Abstract
Objective:
Investigate feasibility of telehealth with remote blood pressure monitoring for management of hypertension in postpartum women at risk of severe hypertension after hospital discharge.
Methods:
In a single-center, prospective single-cohort feasibility study, women with hypertension in pregnancy participated in a postpartum telehealth intervention for blood pressure management after discharge. The primary feasibility outcome measures were recruitment and retention through 6 weeks postpartum. Secondary outcomes included the incidence of severe postpartum hypertension and/or need for blood pressure treatment after discharge, participant satisfaction, and 6-week hospital readmission. Participants received a tablet and equipment to transmit vital signs to a central monitoring site daily. Participants participated in telehealth or telephone visits with a nurse at 48 h and as needed.
Results:
Among 1413 deliveries 263 (19%) women had hypertension in pregnancy and 55/124 (47%) of women approached were consented. The retention rate was 95%. Among study participants, the incidence of severe hypertension after discharge was 9 (16%). 29 (53%) of participants required treatment due to exacerbations in blood pressure after discharge, in which 9(16%) were severe. There were no hospital readmissions. Overall 39 (86%) participants were satisfied with the remote monitoring.
Conclusions:
Feasibility and participant satisfaction were demonstrated. The incidence of severe hypertension and need for blood pressure treatment after discharge and during 6 weeks postpartum was 16% and 53%. Our results indicate telehealth is a promising strategy for postpartum hypertension management to decrease maternal morbidity and hospital readmission.
Keywords: Hypertension, Pregnancy, Telehealth, Hospital readmission
1. Introduction
Approximately 10% of pregnancies in the United States and 22% of pregnancies in Wisconsin are affected by hypertension-related disorders [1–4]. The majority of research has focused on antenatal management of hypertension in pregnancy; therefore, there is very little information on how to best manage postpartum hypertension, regardless of type or severity to optimize maternal safety [5]. Postpartum, it is not uncommon for normotensive women to have a physiologic increase in blood pressure [6]. Furthermore, in an observational study in hypertensive women with or without proteinuria, many of the women had an initial decrease in blood pressure after delivery, followed by a rise to hypertensive levels between days 3–6 postpartum [7]. Approximately, 50% of the participants had a blood pressure of 150/100 mmHg or more recorded on day 5 postpartum and blood pressures of 170/100 mmHg were frequently attained during the first week postpartum by many participants [7]. This increase typically occurs after the woman is discharged from the hospital. The exact reason for exacerbation of hypertension between days 3–5 is not exactly known, but it can have serious consequences such as stroke and rarely death [8–10]. A previous study reported the mean time to regain normotensive values of 5.7 ± 3.7 weeks post-delivery in the postpartum period for women with gestational hypertension and preeclampsia [11].
The American College of Obstetricians and Gynecologists (ACOG) guidelines suggest blood pressure monitoring in the hospital or that equivalent outpatient surveillance be performed for at least 72 h post-partum and 7–10 days after childbirth or earlier in women with symptoms who had gestational hypertension, preeclampsia, or superimposed preeclampsia [1]. Non-pregnant adult hypertension has been recently redefined as Stage 1 hypertension being 130–139 mmHg or 80–89 mmHg and Stage 2 hypertension being ≥140 mmHg or ≥90 mmHg [12]. Non-pregnant, severe hypertension is typically de-fined as blood pressure of > 170 mmHg or > 110 mmHg. During pregnancy and postpartum, severe hypertension is defined as > 160 mmHg or > 110 mmHg and consensus guidelines recommend acute treatment to prevent acute maternal vascular complications [1]. There are no evidence-based guidelines for management of postpartum hypertension; however, it has been suggested to consider treatment of postpartum women with blood pressure values of ≥150 mmHg or ≥100 mmHg, knowing that maternal blood pressure typically rises in the first week postpartum [1].
Postpartum hypertension is a leading indication for hospital read-missions within the first 6 weeks after delivery [2]. Compared with normotensive pregnancies, hypertension-related disorders of pregnancy resulted in an excess 404,800 hospital days and inpatient care costs of $731 million through 42-days postpartum [3]. There is emerging data on the feasibility and satisfaction of home blood pressure monitoring for postpartum women with a hypertension-related pregnancy disorder [13–18]. Cost-effective interventions are needed to identify women at risk for severe postpartum hypertension, support optimal blood pressure follow-up, and self-management.
