HCa-I; generously provided by Luka Milas, University of Texas MD Anderson Cancer Center) was used in this study as a murine in vivo heterotopic tumor model. A heterotopic tumor model was generated in the right thigh of six-week-old male syngeneic C3H/HeN mice, and radiation was delivered as described in our previous study (Lee et al., 2016). Briefly, 1 × 106 HCa-I cells were injected into the right thigh muscles of mice. Two weeks after implantation, three mice were randomly assigned to a radiation treatment group or a control group. Radiation was administered to the implanted tumor as a single dose of 10 Gy with 320 kV X-ray using the X-RAD 320 (Precision X-ray, USA). Irradiated and non-irradiated tumor samples were obtained 1 or 3 days after radiation. All animal experiments were performed in accordance with the Animal Research Committee’s Guidelines at Yonsei University Medical College, and all facilities were approved by the Association for Assessment and Accreditation of Laboratory Animal Care (IACUC-2012-0177).
Establishment of human hepatoma xenograft, coculture models, radiation, and treatment with DNA damaging agents
The human hepatocarcinoma cell line Huh7, normal lung fibroblast cell line IMR90, and WI38 cells (purchased from the American Type Culture Collection) were cultured in DMEM (Welgene, Korea) supplemented with 10% fetal bovine serum (FBS; HyClone, USA) and 1% penicillin/streptomycin (Gibco, USA). Subcutaneous xenotransplantation of hepatoma Huh7 cells (1 × 107 cells) into Balb/c nude mice were generated. The implanted tumor was locally exposed to radiation of 5 Gy. To co-culture two cell lines, 1 × 105 Huh7 cells were cultured in a transmembrane insert (0.3 μm pore; SPL, Korea) for 6 h and the insert was translocated into a well in a 6-well plate where IMR90 cells were cultured until approximately 90% confluent. They grew for another 24 h in the presence of histone demethylase inhibitor (5 μM IOX-1 or 10 μM GSK-J1), histone methyltransferase inhibitor (0.5 μM chaetocin), histone deacetylase inhibitor (0.1 μM TSA), or their vehicles. Huh7 cells were irradiated with a dose of 5 or 10 Gy. For three-dimensional (3D) culture, the grown Huh-7 cells were detached from culture plates using AccumaxTM (Millipore, USA) DPBS and approximately 1 × 106 cells were seeded on a 35 mm-culture dish coated with hydrophilic polymer (PrimeSurface; Akita Sumitomo Bakelite, Japan). Spheroids formed after 24 h before being irradiated. For induction DNA damage, Huh7 cells were treated with 50 μM etoposide (Sigma, USA) or 50 μM phleomycin (Sigma) for 2 h.
RNA isolation and RT-PCR/qRT-PCR
Total RNA was isolated from tumor tissue and normal peritumor liver tissue cells obtained from the heterotopic tumor model and cultured Huh7 cells using RNeasy Mini Kit (Qiagen, USA) according to the manufacturer’s instructions. Reverse transcription polymerase chain reaction (RT-PCR) was carried out using 2 μg of total RNA. Quantitative real-time PCR (qRT-PCR) was performed using Maxima SYBR Green (Thermo Scientific, USA) with Rotor Gene Q (Qiagen). The primers used for qRT-PCR are listed in Supplementary Tables S1 and S2.
Quantitation of CXCL12 proteins using ELISA
The amount of CXCL12 proteins were measured using ELISA (R&D System, USA) according to manufacturer’s protocol.
Chromatin immunoprecipitation
Chromatin immunoprecipitation was carried out using EZ-ChIP Kit according to manufacturer’s protocols (Millipore) with antibodies against H3K4me3, H3K9ac, H3K9me3, or without antibody (input). The immunoprecipitated and input DNAs were analyzed using qPCR. The primer sequences are described in Supplementary Table S2.
Immunoblotting
Cell lysates in RIPA buffer (50 mM Tris-Hcl pH 7.5, 150 mM NaCl, 1% NP-40, 0.1% SDS, protease inhibitor) were immunoblotted using anti-CCAAT/enhancer-binding protein β (c/EBPβ) antibody (Abcam, UK). Immunoreactive bands were normalized to the control anti-actin (Santa Cruz Biotechnology, USA) band.
Invasion assay
Matrigel invasion chambers (24 well and 8 μm pore; BD Biosciences, USA) were used for the invasion assay. 0.5 ml of cell suspensions in culture medium containing 5 × 104 cells/ml were added to each 24 well invasion chamber. Either medium or medium supplemented with the indicated dose of recombinant CXCL12 (Thermo Fisher Scientific) was added to the lower chamber. Chambers were incubated overnight in a humidified incubator at 37°C and 5% CO2. Non-invading cells on the top of the Matrigel matrix in the upper chamber were removed twice using a cotton swab (Sun et al., 2018). The cells on the lower surface of the membrane were stained with hematoxylin-eosin for 5 min and the invaded cells were counted under the microscope in several fields in triplicate (Carl Zeiss, USA). Data was expressed as the percent invasion through the Matrigel matrix and membrane relative to the migration of cells through the uncoated membrane.
C/EBPβ and CXCL12 knockdown and transfection
Double-stranded short hairpin RNA for c/EBPβ was generated using the pSUPER.retro.puro vector (Oligoengine, USA). The siRNA primers were designed to target c/EBPβ (open reading frame, 5′-CCAAGAAGACCGTGGACAA-3′; 3′-UTR, 5′-GAAGAAACGTCTATGTGTA-3′) and CXCL12 (5′-UTR, 5′-CCATGAACGCCAAGGTCGT-3′). Transfections were performed using an Effectene kit (Qiagen) for knock-down in mammalian cells.
Flow cytometry analysis of CD133+/CD24− expression
Expression of CD133 and CD24 in Huh7 cells was determined using flow cytometry at 3 day after γ-irradiation. After staining with anti-CD133-FITC and CD24-PE antibodies (Miltenyi Biotec, Germany), the cells were analyzed using flow cytometry (BD FACSAria III; BD Biosciences). Data are shown as the mean ± SD (n = 3).
Statistical analysis
All experimental data were obtained in at least triplicate unless otherwise mentioned and are presented as mean ± standard deviation. Statistical comparison by analysis of variance was performed at a significance level of P < 0.05 based on the ANOVA t-test and Student’s t-test.
RESULTS
Transcriptional response to radiation in tumor microenvironment models
Both the in vivo murine heterotopic tumor microenvironment model (Lee et al., 2016) and human xenograft tumor model and in vitro coculture system were established to investigate responses to radiation. To analyze the expression of genes, which plays a biologically relevant role in tumor progression, angiogenesis, metastasis, proliferation and survival (Kryczek et al., 2007; Teicher and Fricker, 2010), in murine tumor microenvironment in response to radiation, we used transplantable murine tumor HCa-I cells that are highly radioresistant with a radiation dose yielding a 50% tumor cure rate above 80 Gy (Lee et al., 2016). Expression of the CXCL12-CXCR4/7 axis that is highly expressed in multiple cell types including hematopoietic stem cells and cancer cells (Kryczek et al., 2007), and contributes to tumor growth and spread (Chatterjee et al., 2014; Domanska et al., 2013), were compared before and after γ-irradiation, using HCa-1 cells in a heterotopic tumor model. Higher expression of CXCL12-CXCR4/7 was observed in surviving HCa-1 cells in the murine heterotopic tumor microenvironment after γ-irradiation than in the untreated HCa-1 cells (Fig. 1A). The expression of CXCL12 was prominently increased in surviving HCa-1 cells by approximately 17-fold 72 h after irradiation, and mRNA expression levels of its receptor CXCR4/7 increased by approximately two-fold 72 h after irradiation (Fig. 1A), suggesting that radiation induces expression of the CXCL12-CXCR4/7 axis in the tumor microenvironment. Vimentin, encoded by the VIM gene and a marker of mesenchymal cells or cells undergoing an epithelial-to-mesenchymal transition during metastatic progression, and another oncogenic MTA1 gene were approximately 3-fold higher, while expression of c-myc, CDH1, and CCND1 and other genes varied little compared to the levels in HCa-1 cells from an untreated heterotopic tumor microenvironment (Supplementary Fig. S1). Thus, these expression data in the heterotopic tumor microenvironment indicate that expression of some but not most genes in cancer cells are affected by irradiation.
