Abstract
BACKGROUND:
Since the mid-1980s, the burden of liver cancer in the United States has doubled, with 31,411 new cases and 24,698 deaths occurring in 2014. Foreign-born individuals may be more likely to die of liver cancer than individuals in the general US-born population because of higher rates of hepatitis B infection, a low socioeconomic position, and language barriers that limit the receipt of early cancer detection and effective treatment.
METHODS:
To determine whether liver cancer mortality rates were higher among foreign-born individuals versus US-born individuals in the United States, population-based cancer mortality data were obtained from the National Center for Health Statistics of the Centers for Disease Control and Prevention. Annual population estimates were obtained from the US Census Bureau’s American Community Survey. Age-adjusted mortality rates and rate ratios (RRs) for liver cancer stratified by birth place were calculated, and the average annual percent change (AAPC) was used to evaluate trends.
RESULTS:
A total of 198,557 deaths from liver and intrahepatic bile duct cancer were recorded during 2005–2014, and 16% occurred among foreign-born individuals. Overall, foreign-born individuals had a 24% higher risk of liver cancer mortality than US-born individuals (RR, 1.24; 95% confidence interval [CI], 1.22–1.25). Foreign-born individuals did not have any significant changes in liver cancer mortality rates overall, but among US-born individuals, liver cancer mortality rates significantly increased (AAPC, 2.7; 95% CI, 2.1–3.3).
CONCLUSIONS:
Efforts that address the major risk factors for liver cancer are needed to help to alleviate the health disparities observed among foreign-born individuals and reverse the increasing trend observed in the US-born population.
Keywords: cancer, foreign-born, hepatitis B, hepatitis C, liver, liver and intrahepatic bile duct, liver cancer, mortality, mortality rates, nativity status, US-born
INTRODUCTION
Since the mid-1980s, the liver cancer mortality rate in the United States has doubled,1 with 31,411 new cases and 24,698 deaths occurring in 2014.2 In the US population, liver cancer mortality rates are significantly increasing for nearly all sex, age, and race/ethnicity group stratifications.3,4 Globally, chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV) is the major cause of liver cancer3,5; however, in the United States, obesity, chronic HCV infection, excessive alcohol consumption, and tobacco use are the leading causes of liver cancer.6
Foreign-born individuals represent a rapidly growing segment of the general US population, and from 1970 to 2010, the foreign-born population increased from 9.6 to 40.0 million individuals.7 This group is also more likely to develop and die of liver cancer than US-born individuals.8 According to their country of origin, HBV infection rates in the foreign-born population can range from 1 to 33 times the rates observed in the general US-born population, whereas their risk of HCV infection is lower than or similar to the risk for their US-born counterparts.9–12 Barriers such as limited English proficiency, an undocumented status, a mistrust or fear of an unfamiliar health care system, busy work schedules, and a low socioeconomic position all hinder foreign-born individuals from proper diagnosis and treatment, and this increases their risk of developing and dying of liver cancer.13,14 Although other studies have evaluated excess liver cancer mortality risk in the foreign-born population, the work is not contemporary8 or has been limited in geographic area or the race/ethnicity of the participants.15,16 We analyzed the most recent 10-year national mortality rate data (2005–2014) to provide current liver cancer mortality estimates by birth place, sex, and race/ethnicity and to determine the extent to which these rates are changing over time.
MATERIALS AND METHODS
Data Sources
Population-based cancer mortality data were obtained from the National Center for Health Statistics. The National Center for Health Statistics receives demographic and vital statistics from the National Vital Statistics System. The National Vital Statistics System collects information on sex, race, ethnicity, birth place, age at death, state of residence, and cause of death from death certificates filed in each state. Individuals whose death certificates stated that they were born in 1 of the 50 states, the District of Columbia, or the US territories were categorized as US-born. Remaining cases with a recorded place of birth were categorized as foreign-born.17 Mortality data were selected with code C22 from the International Classification of Diseases, Tenth Revision, to identify all liver and intrahepatic bile duct cancers (called liver cancer in this article). A total of 200,925 deaths from liver cancer were recorded between 2005 and 2014. Cases with no recorded place of birth (1.2%) were excluded from all analyses.
Annual population estimates were obtained from the US Census Bureau’s American Community Survey (ACS). The ACS is a continuous national survey of randomly selected households and collects detailed information on the US population, including information on birth place.18 Information is collected regardless of residency status, so legal and undocumented individuals are surveyed and represented in the estimates.19 Because of this, the ACS is often used as a detailed source of information for the immigrant population in the United States.17 The ACS also provides 5-, 3-, and 1-year estimates of the US population and provides public-use microdata sample files that can be used to estimate the US population with social, housing, and demographic characteristics. We obtained the annual ACS public-use microdata sample files from 2005 to 2014 and estimated the US-born and foreign-born populations for age, race/ethnicity, and sex subgroups with the provided person weights.
