Abstract
The 826 G protein-coupled receptors (GPCRs) in the human proteome regulate key physiological processes, and thus have long been attractive as drug targets. With crystal structure determinations of more than 50 different human GPCRs during the last decade, an initial platform for structure-based rational design has been established for drugs that target GPCRs, which is currently being augmented with cryo-EM structures of higher-order GPCR complexes. Nuclear magnetic resonance (NMR) spectroscopy in solution is one of the key approaches for expanding this platform with dynamic features, which can be accessed at physiological temperature and with minimal modification of the wild-type GPCR covalent structures. Here, we review strategies for the use of advanced biochemistry and NMR techniques with GPCRs, survey projects where crystal or cryo-EM structures have been complemented with NMR investigations, and discuss the impact of this integrative approach on GPCR biology and drug discovery.
More than 30% of all drugs approved by the US Food and Drug Administration target G protein-coupled receptors (GPCRs)1–3, and these drugs are utilized in a wide range of therapeutic areas, including inflammation and diseases of the central nervous system as well as the cardiovascular, respiratory and gastrointestinal systems2,4. Currently more than 300 agents are in clinical trials, of which around 60 target novel GPCRs for which no drug has as yet been approved2. The novel GPCR targets also include orphan GPCRs, for which endogenous ligands have not yet been discovered2. Overall, the drugs approved so far target only 27% of the human non-olfactory GPCRs2, indicating that much excitement still lies ahead.
Identifying new GPCR drugs will need additional detailed knowledge of GPCR biology, especially knowledge from structural biology, given the complex structure–function relationships involved in GPCR signaling. Along this line, recent reviews on GPCRs have covered studies with antibodies5 and nanobodies6, allosteric modulation7–10 biased signaling11–15, methods in GPCR structural biology16–19, GPCR crystal structures20–27 and drug development2,4,28,29, with some reviews addressing specific GPCR families30,31. Complementing the substantial number of GPCR crystal structures that have become available in the past decade, as well as the recent demonstrations of the potential for cryo-EM to provide information on higher-order GPCR complexes32–36, dynamic studies of GPCRs are important for providing new insights into GPCR biology that can assist drug discovery. In this respect, nuclear magnetic resonance (NMR) spectroscopy in solution is a key tool for analysing function-related conformational equilibria in GPCRs as they relate to allosteric coupling, variable efficacies and biased signaling of GPCR ligands, which are of particular interest for their potential as drugs. Furthermore, NMR spectroscopy is also a useful tool for fragment-based lead discovery with GPCR targets.
In this article, we first overview the structural biology of GPCRs based on knowledge from X-ray crystallography, cryo-EM and solution NMR, and then focus on the application of solution NMR to enhance understanding of GPCR biology relevant to drug discovery, as well as in fragment-based lead discovery. Solid-state NMR studies can also be valuable in the study of GPCR and other membrane protein structures, but have been reviewed elsewhere37–43 and are not covered here.
Structural biology of GPCRs
Human GPCR biology.
GPCRs are one of the largest membrane protein families in eukaryotes. There are 826 GPCRs in the human proteome, which are classified into five main families based on sequence homology and phylogenetic analyses: rhodopsin family (Class A), secretin family (Class B), glutamate family (Class C), frizzled family (Class F), and adhesion family22,44. GPCRs function as receptors for various neurotransmitters, hormones, cytokines (chemokines), metabolites, tastants, and odorants. As well as being a key class of drug targets, GPCRs are frequent “off-targets” of drugs that target other proteins, which can result in unanticipated side effects, and mutations of GPCRs have been linked to dozens of monogenic diseases22,29.
Activated GPCRs induce signal transduction mediated by G proteins, GPCR kinases (GRKs) and arrestins12,16,22,24,45 (Fig. 1a). Upon G protein activation, the Gα subunit dissociates from the Gβγ subunits and modulates production of second messengers such as cAMP. Gβγ modulates downstream signaling networks, including ion channels and phospholipases. In addition, the activated GPCRs may be phosphorylated by GRKs, and the phosphorylated GPCRs stimulate G protein-independent signal transduction mediated by arrestins (Fig. 1a), such as receptor internalization and activation of kinase signaling networks.
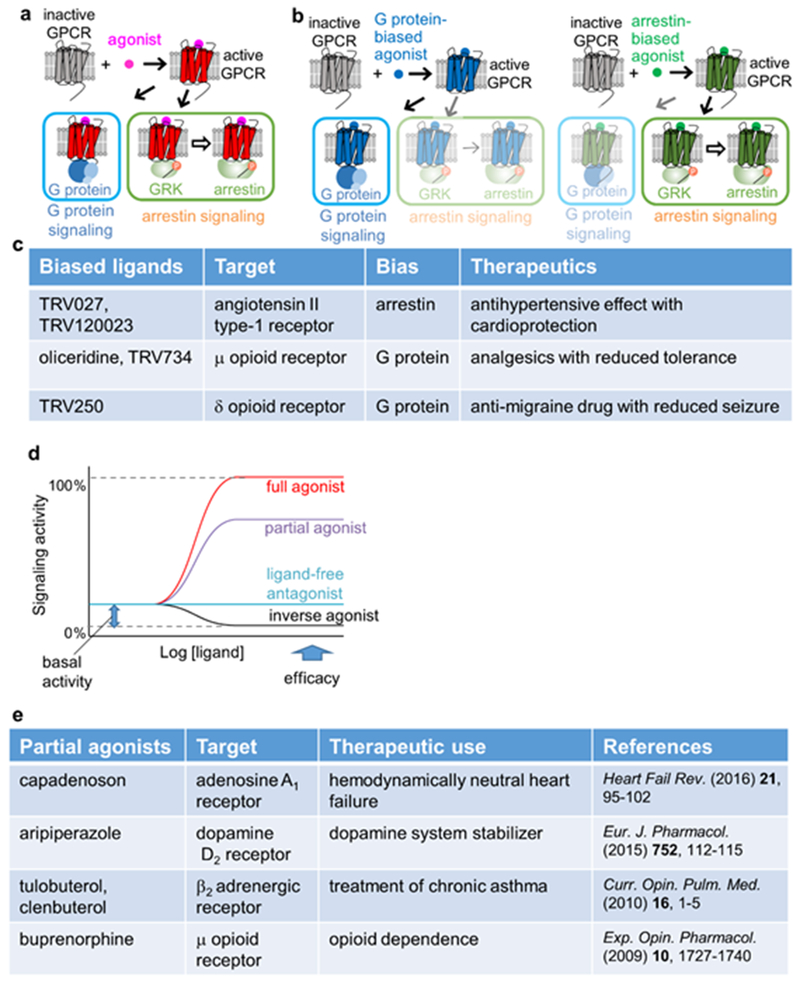
Figure 1 |. Therapeutic use, efficacy, and biased signaling of GPCR ligands.
a | Schematic diagram of GPCR signaling. Activated GPCRs induce signal transduction through independent signaling pathways, either via G proteins or via G protein-coupled receptor kinases (GRKs) and arrestins. b | Schematic diagram of biased signaling. A G protein-biased ligand and an arrestin-biased ligand elicit signaling via the G protein pathway and the arrestin pathway, respectively. c | Examples of clinically effective biased ligands of GPCRs. d | Plots of signaling activity versus ligand concentration, representing different common GPCR efficacies. e | Examples of clinically effective partial agonists of GPCRs.
Different GPCR ligands can modulate signaling through G protein and arrestin pathways differently, and the ligands that preferentially modulate one of the signaling pathways are referred to as ‘biased ligands’11–15,45–49 (Fig. 1b). Differences in biased signaling critically affect the therapeutic properties of GPCR ligands. Drugs can thus produce side effects mediated through the activation of “unwanted” signaling pathways by the targeted GPCR. It is of keen interest to minimize such side effects by designing biased ligands that selectively activate the signaling pathway required to produce the desired therapeutic response11,12,50,51. For example, G protein-biased agonists of the μ-opioid receptor (MOR) increase the analgesia and reduce the on-target adverse effects compared with morphine52–57, and several novel biased ligands are under evaluation in clinical trials12 (Fig. 1c). This work is of great socio-economic interest owing to the crisis caused by the misuse of prescription opiates in the United States58. In addition to the bias between the G protein and arrestin pathways, many other types of bias have been proposed, including those between different G protein subtypes59.
GPCRs exhibit variable basal activities in the absence of bound ligands, and each ligand for a given GPCR has a characteristic level of ability to activate or deactivate its target, which is commonly referred to as its “efficacy”. According to their efficacies, GPCR ligands are classified as full agonists, partial agonists, antagonists and inverse agonists (Fig. 1d)22,60. These differences in the efficacies affect the therapeutic properties of the GPCR ligands, and partial agonists can offer clinical advantages over full agonists and antagonists (Fig. 1e)11,61–64.
Most descriptions of biased agonism were initially based on studies using recombinant cell systems50. Although consistent and robust responses of different signaling pathways can thus be obtained 50, many factors can confound the interpretation of these assays, including off-target effects, cell backgrounds, receptor and effector expression levels, and the kinetic properties of agonists11,50,65. Thus, the integration of studies using recombinant cell systems with structural biology, detecting direct effects on the receptor conformation, is important in studying biased agonism.
X-ray crystal and cryo-EM structures of human GPCRs.
Since the first crystal structure of bovine rhodopsin published in 200066 and the first crystal structure of a human GPCR, β2AR, published in 200767,68, the number of GPCR crystal structures has rapidly increased, so that structures of more than 50 different GPCRs and over 250 of their complexes with different ligands are presently available in the Protein Data Bank, as documented in the GPCRdb69 (Fig. 2). This includes structures of Class A, Class B, Class C, and Class F receptors69. Cryo-EM structures of GPCRs in complexes with partner proteins are also appearing at an increasingly rapid pace. Examples include tertiary complexes of receptors with G proteins and agonists of the glucagon-like peptide-1 receptor (GLP-1R)35, the calcitonin receptor36, the A1 adenosine receptor70, the μ-opioid receptor71, the serotonin 5HT1B receptor33, and rhodopsin34.
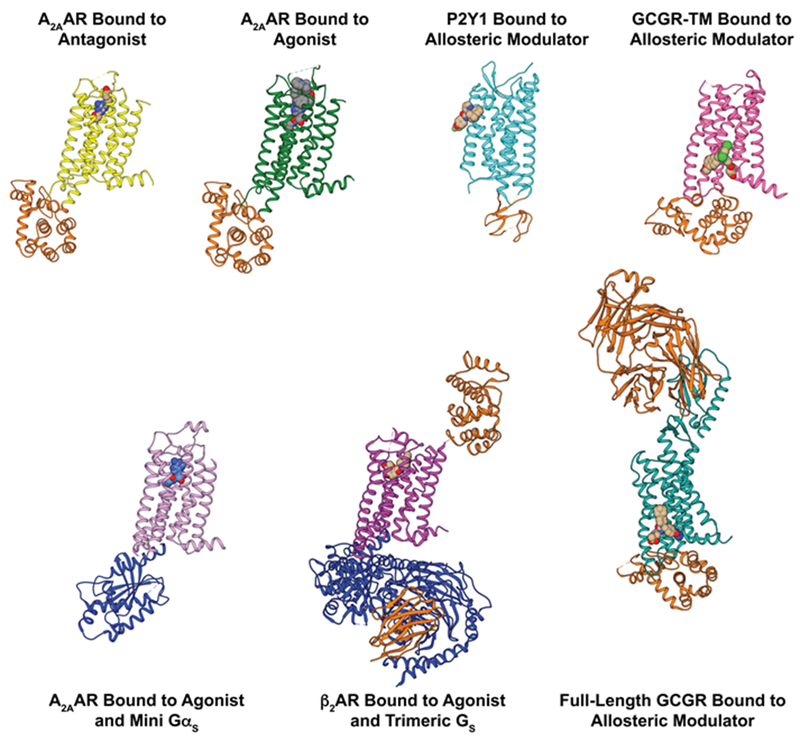
Figure 2 |. Selected crystallography data on GPCR structural architectures and intermolecular interactions.
Side views are shown of crystal structures of GPCRs in binary complexes with orthosteric ligands or allosteric modulators, and in tertiary complexes with intracellular partner proteins and orthosteric ligands or allosteric modulators. Fusion proteins, nanobodies, and antibodies used to facilitate crystallization of the GPCR complexes are shown in orange, and intracellular partner proteins are shown in blue. Different colors are used for different functional states of GPCRs: A2AAR in complex with the antagonist ZM241385 (yellow; PDB 3EML) and the agonist UK432097 (green; PDB 3QAK), P2Y1 receptor bound to the allosteric modulator BPTU (cyan; PDB 4XNV), the transmembrane domain (TM) of GCGR in complex with the allosteric modulator MK-0893 (fuchsia; PDB 5EE7), A2AAR in a tertiary complex with the agonist NECA and a mini Gs protein (salmon; PDB 5G53), β2AR in a tertiary complex with an agonist and a heterotrimeric G protein (purple; PDB 3SN6), and full-length GCGR in complex with the allosteric modulator NNC0640 (marine; PDB 5XEZ).
Crystal structures have revealed the GPCR three-dimensional architectures, locations of bound ligands and atomic details of receptor–ligand interactions. All Class A GPCRs share a structural topology characterized by seven transmembrane helices (TMI to TMVII) linked by three intracellular loops (ICL) and three extracellular loops (ECL). A canonical “orthosteric” ligand binding pocket facing the extracellular surface is formed by residues of transmembrane helices and extracellular loops (Fig. 2)22,72. Crystal structures have also identified ligand binding sites in transmembrane regions outside of the orthosteric pocket of Class A73 and Class B74 receptors, which relate to allosteric modulation of effects from orthosteric ligand binding (Fig. 2). In GPCR crystal structures determined at resolutions of 2.5 Å or higher, small molecules and ions have been observed, including bound sodium75,76, networks of crystallographic water molecules75, lipids and cholesterol77, providing a structural basis for rationalizing the regulation of GPCRs by allosteric modulators. Knowledge about mechanisms underlying allosteric modulators can in turn provide novel insights into the signal transduction through GPCRs. Exogenous allosteric modulators may be useful for the development of GPCR subtype-specific drugs, where different subtypes may have nearly identical orthosteric sites78.
The crystal structures of the β2-adrenergic receptor (β2AR) indicated the following structural mechanism for Class A GPCR activation78–81 (Fig. 3a). Full agonists induce a rearrangement of the interactions between TM helices in the central part of the GPCR structure, i.e., an inward shift of TMV at P2115.50, an axial shift of TMIII at I1213.40, and an outward shift of TMVI starting at F2826.44 (the four-digit superscripts indicate the Ballesteros-Weinstein nomenclature82 for amino acid residues in GPCRs, where the first number indicates the helix in which the residue is located). These three residues are referred to as the P/I/F motif. The rearrangement of the TM helices induces a large outward movement of the cytoplasmic half of TMVI, and thus increases the distance between TMIII and TMVI in the cytoplasmic region and disrupts a salt bridge in an ionic lock, which accompanies the repositioning of the sidechain of Y3267.53 in a second highly conserved motif consisting of residues NPxxY. The residues that exhibit conformational changes upon activation of β2AR are highly conserved among the Class A GPCRs81,83,84, and when comparing antagonist-bound states and ternary complexes with agonists and G protein-mimetics, similar conformational differences were observed for other Class A GPCRs. Similar conformational changes upon activation (Fig. 3a) thus seem to be characteristic among the Class A GPCRs.
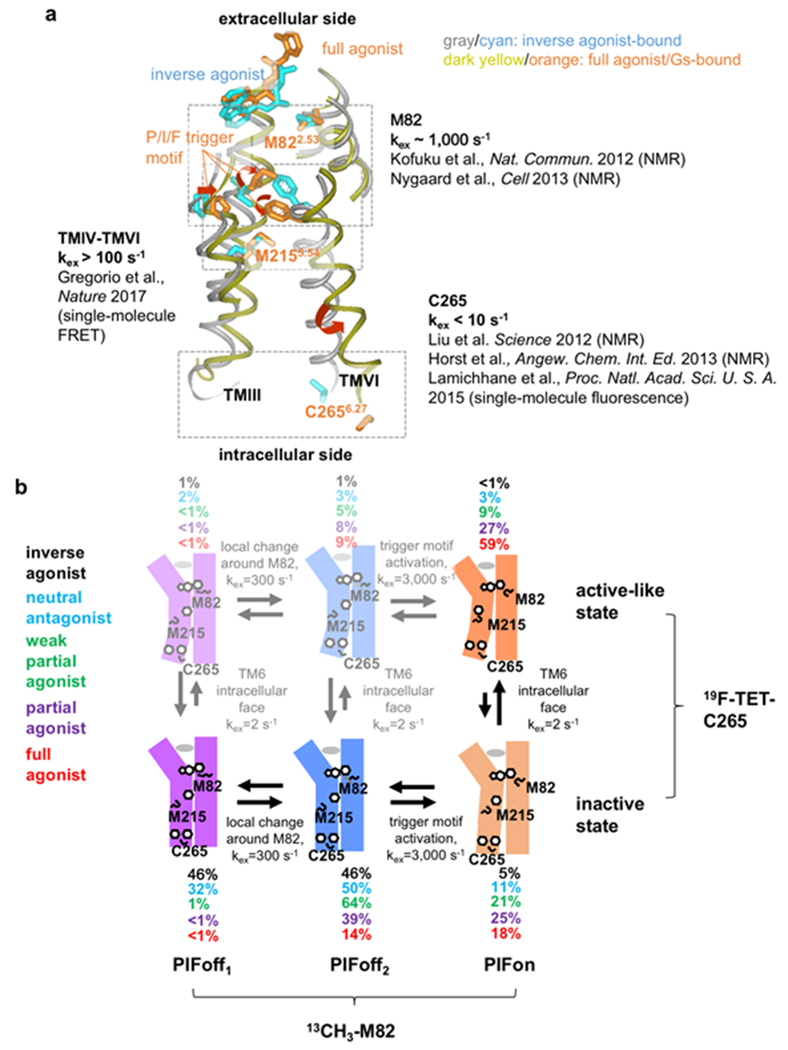
Figure 3 |. Conformational plasticity of β2AR.
a | Exchange rates between simultaneously populated locally different conformations observed with NMR and fluorescence probes introduced in different locations of β2AR. Shown are the crystal structures of β2AR complexes with the inverse agonist carazolol (PDB: 2RH1), and with the full agonist BI-167107 and a G-protein (PDB: 3SN6), overlaid for best fit of TMII; the drawing represents a side view with the extracellular surface at the top. TMII, TMIII, TMV and TMVI are shown as grey or dark-yellow Cα traces, and the sidechains of M822.53, I1213.40, P2115.50, M2155.54, C2656.27 and F2826.44, and the bound ligands are depicted by cyan or orange sticks, as detailed in the figure. The antagonist and full agonist complexes differ by a rearrangement of the TM helices and the P/I/F activation motif of P2115.50, I1213.40 and F2826.44. The rearrangement at the P/I/F motif induces conformational changes near the cytoplasmic region that is involved in G protein binding. The environments around M822.53, M2155.54, and C2656.27 have been shown to be sensitive to the above-described conformational changes upon activation. b | Schematic model of plasticity in β2AR, which rationalizes the wide range of different exchange rates in (a). Vertical arrows indicate the equilibria between ‘inactive’ and ‘active-like’ conformations at the cytoplasmic surface, as observed with NMR probes at C265. Horizontal arrows correspond to equilibria in the local region around M82 (PIFoff1↔PIFoff2) and in the P/I/F motif (PIFoff2↔PIFon), as observed with probes at M82 (the states PIFoff1, PIFoff2 and PIFon correspond to M82D, M82U and M82A in a previous report113). Black, cyan, green, purple and red numbers are the populations of the different states, as observed experimentally for β2AR bound to an inverse agonist, an antagonist, a weak partial agonist, a partial agonist, and a full agonist, respectively. The states with populations below 10% are listed in faint colors.