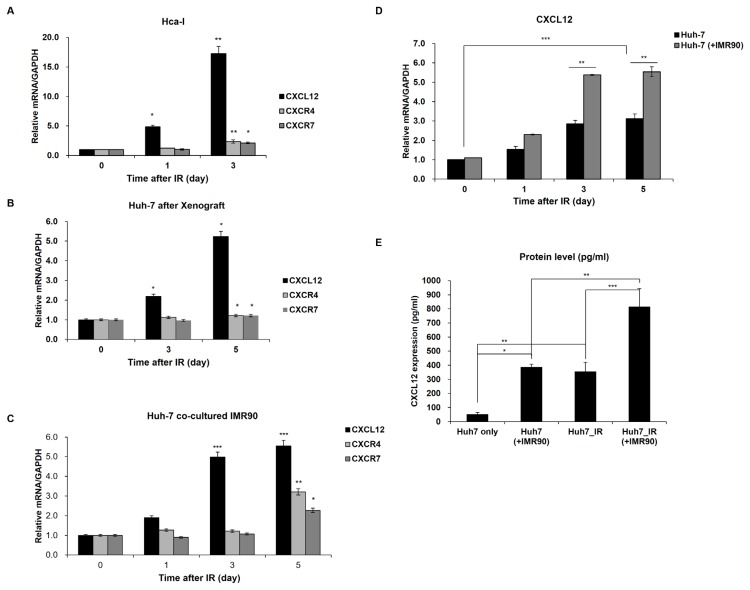
Fig. 1. Radiation induces altered transcription in an in vivo murine heterotopic tumor microenvironment (A), a human hepatoma xenograft (B), and coculture system (C–E).
(A) Transcript levels of CXCL12-CXCR4/7 axis genes were elevated in the heterotopic tumor model after radiation. mRNA levels of CXCL12-CXCR4/7 genes were measured approximately 1 or 3 days after exposure to radiation. The mRNA level of the control sample without radiation was arbitrarily set to one. (B) In the human hepatoma xenograft model, radiation enhanced the CXCL12-CXCR4/7 genes. After irradiation, the mRNA levels were measured 3 to 5 days later in the xenografted Huh7 cells. The levels of the unirradiated samples were arbitrarily set to one. (C) Expression of CXCL12-CXCR4/7 pathway genes increased 3 to 5 days after radiation in human hepatoma Huh7 cells cocultured with IMR90 cells. (D) The coculture (Huh7 + IMR90) effect on the radiation-induced CXCL12 expression was analyzed compared to the single-cultured Huh7 cells. Huh7 cells were grown in single- (Huh7 only) or coculture with IMR90 cells (Huh7 + IMR90) and exposed to radiation (IR) or not. The CXCL12 mRNA levels in the unirradiated single- or cocultured Huh7 cells were set to one. (E) The radiation or coculture-induced CXCL12 proteins were evaluated using ELISA. *P < 0.05, **P < 0.01, ***P < 0.001.
To compare the transcriptional responses to radiation in the murine and human tumor microenvironment models, the human tumor xenograft model, and the co-culture system in a plate with insets were used. Human hepatoma Huh7 cells were transplanted into immunocompromised mice or co-cultured with normal IMR90 cells in insets. In the human hepatoma xenograft model, radiation apparently induced CXCL12 expression 5.2-fold and slightly induced expression of CXCR4/7 1.2-fold 5 days after radiation compared with that in untreated cells (Fig. 1B). Consistent with radiation-induced CXCL12 expression in the xenograft model, the expression of CXCL12 in the cocultured hepatocarcinoma Huh7 cells 3 days after γ-irradiation increased approximately 5-fold compared with the level before irradiation (Fig. 1C). In a similar manner, irradiation enhanced the levels of CXCR4/7 transcripts in Huh7 cells approximately 3.3- and 2.3-fold, respectively, 5 days after radiation (Fig. 1C). Along with the CXCL12-CXCR4/7 transcriptional data obtained from the murine heterotopic hepatoma model, the transcriptional expression of the CXCL12-CXCR4/7 axis was induced in response to radiation in the human hepatoma in vivo xenograft model and in vitro coculture model.
Interestingly, CXCL12 mRNA induction by radiation in the cocultured Huh7 cells appeared to be larger than in the single cultured Huh7 cells (Fig. 1D), indicating that coculture of cancer and normal cells, mimicking tumor microenvironment, affects CXCL12 expression, presumably having benefits for tumor cells. In parallel with the CXCL12 mRNA data, the amounts of CXCL12 protein also increased in the cocultured hepatoma Huh7 cells after γ-irradiation (Fig. 1E). The level of CXCL12 protein was elevated in Huh7 cells cocultured with normal cells without γ-irradiation (approximately 7.4-fold) compared to the single-cultured Huh7 cells. This level was comparable to the single-cultured and irradiated Huh7 cells (Fig. 1E). Also, radiation further increased the CXCL12 proteins approximately 2.1-fold in the cocultured Huh7 cells compared to the cocultured Huh7 cells without radiation or the single-cultured and irradiated Huh7 cells (Fig. 1E). Based on the radiation-induced CXCL12-CXCR4/7 expression in the murine tumor microenvironment model, the human hepatoma xenograft and coculture models showed that tumor microenvironmental conditions or radiation significantly increases CXCL12-CXCR4/7 expression.
Epigenetic histone modifications at the CXCL12 promoter in response to radiation in tumor microenvironment models
Since the CXCL12-CXCR4/7 pathway is a crucial axis in carcinogenesis and angiogenesis linked to tumor progression (Domanska et al., 2013) and metastasis (Chatterjee et al., 2014), we investigated molecular mechanisms leading to a radiation-induced increase in CXCL12 transcription. First, we tested whether histone modification at its promoter could cause the differential expression of the CXCL12 gene following irradiation. We did this because histone modifications can generally alter gene expression, and histone hypoacetylation has been also reported to contribute to its downregulation (Romain et al., 2017). Thus, modifications at lysine 4 (H3K4) and lysine 9 (H3K9) residues in histone 3 (H3), which are important determinants of transcriptional activity in the CXCL12 promoter, were analyzed using chromatin immunoprecipitation with antibodies against specific H3 modifications in hepatoma cells in the murine and human in vivo/in vitro tumor microenvironments with or without γ-irradiation.