Statistical Analysis
Mortality data from the National Center for Health Statistics and population estimates from the ACS for 2005–2014 were formatted with software from the Surveillance, Epidemiology, and End Results (SEER) program (SEER*Prep, version 2.5.3) to create a SEER*Stat database. Performing age adjustments to the 2000 US standard population, we calculated age-adjusted mortality rates for liver cancer among US- and foreign-born individuals. Rate ratios (RRs), standard errors, and 95% confidence intervals (CIs) were calculated with SEER*Stat (version 8.3.4).
The National Cancer Institute’s Joinpoint trend analysis software (version 4.5.0.1) was used to evaluate the annual percent change (APC) and average annual percent change (AAPC) in mortality rates from 2005 to 2014 by birth place (US- vs foreign-born) after stratification by sex, age, and race/ethnicity. APCs were calculated with a maximum of 1 joinpoint. The Monte Carlo permutation method was used to test whether the APC or AAPC was significantly different from 0.
RESULTS
Comparing Liver Cancer Mortality Rates: Foreign-Born Versus US-Born
From 2005 to 2014, 31,097 deaths from liver cancer were recorded for the foreign-born population, whereas 167,460 deaths were recorded among the US-born. Differences in liver cancer mortality rates by birth place, sex, race/ethnicity, and age groups are displayed in Table 1. Overall, foreign-born individuals had a higher risk of liver cancer mortality than US-born individuals (RR, 1.24; 95% CI, 1.22–1.25). This pattern remained after stratification by sex with an RR of 1.17 (95% CI, 1.15–1.19) for males and with an RR of 1.44 (95% CI, 1.42–1.48) for females. With stratification by sex and race/ethnicity, foreign-born males who were non-Hispanic Asian/Pacific Islander (API; RR, 1.71; 95% CI, 1.59–1.84) had higher mortality rates than US-born non-Hispanic APIs. In contrast, foreign-born men who were non-Hispanic black (RR, 0.61; 95% CI, 0.57–0.66) or Hispanic (RR, 0.52; 95% CI, 0.51–0.54) had a significantly lower risk of death than US-born individuals of the same race/ethnicity. Non-Hispanic white and non-Hispanic API foreign-born women had higher liver cancer mortality rates than their US-born counterparts (RR for non-Hispanic whites, 1.16; 95% CI, 1.11–1.20; RR for non-Hispanic APIs, 1.56; 95% CI, 1.41–1.73). Foreign-born women who were non-Hispanic black (RR, 0.91; 95% CI, 0.83–0.99) or Hispanic (RR, 0.92; 95% CI, 0.88–0.96) had a significantly lower risk of death than their US-born counterparts. Foreign-born men and women had significantly higher mortality rates in most age groups examined than US-born men and women with the exception of men 50 to 64 years old.
TABLE 1.
Liver Cancer Mortality Rates in the United States by Demographic Characteristics: 2005–2014
| US-Born | Foreign-Born | ||||
|---|---|---|---|---|---|
| No. of Cases | Average Annual Rate (95% CI)a | No. of Cases | Average Annual Rate (95% CI)a | Rate Ratio (95% CI)b | |
| Overall | 167,460 | 5.69 (5.66–5.71) | 31,097 | 7.04 (6.96–7.13) | 1.24c (1.22–1.25) |
| Sex | |||||
| Male | 113,666 | 8.49 (8.44–8.54) | 19,312 | 9.92 (9.78–10.07) | 1.17° (1.15–1.19) |
| Female | 53,794 | 3.32 (3.29–3.35) | 11,785 | 4.80 (4.71–4.89) | 1.44c (1.42–1.48) |
| Age: male | |||||
| <35 y | 715 | 0.11 (0.10–0.12) | 225 | 0.20 (0.16–0.24) | 1.73c (1.42–2.14) |
| 35–49y | 5,643 | 2.05 (1.99–2.10) | 1,772 | 2.71 (2.58–2.84) | 1.32c (1.25–1.40) |
| 50–64y | 49,996 | 20.48 (20.30–20.66) | 6,871 | 17.24 (16.84–17.65) | 0.84c (0.82–0.86) |
| 65–79 y | 39,344 | 34.46 (34.11–34.80) | 7,250 | 45.60 (44.55–46.68) | 1.32c (1.29–1.36) |
| >80 y | 17,968 | 50.22 (49.48–50.96) | 3,194 | 70.81 (68.37–73.31) | 1.41c (1.36–1.46) |
| Age: female | |||||
| <35 y | 524 | 0.08 (0.08–0.09) | 102 | 0.12 (0.08–0.17) | 1.39 (0.91–2.06) |
| 35–49 y | 2,205 | 0.80 (0.77–0.83) | 583 | 0.90 (0.83–0.97) | 1.12c (1.02–1.23) |
| 50–64 y | 13,520 | 5.20 (5.11–5.29) | 2,447 | 5.55 (5.33–5.77) | 1.07c (1.02–1.11) |
| 65–79 y | 20,263 | 15.07 (14.86–15.28) | 5,027 | 23.80 (23.14–24.47) | 1.58c (1.53–1.63) |
| >80 y | 17,282 | 27.29 (26.88–27.70) | 3,626 | 44.47 (43.03–45.94) | 1.63c (1.57–1.69) |
| Race/ethnicity: male | |||||
| Non-Hispanic white | 83,919 | 7.50 (7.44–7.55) | 4,720 | 7.40 (7.18–7.62) | 0.99 (0.96–1.02) |
| Non-Hispanic black | 17,712 | 13.36 (13.16–13.58) | 1,104 | 8.19 (7.64–8.77) | 0.61c (0.57–0.66) |
| Non-Hispanic API | 843 | 9.44 (8.80–10.12) | 7,755 | 16.17 (15.79–16.56) | 1.71c (1.59–1.84) |
| Hispanic | 9.930 | 17.33 (16.97–17.69) | 5,693 | 9.01 (8.75–9.27) | 0.52c (0.51–0.54) |
| Race/ethnicity: female | |||||
| Non-Hispanic white | 41,609 | 3.05 (3.02–3.08) | 3,261 | 3.53 (3.40–3.66) | 1.16c (1.11–1.20) |
| Non-Hispanic black | 7,389 | 4.43 (4.33–4.53) | 622 | 4.03 (3.71–4.39) | 0.91c (0.83–0.99) |
| Non-Hispanic API | 457 | 4.32 (3.91–4.75) | 3,826 | 6.73 (6.52–6.96) | 1.56c (1.41–1.73) |
| Hispanic | 3,706 | 5.87 (5.68–6.07) | 4,048 | 5.39 (5.21–5.57) | 0.92c (0.88–0.96) |
Abbreviations: API, Asian/Pacific Islander; CI, confidence interval.
