Abstract
Background
We investigated the correlation between pancreatic ductal adenocarcinoma patient prognosis and the presence of tumour infiltrating lymphocytes and expression of 521 immune system genes.
Methods
Intratumoural CD3+, CD8+, and CD20+ lymphocytes were examined by immunohistochemistry in 12 PDAC patients with different outcomes who underwent pancreaticoduodenectomy. The results were correlated with gene expression profile using the digital multiplexed NanoString nCounter analysis system (NanoString Technologies, Seattle, WA, USA).
Results
Twenty immune system genes were significantly differentially expressed in patients with a good prognosis relative to patients with a worse prognosis: TLR2 and TLR7 (Toll-like receptor superfamily); CD4, CD37, FOXP3, PTPRC (B cell and T cell signalling); IRF5, IRF8, STAT1, TFE3 (transcription factors); ANP32B, CCND3 (cell cycle); BTK (B cell development); TNF, TNFRF1A (TNF superfamily); HCK (leukocyte function); C1QA (complement system); BAX, PNMA1 (apoptosis); IKBKE (NFκB pathway). Differential expression was more than twice log 2 for TLR7, TNF, C1QA, FOXP3, and CD37.
Discussion
Tumour infiltrating lymphocytes were present at higher levels in samples from patients with better prognosis. Our findings indicate that tumour infiltrating lymphocyte levels and expression level of the immune system genes listed above influence pancreatic ductal adenocarcinoma prognosis. This information could be used to improve selection of best responders to immune inhibitors.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a lethal disease often with a poor prognosis [1]. PDAC is characterized by a desmoplastic, highly heterogeneous and immune-suppressive microenvironment that hinders antitumour immunity. PDACs tend to recruit immunosuppressive cells including myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs) and tumour-associated macrophages (TAMs). PDACs also inhibit immune effector cells, mainly CD4+ CD8+ T lymphocytes, natural killer (NK) cells and dendritic cells (DCs) [2]. Some PDACs, moreover, overexpress programmed death ligand 1 (PDL1) and secrete inhibitory cytokines such as interleukin 10 (IL10) and tumour growth factor β (TGFβ) [3]. Desmoplasia, another hallmark of PDAC, is characterized by a noticeable proliferation of myofibroblasts and generates a strong barrier against tumour infiltration by both immune cells and drugs [4].
The correlation between tumour infiltrating lymphocytes (TILs) and clinical outcomes has been investigated in several studies involving PDAC patients. Fukunaga et al found that patients with CD8+ tumours had a higher five-year overall survival (OS) rate than patients with CD8- tumours, and similarly for patients with CD4+ tumours versus CD4- tumours [5]. Evaluating the prognostic value of CD3+, CD8+, and CD20+ TILs, Tewary et al found an association between CD3+ and CD20+ TILs and a higher survival rate [6]. Furthermore, Ino et al found that patients with high CD8+ and CD4+ TIL and low Treg counts had a better survival rate than patients with low CD8+ and CD4+ TIL and high Treg counts [7]. Patients with CD4+ tumours were found to have OS and disease-free survival (DFS) higher than patients with CD4- tumours, and a high CD8+/FoxP3+ lymphocyte ratio correlated with better clinical outcomes, but no significant correlation was found between CD8+ TILs and survival or other clinical-pathological features [8]. Karakhanova et al found that CD4+ and CD8+ count correlated with higher OS and DFS [9].
The immune system interacts intimately with tumours over the entire process of disease development. This complex immune system-tumour cross-talk can both inhibit and enhance tumour growth [10]. Major features of the process by which tumours escape the immune system include a reduction in the recognition of cancer antigens by immune cells and the development of an immune suppressive microenvironment. The tumour microenvironment of PDAC has been consistently reported to be capable of promoting immune escape, rendering the immune system ineffective in eliciting an anti-tumour response. Improved understanding of the tumour microenvironment and how it contributes to immune evasion could, therefore, lead to better treatments and outcomes for PDAC patients [11]. This motivated our investigation of differences in TILs and immune-related gene expression between PDAC patients with good and worse clinical outcomes.
Materials & methods
Patients and sample collection
Fresh PDAC specimens were obtained from patients (n = 12) undergoing surgical resection at the Department of Medical, Surgical & Health Sciences, Cattinara Teaching Hospital, Trieste University, between 2005 and 2015. These experiments were approved by Trieste University Institutional Review Board. Tissue specimens were snap frozen in liquid nitrogen and stored at −80°C.
Formalin-fixed, paraffin wax-embedded sections were used for immunohistochemical staining. All 12 paraffin wax blocks were confirmed to contain tumour tissue by two pathologists, comprising six pancreatic adenocarcinomas with a good prognosis and six pancreatic adenocarcinomas with a bad prognosis.
The following clinical data were collected: patient age, gender, and outcome; the presence/absence of metastasis; tumour location, size, margin status, TNM stage, degree of differentiation, invasion degree and location (lymph node, bile duct/duodenal serosa, hepatic, portal vein, vascular, perineural), schedule of chemotherapy, neoadjuvant and/or adjuvant chemotherapy, chemotherapy toxicity, and treatment follow up. Clinical Stadiation of patients ranged from stage IA to III but none of them was metastatic and none of them underwent chemotherapy. Patients were informed about the project and gave written consent for study participation. Of note, a caveat to the study is that sample was relatively scarce because of the intrinsically hard-to-reach nature of the disease plus the requirement to put aside part of every sample for storage at the institution as per legal and ethical regulations.
Follow up
OS was measured from the time of surgery to the time of death or the last follow up visit. Dates of death were obtained from patient hospital records or follow up telephone calls. A more in-depth analysis of the 12 patients revealed two groups with different DFS and/or OS: six patients with an OS between 25 and 66 months were classified as “good cases”, while six with OS between 2 and 9 months or DFS between 1 and 2 months were classified as “worse cases”. Table 1 summarizes the clinical-pathological data of the two groups of patients and Fig 1 shows the Kaplan-Meier curves for DFS and OS for the two groups of patients.
Table 1. Summary of patient clinical-pathological information.