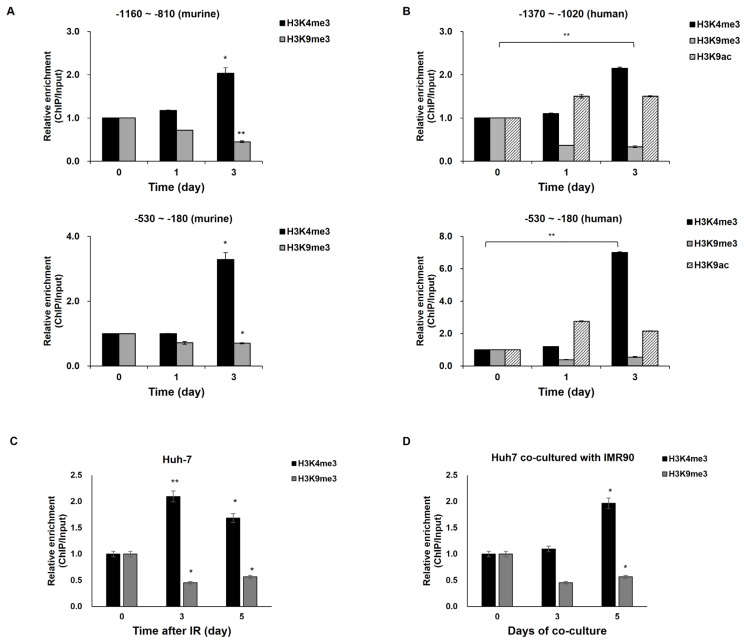
The histone modifications in response to radiation were analyzed at murine and human CXCL12 promoters spanning −2 kb to +1 kb (Supplementary Fig. S2). At five sites within the promoter of murine CXCL12 (−2000 to −1650, −1700 to −1440, −1160 to −810, −530 to −180, and −320 to +20 bps from its transcription start site) in surviving HCa-1 cells after radiotherapy, increased histone tri-methylation at H3K4 (H3K4me3) related to open chromatin mark was observed 3 days after irradiation, but radiation had few effects at the other eight regions (Fig. 2A and Supplementary Fig. S3A). Another histone modification mark for transcriptional activation, acetylation at H3K9 (H3K9ac) increased at two regions (−530 to −180 and −110 to +240) but were little changed at the other regions after irradiation (Fig. 2A and Supplementary Fig. S3B), indicating that H3K9ac increments at the murine CXCL12 promoter are likely to be related to the radiation-induced CXCL12 transcription in the murine tumor microenvironment (Fig. 1A). Also, the transcriptional repressive maker tri-methylation at H3K9 (H3K9me3) decreased at three regions (−1160 to −810, −530 to −180, and −110 to +200); however, no major changes were observed after radiation at the other regions of the CXCL12 promoter in the Hca-1 cells of tumor microenvironment (Fig. 2A and Supplementary Fig. S3C). These results indicate that decreased H3K9me3 is related to radiation-induced CXCL12 transcription. Taken together with all tested histone modifications in response to radiation, the radiation-induced histone modification profile at the murine CXCL12 promoter in the tumor microenvironment (Fig. 2A and Supplementary Fig. S3) appeared to be correlated with CXCL12 upregulation in HCa-1 cells surviving irradiation (Fig. 1A).
Fig. 2. Epigenetic modifications in response to radiation at the CXCL12 promoter in the murine heterotopic hepatoma microenvironment (A), human hepatoma xenograft (B), and coculture (C and D) models.
The levels of the unirradiated samples were arbitrarily set to one. (A) At the murine CXCL12 promoter, alterations in trimethylation at histone 3 lysine 4 (H3K4me3) and lysine 9 (H3K9me3) were monitored 1 to 3 days after radiation. (B) Modifications of H3K4me3, H3K9me3, and acetylation at histone 3 lysine 9 residue (H3K9ac) at the human CXCL12 promoter were evaluated 1 to 3 days after radiation in the xenografted Huh7 cells. (C and D) The experiment in (B) was repeated in single-cultured (C) and cocultured (D) Huh7 cells with or without radiation. Huh7 cells were cultured alone (C; Huh7) or together with IMR90 cells (D; Huh7 co-cultured with IMR90) and treated with or without γ-irradiation. CXCL12 mRNA levels were analyzed 3 or 5 days after irradiation. (E) Huh7 cells were treated with etoposide or phleomycin for 2 h. Three days after treatment with DNA damaging agents, CXCL12 mRNA levels were analyzed and modifications of H3K4me3 and H3K9me3 at the human CXCL12 promoter were evaluated. (F) Huh7 cells were pretreated with 10 μM ATM inhibitor (KU 55933; Sigma) for 30 min, followed by irradiation, CXCL12 mRNA levels were monitored. Modifications in H3K4me3 and H3K9me3 at the human CXCL12 promoter were measured. *P < 0.05, **P < 0.01, ***P < 0.001.
Next, we examined histone modifications at the CXCL12 promoter after radiation in the human tumor microenvironment-mimicking systems. Like the irradiated human tumor microenvironment, higher H3K4me3 modification was detected at two sites (−1160 to −810 and −530 to −180 from its transcription start site) in the human promoter regions of the CXCL12 gene in xenografted Huh7 cells 3 days after irradiation compared to control cells (Fig. 2B and Supplementary Fig. S4A). Also, the transcriptional repressive chromatin mark H3K9me3 was decreased at the sites after irradiation (Fig. 2B and Supplementary Fig. S4B). The increased H3K4me and decreased H3K9me modifications induced by radiation at the CXCL12 promoter in the xenografted Huh7 cells match with active promoters (Choi and Lee, 2013), suggesting that radiation-induced histone modifications at the CXCL12 promoter region may contribute to CXCL12 upregulation.
In further mechanistic studies on human hepatoma Huh7 cells, we decided to focus on the upstream site (−530 to −180) (Figs. 2C and 2D). Consistent with the H3 modifications at the CXCL12 promoter in the irradiated-Huh7 cells of the xenograft model, H3K4me increased and H3K9me decreased at the same CXCL12 promoter site in irradiated Huh7 cells compared to cells before irradiation (Fig. 2C). Because coculture without irradiation could enhance CXCL12 transcription and radiation-induced CXCL12 transcription was likely to be more strongly affected in the coculture, we compared histone modification levels in the coculture to the single-culture (Fig. 2D), in order to see coculture effects on the irradiation-induced histone modifications. The H3K4me3 level at the CXCL12 promoter region in the irradiated single cultured cells increased approximately two-fold 3-days after irradiation. Similarly, radiation induced a H3K4me3 increase at the CXCL12 promoter by approximately two-fold after 5 days in the cocultured Huh7 cells. Taken together with H3K4me3 modification data, it was suggested that the radiation-induced H3K4me3 modification at the CXCL12 promoter may mediate radiation-induced CXCL12 expression.
Because radiation therapeutics generally rely on the DNA damage response, we tested whether the transcriptional induction of CXCL12 and epigenetic changes at its promoter might occur in response to other DNA damaging agents like after radiation. Etoposide and phleomycin could induce CXCL12 transcription with increased H3K4me3 and reduced H3K9me3 at the promoter (Fig. 2E), which is analogous to what is induced by radiation. This suggests that DNA damage responses like the ATM/ATR-mediated DNA damage signaling pathway is implicated in radiation-induced CXCL12 transcription concordant with its epigenetic changes. Thus, we examined if an ATM inhibitor could decrease radiation-induced CXCL12 transcription and alleviate the enriched H3K4me3 and reduced H3K9me3 at its promoter. The radiation-induced CXCL12 transcriptional induction and the H3K4me3 enrichment/H3K9me3 reduction at the CXCL12 promoter region were reversed by the ATM inhibitor KU55933 (Fig. 2F). This supports the conclusion that radiation-induced CXCL12 effects take place in a DNA damage-dependent manner.
Recruitment of transcriptional activator c/EBPβ at the CXCL12 promoter is accompanied by epigenetic histone modification in response to radiation
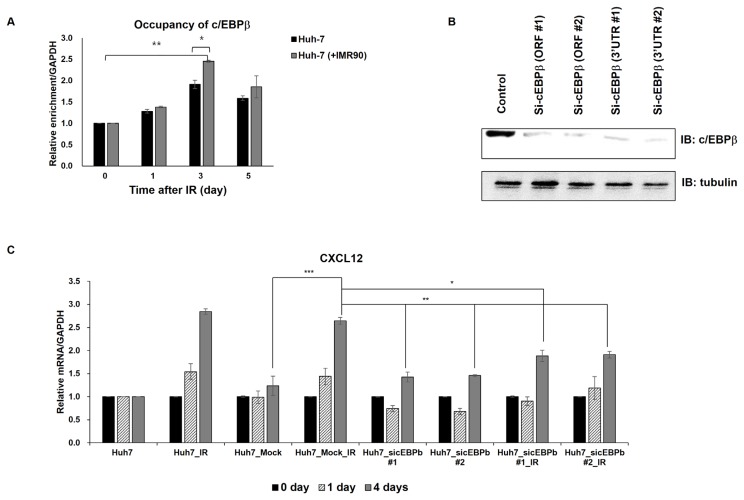
Since c/EBPβ is known to regulate CXCL12 expression (Fu et al., 2014) and c/EBPβ is associated with a wide range of neoplastic disorders (Zahnow, 2011), we decided to investigate engagement of c/EBPβ in the upregulation of CXCL12 in response to irradiation. We analyzed the c/EBPβ occupancy at the promoter following radiation. Recruitment of c/EBPβ was induced in the single- or cocultured Huh7 cells up to 5-days after irradiation, suggesting that changes in transcription factor occupancy are accompanied by epigenetic changes at the CXCL12 promoter in response to irradiation. Coculture and concomitant radiation induced its significant recruitment (approximately 2.4-fold) (Fig. 3A), further suggesting that c/EBPβ may mediate radiation-induced CXCL12 transcription.