The liver cancer mortality rates include liver and intrahepatic bile duct cancers (C22). Values in columns may not sum to the total because of missing values. Underlying mortality rates were provided by the National Center for Health Statistics.
Rates have been age-adjusted to the 2000 US standard population and are shown per 100,000.
The US-born population was used as the reference group to generate rate ratios.
P < .05.
Trends in Liver Cancer Mortality Rates
Overall, liver cancer mortality rates significantly increased in the United States from 2005 to 2014 (AAPC, 2.2; 95% CI, 1.0–3.4; Table 2). In general, rates significantly increased among men and women, among individuals 50 years old or older, and among non-Hispanic whites, non-Hispanic blacks, and Hispanics. Notably, the largest increases overall were observed among men and women aged 50 to 64 years, with identical increases in men (AAPC, 3.9; 95% CI, 2.9–4.8) and women (AAPC, 3.9; 95% CI, 2.6–5.3).
TABLE 2.
Overall Trends in Liver Cancer Mortality Rates by Nativity Status, Sex, Age, and Race/Ethnicity in the United States: 2005–2014
| Trend 1 | Trend 2 | AAPC | ||||||
|---|---|---|---|---|---|---|---|---|
| Group | Years | APC | 95% CI | Years | APC | 95% CI | 2005–2014 | 95% CI |
| US- and foreign-born combined | ||||||||
| Overall | 2005–2007 | −0.84 | −7.15 to 5.90 | 2007–2014 | 3.05a | 2.25 to 3.85 | 2.17a | 0.95 to 3.41 |
| Sex | ||||||||
| Male | 2005–2014 | 2.49a | 1.94 to 3.03 | 2.49a | 1.94 to 3.03 | |||
| Female | 2005–2008 | −1.41 | −3.97 to 1.23 | 2008–2014 | 3.58a | 2.75 to 4.42 | 1.89a | 1.10 to 2.70 |
| Age: male | ||||||||
| <35 y | 2005–2014 | −2.48 | −5.71 to 0.85 | −2.48 | −5.71 to 0.85 | |||
| 35–49 y | 2005–2014 | −2.81a | −3.96 to −1.64 | −2.81a | −3.96 to −1.64 | |||
| 50–64y | 2005–2012 | 5.24a | 4.42 to 6.06 | 2012–2014 | −0.87 | −5.62 to 4.11 | 3.85a | 2.87 to 4.84 |
| 65–79 y | 2005–2007 | −0.74 | −5.04 to 3.75 | 2007–2014 | 2.82a | 2.29 to 3.36 | 2.02a | 1.20 to 2.85 |
| ≥80 y | 2005–2014 | 1.75a | 0.75 to 2.76 | 1.75a | 0.75 to 2.76 | |||
| Age: female | ||||||||
| <35 y | 2005–2014 | −0.22 | −2.89 to 2.53 | −0.22 | −2.89 to 2.53 | |||
| 35–49 y | 2005–2014 | −0.17 | −2.27 to 1.99 | −0.17 | −2.27 to 1.99 | |||
| 50–64 y | 2005–2008 | 0.47 | −3.88 to 5.02 | 2008–2014 | 5.72a | 4.42 to 7.03 | 3.94a | 2.61 to 5.29 |
| 65–79 y | 2005–2007 | −2.13 | −5.78 to 1.66 | 2007–2014 | 2.95a | 2.48 to 3.43 | 1.80a | 1.09 to 2.52 |
| ≥80 y | 2005–2007 | −7.68 | −15.69 to 1.09 | 2007–2014 | 2.86a | 1.69 to 4.04 | 0.42 | −1.26 to 2.12 |
| Race/ethnicity: male | ||||||||
| Non-Hispanic white | 2005–2014 | 2.65a | 2.13 to 3.18 | 2.65a | 2.13 to 3.18 | |||
| Non-Hispanic black | 2005–2014 | 2.06a | 1.34 to 2.79 | 2.06a | 1.34 to 2.79 | |||