In a crystal structure of the ternary complex of β2AR with a full agonist and a G protein85, a cytoplasmic cavity is formed by the outward shift of the cytoplasmic half of TM V and TMVI and the distortion of TMVII, when compared with their conformations in the inverse agonist-bound form68; the C-terminal helix of the G protein is then inserted into the cytoplasmic cavity. Similar cytoplasmic cavity formation was observed for the crystal structures of other GPCRs in tertiary complexes with a full agonist and a G protein mimetic86 as well as for cryo-EM structures of GPCRs in tertiary complexes with an agonist and G protein33,34,70,71.
GPCR crystal structures have been extensively utilized for structure-based drug design, and there are more than 30 reports on in silico screening of GPCR ligands, some of which resulted in identification of GPCR ligands with novel chemical scaffolds22,87. However, information on GPCR features that are of special interest to in drug discovery, such as basal activity, biased signaling, partial agonism, and allosteric modulation by ions and small molecules can only be partially derived from crystallography data. These limitations arise in large part from the well-established practice of making modifications to the wild-type GPCR covalent structure to facilitate crystallization, such as truncation of flexible domains and chain ends, introduction of soluble fusion proteins into the loops or at the receptor N-terminus88,89, and systematic replacement of endogenous amino acids to increase the thermostability90–92. Alternatively, crystallization has also been facilitated by the formation of stable non-covalent complexes with a partner protein, such as an engineered G protein93, antibody94 or nanobody6,81, but this can also affect functionally relevant conformational equilibria.
An illustration of the limitations is provided by the β1 adrenergic receptor (β1AR), which was thermostabilized by modifications that also trap the receptor in an inactive state. The crystal structures then showed nearly identical conformations for complexes with antagonists, a partial agonist, a full agonist, and a biased agonist92,95.
GPCR crystallography also depends on the selection of the orthosterically bound ligand. All small-molecule ligands used so far appear to have similar chemical properties and high binding affinity (Ki ≤10 nM )96, thus probably providing information on only a fraction of the drug scaffolds that can potentially be recognized by GPCRs. Crystal structure determination is particularly challenging for GPCRs that bind endogenous or synthetic peptides, so that only a few crystal structures have so far been determined for GPCRs in complex with peptides23; these include endothelin97, neurotensin98, and the neuropeptide Y1 receptor99. As peptide therapeutics are promising2, especially for Class B GPCRs, other techniques such as NMR may be valuable in providing more comprehensive structural information on GPCR-peptide drug systems.
In summary, the success of GPCR crystallography has also revealed the need to complement crystal structures with data collected with different techniques in milieus that are closer to physiological conditions than the crystalline state and liquid nitrogen temperature, and solution NMR is particularly promising in this respect.
NMR data and GPCR functions.
NMR methods provide information about dynamics of proteins over a wide range of frequencies in aqueous solutions at near-physiological temperature100–102. NMR studies thus revealed that global and local conformational fluctuations of protein structures are tightly related to their functions, such as enzymatic activities103,104 or molecular recognition105, especially also in membrane proteins106–109. Although NMR studies of GPCRs are challenging, observations of the NMR signals of various GPCRs have been accomplished through recent methodological advances, including ultra-high-field NMR instruments, improved cryogenic probes, transverse relaxation-optimized spectroscopy (TROSY)110,111, and refined methods for labeling with stable isotopes and other NMR probes (Box 1).
Box 1 |. Preparation of native-like human GPCRs for solution NMR.
Expression systems
Protein production for most published GPCR NMR studies has been carried out using insect cell-baculovirus expression systems (BVES), which are also routinely used for protein production in GPCR crystallography. More recently, mammalian cell cultures have been employed for expression of GPCRs with large extracellular domains, as typically found in Class B and Class F GPCRs232,233, which also retain human-like glycosylation and other post-translational modifications. Several human GPCRs have also been recombinantly expressed in yeast, particularly in Pichia pastoris234–236, including the histamine H1 receptor237 and adenosine A2A receptor (A2AAR)238. A survey of expression systems used for the preparation of GPCR NMR samples is included in Table 1.
Labeling by post-translational chemical modification
Many GPCR NMR studies so far introduced NMR probes by chemical modification of amino acid side chains during or after protein production. This strategy benefits from the comparatively high expression yields for wild type-like GPCRs, since NMR probes are incorporated in a post-translational step during protein purification at specific locations in the receptor through chemical conjugation to reactive –SH or –NH3 groups of amino acid side chains. Two common extrinsic GPCR NMR probes are 19F-groups chemically conjugated with cysteines, and 13CH3 groups introduced by reductive methylation of lysines. The use of trifluoromethyl probes in integral membrane proteins was pioneered by Khorana for NMR studies of bovine rhodopsin239, and more recently applied to studies of β2AR112,117,119,120,122 and A2AAR125,131. Methylated lysines have been used in NMR studies of β2AR116 and MOR128.
Introduction of NMR probes by chemical modification has made use both of targeting suitably located endogenous cysteines or lysines and of amino acid replacement in judiciously selected sequence locations with non-endogenous cysteines125,178. Generally, post-translational chemical modification is limited to residues at the extracellular or intracellular surfaces; however, unintended labeling of non-surface residues can occur, creating unwanted background signals that interfere with the interpretation of NMR data from the surface-facing probes. For example, in A2AAR the labeling of a cysteine engineered into the intracellular surface resulted in heterogeneous labeling of additional cysteines when the protein was solubilized in detergent micelles. To overcome this problem, the in-membrane chemical modification (IMCM) method was introduced, whereby 19F NMR probes are chemically introduced into GPCRs embedded in membranes isolated from the expression host, i.e., before protein isolation and purfication178. Alternatively, 19F-NMR background signals have been removed by replacement of all but the cysteine(s) of interest by other amino acids.
Post-translational chemical modification has also been used to introduce paramagnetic relaxation enhancement (PRE) agents, such as nitroxide spin labels, into discrete sequence locations in proteins240–242. This approach has been widely used in NMR structure determination efforts and studies of intermolecular interactions of soluble and membrane proteins240,243,244, using cysteine-modifying chemistry similar to the 19F-NMR approach to introduce such spin labels at discrete locations in GPCRs.
Expression in stable-isotope labeled media
Stable isotopes can be used in the cell culture medium for GPCR production, either as stable-isotope-labeled amino acids, such as 13CH3-methionine113,115,118,121,129 and 15N-valine114, or stable-isotope-labeled nutrients, where 15N, 13C, and 2H are then incorporated uniformly throughout the receptor127.
Deuteration of GPCRs is necessary to obtain the full benefits of high resolution in [15N, 1H]-TROSY correlation NMR experiments110,245. Expression of deuterated GPCRs in eukaryotic organisms has historically been a challenge, as insect and mammalian cells do not tolerate D2O, but deuteration of human GPCRs has been achieved by protein production with Pichia pastoris in D2O media126,127,246. Higher levels of protein deuteration were achieved by adding deuterated carbon sources126,246. Illustrations for successful expression in Pichia pastoris include deuterated human A2AAR, enabling of 13C-methyl labeling of isoleucines126,246,, and studies of 1H–15N backbone and side chain signals127. A limitation of deuteration by expression of human GPCRs in P. pastoris grown in D2O media is that back-protonation of amide groups can be incomplete, resulting in a smaller number of NMR signals than one would expect from the amino acid sequence. Deuteration in insect cells and mammalian cells has been achieved by introducing deuterated amino acids or deuterated digests from lower organisms to the cell culture media. Examples are the expression of deuterated GPCRs in BVES by introducing deuterated amino acids in H2O-based insect cell media for β2AR121 and MOR129, and the use of H2O-based insect cell media containing deuterated yeast digests for expression of β1AR247 and the C-C chemokine receptor type 5 (CCR5)248. Using these approaches, the extent of deuteration may be lower than what is achieved with D2O-based expression media for P. pastoris, but back-protonation is expected to be nearly complete247. Selective 13CH3 labeling of alanine methyl groups on a deuterated background has been achieved for β2AR expressed in BVES249.
Introduction of non-proteinogenic amino acids through amber codon suppression technology has been used to introduce NMR probes into GPCRs for residue-specific biophysical characterization of dynamics and ligand binding events250–253. Using this approach, 19F NMR probes such as fluorotyrosine can be introduced at specific interior locations in transmembrane helices that are inaccessible for post-translational chemical modification.
Expression of human GPCRs in E. coli, which is the standard expression system for stable-isotope labeled proteins for NMR studies, has so far found limited applications130,254,255.
Labeling of GPCR ligands and partner proteins
Expression of the isotopically labeled Gα subunit of the trimeric G protein complex has been carried out in E. coli, permitting deuteration and uniform or amino acid-specific labeling of G proteins256. Isotopically labeled G protein mimicking camelid single-chain antibodies, so-called “nanobodies,” for the μ-opioid receptor (MOR) have also been expressed and characterized by solution NMR257.
NMR studies, along with various other spectroscopic studies, have revealed that GPCRs exist in function-related equilibria between multiple locally different conformations that are simultaneously populated. These function-related equilibria are typically abolished by the modifications used for crystallography studies described above. NMR studies thus provide information that is complementary to data from X-ray crystallography and cryo-EM studies, which should be considered in functional interpretations of GPCR crystal structures. NMR can also be used for serial analyses of the conformations of GPCRs bound to various ligands with different efficacies and extent of biased signaling112–115, whereas the determination of the crystal structures in a wider range of ligand-bound states is not straightforward for GPCRs22. Table 1 summarizes recent NMR studies of conformational equilibria in GPCRs that relate to partial agonism and biased signaling112–131, and which aim at facilitating the design of new compounds that can modulate GPCR signaling.
Table 1.
Survey of NMR Studies of GPCRs
| GPCR | NMR Labels | Expression System | Membrane Mimetic | ref. |
|---|---|---|---|---|
| β2AR | 13CH3-Lys | Sf9 | DDM | 116 |
| β2AR | 19F-TET | Sf9 | DDM/CHS | 112 |
| β2AR | 13CH3-Met | expresSF+ | DDM | 113 |
| β2AR | 19F-BTFA | Sf9 | DDM and LMNG | 117 |
| β2AR | 13CH3-Met | Sf9 | DDM | 118 |
| β2AR | 19F-BTFA | Sf9 | LMNG | 119 |
| β2AR | 19F-TET | Sf9 | DDM/CHS | 120 |
| β2AR | 13CH3-Met, 2H | expresSF+ | nanodisc | 121 |
| β2AR | 19F-BTFMA | Sf9 | DDM | 122 |
| β2AR-T4L | 19F-TET | Sf9 | DDM/CHS | 123 |
| β2AR | 13CH3-Met, 2H Cterm-2H, 13C, 15N | expresSF+ + E. coli (segmental label) | nanodisc | 124 |
| β1AR | 15N-Val | High Five | DM | 114 |
| β1AR | 13CH3-Met | Sf9/Sf21 | LMNG | 115 |
| BLT2 | 13CH3-Ile | E. coli | nanodisc | 130 |
| A2AAR | 19F-BTFMA | P. pastoris | LMNG/CHS | 125 |
| A2AAR | 13CH3-Ile, 2H | P. pastoris | DDM | 126 |
| A2AAR | u-15N, ~70% 2H | P. pastoris | LMNG/CHS | 127 |
| A2AAR | 19F-BTFMA | P. pastoris | LMNG/CHS | 131 |
| MOR | 13CH3-Met, 2H | expresSF+ | LMNG/CHS | 128 |
| MOR | 13CH3-Lys | Sf9 | LMNG/CHS | 129 |
In addition to observing GPCRs and their interactions with drugs and partner proteins, NMR has been used to directly observe GPCR ligands in both their unbound and receptor-bound conformations. This enables the determination of structures of GPCR ligands and measurement of their conformational dynamics while bound to the receptor and, in principle, their drug pharmacokinetics. Some examples from a very large array of literature data on this topic include the determination of the structure of the agonist dynorphin bound to the κ-opioid receptor132, the cytokine interleukine-8 and its interactions with the chemokine receptor CXCR1133,134, lipids of the free fatty acid receptor135, and peptide agonists of the bradykinin receptors136. Importantly, NMR can be used to monitor the relatively weak interactions of low-molecular-mass compounds with GPCRs, and therefore can support fragment-based lead discovery approaches. Finally, NMR has been used to study the structure and dynamics of GPCR partner proteins, including G proteins and arrestins, and their interactions with GPCRs and nucleotides137–139. Thus, in addition to information from NMR observation of GPCRs, NMR can provide a comprehensive view of a wide range of molecules that interact with GPCRs and modulate GPCR signaling.
NMR studies of human GPCRs
NMR studies on the dynamics of GPCRs related to their functions have so far typically been performed in the following manner. First, labeled GPCRs are prepared using fluorine and/or stable-isotope labeling methods (Box 1) and reconstituted in detergent micelles or lipid nanodiscs (Box 2). Second, resolved NMR signals are observed and assigned, and structural and dynamics information is obtained from the resonances. For example, the 13C chemical shifts of side-chain methyl signals probe the side-chain conformation140,141, the 1H and 19F chemical shifts of side-chain 13CH3 and CF3 groups probe their local environments, including the ring current effects from neighboring aromatic residues113,142,143, and the solvent accessibility of the observed atoms can be assessed by the addition of paramagnetic probes to the solvent112,144. Exchange rates and species populations in conformational equilibria can be determined with line-shape analysis145, CPMG122, saturation transfer119,120,122 and exchange spectroscopy120, including perturbation of an equilibrium by changing the temperature. The functional relevance of the observed conformational changes can then be explored by investigations of the relationships between the NMR spectra and the signaling activities of the GPCRs.
Box 2 |. Reconstitution of human GPCRs for NMR studies in solution.
Under physiological conditions, GPCRs are embedded in lipid bilayers, whereas for structural studies they have so far in most cases been solubilized by detergents. In detergent micelles, GPCRs do not necessarily retain their full activities258, and a lipid bilayer environment is reportedly preferable for GPCR phosphorylation by GRKs259. Nanodiscs can accommodate membrane proteins within a 10-nm-diameter disc-shaped lipid bilayer, stabilized by α-helical amphipathic membrane scaffold proteins (MSPs)260,261. Nanodiscs thus promise to provide a lipid environment with more native-like properties in terms of the lateral pressure and curvature profiles, since detergent micelles have a strong curvature and different lateral pressure profiles from lipid membranes262. Membrane proteins embedded in nanodiscs are attractive for investigating interacting ligands on one side and signaling partner proteins on the other side. Nanodiscs are water soluble and monodisperse, which has already enabled their use in various GPCR studies121,124,130,151,152,204,205,258,263–271.
Nasr et al. recently reported covalently circularized nanodiscs (cNDs) with enhanced stability and strictly defined diameter sizes, which are obtained by circularizing the MSP using sortase272. The NMR spectra of a β-barrel membrane protein, VDAC-1, embedded in cNDs exhibited enhanced signal intensities and spectral resolution due to reduced sample heterogeneity272, suggesting that cNDs could facilitate future NMR analyses of GPCRs in lipid bilayers. Disc-shaped lipid bilayers have also been formed using the saposin-A scaffold protein (Salipro nanoparticles273,274) or synthetic polymers220,275. Saposin-A scaffold proteins are reportedly helpful for the formation of bilayer discs with different sizes, and the synthetic polymer discs have the advantage of being formed directly from lipid bilayers, without using detergents.
Under physiological conditions, GPCRs can be embedded in the lipid bilayers of tissues with various lipid compositions, and the signaling activities of GPCRs can be affected by the lipid composition of the bilayers. For example, experimental studies using β2AR in nanodiscs indicated that phospholipids can act as allosteric modulators258, and the NMR signals of leukotriene B4 receptor in nanodiscs were found to be affected by the addition of cholesteryl hemisuccinate130. Reconstitution of GPCRs into nanodisc lipid bilayers thus opens avenues to examine effects of various composition of lipid bilayers on the activities and conformational equilibria of GPCRs by solution NMR experiments at near-physiological temperatures.
In the following sections, results obtained by using these approaches with different individual GPCRs are described, with most examples from GPCRs in Class A, which is the largest phylogenetic class of GPCRs79. Among almost 700 Class A GPCRs, 296 are non-olfactory GPCRs which function as receptors of various hormones and neurotransmitters, and are the most commonly exploited GPCRs for pharmacological interventions22. NMR studies of Class A GPCRs have provided insights into the mechanisms enabling partial agonism and biased signaling, with β2AR as the representative of Class A GPCRs with the most extensive set of experimental data. Exploratory NMR measurements with other Class A GPCRs have clearly indicated that all the information gathered for β2AR is not readily applicable for other GPCRs, and increasing interest has recently been focused on structural and functional studies of other Class A GPCRs.
β2-adrenergic receptor.
