Abstract
Background
The associations of individual antiepileptic drugs (AEDs) with pregnancy duration and size at birth, and potential dose relations, are not well characterized.
Methods
This cohort study used nationwide Swedish register data (1996–2013). Adjusting for smoking, epilepsy and other AED indications, we used linear and quantile regression to explore associations with pregnancy duration, and birth weight, length, and head circumference (the last three operationalized as z-scores). We used logistic regression for preterm delivery, small for gestational age, and microcephaly. Lamotrigine was the reference drug.
Results
6,720 infants were exposed to AEDs in utero; AED exposure increased over the study period. Relative to lamotrigine-exposed infants, carbamazepine-exposed infants were born, on average, 1.3 days earlier (mean [95% confidence interval]: -1.3 [-2.3 to -0.3]); were 0.1 standard deviations (SDs) lighter (-0.1 [-0.2 to 0.0]); and had a head circumference that was 0.2 SDs smaller (-0.2 [-0.3 to -0.1]). Pregabalin-exposed infants were born, on average, 1.1 days earlier (-1.1 [-3.0 to 0.8]); were 0.1 SDs lighter (-0.1 [-0.3 to 0.0]); and had the same head circumference as lamotrigine-exposed infants. Levetiracetam-exposed infants were born, on average, 0.5 days earlier (-0.5 [-2.6 to 1.6]); were 0.1 SDs lighter (-0.1 [-0.3 to 0.0]); and had a head circumference 0.1 SDs smaller (-0.1 [-0.3 to 0.1]). Valproic acid–exposed infants had, on average, the same duration of gestation and birth weight z-score as lamotrigine-exposed infants, but had a head circumference 0.2 SDs smaller (-0.2 [-0.2 to -0.1]). Associations between carbamazepine exposure and pregnancy duration and between valproic acid exposure and pregnancy duration and birth weight z-score were more negative at the left than at the right tails of the outcome distributions. Effect-measure modification and dose-response relations were noted for some of the associations.
Conclusions
Relative to lamotrigine, valproic acid and carbamazepine were associated with smaller head circumference.
Introduction
Epilepsy and antiepileptic drugs (AEDs) have been associated with adverse pregnancy, fetal, and neonatal outcomes [1]. AEDs differ in their risk for congenital malformations [2–4], and some associations have been found to be dose dependent [4–6]. Newer AEDs are generally considered safer than the older drugs, with the possible exception of topiramate [7]. Antiepileptic drugs also differ in the magnitude of their associations with adverse neurodevelopmental outcomes in the offspring, which also appear to be dose dependent [8–10]. The exploration of indication and dose is important because some AEDs have several indications, confounding by indication has been a concern, and AED doses are often higher in epilepsy than in other conditions [11].
A meta-analysis has shown elevated point estimates for the association of AEDs, as a group, with shortened pregnancies and reduced birth size [1], but comparative safety evidence for these endpoints is scarce, as demonstrated by a systematic literature search we conducted to inform our decision on which AED to use as a reference drug [12] and to provide context to the present study. We identified 15 papers that provided adjusted comparisons for individual AEDs [13–27], of which 12 used unexposed populations as the reference (details on this literature search are in S1 File).
Furthermore, previous research has assessed associations with dichotomized endpoints or associations using only the mean of the continuous distributions (please see literature review in S1 File). In this study, we sought to explore the comparative safety of individual AEDs on pregnancy duration and birth weight, length, and head circumference and to explore dose relations on these endpoints, adjusting for epilepsy and other indications. To characterize associations thoroughly, we assessed continuous and binary forms of the endpoints and investigated potential AED effects in both tails of the endpoint distributions. We used a comparative safety design with lamotrigine as the reference instead of no AED use. This approach has the advantage of reducing confounding by indication; in addition, the study results will address more directly the choices faced by patients and clinicians when treatment with these drugs, for epilepsy or other indications is indicated.
Methods
Overview
We conducted a cohort study based on nationwide Swedish register data from 1996 through 2013 to explore the association between maternal use of individual AEDs and pregnancy duration and fetal size. We chose lamotrigine as the reference AED because it is commonly used, providing a statistical advantage as well as a reasonable clinical comparison, and has been considered to have fewer adverse fetal effects than other AEDs based on information available at the time this study was conducted [2, 12, 28, 29].
Data sources
In Sweden, tax-funded health care is provided to all citizens. Information arising from contacts with the health care system is collected in registries that can be linked through a unique personal registration number assigned to all individuals residing in Sweden. Drugs are coded in the Anatomic Therapeutic Chemical classification system, and diagnoses are coded using the International Classification of Diseases (10th revision since 1997).
The Swedish Medical Birth Register [30] collects information from prenatal care, including self-reported medication use at first and subsequent visits, and from standardized delivery charts, including gestational age at birth, birth weight, length, and head circumference. Information on medication use in the first visit is more complete than in subsequent visits. Medications noted only in free-text comments have been coded and incorporated in the structured drug fields. The Prescribed Drug Register records all prescription medications dispensed by pharmacies since 1 July 2005. Information available from prescriptions include drug name, drug strength, number of packages dispensed, and number of defined daily doses (DDDs) per package [31]; indication is not specifically recorded. The National Patient Register includes all discharge records from hospitalizations since 1987 and 75%-80% of visits to specialists, including psychiatric care, since 2001. The Swedish Register of Education contains information on the maximum education level attained per year [32]. The Total Population Register contains demographic and administrative information including nationality and birth and migration dates [33].
Study population
The study population included all women with records for AEDs in pregnancy who delivered a live infant with gestational age of 24 to 42 completed weeks in 1996–2013 and their newborns. Infants born from women who immigrated less than 12 months before pregnancy and infants with chromosomal abnormalities were excluded (139 [2%] and 22 [0.3%] infants, respectively, out of 6,881 infants before exclusion criteria were applied). Infants with congenital malformations and no chromosomal abnormalities and infants from multiple pregnancies were included. All eligible infants per woman were included.
Exposure
We report on the five AEDs that were most commonly used in pregnancy in the last year of our study period: carbamazepine, valproic acid, pregabalin, levetiracetam, and lamotrigine. In Europe, carbamazepine is indicated for the treatment of epilepsy and pain of trigeminal neuralgia and for the prevention of manic-depressive psychosis in patients with bipolar disorder [34]. Valproic acid is indicated for the treatment of epilepsy and bipolar disorder, and, in some European countries, for migraine prevention [35]. Pregabalin is indicated for the treatment of neuropathic pain, epilepsy, and generalized anxiety disorder [36]. Levetiracetam is indicated for the treatment of epilepsy [35]. Lamotrigine is indicated for the treatment of epilepsy and bipolar disorder [37].
We defined three exposure windows for analysis: any time in pregnancy, first trimester (regardless of whether treatment was later discontinued), and first and second/third trimesters (“continuers”). To create the exposure variables, information on first-trimester exposure was obtained from prescriptions dispensed between the first day of the last menstrual period and gestational day 89 and from self-report in the first prenatal visit in women who started prenatal care by gestational week 15. Information on second-/third-trimester exposure was obtained from prescriptions dispensed between day 90 and the day before delivery, from self-reports in the first prenatal visit in women who started prenatal care after gestational week 15, and from self-reports in subsequent prenatal visits (self-reports did not allow a clear differentiation of second- versus third-trimester exposure; thus, we combined both periods). Because of incomplete capture of self-reports after the first prenatal visit, exposure in continuers was defined only for the period for which dispensing data were available (deliveries in 2006–2013). Women and infants exposed to more than one AED were considered to be exposed to each of them.
Dose was derived from dispensed prescriptions (deliveries in 2006–2013). For each prescription, dose was calculated by multiplying the number of packs dispensed by the number of DDDs per pack and by the number of milligrams in a DDD [31]. The mean daily dose was calculated separately for each AED per infant by dividing the dose in prescriptions dispensed between the first day of the last menstrual period and the day before delivery over the number of days in the same period.
Characteristics of the study population
We extracted medical and obstetric information from the national health registers, which derive their information from prenatal care records, hospitalization records, outpatient specialist care records, and dispensed prescriptions. Codes, source of data, timing of ascertainment, categorization, and other details for medical and other characteristics are presented in S2 File.
Endpoints
Study endpoints were duration of pregnancy, preterm delivery, birth weight, small for gestational age (SGA), length at birth, head circumference at birth, and microcephaly, all ascertained from the Medical Birth Register. Duration of pregnancy is predominantly based on ultrasound estimation [38] and is recorded in days; preterm delivery was defined as delivery before 37 completed weeks. Birth weight, length, and head circumference were operationalized as z-scores to assess size independently from gestational age at birth; the birth weight z-score for each infant is the observed birth weight minus the reference mean birth weight, divided by the reference birth weight standard deviation (SD), where the mean and SD were those for infants born at the same gestational age, using a local standard [39]. Small for gestational age was defined within the Medical Birth Register from standard growth curves based on ultrasound-derived fetal weights for singletons only [40]. Microcephaly was defined within the Medical Birth Register as a head circumference of two or more SDs below the mean for gestational age at birth, using a local standard [39].
Statistical analyses
In the main analysis, continuous endpoints were analyzed using linear regression and quantile regression for the 10th, 50th, and 90th percentiles [41]. We produced unadjusted results and results adjusted for maternal age at delivery, education, country of origin, marital status, early pregnancy body mass index, smoking in current pregnancy, alcohol dependence, diabetes (preexisting and gestational), hypertension (preexisting and gestational), epilepsy, depression, bipolar disorder, migraine, chronic pain, other psychiatric disorders, and year of delivery. Variable definitions are presented in S2 File. Missing values (Table 1) were imputed for analysis as the most commonly observed value in the study population; multiple imputation had been planned for variables with missing values in 10% or more of the observations, but missingness was consistently below that threshold. Binary endpoints were analyzed using logistic regression. We conducted adjusted analyses in comparisons with five or more events in the smallest cell (i.e., exposed cases, exposed noncases, unexposed cases, unexposed noncases), adjusting for the variables listed above. We used the weighted copy method to facilitate the convergence of logistic regression models. With this method, analyses are conducted on an expanded data set that consists of the original data set and a copy of the data with the outcomes reversed, the latter receiving low weight, to allow the iterations to converge without distorting the estimates. Confidence intervals are adjusted by the use of weights in the code [42–44]. We weighted the original data 999 times that of the data with reversed outcomes. The unit of analysis was pregnancy for the endpoints duration of pregnancy and preterm delivery; for other endpoints, the unit of analysis was the infant.