| Non-Hispanic API | 2005–2014 | 0.17 | −0.49 to 0.84 | 0.17 | −0.49 to 0.84 | |||
| Hispanic | 2005–2014 | 1.59a | 0.60 to 2.58 | 1.59a | 0.60 to 2.58 | |||
| Race/ethnicity: female | ||||||||
| Non-Hispanic white | 2005–2008 | −1.50 | −4.56 to 1.67 | 2008–2014 | 3.65a | 2.64 to 4.68 | 1.91a | 0.95 to 2.88 |
| Non-Hispanic black | 2005–2007 | −2.10 | −12.06 to 8.98 | 2007–2014 | 3.44a | 2.16 to 4.74 | 2.18a | 0.20 to 4.21 |
| Non-Hispanic API | 2005–2014 | −0.26 | −2.02 to 1.54 | −0.26 | −2.02 to 1.54 | |||
| Hispanic | 2005–2014 | 1.46a | 0.37 to 2.57 | 1.46a | 0.37 to 2.57 | |||
| Foreign-born only | ||||||||
| Overall | 2005–2007 | −4.04 | −14.25 to 7.38 | 2007–2014 | 1.86a | 0.53 to 3.21 | 0.52 | −1.53 to 2.61 |
| Sex | ||||||||
| Male | 2005–2014 | 1.15a | 0.12 to 2.19 | 1.15a | 0.12 to 2.19 | |||
| Female | 2005–2007 | −7.23 | −17.89 to 4.82 | 2007–2014 | 2.27a | 0.86 to 3.69 | 0.08 | −2.13 to 2.33 |
| Age: male | ||||||||
| <35 y | 2005–2014 | −2.34 | −8.64 to 4.40 | −2.34 | −8.64 to 4.40 | |||
| 35–49 y | 2005–2014 | −2.39a | −3.86 to −0.89 | −2.39a | −3.86 to −0.89 | |||
| 50–64 y | 2005–2014 | 1.46a | 0.38 to 2.56 | 1.46a | 0.38 to 2.56 | |||
| 65–79 y | 2005–2014 | 0.85 | −0.36 to 2.08 | 0.85 | −0.36 to 2.08 | |||
| ≥80 y | 2005–2014 | 2.53a | 1.36 to 3.72 | 2.53a | 1.36 to 3.72 | |||
| Age: female | ||||||||
| <35 y | 2005–2014 | −11.51a | −18.33 to −4.12 | −11.51a | −18.33 to −4.12 | |||
| 35–49 y | 2005–2014 | −3.18 | −6.82 to 0.60 | −3.18 | −6.82 to 0.60 | |||
| 50–64 y | 2005–2014 | 1.52 | −0.50 to 3.58 | 1.52 | −0.50 to 3.58 | |||
| 65–79 y | 2005–2014 | 0.97 | −0.58 to 2.55 | 0.97 | −0.58 to 2.55 | |||
| ≥80 y | 2005–2007 | −11.02 | −29.67 to 12.57 | 2007–2014 | 3.65a | 0.84 to 6.54 | 0.19 | −4.03 to 4.60 |
| Race/ethnicity: male | ||||||||
| Non-Hispanic white | 2005–2014 | 2.13a | 0.80 to 3.48 | 2.13a | 0.80 to 3.48 | |||
| Non-Hispanic black | 2005–2014 | −3.82a | −5.88 to −1.71 | −3.82a | −5.88 to −1.71 | |||
| Non-Hispanic API | 2005–2014 | −0.45 | −1.21 to 0.31 | −0.45 | −1.21 to 0.31 | |||
| Hispanic | 2005–2014 | 2.07 | −0.09 to 4.28 | 2.07 | −0.09 to 4.28 | |||
| Race/ethnicity: female | ||||||||
| Non-Hispanic white | 2005–2014 | 1.32 | −0.82 to 3.50 | 1.32 | −0.82 to 3.50 | |||
| Non-Hispanic black | 2005–2014 | −0.49 | −5.24 to 4.50 | −0.49 | −5.24 to 4.50 | |||
| Non-Hispanic API | 2005–2007 | −12.28 | −29.74 to 9.52 | 2007–2014 | 1.06 | −1.58 to 3.77 | −2.07 | −5.98 to 2.00 |
| Hispanic | 2005–2014 | 1.58 | −0.08 to 3.27 | 1.58 | −0.08 to 3.27 | |||
| US-born only | ||||||||
| Overall | 2005–2014 | 2.72a | 2.11 to 3.34 | 2.72a | 2.11 to 3.34 | |||
| Sex | ||||||||
| Male | 2005–2014 | 2.69a | 2.14 to 3.25 | 2.69a | 2.14 to 3.25 | |||