The conformational dynamics of β2AR, a Class A GPCR stimulated by adrenaline and various bronchodilators, has been extensively studied by multiple groups (Table 1). NMR signals have been observed for CF3 groups of 2,2,2-trifluoroethanethiol (TET)112,120, 3-bromo-1,1,1-trifluoroacetone (BTFA)117,119, and 2-bromo-4-trifluoromethyl-acetanilide (BTFMA)122,146, which were covalently linked to cysteine residues near to the cytoplasmic surface. Additional studies were based on observation of 13CH3 signals of reductively 13C-methylated lysine residues near the extracellular surface116 and for 13CH3-labeled methionine residues113,118,121, which are widely distributed in the transmembrane region. Overall, NMR data are now available from observation of probes in strategic locations distributed over the entire three-dimensional molecular structure. In addition, other methods, including fluorescence spectroscopy147–154, electron paramagnetic resonance (EPR)122, chemical labeling155,156, and hydrogen–deuterium exchange157, have been utilized for studying the conformation of β2AR. These data have enabled examinations of the global conformational dynamics that determines the efficacies and biased signaling of β2AR.
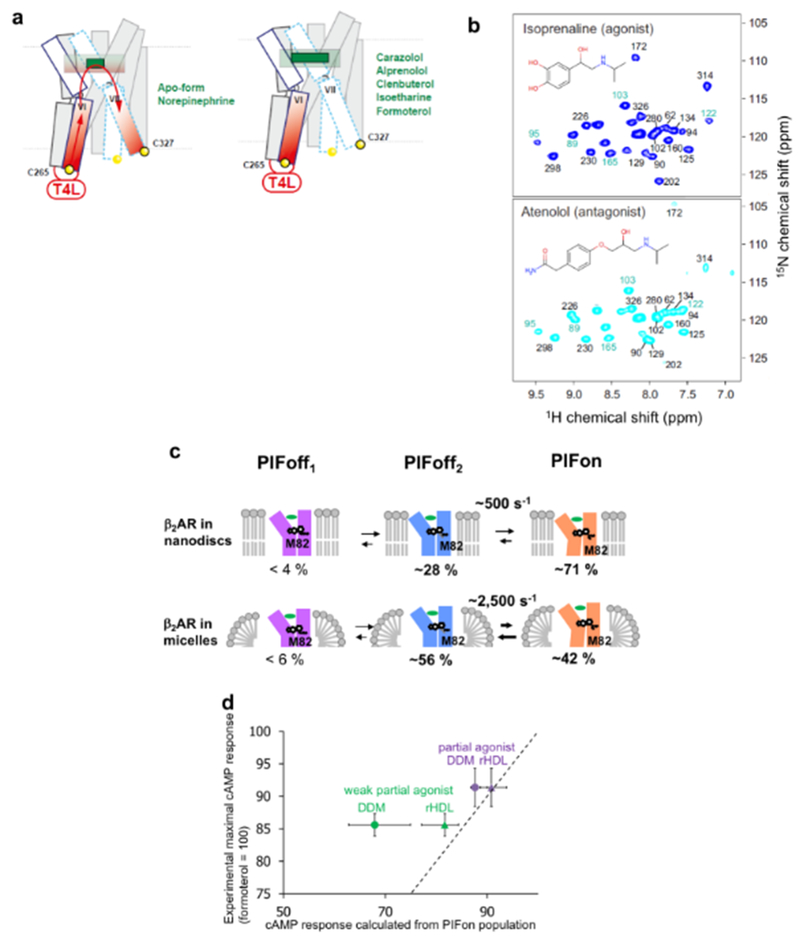
Conformational equilibria related to variations in ligand efficacy have been observed in various regions of β2AR, using NMR probes located primarily in positions where large differences were observed between crystal structures of inactive and active-like states68,85. With CF3 probes at C2656.27 and C3277.54 near the cytoplasmic tips of TMVI and TMVII, respectively, equilibria between inactive and active-like conformations were observed, with exchange rates slower than 10 s−1,112,120, and the population of the active-like state correlated with the ligand efficacy112 (Fig. 4). The exchange rates observed using a single-molecule fluorescence probe at C2656.27 were similar to those observed using the CF3 probe152.
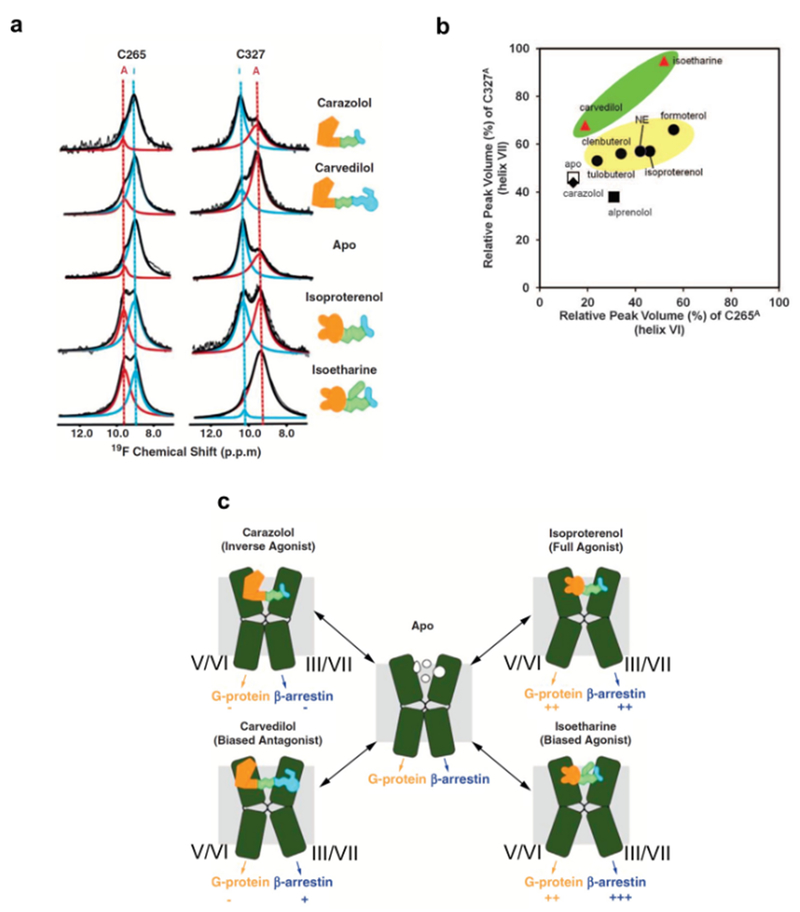
Figure 4 |. NMR-observable conformational equilibria related to biased signaling of β2AR.
a | 19F-NMR signals of CF3 probes attached to C265 and C327 in the apo-form and in four drug complexes of β2AR. The experimental spectra (thin black line showing noise) have been deconvoluted into signals of an active-like state (red, A) and an inactive state (blue, I) of β2AR. The thick black line represents the sum of the signals I and A. b | Plot of the relative peak volumes for an active-like state of β2AR observed at C265 (C265A) versus the relative peak volumes for an active-like state at C327 (C327A). The relative peak volumes are the ratios of the volume of peak A and the sum of the volumes of peaks A and I. The data for the complexes with the agonists tulobuterol, clenbuterol, norepinephrine (NE), isoproterenol, and formoterol are shown as black circles highlighted by a yellow background. The data with the biased ligands carvedilol and isoetharine are shown as red triangles highlighted by a green background. The data with the neutral antagonist alprenolol, the inverse agonist carazolol, and the apo state are shown as a black square, a black diamond, and an open square, respectively. c | Signaling intensities through two β2AR pathways suggested by the 19F-NMR experiments. TMVI and TMVII of β2AR are shown as green kinked cylinders. The structures of the four bound ligands are schematically drawn (yellow, green, and cyan) and their functionalities are indicated. The arrows at the lower end of the cylinders indicate the signaling to the downstream effectors, with plus and minus signs indicating the signaling levels relative to the basal state.
Additionally, faster intramolecular rate processes were discovered by observation of the M82 side chain, which reflects the conformation of the P/I/F motif in the central transmembrane region113. Equilibria among two conformations containing the inactive P/I/F motif, PIFoff1 and PIFoff2, and one conformation with the active P/I/F motif, PIFon, were observed (Fig. 3), and the population of the latter correlated with different ligand efficacies113. Remarkably, the exchange rates seen for the P/I/F motif were larger than those observed for the CF3 probe at C2656.27 i.e. ~2,000 s-1. This exchange rate is in closer agreement with kex >100 s−1 observed using single-molecule spectroscopy with FRET probes at the positions N148C4.40 and L266C6.28. FRET is sensitive to variations of the TMIV–TMVI distance in the interior region, which thus appears to also reflect the conformation of the P/I/F motif. In the case of M2155.54, for which the chemical shifts of the peripheral 13CH3 group reflect both the activation state of the P/I/F motif and the crystallographically observed conformational changes near the intracellular surface113, the NMR signals are affected by both of the aforementioned equilibria.
In order to integrate the NMR data with information obtained from other techniques and thus to generate a global view of the structure and conformational dynamics of β2AR, simulations of the resonances from M822.53, M2155.54, and the CF3 probe at C2656.27 were performed. A model that was found to be in agreement with the NMR experiments and the ligand efficacies is depicted in Fig. 3b, where the exchange rates of the P/I/F motif activation are different from those of the conformational exchange near the intracellular surface, as manifested by the 19F probe at C2656.27. CPMG experiments with another CF3 probe, 2-bromo-4-trifluoromethyl-acetanilide, at C265 of β2AR in the apo and inverse agonist-bound states indicated that C265 underwent conformational rate processes with exchange rates > 1,000 s−1 122,146, suggesting that in the inactive state the cytoplasmic surface region of β2AR is characterized as a manifold of rapidly exchanging substates.
NMR studies of β2AR provided a structural basis for biased signaling of GPCRs112. Carvedilol and isoetharine share similar chemical structures with the inverse agonist carazolol and the full agonist isoproterenol, respectively, and have been classified as β-arrestin–biased ligands of β-adrenergic receptors158,159. Arrestin signaling through β2AR promotes cardiomyocyte survival, and the arrestin-biased β2AR ligands represent a potentially useful therapeutic approach160. Liu et al. observed the resonances of 19F labels introduced at C2656.27 and C3277.54, which are at the cytoplasmic ends of TMVI and TMVII of β2AR, respectively, and demonstrated that β-arrestin-biased ligands predominantly impacted the conformation of C3277.54,112 (Fig. 4, a and b). These results suggested that β-arrestin-biased ligands of β2AR induce conformational changes of TMIII and TMVII, where the populations of inactive and active-like states seen in this location are not paralleled by those seen at C2656.27 for TMV and TMVI (Fig. 4c).
Experiments with β-adrenergic receptors have also provided useful lessons for improving the technologies for structural studies of GPCRs. NMR was used to study the effects of the insertion of the fusion protein utilized in crystallographic studies of β2AR16 on the function-related conformational equilibria123. To this end, the 19F-NMR signals of CF3 groups introduced at the cytoplasmic ends of TMVI and TMVII were compared for β2AR with and without T4 lysozyme fused into the ICL3. The fusion of T4 lysozyme into the ICL3 was thus found to block the conformational equilibrium seen at the cytoplasmic tip of TMVI in the active-like state, independent of the ligand bound to β2AR123 (Fig. 5a). In addition, in the apo and norepinephrine-bound states, the fusion of T4 lysozyme induced a long-range effect via the orthosteric cavity on the conformational equilibrium at the cytoplasmic tip of TMVII (Fig. 5a)123. The observation at C265 is in apparent contrast with data from earlier fluorescence experiments with β2AR-T4L67, which measured the overall effect of the fusion protein on labels in both positions 265 and 327. In contrast, by individually labeling C265 and C327 in the 19F-NMR study, the separate response at each of the cytoplasmic ends of helices VI and VII could be measured. The NMR results now provide a rationale for the fact that the fluorescence experiments, which were designed before the crystal structure had been known, did not reveal the status of TM VI, because the changes in the fluorescence measurements between agonist and antagonist complexes were dominated by the probe at 32753. The results obtained by measurements in solution emphasize that effects of ICL3 fusions and/or thermostabilizing mutations on the conformational equilibria in GPCRs should be duly considered in functional interpretations of GPCR crystal structures obtained with such protein modifications.
Figure 5 |. Effects on local conformational equilibria of β-adrenergic receptors from different experimental set-ups.
a | Schematic side view representations of β2AR-T4L visualizing the effects of T4L-fusion into ICL3 on local conformational equilibria related to the G-protein and arrestin signaling pathways, as observed with 19F-NMR probes at positions 265 and 327123 (see also Fig. 4). The transmembrane helices TMI to TMV are shaded in gray. TMVII, which is in equilibrium between two conformational states manifested by different NMR chemical shifts of a 19F probe attached to C327, is represented as dashed cyan rectangles. TMVI, which is blocked in an active-like state by the T4L fusion, is represented by solid blue rectangles. The T4L fusion protein is depicted as a small red oval, the direction of its impact on local conformational equilibria is indicated by red arrows, and increased intensity of its impact is indicated by increased density of the red shading. The positions of the 19F-NMR labels are shown by yellow spheres, where the active-like state is further identified by a black circle. The orthosteric ligand binding cavity is indicated by shading. The left panel represents the ligand-binding cavity in the complex with the small ligand norepinephrine (small green rectangle; in the apo-form the cavity does not contain a ligand); the red arrows indicate that a long-range effect from T4L via the orthosteric ligand binding cavity causes a shift in the conformational equilibrium at the intracellular tip of TMVII. The right panel shows the orthosteric binding site containing one of the listed larger ligands (green rectangle); the long-range effect from T4L to TMVII is suppressed by the presence of these ligands, which carry a hydrophobic substituent at the ethanolamine end. b | [15N,1H]-TROSY correlation NMR spectra of thermostabilized turkey β1AR (TS-β1AR) containing selective 15N-labeling of its 28 valines in antagonist-bound and agonist-bound states. Resonances are marked with assignment information (black, firm; cyan, tentative). The ligand chemical structures are shown as inserts. c | Differences between corresponding exchange rates and populations of conformational substates in β2AR in nanodiscs and in DDM micelles121. The upper and lower panels visualize conformational equilibria of the β2AR complex with the weak partial agonist tulobuterol in nanodiscs and in DDM micelles, respectively. Approximate relative populations of the three states are indicated below the drawings. d | Correlation between the simulated cAMP response based on the PIFon populations observed in the M82 NMR signal and the experimental maximal cAMP responses. Plots of the relative amounts, in percent, of cAMP formed upon activation of CHO-K1 cells by the partial agonists tulobuterol and clenbuterol, which were previously identified as such by cAMP accumulation assays121, versus the relative concentration of cAMP in activated cells calculated from the measured populations of PIFon. Both values were normalized to those in the fully active states, and thus are expected to be close to each other whenever the PIFon populations determined by NMR were similar to those in vivo. Circles and triangles represent the cAMP responses based on the PIFon populations of β2AR in DDM micelles and in nanodiscs, respectively. The dotted line represents the hypothetical situation where the simulated cAMP response would be equal to the experimental maximal cAMP response. The error bars along the horizontal axis represent the cAMP concentrations calculated based on the lowest and highest PIFon populations estimated from the M82 NMR signals.
A thermostabilized variant of the β1-adrenergic receptor (β1AR), which has 56% sequence identity to β2AR, was prepared with 15N-labeling of its 28 valines. The [15N,1H]-TROSY correlation NMR spectra (Fig. 5b) contain more than 20 signals, of which a large portion were assigned. Corresponding signals of the antagonist and agonist complexes have closely similar chemical shifts (Fig. 5b), which reflects the previously mentioned fact that the thermostabilization used locks the receptor in the inactive conformation, as confirmed by x-ray crystallography92. Nonetheless, NMR spectroscopy revealed subtle differences in line shapes and chemical shifts (Fig. 5b), which were used to obtain novel insights. For example, chemical shift changes upon formation of a ternary complex with the G protein-mimicking nanobody Nb80 were reported for valines located throughout the receptor, representing “reverse” allosteric effects near the extracellular surface from complex formation with the nanobody at the intracellular signaling surface99. Allosteric effects of β1AR complex formation with G protein-mimicking nanobodies were also observed with the use of 13C-methyl-methionine labels115.
Comparative NMR studies of a β2AR complex with a partial agonist in nanodiscs and in DDM micelles revealed that the exchange rates between the conformations with inactive and active P/I/F motifs are lower in nanodiscs than in the detergent micelles (Box 2), and that the population of the conformation with an active P/I/F motif of β2AR is higher in nanodiscs than in DDM micelles (Fig. 5c)121. NMR analyses of other membrane proteins embedded in nanodiscs also showed modulation of the conformational equilibria and the functions of membrane proteins by the lipid bilayer environment108,161. The maximum cAMP response, calculated using the populations of the conformations with the active P/I/F motif of β2AR in nanodiscs, correlated better with the experimental values than those calculated for β2AR in DDM micelles121 (Fig. 5d). These NMR investigations of GPCRs, and work with other membrane proteins, indicate that data on membrane proteins in detergent micelles need to be carefully evaluated, since different values for relative populations of the different conformational states and the exchange rates between them were measured in the lipid bilayer environment of nanodiscs.
μ-opioid receptor (MOR).
This Class A GPCR is stimulated by various opioid drugs, including morphine. Studies have indicated that MOR signaling through Gi (the inhibitory G protein for adenylyl cyclase) has a key role in the desired analgesic properties of morphine, whereas signaling through β-arrestin is associated with adverse side effects such as respiratory depression162,163. This knowledge has led to efforts to identify biased MOR ligands that show enhanced Gi signaling and reduced β-arrestin signaling compared with morphine, including oliceridine (TRV130)164, PZM2157 and SR1701855,56, which result in increased analgesia with reduced on-target adverse effects52,53,57. Further understanding of the mechanisms underlying biased MOR signaling are therefore of high interest for the development of much-needed novel analgesics.