ECOG: Eastern Cooperative Oncology Group (Performance Status); TNM Stadiation: T = Tumour (0–4), N = Nodes (0–2), M = Metastasized (0–1).
| Good cases | Worse cases | |
|---|---|---|
| Num. Pts | 6 | 6 |
| Age | 65.3 | 63.8 |
| Gender | ||
| Male | 4 | 3 |
| Female | 2 | 3 |
| ECOG | ||
| 0 | 2 | 0 |
| 1 | 2 | 3 |
| 2 | 1 | 2 |
| Not reported | 1 | 1 |
| TNM | ||
| T | ||
| T0 | 0 | 0 |
| T1 | 1 | 2 |
| T2 | 0 | 4 |
| T3 | 4 | 0 |
| T4 | 1 | 0 |
| N | ||
| N0 | 2 | 2 |
| N1 | 4 | 4 |
| M | ||
| M0 | 6 | 6 |
| STAGE | IA/IIA/IIB/III | IB/IIA/IIB |
Fig 1. Worse and good prognosis PDAC patient groups.
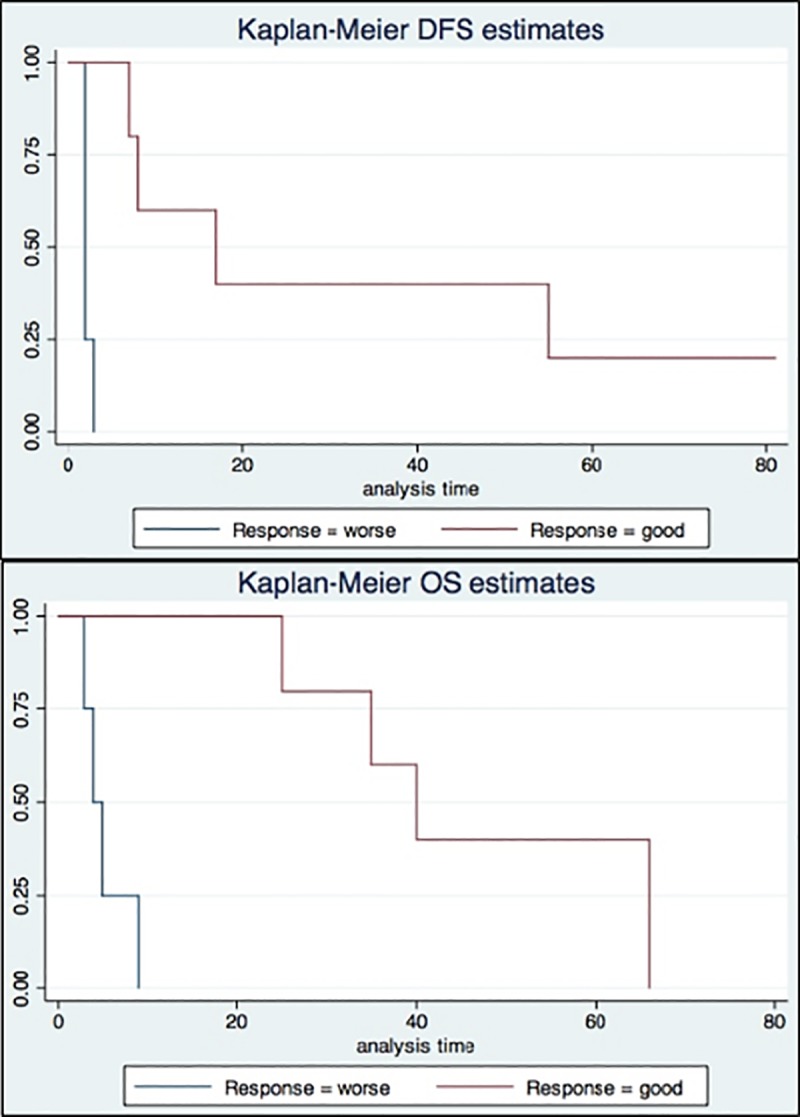
Kaplan-Meier curves show the difference of OS or DFS between two groups of patients: one group with a worse prognosis (“worse”, blue line) and the other with a better prognosis (“good”, red line).
PanCancer immune profile panel multiplex gene expression profiling
Three patients were randomly selected from each of the good and worse prognosis groups. In accord with Koti et al [12], pathologists in our department selected and extracted two areas of at least 70% of cellularity from each formalin-fixed, paraffin wax-embedded tumour tissues, and total RNA was extracted using Qiagen RNeasy (Qiagen Inc., Toronto, ON, Canada) as per the manufacturer’s instructions. A NanoDrop ND-100 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) was used to measure RNA concentration and purity. All RNA samples included in the study passed the quality control requirements (as verified by RNA integrity number or OD 260/280 ratio) of the platform. Using 100 ng total RNA from each sample as input, according to the manufacturer’s instructions, the digital multiplexed NanoString nCounter analysis system (NanoString Technologies, Seattle, WA, USA) was used for gene expression profiling. Tumour RNA samples were analysed using nCounter PanCancer immune profile panel consisting of 770 human immune-related genes (Nanostring Technologies).
In this assay, colour-coded barcodes are used to represent single-target transcripts in the reaction. An overnight hybridisation reaction was used to incorporate the resulting material, carried out by combining 20 ml of nCounter Reporter probes in hybridisation buffer, 5 ml of nCounter Capture probes and 5 ml of the total RNA sample for a total reaction volume of 30 ml. The hybridisations were incubated at 65°C for 16–20 h. An excess of probes is provided during overnight hybridization to ensure that each target finds a probe pair. Target abundance values can then be determined through the nCounter Digital Analyzer by counting the individual fluorescent barcodes. A high-density scan was performed for each assay (encompassing 600 fields of view). After hybridisation, the cartridges were analysed in the Digital Analyzer that counts (representing the number of molecules) and arranges the barcodes.