2. Methods
2.1. Objective
The purpose of this study was to investigate the feasibility of tele-health with remote blood pressure monitoring for management of hypertension in postpartum women at risk of severe or exacerbation in hypertension after hospital discharge, including recruitment, consent and retention.
2.2. Study population
The study population included women admitted to the labor and delivery unit of a Midwestern academic hospital with the following inclusion criteria: ≥18 years old, with one of the following hypertension-related diagnoses during pregnancy: chronic, gestational, preeclampsia, or eclampsia, or a new hypertension diagnosis postpartum. These diagnoses were made using clinical data during hospitalization and verified in the electronic medical record by the research coordinator prior to eligible women being approached for study enrollment/consent. Regardless of the hypertension diagnosis, the blood pressure criteria for defining a new hypertension diagnosis during pregnancy required a SBP ≥ 140 mmHg or DBP ≥ 90 mmHg on two separate occasions, at least 4 h apart. Women were excluded if they had been readmitted after their primary hospital admission for delivery of their neonate. We obtained maternal and obstetrical sociodemographic data from the electronic medical records, including parity, maternal age, race/ethnicity, insurance status, body mass index (BMI), and gestational age at delivery. Women were informed of this study by a physician with obstetric privileges or midwife provider. If they were interested, they were placed on a list to be approached by the study coordinator for possible enrollment and if they were accepting of enrollment, they were consented. Reasons women did not consent included: 1) ineligibility, 2) unwilling to participate, 3) insufficient staffing for recruitment, and 4) equipment availability. Incentives for participation included a 20-package of diapers at enrollment and a $15 gift card at 6 weeks postpartum.
The primary feasibility outcome measures were recruitment, consent and retention through 42 days postpartum. The secondary outcomes were the incidence of severe hypertension (defined using ACOG criteria blood pressure values of ≥160 or ≥110 mmHg) after discharge, postpartum blood pressure requiring treatment (defined using ACOG criteria blood pressure values of ≥150 mmHg or ≥100 mmHg), participant evaluation of the equipment and satisfaction, total participant contacts required, and hospital readmission at 6 weeks postpartum. The study was approved by the University of Wisconsin Institutional Review Board with written informed consent.
2.3. Intervention
Upon enrollment participants received a tablet device (The Genesis Touch® remote participant monitor, which leverages a Samsung Galaxy Tab®), blood pressure cuff (A&D 787), weight scale (Honeywell®), and oxygen sensor (Honeywell Life Care Solutions, Brookfield WI). Equipment training was provided to all participants prior to discharge from the hospital. The equipment was registered to a specific participant that synced to a central monitoring platform allowing for Bluetooth transmission of all home vitals. After hospital discharge, the participant transmitted their vitals daily and had scheduled telehealth visits and/or telephone calls with a research nurse 48 h after discharge, and later as needed. One of two nurses was available for each participant daily. The nurses were required for participant care and follow-up approximately 20 h per week.