Table 1. Characteristics of study population and mean daily dose by antiepileptic drug.
| Characteristic | Lamotrigine | Carbamazepine | Pregabalin | Levetiracetam | Valproic acid |
|---|---|---|---|---|---|
| Number of exposed women | 1,757 | 1,529 | 542 | 245 | 809 |
| Number of exposed infants | 2,254 | 2,095 | 562 | 307 | 1,137 |
| Age at delivery (years) | |||||
| 24 or less | 427 (18.9%) | 249 (11.9%) | 119 (21.2%) | 52 (16.9%) | 206 (18.1%) |
| 25–29 | 691 (30.7%) | 626 (29.9%) | 160 (28.5%) | 92 (30.0%) | 350 (30.8%) |
| 30–34 | 721 (32.0%) | 728 (34.7%) | 151 (26.9%) | 114 (37.1%) | 374 (32.9%) |
| 35 or more | 415 (18.4%) | 492 (23.5%) | 132 (23.5%) | 49 (16.0%) | 207 (18.2%) |
| Mother’s country of origin | |||||
| Nordic countries | 2,040 (90.5%) | 1,812 (86.5%) | 486 (86.5%) | 257 (83.7%) | 995 (87.5%) |
| Other European countries | 86 (3.8%) | 72 (3.4%) | 21 (3.7%) | 16 (5.2%) | 59 (5.2%) |
| Asia | 82 (3.6%) | 128 (6.1%) | 39 (6.9%) | 24 (7.8%) | 60 (5.3%) |
| Others | 46 (2.0%) | 83 (4.0%) | 16 (2.8%) | 10 (3.3%) | 23 (2.0%) |
| Maternal education | |||||
| Up to 12 years | 1,396 (61.9%) | 1,340 (64.0%) | 461 (82.0%) | 182 (59.3%) | 759 (66.8%) |
| 13 years or more | 826 (36.6%) | 717 (34.2%) | 96 (17.1%) | 115 (37.5%) | 364 (32.0%) |
| No information | 32 (1.4%) | 38 (1.8%) | 5 (0.9%) | 10 (3.3%) | 14 (1.2%) |
| Maternal marital status | |||||
| Lives with child´s father | 1,926 (85.4%) | 1,854 (88.5%) | 382 (68.0%) | 275 (89.6%) | 1,004 (88.3%) |
| Does not live with child´s father | 253 (11.2%) | 184 (8.8%) | 157 (27.9%) | 25 (8.1%) | 93 (8.2%) |
| No information | 75 (3.3%) | 57 (2.7%) | 23 (4.1%) | 7 (2.3%) | 40 (3.5%) |
| Early pregnancy BMI (kg/m2) | |||||
| Less than 18.5 | 44 (2.0%) | 33 (1.6%) | 9 (1.6%) | 8 (2.6%) | 22 (1.9%) |
| 18.5 to less than 25 | 1,097 (48.7%) | 1,066 (50.9%) | 243 (43.2%) | 174 (56.7%) | 513 (45.1%) |
| 25 to less than 30 | 595 (26.4%) | 500 (23.9%) | 145 (25.8%) | 72 (23.5%) | 318 (28.0%) |
| 30 or more | 341 (15.1%) | 287 (13.7%) | 120 (21.4%) | 36 (11.7%) | 179 (15.7%) |
| Obese (codes for obesity) | 2 (0.1%) | 2 (0.1%) | 2 (0.4%) | 0 (0.0%) | 2 (0.2%) |
| No information | 175 (7.8%) | 207 (9.9%) | 43 (7.7%) | 17 (5.5%) | 103 (9.1%) |
| Smoking during pregnancy | |||||
| Smoker | 386 (17.1%) | 258 (12.3%) | 234 (41.6%) | 26 (8.5%) | 211 (18.6%) |
| Nonsmoker | 1,809 (80.3%) | 1,787 (85.3%) | 310 (55.2%) | 277 (90.2%) | 894 (78.6%) |
| No information | 59 (2.6%) | 50 (2.4%) | 18 (3.2%) | 4 (1.3%) | 32 (2.8%) |
| Alcohol dependence | 123 (5.5%) | 38 (1.8%) | 69 (12.3%) | 1 (0.3%) | 25 (2.2%) |
| AED indications/uses | |||||
| Epilepsy | 1,559 (69.2%) | 1,774 (84.7%) | 37 (6.6%) | 303 (98.7%) | 939 (82.6%) |
| Depression | 445 (19.7%) | 106 (5.1%) | 273 (48.6%) | 19 (6.2%) | 86 (7.6%) |
| Bipolar disorder | 460 (20.4%) | 27 (1.3%) | 57 (10.1%) | 2 (0.7%) | 74 (6.5%) |
| Other psychiatric disorders | 645 (28.6%) | 179 (8.5%) | 371 (66.0%) | 37 (12.1%) | 162 (14.2%) |
| Migraine | 224 (9.9%) | 105 (5.0%) | 132 (23.5%) | 24 (7.8%) | 62 (5.5%) |
| Chronic pain | 575 (25.5%) | 283 (13.5%) | 387 (68.9%) | 78 (25.4%) | 172 (15.1%) |
| Restless legs syndrome | 9 (0.4%) | 6 (0.3%) | 13 (2.3%) | 1 (0.3%) | 2 (0.2%) |
| None of the above | 63 (2.8%) | 222 (10.6%) | 30 (5.3%) | 3 (1.0%) | 93 (8.2%) |
| Diabetes (preexisting or gestational) | 76 (3.4%) | 56 (2.7%) | 24 (4.3%) | 10 (3.3%) | 43 (3.8%) |
| Hypertension (preexisting or gestational) | 100 (4.4%) | 100 (4.8%) | 48 (8.5%) | 12 (3.9%) | 65 (5.7%) |
| Medications in current pregnancy | |||||
| AED polytherapy | 442 (19.6%) | 269 (12.8%) | 63 (11.2%) | 180 (58.6%) | 256 (22.5%) |
| Antidepressants | 458 (20.3%) | 107 (5.1%) | 284 (50.5%) | 15 (4.9%) | 117 (10.3%) |
| Antipsychotics | 157 (7.0%) | 31 (1.5%) | 76 (13.5%) | 3 (1.0%) | 50 (4.4%) |
| Migraine treatment | 51 (2.3%) | 21 (1.0%) | 43 (7.7%) | 6 (2.0%) | 21 (1.8%) |
| Opioids | 165 (7.3%) | 79 (3.8%) | 195 (34.7%) | 27 (8.8%) | 55 (4.8%) |
| Female infant | 1,178 (52.3%) | 982 (46.9%) | 285 (50.7%) | 144 (46.9%) | 557 (49.0%) |
| High dose (mean, mg/day)a | 454 | 905 | 384 | 2,489 | 1,349 |
| Low dose (mean, mg/day)b | 41 | 186 | 12 | 402 | 211 |
AED, antiepileptic drug; BMI, body mass index.
Note = The denominator for calculations is the number of infants.
a Mean dose in top tertile of pregnancy daily dose.
b Mean dose in bottom tertile of pregnancy daily dose.
The Regional Ethical Review Board in Stockholm, Sweden, approved the linkage of registers to perform this type of study (DNR 2013/862-31/5). This study was judged to be exempt from review by the RTI International institutional review board.
As secondary and sensitivity analyses, to better understand the influence of the underlying maternal health problem being treated, we repeated the main analysis in mothers with a diagnosis of epilepsy or chronic pain. We also explored the influence of monotherapy versus polytherapy (e.g., carbamazepine in polytherapy [not including lamotrigine] vs. lamotrigine in polytherapy [not including carbamazepine]). To address potential exposure misclassification and biases related to missing data, we conducted analyses on women with definite exposure (women in whom AED use from self-reports and dispensed prescriptions were consistent) and a complete case analysis. Addressing whether associations might be driven by in-utero crowding or malformations, we repeated analyses in singletons with no major congenital malformations. We repeated the analyses in the first pregnancy or infant per woman to gain understanding on any statistical effect free from the correlation among siblings. We also explored associations separately in female and male infants. We explored effect-measure modification separately by smoking and use of selective serotonin reuptake inhibitors (SSRIs) in pregnancy in linear regression analyses by incorporating an appropriate interaction term into the regression models.
In dose analyses, we compared the top tertile of mean daily dose with the bottom tertile (which served as the reference) for each individual AED, using linear regression. All models were adjusted as in the main analysis, and the weighted copy method was used for binary endpoints.
We present results from a subset of analyses in the body of this paper; others, including analyses on birth weight, length, and head circumference as recorded (in grams or centimeters, as opposed to z-scores), are included in S3 File.
Results
Study population
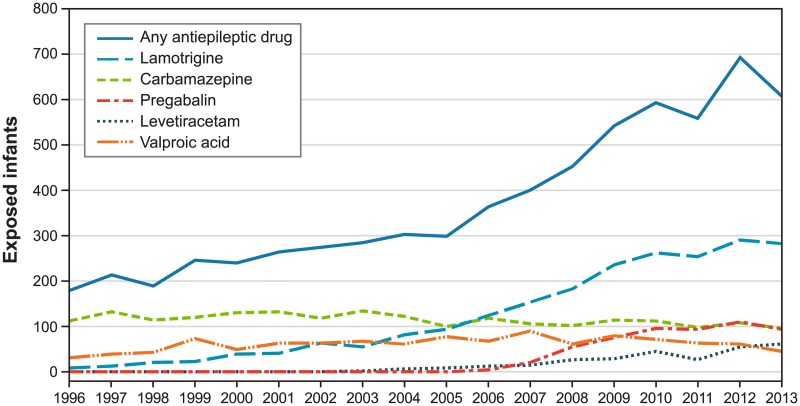
The study population comprised 6,720 infants born to 5,112 women. Antiepileptic drug use in pregnancy increased from 181 exposed infants in 1996 to 607 in 2013 (Fig 1). In 2013, the most commonly used AEDs were lamotrigine (47%), carbamazepine (16%), pregabalin (16%), levetiracetam (10%), and valproic acid (8%); we present results on these drugs. The prevalences of most maternal characteristics were quite homogeneous across users of individual study AEDs (Table 1), except for the medical conditions for which study AEDs are prescribed. Means and percentages of study outcomes in infants exposed to the individual study drugs are shown in Table A in S3 File.
Fig 1. Use of antiepileptic drugs in pregnancy, Sweden 1996–2013.
Note = Year represents year of delivery. The curve labeled "any antiepileptic drug" includes all drugs in chapter N03 of the Anatomical Therapeutic Chemical (ATC) classification system.
Carbamazepine
Carbamazepine use decreased over the study period from 63% of AED-exposed infants in 1996 to 16% in 2013 (Fig 1); mothers of 85% of carbamazepine-exposed infants had an epilepsy diagnosis, and 13% of infants were exposed to AED polytherapy (Table 1).
We observed a pattern of slightly shorter pregnancies with linear regression models (mean [95% confidence interval]: -1.3 [-2.3 to -0.3] days) and smaller infants after exposure to carbamazepine, relative to lamotrigine, with an asymmetrical effect in which the head circumference z-score was somewhat more affected (-0.2 [-0.3 to -0.1] SDs) than birth weight or birth length z-scores (both at -0.1 [-0.2 to 0] SDs) (Table 2 and Table B in S3 File). Associations at the 10th percentile of pregnancy duration were generally more negative than associations at the 90th percentile (i.e., regression coefficients from quantile regression models for carbamazepine indicated that exposure to carbamazepine was associated with a shorter pregnancy duration when assessed at the 10th percentile of pregnancy duration than when assessed at the 90th percentile). Most odds ratios (ORs) from logistic regression models for preterm delivery, SGA, and microcephaly ranged between 1.1 and 1.5; associations were larger in infants exposed to polytherapy. Odds ratios for SGA and microcephaly in women with chronic pain were also larger. Exposure to SSRIs operated as an effect-measure modifier for duration of gestation, with shorter pregnancies (mean -5.8 [-9.7 to -2.0] days) in women exposed to both carbamazepine and SSRIs (Table C in S3 File). Relative to low doses of carbamazepine, high doses of carbamazepine were associated with shorter duration of pregnancy, smaller head circumference, and higher risk for preterm birth and SGA (Table 2 and Table B in S3 File).