Immunohistochemistry
An automatic stainer (BenchMark ULTRA, Ventana Medical System, Inc.) was used for the immunohistochemical test. The antigen was retrieved with cell conditioning buffer 1. Next, endogenous peroxidase was inhibited with H2O2 at 3% (Bioptica) for 10 min. Samples were incubated with primary antibody anti-CD3 (2GV6) (Roche-Ventana), anti-CD8 (SP57) (Roche-Ventana), Rabbit Monoclonal Pre-diluted (0.4μg/mL), for 20 min at 36°C; anti-CD20 (L26) (Roche-Ventana), Mouse Monoclonal Pre-Diluted (0.4μg/mL) for 24 min at 36°C. The antibody was exposed with ultraView Universal DAB Detection Kit (Cat No. 760–500). As counterstain, Mayer haematoxylin was used for 4 min.
TIL levels were assessed by two investigators blind to the patients’ clinical-pathological data using the standardized method coded in 2015 by the International TILs Working Group [13]. TILs were investigated per microscopic field (5X and 10X) and an average over ten independent regions having the most abundant immunoreactive cells was calculated for each slide.
Immunohistochemistry statistical analysis
For immunohistochemistry statistical analysis, a preliminary data exploration was performed. Numerical variables were expressed as median and range and were compared by non-parametric tests (Mann–Whitney U-test). Qualitative data were expressed as frequencies and organized into contingency tables; the association between categorical variables was investigated by means of Fisher’s exact test or Pearson’s Chi-square. Time-dependent variables were calculated according to the Kaplan–Meier method. For the entire statistical analysis, the significance levels were established at p<0.05. All data were analysed with STATA software.
Immune profile panel multiplex nanoString statistical analyses
NanoString data analysis
nSolver (NanoString Technologies) was used for the normalization of raw data as previously reported [14]. The raw NanoString counts were initially subjected to normalization for all target RNAs in all samples based on built-in positive controls. This step accounts for post-hybridization processing, inter-sample and experimental variation such as hybridization efficiency. The geometric mean of each of the controls was calculated, indicating the overall assay efficiency. For the mRNA content normalisation, housekeeping genes were then used. To facilitate downstream statistical analysis, values < 0 were blanketed and considered equal to 1. After initial normalisation steps, data were imported to GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA) to be processed for statistical analysis. Multiple t-tests were performed with correction for multiple comparisons through the Sidak–Bonferroni method. A difference in expression with a P-value of ≤ 0.05 was considered statistically significant.
Results
Immune-related gene expression analysis in pancreatic adenocarcinomas with good versus worse prognosis
Prognosis of primary PDAC patients was determined using clinical data and Kaplan-Meier curves (Fig 1). Three primary PDAC patients with a good prognosis and three with a worse prognosis were then chosen for mRNA analysis by PanCancer Immune Profile Panel multiplex gene expression analysis. Fig 2 shows the differential gene expression. Among the immune system genes showing statistically significant (p <0.01) differential expression between pancreatic adenocarcinoma with a good and worse prognosis, differential expression of TLR7, TNF, C1QA, FOXP3, and CD37 was at least twice log 2: +2.76 log 2 ± 0.58 (p <0.00896), +2.39 log 2 ± 0.389 (p <0.00356), +2.19 log 2 ± 0.43 (p <0.00697), +2.07 log 2 ± 0.372 (p <0.00513), and +2 log 2 ± 0.297 (p <0.00254), respectively (S1 Fig). BTK (+1.91 log 2 ± 0.309 (p<0.0035)), CD4 (+1.86 log 2 ± 0.235 (p<0.00138)), HCK (+1.86 log 2 ± 0.304 (p<0.00364)), PTPRC (+1.83 log 2 ± 0.259 (p<0.00211)), CCND3 (+1.67 log 2 ± 0.337 (p<0.00777)), STAT1 (+1.59 log 2 ± 0.238 (p<0.00626)), IKBKE (+1.51 log 2 ± 0.282 (p< 0.00585)), IRF8 (+1.43 log 2 ± 0.246 (p<0.00439)), TNFRF1A (+1.39 log 2 ± 0.298 (p<0.00954)), TLR2 (+1.34 log 2 ± 0.147 (p<0.000799)), BAX (+1.31 log 2 ± 0.246 (p<0.00598)), IRF5 (+1.27 log 2 ± 0.193 (p<0.00272)), PNMA1 (+0.986 log 2 ± 0.201 (p<0.00799)), ANP32B (+0.92 log 2 ± 0.163 (p<0.00484)), TFE3 (-0.37 log 2 ± 0.0783 (p<0.00919)), and mRNA also showed statistically significant (p <0.01), but less than twice log 2, differential expression between pancreatic adenocarcinomas with good and worse prognosis (Table 2).
Fig 2. Differential gene expression between “good prognosis cases” and “worse prognosis cases” of PDAC.
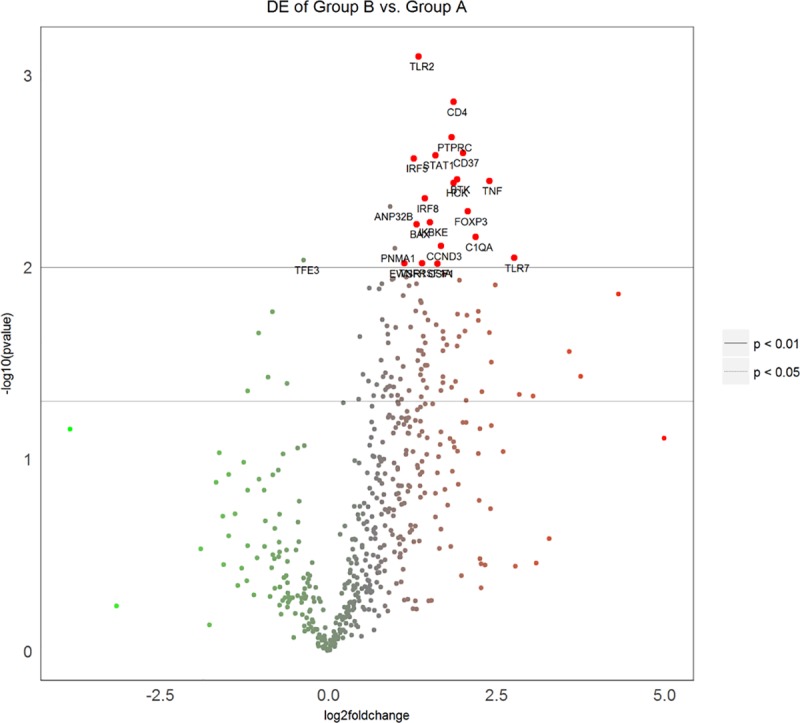
Volcano plot in which the larger red dots represent only those genes whose expression is at least twice log2 higher in “good cases” (Group B) relative to “worse cases” (Group A) with p-value < 0.01. Genes with highly statistically significant differential expression fall at the top of the plot above the horizontal lines, and highly differentially expressed genes fall to either side depending on whether they are negatively or positively differentially expressed. Horizontal lines indicate various False Discovery Rate (FDR) thresholds or p-value thresholds if there is no adjustment to the p-values. Genes are red if the resulting p-value is below the given FDR or p-value threshold. The 20 genes showing the most statistically significant differential expression are labelled in the plot.