| Female | 2005–2008 | −1.19 | −4.27 to 1.99 | 2008–2014 | 3.74a | 2.73 to 4.75 | 2.07a | 1.11 to 3.04 |
| Age: male | ||||||||
| <35 y | 2005–2014 | −1.76 | −5.33 to 1.95 | −1.76 | −5.33 to 1.95 | |||
| 35–49 y | 2005–2014 | −3.16a | −4.61 to −1.70 | −3.16a | −4.61 to −1.70 | |||
| 50–64 y | 2005–2012 | 5.66a | 4.90 to 6.43 | 2012–2014 | −0.52 | −4.94 to 4.11 | 4.26a | 3.34 to 5.18 |
| 65–79 y | 2005–2014 | 2.53a | 1.95 to 3.11 | 2.53a | 1.95 to 3.11 | |||
| ≥80 y | 2005–2014 | 1.52a | 0.43 to 2.62 | 1.52a | 0.43 to 2.62 | |||
| Age: female | ||||||||
| <35 y | 2005–2014 | 0.61 | −1.68 to 2.96 | 0.61 | −1.68 to 2.96 | |||
| 35–49 y | 2005–2014 | 0.56 | −1.47 to 2.62 | 0.56 | −1.47 to 2.62 | |||
| 50–64y | 2005–2008 | 1.43 | −3.35 to 6.43 | 2008–2014 | 6.08a | 4.67 to 7.51 | 4.50a | 3.05 to 5.98 |
| 65–79 y | 2005–2008 | −0.50 | −1.97 to 0.99 | 2008–2014 | 3.38a | 2.91 to 3.85 | 2.07a | 1.62 to 2.52 |
| ≥80 y | 2005–2007 | −7.04 | −15.95 to 2.82 | 2007–2014 | 2.42a | 1.12 to 3.75 | 0.24 | −1.62 to 2.13 |
| Race/ethnicity: male | ||||||||
| Non-Hispanic white | 2005–2014 | 2.67a | 2.11 to 3.23 | 2.67a | 2.11 to 3.23 | |||
| Non-Hispanic black | 2005–2014 | 2.47a | 1.79 to 3.16 | 2.47a | 1.79 to 3.16 | |||
| Non-Hispanic API | 2005–2014 | 3.07 | −0.42 to 6.68 | 3.07 | −0.42 to 6.68 | |||
| Hispanic | 2005–2014 | 1.51a | 0.63 to 2.39 | 1.51a | 0.63 to 2.39 | |||
| Race/ethnicity: female | ||||||||
| Non-Hispanic white | 2005–2008 | −1.29 | −4.82 to 2.37 | 2008–2014 | 3.66a | 2.49 to 4.84 | 1.98a | 0.88 to 3.10 |
| Non-Hispanic black | 2005–2007 | −2.99 | −13.28 to 8.52 | 2007–2014 | 3.92a | 2.56 to 5.29 | 2.34a | 0.26 to 4.47 |
| Non-Hispanic API | 2005–2014 | 2.65 | −1.07 to 6.51 | 2.65 | −1.07 to 6.51 | |||
| Hispanic | 2005–2009 | −0.89 | −3.99 to 2.30 | 2009–2014 | 2.81a | 0.78 to 4.88 | 1.15 | −0.23 to 2.54 |
Abbreviations: AAPC, average annual percent change; APC, annual percent change; API, Asian/Pacific Islander; CI, confidence interval. The liver cancer mortality rates include liver and intrahepatic bile duct cancers (C22). A maximum of 1 joinpoint was allowed.
The APC or AAPC was significantly different from 0 at α = .05.
Among foreign-born individuals overall, no significant changes were observed in liver cancer mortality rates from 2005 to 2014 (AAPC, 0.5; 95% CI, −1.5 to 2.6; Table 2). When they were stratified by sex, a significant increase in mortality rates was observed among men overall (AAPC, 1.2), with rates specifically increasing among men aged 50 to 64 years (AAPC, 1.5) and men aged ≥80 years (AAPC, 2.5) and among men of non-Hispanic white race/ethnicity (AAPC, 2.1). Among foreign-born women, no significant increases in mortality rates were observed overall, by age, or by race/ethnicity stratifications.