Table 2. Association between in-utero carbamazepine exposure and the endpoints duration of pregnancy and size at birth.
| Exposed to Carbamazepine /Reference, n/n | Difference (95% CI) | Odds Ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| Mean | 10th Percentile | 50th Percentile | 90th Percentile | |||
| Pregnancy duration (days) | Preterm birth | |||||
| Use any time in pregnancya | 1,975 / 2,123 | -1.3 (-2.3 to -0.3) | -1.1 (-3.1 to 0.9) | -0.9 (-1.8 to 0.1) | -0.1 (-1.3 to 1.0) | 1.2 (0.9 to 1.5) |
| Use in first trimestera | 1,686 / 1,930 | -1.6 (-2.7 to -0.5) | -2.3 (-4.5 to -0.1) | -0.9 (-1.8 to 0.0) | -0.5 (-1.5 to 0.6) | 1.3 (1.0 to 1.8) |
| Continuersa | 459 / 1,013 | -1.3 (-3.0 to 0.3) | 0.0 (-3.8 to 3.8) | -0.3 (-2.0 to 1.3) | -0.5 (-1.9 to 0.9) | 1.1 (0.7 to 1.7) |
| Mother with epilepsya | 1,665 / 1,447 | -1.3 (-2.4 to -0.2) | -1.6 (-3.5 to 0.3) | -0.5 (-1.5 to 0.5) | -0.2 (-1.3 to 0.9) | 1.3 (0.9 to 1.7) |
| Mother with chronic paina | 259 / 541 | -1.5 (-4.2 to 1.1) | -4.5 (-10.5 to 1.5) | -0.7 (-2.7 to 1.4) | 0.1 (-2.7 to 2.8) | 1.3 (0.7 to 2.3) |
| Polytherapya | 167 / 336 | -2.4 (-5.8 to 1.0) | -6.1 (-15.1 to 2.8) | -2.0 (-5.4 to 1.4) | -1.5 (-4.0 to 1.0) | 1.7 (0.9 to 3.3) |
| High vs. low dose of carbamazepine | 264 / 275 | -4.6 (-7.5 to -1.6) | -6.8 (-12.6 to -0.9) | -3.4 (-5.8 to -0.9) | -2.1 (-4.7 to 0.4) | 2.8 (1.3 to 6.0) |
| Birth weight z-score | SGA | |||||
| Use any time in pregnancya | 1,988 / 2,147 | -0.1 (-0.2 to -0.0) | -0.0 (-0.1 to 0.1) | -0.1 (-0.2 to -0.0) | -0.2 (-0.3 to -0.1) | 1.4 (0.9 to 2.1) |
| Use in first trimestera | 1,699 / 1,953 | -0.1 (-0.2 to -0.0) | -0.1 (-0.2 to 0.1) | -0.1 (-0.2 to -0.0) | -0.2 (-0.3 to -0.1) | 1.7 (1.0 to 2.6) |
| Continuersa | 466 / 1,021 | -0.1 (-0.2 to -0.0) | -0.1 (-0.3 to 0.1) | -0.1 (-0.2 to 0.0) | -0.2 (-0.3 to -0.0) | 1.3 (0.7 to 2.6) |
| Mother with epilepsya | 1,676 / 1,459 | -0.1 (-0.2 to -0.0) | 0.0 (-0.1 to 0.1) | -0.1 (-0.2 to -0.1) | -0.2 (-0.3 to -0.0) | 1.2 (0.8 to 1.9) |
| Mother with chronic paina | 263 / 552 | -0.2 (-0.3 to 0.0) | -0.1 (-0.4 to 0.3) | -0.1 (-0.3 to 0.1) | -0.1 (-0.4 to 0.1) | 1.8 (0.8 to 4.2) |
| Polytherapya | 167 / 339 | -0.5 (-0.7 to -0.3) | -0.6 (-0.9 to -0.3) | -0.5 (-0.7 to -0.2) | -0.3 (-0.8 to 0.1) | 4.2 (1.2 to 14.4) |
| High vs. low dose of carbamazepine | 267 / 275 | -0.1 (-0.3 to 0.1) | -0.1 (-0.4 to 0.2) | -0.1 (-0.3 to 0.1) | -0.1 (-0.4 to 0.1) | 2.0 (0.7 to 5.6) |
| Birth length z-score | ||||||
| Use any time in pregnancya | 1,963 / 2,119 | -0.1 (-0.2 to -0.0) | -0.1 (-0.2 to 0.0) | -0.1 (-0.2 to 0.0) | -0.2 (-0.3 to -0.0) | |
| Use in first trimestera | 1,681 / 1,930 | -0.1 (-0.2 to -0.0) | -0.1 (-0.2 to 0.0) | -0.1 (-0.2 to -0.0) | -0.2 (-0.3 to -0.1) | |
| Continuersa | 461 / 1,006 | -0.2 (-0.3 to -0.1) | -0.2 (-0.4 to 0.0) | -0.2 (-0.3 to -0.1) | -0.2 (-0.4 to -0.1) | |
| Mother with epilepsya | 1,655 / 1,441 | -0.1 (-0.2 to -0.0) | -0.1 (-0.3 to -0.0) | -0.1 (-0.2 to 0.0) | -0.1 (-0.3 to -0.0) | |
| Mother with chronic paina | 260 / 542 | -0.2 (-0.4 to -0.0) | -0.1 (-0.3 to 0.2) | -0.2 (-0.4 to 0.1) | -0.3 (-0.6 to -0.0) | |
| Polytherapya | 163 / 331 | -0.3 (-0.5 to -0.1) | -0.2 (-0.5 to 0.1) | -0.2 (-0.5 to 0.0) | -0.6 (-0.8 to -0.3) | |
| High vs. low dose of carbamazepine | 260 / 273 | -0.1 (-0.3 to 0.0) | -0.1 (-0.4 to 0.1) | -0.2 (-0.4 to -0.0) | -0.0 (-0.3 to 0.2) | |
| Birth head circumference z-score | Microcephaly | |||||
| Use any time in pregnancya | 1,883 / 2,096 | -0.2 (-0.3 to -0.1) | -0.2 (-0.3 to -0.0) | -0.2 (-0.3 to -0.1) | -0.2 (-0.3 to -0.1) | 1.2 (0.7 to 1.9) |
| Use in first trimestera | 1,605 / 1,906 | -0.2 (-0.3 to -0.2) | -0.2 (-0.4 to -0.1) | -0.3 (-0.4 to -0.2) | -0.3 (-0.4 to -0.2) | 1.3 (0.8 to 2.1) |
| Continuersa | 456 / 1,002 | -0.3 (-0.4 to -0.2) | -0.3 (-0.5 to -0.1) | -0.4 (-0.5 to -0.2) | -0.4 (-0.6 to -0.2) | 1.3 (0.6 to 3.3) |
| Mother with epilepsya | 1,585 / 1,421 | -0.2 (-0.3 to -0.1) | -0.2 (-0.4 to -0.1) | -0.2 (-0.3 to -0.1) | -0.2 (-0.3 to -0.1) | 1.2 (0.7 to 1.9) |
| Mother with chronic paina | 256 / 543 | -0.2 (-0.4 to -0.0) | -0.2 (-0.6 to 0.1) | -0.2 (-0.4 to -0.1) | 0.0 (-0.2 to 0.3) | 2.7 (0.8 to 9.1) |
| Polytherapya | 155 / 329 | -0.6 (-0.8 to -0.4) | -0.5 (-0.8 to -0.2) | -0.6 (-0.8 to -0.3) | -0.7 (-1.0 to -0.4) | 2.6 (0.9 to 7.3) |
| High vs. low dose of carbamazepine | 256 / 271 | -0.2 (-0.4 to 0.0) | -0.2 (-0.6 to 0.1) | -0.3 (-0.6 to -0.0) | -0.2 (-0.5 to 0.1) | Not applicable |
AED, antiepileptic drug; CI, confidence interval; SGA, small for gestational age.
a The reference is lamotrigine in the same exposure window.
Note = AED use was ascertained at any time in pregnancy, except where noted (indented rows). Analyses on continuers used data from deliveries in 2006–2013. In dose-response analyses, the reference was the bottom tertile of mean daily dose of carbamazepine (2006–2013). All results were adjusted for birth year, maternal age at delivery, education, country of origin, marital status, body mass index, smoking in current pregnancy, alcohol dependence, diabetes, hypertension, epilepsy, depression, bipolar disorder, migraine, chronic pain, and other psychiatric disorders. When the smallest cell count was < 5, we did not produce adjusted results (“not applicable”). Models restricted to polytherapy compared infants exposed to carbamazepine and another AED (except lamotrigine) with those exposed to lamotrigine and another AED (except carbamazepine).
Pregabalin
Despite first appearing in 2006, pregabalin was the third most commonly used AED in this cohort in 2013 (16% of infants). Pregabalin users differed from users of other AEDs: pregabalin users were younger, had fewer years of education, lived less frequently with the infant’s father, and were more likely to be obese or smokers. Chronic pain was common among mothers of pregabalin-exposed infants (69% of pregabalin-exposed infants), as were psychiatric conditions comprising psychoses, panic attacks, and other conditions (“other psychiatric disorders” in Table 1, 66%); mothers of 7% of infants had an epilepsy diagnosis, and mothers of 11% were on AED polytherapy (Table 1).
Pregabalin-exposed pregnancies were slightly shorter than lamotrigine-exposed pregnancies (-1.1 [-3.0 to 0.8] days on average), which was more notable in women with a diagnosis of epilepsy (-5.6 [-10.7 to -0.4] days on average) (Table 3 and Table D in S3 File). Birth weight and length z-scores were slightly lower in pregabalin-exposed than in lamotrigine-exposed infants (-0.1 [-0.3 to 0] and -0.1 [-0.2 to 0] SDs on average, respectively), and head circumference z-score was less affected (0 [-0.1 to 0.1] SDs on average). Among continuers, though, the OR for microcephaly was 5.3 (0.9 to 30.8). The association with pregnancy duration appeared to be more pronounced when the fetus was female, while the opposite was true for head circumference. No clear effect-measure modification with smoking or SSRI use, and no dose-response relation were observed (Table E in S3 File).