Table 2. Top 20 genes differentially expressed between “good cases” and “worse cases”.
| Genes | Differential expression between “good cases” and “worse cases” (log2 fold change) | Std error | P-Value |
|---|---|---|---|
| TLR7-mRNA | 2.76 | 0.58 | 0.00896 |
| TNF-mRNA | 2.39 | 0.389 | 0.00356 |
| C1QA-mRNA | 2.19 | 0.43 | 0.00697 |
| FOXP3-mRNA | 2.07 | 0.372 | 0.00513 |
| CD37-mRNA | 2 | 0.297 | 0.00254 |
| BTK-mRNA | 1.91 | 0.309 | 0.0035 |
| CD4-mRNA | 1.86 | 0.235 | 0.00138 |
| HCK-mRNA | 1.86 | 0.304 | 0.00364 |
| PTPRC-mRNA | 1.83 | 0.259 | 0.00211 |
| CCND3-mRNA | 1.67 | 0.337 | 0.00777 |
| STAT1-mRNA | 1.59 | 0.238 | 0.00262 |
| IKBKE-mRNA | 1.51 | 0.282 | 0.00585 |
| IRF8-mRNA | 1.43 | 0.246 | 0.00439 |
| TNFRSF1A-mRNA | 1.39 | 0.298 | 0.00954 |
| TLR2-mRNA | 1.34 | 0.147 | 0.000799 |
| BAX-mRNA | 1.31 | 0.246 | 0.00598 |
| IRF5-mRNA | 1.27 | 0.193 | 0.00272 |
| PNMA1-mRNA | 0.986 | 0.201 | 0.00799 |
| ANP32B-mRNA | 0.92 | 0.163 | 0.00484 |
| TFE3-mRNA | -0.37 | 0.0783 | 0.00919 |
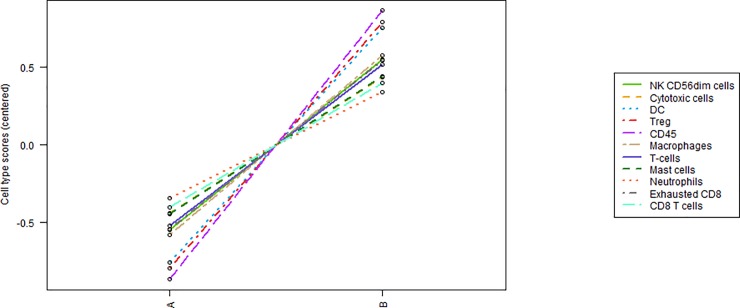
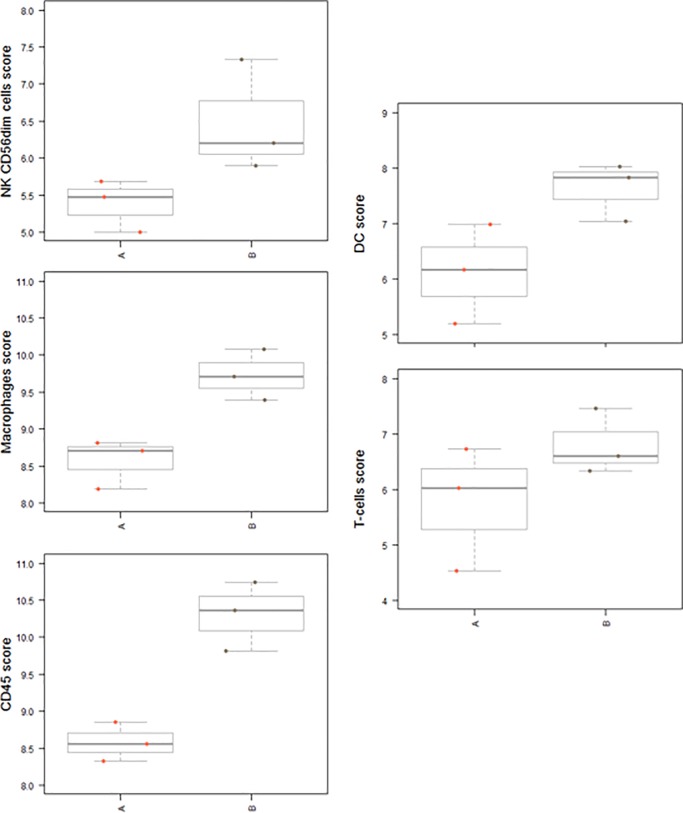
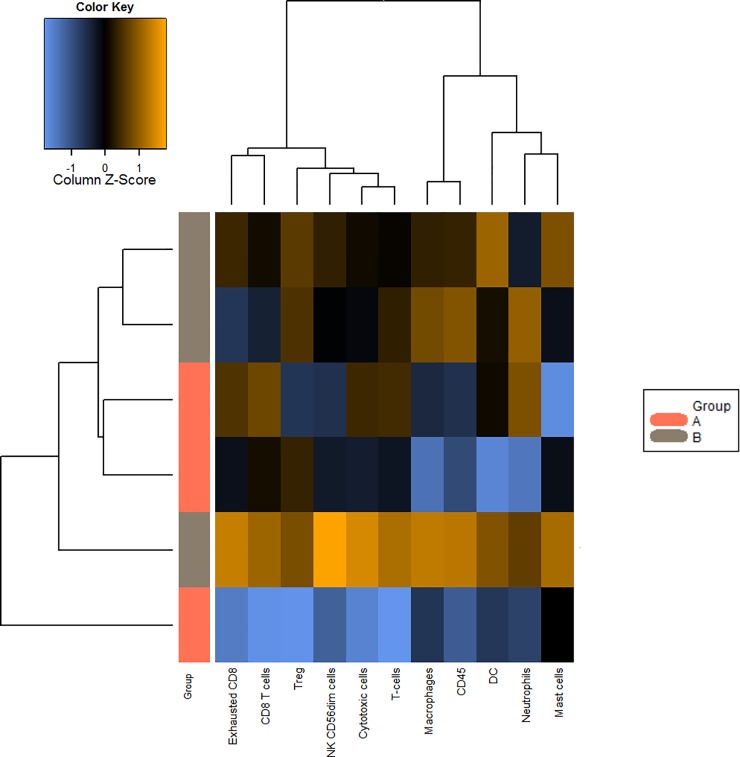
Gene expression analysis indicated that the pancreatic adenocarcinoma group with a good prognosis showed higher levels of the following cell types compared to the group with a worse prognosis (Figs 3–5): CD45-expressing cells, Tregs, DCs, macrophages, NK CD56dim cells, T cells, exhausted CD8+ cells, cytotoxic cells, mast cells, CD8+ T cells and neutrophils (Figs 3 and 4). Box plot representations indicate that the following subtypes of cells exhibit particularly different levels: CD45-expressing cells, dendritic cells, macrophages, natural killer cells, the family of T cells (Fig 5), and exhausted CD8+ and Treg cells (S1–S3 Figs).