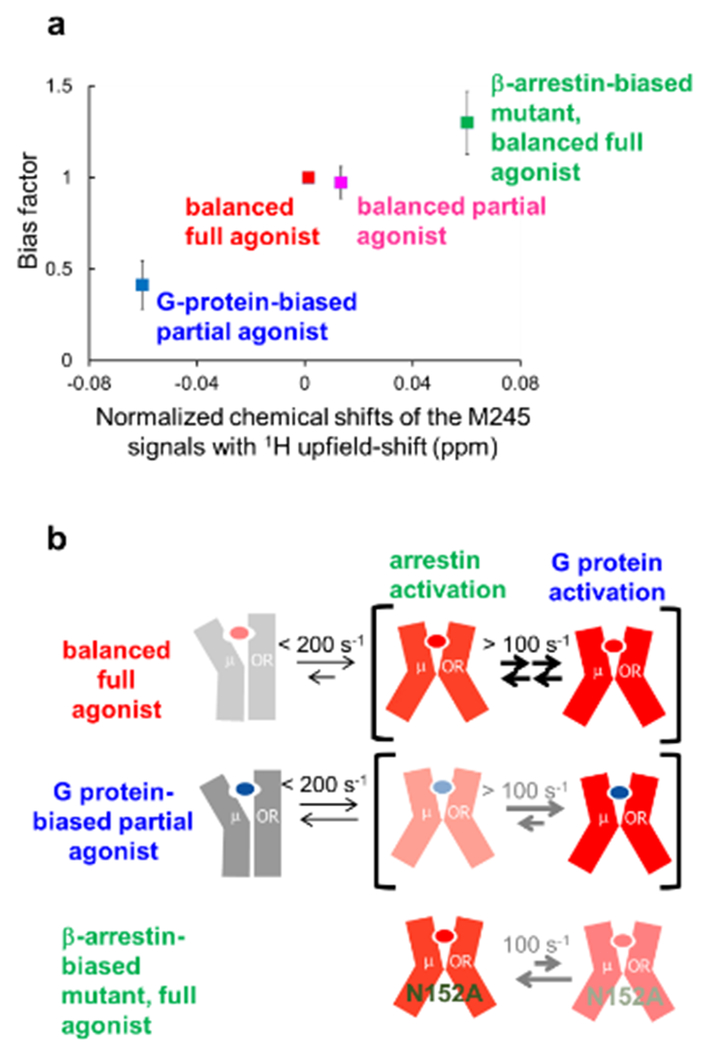
Crystal structures of MOR have been solved in various forms, including those bound to inverse agonists and to a full agonist with a G protein-mimicking nanobody165,166. Activation-induced conformational changes of the structural motifs that are widely conserved among Class A GPCRs, including the cytoplasmic cavity formation, were also observed for the MOR structures. These crystal structures were complemented in important ways by NMR studies. Sounier et al. used NMR signals originating from 13CH3 probes attached to the solvent-accessible lysines of MOR in the apo state, an agonist-bound state, and the ternary complex with an agonist and a G protein-mimicking nanobody128. Okude et al. observed the NMR signals of M1633.46, M2455.49 M2575.61 and M2836.36 of MOR, which are sensitive to conformational changes of TMIII, V, VI, and VII, in the balanced and biased ligand-bound states129. The chemical shifts of these residues correlated well with the bias factors, which are the ratios of the β-arrestin signaling efficacies to the G protein signaling efficacies in each state (Fig. 6a). These results, along with temperature-dependent shifts of these resonances, suggested that the intracellular cavity of MOR exists in an equilibrium between a closed and multiple open conformations, with coupled rearrangements of the transmembrane helices III, V, VI and VII, and that the relative populations of the open conformations determine the G protein- and arrestin-mediated signaling levels in different ligand-bound states (Fig. 6b).
Figure 6 |. NMR-observable conformational equilibria related to biased signaling of MOR.
a | Correlation between the normalized chemical shifts of the M245 NMR signals and the value of the bias factor, which is the ratio of the β-arrestin signaling efficacy to the G protein signaling efficacy. b | Mechanisms leading to functional selectivity of MOR for different ligands. In the balanced full agonist state with DAMGO bound, MOR primarily adopts the active conformation (red), in which the intracellular surface is characterized by multiple substates, with exchange rates larger than 100 s−1. In the G protein-biased partial agonist TRV130-bound state, MOR exists in an equilibrium between the inactive (grey) and active conformations, with exchange rates smaller than 200 s−1, and the equilibrium within the active substates is shifted toward the conformation preferred for G protein activation. In the DAMGO-bound state of the MOR N1523.35A mutant, where MOR adopts only the active conformation, the equilibrium among the active substates is shifted toward the conformation preferred for arrestin activation.
A2A adenosine receptor.
A2AAR plays myriad roles in human health, including regulating myocardial blood flow, and it is heavily expressed in the central nervous system, where it is involved with dopamine and glutamine release167,168. A2AAR has recently also become a promising target for developing cancer immunotherapies169–173. Similar to β2AR, A2AAR is structurally well characterized. Crystal structures are available of antagonist complexes90,174, agonist complexes175,176, and a ternary complex with an agonist and a “mini” G protein177. For NMR spectroscopy it is of interest that A2AAR has been expressed in multiple cell types, including Pichia pastoris, insect cells, and mammalian cells, permitting many options for stable-isotope labeling (Box 1).
19F-NMR has been used to study A2AAR by post-translational chemical modification of non-endogenous cysteines introduced at positions near the intracellular surface, which were labeled with 19F-BTFMA125, or via in-membrane chemical modification with TET178,179, and then studied in detergent micelles. One-dimensional 19F-NMR spectra were measured for A2AAR complexes with ligands of different efficacies. Efficacy-dependent changes of the relative populations of different conformational states were observed125,179, which were also shown to respond to variable salt concentrations131.
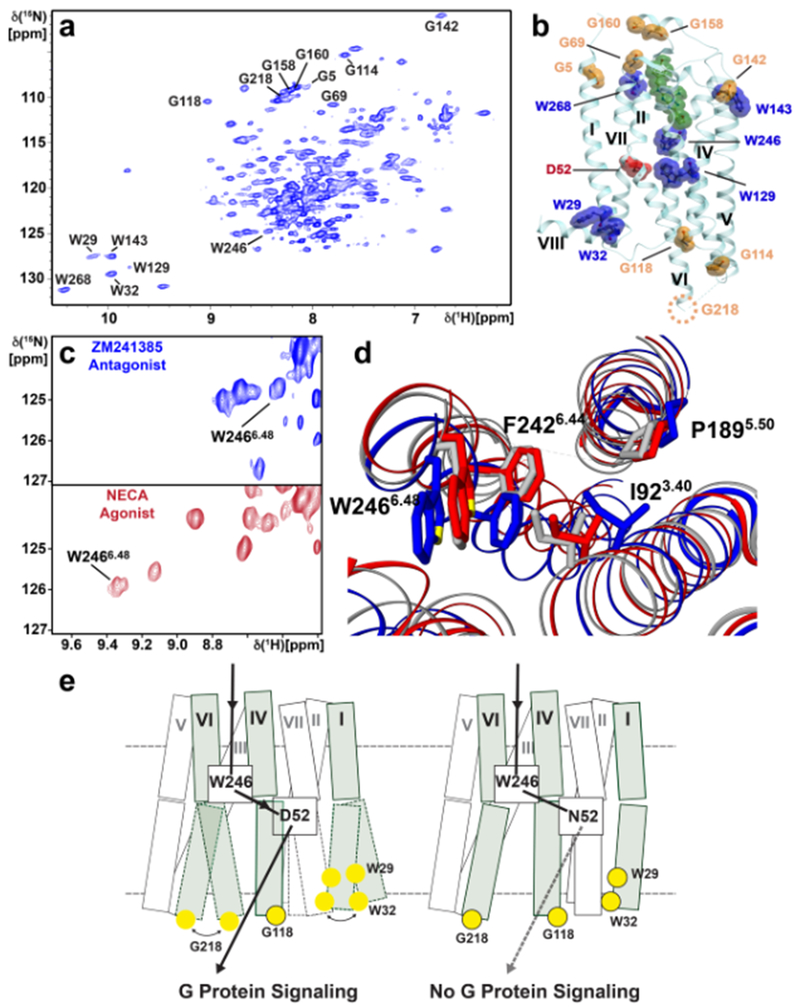
[u-15N, ~70% 2H]-human A2AAR was expressed in Pichia pastoris, and single-amino acid replacements were used for the assignment of all six tryptophan indole 15N–1H signals and eight glycine backbone 15N-1H signals distributed on the extracellular and intracellular surfaces (Fig 7a)127. The distribution of NMR probes (Fig. 7b) permitted a global view of the response of A2AAR structure and dynamics to variable efficacy of bound drugs. Effects of the inactivation of an allosteric center by replacement of residue Asp522.50 with Asn resulted in local changes of the orientation of the toggle switch Trp2466.48 relative to the P/I/F activation motif (Fig. 7,c and d), which are directly correlated with both a change of conformational dynamics at the intracellular surface and loss of G protein signaling127 (Fig. 7e).
Figure 7. NMR affords a global view of A2AAR response to variable drug efficacy and inactivation of an allosteric center.
a | [15N,1H]-TROSY NMR correlation spectrum of [u-15N, ~70% 2H]-A2AAR in complex with the antagonist ZM241385. Assigned backbone 15N–1H glycine and indole 15N–1H tryptophan signals are annotated. b | Locations of assigned glycine (orange) and tryptophan (blue) signals in the crystal structure of A2AAR in complex with the antagonist ZM241385 (PDB 6AQF). ZM241385 is shown in green. D522.50, a critical residue in the allosteric center, is shown in red. Helices are labeled with Roman numerals. The position of G218 is indicated by a dotted circle, as it was replaced with a fusion protein to facilitate crystallization. c | The indole 15N–1H signal of W2466.48, the so-called “toggle switch” tryptophan276,277, is highly responsive to variable drug efficacy, as observed by comparing NMR spectra for A2AAR in complex with the antagonist ZM241385 (blue) and agonist NECA (red). d | The response of the W2466.48 indole 15N–1H signal to variable drug efficacy can be rationalized by reorientation of the nearby F2426.44 in the P/I/F activation motif, which is highly conserved among Class A GPCRs. e | Schematic side views of A2AAR (left) and A2AAR[D52N] (right). Each transmembrane helix is represented by two adjoining rectangles. The three helices carrying NMR reporter groups near the intracellular surface are shaded, and the residues with assigned NMR lines are indicated by yellow spheres, where black framed spheres indicate that a single NMR line was observed, and unframed spheres correspond to multiple-component signals. The helices drawn with broken lines indicate local polymorphisms seen in the NMR spectra of the residues with unframed yellow spheres. The broken horizontal lines indicate the extracellular and intracellular membrane surfaces. The black arrow indicates the signaling pathway from the orthosteric drug binding site to the intra- cellular surface. In A2AAR, signaling has been correlated with local polymorphisms at the intracellular tips of the helices I and VI. In A2AAR[D52N], signaling to the intracellular surface is quenched. The broken arrow indicates loss of signaling to the G protein, which correlates with the abolishment of the dynamic polymorphisms at the intracellular surface.
[1H,13C-Ile, u-2H]-A2AAR was expressed in Pichia pastoris for methyl TROSY NMR experiments, where residues I923.40 and I2928.47 were assigned by amino acid replacement126. NMR experiments for A2AAR complexes with ligands of different efficacies indicated that inverse agonists suppressed high-frequency motions on the intracellular signaling surface, and large conformational changes were observed depending on the sodium concentration in the A2AAR solutions126.
A2AAR has an exceptionally long C-terminal polypeptide “tail” when compared with Class A GPCRs at large, i.e. 122 residues rather than 30 to 40 residues. In crystallization constructs, the A2AAR amino acid sequence was truncated at position 316 in order to facilitate crystallization. NMR studies of the isolated C-terminal polypeptide showed that it is intrinsically disordered180,181, and additional biophysical data suggested that this intrinsic flexibility is important for complex formation with various partner proteins, including calmodulin180. Intrinsically disordered polypeptide segments in GPCRs have been shown to be subject to posttranslational modifications and thought to be critical for interactions with G proteins and other partner proteins182. NMR is especially well suited for studying intrinsically disordered polypeptides183,184.
Class B GPCRs.
Class B GPCRs are composed of a 100- to 150-residue N-terminal extracellular domain (NTD or ECD) and a canonical transmembrane domain with seven transmembrane α-helices. This class comprises 15 receptors, which all bind polypeptide hormones30,31. Class B GPCRs are involved in key physiological processes and associated diseases, including diabetes, osteoporosis, cancer, neurodegeneration, cardiovascular disease, migraine and psychiatric disorders31 and so are attractive drug targets185. Examples include numerous glucagon-like peptide 1 receptor (GLP1R) agonists that are in use for the treatment of diabetes, and the peptides tesamorelin, a growth hormone-releasing hormone receptor (GHRHR) agonist, and teduglutide, a glucagon-like peptide 2 receptor (GLP2R) agonist4,186. However, no small-molecule agonist drugs directed at Class B receptors have so far been identified, and thus new structural information about Class B GPCRs is expected to open exciting new perspectives4. For NMR applications it is of interest that various Class B GPCRs have been reported to exhibit biased signaling and allosteric modulation, and there is accumulating evidence for the importance of these features for the development of drugs that target Class B GPCRs30. Differences to Class A GPCRs arise because the residues that exhibit the largest conformational changes upon activation in Class A GPCRs, such as the P/I/F, NPxxY, and D(E)RY motifs60, are not conserved in Class B GPCRs31, and furthermore the extracellular N-terminal domain of Class B GPCRs may also be directly involved in the regulation of signaling. Overall, the extensive knowledge on Class A GPCR structures is thus not directly applicable to Class B GPCRs.
Class B GPCRs offer new possibilities for applying NMR to study mechanisms of ligand recognition and intracellular signaling. Most endogenous Class B GPCR ligands are polypeptides, which can be labeled with stable isotopes187 to study their detailed atomic interactions with receptors by NMR. The possibility also arises to use NMR in parallel with intein chemistry to selectively label different segments of the GPCR sequence with 2H, 15N and/or 13C. Intein chemistry has been applied to study a C-terminal polypeptide segment of β2AR124, and studies of selectively labeled Class B receptor extracellular domains within the full-length receptor would be an obvious extension of this work. NMR can thus provide information by observation of either the endogenous ligand, the GPCR, intracellular partner proteins, or by combinations thereof, since all these components can be individually labeled with NMR-observable probes that provide direct experimental access to ligand and partner protein binding mechanisms that underlie the physiological signaling by Class B GPCRs.
NMR in GPCR drug discovery
The above sections summarize how NMR studies on GPCRs have complemented structural understanding from other biophysical techniques and highlighted opportunities for drug discovery. Here, we discuss the two main ways in which NMR studies can be used in pursuing such opportunities: applications in fragment-based screening to identify starting points for drug discovery, and understanding and optimizing the pharmacological characteristics of GPCR-targeted drugs.
NMR support of fragment-based lead discovery.
Fragment-based screening (FBS) uses libraries of thousands of small molecular fragments to find starting points for developing novel drugs. This contrasts with high-throughput screening (HTS) of millions of compounds to find drug-sized starting compounds. Sampling of the chemical space tends to be more efficient with molecular fragments than with larger molecules, and as they form fewer interactions, small fragments bind to a wider range of sites on a greater number of proteins, leading to higher hit rates188.
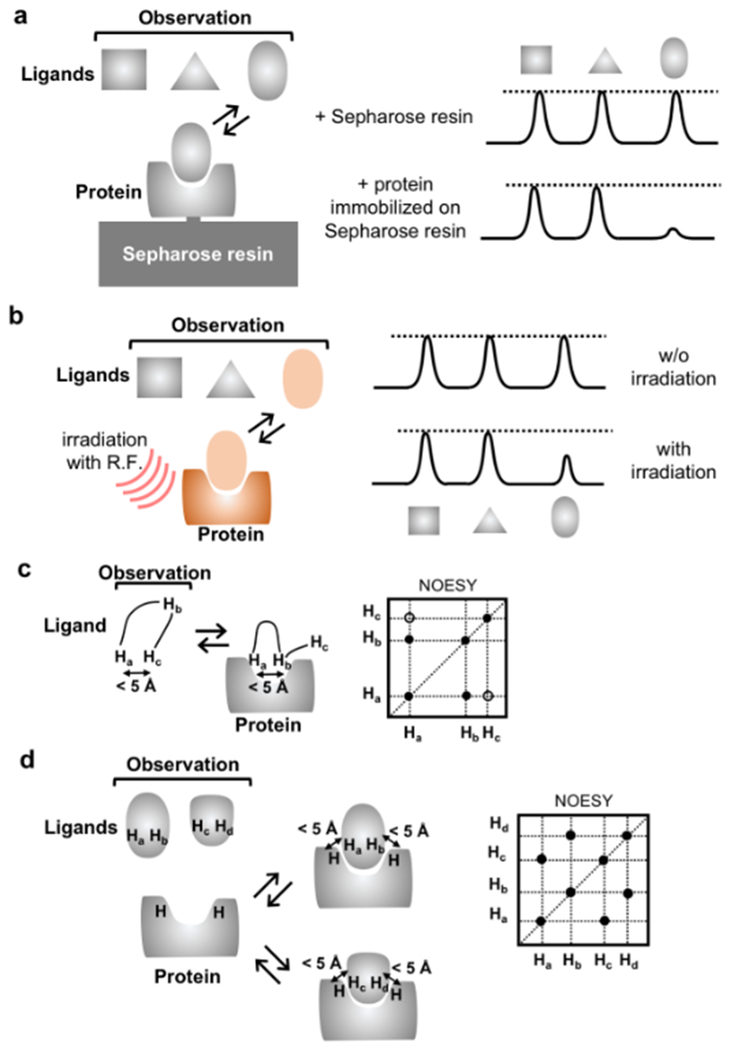
However, the binding affinities of small molecular fragments are likely to be lower than those for larger molecules, and therefore high assay sensitivity is needed in FBS. NMR spectroscopy was the first method used successfully to screen fragments189, and it is still a commonly used method17. FBS has been applied to GPCRs with thermostabilization190, and NMR has been utilized for FBS targeting β1AR191 and A2AAR192,193. Ligand-observed NMR methods, such as target immobilized NMR screening (TINS) and saturation transfer difference spectroscopy (STD) (Fig. 8, a and b), have been utilized for FBS with β1AR191 and A2AAR192,193; fragments have been identified that inhibited binding of tritium-labeled ligands with IC50 values ranging from 5 µM to 5 mM. Special advantages of these methods include that they can detect weak binders, require less protein than protein-observed NMR experiments, do not require isotope labels, and have no upper size limit for the protein188,194. The main challenge in FBS is in combining fragments to molecules for lead optimization, where structural information is required to decide on where to grow, merge or link a given fragment195. NMR methods can provide such information. Using forbidden coherence transfer (FCT) measurements196, which is sensitive to the degree of surface complementarity, the substitution position of the ligands to grow can be identified. Transferred NOE spectroscopy132,197,198 (Fig. 9c) and interligand NOEs for pharmacophore mapping (INPHARMA)135,199 (Fig. 9d) provide information about how to merge or link fragments in lead optimization processes. STD NMR200–202 has also been applied for epitope mapping and determination of the bound conformations of GPCR ligands203–206. The bound conformations of ligands for kappa opioid receptor, pituitary adenylate cyclase activating polypeptide receptor, and leukotriene receptor BLT2 have been determined by transferred NOE spectroscopy132,198,206, and INPHARMA has been applied for the generation of docking models of A2AAR and GPR40135,199.