Table 3. Association between in-utero pregabalin exposure and the endpoints duration of pregnancy and size at birth.
| Exposed to Pregabalin /Reference, n/n | Difference (95% CI) | Odds Ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| Mean | 10th Percentile | 50th Percentile | 90th Percentile | |||
| Pregnancy duration (days) | Preterm birth | |||||
| Use any time in pregnancya | 522 / 2,190 | -1.1 (-3.0 to 0.8) | -2.7 (-6.7 to 1.2) | -0.5 (-2.5 to 1.4) | 0.3 (-1.6 to 2.3) | 1.5 (1.0 to 2.4) |
| Use in first trimestera | 484 / 1,977 | -1.8 (-3.7 to 0.2) | -3.2 (-7.5 to 1.1) | -0.5 (-2.4 to 1.3) | -0.1 (-2.2 to 2.0) | 1.9 (1.2 to 3.0) |
| Continuersa | 142 / 1,025 | -1.2 (-4.7 to 2.3) | -0.5 (-7.2 to 6.2) | 0.4 (-3.4 to 4.2) | -0.6 (-4.7 to 3.5) | 2.3 (1.0 to 5.3) |
| Mother with epilepsya | 33 / 1,537 | -5.6 (-10.7 to -0.4) | -11.2 (-35.7 to 13.3) | -4.2 (-10.0 to 1.6) | 3.3 (-4.7 to 11.2) | 4.2 (1.6 to 11.4) |
| Female infantsa | 265 / 1,146 | -2.0 (-4.6 to 0.7) | -3.2 (-8.7 to 2.2) | -1.9 (-4.2 to 0.5) | -2.4 (-5.1 to 0.3) | 1.9 (1.0 to 3.4) |
| Male infantsa | 257 / 1,044 | -0.2 (-3.1 to 2.6) | 0.4 (-4.6 to 5.4) | -0.2 (-3.5 to 3.1) | 1.2 (-1.7 to 4.2) | 1.2 (0.6 to 2.4) |
| High vs. low dose of pregabalin | 175 / 174 | 0.6 (-2.7 to 3.9) | 0.4 (-7.0 to 7.7) | 1.1 (-1.9 to 4.2) | 1.2 (-2.3 to 4.7) | 1.1 (0.6 to 2.3) |
| Birth weight z-score | SGA | |||||
| Use any time in pregnancya | 528 / 2,215 | -0.1 (-0.3 to 0.0) | -0.0 (-0.3 to 0.2) | -0.2 (-0.3 to 0.0) | -0.2 (-0.4 to 0.0) | 1.3 (0.6 to 3.0) |
| Use in first trimestera | 489 / 2,001 | -0.2 (-0.3 to -0.0) | -0.0 (-0.3 to 0.2) | -0.2 (-0.4 to -0.0) | -0.2 (-0.5 to -0.0) | 1.3 (0.6 to 3.1) |
| Continuersa | 142 / 1,033 | -0.1 (-0.4 to 0.1) | -0.2 (-0.7 to 0.3) | -0.1 (-0.4 to 0.2) | -0.2 (-0.6 to 0.1) | 0.6 (0.1 to 3.0) |
| Mother with epilepsya | 33 / 1,550 | 0.1 (-0.3 to 0.5) | 0.2 (-0.9 to 1.2) | 0.2 (-0.2 to 0.6) | -0.3 (-0.7 to 0.2) | Not applicable |
| High vs. low dose of pregabalin | 177 / 176 | 0.0 (-0.2 to 0.3) | -0.3 (-0.8 to 0.1) | 0.2 (-0.1 to 0.5) | 0.2 (-0.1 to 0.6) | 1.3 (0.4 to 4.6) |
| Birth length z-score | ||||||
| Use any time in pregnancya | 521 / 2,186 | -0.1 (-0.2 to 0.0) | -0.0 (-0.3 to 0.2) | -0.1 (-0.3 to 0.0) | -0.1 (-0.4 to 0.1) | |
| Use in first trimestera | 484 / 1,977 | -0.1 (-0.2 to 0.0) | -0.1 (-0.4 to 0.1) | -0.2 (-0.4 to 0.0) | -0.1 (-0.3 to 0.2) | |
| Continuersa | 140 / 1,018 | -0.1 (-0.4 to 0.1) | -0.1 (-0.5 to 0.4) | -0.1 (-0.4 to 0.3) | -0.2 (-0.7 to 0.2) | |
| Mother with epilepsya | 32 / 1,530 | -0.1 (-0.5 to 0.3) | -0.1 (-0.9 to 0.8) | 0.1 (-0.3 to 0.6) | -0.1 (-0.7 to 0.5) | |
| High vs. low dose of pregabalin | 172 / 175 | 0.0 (-0.2 to 0.2) | -0.3 (-0.7 to 0.0) | -0.0 (-0.3 to 0.2) | 0.1 (-0.2 to 0.5) | |
| Birth head circumference z-score | Microcephaly | |||||
| Use any time in pregnancya | 516 / 2,160 | -0.0 (-0.1 to 0.1) | 0.1 (-0.1 to 0.3) | -0.0 (-0.2 to 0.1) | -0.1 (-0.3 to 0.1) | 1.2 (0.5 to 2.9) |
| Use in first trimestera | 480 / 1,951 | -0.0 (-0.2 to 0.1) | 0.1 (-0.1 to 0.4) | -0.1 (-0.2 to 0.1) | 0.0 (-0.2 to 0.3) | 1.3 (0.5 to 3.4) |
| Continuersa | 136 / 1,012 | -0.1 (-0.3 to 0.2) | -0.1 (-0.7 to 0.6) | -0.0 (-0.3 to 0.3) | -0.1 (-0.5 to 0.2) | 5.3 (0.9 to 30.8) |
| Mother with epilepsya | 32 / 1,508 | -0.0 (-0.4 to 0.4) | 0.0 (-1.0 to 1.1) | 0.1 (-0.3 to 0.4) | 0.4 (-0.5 to 1.3) | Not applicable |
| Female infantsa | 264 / 1,128 | 0.0 (-0.2 to 0.2) | 0.3 (-0.1 to 0.6) | -0.1 (-0.3 to 0.1) | 0.0 (-0.3 to 0.3) | 1.2 (0.3 to 4.5) |
| Male infantsa | 252 / 1,032 | -0.0 (-0.3 to 0.2) | -0.3 (-0.6 to 0.1) | 0.0 (-0.2 to 0.2) | -0.1 (-0.4 to 0.2) | 1.6 (0.5 to 5.7) |
| High vs. low dose of pregabalin | 170 / 174 | 0.0 (-0.2 to 0.2) | 0.1 (-0.2 to 0.5) | 0.1 (-0.2 to 0.4) | -0.3 (-0.8 to 0.1) | Not applicable |
AED, antiepileptic drug; CI, confidence interval; SGA, small for gestational age.
a The reference is lamotrigine in the same exposure window.
AED use was ascertained at any time in pregnancy, except where noted (indented rows). Analyses on continuers are based on data from deliveries in 2006–2013. In dose-response analyses, the reference was the bottom tertile of mean daily dose of pregabalin (2006–2013). All results are adjusted for birth year, maternal age at delivery, education, country of origin, marital status, body mass index, smoking in current pregnancy, alcohol dependence, diabetes, hypertension, epilepsy, depression, bipolar disorder, migraine, chronic pain, and other psychiatric disorders. When the smallest cell count was < 5, we did not produce adjusted results (“not applicable”).
Levetiracetam
First appearing in this cohort in 2002, levetiracetam use increased to be the fourth most commonly used AED in 2013 (10% of infants, Fig 1). Mothers of 99% of levetiracetam-exposed infants had a diagnosis of epilepsy; 59% of infants were exposed AED polytherapy (Table 1). Common polytherapies involved lamotrigine (91 of 180 infants), carbamazepine (48), and valproic acid (33).
On average, pregnancy duration was half a day shorter (-0.5 [-2.6 to 1.6]), birth weight was 0.1 SDs lighter (-0.1 [-0.3 to 0.0] SD), length was similar (0.0 [-0.1 to 0.1] SDs), and head circumference was 0.1 SD smaller (-0.1 [-0.3 to 0.1] SD) in pregnancies and infants exposed to levetiracetam than in those exposed to lamotrigine (Table 4 and Table F in S3 File). In women with chronic pain, levetiracetam-exposed pregnancies were longer than lamotrigine-exposed pregnancies. Most ORs for preterm delivery were slightly above 1; adjusted ORs for SGA and microcephaly were often not estimable due to cell counts below five. Infants exposed to polytherapy had reduced head circumference (-0.6 [-0.9 to -0.3] SDs on average). Exposure to an SSRI operated as an effect-measure modifier for duration of gestation, with shorter pregnancies (-11.5 [-22.3 to -0.6]) days) in women exposed to both levetiracetam and SSRIs (Table G in S3 File). No clear dose-response relations were observed.
Table 4. Association between in-utero levetiracetam exposure and the endpoints duration of pregnancy and size at birth.