Fig 3. Tumour immune cell profiling by immunohistochemical analysis.
Trend plot summarizing the change in abundance of cell types from “worse cases” (A) to “good cases” (B).
Fig 5. Box plots.
Box plots of measurements of CD45-expressing cells, dendritic cells, macrophages, NK, and T cells, in “good cases” (B) and “worse cases” (A).
Fig 4. Heat map showing the levels of the different cell types in “worse cases” (pink) and “good cases” (grey).
Yellow-orange indicates high abundance, blue indicates low abundance.
Tumour immune cell profiling in pancreatic adenocarcinomas with good versus worse prognosis
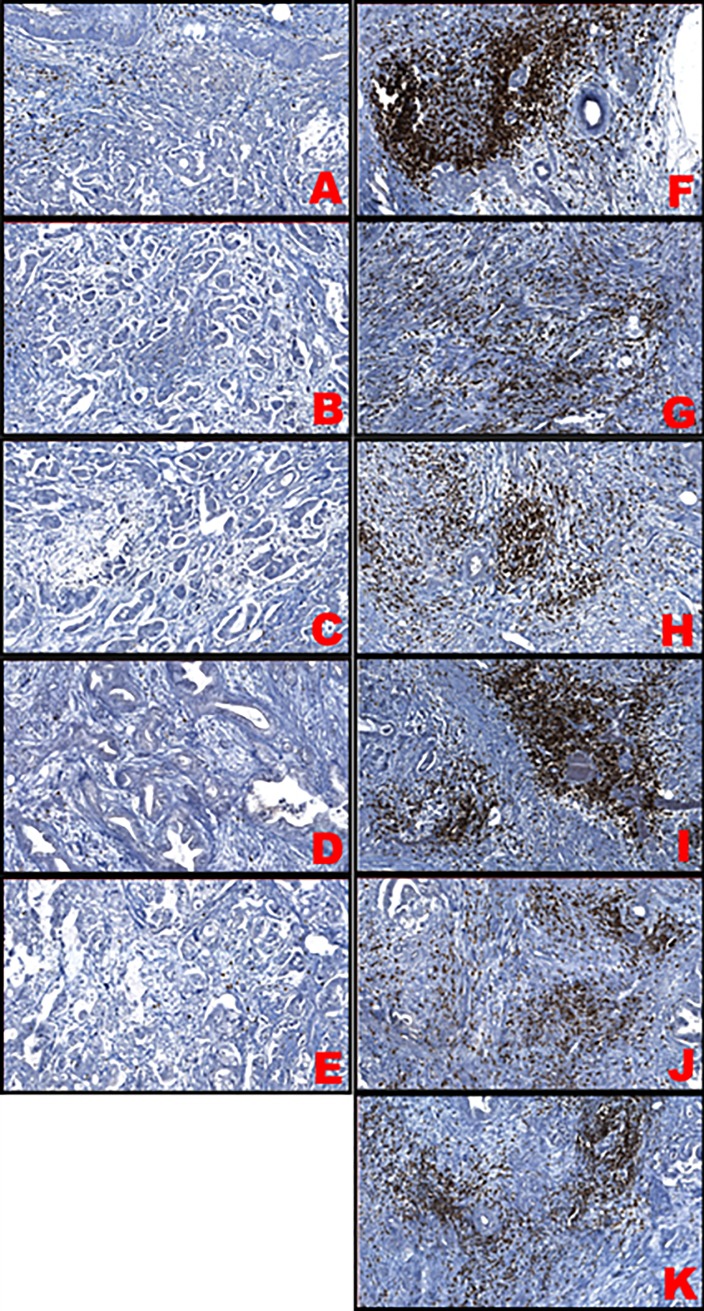
In agreement with existing data [5–9], tumour immune cell marker levels were higher in good prognosis cases compared to worse prognosis cases (Table 3). The CD3 level was statistically higher in the good prognosis group compared to the worse prognosis group (p = 0.0267, Table 4), (Fig 6). Despite the fact that the number of CD8+ and CD20+ cells has been found to be higher in patients with good prognosis in our study, no statistically significant difference was found between the two subgroups of immune cells (p = 0.119 and p = 0.925, respectively) (Table 4), (Fig 6). TIL marker levels were not assessed in one case due to calcification in pancreatic ductal tissue. Fig 7 shows the detection of TILs in the two sets of six PDAC patients. Staining for TILs was visually negative in the adenocarcinomas with a worse prognosis while it was positive for the adenocarcinomas with a good prognosis.