Fig. 3. The transcriptional factor c/EBPβ is required for radiation-induced CXCL12 expression.
(A) Radiation induces c/EBPβ recruitment to the CXCL12 promoter in both single- or cocultured Huh7 cells. ChIP assays were carried out using anti-c/EBPβ antibody 1 to 5 days after treating Huh7 cells with or without γ-irradiation. The pulled-down chromatin pools were PCR amplified with primers targeting the CXCL12 promoter. The pulled-down chromatin samples immediately before radiation (0 day) were arbitrarily set to one. (B) The c/EBPβ knockdown levels were confirmed using western blotting. (C) The c/EBPβ knockdown alleviated radiation-induced CXCL12 expression. Time course analysis of CXCL12 mRNA levels were performed following radiation in Huh7 cells transfected with an empty (mock) or c/EBPβ silencing (sic/EBPβ) vectors one day earlier. The CXCL12 mRNA levels in non-transfected and non-irradiated Huh7 cells were arbitrarily set to one. *P < 0.05, **P < 0.01, ***P < 0.001.
To further confirm the involvement of c/EBPβ in radiation-induced CXCL12 expression, we monitored CXCL12 expression after radiation in the c/EBPβ knockdown cells (Figs. 3B and 3C, Supplementary Fig. S5). c/EBPβ knockdown ameliorated the radiation-induced CXCL12 expression (Fig. 3C), indicating that c/EBPβ is required for radiation-induced CXCL12 upregulation.
Based on the epigenetic data from murine and human tumor microenvironment models, we concluded that radiation induces changes in histone modifications and transcription factor occupancy at specific sites in the CXCL12 promoter in the hepatoma tumor microenvironment, possibly leading to upregulation.
Enhanced CXCL12-CXCR4 loop in response to radiation
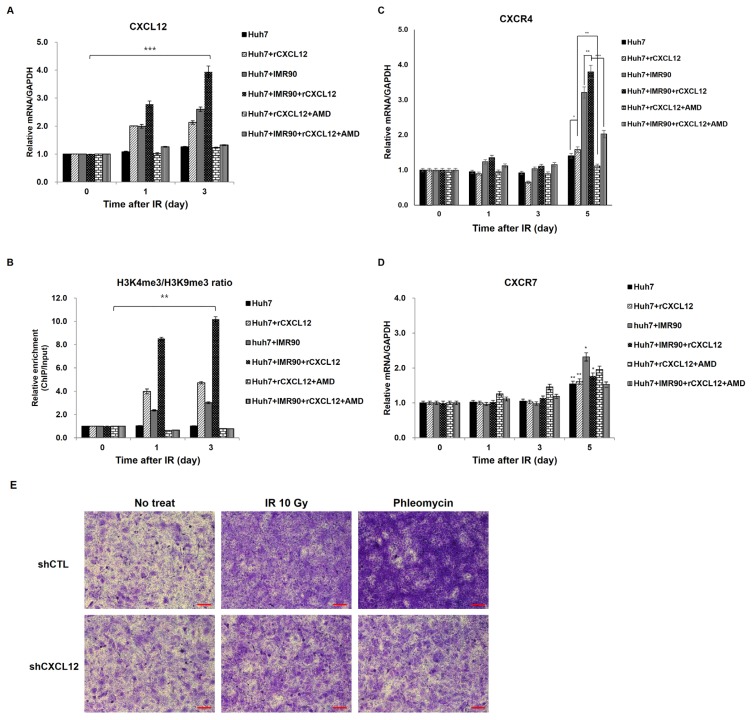
Because CXCR4/7, the receptors of CXCL12, were also enhanced by approximately two-fold 3 days after irradiation in an in vivo tumor microenvironment model (Fig. 1A), we examined whether increased CXCL12 after radiation could act on the tumor cells within the tumor microenvironment in a positive feedback loop. To do this, we monitored the effects after adding recombinant CXCL12 ligand (rCXCL12) and a chemokine receptor antagonist for CXCR4 and CXCL12-mediated chemotaxis, plerixafor octahydrochloride (AMD3100 8HCL) on CXCL12 mRNA expression and histone modifications at the promoter during a time course. Addition of rCXCL12 induced CXCL12 mRNA production (Fig. 4A) and higher ratio of H3K4me3 to H3K9me3 in the single- or co-cultured Huh7 cells (Fig. 4B) compared to untreated cells. In single- or cocultured Huh7 cells exposed to rCXCL12, CXCL12 mRNA increased up to two- and four-times, and the ratio of H3K4me3 to H3K9me3 increased up to four- and ten-times, respectively, compared to untreated control cells (Figs. 4A and 4B). The data from rCXCL12 experiments show correlation between CXCL12 mRNA expression and histone modification at the promoter. Also, the CXCR4 antagonist AMD3100 abrogated the rCXCL12 effects on CXCL12 mRNA expression and histone modifications in both single- and cocultured Huh7 cells (Fig. 4A). However, unlike the modulation of CXCL12 expression, CXCR4/7 expression was only hardly or slightly modulated by rCXCL12 and CXCR4 antagonist 3 days after γ-irradiation (Figs. 4C and 4D). On the fifth day after γ-irradiation, rCXCL12 modestly enhanced CXCR4 expression, but AMD3100 reversed the rCXCL12-induced CXCR4 expression in single and cocultured Huh7 cells (Fig. 4C). In contrast, CXCR7 expression changed litter after the rCXCL12 and the CXCR4 antagonist (Fig. 4D). These results suggest that the radiation-induced CXCL12 increase can act as an autocrine signal, leading to reinforcing a positive CXCL12-CXCR4 circuit.
Fig. 4. Modulation of CXCL12 transcription by recombinant CXCL12 (rCXCL12) or a CXCR4 inhibitor AMD3100.
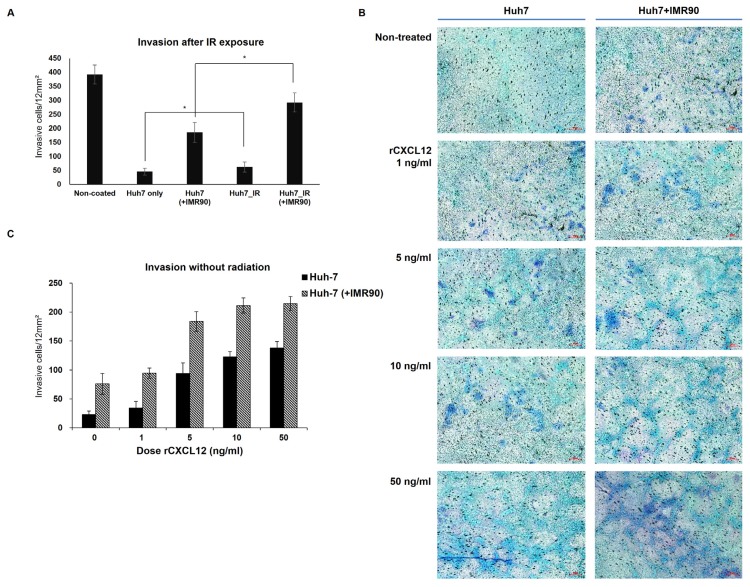
(A) Recombinant CXCL12 (rCXCL12) induces expression of CXCL12, but AMD3100 inhibits the rCXCL12-induced CXCL12 expression in the single- (Huh7) and cocultured (Huh7 + IMR90) Huh7 cells. Huh7 cells were cultured alone (Huh7) or together with IMR90 cells (Huh7 + IMR90) and treated with rCXCL12 or AMD3100 for 24 to 72 h as described. CXCL12 mRNA levels were analyzed. (B) Modifications of H3 at CXCL12 promoters by rCXCL12 or AMD3100 were analyzed in Huh7 cells for 24 to 72 h after treatment with rCXCL12 or AMD3100. ChIP assays were performed using anti-H3K4me and H3K9me antibodies. (C and D) The experiments in (A) were repeated for CXCR4 (C) and CXCR7 expression (D). *P < 0.05, **P < 0.01, ***P < 0.001. (E) Huh7 cells transfected with an empty (mock) or CXCL12 silencing (shCXCL12) vectors one day earlier then irradiation or phleomycin treatment. Scale bar = 100 μm. Invasion was evaluated 3 days after irradiation or phleomycin treatment by incubating Huh7 cells on a Matrigel-coated chamber.