| Exposed to Levetiracetam/Reference, n/n | Difference (95% CI) | Odds Ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| Mean | 10th Percentile | 50th Percentile | 90th Percentile | |||
| Pregnancy duration (days) | Preterm birth | |||||
| Use any time in pregnancya | 213 / 2,133 | -0.5 (-2.6 to 1.6) | -1.0 (-6.3 to 4.3) | 0.6 (-1.2 to 2.4) | 1.6 (-0.2 to 3.3) | 1.3 (0.8 to 2.3) |
| Use in first trimestera | 184 / 1,938 | -0.7 (-2.9 to 1.5) | -1.7 (-7.3 to 4.0) | 0.3 (-1.8 to 2.5) | 1.8 (0.1 to 3.6) | 1.6 (0.9 to 2.8) |
| Continuersa | 144 / 990 | -1.1 (-3.5 to 1.4) | -1.0 (-7.7 to 5.7) | 1.0 (-1.7 to 3.6) | 0.2 (-2.5 to 2.9) | 1.3 (0.6 to 2.6) |
| Mother with chronic paina | 52 / 536 | 2.6 (-2.1 to 7.4) | 5.3 (-5.3 to 16.0) | 2.1 (-1.9 to 6.1) | 5.0 (-0.5 to 10.5) | Not applicable |
| Polytherapya | 87 / 346 | -0.1 (-4.0 to 3.8) | -0.5 (-8.4 to 7.4) | 0.3 (-3.7 to 4.2) | -1.1 (-4.4 to 2.3) | 1.0 (0.4 to 2.7) |
| High vs. low dose of levetiracetam | 89 / 89 | -0.2 (-4.6 to 4.3) | -5.7 (-18.1 to 6.8) | -0.0 (-4.4 to 4.4) | 1.1 (-3.4 to 5.6) | 0.4 (0.1 to 1.6) |
| Birth weight z-score | SGA | |||||
| Use any time in pregnancya | 215 / 2,157 | -0.1 (-0.3 to 0.0) | -0.1 (-0.4 to 0.1) | 0.0 (-0.1 to 0.2) | -0.2 (-0.4 to 0.0) | 1.3 (0.5 to 3.0) |
| Use in first trimestera | 186 / 1,961 | -0.1 (-0.3 to 0.0) | -0.2 (-0.5 to 0.0) | 0.0 (-0.1 to 0.2) | -0.1 (-0.3 to 0.1) | 1.8 (0.7 to 4.3) |
| Continuersa | 146 / 998 | -0.1 (-0.3 to 0.1) | -0.2 (-0.6 to 0.2) | -0.0 (-0.2 to 0.1) | -0.2 (-0.5 to 0.1) | 1.7 (0.6 to 4.5) |
| Mother with chronic paina | 51 / 546 | -0.1 (-0.4 to 0.3) | 0.2 (-0.8 to 1.3) | 0.2 (-0.2 to 0.5) | -0.4 (-0.9 to 0.1) | Not applicable |
| Polytherapya | 88 / 349 | -0.5 (-0.7 to -0.2) | -0.5 (-1.0 to 0.0) | -0.5 (-0.9 to -0.1) | -0.4 (-0.8 to 0.1) | Not applicable |
| High vs. low dose of levetiracetam | 90 / 91 | 0.1 (-0.1 to 0.4) | 0.2 (-0.7 to 1.2) | 0.1 (-0.2 to 0.4) | 0.4 (-0.1 to 0.9) | Not applicable |
| Birth length z-score | ||||||
| Use any time in pregnancya | 213 / 2,128 | -0.0 (-0.1 to 0.1) | -0.1 (-0.3 to 0.2) | 0.1 (-0.0 to 0.3) | -0.1 (-0.4 to 0.2) | |
| Use in first trimestera | 184 / 1,937 | -0.0 (-0.2 to 0.1) | -0.0 (-0.3 to 0.3) | 0.1 (-0.0 to 0.3) | -0.2 (-0.4 to 0.1) | |
| Continuersa | 144 / 983 | 0.1 (-0.1 to 0.2) | 0.2 (-0.2 to 0.5) | 0.1 (-0.0 to 0.3) | -0.2 (-0.4 to 0.1) | |
| Mother with chronic paina | 51 / 536 | 0.1 (-0.2 to 0.4) | 0.4 (-0.3 to 1.1) | 0.3 (0.1 to 0.5) | 0.1 (-0.5 to 0.7) | |
| Polytherapya | 88 / 340 | -0.2 (-0.5 to 0.0) | -0.5 (-0.9 to -0.1) | -0.0 (-0.3 to 0.3) | -0.3 (-0.7 to 0.2) | |
| High vs. low dose of levetiracetam | 89 / 90 | 0.1 (-0.1 to 0.4) | 0.1 (-0.5 to 0.7) | 0.0 (-0.3 to 0.4) | 0.3 (-0.2 to 0.8) | |
| Birth head circumference z-score | Microcephaly | |||||
| Use any time in pregnancya | 206 / 2,103 | -0.1 (-0.3 to 0.1) | -0.1 (-0.4 to 0.2) | -0.1 (-0.3 to 0.1) | -0.1 (-0.4 to 0.1) | 1.4 (0.6 to 3.5) |
| Use in first trimestera | 178 / 1,912 | -0.1 (-0.3 to 0.1) | 0.0 (-0.4 to 0.4) | -0.1 (-0.3 to 0.1) | -0.1 (-0.4 to 0.1) | 1.6 (0.6 to 4.4) |
| Continuersa | 140 / 978 | -0.0 (-0.2 to 0.1) | 0.1 (-0.3 to 0.5) | -0.0 (-0.3 to 0.2) | -0.0 (-0.4 to 0.4) | Not applicable |
| Mother with chronic paina | 50 / 536 | -0.0 (-0.3 to 0.3) | -0.2 (-0.9 to 0.6) | -0.1 (-0.3 to 0.2) | -0.3 (-0.9 to 0.2) | Not applicable |
| Polytherapya | 84 / 336 | -0.6 (-0.9 to -0.3) | -0.4 (-1.0 to 0.2) | -0.8 (-1.0 to -0.5) | -0.5 (-0.9 to -0.0) | 2.7 (0.7 to 9.6) |
| High vs. low dose of levetiracetam | 87 / 89 | -0.0 (-0.3 to 0.3) | 0.4 (-0.2 to 1.0) | 0.1 (-0.3 to 0.4) | -0.4 (-1.0 to 0.2) | Not applicable |
AED, antiepileptic drug; CI, confidence interval; SGA, small for gestational age.
a The reference is lamotrigine in the same exposure window.
AED use was ascertained at any time in pregnancy, except where noted (indented rows). Analyses on continuers used data from deliveries in 2006–2013. In dose-response analyses, the reference was the bottom tertile of mean daily dose of levetiracetam (2006–2013). All results are adjusted for birth year, maternal age at delivery, education, country of origin, marital status, body mass index, smoking in current pregnancy, alcohol dependence, diabetes, hypertension, epilepsy, depression, bipolar disorder, migraine, chronic pain, and other psychiatric disorders. When the smallest cell count was < 5, we did not produce adjusted results (“not applicable”).
Valproic acid
Valproic acid exposure decreased from 18% of infants in 1996 to 8% in 2013 (Fig 1). Commonly, mothers of exposed infants had a diagnosis of epilepsy (83%); 23% were on polytherapy (Table 1).
On average, valproic acid–exposed pregnancies had a duration similar to lamotrigine-exposed pregnancies (0 [-1.2 to 1.2] days), and infants were born with the same weight for gestational age (0 [-0.1 to 0] SDs) (Table 5 and Table H in S3 File). However, we observed a gradient in which associations assessed at the 10th percentile were in the direction of the left tail (i.e., shorter pregnancies, infants lighter for gestational age) and in the direction of the right when assessed at the 90th percentile (i.e., longer pregnancies, infants heavier for gestational age). This was also true for the comparison of high versus low valproic acid doses. The association with pregnancy duration was toward longer pregnancies when the fetus was female, opposite to what was observed in pregnancies with male fetuses: the difference was 5.4 days at the 10th percentile. We observed effect-measure modification for duration of pregnancy by smoking and use of SSRIs, which resulted in valproic acid use and smoking or SSRI use being associated with shorter pregnancies (-3.1 [-6.1 to -0.2] and -3.9 [-7.7 to -0.1] days, respectively; Table I in S3 File). Birth length did not seem to be adversely affected. Valproic acid–exposed infants had a smaller head circumference relative to lamotrigine-exposed infants, and continuers were more strongly affected (OR for microcephaly: 3.9 [1.7 to 9.0]). For all endpoints except birth length, polytherapy-exposed infants were more severely affected, with a difference in duration of 10 days at the 10th percentile. Odds ratios were generally higher for valproic acid than for other study AEDs.
Table 5. Association between in-utero valproic acid exposure and the endpoints duration of pregnancy and size at birth.
| Exposed to Valproic Acid/Reference, n/n | Difference (95% CI) | Odds Ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| Mean | 10th Percentile | 50th Percentile | 90th Percentile | |||
| Pregnancy duration (days) | Preterm birth | |||||
| Use any time in pregnancya | 985 / 2,086 | -0.0 (-1.2 to 1.2) | -1.9 (-5.3 to 1.4) | 1.0 (-0.3 to 2.3) | 1.6 (0.4 to 2.8) | 1.5 (1.1 to 2.0) |
| Use in first trimestera | 845 / 1,902 | -0.1 (-1.3 to 1.2) | -1.3 (-4.9 to 2.2) | 0.8 (-0.4 to 2.0) | 1.4 (0.3 to 2.5) | 1.6 (1.1 to 2.2) |
| Continuersa | 253 / 996 | -0.0 (-2.0 to 2.0) | -3.9 (-10.6 to 2.7) | 1.8 (-0.4 to 3.9) | 2.4 (0.7 to 4.1) | 1.7 (1.1 to 2.8) |
| Polytherapya | 115 / 299 | -3.4 (-6.9 to 0.2) | -10.0 (-19.5 to -0.5) | 0.1 (-3.4 to 3.5) | 2.2 (-1.1 to 5.4) | 3.0 (1.5 to 6.2) |
| Female infantsa | 480 / 1,094 | 0.6 (-1.1 to 2.4) | 1.6 (-2.3 to 5.5) | 1.1 (-0.6 to 2.9) | 1.7 (0.2 to 3.3) | 1.1 (0.7 to 1.8) |
| Male infantsa | 505 / 992 | -0.7 (-2.4 to 1.0) | -3.8 (-7.6 to -0.0) | -0.1 (-1.6 to 1.5) | 0.9 (-0.6 to 2.4) | 1.9 (1.2 to 2.9) |
| High vs. low dose of valproic acid | 165 / 167 | -1.0 (-4.9 to 2.9) | -2.4 (-10.1 to 5.3) | -0.5 (-3.9 to 3.0) | 1.0 (-1.4 to 3.5) | 1.4 (0.5 to 3.4) |
| Birth weight z-score | SGA | |||||
| Use any time in pregnancya | 992 / 2,110 | -0.0 (-0.1 to 0.0) | -0.1 (-0.3 to 0.1) | -0.1 (-0.2 to 0.1) | 0.1 (-0.1 to 0.2) | 1.9 (1.2 to 2.9) |
| Use in first trimestera | 852 / 1,924 | -0.1 (-0.2 to 0.0) | -0.1 (-0.3 to 0.1) | -0.0 (-0.2 to 0.1) | 0.1 (-0.1 to 0.2) | 2.4 (1.5 to 3.8) |
| Continuersa | 257 / 1,004 | -0.1 (-0.3 to 0.1) | -0.3 (-0.6 to 0.1) | -0.0 (-0.2 to 0.2) | 0.3 (0.0 to 0.5) | 2.5 (1.3 to 5.0) |
| Polytherapya | 116 / 302 | -0.2 (-0.5 to 0.0) | -0.2 (-0.6 to 0.2) | -0.1 (-0.4 to 0.1) | -0.4 (-0.7 to -0.1) | 2.6 (0.6 to 11.0) |
| High vs. low dose of valproic acid | 169 / 168 | -0.1 (-0.3 to 0.2) | -0.4 (-0.9 to 0.1) | -0.1 (-0.4 to 0.2) | 0.4 (-0.0 to 0.8) | Not applicable |
| Birth length z-score | ||||||
| Use any time in pregnancya | 966 / 2,083 | 0.1 (0.0 to 0.2) | 0.0 (-0.1 to 0.2) | 0.1 (-0.0 to 0.2) | 0.2 (0.1 to 0.3) | |
| Use in first trimestera | 828 / 1,901 | 0.1 (-0.0 to 0.2) | 0.0 (-0.1 to 0.2) | 0.1 (-0.0 to 0.2) | 0.2 (0.1 to 0.4) | |
| Continuersa | 254 / 989 | 0.1 (-0.0 to 0.3) | 0.0 (-0.3 to 0.4) | 0.2 (-0.0 to 0.4) | 0.3 (0.0 to 0.5) | |
| Polytherapya | 112 / 295 | 0.0 (-0.2 to 0.3) | 0.2 (-0.2 to 0.5) | 0.2 (0.0 to 0.4) | -0.1 (-0.5 to 0.3) | |
| High vs. low dose of valproic acid | 167 / 167 | 0.2 (-0.1 to 0.4) | 0.3 (-0.2 to 0.8) | 0.1 (-0.1 to 0.4) | 0.3 (-0.2 to 0.8) | |
| Birth head circumference z-score | Microcephaly | |||||
| Use any time in pregnancya | 931 / 2,059 | -0.2 (-0.2 to -0.1) | -0.1 (-0.3 to 0.0) | -0.1 (-0.2 to -0.1) | -0.2 (-0.3 to -0.0) | 1.7 (1.0 to 2.8) |
| Use in first trimestera | 802 / 1,877 | -0.1 (-0.2 to -0.0) | -0.1 (-0.2 to 0.1) | -0.2 (-0.3 to -0.1) | -0.2 (-0.3 to -0.0) | 1.8 (1.0 to 3.1) |
| Continuersa | 252 / 983 | -0.2 (-0.3 to -0.0) | -0.2 (-0.5 to 0.1) | -0.2 (-0.3 to -0.0) | -0.2 (-0.4 to 0.1) | 3.9 (1.7 to 9.0) |
| Polytherapya | 107 / 292 | -0.5 (-0.7 to -0.2) | -0.5 (-0.9 to -0.1) | -0.4 (-0.6 to -0.1) | -0.4 (-0.9 to 0.0) | 3.1 (1.0 to 9.8) |
| High vs. low dose of valproic acid | 166 / 162 | -0.2 (-0.5 to 0.0) | -0.4 (-1.0 to 0.2) | -0.1 (-0.4 to 0.2) | 0.1 (-0.3 to 0.6) | Not applicable |
AED, antiepileptic drug; CI, confidence interval; SGA, small for gestational age.
a The reference is lamotrigine in the same exposure window.