Table 3. Levels of CD3, CD8 and CD20 TIL subpopulations.
Data are reported as a percentage value of stroma TILs and are related to the sub-group assessed (CD3, CD8, CD20). For every antigen, two columns of values are reported, each corresponding to a different pathologist’s evaluation. The final score has been given in a semi-quantitative way by evaluating cell density in 10 digital images (20x enlargement) and calculating the average of all scores. The scores have been given following the guidelines of the “International TILs Working Group 2014”.
| Sample number | CD3 | CD8 | CD20 | ||||
|---|---|---|---|---|---|---|---|
| Worse cases | 1 | 6 | 7 | 5 | 9 | 1 | 2 |
| 2 | Not performed | ||||||
| 3 | 13 | 11 | 10 | 6 | 2 | 3 | |
| 4 | 9 | 8 | 13 | 13 | 1 | 2 | |
| 5 | 9 | 4 | 3 | 4 | 2 | 3 | |
| 6 | 9 | 5 | 1 | 3 | 0 | 0 | |
| Good cases | 1 | 10 | 8 | 10 | 9 | 2 | 3 |
| 2 | 10 | 8 | 5 | 3 | 0 | 1 | |
| 3 | 23 | 16 | 6 | 12 | 0 | 0 | |
| 4 | 10 | 8 | 10 | 8 | 0 | 1 | |
| 5 | 27 | 16 | 15 | 13 | 5 | 4 | |
| 6 | 16 | 15 | 15 | 11 | 2 | 3 | |
Table 4. Statistical difference of TIL levels between the two groups.
The table summarizes the statistical difference of TIL levels between the “worse case” and “good case” groups (non-parametric Mann Whitney test or “U-test”).
| Median “worse cases” | Median “good cases” | p-value | |||
|---|---|---|---|---|---|
| N. pts | N. pts | ||||
| CD3 | 5 | 7 (6.5–12) | 6 | 12.25 (9–35) | 0.0267 |
| CD8 | 5 | 7 (2–13) | 6 | 9.25 (4–14) | 0.119 |
| CD20 | 5 | 1.5 (0–2.5) | 6 | 1.5 (0–4.5) | 0.925 |
Fig 6. Levels of TIL markers in “worse cases” (Worse) and “good cases” (Good) of pancreatic adenocarcinoma.
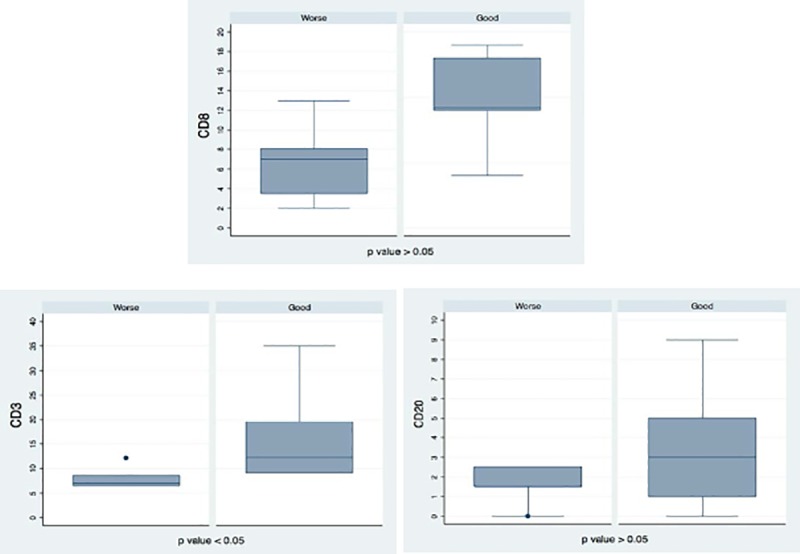
The box plots represent the expression levels of CD3, CD8 and CD20 TIL subpopulations comparing the “worse case” and “good case” groups. The lower table summarizes the statistical difference of TIL levels between the “worse case” and “good case” groups (non-parametric Mann Whitney test or “U-test”).
Fig 7. TILs stained for CD3 expression across the two groups of patients.
Samples A-E were collected from “worse cases”, samples F-K from “good cases”.
Discussion
Immune cells within the cancer infiltrate may have a role in fighting cancer growth via antigen restricted tumouricidal responses or they may promote tumour progression by suppressing the immune system [15]. There are three major barriers impeding immune therapy in PDAC: 1. The mutational load in PDAC is much lower than that of lung cancers and melanoma; 2. PDAC has a strong immunosuppressive microenvironment which is composed of a dense desmoplastic reaction having remarkable infiltration of tumourigenic MDSCs and macrophages [16]; 3. The PDAC microenvironment has a very low number of infiltrating T cells, insufficient to provide a significant T cell response.
In the current study, PDAC samples from patients with a good prognosis had higher levels of TILs compared to a group of patients with a worse prognosis, as assessed via immune marker levels. Even though the patient numbers are small and the selection of good prognosis or worse prognosis somewhat arbitrary (based on clinical data and Kaplan-Meier curves) (Fig 1), the correlation is consistent with previous reports suggesting that TIL levels provide a robust predictor of outcome in pancreatic cancer [8,17]. Consistent with data reported by Stromness et al, we point out that in some samples of the “Good” prognosis group, CD3+ cells tend to organise in tertiary lymphoid structures (TLS) within tumour stroma [18]. Although there is limited knowledge of TLS, these formations are commonly found in solid tumour with a better prognosis, suggesting their possible role in T cell regulation of in-situ immune response[18]. Furthermore, our study revealed a significant (p-value <0.001) differential expression of 20 immune system genes between PDAC patients with good and worse prognoses. Among these genes, the expression of five (TLR7, TNF, C1QA, FOXP3, CD37) was at least twice log 2 higher in the good prognosis group relative to the worse prognosis group. Expression levels of these five genes could constitute a molecular signature of likely outcome and could therefore be useful for clinical applications.