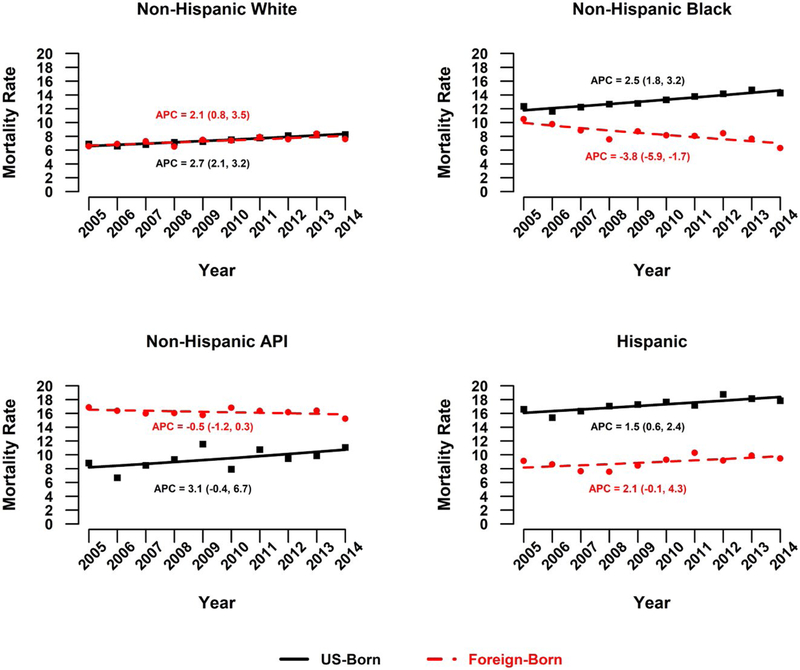
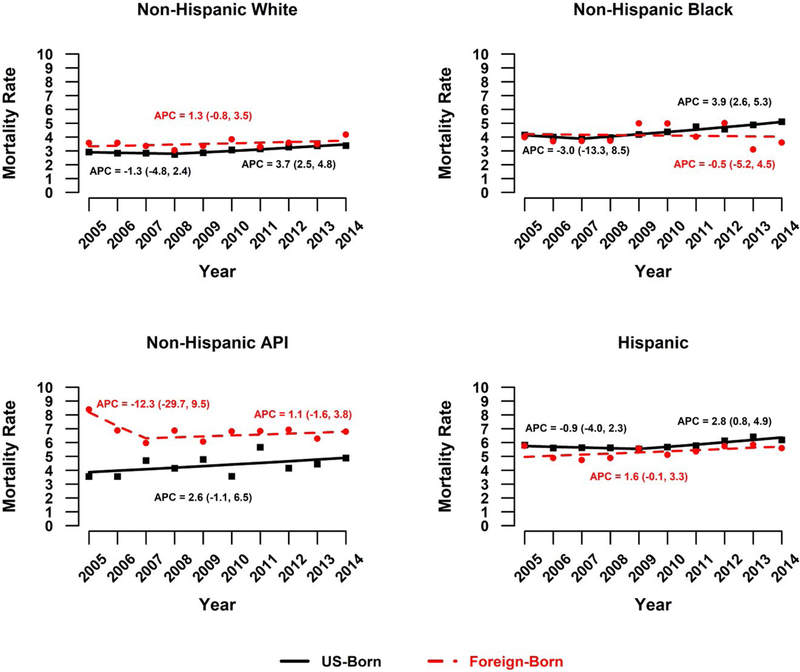
Liver cancer mortality rates significantly increased from 2005 to 2014 among US-born individuals (AAPC, 2.7; 95% CI, 2.1–3.3; Table 2). Among US-born men, liver cancer mortality rates significantly increased overall (AAPC, 2.7), with rates specifically increasing among men 50 years old or older and among non-Hispanic white, non-Hispanic black, and Hispanic men (Fig. 1). The trends for US-born women varied; significant increases were observed overall (AAPC, 2.1), among women 50 to 79 years old (AAPC for 50–64 years, 4.5; AAPC for 65–79 years, 2.1), and among non-Hispanic white women (AAPC, 2.0) and non-Hispanic black women (AAPC, 2.3; Fig. 2 and Table 2).
Figure 1.
APCs in liver cancer mortality rates among men by nativity status: United States, 2005–2014. APC indicates annual percent change; API, Asian/Pacific Islander.
Figure 2.
APCS in liver cancer mortality rates among women by nativity status: United States, 2005–2014. APC indicates annual percent change; API, Asian/Pacific Islander.
DISCUSSION
Foreign-born individuals in the United States have an elevated risk of liver cancer mortality in comparison with the US-born population; however, their risk varies by age, sex, and race/ethnicity. In both foreign- and US-born populations, liver cancer mortality rates were higher for men than women and for older individuals than younger age groups. The findings from this study are consistent with other studies that have found higher liver cancer mortality rates among foreign-born individuals,8 men,3 and older age groups3,4,20,21 in comparison with their respective counterparts. When we analyzed trends over time, we found that the foreign-born population had few significant changes in liver cancer mortality rates, whereas a significant increasing trend was observed in the US-born population for most race/ethnicities, age groups, and sex stratifications.
Although foreign-born individuals generally had an elevated risk of liver cancer mortality, when they were stratified by ethnicity and sex, foreign-born non-Hispanic APIs were the only foreign-born group to have significantly higher liver cancer mortality for both sexes in comparison with their US-born counterparts. The high risk of liver cancer mortality observed among foreign-born APIs is likely to be partially explained by an elevated risk of acquiring an HBV infection before their immigration to the United States.22 HBV infection rates among foreign-born individuals can range from 1 to 33 times the observed rates among the general US-born population according to their country of origin and subsequent HBV vaccination rates.9,10 As childhood HBV vaccination rates continue to improve globally, it is likely that the liver cancer mortality rates among foreign-born individuals in the United States will decline.3 In contrast to foreign-born APIs, foreign-born Hispanic and non-Hispanic black men had significantly lower rates of liver cancer mortality than their US-born counterparts. This may partly be explained by the higher rates of metabolic disorders, alcoholic liver disease, and HCV infection among US-born Hispanic men in comparison with their foreign-born counterparts,22 and it is likely that a similar trend is occurring among US-born non-Hispanic blacks.8 In contrast to previous work, this study did not find a significant decrease in liver cancer mortality rates among US- or foreign-born APIs.3,4 Although this finding could be due to fluctuating mortality rates, these rates should be monitored and elucidated to improve interventions in this population.