Figure 8. |. NMR methods for studies of GPCR–ligand interactions.
a | Target-immobilized NMR screening (TINS). Resonance broadening of ligands upon binding to proteins immobilized on Sepharose resin is observed. The bound ligand can be identified by the broadening of its ligand signals. b | Saturation transfer difference (STD) NMR. The protein–ligand complex is irradiated at a frequency corresponding to hydrogen atoms of the protein, so that saturation can be transferred from the protein to the ligand. If the complex has sufficiently large ligand exchange rates, then this saturation is transferred to the bulk free ligands. The bound ligand can be identified by the transferred saturation. c | Transfer NOE (trNOE). Similar to (b): negative NOEs on the bound ligand are transferred by rapid ligand exchange to the bulk of free ligands. Therefore, trNOEs provide information on distances between protons of the ligand in the receptor-bound state. d | Interligand NOEs for pharmacophore mapping (INPHARMA). Protein-mediated NOEs between two or multiple simultaneously bound ligands are observed. Thus, INPHARMA peaks describe the orientation of the two ligands relative to each other in the receptor binding pocket.
Figure 9 |. NMR screening of biased ligands.
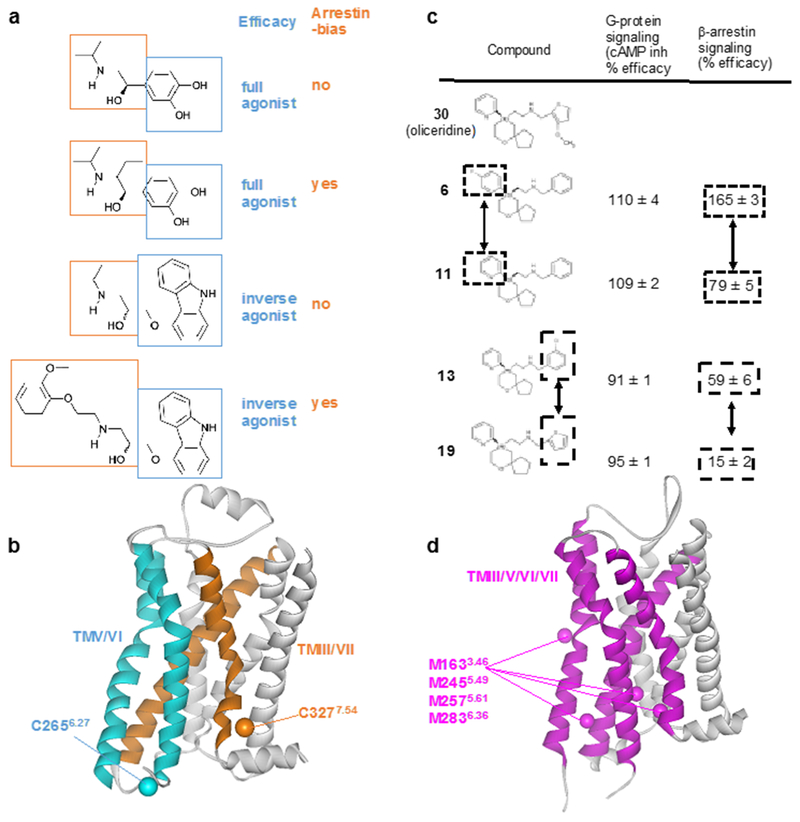
a | Structures and functions of β2AR ligands. Modifications of the ethanolamine tail moieties (highlighted in orange boxes) result in selective modulation of the efficacies for β-arrestin signaling, whereas modifications of the aromatic head groups (highlighted in blue boxes) affect the efficacies of both G protein-mediated signaling and β-arrestin-mediated signaling. b | Crystal structure of the β2AR complex with the inverse agonist carazolol (PDB: 2RH1). TMV and TMVI (cyan) form the binding site for the aromatic head groups shown in (a). TMIII and TMVII (orange) form the binding site for the ethanolamine tail moieties shown in (a). 19F-NMR probes introduced at C2656.27 (Cβ is shown cyan) enable studies of the roles of TMV and TMVI for G protein signaling efficacy. 19F-NMR probes introduced at C3277.54 (Cβ is shown orange) enable studies of TMIII and TMVII in arrestin bias. c | Structure–function relationships of derivatives of the MOR ligand oliceridine. Modification of either of the two aromatic rings that are highlighted in boxes results in selective modulation of β-arrestin signaling. d | Crystal structure of MOR with a morphinan antagonist (PDB: 4DKL). TMIII, TMV, TMVI and TMVII are shown as purple ribbons, and Cε atoms from M1633.46, M2455.49, M2575.61, M2836.36 are shown as purple spheres. NMR studies observing 13CH3 groups of these four methionines suggest that TMIII, TMV, TMVI and TMVII are involved in G protein/arrestin signaling bias.
NMR for optimization of drug pharmacological characteristics.
An attractive goal for structure-based drug design is to improve specificity of receptor–ligand interactions while increasing ligand affinities, efficacies and desired biased signaling. It has been proposed that structures of mutant GPCRs with increased thermostability in the agonist-bound state can be utilized for the development of agonists4, and biased ligands of opioid receptors were identified by a structure-based approach57,207, using inactive crystal structures in the antagonist-bound state. In this respect, NMR can provide structural information about conformational equilibria that are related to both the ligand efficacies and biased signaling in GPCRs. In addition, NMR studies revealed relationships between the chemical structures of GPCR ligands and their biased signaling, indicating that NMR studies would be helpful for the design of biased ligands.
For example, conformational changes of TMIII and TMVII in β2AR that are not paralleled by those of TMV and TMVI in complexes with β-arrestin-biased ligands are in agreement with the following structure–activity relationships of β2AR ligands (Fig. 9a and b). Modifications of the ethanolamine tail moieties, which are bound to TMIII and TMVII in the crystal structures68,81,208, result in selective modulation of the efficacies for β-arrestin signaling, whereas modifications of the aromatic head groups, which bind to TMV and TMVI in the crystal structures81,85,208, affect the efficacies of both G protein-mediated signaling and β-arrestin-mediated signaling112. Results from crystallography of the serotonin 5-HT1B and 5-HT2B receptors bound to ergotamine209, and from fluorescence studies of the ghrelin receptor GHS-R1a210 and the arginine vasopressin type 2 receptor211 are also in agreement with selective activation of G protein- and β-arrestin-mediated signaling by localized conformational changes of TMV and TMVI, and of TMIII and TMVII, respectively.
In MOR, a comparison of olceridine derivatives, composed of the 2-[6-oxaspiro[4.5]decan-9-yl]ethylamine scaffold and two aromatic groups attached to the oxaspiro[4,5]decan and ethanolamine moieties (Fig. 9c), including compounds 6 to 11 and 13 to 19, revealed that the modification of either of the two aromatic rings results in selective modulation of β-arrestin signaling164. These structure–activity relationships of the olceridine derivatives are in agreement with a population shift of the conformational equilibria that accompanies the global conformational changes in TMIII, TMV, TMVI and TMVII upon the β-arrestin-biased N152A3.35 mutation, as revealed by NMR (Fig. 9d)129. The coupled conformational changes of TMIII, TMV, TMVI and TMVII are also in agreement with the structure–activity relationships of the D2 dopamine receptor: the modification of both the head and tail groups of the ligands for D2 dopamine receptor resulted in selective modulation of the β-arrestin signaling212.
The mobility of the extracellular loops of GPCRs can affect the binding kinetics of ligands that attach to the binding pocket, and the binding kinetics correlates with the duration of drug action78. In molecular dynamics simulations of M2 and M3 muscarinic acetylcholine receptors bound to the ligand tiotropium, the ECL2 of the M3 receptor bound to tiotropium exhibited lower mobility than that of the M2 receptor213. The reduced mobility of the M3 receptor appears to parallel the slow association and dissociation of the ligand, and slow dissociation from the M3 receptor is responsible for the clinically preferable long-acting profile of tiotropium. In addition, ligands may adopt multiple different binding modes to their target molecules214, and heterobivalent or non-bivalent ligands that are able to bind in two different orientations to the GPCRs have been identified for MOR, muscarinic M2 receptor, histamine H1 receptor, and dopamine D3 receptor215,216. In the case of the bipharmacophoric ligands of muscarinic M2 receptor216, consisting of an active moiety and an inactive moiety, the ligand binding modes dynamically switch between an active signaling-compotent pose and an inactive binding pose. The population of the active pose is related to the efficacies and is affected by the affinity ratio between the active and inactive moieties216. We conclude that dynamic ligand binding in multiple binding modes is a novel promising feature to be considered for the rational design of agonists with predictable efficacies. Finally, NMR has been utilized for investigations of the conformational equilibria in the ligand-binding sites of GPCRs132, which are important for structure-guided drug design217. In the NMR study of kappa opioid receptor and its peptide ligand, motion on the sub-nanosecond timescale was observed for the N terminal region of the ligand, which is known to be crucial for activation132. Sub-nanosecond timescale motion in the ligand-binding site is correlated to the surface complementarity196,218; observation and optimization of ligand mobility thus promises to provide important input for the design of ligands with improved affinities.
Outlook
Since 2007, X-ray crystallography has set high standards for structure determination of human GPCR complexes with a wide range of drug molecules and other ligands, and the potential of cryo-EM to provide structure determinations of higher-order complexes of GPCRs, with ligands, modulators and partner proteins is now rapidly developing. While this review primarily discusses integrative use of X-ray crystal structures and NMR spectroscopy in solution, the inclusion of cryo-EM results is an exciting prospect, which may dominate the field in the future. Compared to X-ray crystallography, cryo-EM has greatly increased potential to provide structures of multiple simultaneously populated conformations in a given sample preparation219. It is exciting to look forward to combining this information on static arrays of different structures with the potential of solution NMR to characterize local and global three-dimensional structural polymorphisms in GPCRs, and to provide information on the relative populations of the different macromolecular species and the exchange kinetics among them.
In this review, we have emphasized the ability of NMR to study wild type or near-wild type GPCRs in aqueous media at physiological temperatures. For the future it will be important to further enable studies of GPCRs in sample preparations that also represent close mimics of other aspects of the physiological environment. In this context, the development of specialized, sophisticated GPCR biochemistry is called for. This may include the formation of lipid bilayer discs directly from cell membranes220 or the preparation of size-controlled liposomes using a DNA nanotemplate221, enabling NMR spectroscopy with GPCRs reconstituted in lipid mixtures that closely mimic those found in living cells. Combined with these improvements of GPCR reconstitution (Box 2), refined stable-isotope labeling (Box 1) will be needed, such as the use of non-native amino acids to introduce NMR probes at discrete locations in GPCRs expressed in mammalian or insect cells, and the use of segmental labeling for NMR studies of selected polypeptide segments in intact GPCRs124. However, refined GPCR biochemistry for NMR may come at the cost of lower expression and purification yields, and so the success of new approaches will also require advances in NMR technologies. Dynamic nuclear polarization (DNP) NMR, which can provide an orders-of-magnitude increase in experimental sensitivity for studies of challenging systems, appears particularly promising222,223, and which has also been extensively used in solid state NMR136,224–227.
Overall, there is the promise that NMR studies in solution environments that are ever closer to the physiological milieu will be used to investigate the structural basis of impacts by cellular traits on GPCR function12. Specifically, this will address phenomena such as GPCR oligomerization228,229, posttranslational modifications230, and complex formation with other partners such as receptor–activity modifying proteins (RAMP)231. Awaiting these technical advances and an abundance of new data from cryo-EM, we look forward to exciting times for the use of solution NMR in integrative GPCR structural biology projects. Each step forward in structural biology will support discovery of new drugs as well as improvement of existing drugs and their application.
Acknowledgements
This work is supported by The Ministry of Education, Culture, Sports, Science and Technology (MEXT)/Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers JP17H06097 (I.S.), JP18H04540 (T.U.), JP17H04999 (Y.K.); the development of core technologies for innovative drug development based upon IT and the development of innovative drug discovery technologies for middle-sized molecules, from the Japan Agency for Medical Research and Development, AMED (I.S.); an American Cancer Society Postdoctoral Fellowship (M.T.E); NIH/NIGMS R01GM115825 (K.W.); K.W. is the Cecil H. and Ida M. Green Professor of Structural Biology at The Scripps Research Institute
Glossary
- Allosteric modulators
Molecules that bind to sites on GPCRs that are spatially distinct from the orthosteric binding pocket and modulate the affinity and/or efficacy of drugs bound to the orthosteric site. Allosteric modulators can be synthetic or endogenous compounds or metal ions in the cellular environment
- Biased ligand
GPCR agonists can activate both G protein and β-arrestin signaling pathways (see Figure 1). Agonists that activate predominantly one of intracellular pathways are referred to as “biased ligands”. Drugs functioning as agonists may produce unwanted side effects mediated through the activation of multiple signaling pathways. Such side effects can be minimized by designing biased ligands that selectively activate only the signaling pathway required to produce the desired therapeutic response.
- Conformational equilibria
Solution NMR studies established that GPCRs in near physiological environments exist in multiple, locally different conformers that are simultaneously populated in function-related equilibria. It has been shown that the relative populations of these conformers are related to the efficacies and the bias of bound drugs
- GPCR Classes
GPCRs have been grouped into six different classes, labeled A through F, based on sequence homologies and physiological functions
- Efficacy
Efficacy refers to the extent to which a GPCR ligand changes the receptor signaling intensity relative to its basal level. The efficacy is a key determinant of the therapeutic properties of a GPCR-targeting drug
- Sequence motif
Polypeptide segments of 2 or several amino acids which are highly conserved among GPCRs of a given class and have been identified as key components of activation centers
- Activation center
Clusters of closely spaced amino acids in the three-dimensional structure. Sequence motifs are often part of activation centers
- Stable-isotope labeling
NMR spectroscopy with complex biomacromolecular systems is routinely based on labeling of proteins or other components with stable NMR-observable isotopes. Widely used stable isotopes in GPCR research are 2H, 13C, 15N and 19F
- TROSY (transverse relaxation-optimized spectroscopy)
An experiment that enables solution NMR studies of large macromolecules or supramolecular structures, in particular of membrane proteins reconstituted into micelles, bicelles or nanodiscs
References
- 1.Rask-Andersen M, Almén MS & Schiöth HB Trends in the exploitation of novel drug targets. Nat. Rev. Drug Discov. 10, 579–590, doi: 10.1038/nrd3478 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB & Gloriam DE Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 16, 829–842, doi: 10.1038/nrd.2017.178 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santos R et al. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 16, 19–34, doi: 10.1038/nrd.2016.230 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jazayeri A, Andrews SP & Marshall FH Structurally enabled discovery of adenosine A2A receptor antagonists. Chem. Rev. 117, 21–37, doi: 10.1021/acs.chemrev.6b00119 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Hutchings CJ, Koglin M, Olson WC & Marshall FH Opportunities for therapeutic antibodies directed at G-protein-coupled receptors. Nat. Rev. Drug Discov. 16, 787–810, doi: 10.1038/nrd.2017.91 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Steyaert J & Kobilka BK Nanobody stabilization of G protein-coupled receptor conformational states. Curr. Opin. Struct. Biol. 21, 567–572, doi: 10.1016/j.sbi.2011.06.011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Congreve M, Oswald C & Marshall FH Applying structure-based drug design approaches to allosteric modulators of GPCRs. Trends Pharmacol. Sci. 38, 837–847, doi: 10.1016/j.tips.2017.05.010 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Conn PJ, Lindsley CW, Meiler J & Niswender CM Opportunities and challenges in the discovery of allosteric modulators of GPCRs for treating CNS disorders. Nat. Rev. Drug Discov. 13, 692–708, doi: 10.1038/nrd4308 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Changeux J-P & Christopoulos A Allosteric Modulation as a Unifying Mechanism for Receptor Function and Regulation. Cell 166, 1084–1102, doi: 10.1016/j.cell.2016.08.015 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Changeux J-P 50 years of allosteric interactions: the twists and turns of the models. Nature reviews Molecular cell biology 14, 819 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Violin JD, Crombie AL, Soergel DG & Lark MW Biased ligands at G-protein-coupled receptors: promise and progress. Trends Pharmacol. Sci. 35, 308–316 (2014). [DOI] [PubMed] [Google Scholar]