AED use was ascertained at any time in pregnancy, except where noted (indented rows). Analyses on continuers used data from deliveries in 2006–2013. In dose-response analyses, the reference was the bottom tertile of mean daily dose of valproic acid (2006–2013). All results were adjusted for birth year, maternal age at delivery, education, country of origin, marital status, body mass index, smoking in current pregnancy, alcohol dependence, diabetes, hypertension, epilepsy, depression, bipolar disorder, migraine, chronic pain, and other psychiatric disorders. When the smallest cell count was < 5, we did not produce adjusted results (“not applicable”). Models restricted to polytherapy compared infants exposed to valproic acid and another AED (except lamotrigine) with those exposed to lamotrigine and another AED (except valproic acid).
Lamotrigine
Lamotrigine use in pregnancy increased over the study period from 6% in 1996 to 47% in 2013 (Fig 1). Mothers of exposed infants often had a diagnosis of epilepsy (69%); 20% of women were on polytherapy.
In dose-response analyses, pregnancies exposed to high doses were, on average, 1.8 days shorter (-1.8 [-3.8 to 0.2]) than those exposed to low doses; the OR for preterm birth was 1.3 (0.7 to 2.2) (Table 6 and Table J in S3 File). We did not observe meaningful associations between higher doses and lower z-scores.
Table 6. Association between in-utero lamotrigine exposure and the endpoints duration of pregnancy and size at birth.
| Exposed to High/Low Dose, n/n | Difference (95% CI) | Odds Ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| Mean | 10th Percentile | 50th Percentile | 90th Percentile | |||
| Pregnancy duration (days) | Preterm birth | |||||
| High vs. low dose of lamotrigine | 551 / 547 | -1.8 (-3.8 to 0.2) | -0.9 (-5.1 to 3.3) | -0.6 (-2.6 to 1.3) | -1.1 (-3.2 to 1.0) | 1.3 (0.7 to 2.2) |
| Birth weight z-score | SGA | |||||
| High vs. low dose of lamotrigine | 557 / 557 | 0.1 (-0.1 to 0.2) | -0.0 (-0.3 to 0.2) | 0.1 (-0.0 to 0.3) | 0.1 (-0.1 to 0.3) | 0.9 (0.3 to 2.1) |
| Birth length z-score | ||||||
| High vs. low dose of lamotrigine | 548 / 551 | 0.1 (-0.1 to 0.2) | -0.1 (-0.3 to 0.2) | -0.0 (-0.2 to 0.1) | 0.3 (0.1 to 0.5) | |
| Birth head circumference z-score | Microcephaly | |||||
| High vs. low dose of lamotrigine | 543 / 550 | 0.0 (-0.1 to 0.2) | -0.1 (-0.4 to 0.1) | -0.0 (-0.2 to 0.2) | 0.0 (-0.2 to 0.3) | 0.5 (0.2 to 1.6) |
AED, antiepileptic drug; CI, confidence interval; SGA, small for gestational age.
AED use was ascertained at any time in pregnancy. The reference was the bottom tertile of mean daily dose of lamotrigine (2006–2013). All results were adjusted for birth year, maternal age at delivery, education, country of origin, marital status, body mass index, smoking in current pregnancy, alcohol dependence, diabetes, hypertension, epilepsy, depression, bipolar disorder, migraine, chronic pain, and other psychiatric disorders.
Other key variables: Smoking, diabetes, and epilepsy
To put results on individual AEDs in perspective, we considered the size of the point estimates for other variables obtained from the main analysis. In all linear regression analyses for exposure at any time in pregnancy, the estimated effect of smoking was more negative than the estimated effect for all study AEDs on all study outcomes (Table K in S3 File). For example, birth weight z-score point estimates for study AEDs were between 0 and -0.1 SDs, while, for smoking, they were between -0.4 and -0.5 SDs. Diabetes was associated with a shorter duration of pregnancy of over 1 week in analyses of all study AEDs, an association several times larger than that of study AEDs. Point estimates for epilepsy were small or null.
Discussion
In this population-based, comparative safety cohort study involving 6,720 infants exposed to AEDs in pregnancy in Sweden during 1996–2013, we observed an increase in AED use in pregnancy over time and an evolution in preference from older to newer AEDs. With the possible exception of pregabalin, maternal characteristics were comparable across users of individual AEDs, except for the indications or uses for each drug: in the extremes, levetiracetam was used almost exclusively in women with an epilepsy diagnosis, and pregabalin was used mostly in women with chronic pain or psychiatric diagnoses. These diagnoses, which may have acted as confounders despite the comparative safety design, were controlled in the analyses. Analyses comparing individual AEDs to lamotrigine showed generally small associations (e.g., mean changes in duration of pregnancy smaller than 3 days, changes in z-scores mostly up to 0.2 SDs), which were generally milder than those observed for smoking or diabetes. Below, we contextualize our findings within what was previously known about the associations between the study AEDs and size at birth, congenital malformations and cognitive outcomes.
Carbamazepine
On the basis of mean results from the main analysis for AED exposure at any time in pregnancy, carbamazepine-exposed infants were born 1 day earlier, were 0.1 SDs lighter and shorter, and had a head circumference that was 0.2 SDs smaller for their gestational age than infants exposed to lamotrigine; estimated effects were dose dependent. For carbamazepine versus lamotrigine in monotherapy, our literature search identified a relative risk for SGA of 1.3 (1.0 to 1.7) [16] and an OR of 3.1 (0.9 to 10.9) [22], compared with an OR of 1.3 (0.8 to 2.0) from our study. In a myriad of statistical comparisons identified in the literature search, relative to unexposed populations, carbamazepine has been associated with shorter pregnancies and lower birth weight, length, and/or head circumference, sometimes with wide confidence intervals [13, 14, 18–21, 23, 24, 26]. Maternal exposure to carbamazepine has been associated with major congenital malformations [4] in a dose-dependent manner [5]; the association with adverse developmental, cognitive, and behavioral outcomes is less clear [8, 10].
On the basis of these reports, fetal head size measurements in pregnant women who use carbamazepine should be taken into consideration in planning future maternal and infant care. Lower doses appear to be preferable with regard to several offspring outcomes.
Pregabalin
We observed that pregabalin-exposed infants were born, on average, 1 day earlier; were 0.1 SDs lighter and shorter; and had similar head circumference for their gestational age than infants exposed to lamotrigine; no clear dose effects were seen. Because pregabalin is a relatively new AED, the literature on its safety in pregnancy is limited. Our literature search identified one study that reported elevated risk, with wide confidence intervals, for preterm delivery and SGA based on a small number of pregnancies exposed to pregabalin compared to unexposed pregnancies [45, 46]. Its association with congenital malformations is contested [11, 27, 47], and not much is known on any potential association with adverse neurodevelopmental outcomes [8].
Levetiracetam
In our study, levetiracetam-exposed infants were born, on average, 0.5 days earlier; were 0.1 SDs lighter, with similar length; and were 0.1 SDs smaller in head circumference for their gestational age than those exposed to lamotrigine. One study identified in our literature search reported that the relative risk for the association between levetiracetam versus lamotrigine monotherapy and SGA was 1.3 (1.0 to 1.7) [16], which compares with the OR in our study for monotherapy or polytherapy combined: 1.3 (0.5 to 3.0). Comparisons with women unexposed to AEDs were less clear: one study reported that levetiracetam exposure was associated with shorter pregnancies and lighter infants [14], one reported lighter infants but practically null association with duration of pregnancy and head circumference [20], and one reported protective effects for SGA and microcephaly [24]. The pooled risk for congenital malformations in subjects exposed to levetiracetam has been reported as similar to that for the unexposed, although some individual studies reported increased risk [4]. Developmental outcomes appear not to be negatively affected based on a single cohort [8, 10].
Valproic acid
In our study, valproic acid–exposed infants had, on average, the same duration of gestation and birth weight for gestational age but were 0.2 SDs smaller in head circumference for gestational age than infants exposed to lamotrigine. Null mean associations masked opposite results in the two tails of the distributions of pregnancy duration and birth weight z-scores. Outcomes were worse in infants exposed to valproate in polytherapy in pregnancy, which has also been reported for congenital malformations [48]. Our literature search identified studies reporting an association of valproic acid versus lamotrigine monotherapy and SGA (relative risk: 1.5 [1.0 to 2.2] [16] and OR: 4.1 [1.1 to 15.0] [22]) that compares with that in our study (OR: 1.9 [1.2 to 3.2]). In comparison with unexposed subjects, results have been mixed: exposure to valproic acid has been reported to have a practically null association with mean pregnancy duration [20], conferring a null [23] or increased risk for preterm delivery [14, 20]; to decrease mean birth weight [19, 20], conferring a null [23] or increased [14, 20] risk for low birth weight but not for very low birth weight [25]; to confer a lower [14, 24] or increased risk for SGA [20]; and to reduce head circumference [13, 20]. Valproic acid is a known teratogen [49], and a dose-response relation has been reported for this association [5], with variations across types of major congenital malformations [50]. In-utero exposure to valproic acid has also been reported to be associated with hearing impairment [51] and to have a dose-response relation with adverse developmental, cognitive, and behavioral outcomes [8, 10, 52]. In 2014, the European Medicines Agency (EMA) conducted a review on the pregnancy safety of valproic acid, after which it imposed a number of risk minimization activities in Europe [53]. Subsequent studies in France, the first country in which valproic acid was approved to treat epilepsy [54], showed that valproic acid use continued to be high [11, 55]. This triggered a second review by EMA, which then strengthened its risk minimization measures, now including a pregnancy prevention program [56].
Recommendations restricting the use of valproic acid in pregnancy are already in place [56], and our study supports those recommendations. Our results also confirm that monotherapy appears to be preferable to polytherapy for these outcomes.