Radiation-induced CXCL12-CXCR4/7 expression contributes to tumor malignancy in surviving cells
We investigated the physiological changes in hepatoma cells with respect to changes in CXCL12-CXCR4/7 expression in the tumor microenvironment after radiation. The CXCL12-CXCR4/7 axis plays a variety of roles in tumor progression and malignancy including invasion (Domanska et al., 2013). Thus, we studied the relationship between the radiation-induced CXCL12-CXCR4/7 axis and invasion. To do this, we measured invasiveness of Huh7 cells with or without radiation. Higher invasion was observed in the irradiated single cultured Huh7 cells compared to non-radiated cells (Fig. 5A). In a similar manner, radiation induced invasion of cocultured Huh7 cells compared to unirradiated control cells (Fig. 5A). Interestingly, invasion was enhanced in the cocultured Huh7 cells without radiation compared to single cultured Huh7 cells, indicating that the tumor microenvironment enables cancer cells to gain invasive and metastatic properties.
Fig. 5. Radiation or coculture increased invasiveness of Huh7 cells.
(A) Invasion was evaluated by incubating Huh7 cells on a Matrigel-coated chamber at the indicated times after coculture or irradiation. (B and C) rCXCL12 treatment induced invasion of Huh7 cells. Scale bar = 100 μm. (D) Expression of MMP-2 and -9 in Huh7 cells was induced by radiation or coculture. MMP-2 and -9 mRNA were measured at indicated times. The levels of MMP-2 and -9 mRNAs in the single-cultured and unirradiated Huh7 cells were arbitrarily set to one. (E) rCXCL12 induced MMP-2 and -9 expression like irradiation. In contrast, AMD3100 blocked rCXCL12-induced MMP-2 and -9 expression. The MMP-2 and -9 mRNA levels at indicated times were measured after treatment with rCXCL12 with or without AMD3100. *P < 0.05, **P < 0.01.
To examine whether radiation-induced CXCL12 expression can mediate this radiation-induced invasion, invasiveness was monitored after treatment with recombinant CXCL12 (rCXCL12) without radiation (Figs. 5B and 5C). Interestingly, rCXCL12 addition without radiation induced invasion in both single- and cocultured Huh7 cells in a dose-dependent manner. Together with the radiation-induced invasion results, this correlation between rCXCL12 and invasion data indicate that radiation-induced CXCL12 can promote invasion of tumor cells after radiation. To see whether radiation-induced CXCL12 plays a role in the invasion of radiation-treated tumor cells, we evaluated the invasiveness of CXCL12-deficient hepatoma cells after irradiation. CXCL12 knockdown alleviated the radiation-enhanced invasion (Fig. 4E), indicating that the radiation or DNA damaging agent (phleomycin)-induced CXCL12 contributes to malignancy of radiation-treated cancer cells.
Because ECM proteins are required for invasion, we next investigated radiation effects on the invasive and metastatic ECM proteins. MMP2/9 expression was compared to non-irradiated control cells. Coculture of Huh7 cells with normal cells slightly induced MMP2/9 proteins (about 40%) compared to the single culture. Radiation further intensified MMP2/9 expression in the cocultured Huh7 cells by approximately 2.6- and 2.8-fold, respectively, 5-days after irradiation compared to cocultured and non-irradiated cells (Fig. 5D). Together, these data show that treatment with γ-irradiation results in increased expression of MMP2/9 and CXCL12-CXCR4/7 genes in human hepatoma microenvironments.
To finally investigated whether radiation-induced MMP2/9 expression was mediated by CXCL12. MMP2/9 expression was monitored after rCXCL12 or antagonist treatment without radiation. Coculture of Huh7 cells mimicking the tumor microenvironment with rCXCL 12 treatment enhanced the expression of MMP2/9 genes (Fig. 5E). However, AMD 3100, an antagonist of the CXCL12-CXCR4/7 axis, abrogated the coculture- or rCXC12-mediated upregulation of MMP2/9 genes (Fig. 5E). These data indicate that the radiation-induced upregulation of CXCL12/CXCR4/7 axis can mediate upregulation of MMP2/9 genes and invasion.
Epigenetic inhibitor can undermine malignant cancer subpopulation expressing CSC markers, in conjunction with attenuation of the radiation-induced CXCL12 expression
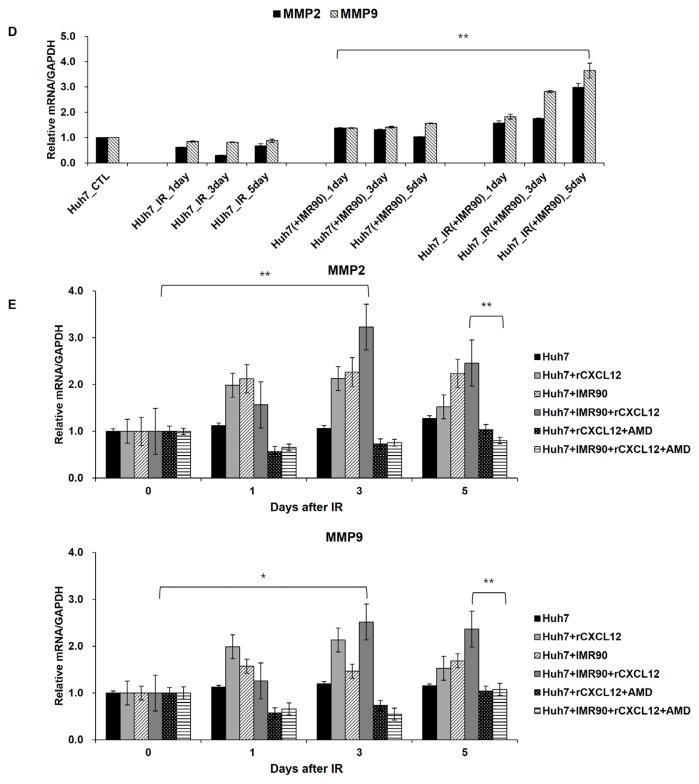
Activation of the CXCL12-CXCR4/7 pathway promotes metastasis (Kryczek et al., 2007) and survival of cancer cells (Teicher and Fricker, 2010; Uy et al., 2012) and is involved in stemness (Würth et al., 2014) and drug resistance (Jung et al., 2013), resulting in gaining CSC properties (Domanska et al., 2013; Trautmann et al., 2014; Würth et al., 2014). Also, CXCR4 was identified as a prognostic marker for radiation resistant CSCs (Trautmann et al., 2014). Thus, we investigated whether the radiation-induced CXCL12-CXCR4/7 pathway affects the expression of the CSC markers CD133+/CD24− (Choi and Lee, 2013) in a population of Huh7 cells after irradiation. The Huh7 cells have a very small population expressing CSC markers for CD133+/CD24− cells (Fig. 6). Radiation affected the expression of these markers and changed the possibly malignant subpopulation by approximately 2.3 to 3.8-fold in the irradiated Huh7 cells (Fig. 6A). Moreover, the CSC marker-expressing subpopulation increased more strongly to approximately 3.68-fold in the cocultured Huh7 cells with normal IMR80 cells (comparable to 3.8-fold in the irradiated single cultured cell population) (Fig. 6A), suggesting that the tumor microenvironment is important for the proportion of cell subpopulation expressing CSC markers. In the coculture system, radiation had a further impact on the expression of these markers in the Huh7 cells up to 6.8 to 9.9-fold, further supporting the importance of the tumor microenvironment in expressing CSC markers.