- 12.Smith JS, Lefkowitz RJ & Rajagopal S Biased signalling: from simple switches to allosteric microprocessors. Nat. Rev. Drug Discov, doi: 10.1038/nrd.2017.229 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane JR, May LT, Parton RG, Sexton PM & Christopoulos A A kinetic view of GPCR allostery and biased agonism. Nat. Chem. Biol. 13, 929–937, doi: 10.1038/nchembio.2431 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Rankovic Z, Brust TF & Bohn LM Biased agonism: An emerging paradigm in GPCR drug discovery. Bioorg. Med. Chem. Lett. 26, 241–250, doi: 10.1016/j.bmcl.2015.12.024 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reiter E, Ahn S, Shukla AK & Lefkowitz RJ Molecular mechanism of β-arrestin-biased agonism at seven-transmembrane receptors. Annu. Rev. Pharmacol. Toxicol. 52, 179–197 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh E, Kumari P, Jaiman D & Shukla AK Methodological advances: the unsung heroes of the GPCR structural revolution. Nat. Rev. Mol. Cell Biol. 16, 69–81, doi: 10.1038/nrm3933 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Renaud JP et al. Biophysics in drug discovery: impact, challenges and opportunities. Nat. Rev. Drug Discov. 15, 679–698, doi: 10.1038/nrd.2016.123 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Didenko T, Liu JJ, Horst R, Stevens RC & Wüthrich K Fluorine-19 NMR of integral membrane proteins illustrated with studies of GPCRs. Curr. Opin. Struct. Biol. 23, 740–747, doi: 10.1016/j.sbi.2013.07.011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prosser RS & Kim TH Nuts and bolts of CF3 and CH3 NMR toward the understanding of conformational exchange of GPCRs. Methods Mol. Biol. 1335, 39–51, doi: 10.1007/978-1-4939-2914-6_4 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Ranjan R, Dwivedi H, Baidya M, Kumar M & Shukla AK Novel structural insights into GPCR-β-arrestin interaction and signaling. Trends Cell Biol. 27, 851–862, doi: 10.1016/j.tcb.2017.05.008 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Audet M & Bouvier M Restructuring G-protein- coupled receptor activation. Cell 151, 14–23, doi: 10.1016/j.cell.2012.09.003 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Wacker D, Stevens RC & Roth BL How ligands illuminate GPCR molecular pharmacology. Cell 170, 414–427, doi: 10.1016/j.cell.2017.07.009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu F, Song G, de Graaf C & Stevens RC Structure and function of peptide-binding G protein-coupled receptors. J. Mol. Biol. 429, 2726–2745, doi: 10.1016/j.jmb.2017.06.022 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Hilger D, Masureel M & Kobilka BK Structure and dynamics of GPCR signaling complexes. Nat. Struct. Mol. Biol. 25, 4–12, doi: 10.1038/s41594-017-0011-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpenter B & Tate CG Active state structures of G protein-coupled receptors highlight the similarities and differences in the G protein and arrestin coupling interfaces. Curr. Opin. Struct. Biol. 45, 124–132, doi: 10.1016/j.sbi.2017.04.010 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Zhou XE, Melcher K & Xu HE Understanding the GPCR biased signaling through G protein and arrestin complex structures. Curr. Opin. Struct. Biol. 45, 150–159, doi: 10.1016/j.sbi.2017.05.004 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Scheerer P & Sommer ME Structural mechanism of arrestin activation. Curr. Opin. Struct. Biol. 45, 160–169, doi: 10.1016/j.sbi.2017.05.001 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Jazayeri A, Dias JM & Marshall FH From G Protein-coupled receptor structure resolution to rational drug design. J. Biol. Chem. 290, 19489–19495, doi: 10.1074/jbc.R115.668251 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauser AS et al. Pharmacogenomics of GPCR drug targets. Cell 172, 41–54. e19, doi: 10.1016/j.cell.2017.11.033 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wootten D, Miller LJ, Koole C, Christopoulos A & Sexton PM Allostery and biased agonism at class B G protein-coupled receptors. Chem. Rev. 117, 111–138, doi: 10.1021/acs.chemrev.6b00049 (2017). [DOI] [PubMed] [Google Scholar]
- 31.de Graaf C et al. Extending the structural view of class B GPCRs. Trends Biochem. Sci. 42, 946–960, doi: 10.1016/j.tibs.2017.10.003 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Renaud J-P et al. Cryo-EM in drug discovery: achievements, limitations and prospects. Nat. Rev. Drug Discov. (2018). [DOI] [PubMed] [Google Scholar]
- 33.García-Nafría J, Nehmé R, Edwards PC & Tate CG Cryo-EM structure of the serotonin 5-HT1B receptor coupled to heterotrimeric Go. Nature 558, 620–623, doi: 10.1038/s41586-018-0241-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang Y et al. Cryo-EM structure of human rhodopsin bound to an inhibitory G protein. Nature 558, 553–558, doi: 10.1038/s41586-018-0215-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y et al. Cryo-EM structure of the activated GLP-1 receptor in complex with a G protein. Nature 546, 248–253, doi: 10.1038/nature22394 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang YL et al. Phase-plate cryo-EM structure of a class B GPCR-G-protein complex. Nature 546, 118–123, doi: 10.1038/nature22327 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eddy MT & Yu T-Y Membranes, peptides, and disease: unraveling the mechanisms of viral proteins with solid state nuclear magnetic resonance spectroscopy. Solid state NMR 61–62, 1–7, doi: 10.1016/j.ssnmr.2014.04.002 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Brown LS & Ladizhansky V Membrane proteins in their native habitat as seen by solid-state NMR spectroscopy. Protein Sci. 24, 1333–1346, doi: 10.1002/pro.2700 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Opella SJ & Marassi FM Applications of NMR to membrane proteins. Arch. Biochem. Biophys. 628, 92–101, doi: 10.1016/j.abb.2017.05.011 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplan M, Pinto C, Houben K & Baldus M Nuclear magnetic resonance (NMR) applied to membrane–protein complexes. Q. Rev. Biophys. 49, 1010, doi: 10.1017/S003358351600010X (2016). [DOI] [PubMed] [Google Scholar]
- 41.Mandala VS, Williams JK & Hong M Structure and Dynamics of Membrane Proteins from Solid-State NMR. Annu. Rev. Biophys. 47, 201–222, doi: 10.1146/annurev-biophys-070816-033712 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wylie BJ, Do HQ, Borcik CG & Hardy EP Advances in solid-state NMR of membrane proteins. Mol. Phys. 114, 3598–3609, doi: 10.1080/00268976.2016.1252470 (2016). [DOI] [Google Scholar]
- 43.Judge PJ & Watts A Recent contributions from solid-state NMR to the understanding of membrane protein structure and function. Curr. Opin. Chem. Biol. 15, 690–695, doi: 10.1016/j.cbpa.2011.07.021 (2011). [DOI] [PubMed] [Google Scholar]
- 44.Alexander SP et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: G protein-coupled receptors. Br. J. Pharmacol. 174 Suppl 1, S17–S129, doi: 10.1111/bph.13878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bologna Z, Teoh JP, Bayoumi AS, Tang Y & Kim IM Biased G Protein-coupled receptor signaling: new player in modulating physiology and pathology. Biomol. Ther. (Seoul) 25, 12–25, doi: 10.4062/biomolther.2016.165 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benredjem B, Dallaire P & Pineyro G Analyzing biased responses of GPCR ligands. Curr. Opin. Pharmacol. 32, 71–76, doi: 10.1016/j.coph.2016.11.008 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Urban JD et al. Functional selectivity and classical concepts of quantitative pharmacology. J. Pharmacol. Exp. Ther. 320, 1–13 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Pupo AS et al. Recent updates on GPCR biased agonism. Pharmacol. Res. 112, 49–57, doi: 10.1016/j.phrs.2016.01.031 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Reiter E et al. β-arrestin signalling and bias in hormone-responsive GPCRs. Mol. Cell. Endocrinol. 449, 28–41, doi: 10.1016/j.mce.2017.01.052 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Thompson GL et al. Systematic analysis of factors influencing observations of biased agonism at the mu-opioid receptor. Biochem. Pharmacol. 113, 70–87, doi: 10.1016/j.bcp.2016.05.014 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Kenakin T Functional selectivity and biased receptor signaling. J. Pharmacol. Exp. Ther. 336, 296–302, doi: 10.1124/jpet.110.173948 (2011). [DOI] [PubMed] [Google Scholar]
- 52.Soergel DG et al. First clinical experience with TRV130: pharmacokinetics and pharmacodynamics in healthy volunteers. J. Clin. Pharmacol. 54, 351–357 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Soergel DG et al. Biased agonism of the μ-opioid receptor by TRV130 increases analgesia and reduces on-target adverse effects versus morphine: A randomized, double-blind, placebo-controlled, crossover study in healthy volunteers. Pain 155, 1829–1835 (2014). [DOI] [PubMed] [Google Scholar]
- 54.DeWire SM et al. A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine. J. Pharmacol. Exp. Ther. 344, 708–717 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Schmid CL et al. Bias factor and therapeutic window correlate to predict safer opioid analgesics. Cell 171, 1165–1175. e1113, doi: 10.1016/j.cell.2017.10.035 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Majumdar S & Devi LA Strategy for making safer opioids bolstered. Nature 553, 286–288, doi: 10.1038/d41586-018-00045-1 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Manglik A et al. Structure-based discovery of opioid analgesics with reduced side effects. Nature 537, 185–190, doi: 10.1038/nature19112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volkow ND & Collins FS The role of science in addressing the opioid crisis. N. Engl. J. Med. 377, 391–394, doi: 10.1056/NEJMsr1706626 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Sivertsen B, Holliday N, Madsen AN & Holst B Functionally biased signalling properties of 7TM receptors - opportunities for drug development for the ghrelin receptor. Br. J. Pharmacol. 170, 1349–1362, doi: 10.1111/bph.12361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenbaum DM, Rasmussen SG & Kobilka BK The structure and function of G-protein-coupled receptors. Nature 459, 356–363 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hanania NA, Dickey BF & Bond RA Clinical implications of the intrinsic efficacy of β-adrenoceptor drugs in asthma: full, partial and inverse agonism. Curr. Opin. Pulm. Med. 16, 1–5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stotts AL, Dodrill CL & Kosten TR Opioid dependence treatment: options in pharmacotherapy. Expert Opin. Pharmacother. 10, 1727–1740, doi: 10.1517/14656560903037168 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pich EM & Collo G Pharmacological targeting of dopamine D3 receptors: Possible clinical applications of selective drugs. Eur. Neuropsychopharmacol. 25, 1437–1447, doi: 10.1016/j.euroneuro.2015.07.012 (2015). [DOI] [PubMed] [Google Scholar]
- 64.Greene SJ et al. Partial adenosine A1 receptor agonism: a potential new therapeutic strategy for heart failure. Heart Fail Rev. 21, 95–102, doi: 10.1007/s10741-015-9522-7 (2016). [DOI] [PubMed] [Google Scholar]
- 65.da Silva Junior ED et al. Factors influencing biased agonism in recombinant cells expressing the human α1A -adrenoceptor. Br. J. Pharmacol. 174, 2318–2333, doi: 10.1111/bph.13837 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palczewski K et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science 289, 739–745 (2000). [DOI] [PubMed] [Google Scholar]
- 67.Rosenbaum DM et al. GPCR engineering yields high-resolution structural insights into β2-adrenergic receptor function. Science 318, 1266–1273 (2007). [DOI] [PubMed] [Google Scholar]
- 68.Cherezov V et al. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science 318, 1258–1265 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pándy-Szekeres G et al. GPCRdb in 2018: adding GPCR structure models and ligands. Nucleic Acids Res. 46, D440–D446, doi: 10.1093/nar/gkx1109 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Draper-Joyce CJ et al. Structure of the adenosine-bound human adenosine A1 receptor–Gi complex. Nature 558, 559–563, doi: 10.1038/s41586-018-0236-6 (2018). [DOI] [PubMed] [Google Scholar]
- 71.Koehl A et al. Structure of the µ-opioid receptor–Gi protein complex. Nature 558, 547–552, doi: 10.1038/s41586-018-0219-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghosh E, Nidhi K & Shukla AK SnapShot: GPCR-ligand interactions. Cell 159, 1712, 1712.e1711, doi: 10.1016/j.cell.2014.12.008 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Zhang D et al. Two disparate ligand-binding sites in the human P2Y1 receptor. Nature 520, 317–321, doi: 10.1038/nature14287 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jazayeri A et al. Extra-helical binding site of a glucagon receptor antagonist. Nature 533, 274–277, doi: 10.1038/nature17414 (2016). [DOI] [PubMed] [Google Scholar]
- 75.Liu W et al. Structural basis for allosteric regulation of GPCRs by sodium ions. Science 337, 232–236, doi: 10.1126/science.1219218 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fenalti G et al. Molecular control of δ-opioid receptor signalling. Nature 506, 191–196, doi: 10.1038/nature12944 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hanson MA et al. A specific cholesterol binding site is established by the 2.8 A structure of the human β2-adrenergic receptor. Structure 16, 897–905, doi: 10.1016/j.str.2008.05.001 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Latorraca NR, Venkatakrishnan AJ & Dror RO GPCR dynamics: structures in motion. Chem. Rev. 117, 139–155, doi: 10.1021/acs.chemrev.6b00177 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Cong X, Topin J & Golebiowski J Class A GPCRs: structure, function, modeling and structure-based ligand design. Curr. Pharm. Des. 23, 4390–4409, doi: 10.2174/1381612823666170710151255 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Tautermann CS GPCR structures in drug design, emerging opportunities with new structures. Bioorg. Med. Chem. Lett. 24, 4073–4079, doi: 10.1016/j.bmcl.2014.07.009 (2014). [DOI] [PubMed] [Google Scholar]
- 81.Rasmussen SG et al. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 469, 175–180, doi: 10.1038/nature09648 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ballesteros JA & Weinstein H Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G-protein coupled receptors. Methods Neurosci. 25, 366–428 (1995). [Google Scholar]
- 83.Tehan BG, Bortolato A, Blaney FE, Weir MP & Mason JS Unifying family A GPCR theories of activation. Pharmacol. Ther. 143, 51–60, doi: 10.1016/j.pharmthera.2014.02.004 (2014). [DOI] [PubMed] [Google Scholar]
- 84.Park JH, Scheerer P, Hofmann KP, Choe HW & Ernst OP Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature 454, 183–187 (2008). [DOI] [PubMed] [Google Scholar]
- 85.Rasmussen SG et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477, 549–555, doi: 10.1038/nature10361 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manglik A & Kruse AC Structural basis for G Protein-coupled receptor activation. Biochemistry 56, 5628–5634, doi: 10.1021/acs.biochem.7b00747 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumari P, Ghosh E & Shukla AK Emerging approaches to GPCR ligand screening for drug discovery. Trends Mol. Med. 21, 687–701, doi: 10.1016/j.molmed.2015.09.002 (2015). [DOI] [PubMed] [Google Scholar]
- 88.Zou Y, Weis WI & Kobilka BK N-terminal T4 lysozyme fusion facilitates crystallization of a G protein coupled receptor. PLoS One 7, e46039, doi: 10.1371/journal.pone.0046039 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson AA et al. Structure of the nociceptin/orphanin FQ receptor in complex with a peptide mimetic. Nature 485, 395 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doré AS et al. Structure of the adenosine A2A receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 19, 1283–1293, doi: 10.1016/j.str.2011.06.014 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lebon G, Bennett K, Jazayeri A & Tate CG Thermostabilisation of an agonist-bound conformation of the human adenosine A2A receptor. J. Mol. Biol. 409, 298–310, doi: 10.1016/j.jmb.2011.03.075 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Warne T et al. The structural basis for agonist and partial agonist action on a β1-adrenergic receptor. Nature 469, 241–244, doi: 10.1038/nature09746 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Carpenter B, Nehmé R, Warne T, Leslie AGW & Tate CG Structure of the adenosine A2A receptor bound to an engineered G protein. Nature 536, 104–107, doi: 10.1038/nature18966 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hino T et al. G-protein-coupled receptor inactivation by an allosteric inverse-agonist antibody. Nature 482, 237–240, doi: 10.1038/nature10750 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Warne T, Edwards PC, Leslie AG & Tate CG Crystal structures of a stabilized β1-adrenoceptor bound to the biased agonists bucindolol and carvedilol. Structure 20, 841–849, doi: 10.1016/j.str.2012.03.014 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang X, Stevens RC & Xu F The importance of ligands for G protein-coupled receptor stability. Trends Biochem. Sci. 40, 79–87, doi: 10.1016/j.tibs.2014.12.005 (2015). [DOI] [PubMed] [Google Scholar]
- 97.Shihoya W et al. Activation mechanism of endothelin ET. Nature 537, 363–368, doi: 10.1038/nature19319 (2016). [DOI] [PubMed] [Google Scholar]
- 98.White JF et al. Structure of the agonist-bound neurotensin receptor. Nature 490, 508–513, doi: 10.1038/nature11558 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang Z et al. Structural basis of ligand binding modes at the neuropeptide Y Y1 receptor. Nature, 1–22, doi: 10.1038/s41586-018-0046-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mittermaier A & Kay L Observing biological dynamics at atomic resolution using NMR. Trends Biochem. Sci. 34, 601–611, doi: 10.1016/j.tibs.2009.07.004 (2009). [DOI] [PubMed] [Google Scholar]
- 101.Osawa M, Takeuchi K, Ueda T, Nishida N & Shimada I Functional dynamics of proteins revealed by solution NMR. Curr. Opin. Struct. Biol. 22, 660–669, doi: 10.1016/j.sbi.2012.08.007 (2012). [DOI] [PubMed] [Google Scholar]
- 102.Ikeya T et al. Solution NMR views of dynamical ordering of biomacromolecules. Biochim. Biophys. Acta 1862, 287–306, doi: 10.1016/j.bbagen.2017.08.020 (2018). [DOI] [PubMed] [Google Scholar]
- 103.Bhabha G et al. A dynamic knockout reveals that conformational fuctuations influence the chemical step of enzyme catalysis. Science 332, 234–238 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kerns SJ et al. The energy landscape of adenylate kinase during catalysis. Nat. Struct. Mol. Biol. 22, 124–131, doi: 10.1038/nsmb.2941 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lange OF et al. Recognition dynamics up to microseconds revealed from an RDC-derived ubiquitin ensemble in solution. Science 320, 1471–1475, doi: 10.1126/science.1157092 (2008). [DOI] [PubMed] [Google Scholar]
- 106.Morrison EA et al. Antiparallel EmrE exports drugs by exchanging between asymmetric structures. Nature 481, 45–50, doi: 10.1038/nature10703 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Brüschweiler S, Yang Q, Run C & Chou JJ Substrate-modulated ADP/ATP-transporter dynamics revealed by NMR relaxation dispersion. Nat. Struct. Mol. Biol. 22, 636–641, doi: 10.1038/nsmb.3059 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Minato Y et al. Conductance of P2X4 purinergic receptor is determined by conformational equilibrium in the transmembrane region. Proc. Natl. Acad. Sci. U. S. A. 113, 4741–4746, doi: 10.1073/pnas.1600519113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nishida N et al. Functional dynamics of cell surface membrane proteins. J. Magn. Reson. 241, 86–96, doi: 10.1016/j.jmr.2013.11.007 (2014). [DOI] [PubMed] [Google Scholar]
- 110.Pervushin K, Riek R, Wider G & Wüthrich K Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc. Natl. Acad. Sci. U. S. A. 94, 12366–12371 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tugarinov V, Hwang PM, Ollerenshaw JE & Kay LE Cross-correlated relaxation enhanced 1H-13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J. Am. Chem. Soc. 125, 10420–10428 (2003). [DOI] [PubMed] [Google Scholar]