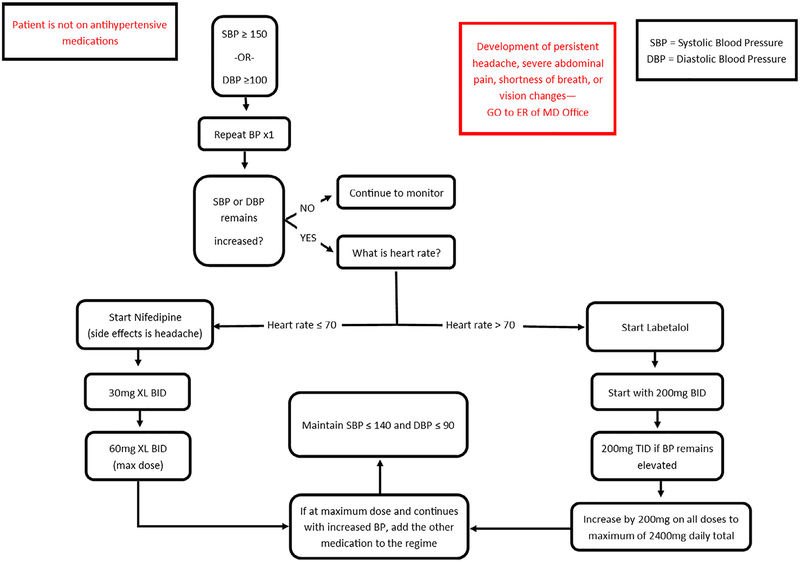
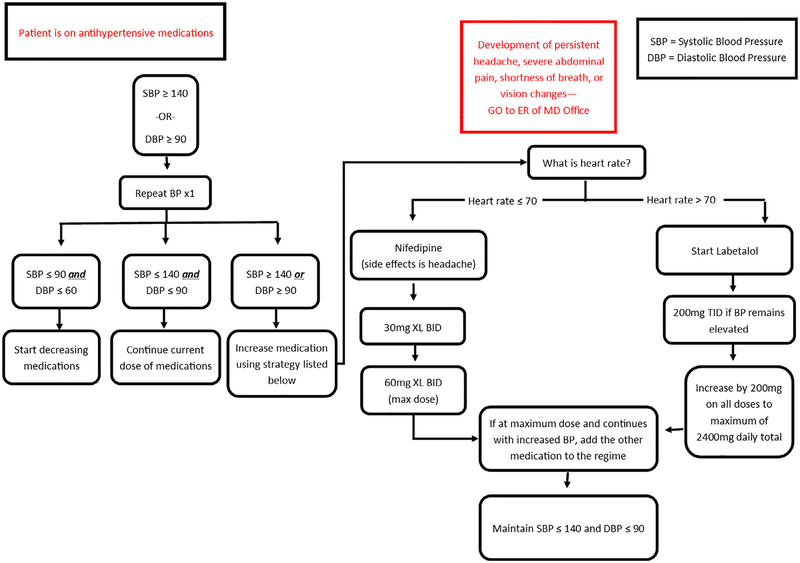
We designed a nurse-driven blood pressure management and treatment algorithm which allowed for early recognition and prompt treatment of severe hypertension (Figs. 1 and 2). Participants reporting symptoms of severe hypertension (e.g., headache, visual disturbances, abdominal pain, shortness of breath) were directed to the hospital triage/emergency room for evaluation. Each participant completed a routine 6-week postpartum clinic visit, which was the study endpoint. Hypertension management was then assumed by their obstetrician or they were referred to their primary care provider.
Fig. 1.
Management algorithm for severe postpartum hypertension in participants not on an antihypertensive medication.
Fig. 2.
Management algorithm for participants on an antihypertensive medication.
In collaboration with the University of Wisconsin Survey Center, we developed a 37-question self-administered questionnaire (SAQ) satisfaction survey to all participants at the 6-week postpartum clinic visit. The participants provided an email address at the time of consent, which allowed the survey to be emailed to all participants through the Qualtrics Survey Service (Qualtrics, Provo, UT). The dimensions of the questions covered: perception of quality of care received, ease of use/ease to learn the telehealth program, effective orientation of the equipment, level of perceived security/privacy utilizing telehealth, and problems encountered using the health equipment devices. For this analysis, we utilized these specific questions: “To what extent would you prefer going to the hospital or clinic instead of using the telehealth technology at home?”; “How much would you recommend the telehealth technology to other women in your situation?”; “How complicated were the instructions for using the telehealth technology?”; “How much mental effort is required to interact with the telehealth technology?”; “How hard is it to use the Genesis Touch monitor?”; “How easy is it to use the blood pressure cuff to measure your blood pressure?”; “How hard is it to use the scale to weigh yourself?”; “How long does it take to measure and record your blood pressure?”; “How secure do you feel submitting your vitals using the telehealth equipment?”; and “Overall, how satisfied are you with the telehealth devices?”. The survey utilized a variety of question formats including Likert scales with response ranging from 1 to 5 (“Not at all”; “A little”; “Somewhat”; “Quite a bit”; “A great deal” or “Never”; “Rarely”; “Sometimes”; “Very often”; “Extremely often”), dichotomous questions with a Yes/No response, and free text.