FOXP3 is a well known marker of Tregs, with a pivotal role in the development and differentiation of these cells to promote tumour immune escape [19,20]. Conversely, FOXP3 has been reported to be an important tumour suppressor gene in breast cancer [21–25], gastric adenocarcinoma [26,27], prostate cancer [28], and non-small cell lung cancer [29]. These findings indicate that the roles of FOXP3 in tumours are diverse and situation-dependent.
C1QA encodes the A-chain polypeptide of complement subcomponent C1q and plays an important role in counteracting tumour cells [30,31]. Teschendorff and Caldas et al showed that overexpression of C1QA in ER-negative basal-like breast cancer patients is associated with better prognosis [32]. It was shown more recently that lower C1QA expression could be linked with worse outcomes in patients with ER-negative breast cancer [33]. Nonetheless, Bulla et al recently showed that C1q can exert functions unrelated to complement activation, contributing to extracellular changes within the tumour microenvironment and supporting tumour growth and invasion [34]. This last finding is supported by Winslow et al [35].
TNF has long been considered a key regulator of the inflammatory and immune response to cancer, promoting either death or survival under different circumstances [36]. Although several anti-TNF therapies have been developed with different binding and pharmacokinetic profiles [37], TNF is used in current therapies to fight cancer, notwithstanding its toxicity [38]. TNF has proved to have an effect on metastatic melanoma treatment and unresectable soft tissue therapies [39,40]. There is evidence of TNF’s role in promoting regression of unresectable hepatic metastasis from colorectal cancer [41] and in causing tumour necrosis via its pro-coagulant effect [42].
TLR7 is of special interest in cancer therapy on account of its strong stimulation of IL-12 and type-I interferons, which are important cytokines and effectors of T and NK cell functions [43,44]. TLR7 ligands can not only activate directly NK cells and cytotoxic T-cells [45,46], but also hamper the suppressive function of myeloid-derived suppressor cells [47,48] and interfere with the migration of Tregs into the tumour [49]. TLR agonists are clinically approved or under clinical evaluation for cancer immunotherapy [50–52].
CD37 belongs to the tetraspanin superfamily of transmembrane proteins that regulate protein adhesion, trafficking, and migration and that are emerging controllers of both humoral and immune control, especially stimulating dendritic cell migration and B cell survival [53–55]. The contribution of CD37 to antitumour immunity has been known since the finding that CD37-/- mice have impaired antitumour responses [56]; however, the role of CD37 in the tumour microenvironment is not clear and further investigations are needed. Tetraspanins in the tumour microenvironment may have therapeutic potential via stimulation or inhibition of immune cell functions, depending on the immune cell type [57].
There are numerous biomarkers that have proved to be clinically useful for other cancers such as lung cancer [58], colorectal cancer [59], breast cancer [60] and melanoma [61], but clinical application of biomarkers for PDAC has been somewhat limited. Indeed, the only FDA-approved PDAC marker, the serum protein CA 19–9, was approved in the 1980s [62]. At least 10% of patients do not express CA 19–9, however, and its level is easily affected by metabolic abnormalities. Several studies have identified biomarkers that could be used as predictors of clinical outcome for PDAC [63–66], but none of these involves the immune-related gene signature revealed here.
The key findings from this study, that longer-surviving PDAC patients had higher levels of intratumoural TILs and overexpressed five immune markers (TLR7, TNF, C1QA, FOXP3, CD37), could have two main uses. Firstly, TIL levels and marker gene panel expression could be used for clinical outcome prediction, stratification and treatment design for PDAC patients. A previous study showed that a signature comprising another 15 genes was an independent prognostic factor in two cohorts of PDAC patients. In contrast to our results, higher expression of these 15 genes was associated with poor OS [63]. Similarly, Sergeant et al identified high co-expression of TGF-β1 and a panel of cell motility genes as independent predictors of worse clinical outcome [64], while Van den Broek et al discovered that high expression of ABCB1 and CXCR4 correlated with worse clinical outcome [65]. Furthermore, decreased levels of DPEP1 and increased expression of TPX2 were independently associated with poor survival [66]. Presumably, a wide panel of validated gene signatures would be most useful for outcome prediction, stratification and therapeutic decision making.
Secondly, our findings could be useful in developing new PDAC treatments, for example in combination with current immunotherapeutic strategies. Expression of the target genes identified here could be induced together with therapies modulating the tumour microenvironment to relieve immunosuppression, and/or approaches to break down the desmoplastic barrier surrounding PDAC to facilitate target access for infiltrating T cells or therapeutic molecules [15]. Such strategies could be effected in combination with recently reported gene therapy and oncologic vaccination approaches [67–69].
In summary, our data indicate that a gene signature comprising at least TLR7, TNF, C1QA, FOXP3, and CD37 could be useful to improve the prediction of OS in PDAC patients. Together with an assessment of TIL levels, such an immune system gene panel constitutes a potential prognostic tool to permit a risk-based stratification of pancreatic tumour patients into personalized treatment protocols towards improving the current abysmal clinical outcome of these patients.
Future perspective
The findings in the paper might be useful in stratifying patients and indicating the best treatment available for pancreatic cancer patients. In addition, our study could pave the way for finding novel targets for the development of new drugs for this disease.
Supporting information
Volcano plot displaying each gene's -log10(p-value) against log2 fold change: a) TLR7, b) TNF, c) C1QA, d) FOXP3 and e) CD37. Highly statistically significant genes fall at the top of the plot, and highly differentially expressed genes fall to either side. Genes within the selected gene set are highlighted in orange. Horizontal lines indicate various False Discovery Rate (FDR) thresholds.
(TIFF)
Box plots show levels of exhausted CD8+ cells (a) and Tregs (b) in patients with worse prognosis (group A) and patients with worse prognosis (group B). Even though scores seem overlapping, the average score for both sets of cells is higher in group B than in group A, probably due to the fact that longer-surviving PDAC patients had higher levels of intratumoral TILs.
(TIFF)
The diagram shows the abundance of exhausted CD8+ cells and Tregs compared to levels of CD8+ cells. In agreement with the previous figure, levels of exhausted CD8+ cells (green line) and Tregs (dashed orange line) are reported to be lower in the group with a worse prognosis (group A) than in the group with a better prognosis (group B) when compared with the total level of CD8+ cells.