Lamotrigine
This study assessed the safety of lamotrigine, used as the reference for drug-to-drug comparisons, in dose analyses. We observed an association between high doses of lamotrigine and shorter pregnancies (1.8 days on average). In comparisons of women exposed to lamotrigine with those unexposed, published studies estimated null or adverse effects on pregnancy duration and birth weight [14, 20, 23], protective or null effects on SGA [14, 20, 24], and null effects on head circumference [13, 20]. A recent systematic review that focused on lamotrigine concluded that there was no association between lamotrigine in monotherapy and congenital malformations, preterm delivery, or SGA [28, 29]; but a dose dependency was reported for congenital malformations.[5]. Studies assessing neurodevelopmental outcomes have reported outcomes similar to those of the general population, but also a potentially increased risk for some specific deficits [8, 10]. On the basis of these reports, lower doses appear to be preferable to higher doses for these offspring outcomes.
Secondary and sensitivity analyses, strengths, and limitations
We treated all pregnancies as independent observations because statistical models incorporating within-woman correlation would not converge; results from a sensitivity analysis including only the first infant per woman (S3 File) generally shows, as expected, wider confidence intervals. They also show some variability in point estimates, because this sensitivity analysis excluded fewer infants exposed to pregabalin but more infants exposed to valproic acid than those exposed to lamotrigine. Twelve percent of study infants had missing data, although less than 10% of patients had missing data for any given variable, with missingness decreasing over time; the complete case analysis (S3 File) was consistent with the main analysis. We only ascertained prescriptions dispensed during pregnancy due to the lack of information on duration of use of prescribed medications; while this could have caused under-ascertainment of prescription-based exposure, we expect we captured AED use when it extended into pregnancy, from self-report during prenatal care.
This is an observational study that used existing data sources, with the strengths and limitations of this type of study. Limitations include that we cannot be certain that a woman used the medications that she was dispensed or certain of the timing of the use of the medication, both of which can result in misclassification of exposure. Diagnoses, including the outcomes, were not confirmed or validated for this study, which could result in misclassification of confounders and outcomes. A review of validation studies reported positive predictive values above 80% and often above 90% for a wide range of discharge diagnoses, including psychiatric and neurologic diagnoses [57]. Despite study design features introduced to reduce confounding, namely the comparative safety design and extensive multivariate adjustment, some residual confounding is possible. This is especially true for pregabalin analyses, given the distinct profile of its users.
Strengths of the study design include the fact that data are collected prospectively as patients seek and receive health care, as well as our ability to incorporate exposure from both self-reports and dispensed prescriptions. Results from analyses that defined exposure based on concordant self-reports and dispensed prescriptions are consistent with the main analysis. We were able to adjust for multiple AED indications or uses and to explore associations in the tails of study outcomes using quantile regression, a method not often used in studies on drug safety. We thus identified that a zero association at the mean (i.e., results from linear regression) can mask associations at the tails of the outcome distribution, as was seen in this study for valproic acid, and duration of pregnancy and birth weight z-score using quantile regression. Another strength of this study is our ability to define our endpoints as z-scores, which we preferred because z-scores enable assessing size independently of any effect on pregnancy duration. Because other researchers may be interested in results on birth weight, length, and head circumference without this transformation, we included those results in S3 File.
We observed different estimated effects on pregnancies with female and male fetuses for some associations, without a clear pattern. While these may reflect true effects of AEDs, they may also reflect differential fetal survival by sex perhaps in relation to sex-specific congenital malformations [58]. Table 1 shows some variation in the percentage of female infants across AEDs. We hope future research will help clarify this aspect.
The body of evidence on the associations between in-utero exposure to AEDs and maternal, pregnancy, fetal, and infant outcomes argue against combining all AEDs into a single group for safety pregnancy research. The relative prevalence of AED use in pregnancy has evolved over time, and drugs have different safety profiles, making results on the combined AEDs not comparable from one study to another and not reflective of the risk of any specific AED.
Conclusions
We observed that commonly used AEDs have distinct safety profiles regarding duration of pregnancy and size at birth. In comparison with lamotrigine, valproic acid and carbamazepine had a more negative association with head circumference than other study AEDs, suggesting that fetal head size in exposed pregnancies should be monitored. To put our results in perspective, we note that the adverse effects of AEDs on the outcomes studied were generally smaller than those of smoking. Although no mean effect was observed, associations between valproic acid and the endpoints duration of pregnancy and birth weight for gestational age in the left tail of the distributions were toward shorter pregnancies and smaller infants.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
Editorial help was provided by John Forbes, and graphic services were provided by Jason Mathes, both from RTI Health Solutions (RTI-HS). Abenah Harding, from RTI-HS, provided helpful comments on a previous version of this manuscript.
Data Availability
The data used in this study are national register information made available under ethical permission. The original data sets from which the authors’ extraction was made are as follows: Swedish Medical Birth Register; Prescribed Drug Register; National Patient Register; Swedish Register of Education; and Total Population Register. The authors had no special privileges in accessing the data. Dissemination of personal information is regulated by the Swedish Secrecy Act and researchers wishing access to individual-level data will need to apply for permission through a Research Ethics Board (etikprovningsmyndigheten.se) and from the primary data owners Statistics Sweden (https://www.scb.se/en/services/guidance-for-researchers-and-universities) and the National Board of Health and Welfare (https://www.socialstyrelsen.se/en/statistics-and-data/statistics/), in accordance with Swedish law.
Funding Statement
ASO, CA and BMD received funds from the Swedish Research Council through the Swedish Initiative for Research on Microdata in the Social And Medical Sciences (SIMSAM) framework grant no. 340-2013-5867. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. For AVM, KJR, and EP: this project was internally funded. TM received no specific funding for this work.
References
- 1.Viale L, Allotey J, Cheong-See F, Arroyo-Manzano D, McCorry D, Bagary M, et al. Epilepsy in pregnancy and reproductive outcomes: a systematic review and meta-analysis. Lancet. 2015; 386 (10006): 1845–1852. 10.1016/S0140-6736(15)00045-8 [DOI] [PubMed] [Google Scholar]
- 2.Veroniki AA, Cogo E, Rios P, Straus SE, Finkelstein Y, Kealey R, et al. Comparative safety of anti-epileptic drugs during pregnancy: a systematic review and network meta-analysis of congenital malformations and prenatal outcomes. BMC Med. 2017; 15 (1): 95 10.1186/s12916-017-0845-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bromley RL, Weston J, Marson AG. Maternal Use of Antiepileptic Agents During Pregnancy and Major Congenital Malformations in Children. JAMA. 2017; 318 (17): 1700–1701. 10.1001/jama.2017.14485 [DOI] [PubMed] [Google Scholar]
- 4.Weston J, Bromley R, Jackson CF, Adab N, Clayton-Smith J, Greenhalgh J, et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database Syst Rev. 2016; 11: CD010224 10.1002/14651858.CD010224.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Perucca E, et al. Comparative risk of major congenital malformations with eight different antiepileptic drugs: a prospective cohort study of the EURAP registry. Lancet Neurol. 2018; 17 (6): 530–538. 10.1016/S1474-4422(18)30107-8 [DOI] [PubMed] [Google Scholar]
- 6.Koren G, Berkovitch M, Ornoy A. Dose-Dependent Teratology in Humans: Clinical Implications for Prevention. Paediatr Drugs. 2018; 20 (4): 331–335. 10.1007/s40272-018-0294-0 [DOI] [PubMed] [Google Scholar]
- 7.de Jong J, Garne E, de Jong-van den Berg LT, Wang H. The Risk of Specific Congenital Anomalies in Relation to Newer Antiepileptic Drugs: A Literature Review. Drugs Real World Outcomes. 2016; 3 (2): 131–143. 10.1007/s40801-016-0078-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerard EE, Meador KJ. An Update on Maternal Use of Antiepileptic Medications in Pregnancy and Neurodevelopment Outcomes. J Pediatr Genet. 2015; 4 (2): 94–110. 10.1055/s-0035-1556741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veroniki AA, Rios P, Cogo E, Straus SE, Finkelstein Y, Kealey R, et al. Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: a systematic review and network meta-analysis. BMJ Open. 2017; 7 (7): e017248 10.1136/bmjopen-2017-017248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bromley RL, Baker GA. Fetal antiepileptic drug exposure and cognitive outcomes. Seizure. 2017; 44: 225–231. 10.1016/j.seizure.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 11.Raguideau F, Zureik M, Dray-Spira R, Blotière P-O, Weill A, Coste J. Exposition in utero à l’acide valproïque et aux autres traitements de l’épilepsie et des troubles bipolaires et risque de malformations congénitales majeures (MCM) en France. France2017 [cited 2 August 2018]. https://ansm.sante.fr/var/ansm_site/storage/original/application/fb3faa8c4a5c5c5dedfc1423213c219d.pdf.