2.4. Statistical considerations
A sample size of 55 was estimated to achieve a desired precision in the estimated recruitment and consent rates to within a 95% confidence interval of ± 13% or 14% assuming 10% drop-out [18–21]. Baseline demographic variables were collected from the electronic medical records on all participants. The primary outcome variables included recruitment and retention in telehealth for postpartum hypertension. Secondary outcomes included the incidence of severe hypertension after discharge, postpartum blood pressures requiring treatment, number of blood pressure readings submitted and number of participant contacts required, participant evaluation of the equipment and satisfaction, and 6-week hospital emergency room visits and hospital readmission after discharge. The participants who opted out of the study prior to the 6-week postpartum visit were included in the analysis when data were available. We analyzed the demographic characteristics outcomes using a descriptive analysis with mean (standard deviation), and frequency (percentage) where appropriate. All data were analyzed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
3. Results
Amongst 1413 women who were delivered during March 23, 2017-July 14, 2017, 263 (18%) had a hypertensive disorder affecting their pregnancy, and 124 of them agreed to be approached for this study, resulting in a recruitment rate of 47%. A total of 55 out of 124 approached participants gave informed consent, resulting in a consent rate of 44%. The most common reasons participants declined were: 1) overwhelmed with the postpartum period, 2) perception that participation was too time consuming, and 3) inability to return equipment at the end of the study. Over the course of the study period, 52 participants completed the 6-week clinic visit, resulting in a retention rate of 95%. All three women who opted out of the study before week 6 reported being overwhelmed with the postpartum period and 2 had neonates in the neonatal intensive care unit.
Baseline demographics and clinical characteristics of the participants at discharge are summarized in Table 1. Among the hypertensive women who enrolled, the mean (SD) maternal age was 31.8 ± 4.9; the mean (SD) pre-pregnancy body mass index (BMI) was 32.6 ± 8.3, 45 (82%) were married; 34 (62%) were nulliparous; 51 (93%) were white; 5 (9%) were Hispanic; and 4 (7%) currently used tobacco. The mean (SD) gestational age at delivery was 37.3 ± 3.1 weeks; the mode of delivery was vaginal in 23 (42%) and cesarean in 32 (58%); and 16 neonates (29%) were admitted to the neonatal intensive care unit. Type of maternal hypertension included 6 (11%) chronic including three with superimposed preeclampsia, 17 (31%) gestational, and 35 (64%) preeclampsia. 47 (85%) participants received non-steroidal anti-inflammatory drugs during their postpartum admission. The mean (SD) postpartum day of hospital discharge was 3.2 ± 1.0. Overall, 22 (40%) participants were discharged with at least one antihypertensive medication including 12 with labetalol. The mean (SD) blood pressure at discharge was 131/77.1 ± 10.5/8.9 mmHg. 44 (80%) of the participants lived within 20 miles of the Midwestern academic hospital. The remaining 10% of study participants all lived within 50 miles of the hospital.
Table 1.
Maternal demographic and baseline characteristics.
| N = 55 | |
|---|---|
| Maternal age in years | 31.8 ± 4.9 |
| Pre-pregnancy body mass index | 32.6 ± 8.3 |
| Married | 45 (82) |
| Parity, Nulliparous | 34 (62) |
| Race, White | 51 (93) |
| Ethnicity, Hispanic | 5 (9.1) |
| Current tobacco use | 4(7) |
| Gestational age at delivery in weeks | 37.3 ± 3.1 |
| Mode of Delivery | |
| Vaginal, spontaneous or operative | 23 (42) |
| Cesarean | 32 (58) |
| Neonatal intensive care unit admission | 16 (29) |
| Type of Hypertension | |
| Chronic | 6 (11) |
| Superimposed preeclampsia | 3 (5.5) |
| Gestational | 17 (31) |
| Preeclampsia | 35 (64) |
| Severe features | 19 (35) |
| Inpatient NSAID use | 47 (85) |
| Postpartum day of discharge | 3.2 ± 1.0 |
| Systolic blood pressure mmHg at discharge | 131 ± 10.5 |
| Diastolic blood pressure mmHg at discharge | 77.1 ± 8.9 |
| Antihypertensive medication at discharge | 22 (40) |
| Labetalol | 12 (55) |
| Nifedipine | 4 (18) |
| Labetalol & Nifedipine | 6 (27) |
n, number; %, percent; SD, standard deviation; NSAID, non-steroidal anti-inflammatory drugs; mmHg, millimeters Mercury.