- 112.Liu JJ, Horst R, Katritch V, Stevens RC & Wüthrich K Biased signaling pathways in β2-adrenergic receptor characterized by 19F-NMR. Science 335, 1106–1110, doi: 10.1126/science.1215802 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kofuku Y et al. Efficacy of the β2-adrenergic receptor is determined by conformational equilibrium in the transmembrane region. Nat. Commun. 3, 1045, doi: 10.1038/ncomms2046 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Isogai S et al. Backbone NMR reveals allosteric signal transduction networks in the β1-adrenergic receptor. Nature 530, 237–241, doi: 10.1038/nature16577 (2016). [DOI] [PubMed] [Google Scholar]
- 115.Solt AS et al. Insight into partial agonism by observing multiple equilibria for ligand-bound and Gs-mimetic nanobody-bound β1-adrenergic receptor. Nat. Commun. 8, 1795, doi: 10.1038/s41467-017-02008-y (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bokoch MP et al. Ligand-specific regulation of the extracellular surface of a G-protein-coupled receptor. Nature 463, 108–112 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chung KY et al. Role of detergents in conformational exchange of a G protein-coupled receptor. J. Biol. Chem. 287, 36305–36311, doi: 10.1074/jbc.M112.406371 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nygaard R et al. The dynamic process of β2-adrenergic receptor activation. Cell 152, 532–542, doi: 10.1016/j.cell.2013.01.008 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim TH et al. The role of ligands on the equilibria between functional states of a G protein-coupled receptor. J. Am. Chem. Soc. 135, 9465–9474, doi: 10.1021/ja404305k (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Horst R, Liu JJ, Stevens RC & Wüthrich K β2-adrenergic receptor activation by agonists studied with 19F NMR spectroscopy. Angew. Chem. Int. Ed. Engl. 52, 10762–10765, doi: 10.1002/anie.201305286 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kofuku Y et al. Functional dynamics of deuterated β2-adrenergic receptor in lipid bilayers revealed by NMR spectroscopy. Angew. Chem. Int. Ed. 53, 13376–13379, doi: 10.1002/anie.201406603 (2014). [DOI] [PubMed] [Google Scholar]
- 122.Manglik A et al. Structural insights into the dynamic process of β2-adrenergic receptor signaling. Cell 161, 1101–1111, doi: 10.1016/j.cell.2015.04.043 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Eddy MT, Didenko T, Stevens RC & Wüthrich K β2-adrenergic receptor conformational response to fusion protein in the third intracellular loop. Structure 24, 2190–2197, doi: 10.1016/j.str.2016.09.015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shiraishi Y et al. Phosphorylation-induced conformation of β2-adrenoceptor related to arrestin recruitment revealed by NMR. Nat. Commun. 9, 194, doi: 10.1038/s41467-017-02632-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ye L, Van Eps N, Zimmer M, Ernst OP & Prosser RS Activation of the A2A adenosine G-protein-coupled receptor by conformational selection. Nature 533, 265–268, doi: 10.1038/nature17668 (2016). [DOI] [PubMed] [Google Scholar]
- 126.Clark LD et al. Ligand modulation of sidechain dynamics in a wild-type human GPCR. Elife 6, doi: 10.7554/eLife.28505 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eddy MT et al. Allosteric coupling of drug binding and intracellulars signaling in the A2A adenosine receptor. Cell 172, 68–80. e12, doi: 10.1016/j.cell.2017.12.004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sounier R et al. Propagation of conformational changes during μ-opioid receptor activation. Nature 524, 375–378, doi: 10.1038/nature14680 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Okude J et al. Identification of a conformational equilibrium that determines the efficacy and functional selectivity of the μ-opioid receptor. Angew. Chem. Int. Ed. Engl. 54, 15771–15776, doi: 10.1002/anie.201508794 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Casiraghi M et al. Functional modulation of a G protein-coupled receptor conformational landscape in a lipid bilayer. J. Am. Chem. Soc. 138, 11170–11175, doi: 10.1021/jacs.6b04432 (2016). [DOI] [PubMed] [Google Scholar]
- 131.Ye L et al. Mechanistic insights into allosteric regulation of the A2A adenosine G protein-coupled receptor by physiological cations. Nat. Commun. 9, 1372, doi: 10.1038/s41467-018-03314-9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.O’Connor C et al. NMR structure and dynamics of the agonist dynorphin peptide bound to the human kappa opioid receptor. Proc. Natl. Acad. Sci. U. S. A. 112, 11852–11857, doi: 10.1073/pnas.1510117112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Park SH, Berkamp S, Radoicic J, De Angelis AA & Opella SJ Interaction of monomeric interleukin-8 with CXCR1 mapped by proton-detected fast MAS solid-state NMR. Biophys. J. 113, 2695–2705, doi: 10.1016/j.bpj.2017.09.041 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Berkamp S, Park SH, De Angelis AA, Marassi FM & Opella SJ Structure of monomeric Interleukin-8 and its interactions with the N-terminal Binding Site-I of CXCR1 by solution NMR spectroscopy. J. Biomol. NMR 69, 111–121, doi: 10.1007/s10858-017-0128-3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bartoschek S et al. Drug design for G-protein-coupled receptors by a ligand-based NMR method. Angew. Chem. 49, 1426–1429, doi: 10.1002/anie.200905102 (2010). [DOI] [PubMed] [Google Scholar]
- 136.Joedicke L et al. The molecular basis of subtype selectivity of human kinin G-protein-coupled receptors. Nat. Chem. Biol. 14, 284–290, doi: 10.1038/nchembio.2551 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goricanec D et al. Conformational dynamics of a G-protein α subunit is tightly regulated by nucleotide binding. Proc. Natl. Acad. Sci. U. S. A. 113, E3629–3638, doi: 10.1073/pnas.1604125113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Toyama Y et al. Dynamic regulation of GDP binding to G proteins revealed by magnetic field-dependent NMR relaxation analyses. Nat. Commun. 8, 14523, doi: 10.1038/ncomms14523 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhuang T et al. Involvement of distinct arrestin-1 elements in binding to different functional forms of rhodopsin. Proc. Natl. Acad. Sci. U. S. A. 110, 942–947, doi: 10.1073/pnas.1215176110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.London RE, Wingad BD & Mueller GA Dependence of amino acid side chain 13C shifts on dihedral angle: application to conformational analysis. J. Am. Chem. Soc. 130, 11097–11105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Butterfoss GL et al. Conformational dependence of 13C shielding and coupling constants for methionine methyl groups. J. Biomol. NMR 48, 31–47 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Perkins SJ & Wüthrich K Ring current effects in the conformation dependent NMR chemical shifts of aliphatic protons in the basic pancreatic trypsin inhibitor. Biochim. Biophys. Acta 576, 409–423 (1979). [DOI] [PubMed] [Google Scholar]
- 143.Liu D & Wüthrich K Ring current shifts in 19F-NMR of membrane proteins. J. Biomol. NMR 65, 1–5, doi: 10.1007/s10858-016-0022-4 (2016). [DOI] [PubMed] [Google Scholar]
- 144.Pintacuda G & Otting G Identification of protein surfaces by NMR measurements with a pramagnetic Gd(III) chelate. J. Am. Chem. Soc. 124, 372–373 (2002). [DOI] [PubMed] [Google Scholar]
- 145.Bloembergen N, Purcell EM & Pound RV Relaxation effects in nuclear magnetic resonance absorption. Phys. Rev. 73, 679–712, doi: 10.1103/PhysRev.73.679 (1948). [DOI] [Google Scholar]
- 146.Staus DP et al. Allosteric nanobodies reveal the dynamic range and diverse mechanisms of G-protein-coupled receptor activation. Nature 535, 448–452, doi: 10.1038/nature18636 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gether U et al. Agonists induce conformational changes in transmembrane domains III and VI of the β2 adrenoceptor. EMBO J. 16, 6737–6747 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ghanouni P, Steenhuis JJ, Farrens DL & Kobilka BK Agonist-induced conformational changes in the G-protein-coupling domain of the β2 adrenergic receptor. Proc. Natl. Acad. Sci. U. S. A. 98, 5997–6002, doi: 10.1073/pnas.101126198 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yao X et al. Coupling ligand structure to specific conformational switches in the β2-adrenoceptor. Nat. Chem. Biol. 2, 417–422 (2006). [DOI] [PubMed] [Google Scholar]
- 150.Yao XJ et al. The effect of ligand efficacy on the formation and stability of a GPCR-G protein complex. Proc. Natl. Acad. Sci. U. S. A. 106, 9501–9506 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gregorio GG et al. Single-molecule analysis of ligand efficacy in β2AR-G-protein activation. Nature 547, 68–73, doi: 10.1038/nature22354 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lamichhane R et al. Single-molecule view of basal activity and activation mechanisms of the G protein-coupled receptor β2AR. Proc. Natl. Acad. Sci. U. S. A. 112, 14254–14259, doi: 10.1073/pnas.1519626112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stumpf AD & Hoffmann C Optical probes based on G protein-coupled receptors - added work or added value? Br. J. Pharmacol. 173, 255–266, doi: 10.1111/bph.13382 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rahmeh R et al. Structural insights into biased G protein-coupled receptor signaling revealed by fluorescence spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 109, 6733–6738 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kahsai AW et al. Multiple ligand-specific conformations of the β2-adrenergic receptor. Nat. Chem. Biol. 7, 692–700 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xiao K, Chung J & Wall A The power of mass spectrometry in structural characterization of GPCR signaling. J. Recept. Signal Transduct. Res. 35, 213–219, doi: 10.3109/10799893.2015.1072979 (2015). [DOI] [PubMed] [Google Scholar]
- 157.West GM et al. Ligand-dependent perturbation of the conformational ensemble for the GPCR β2 adrenergic receptor revealed by HDX. Structure 19, 1424–1432 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wisler JW et al. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc. Natl. Acad. Sci. U. S. A. 104, 16657–16662, doi: 10.1073/pnas.0707936104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Drake MT et al. β-arrestin-biased agonism at the β2-adrenergic receptor. J. Biol. Chem. 283, 5669–5676, doi: 10.1074/jbc.M708118200 (2008). [DOI] [PubMed] [Google Scholar]
- 160.Carr R et al. β-arrestin-biased signaling through the β2-adrenergic receptor promotes cardiomyocyte contraction. Proc. Natl. Acad. Sci. U. S. A. 113, E4107–4116, doi: 10.1073/pnas.1606267113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Imai S et al. Functional equilibrium of the KcsA structure revealed by NMR. J. Biol. Chem. 287, 39634–39641, doi: 10.1074/jbc.M112.401265 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Boom M et al. Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr. Pharm. Des. 18, 5994–6004 (2012). [DOI] [PubMed] [Google Scholar]
- 163.Dahan A et al. Anesthetic potency and influence of morphine and sevoflurane on respiration in μ-opioid receptor knockout mice. Anesthesiology 94, 824–832 (2001). [DOI] [PubMed] [Google Scholar]
- 164.Chen XT et al. Structure-activity relationships and discovery of a G protein biased μ opioid receptor ligand, [(3-methoxythiophen-2-yl)methyl]({2-[(9R)-9-(pyridin-2-yl)-6-oxaspiro-[4.5]decan- 9-yl]ethyl})amine (TRV130), for the treatment of acute severe pain. J. Med. Chem. 56, 8019–8031 (2013). [DOI] [PubMed] [Google Scholar]
- 165.Huang W et al. Structural insights into µ-opioid receptor activation. Nature 524, 315–321, doi: 10.1038/nature14886 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Manglik A et al. Crystal structure of the μ-opioid receptor bound to a morphinan antagonist. Nature 485, 321–326 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ferré S et al. Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry implications for drug addiction, sleep and pain. Prog. Neurobiol. 83, 332–347, doi: 10.1016/j.pneurobio.2007.04.002 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schiffmann SN, Fisone G, Moresco R, Cunha RA & Ferré S Adenosine A2A receptors and basal ganglia physiology. Prog. Neurobiol. 83, 277–292, doi: 10.1016/j.pneurobio.2007.05.001 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Young A et al. Co-inhibition of CD73 and A2AR adenosine signaling improves anti-tumor immune responses. Cancer Cell 30, 391–403, doi: 10.1016/j.ccell.2016.06.025 (2016). [DOI] [PubMed] [Google Scholar]
- 170.Hatfield SM & Sitkovsky M A2A adenosine receptor antagonists to weaken the hypoxia-HIF-1α driven immunosuppression and improve immunotherapies of cancer. Curr. Opin. Pharmacol. 29, 90–96, doi: 10.1016/j.coph.2016.06.009 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Leone RD, Lo YC & Powell JD A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput. Struct. Biotechnol. J. 13, 265–272, doi: 10.1016/j.csbj.2015.03.008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mediavilla-Varela M et al. Antagonism of adenosine A2A receptor expressed by lung adenocarcinoma tumor cells and cancer associated fibroblasts inhibits their growth. Cancer Biol. Ther. 14, 860–868, doi: 10.4161/cbt.25643 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mittal D et al. Antimetastatic effects of blocking PD-1 and the adenosine A2A receptor. Cancer Res. 74, 3652–3658, doi: 10.1158/0008-5472.CAN-14-0957 (2014). [DOI] [PubMed] [Google Scholar]
- 174.Jaakola VP et al. The 2.6 angstrom crystal structure of a human A2A adenosine receptor bound to an antagonist. Science 322, 1211–1217 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xu F et al. Structure of an agonist-bound human A2A adenosine receptor. Science 332, 322–327 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lebon G et al. Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature 474, 521–525 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Carpenter B, Nehmé R, Warne T, Leslie AG & Tate CG Structure of the adenosine A2A receptor bound to an engineered G protein. Nature 536, 104–107, doi: 10.1038/nature18966 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sušac L, O’Connor C, Stevens RC & Wüthrich K In-Membrane Chemical Modification (IMCM) for site-specific chromophore labeling of GPCRs. Angew. Chem. Int. Ed. Engl. 54, 15246–15249, doi: 10.1002/anie.201508506 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Susac L NMR studies of GPCR structure and function Ph. D. thesis, The Scripps Research Institute, (2015). [Google Scholar]
- 180.Piirainen H et al. Human adenosine A2A receptor binds calmodulin with high affinity in a calcium-dependent manner. Biophys. J. 108, 903–917, doi: 10.1016/j.bpj.2014.12.036 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tossavainen H, Hellman M, Piirainen H, Jaakola V & Permi P H-N, N, C-a, C-β and C’ assignments of the intrinsically disordered C-terminus of human adenosine A2A receptor. Biomol. NMR Assign. 9, 403–406, doi: 10.1007/s12104-015-9618-y (2015). [DOI] [PubMed] [Google Scholar]
- 182.Venkatakrishnan AJ et al. Structured and disordered facets of the GPCR fold. Curr. Opin. Struct. Biol. 27, 129–137, doi: 10.1016/j.sbi.2014.08.002 (2014). [DOI] [PubMed] [Google Scholar]
- 183.Berlow RB, Dyson HJ & Wright PE Expanding the paradigm: intrinsically disordered proteins and allosteric regulation. J. Mol. Biol, doi: 10.1016/j.jmb.2018.04.003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wright PE & Dyson HJ Intrinsically disordered proteins in cellular signalling and regulation. Nature 16, 18–29, doi: 10.1038/nrm3920 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Oh DY & Olefsky JM G protein-coupled receptors as targets for anti-diabetic therapeutics. Nat. Rev. Drug Discov. 15, 161–172, doi: 10.1038/nrd.2015.4 (2016). [DOI] [PubMed] [Google Scholar]
- 186.Prasad-Reddy L & Isaacs D A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context 4, 212283, doi: 10.7573/dic.212283 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hou Y et al. Solvent-accessibility of discrete residue positions in the polypeptide hormone glucagon by 19F-NMR observation of 4-fluorophenylalanine. J. Biomol. NMR 68, 1–6, doi: 10.1007/s10858-017-0107-8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Erlanson DA, Fesik SW, Hubbard RE, Jahnke W & Jhoti H Twenty years on: the impact of fragments on drug discovery. Nature Rev. Drug Discov. 15, 605–619, doi: 10.1038/nrd.2016.109 (2016). [DOI] [PubMed] [Google Scholar]
- 189.Shuker SB, Hajduk PJ, Meadows RP & Fesik SW Discovering high-affinity ligands for proteins: SAR by NMR. Science 274, 1531–1534 (1996). [DOI] [PubMed] [Google Scholar]
- 190.Andrews SP, Brown GA & Christopher JA Structure-Based and Fragment-Based GPCR Drug Discovery. ChemMedChem 9, 256–275, doi: 10.1002/cmdc.201300382 (2013). [DOI] [PubMed] [Google Scholar]
- 191.Congreve M et al. Fragment screening of stabilized G-protein-coupled receptors using biophysical methods. Methods Enzymol. 493, 115–136, doi: 10.1016/B978-0-12-381274-2.00005-4 (2011). [DOI] [PubMed] [Google Scholar]
- 192.Chen D et al. Fragment screening of GPCRs using biophysical methods: identification of ligands of the adenosine A2A receptor with novel biological activity. ACS Chem. Biol. 7, 2064–2073, doi: 10.1021/cb300436c (2012). [DOI] [PubMed] [Google Scholar]
- 193.Igonet S et al. Enabling STD-NMR fragment screening using stabilized native GPCR: A case study of adenosine receptor. Sci. Rep. 8, 8142, doi: 10.1038/s41598-018-26113-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pellecchia M, Sem DS & Wüthrich K NMR in drug discovery. Nat. Rev. Drug Discov. 1, 211–219, doi: 10.1038/nrd748 (2002). [DOI] [PubMed] [Google Scholar]
- 195.Hajduk PJ & Greer J A decade of fragment-based drug design: strategic advances and lessons learned. Nat. Rev. Drug Discov. 6, 211–219, doi: 10.1038/nrd2220 (2007). [DOI] [PubMed] [Google Scholar]
- 196.Mizukoshi Y et al. Improvement of ligand affinity and thermodynamic properties by NMR‐based evaluation of local dynamics and surface complementarity in the receptor‐bound state. Angew. Chem. 55, 14606–14609 (2016). [DOI] [PubMed] [Google Scholar]
- 197.Brancaccio D et al. Ligand-Based NMR Study of C-X-C Chemokine Receptor Type 4 (CXCR4)–Ligand Interactions on Living Cancer Cells. J. Med. Chem. 61, 2910–2923, doi: 10.1021/acs.jmedchem.7b01830 (2018). [DOI] [PubMed] [Google Scholar]