- 12.Margulis AV, Oberg AS, Hernandez-Diaz S. Antiepileptic drugs in pregnancy: searching for a reference drug for comparative safety. Pharmacoepidemiol Drug Saf. 2017; 26 (Suppl 2): 408–409. [Google Scholar]
- 13.Almgren M, Kallen B, Lavebratt C. Population-based study of antiepileptic drug exposure in utero—influence on head circumference in newborns. Seizure. 2009; 18 (10): 672–675. 10.1016/j.seizure.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 14.Artama M, Gissler M, Malm H, Ritvanen A. Effects of maternal epilepsy and antiepileptic drug use during pregnancy on perinatal health in offspring: nationwide, retrospective cohort study in Finland. Drug Saf. 2013; 36 (5): 359–369. 10.1007/s40264-013-0052-8 [DOI] [PubMed] [Google Scholar]
- 15.Battino D, Kaneko S, Andermann E, Avanzini G, Canevini MP, Canger R, et al. Intrauterine growth in the offspring of epileptic women: a prospective multicenter study. Epilepsy Res. 1999; 36 (1): 53–60. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez-Diaz S, McElrath TF, Pennell PB, Hauser WA, Yerby M, Holmes LB. Fetal growth and premature delivery in pregnant women on antiepileptic drugs. Ann Neurol. 2017; 82 (3): 457–465. 10.1002/ana.25031 [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Diaz S, Mittendorf R, Smith CR, Hauser WA, Yerby M, Holmes LB, et al. Association between topiramate and zonisamide use during pregnancy and low birth weight. Obstet Gynecol. 2014; 123 (1): 21–28. 10.1097/AOG.0000000000000018 [DOI] [PubMed] [Google Scholar]
- 18.Hiilesmaa VK, Teramo K, Granstrom ML, Bardy AH. Fetal head growth retardation associated with maternal antiepileptic drugs. Lancet. 1981; 2 (8239): 165–167. 10.1016/s0140-6736(81)90354-8 [DOI] [PubMed] [Google Scholar]
- 19.Hvas CL, Henriksen TB, Ostergaard JR, Dam M. Epilepsy and pregnancy: effect of antiepileptic drugs and lifestyle on birthweight. BJOG. 2000; 107 (7): 896–902. [DOI] [PubMed] [Google Scholar]
- 20.Kilic D, Pedersen H, Kjaersgaard MI, Parner ET, Vestergaard M, Sorensen MJ, et al. Birth outcomes after prenatal exposure to antiepileptic drugs—a population-based study. Epilepsia. 2014; 55 (11): 1714–1721. 10.1111/epi.12758 [DOI] [PubMed] [Google Scholar]
- 21.Kolstad E, Veiby G, Gilhus NE, Bjork M. Overweight in epilepsy as a risk factor for pregnancy and delivery complications. Epilepsia. 2016; 57 (11): 1849–1857. 10.1111/epi.13573 [DOI] [PubMed] [Google Scholar]
- 22.Pennell PB, Klein AM, Browning N, Baker GA, Clayton-Smith J, Kalayjian LA, et al. Differential effects of antiepileptic drugs on neonatal outcomes. Epilepsy Behav. 2012; 24 (4): 449–456. 10.1016/j.yebeh.2012.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Pregnancy, delivery, and outcome for the child in maternal epilepsy. Epilepsia. 2009; 50 (9): 2130–2139. 10.1111/j.1528-1167.2009.02147.x [DOI] [PubMed] [Google Scholar]
- 24.Veiby G, Daltveit AK, Engelsen BA, Gilhus NE. Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. J Neurol. 2014; 261 (3): 579–588. 10.1007/s00415-013-7239-x [DOI] [PubMed] [Google Scholar]
- 25.Wen X, Hartzema A, Delaney JA, Brumback B, Liu X, Egerman R, et al. Combining adverse pregnancy and perinatal outcomes for women exposed to antiepileptic drugs during pregnancy, using a latent trait model. BMC Pregnancy Childbirth. 2017; 17 (1): 10 10.1186/s12884-016-1190-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wide K, Winbladh B, Tomson T, Kallen B. Body dimensions of infants exposed to antiepileptic drugs in utero: observations spanning 25 years. Epilepsia. 2000; 41 (7): 854–861. [DOI] [PubMed] [Google Scholar]
- 27.Winterfeld U, Merlob P, Baud D, Rousson V, Panchaud A, Rothuizen LE, et al. Pregnancy outcome following maternal exposure to pregabalin may call for concern. Neurology. 2016; 86 (24): 2251–2257. 10.1212/WNL.0000000000002767 [DOI] [PubMed] [Google Scholar]
- 28.Pariente G, Leibson T, Shulman T, Adams-Webber T, Barzilay E, Nulman I. Erratum to: Pregnancy Outcomes Following In Utero Exposure to Lamotrigine: A Systematic Review and Meta-Analysis. CNS Drugs. 2017; 31 (6): 451 10.1007/s40263-017-0439-7 [DOI] [PubMed] [Google Scholar]
- 29.Pariente G, Leibson T, Shulman T, Adams-Webber T, Barzilay E, Nulman I. Pregnancy Outcomes Following In Utero Exposure to Lamotrigine: A Systematic Review and Meta-Analysis. CNS Drugs. 2017; 31 (6): 439–450. 10.1007/s40263-017-0433-0 [DOI] [PubMed] [Google Scholar]
- 30.Källén B, Källén K. The Swedish Medical Birth Register—a summary of content and quality. 2003 [cited 5 June 2016]. https://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/10655/2003-112-3_20031123.pdf.
- 31.WHO Collaborating Center for Drug Statistics Methodology. Defined daily dose, definition and general considerations 2009. http://www.whocc.no/ddd/definition_and_general_considera/. Cited 14 February 2016.
- 32.Abrahamsson K. Swedish Register of Education. 2018 [cited 22 July 2018]. http://www.jpi-dataproject.eu/Home/Database/348?topicId=4.
- 33.Ludvigsson JF, Almqvist C, Bonamy AK, Ljung R, Michaelsson K, Neovius M, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016; 31 (2): 125–136. 10.1007/s10654-016-0117-y [DOI] [PubMed] [Google Scholar]
- 34.EMA. Public summary of opinion on orphan designation. Carbamazepine for the treatment of metaphyseal chondrodysplasia, Schmid type. 2016 [cited 26 May 2019]. https://www.ema.europa.eu/en/documents/orphan-designation/eu/3/16/1746-public-summary-positive-opinion-orphan-designation-carbamazepine-treatment-metaphyseal_en.pdf.
- 35.European Commission. Public Health—Union Register of medicinal products—Procedures for nationally authorised products for human use. 2019 [cited 26 May 2019]. http://ec.europa.eu/health/documents/community-register/html/reg_hum_refer.htm.
- 36.EMA. Lamictal—Summary of product characteristics. 2017 [cited 26 May 2019]. https://www.ema.europa.eu/en/medicines/human/EPAR/lyrica.
- 37.EMA. Annex II—Scientific conclusions and grounds for amendment of the summaries of product characteristics, labelling and package leaflet presented by the EMEA. 2008 [cited 26 May]. https://www.ema.europa.eu/en/documents/referral/lamictal-article-30-referral-annex-i-ii-iii_en.pdf.
- 38.Hagenfeldt K, Alton V, Axelsson O, Blennow M, Bojö F, Bygdeman M, et al. Routine Ultrasound Examination During Pregnancy [in Swedish]. Stockholm, Sweden1998 [cited 22 July 2018]. https://www.sbu.se/en/publications/sbu-assesses/routine-ultrasound-examination-during-pregnancy/.
- 39.Niklasson A, Albertsson-Wikland K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr. 2008; 8: 8 10.1186/1471-2431-8-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr. 1996; 85 (7): 843–848. [DOI] [PubMed] [Google Scholar]
- 41.Hao L, Naiman DQ. Quantile Regression: SAGE Publications; 2007. 140 p. [Google Scholar]
- 42.McInerney KA, Hahn KA, Hatch EE, Mikkelsen EM, Steiner AZ, Rothman KJ, et al. Lubricant use during intercourse and time to pregnancy: a prospective cohort study. BJOG. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen MR, Deddens JA. Re: "Easy SAS calculations for risk or prevalence ratios and differences". Am J Epidemiol. 2006; 163 (12): 1158–1159; author reply 1159–1161. 10.1093/aje/kwj162 [DOI] [PubMed] [Google Scholar]
- 44.Petersen MR, Deddens JA. A comparison of two methods for estimating prevalence ratios. BMC Med Res Methodol. 2008; 8: 9 10.1186/1471-2288-8-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mostacci B, Poluzzi E, D’Alessandro R, Cocchi G, Tinuper P, Espea Study Group. Correction: Adverse pregnancy outcomes in women exposed to gabapentin and pregabalin: data from a population-based study. J Neurol Neurosurg Psychiatry. 2018; 89 (5): e1 10.1136/jnnp-2017-316143corr1 [DOI] [PubMed] [Google Scholar]
- 46.Mostacci B, Poluzzi E, D’Alessandro R, Cocchi G, Tinuper P, Espea Study Group. Adverse pregnancy outcomes in women exposed to gabapentin and pregabalin: data from a population-based study. J Neurol Neurosurg Psychiatry. 2018; 89 (2): 223–224. 10.1136/jnnp-2017-316143 [DOI] [PubMed] [Google Scholar]
- 47.Patorno E, Bateman BT, Huybrechts KF, MacDonald SC, Cohen JM, Desai RJ, et al. Pregabalin use early in pregnancy and the risk of major congenital malformations. Neurology. 2017; 88 (21): 2020–2025. 10.1212/WNL.0000000000003959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vajda FJE, O’Brien TJ, Graham JE, Hitchcock AA, Lander CM, Eadie MJ. Antiepileptic drug polytherapy in pregnant women with epilepsy. Acta Neurol Scand. 2018; 138 (2): 115–121. 10.1111/ane.12965 [DOI] [PubMed] [Google Scholar]
- 49.Jentink J, Loane MA, Dolk H, Barisic I, Garne E, Morris JK, et al. Valproic acid monotherapy in pregnancy and major congenital malformations. N Engl J Med. 2010; 362 (23): 2185–2193. 10.1056/NEJMoa0907328 [DOI] [PubMed] [Google Scholar]
- 50.Vajda FJ, O’Brien TJ, Graham JE, Lander CM, Eadie MJ. Dose dependence of fetal malformations associated with valproate. Neurology. 2013; 81 (11): 999–1003. 10.1212/WNL.0b013e3182a43e81 [DOI] [PubMed] [Google Scholar]
- 51.Foch C, Araujo M, Weckel A, Damase-Michel C, Montastruc JL, Benevent J, et al. In utero drug exposure and hearing impairment in 2-year-old children A case-control study using the EFEMERIS database. Int J Pediatr Otorhinolaryngol. 2018; 113: 192–197. 10.1016/j.ijporl.2018.07.035 [DOI] [PubMed] [Google Scholar]
- 52.Elkjaer LS, Bech BH, Sun Y, Laursen TM, Christensen J. Association Between Prenatal Valproate Exposure and Performance on Standardized Language and Mathematics Tests in School-aged Children. JAMA Neurol. 2018; 75 (6): 663–671. 10.1001/jamaneurol.2017.5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pharmacovigilance Risk Assessment Committee. Procedure under Article 31 of Directive 2001/83/EC resulting from pharmacovigilance data: substances related to valproate. 2014 [cited 18 November 2018]. https://www.ema.europa.eu/documents/referral/valproate-related-substances-article-31-referral-prac-assessment-report_en.pdf.
- 54.Shorvon SD. Drug treatment of epilepsy in the century of the ILAE: the second 50 years, 1959–2009. Epilepsia. 2009; 50 Suppl 3: 93–130. [DOI] [PubMed] [Google Scholar]
- 55.Raguideau F, Ehrhardt C, Dray-Spira R, Zureik M, Blotière P-O, Weill A, et al. Exposition à l’acide valproïque et ses dérivés au cours de la grossesse en France de 2007 à 2014: une étude observationnelle sur les données du SNIIRAM. France2016 [cited 11 August 2018]. https://ansm.sante.fr/afssaps/content/download/91481/1148883/version/1/file/Rapport_EtudeVPA_24.08-def.pdf.
- 56.EMA. New measures to avoid valproate exposure in pregnancy endorsed. 2018 [cited 11 August 2018]. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Valproate_2017_31/European_Commission_final_decision/WC500250216.pdf.
- 57.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011; 11: 450 10.1186/1471-2458-11-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bruckner TA, Karasek D, Yang W, Shaw GM, Catalano RA. Cohort Variation in Selection During Pregnancy and Risk of Selected Birth Defects Among Males. Epidemiology. 2017; 28 (4): 580–586. 10.1097/EDE.0000000000000661 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The data used in this study are national register information made available under ethical permission. The original data sets from which the authors’ extraction was made are as follows: Swedish Medical Birth Register; Prescribed Drug Register; National Patient Register; Swedish Register of Education; and Total Population Register. The authors had no special privileges in accessing the data. Dissemination of personal information is regulated by the Swedish Secrecy Act and researchers wishing access to individual-level data will need to apply for permission through a Research Ethics Board (etikprovningsmyndigheten.se) and from the primary data owners Statistics Sweden (https://www.scb.se/en/services/guidance-for-researchers-and-universities) and the National Board of Health and Welfare (https://www.socialstyrelsen.se/en/statistics-and-data/statistics/), in accordance with Swedish law.