Data are expressed as mean ± SD or n (%).
The clinical outcomes from the participants after discharge including survey results at 6 weeks postpartum are summarized in Table 2. The mean (SD) number of blood pressure measurements received from patients through 6 weeks postpartum were 33.4 ± 11.1. The mean (SD) number of contacts nurses made with the participants was 6.65 ± 4.5.
Table 2.
Outcomes of participants after hospital discharge.
| N = 55 | |
|---|---|
| Total participants with severe hypertension after discharge* | 9 (16) |
| Time to first postpartum day severe hypertension | 5.7 ± 1.7 |
| Systolic blood pressure mmHg | 165 ± 7.1 |
| Diastolic blood pressure mmHg | 108 ± 11.1 |
| Total participants with increased blood pressure requiring treatment after discharge** | 29 (53) |
| 1st Postpartum day blood pressure required treatment after discharge** | 7.2 ± 7.7 |
| Systolic blood pressure mmHg | 149 ± 11.1 |
| Diastolic blood pressure mmHg | 101 ± 9.1 |
| Requiring antihypertensive medication over 6 weeks postpartum period | 33 (60) |
| Initiated a hypertensive medication after discharge | 11 (20) |
| Increased dosage of medications | 14 (25) |
| Emergency/triage room visit | 6 (11) |
| Hospital readmission | 0 (0) |
Data are expressed as mean ± SD or n (%).
n, number; %, percent; SD, standard deviation; NSAID, non-steroidal anti-inflammatory drugs; mmHg, millimeters Mercury.
Severe hypertension defined using ACOG criteria in pregnancy: ≥160 mmHg or ≥110 mmHg.
Blood pressure requiring postpartum treatment defined using ACOG recommendations of ≥150 mmHg or ≥100 mmHg.
After hospital discharge, 9 (16%) participants developed severe hypertension. Among these 9 participants, the mean (SD) blood pressure was 165/108 ± 7.1/11.1 mmHg; severe hypertension developed at a mean (SD) of 5.7 ± 1.7 days after discharge. Furthermore 33 (60%) of participants had blood pressures exceeding the ACOG recommendations for blood pressure treatment (150/100 mmHg). 14 (25%) of participants were prescribed an increased dose of an anti-hypertensive medication and 11 (20%) were started on their first antihypertensive. We referred 6 (11%) to the emergency department for evaluation of symptomatic severe hypertension (e.g., headache, blurry vision, abdominal pain, shortness of breath). There were no hospital readmissions.
3.1. Study/equipment evaluation
We had an 81% (N = 45) response rate for the participant satisfaction survey. Among the respondents, 38 (84%) would prefer telehealth to going to a hospital or clinic, 42 (93%) would recommend telehealth to other women, 41 (91%) reported the amount of mental effort required to interact with the telehealth technology was “not at all” or “a little,” 42 (93%) felt “quite a bit” or a “great deal” of security in submitting their health information via the telehealth device, 44 (98%) of participants reported it took 1 min to record their blood pressure. Overall 39 (87%) were “very” or “extremely” satisfied with the remote monitoring.
4. Discussion
Our feasibility study demonstrated that telehealth for postpartum blood pressure surveillance and treatment of hypertension is feasible, as assessed by recruitment, consent and retention. The majority of participants expressed satisfaction and preferred telehealth visits rather than going to a clinic for management of their postpartum hypertension. All patients had adequate blood pressure control prior to hospital discharge. Furthermore, using telehealth with remote patient monitoring technology in our Midwestern academic institution, we confirmed previous findings [6,7] that postpartum blood pressures indeed increase after hospital discharge. More importantly, we were able to identify severe hypertension early, provide prompt treatment, and eliminate hypertension-related hospital readmissions in our study over 42-days postpartum.