Notably, our study also shows a continuation of the rapidly rising trend in liver cancer mortality rates in the US-born population. In the current study, liver cancer mortality rates significantly increased for nearly all sex, age, and race/ethnicity groups in the US-born population, and this aligned with previous findings.3,4 It is likely that this increase will continue, and it has been estimated that by 2030, liver cancer will the third leading cause of cancer mortality among men and women.23 Because of the limited treatment options available after diagnosis, the 5-year relative survival rate of patients with liver cancer is only 14.8%.24 To alleviate the higher liver cancer mortality burden observed among foreign-born individuals and reverse the increasing trend observed in the US-born population, a combination of behavioral change programs for weight management, smoking cessation, reductions in alcohol consumption, and risk reduction for people who inject drugs as well as screening and treatment efforts for HBV and HCV will be needed.
Chronic infection with HBV or HCV is a well- documented risk factor for the development of liver cancer; currently in the United States, upward of 2.2 million individuals have chronic HBV,10,25 and 3.5 million have an HCV infection.26 HBV vaccination is 80% to 100% effective in preventing disease. For patients with chronic HBV, early diagnosis and treatment can reduce the risk of liver cancer by 50% to 80%.27,28 There are curative treatment options for HCV infection that can reduce the risk of liver cancer by 75%.29,30 Improved hepatitis screening, HBV vaccination, and hepatitis treatment services for at-risk groups would help to alleviate the growing burden of liver cancer in the United States. The Centers for Disease Control and Prevention and the US Preventive Services Task Force both currently recommend HBV and HCV testing for individuals at high risk for infection. For HBV infection, foreign-born individuals whose country of origin has an HBV infection prevalence higher than 2% are considered a high-risk group.31 For HCV, the following factors are associated with a high risk of infection: current or past injection drug use, long-term hemodialysis, a blood transfusion before 1992, high-risk sexual behaviors, and birth between 1945 and 1965.
Globally, chronic HBV and HCV infections account for a majority of liver cancer cases, but in the United States, the risk factors for liver cancer have been gradually changing.3,10 Historically in the United States, HBV or HCV infections were the leading cause of liver cancer mortality. However, with the implementation of the childhood HBV vaccine and with the elimination of HCV from the nation’s blood supply, the prevalence of these infections in the United States has declined, whereas the incidence and mortality rate of liver cancer continue to increase.6 In a recent study, Makarova-Rusher et al6 calculated the population attributable fraction (PAF) for each of the major risk factors for liver cancer and showed how each risk factor contributed to the overall liver cancer burden in the United States. Their work found that metabolic disorders, including obesity, were responsible for 32% of the liver cancer burden in the United States, and they were followed by HCV (PAF, 20.5%), alcohol (PAF, 13.4%), smoking (PAF, 9%), HBV (PAF, 4.3%), and genetic disorders (PAF, 1.5%); these findings were consistent with another study.32 These results highlight that despite the low liver cancer risk conferred by some risk factors (eg, metabolic disorders or smoking), because of the high prevalence of these conditions in the population, they can account for a substantial number of cancer cases.
The main strength of this study is that it combines validated data from multiple sources to provide the most up-to-date comprehensive examination of liver cancer mortality rates by place of birth for the entire US population over the past decade. In addition, because the place of birth is frequently missing from cancer incidence data, this analysis approach provides the only way to accurately estimate the burden of cancer in the immigrant population at a national level, with only 1.2% of cases missing data for the place of birth. One limitation of this study is that mortality data were based on information obtained from death certificates, and this may have resulted in the undercounting of liver cancer deaths if the causes of death were not fully recorded. In addition, cancers frequently metastasize and spread to the liver, and this leaves the possibility that metastasized cancers from other sites could have been recorded as primary liver cancer. These issues were likely eliminated with the trend analysis because these issues are likely to occur at a constant rate over time.33 In addition, we did not have access to all variables relevant to liver cancer mortality (specifically, socioeconomic attributes, etiologic risk factors, and health insurance status). Finally, we did not differentiate between hepatocellular carcinoma and intrahepatic bile duct cancer in this article because of concerns about misclassification.34,35
In summary, foreign-born individuals in the United States have an elevated risk of liver cancer mortality in comparison with the US-born population. However, although the liver cancer mortality rates among foreign-born individuals have remained fairly stable from 2005 to 2014, the mortality rates among US-born individuals have significantly increased for nearly all race and sex subgroups. Efforts to address the major risk factors for liver cancer are needed to address the health disparities observed among foreign-born individuals and reverse the increasing trend observed in the US-born population.
FUNDING SUPPORT
No specific funding was disclosed.