Fig. 6. Radiation or coculture increased the Huh7 cell subpopulation expressing stem cell markers (CD133+/CD24−).
(A) A representative flow cytometry analysis of the Huh7 cell subpopulation expressing CD133+ CD24−. (B) 3D culture Huh7 cells were analyzed using flow cytometry for a subpopulation expressing CD133+ CD24−. Three days after irradiation or not (control), Huh7 cells were immunostained with CD133-FITC and CD24-PE and analyzed. All analyses were performed in three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
Next, we tested radiation effects on the subpopulation expressing CSC markers in tumor microenvironment using 3D Huh7 culture (Fig. 6B). In a similar manner, radiation increased the CSC subpopulation up to approximately 2.3-fold in 3D culture of Huh7 cells (Fig. 6B). The increase in the subpopulation displaying CSC markers in the 3D culture system after radiation indicates that surviving cancer cells after radiotherapy can exhibit a malignant phenotype.
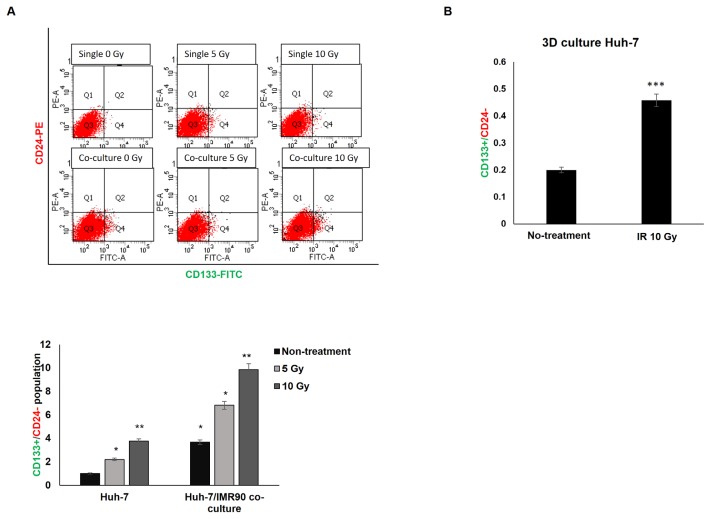
Since radiation induced expression of the CXCL12-CXCR4/7 pathway along with epigenetic histone modification changes at the CXCL12 promoter in the tumor microenvironment, we tested whether and which epigenetic inhibitors could abrogate the radiation-induced CXCL12 expression. Next, we investigated whether this abrogative epigenetic inhibitor could diminish the subpopulation of CSC marker-expressing cancer cells after radiation. First, we used two histone demethylase inhibitors (GSK-J1, a highly potent Jumonji H3K27 histone demethylase inhibitor, and IOX1, a broad spectrum inhibitor of 2-oxoglutarate oxygenases including JmjC demethylase), a histone methyltransferase inhibitor (chaetocin, a nonspecific histone methyltransferase inhibitor), and a histone deacetylase inhibitor (trichostatin A). IOX1 could suppress radiation- or coculture-induced CXCL12 expression (Fig. 7A), and the other epigenetic inhibitors had little effect or enhanced the radiation-induced CXCL12 transcription in the coculture and 3D tumor microenvironments (Supplementary Fig. S6).
Fig. 7. Modulation of CXCL12 expression and CSC marker by inhibitors of histone modifying enzymes (IOX1, GSK-J1, and TSA).
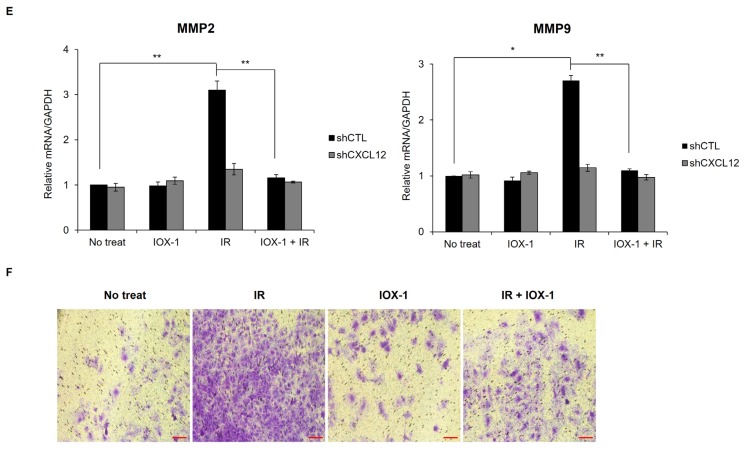
(A) Huh-7 cells were cultured with (cocultured) or without IMR90 cells and pretreated with IOX-1 inhibitor for 24 h followed by IR exposure. The CXCL12 mRNA level of the unpretreated and unirradiated sample was arbitrarily set to one and compared to treated samples. (B and C) Expression of CD133+ CSC marker was also affected by inhibitors after treatment with IR. (D–F) Huh-7 cells were pretreated with IOX-1 inhibitor for 24 h before irradiation. (D) In 3 days, modifications of H3K4me3 and H3K9me3 at the human CXCL12 promoter were evaluated. (E) MMP2 and MMP9 mRNA were measured. *P < 0.05, **P < 0.01, ***P < 0.001. (F) Invasion was evaluated by incubating Huh7 cells in a Matrigel-coated chamber. Scale bar = 100 μm.
Based on the IOX1 activity for the suppression of radiation-induced CXCL12 in tumor microenvironment (Fig. 7A), we also examined whether IOX1 could ameliorate the subpopulation of CSC marker-expressing cancer cells after radiation. IOX1 pretreatment reduced the subpopulation of CSC cells in Huh7 cells surviving radiation in the single-culture and coculture tumor microenvironment (Fig. 7B). Together with CXCL12’s transcription repression effect of IOX1 (Fig. 7A), these results suggested that epigenetic inhibitors such as IOX1 can attenuate and control the adverse effects of radiation in surviving cancer cells after radiotherapy and inhibit CXCL12 gene expression by modulating histone modifications to promote the effectiveness of radiation treatment by eliminating malignant cancer cells. Concurrently, correlated expression of the CSC marker, CD133 at the RNA level was also affected by the inhibitors (Fig. 7C).
We also examined whether IOX1 could attenuate the radiation-induced H3K4me3 and decrease H3K9me3 at CXCL12 promoter leading to enhanced CXCL12 expression. IOX1 could efficiently reverse radiation-induced H3K4me3, but it did not decrease H3K9me3 modifications at the CXCL12 promoter (Fig. 7D). This suggested that IOX1 may exert its alleviating effects on radiation-induced CXCL12 expression by reversing H3K4me3 modification at the CXCL12 promoter.
Because IOX1 could suppress the radiation-induced CXCL12 (Fig. 7A), we asked whether IOX1 could also reverse radiation-enhanced MMP2/9 expression (Fig. 7E) and invasion (Fig. 7F). IOX1 cotreatment with radiation abrogated the upregulation of MMP2/9 induced by radiation and attenuated radiation-enhanced invasiveness (Figs. 7E and 7F). Remarkably, CXCL12 knockdown abrogated the IR-induced MMP2/9 upregulation (Fig. 7E) and invasion (Figs. 4E and 7F), supporting the conclusion that radiation-induced CXCL12 plays an important role in the malignancy of IR-treated cancer cells.