A prior study by Rhoads, et al identified and examined the potential factors that influenced the use of telehealth and adherence to self-monitoring among postpartum women with preeclampsia during the initial 2-week postpartum period [15]. On average, participants electing to use telehealth technology had lower levels of perceived technologic barriers, higher facilitating condition scores, and higher levels of perceived benefits of the technology, compared to non-users. A second study assessed suitability of remote blood pressure self-monitoring using a Bluetooth-enabled blood pressure machine and Android-based mobile phone [16]. The outcome of interest was technologic feasibility and acceptance by women. In this study, 90% of women agreed the kit was simple to use and 78% would prefer this model of testing at home. Our pilot study extends findings of prior studies and demonstrates feasibility of home blood pressure monitoring utilizing telehealth technology for management of postpartum hypertension disorders including; chronic hypertension, mild and severe preeclampsia, superimposed preeclampsia, and HELLP syndrome.
Our results demonstrate that participants view telehealth as a secure and easy monitoring technique. The majority of participants were satisfied with telehealth, preferred it to in-clinic care, and would recommend it to others. Collectively, our pilot study indicates that telehealth technology has high participant acceptance and the potential to be a viable alternative and/or supplement to routine outpatient visits for postpartum blood pressure monitoring. Our findings of strong participant satisfaction are similar to prior published data [16,17]. Larger scale studies are needed to support our findings and establish comparative effectiveness of home blood pressure monitoring with telehealth technology as compared to standard surveillance.
The exact incidence of postpartum hypertension is difficult to ascertain as there continues to be no reliable evidence-based clinical guidelines regarding prevention or treatment of postpartum hypertension [5,10]. Our pilot data has allowed us to start defining the natural history of postpartum hypertension. All of the participants achieved adequate blood pressure within the immediate 72 h post-delivery period, in which ACOG recommends close surveillance [1]. Post-delivery pain management of the study participants was managed pragmatically and at the discretion of the delivery provider. Our hospital policy regarding hypertension in pregnancy follows ACOG’s recommendations and does address the phenomenon that non-steroidal anti-inflammatory drugs (NSAID) are known to cause elevations in blood pressures and recommends consideration of avoidance for post-partum analgesia. Despite these recommendations, the majority (85%) of our participants received NSAIDs while in the hospital. In two recent retrospective studies, the impact of hospital administered postpartum NSAID use amongst women with severe hypertensive disorders of pregnancy have been evaluated and there was no increase in post-partum blood pressures, the requirement for antihypertensive medications, or the rate of adverse postpartum events [22,23]. In a recent randomized controlled trial of ibuprofen versus acetaminophen as a postpartum analgesic for women with severe preeclampsia they did conclude that ibuprofen significantly elevated blood pressures in the postpartum period [25]. Further research is needed to evaluate the effect of NSAID use inpatient and extended use of NSAIDs after hospital discharge.
After discharge we demonstrated 16% of participants had severe hypertension and 53% of participants had increasing blood pressures requiring treatment after the hospital discharge (mean postpartum day of discharge was 3.2 ± 1.0) through 6-week postpartum. Our program allowed for rapid recognition of severe hypertension and prompt treatment in women without concerning symptoms which likely successfully prevented hospital readmissions. Additionally, we identified symptomatic women who were brought in for evaluation to rule out and prevent adverse outcomes related to severe hypertension. The significance of understanding the natural time course of the development and resolution of pregnancy-related hypertension is important in developing meaningful immediate and long-term surveillance and treatment programs for postpartum women with hypertension. We suggest that the time women are at-risk for developing adverse events related to hypertension is beyond the currently recommended 72 h of initial surveillance and prior to the 7–10 days for a follow-up evaluation. We believe this explains why postpartum hypertension is amongst the most common cause for readmission and severe maternal morbidity/mortality. There is growing evidence that remote patient monitoring with a standardized central surveillance program has the ability to provide earlier diagnosis and prompt treatment of severe postpartum hypertension while improving maternal outcomes and decreasing the health care burden and cost of readmission, however larger clinical trials are needed.