We thank Jessica King and Trevor Thompson for their help in building the data set for this article.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Islami F, Miller KD, Siegel RL, Fedewa SA, Ward EM, Jemal A. Disparities in liver cancer occurrence in the United States by race/ ethnicity and state. CA Cancer J Clin. 2017;67:273–289. [DOI] [PubMed] [Google Scholar]
- 2.US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2014 Incidence and Mortality Web-Based Report. Atlanta, GA: National Cancer Institute; 2017. [Google Scholar]
- 3.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual report to the nation on the status of cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109:542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Martel C, Maucort-Boulch D, Plummer M, Franceschi S. Worldwide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015;62:1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makarova-Rusher OV, Altekruse SF, McNeel TS, et al. Population attributable fractions of risk factors for hepatocellular carcinoma in the United States. Cancer. 2016;122:1757–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Census Bureau. The foreign-born population in the United States: 2010. American Community Survey Reports. 2012. www2.census.gov/library/publications/2012/acs/acs-19.pdf. [Google Scholar]
- 8.Singh GK, Hiatt RA. Trends and disparities in socioeconomic and behavioural characteristics, life expectancy, and cause-specific mortality of native-born and foreign-born populations in the United States, 1979–2003. Int J Epidemiol. 2006;35:903–919. [DOI] [PubMed] [Google Scholar]
- 9.Pollack HJ, Kwon SC, Wang SH, Wyatt LC, Trinh-Shevrin C. Chronic hepatitis B and liver cancer risks among Asian immigrants in New York City: results from a large, community-based screening, evaluation, and treatment program. Cancer Epidemiol Biomarkers Prev. 2014;23:2229–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56:422–433. [DOI] [PubMed] [Google Scholar]
- 11.Cokkinides VE, Bandi P, Siegel RL, Jemal A. Cancer-related risk factors and preventive measures in US Hispanics/Latinos. CA Cancer J Clin. 2012;62:353–363. [DOI] [PubMed] [Google Scholar]
- 12.Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341:556–562. [DOI] [PubMed] [Google Scholar]
- 13.Tarraf W, Miranda PY, Gonzalez HM. Medical expenditures among immigrant and non-immigrant groups in the US: findings from the Medical Expenditures Panel Survey (2000–2008). Med Care. 2012;50:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han H, Perumalswami PV, Kleinman LC, Jandorf LH. Voices of multi-ethnic providers in NYC: health care for viral hepatitis to prevent hepatocellular carcinoma. J Cancer Educ. 2014;29:214–223. [DOI] [PubMed] [Google Scholar]
- 15.Pinheiro PS, Callahan KE, Gomez SL, et al. High cancer mortality for US-born Latinos: evidence from California and Texas. BMC Cancer. 2017;17:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Setiawan VW, Wei PC, Hernandez BY, et al. Disparity in liver cancer incidence and chronic liver disease mortality by nativity in Hispanics: the multiethnic cohort. Cancer. 2016;122:1444–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh GK, Rodriguez-Lainz A, Kogan MD. Immigrant health inequalities in the United States: use of eight major national data systems. ScientificWorldJournal. 2013;2013:512313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Census Bureau. American Community Survey: Design and Methodology Report. [Google Scholar]
- 19.Seeff LC, McKenna MT. Cervical cancer mortality among foreign-born women living in the United States, 1985 to 1996. Cancer Detect Prev. 2003;27:203–208. [DOI] [PubMed] [Google Scholar]
- 20.Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012;55(suppl 1):S10–S15. [DOI] [PubMed] [Google Scholar]
- 21.King DE, Matheson E, Chirina S, Shankar A, Broman-Fulks J. The status of baby boomers’ health in the United States: the healthiest generation? JAMA Int Med. 2013;173:385–386. [DOI] [PubMed] [Google Scholar]
- 22.El-Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167:1983–1989. [DOI] [PubMed] [Google Scholar]
- 23.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 24.Momin BR, Pinheiro PS, Carreira H, Li C, Weir HK. Liver cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123(123 suppl 24):5059–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts H, Kruszon-Moran D, Ly KN, et al. Prevalence of chronic hepatitis B virus (HBV) infection in US households: National Health and Nutrition Examination Survey (NHANES), 1988–2012. Hepatology. 2016;63:388–397. [DOI] [PubMed] [Google Scholar]
- 26.Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62:1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordon SC, Lamerato LE, Rupp LB, et al. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol. 2014;12:885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai CL, Yuen MF. Prevention of hepatitis B virus–related hepatocellular carcinoma with antiviral therapy. Hepatology. 2013;57:399–408. [DOI] [PubMed] [Google Scholar]
- 29.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 part 1):329–337. [DOI] [PubMed] [Google Scholar]
- 30.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308:2584–2593. [DOI] [PubMed] [Google Scholar]
- 31.LeFevre ML. Screening for hepatitis B virus infection in non-pregnant adolescents and adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2014; 161:58–66. [DOI] [PubMed] [Google Scholar]
- 32.Welzel TM, Graubard BI, Quraishi S, et al. Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol. 2013;108:1314–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–278. [DOI] [PubMed] [Google Scholar]
- 34.Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56:848–854. [DOI] [PubMed] [Google Scholar]
- 35.Tyson GL, Ilyas JA, Duan Z, et al. Secular trends in the incidence of cholangiocarcinoma in the USA and the impact of misclassification. Dig Dis Sci. 2014;59:3103–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]