DISCUSSION
Besides chemotherapy and surgery, approximately half of cancer patients undergo radiation therapy to decrease the bulk tumor mass and inhibit the spread of cancer cells. However, due to tumor heterogeneity depending on genetic, epigenetic, phenotypic, and environmental differences, it is hard to accurately predict treatment responses. The interaction of cancer cells with their microenvironment is known to induce the consistent occurrence of tumor heterogeneity (Lee et al., 2016), which occasionally leads to the generation and maintenance of therapy resistance and tumor relapse (Junttila and Sauvage, 2013). Radiotherapy interrupts cancer cells and has systemic effects on the tumor microenvironment by modifying the microenvironment (Lumniczky and Sáfrány, 2015) and a complex biological network comprising the extracellular matrix and various cell types, including endothelial cells, fibroblasts, and immune cells (Kuonen et al., 2012) that can modify radiotherapy responsiveness. Changes within the irradiated tumor can include hypoxia formation and adaptation, angiogenesis, and enhanced metastasis (Imaizumi et al., 2010). These vicious processes involve signaling pathways such as CXCL12-CXCR4, cKit, SCF, MAP kinase signaling, and integrin signaling (Eke and Cordes, 2011; Imaizumi et al., 2010; Kong et al., 2014; Kryczek et al., 2007; Kuonen et al., 2012; Lagadec et al., 2012; Würth et al., 2014). However, underlying mechanisms in which radiation induces these microenvironmental modifications (Eke and Cordes, 2011; Lagadec et al., 2012), resulting in modulation of therapy responsiveness and tumor progression, remain unclear. The question regarding the epigenetic effects of radiation on microenvironmental modifications warrants investigation.
Here, we provide new evidence that radiation induces epigenetic modifications to the promoter of the CXCL12 gene in hepatoma cells, resulting in higher expression and activation of its downstream pathway, CXCL12-CXCR4/7. Additionally, radiation was also shown to induce CXCR4 expression, a receptor for the CXCL12 ligand in surviving cancer cells, compared to non-irradiated cancer cells or purely cultured cancer cells. These findings are consistent with previous studies showing that ionizing radiation induces inflammatory, growth factor, and cytokine signaling pathways like the CXCL12-CXCR4 axis (Haubner et al., 2012; Lee et al., 2016). Furthermore, our data show that epigenetic inhibitors mimicking or reversing radiation-induced modifications at the CXCL12 promoter synergize or abrogate radiation-induced CXCL12 expression, indicating that radiation-induced epigenetic modifications are critical molecular mechanisms for radiation effects in the tumor microenvironment.
Previous work shows that radioresistant cancer subline cells exhibit higher CXCR4 expression compared to isogenic radiation-sensitive sublines (Haubner et al., 2012). Also, recent findings showed that the CXCL12-CXCR4/7 pathway is highly activated in CSCs originating from breast, lung, and prostate cancers (Dubrovska et al., 2012; Jung et al., 2013). These previous studies clearly describe correlation between CXCL12 signaling, cancer malignancy, and resistance/relapse. Targeting and eliminating CSC is difficult with standard radiotherapy regimens because they are strongly resistant to radiation and adapt to the tumor microenvironment. This radioresistance mechanism can partially explain our experimental data showing the CXCL12-CXCR4/7 upregulation by irradiation is due to expression changes induced by irradiation and the enrichment for radioresistant cells expressing higher CXCL12-CXCR4/7. Here, we analyzed the surviving residual cancer cells after radiation treatment for radiation-induced genes and their epigenetic signatures. Thus, our results together with recent reports on the role of the CXCL12-CXCR4 pathway in the modulation of responsiveness to irradiation, resistance, and tumor relapse (Krause et al., 2011; Trautmann et al., 2014; Würth et al., 2014) propose that this axis plays an important role in generating and maintaining resistance in residual cancer cells after radiotherapy, leading to resistance, spreading, recurrence, and metastasis.
There is an urgent need to identify the molecular mechanisms regulating responsiveness to radiotherapy that are developed during therapy, in addition to preexisting resistance mechanisms before therapy. Taking into account the involvement of the CXCL12-CXCR4 axis in resistant CSC (Dubrovska et al., 2012; Jung et al., 2013; Krause et al., 2011; Lagadec et al., 2012) and higher expression of CXCR4 in radioresistant cancer subpopulations (Trautmann et al., 2014), our experimental data showing CXCL12-CXCR4 induction by radiation and its epigenetic molecular mechanisms show that irradiation kills bulk cancer cells and also generates resistant and more aggressive phenotypes in a small minority of cancer cells via epigenetic modulations. If this is the case, adoption of epigenetic inhibitors reversing radiation-induced epigenetic effects in combination with radiotherapy is needed to improve patient outcomes. Moreover, epigenetic anticancer strategies in combination with current therapy can target other mechanisms and pathways supporting resistance, relapse, metastasis, and cancer-favorable environmental factors, in addition to CXCL12-CXCR4/7 axis, by reprogramming gene expression alterations toward a normal state by reversing epigenetic abnormalities in cancer cells. Surprisingly, bulk gene expression associated with carcinogenesis, malignancy, and resistance result from epigenetic and genetic alterations. Accordingly, many agents targeting cancer epigenetic abnormalities are under development and in clinical trials. The key potential for epigenetic therapies relies on the fact that epigenetic changes are reversible, allowing for recovery of the expression of epigenetically affected genes to their normal levels (Ahuja et al., 2014). An inhibitor of histone demethylase, IOX1, can also reverse expression of specific genes to a more normal state via changes int he histone modification pattern (Dobrynin et al., 2017; Hu et al., 2015). IOX1 can suppress cyclin D1 expression (Hu et al., 2015), and it can also lead to a decrease in Hif1α expression (Dobrynin et al., 2017). Consistent with this, we found that the epigenetic inhibitor IOX1 can reverse radiation-induced H3K4me3 at the CXCL12 promoter, which is causally related to the attenuation of CXCL12 expression. Due to the fact that CXCL12/CXCR4 is involved in radiation-resistance (Trautmann et al., 2014) and cancer stemness (Domanska et al., 2013; Kuonen et al., 2012) and our findings show that the epigenetic inhibitor IOX1 with radiation can block invasion and enrichment of cells expressing CSC marker (CD133+/CD24−), additional studies are needed to evaluate the potential therapeutic use of epigenetic targeting reagents in combination with radiotherapy.
In conclusion, we have shown that radiation modifies the expression of genes in the CXCL12-CXCR4 axis and epigenetic state of the CXCL12 promoter in tumor microenvironment, indicating a molecular mechanistic link between gene expression and epigenetic modifications in response to radiation. Our studies also suggest that radiation induces a change in the expression of CSC markers, resulting in an increase of the CSC-like subpopulation expressing CD133+/CD24− after radiation. Intriguingly, histone demethylase inhibitors or antagonists of the CXCL12-CXCR4 axis can reverse these radiation-induced effects, supporting the conclusion some poor radiation effects occur in an epigenetic modification-dependent manner. Thus, it seems that the therapeutic inhibition of the CXCL12 signaling pathway via an epigenetic (histone demethylase inhibitor) or direct (antagonist of CXCL12-CXCR4/7) strategy is a promising therapeutic option to refine radiotherapy.
Supplementary Material
ACKNOWLEDGMENTS
We thank all members of Lee’s laboratory for their helpful advice. This work was supported by the grants from the National Research Foundation of Korea (NRF) (2017M2A2A7A01021034 and 2017R1A2B4010146).
Footnotes
Note: Supplementary information is available on the Molecules and Cells website (www.molcells.org).
Disclosure
The authors have no potential conflicts of interest to disclose.