4.1. Strengths and limitations to our study
Many limitations of this study reflect the feasibility design including a single clinical site and small sample size. However, our findings provide estimates needed to design an adequately powered multi-center randomized controlled trial to evaluate the comparative effectiveness and cost of telehealth monitoring. Our study is limited in the ability to understand the increased incidence of hypertension in pregnancy at our institution and across the state of Wisconsin, however this may be explained by the increased incidence of obesity, which was estimated at 29.8% in 2015 [26]. A strength of our admission data was collection of NSAID use inpatient, which did not appear to affect the control of hypertension prior to discharge. We were limited in the ability to adequately track NSAID use outpatient and we intend to track this further to assess any association of the experienced increase in outpatient postpartum blood pressures. We had limited racial/ethnic diversity due to the geographic location of the selected clinical site; however, our future trial will be designed for the recruitment and retention of a racial/ethnically and geographically diverse population. Our survey was developed for evaluation of the participants experience utilizing this specific telehealth with remote patient monitoring intervention. We intend to validate the survey to ensure that the questions included truly measure the issues of importance in assessing telehealth with remote patient monitoring that can be used in future research studies.
5. Conclusions
We demonstrated feasibility of telehealth monitoring for post-partum hypertension-related disorders. We observed important temporal trends in the natural history of postpartum hypertension from discharge through 6 weeks postpartum which will guide larger clinical trials. Telehealth monitoring is a promising outpatient treatment strategy for postpartum hypertension to reduce readmissions and decrease maternal morbidity which needs future research.
6. Prior Presentations
This manuscript was presented at the American Heart Association Council on Hypertension and American Society of Hypertension Joint Scientific Sessions, September 14–17, 2017 in San Francisco, CA.
Acknowledgments
The authors gratefully acknowledge the UnityPoint Health-Meriter Foundation for funding this research. Emmalee Davis, MS and Jamie LaMantia, BS for manuscript preparation.
7. Sources of Funding
This project was supported by the UnityPoint Health-Meriter Foundation and the University of Wisconsin Department of Obstetrics & Gynecology intramural departmental funding.
Footnotes
Potential Conflicts of Interest
None.
ClinicalTrials.gov identification number
.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.preghy.2018.12.007.
References
- [1].American College of Obstetrics and Gynecologists, Task force on hypertension in P. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy, Obstet. Gynecol 122 (2013) 1122–1131. [DOI] [PubMed] [Google Scholar]
- [2].Clapp MA, Little SE, Zheng J, Robinson JN, A multi-state analysis of post-partum readmissions in the United States, Am. J. Obstet. Gynecol 215 (113) (2016) e111–113 e110. [DOI] [PubMed] [Google Scholar]
- [3].Mogos MF, Salemi JL, Spooner KK, McFarlin BL, Salihu HH, Hypertensive disorders of pregnancy and postpartum readmission in the United States: national surveillance of the revolving door, J. Hypertens 36 (3) (2018) 608–618. [DOI] [PubMed] [Google Scholar]
- [4].Wisconsin Perinatal Quality Collaborative. Maternal Hypertension Initiative Madison, WI, USA: Available at: Accessed December 12, 2017. [Google Scholar]
- [5].Magee L, von Dadelszen P, Prevention and treatment of postpartum hypertension, Cochrane Database Syst. Rev (2013) CD004351. [DOI] [PubMed] [Google Scholar]
- [6].Walters BN, Walters T, Blood pressure in the puerperium, Clin. Sci. (Lond.) 71 (5) (1986) 589–594. [DOI] [PubMed] [Google Scholar]
- [7].Walters BNJ, Walters T, Hypertension in the puerperium, Lancet 2 (1987) 330. [DOI] [PubMed] [Google Scholar]