- 198.Inooka H et al. Conformation of a peptide ligand bound to its G-protein coupled receptor. Nat. Struct. Biol. 8, 161–165, doi: 10.1038/84159 (2001). [DOI] [PubMed] [Google Scholar]
- 199.Fredriksson K et al. Nanodiscs for INPHARMA NMR characterization of GPCRs: ligand binding to the Human A2A adenosine receptor. Angew. Chem. Int. Ed. Engl. 56, 5750–5754, doi: 10.1002/anie.201612547 (2017). [DOI] [PubMed] [Google Scholar]
- 200.Cox BD et al. Structural analysis of CXCR4 - Antagonist interactions using saturation-transfer double-difference NMR. Biochem. Biophys. Res. Commun. 466, 28–32, doi: 10.1016/j.bbrc.2015.08.084 (2015). [DOI] [PubMed] [Google Scholar]
- 201.Yong KJ et al. Determinants of ligand subtype-selectivity at α1A-adrenoceptor revealed using saturation transfer difference (STD) NMR. ACS Chem. Biol. 13, 1090–1102, doi: 10.1021/acschembio.8b00191 (2018). [DOI] [PubMed] [Google Scholar]
- 202.Assadi-Porter FM et al. Direct NMR detection of the binding of functional ligands to the human sweet receptor, a heterodimeric family 3 GPCR. J. Am. Chem. Soc. 130, 7212–7213, doi: 10.1021/ja8016939 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kofuku Y et al. Structural Basis of the Interaction between Chemokine Stromal Cell-derived Factor-1/CXCL12 and Its G-protein-coupled Receptor CXCR4. J. Biol. Chem. 284, 35240–35250, doi: 10.1074/jbc.M109.024851 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yoshiura C et al. Elucidation of the CCR1- and CCR5-binding modes of MIP-1α by application of an NMR spectra reconstruction method to the transferred cross-saturation experiments. J. Biomol. NMR 63, 333–340, doi: 10.1007/s10858-015-9992-x (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yoshiura C et al. NMR analyses of the interaction between CCR5 and its ligand using functional reconstitution of CCR5 in lipid bailayers. J. Am. Chem. Soc. 132, 6768–6777, doi: 10.1021/ja100830f (2010). [DOI] [PubMed] [Google Scholar]
- 206.Catoire LJ et al. Structure of a GPCR ligand in its receptor-bound state: leukotriene B4 adopts a highly constrained conformation when associated to human BLT2. J. Am. Chem. Soc. 132, 9049–9057, doi: 10.1021/ja101868c (2010). [DOI] [PubMed] [Google Scholar]
- 207.Zheng Z et al. Structure-based discovery of new antagonist and biased agonist chemotypes for the kappa opioid receptor. J. Med. Chem. 60, 3070–3081, doi: 10.1021/acs.jmedchem.7b00109 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ring AM et al. Adrenaline-activated structure of β2-adrenoceptor stabilized by an engineered nanobody. Nature 502, 575–579, doi: 10.1038/nature12572 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wacker D et al. Structural features for functional selectivity at serotonin receptors. Science 340, 615–619 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mary S et al. Ligands and signaling proteins govern the conformational landscape explored by a G protein-coupled receptor. Proc. Natl. Acad. Sci. U S A 109, 8304–8309 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rahmeh R et al. Structural insights into biased G protein-coupled receptor signaling revealed by fluorescence spectroscopy. Proc. Natl. Acad. Sci. U S A 109, 6733–6738 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shonberg J et al. A structure-activity analysis of biased agonism at the dopamine D2 receptor. J. Med. Chem. 56, 9199–9221, doi: 10.1021/jm401318w (2013). [DOI] [PubMed] [Google Scholar]
- 213.Kruse AC et al. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature 482, 552–556, doi: 10.1038/nature10867 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lewi PJ et al. On the detection of multiple-binding modes of ligands to proteins, from biological, structural, and modeling data. J. Comput. Aided Mol. Des. 17, 129–134 (2003). [DOI] [PubMed] [Google Scholar]
- 215.Wittmann HJ & Strasser A Competitive association binding kinetic assays: a new tool to detect two different binding orientations of a ligand to its target protein under distinct conditions? Naunyn Schmiedebergs Arch. Pharmacol. 390, 595–612, doi: 10.1007/s00210-017-1362-7 (2017). [DOI] [PubMed] [Google Scholar]
- 216.Bock A et al. Dynamic ligand binding dictates partial agonism at a G protein-coupled receptor. Nat. Chem. Biol. 10, 18–20, doi: 10.1038/nchembio.1384 (2014). [DOI] [PubMed] [Google Scholar]
- 217.Bruchas MR & Roth BL New technologies for elucidating opioid receptor function. Trends Pharmacol. Sci. 37, 279–289, doi: 10.1016/j.tips.2016.01.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sharp KA, O’Brien E, Kasinath V & Wand AJ On the relationship between NMR‐derived amide order parameters and protein backbone entropy changes. Proteins: Struct., Funct., Bioinf. 83, 922–930 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Renaud J-P et al. Cryo-EM in drug discovery: achievements, limitations and prospects. Nat. Rev. Drug Discov. 17, 471–492, doi: 10.1038/nrd.2018.77 (2018). [DOI] [PubMed] [Google Scholar]
- 220.Yasuhara K et al. Spontaneous lipid nanodisc fomation by amphiphilic polymethacrylate copolymers. J. Am. Chem. Soc. 139, 18657–18663, doi: 10.1021/jacs.7b10591 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yang Y et al. Self-assembly of size-controlled liposomes on DNA nanotemplates. Nat. Chem. 8, 476–483, doi: 10.1038/nchem.2472 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang G & Hilty C Applications of dissolution dynamic nuclear polarization in chemistry and biochemistry. Mag. Res. Chem. 56, 566–582, doi: 10.1002/mrc.4735 (2018). [DOI] [PubMed] [Google Scholar]
- 223.Ragavan M, Chen H-Y, Sekar G & Hilty C Solution NMR of Polypeptides Hyperpolarized by Dynamic Nuclear Polarization. Analyt. Chem. 83, 6054–6059, doi: 10.1021/ac201122k (2011). [DOI] [PubMed] [Google Scholar]
- 224.Bajaj VS, Mak-Jurkauskas ML, Belenky M, Herzfeld J & Griffin RG Functional and shunt states of bacteriorhodopsin resolved by 250 GHz dynamic nuclear polarization-enhanced solid-state NMR. Proc. Natl. Acad. Sci. U. S. A. 106, 9244–9249, doi: 10.1073/pnas.0900908106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mak-Jurkauskas ML et al. Energy transformations early in the bacteriorhodopsin photocycle revealed by DNP-enhanced solid-state NMR. Proc. Natl. Acad. Sci. U. S. A. 105, 883–888, doi: 10.1073/pnas.0706156105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ni QZ et al. Primary Transfer Step in the Light-Driven Ion Pump Bacteriorhodopsin: An Irreversible U-Turn Revealed by Dynamic Nuclear Polarization-Enhanced Magic Angle Spinning NMR. J. Am. Chem. Soc. 140, 4085–4091, doi: 10.1021/jacs.8b00022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Frederick KK et al. Sensitivity-Enhanced NMR Reveals Alterations in Protein Structure by Cellular Milieus. Cell 163, 620–628, doi: 10.1016/j.cell.2015.09.024 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.George SR, O’Dowd BF & Lee SP G-Protein-coupled receptor oligomerization and its potential for drug discovery. Nat. Rev. Drug Discov. 1, 808–820, doi: 10.1038/nrd913 (2002). [DOI] [PubMed] [Google Scholar]
- 229.Gurevich VV & Gurevich EV GPCRs and Signal Transducers: Interaction Stoichiometry. Trends Pharmacol. Sci. 39, 672–684, doi: 10.1016/j.tips.2018.04.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nørskov-Lauritsen L & Bräuner-Osborne H Role of post-translational modifications on structure, function and pharmacology of class C G protein-coupled receptors. Eur. J. Pharmacol. 763, 233–240, doi: 10.1016/j.ejphar.2015.05.015 (2015). [DOI] [PubMed] [Google Scholar]
- 231.Klein KR, Matson BC & Caron KM The expanding repertoire of receptor activity modifying protein (RAMP) function. Crit. Rev. Biochem. Mol. Biol. 51, 65–71, doi: 10.3109/10409238.2015.1128875 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhang H et al. Structure of the full-length glucagon class B G-protein-coupled receptor. Nature 546, 259–264, doi: 10.1038/nature22363 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zhang X et al. Crystal structure of a multi-domain human smoothened receptor in complex with a super stabilizing ligand. Nat. Commun. 8, 15383, doi: 10.1038/ncomms15383 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Noguchi S & Satow Y Purification of human β2-adrenergic receptor expressed in methylotrophic yeast Pichia pastoris. J. Biochem. 140, 799–804, doi: 10.1093/jb/mvj211 (2006). [DOI] [PubMed] [Google Scholar]
- 235.Krettler C, Reinhart C & Bevans CG Expression of GPCRs in Pichia pastoris for structural studies. Methods Enzymol. 520, 1–29, doi: 10.1016/B978-0-12-391861-1.00001-0 (2013). [DOI] [PubMed] [Google Scholar]
- 236.Yurugi-Kobayashi T et al. Comparison of functional non-glycosylated GPCRs expression in Pichia pastoris. Biochem. Biophys. Res. Commun. 380, 271–276, doi: 10.1016/j.bbrc.2009.01.053 (2009). [DOI] [PubMed] [Google Scholar]
- 237.Shimamura T et al. Structure of the human histamine H1 receptor complex with doxepin. Nature 475, 65–70, doi: 10.1038/nature10236 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Eddy MT et al. Extrinsic tryptophans as NMR probes of allosteric coupling in membrane proteins: application to the A2A adenosine receptor. J. Am. Chem. Soc. 140, 8228–8235, doi: 10.1021/jacs.8b03805 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Klein-Seetharaman J, Getmanova EV, Loewen MC, Reeves PJ & Khorana HG NMR spectroscopy in studies of light-induced structural changes in mammalian rhodopsin: applicability of solution 19F NMR. Proc. Natl. Acad. Sci. U. S. A. 96, 13744–13749 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Otting G Protein NMR using paramagnetic ions. Annu. Rev. Biophys. 39, 387–405, doi: 10.1146/annurev.biophys.093008.131321 (2010). [DOI] [PubMed] [Google Scholar]
- 241.Clore GM & Iwahara J Theory, practice, and applications of paramagnetic relaxation enhancement for the characterization of transient low-population states of biological macromolecules and their complexes. Chem. Rev. 109, 4108–4139, doi: 10.1021/cr900033p (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Iwahara J, Tang C & Clore G Practical aspects of 1H transverse paramagnetic relaxation enhancement measurements on macromolecules. J. Mag. Reson. 184, 185–195, doi: 10.1016/j.jmr.2006.10.003 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Su X-C & Otting G Paramagnetic labelling of proteins and oligonucleotides for NMR. J. Biomol. NMR 46, 101–112, doi: 10.1007/s10858-009-9331-1 (2009). [DOI] [PubMed] [Google Scholar]
- 244.Huang S et al. Utilization of paramagnetic relaxation enhancements for structural analysis of actin-binding proteins in complex with actin. Sci. Rep. 6, 33690, doi: 10.1038/srep33690 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Salzmann M, Wider G, Pervushin K, Senn H & Wüthrich K TROSY-type triple-resonance experiments for sequential NMR assignments of large proteins. J. Am. Chem. Soc. 121, 844–848, doi: 10.1021/ja9834226 (1999). [DOI] [Google Scholar]
- 246.Clark L et al. Methyl labeling and TROSY NMR spectroscopy of proteins expressed in the eukaryote Pichia pastoris. J. Biomol. NMR 62, 239–245, doi: 10.1007/s10858-015-9939-2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Opitz C, Isogai S & Grzesiek S An economic approach to efficient isotope labeling in insect cells using homemade 15N-, 13C- and 2H-labeled yeast extracts. J. Biomol. NMR 62, 373–385, doi: 10.1007/s10858-015-9954-3 (2015). [DOI] [PubMed] [Google Scholar]
- 248.Franke B et al. Production of isotope-labeled proteins in insect cells for NMR. J. Biomol. NMR 22, 1583–1512, doi: 10.1007/s10858-018-0172-7 (2018). [DOI] [PubMed] [Google Scholar]
- 249.Kofuku Y et al. Deuteration and selective labeling of alanine methyl groups of β2-adrenergic receptor expressed in a baculovirus-insect cell expression system. J. Biomol. NMR, doi: 10.1007/s10858-018-0174-5 (2018). [DOI] [PubMed] [Google Scholar]
- 250.Huber T, Naganathan S, Tian H, Ye S & Sakmar TP Unnatural amino acid mutagenesis of GPCRs using amber codon suppression and bioorthogonal labeling. Methods Enzymol. 520, 281–305, doi: 10.1016/B978-0-12-391861-1.00013-7 (2013). [DOI] [PubMed] [Google Scholar]
- 251.Grunbeck A, Huber T, Sachdev P & Sakmar TP Mapping the ligand-binding site on a G protein-coupled receptor (GPCR) using genetically encoded photocrosslinkers. Biochemistry 50, 3411–3413, doi: 10.1021/bi200214r (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Daggett KA & Sakmar TP Site-specific in vitro and in vivo incorporation of molecular probes to study G-protein-coupled receptors. Curr. Opin. Chem. Biol. 15, 392–398, doi: 10.1016/j.cbpa.2011.03.010 (2011). [DOI] [PubMed] [Google Scholar]
- 253.Ye S et al. Tracking G-protein-coupled receptor activation using genetically encoded infrared probes. Nature 464, 1386–1389, doi: 10.1038/nature08948 (2010). [DOI] [PubMed] [Google Scholar]
- 254.Egloff P et al. Structure of signaling-competent neurotensin receptor 1 obtained by directed evolution in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 111, E655–662, doi: 10.1073/pnas.1317903111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Casiraghi M, Damian M, Lescop E, Banères J-L & Catoire LJ Illuminating the energy landscape of GPCRs: the key contribution of solution-state NMR associated with Escherichia coli as an expression host. Biochemistry 57, 2297–2307, doi: 10.1021/acs.biochem.8b00035 (2018). [DOI] [PubMed] [Google Scholar]
- 256.Maly J & Crowhurst KA Expression, purification and preliminary NMR characterization of isotopically labeled wild-type human heterotrimeric G protein αi1. Protein Expr. Purif. 84, 255–264, doi: 10.1016/j.pep.2012.06.003 (2012). [DOI] [PubMed] [Google Scholar]
- 257.Sounier R, Yang Y, Hagelberger J, Granier S & Déméné H 1H, 13C, and 15N backbone chemical shift assignments of camelid single-domain antibodies against active state μ -opioid receptor. Biomol. NMR Assign. 11, 117–121, doi: 10.1007/s12104-017-9733-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Dawaliby R et al. Allosteric regulation of G protein-coupled receptor activity by phospholipids. Nat. Chem. Biol. 12, 35–39, doi: 10.1038/nchembio.1960 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pei G, Tiberi M, Caron MG & Lefkowitz RJ An approach to the study of G-protein-coupled receptor kinases: an in vitro-purified membrane assay reveals differential receptor specificity and regulation by G beta gamma subunits. Proc. Natl. Acad. Sci. U. S. A. 91, 3633–3636 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Bayburt TH, Grinkova YV & Sligar SG Self-assembly of discoidal phospholipid bilayer nanoparticles with membrane scaffold proteins. Nano Lett. 2, 853–856, doi: 10.1021/nl025623k (2002). [DOI] [Google Scholar]
- 261.Denisov IG & Sligar SG Nanodiscs in membrane biochemistry and biophysics. Chem. Rev. 117, 4669–4713, doi: 10.1021/acs.chemrev.6b00690 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Denisov IG, Grinkova YV, Lazarides AA & Sligar SG Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. J. Am. Chem. Soc. 126, 3477–3487, doi: 10.1021/ja0393574 (2004). [DOI] [PubMed] [Google Scholar]
- 263.Leitz AJ, Bayburt TH, Barnakov AN, Springer BA & Sligar SG Functional reconstitution of β2-adrenergic receptors utilizing self-assembling Nanodisc technology. BioTechniques 40, 601–602, 604, 606, passim (2006). [DOI] [PubMed] [Google Scholar]
- 264.Bocquet N et al. Real-time monitoring of binding events on a thermostabilized human A2A receptor embedded in a lipid bilayer by surface plasmon resonance. Biochim. Biophys. Acta 1848, 1224–1233, doi: 10.1016/j.bbamem.2015.02.014 (2015). [DOI] [PubMed] [Google Scholar]
- 265.Van Eps N et al. Conformational equilibria of light-activated rhodopsin in nanodiscs. Proc. Natl. Acad. Sci. U. S. A. 114, E3268–E3275, doi: 10.1073/pnas.1620405114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Dijkman PM & Watts A Lipid modulation of early G protein-coupled receptor signalling events. Biochim. Biophys. Acta 1848, 2889–2897, doi: 10.1016/j.bbamem.2015.08.004 (2015). [DOI] [PubMed] [Google Scholar]
- 267.Bayburt TH et al. Monomeric rhodopsin is sufficient for normal rhodopsin kinase (GRK1) phosphorylation and arrestin-1 binding. J. Biol. Chem. 286, 1420–1428 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Inagaki S et al. Modulation of the interaction between neurotensin receptor NTS1 and Gq protein by lipid. J. Mol. Biol. 417, 95–111, doi: 10.1016/j.jmb.2012.01.023 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Mitra N et al. Calcium-dependent ligand binding and G-protein signaling of family B GPCR parathyroid hormone 1 receptor purified in nanodiscs. ACS Chem. Biol. 8, 617–625, doi: 10.1021/cb300466n (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.El Moustaine D et al. Distinct roles of metabotropic glutamate receptor dimerization in agonist activation and G-protein coupling. Proc. Natl. Acad. Sci. U. S. A. 109, 16342–16347, doi: 10.1073/pnas.1205838109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Schuler MA, Denisov IG & Sligar SG Nanodiscs as a new tool to examine lipid-protein interactions. Methods Mol. Biol. 974, 415–433, doi: 10.1007/978-1-62703-275-9_18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Nasr ML et al. Covalently circularized nanodiscs for studying membrane proteins and viral entry. Nat. Methods 14, 49–52, doi: 10.1038/nmeth.4079 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Chien CH et al. An adaptable phospholipid membrane mimetic system for solution NMR studies of membrane proteins. J. Am. Chem. Soc. 139, 14829–14832, doi: 10.1021/jacs.7b06730 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Frauenfeld J et al. A saposin-lipoprotein nanoparticle system for membrane proteins. Nat. Methods 13, 345–351, doi: 10.1038/nmeth.3801 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Orwick MC et al. Detergent-free formation and physicochemical characterization of nanosized lipid-polymer complexes: Lipodisq. Angew. Chem. Int. Ed. Engl. 51, 4653–4657, doi: 10.1002/anie.201201355 (2012). [DOI] [PubMed] [Google Scholar]
- 276.Shi L et al. β2 adrenergic receptor activation. Modulation of the proline kink in transmembrane 6 by a rotamer toggle switch. J. Biol. Chem. 277, 40989–40996, doi: 10.1074/jbc.M206801200 (2002). [DOI] [PubMed] [Google Scholar]
- 277.Schwartz TW, Frimurer TM, Holst B, Rosenkilde MM & Elling CE Molecular mechanism of 7TM receptor activation--a global toggle switch model. Annu. Rev. Pharmacol. Toxicol. 46, 481–519, doi: 10.1146/annurev.pharmtox.46.120604.141218 (2006). [DOI] [PubMed] [Google Scholar]