REFERENCES
- Ahuja N., Easwaran H., Baylin S.B. Harnessing the potential of epigenetic therapy to target solid tumors. J Clin Invest. 2014;124:56–63. doi: 10.1172/JCI69736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S., Wu Q., McLendon R.E., Hao Y., Shi Q., Hjelmeland A.B., Dewhirst M.W., Binger D.D., Rich J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Behnam Azad B., Nimmagadda S. The intricate role of CXCR4 in cancer. Adv Cancer Res. 2014;124:31–82. doi: 10.1016/B978-0-12-411638-2.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.D., Lee J.S. Interplay between epigenetics and genetics in cancer. Genomics Inform. 2013;11:164–173. doi: 10.5808/GI.2013.11.4.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrynin G., McAllister T.E., Leszczynska K.B., Ramachandran S., Krieg A.J., Kawamura A., Hammond E.M. KDM4A regulates HIF-1 levels through H3K9me3. Sci Rep. 2017;11:11094. doi: 10.1038/s41598-017-11658-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanska U.M., Kruizinga R.C., Nagengast W.B., Timmer-Bosscha H., Huls G., de Vries E.G., Walenkamp A.M. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer. 2013;49:219–230. doi: 10.1016/j.ejca.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Dubrovska A., Elliott J., Salamone R.J., Telegeev G.D., Stakhovsky A.E., Schepotin I.B., Yan F., Wang Y., Bouchez L.C., Kularatne S.A., et al. CXCR4 expression in prostate cancer progenitor cells. PLoS One. 2012;7:e31226. doi: 10.1371/journal.pone.0031226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke I., Cordes N. Radiobiology goes 3D: how ECM and cell morphology impact on cell survival after irradiation. Radiother Oncol. 2011;99:271–278. doi: 10.1016/j.radonc.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Fu S.L., Pang H., Xu J.Z., Wu X.H. C/EBPβ mediates osteoclast recruitment by regulating endothelial progenitor cell expression of SDF-1α. PLoS One. 2014;9:e91217. doi: 10.1371/journal.pone.0091217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot C., Favaudon V., Herceg Z., Sagne C., Sauvaigo S., Merle P., Chemin I. PARP inhibition and the radiosensitizing effects of the PARP inhibitor ABT-888 in in vitro hepatocellular carcinoma models. BMC Cancer. 2014;14:603. doi: 10.1186/1471-2407-14-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubner F., Ohmann E., Pohl F., Struts J., Gassner H.G. Wound healing after radiation therapy: review of the literature. Radiat Oncol. 2012;7:162. doi: 10.1186/1748-717X-7-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.W., Hur W., Choi J.E., Kim J.H., Hwang D., Yoon S.K. Role of ADAM17 in invasion and migration of CD133-expressing liver cancer stem cells after irradiation. Oncotarget. 2016;7:23482–23497. doi: 10.18632/oncotarget.8112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q., Chen J., Zhang J., Xu C., Yang S., Jiang H. IOX1, a JMJD2A inhibitor, suppresses the proliferation and migration of vascular smooth muscle cells induced by angiotensin II by regulating the expression of cell cycle-related proteins. Int J Mol Med. 2015;37:189–196. doi: 10.3892/ijmm.2015.2393. [DOI] [PubMed] [Google Scholar]
- Imaizumi N., Monnier Y., Hegi M., Mirimanoff R.O., Rüegg C. Radiotherapy suppresses angiogenesis in mice through TGF-βRI/ALK5-dependent inhibition of endothelial cell sprouting. PLos One. 2010;5:e11084. doi: 10.1371/journal.pone.0011084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M.J., Rho J.K., Kim Y.M., Jung J.E., Jin Y.B., Ko Y.G., Lee J.S., Lee S.J., Lee J.C., Park M.J. Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene. 2013;32:209–221. doi: 10.1038/onc.2012.37. [DOI] [PubMed] [Google Scholar]
- Junttila M.R., Sauvage F.J. Influence of tumor microenvironment heterogeneity on therapeutic response. Nature. 2013;501:346–354. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- Kong F.M., Cuneo K.C., Wang L., Bonner J.A., Gaspar L.E., Komaki R., Sun A., Morris D.E., Sandler H.M., Movas B. Patterns of practice in radiation therapy for non-small cell lung cancer among members of the American Society for Radiation Oncology. Pract Radiat Oncol. 2014;4:e133–e141. doi: 10.1016/j.prro.2013.05.002. [DOI] [PubMed] [Google Scholar]
- Krause M., Yaromina A., Eicheler W., Koch U., Baumann M. Cancer stem cells: targets and potential biomarkers for radiotherapy. Clin Cancer Res. 2011;17:7224–7229. doi: 10.1158/1078-0432.CCR-10-2639. [DOI] [PubMed] [Google Scholar]
- Kryczek I., Wei S., Keller E., Liu R., Zou W. Stromal derived factor (SDF-1/CXCL12) and human tumor pathogenesis. Am J Physiol Cell Physiol. 2007;292:C987–C995. doi: 10.1152/ajpcell.00406.2006. [DOI] [PubMed] [Google Scholar]
- Kuonen F., Secondini C., Rüegg C. Molecular pathways: emerging pathways mediating growth, invasion, and metastasis of tumors progressing in an irradiated microenvironment. Clin Cancer Res. 2012;18:5196–5202. doi: 10.1158/1078-0432.CCR-11-1758. [DOI] [PubMed] [Google Scholar]
- Lagadec C., Vlashi E., Della Donna L., Dekmezian C., Pajonk F. Radiation-induced reprogramming of breast cancer cells. Stem Cells. 2012;30:833–844. doi: 10.1002/stem.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I.J., Lee E.J., Park H., Kim W., Ha S.J., Shin Y.K., Seong J. Altered biological potential and radioresponse of murine tumors in different microenvironments. Cancer Res Treat. 2016;48:727–737. doi: 10.4143/crt.2014.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumniczky K., Sáfrány G. The impact of radiation therapy on the antitumor immunity: local effects and systemic consequences. Cancer Lett. 2015;356:114–125. doi: 10.1016/j.canlet.2013.08.024. [DOI] [PubMed] [Google Scholar]
- Rich J.N. Cancer stem cells in radiation resistance. Cancer Res. 2007;67:8980–8984. doi: 10.1158/0008-5472.CAN-07-0895. [DOI] [PubMed] [Google Scholar]
- Romain B., Benbrika-Nehmar R., Marisa L., Legrain M., Lobstein V., Oravecz A., Poidevin L., Bour C., Freund J.N., Duluc I., et al. Histone hypoacetylation contributes to CXCL12 downregulation in colon cancer: impact of tumor and cell migration. Oncotarget. 2017;8:38351–38366. doi: 10.18632/oncotarget.16323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Zhang T., Deng Q., Zhou Q., Sun X., Li E., Yu D., Zhong C. Benzidine induces epithelial-mesenchymal transition of human bladder cancer cells through activation of ERK5 pathway. Mol Cells. 2018;41:188–197. doi: 10.14348/molcells.2018.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher B.A., Fricker S.P. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- Trautmann F., Cojoc M., Kurth I., Melin N., Bouchez L.C., Dubrovska A., Peitzsch C. CXCR4 as biomarker for radioresistant cancer stem cells. Int J Radiat Biol. 2014;90:687–699. doi: 10.3109/09553002.2014.906766. [DOI] [PubMed] [Google Scholar]
- Uy G.L., Rettig M.P., Motabi I.H., McFarland K., Trinkaus K.M., Hladnik L.M., Kulkarni S., Abboud C.N., Cashen A.F., Stockerl-Goldstein K.E., et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012;119:3917–3924. doi: 10.1182/blood-2011-10-383406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Würth R., Bajetto A., Harrison J.K., Barbieri F., Florio T. CXCL12 modulation of CXCR4 and CXCR7 activity in human glioblastoma stem-like cells and regulation of the tumor microenvironment. Front Cell Neurosci. 2014;8:144. doi: 10.3389/fncel.2014.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahnow C.A. CCAAT/enhancer-binding protein β: its role in breast cancer and associations with receptor tyrosine kinases. Expert Rev Mol Med. 2011;11:e12. doi: 10.1017/S1462399409001033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.