- [8].Peterson E, Craigo S, House M, Risk factors for postpartum antihypertensive mediation requirement in severe preeclampsia, Hypertens. Pregnancy 29 (2010) 350–356. [DOI] [PubMed] [Google Scholar]
- [9].Ferrazzani S, De Carolis S, Pomini F, et al. , The duration of hypertension in the puerperium of preeclamptic women:relationship with renal impairment and week of delivery, Am. J. Obstet. Gynecol 171 (2) (1994) 506–512. [DOI] [PubMed] [Google Scholar]
- [10].Sibai BM, Etiology and management of postpartum hypertension-preeclampsia, Am. J. Obstet. Gynecol 206 (2012) 470–475. [DOI] [PubMed] [Google Scholar]
- [11].Podymow T, August P, Postpartum course of gestational hypertension and preeclampsia, Hypertens. Pregnancy 29 (2010) 294–300. [DOI] [PubMed] [Google Scholar]
- [12].Welton PK, et al. , High blood pressure clinical practice guideline: executive summary. Am College of Cardiology & Am Heart Association Taskforce on Clinical Practice Guidelines, 2017. [Google Scholar]
- [13].Brown MA, Is there a role for ambulatory blood pressure monitoring in pregnancy? Clin. Exp. Pharmacol. Physiol 41 (2014) 16–21. [DOI] [PubMed] [Google Scholar]
- [14].Magee LA, von Dadelszen P, Chan S, Gafni A, Gruslin A, Helewa M, et al. , Women’s views of their experiences in the CHIPS (Control of Hypertension in Pregnancy Study) Pilot Trial, Hypertens. Pregnancy 26 (2007) 371–387. [DOI] [PubMed] [Google Scholar]
- [15].Taylor RS, Freeman L, North RA, Evaluation of ambulatory and self-initiated blood pressure monitors by pregnant and postpartum women, Hypertens. Pregnancy 20 (2001) 25–33. [DOI] [PubMed] [Google Scholar]
- [16].Rhoads SJ, Serrano CI, Lynch CE, Ounpraseuth ST, Gauss CH, Payakachat N, et al. , Exploring implementation of m-health monitoring in postpartum women with hypertension, Telemed. J. E Health 23 (2017) 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ganapathy R, Grewal A, Castleman JS, Remote monitoring of blood pressure to reduce the risk of preeclampsia related complications with an innovative use of mobile technology, Pregnancy Hypertens 6 (2016) 263–265. [DOI] [PubMed] [Google Scholar]
- [18].Marko KI, Krapf JM, Meltzer AC, Oh J, Ganju N, Martinez AG, et al. , Testing the feasibility of remote participant monitoring in prenatal care using a mobile app and connected devices: a prospective observational trial, JMIR Res. Protoc 5 (2016) e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bowen DJ, Kreuter M, Spring B, How we design feasibility studies, Am. J. Prev. Med 36 (5) (2009. May) 452–457, 10.1016/j.amepre.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lancaster GA, Pilot and feasibility studies come of age!, Pilot Feasibility Stud 1 (2015), 10.1186/2055-5784-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sim J, Lewis M, The size of a pilot study should be calculated in relation to considerations of precision and efficiency, J. Clin. Epidemiol 6 (2012) 301–308. [DOI] [PubMed] [Google Scholar]
- [22].NIHR. Feasibility and pilot studies, in NIHR NETSCC glossary. 222.netscc.ac.uk/glossary/#glos6
- [23].Wasden SW, Ragsdale ES, Chasen ST, Skupski DW, Impact of non-steroidal anti-inflammatory drugs on hypertensive disorders of pregnancy, Pregnancy Hypertens 4 (2014) 259–263. [DOI] [PubMed] [Google Scholar]
- [25].Vigil-De Gracia P, Solis V, Ortega N, Ibuprofen versus acetaminophen as a post-partum analgesic for women with severe pre-eclampsia: randomized clinical study, J. Matern. Fetal Neonatal Med 30 (2017) 1279–1282. [DOI] [PubMed] [Google Scholar]
- [26].Deputy NP, Dub B, Sharma AJ, Prevalence and trends in prepregnancy normal weight – 48 states, New York City, and District of Columbia, 2011–2015, MMWR Morb. Mortal. Wkly Rep 66 (2018) 1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- [24].Viteri OA, England JA, Alrais MA, Lash KA, Villegas MI, Ashimi Balogun OA, et al. , Association of non-steroidal antinflammatory drugs and postpartum hypertension in women with preeclampsia with severe features, Obstet. Gynecol 130 (2017) 830–835. [DOI] [PubMed] [Google Scholar]