(TIFF)
(PDF)
Data Availability
The fully comprehensive gene expression data generated by the Nanostring assay have been deposited in the NCBI Gene Expression Omnibus (GEO) and are accessible via GEO accession number GSE132946. All other relevant data are within the manuscript and Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018. September; [DOI] [PubMed] [Google Scholar]
- 2.Protti MP, De Monte L. Immune infiltrates as predictive markers of survival in pancreatic cancer patients. Front Physiol [Internet]. 2013. [cited 2017 Oct 18];4:210 Available from: http://www.ncbi.nlm.nih.gov/pubmed/23950747 10.3389/fphys.2013.00210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sideras K, Braat H, Kwekkeboom J, van Eijck CH, Peppelenbosch MP, Sleijfer S, et al. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat Rev. 2014. May;40(4):513–22. 10.1016/j.ctrv.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 4.Schober M, Jesenofsky R, Faissner R, Weidenauer C, Hagmann W, Michl P, et al. Desmoplasia and Chemoresistance in Pancreatic Cancer. Cancers (Basel). 2014. October;6(4):2137–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004. January;28(1):e26–31. [DOI] [PubMed] [Google Scholar]
- 6.Tewari N, Zaitoun AM, Arora A, Madhusudan S, Ilyas M, Lobo DN. The presence of tumour-associated lymphocytes confers a good prognosis in pancreatic ductal adenocarcinoma: an immunohistochemical study of tissue microarrays. BMC Cancer. 2013. December;13(1):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer [Internet]. 2013;108(4):914–23. Available from: 10.1038/bjc.2013.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nejati R, Goldstein JB, Halperin DM, Wang H, Hejazi N, Rashid A, et al. Prognostic Significance of Tumor-Infiltrating Lymphocytes in Patients With Pancreatic Ductal Adenocarcinoma Treated With Neoadjuvant Chemotherapy. Pancreas [Internet]. 2017. October [cited 2017 Oct 18];46(9):1180–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28902789 10.1097/MPA.0000000000000914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karakhanova S, Ryschich E, Mosl B, Harig S, Jäger D, Schmidt J, et al. Prognostic and predictive value of immunological parameters for chemoradioimmunotherapy in patients with pancreatic adenocarcinoma. Br J Cancer. 2015. March;112(6):1027–36. 10.1038/bjc.2015.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D WR. Hallmarks of cancer: the next generation. 2011. 646–674 p. [DOI] [PubMed] [Google Scholar]
- 11.Torphy RJ, Zhu Y, Schulick RD. Immunotherapy for pancreatic cancer: Barriers and breakthroughs. Ann Gastroenterol Surg [Internet]. 2018. July [cited 2018 Jul 31];2(4):274–81. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30003190 10.1002/ags3.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koti M, Siu A, Clément I, Bidarimath M, Turashvili G, Edwards A, et al. A distinct pre-existing inflammatory tumour microenvironment is associated with chemotherapy resistance in high-grade serous epithelial ovarian cancer. Br J Cancer [Internet]. 2015;112(7):1215–22. Available from: http://www.nature.com.ezproxy.library.ubc.ca/bjc/journal/v112/n7/full/bjc201581a.html 10.1038/bjc.2015.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015. February;26(2):259–71. 10.1093/annonc/mdu450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin JW, Chilton-MacNeill S, Koti M, van Wijnen AJ, Squire JA, Zielenska M. Digital Expression Profiling Identifies RUNX2, CDC5L, MDM2, RECQL4, and CDK4 as Potential Predictive Biomarkers for Neo-Adjuvant Chemotherapy Response in Paediatric Osteosarcoma. Loeb D, editor. PLoS One. 2014. May;9(5):e95843 10.1371/journal.pone.0095843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo S, Contratto M, Miller G, Leichman L, Wu J. Immunotherapy in pancreatic cancer: Unleash its potential through novel combinations. World J Clin Oncol [Internet]. 2017. June 10 [cited 2018 Jul 31];8(3):230–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28638792 10.5306/wjco.v8.i3.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol. 2013. April;25(2):200–5. 10.1016/j.coi.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lianyuan T, Dianrong X, Chunhui Y, Zhaolai M, Bin J. The predictive value and role of stromal tumor-infiltrating lymphocytes in pancreatic ductal adenocarcinoma (PDAC). Cancer Biol Ther [Internet]. 2018. April 3 [cited 2018 Aug 1];19(4):296–305. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29313457 10.1080/15384047.2017.1416932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stromnes IM, Hulbert A, Pierce RH, Greenberg PD, Hingorani SR. T-cell Localization, Activation, and Clonal Expansion in Human Pancreatic Ductal Adenocarcinoma. Cancer Immunol Res [Internet]. 2017. November [cited 2019 Feb 22];5(11):978–91. Available from: 10.1158/2326-6066.CIR-16-0322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rudensky AY. Regulatory T cells and Foxp3. Immunol Rev [Internet]. 2011. May [cited 2018 Aug 14];241(1):260–8. Available from: 10.1111/j.1600-065X.2011.01018.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol [Internet]. 2007. May 1 [cited 2018 Aug 14];8(5):457–62. Available from: http://www.nature.com/articles/ni1455 10.1038/ni1455 [DOI] [PubMed] [Google Scholar]
- 21.Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, et al. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell [Internet]. 2007. June 29 [cited 2018 Aug 14];129(7):1275–86. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0092867407005454 10.1016/j.cell.2007.04.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Douglass S, Ali S, Meeson AP, Browell D, Kirby JA. The role of FOXP3 in the development and metastatic spread of breast cancer. Cancer Metastasis Rev [Internet]. 2012. December 26 [cited 2018 Aug 14];31(3–4):843–54. Available from: 10.1007/s10555-012-9395-3 [DOI] [PubMed] [Google Scholar]
- 23.Li X, Gao Y, Li J, Zhang K, Han J, Li W, et al. FOXP3 inhibits angiogenesis by downregulating VEGF in breast cancer. Cell Death Dis [Internet]. 2018. July 3 [cited 2018 Aug 11];9(7):744 Available from: http://www.ncbi.nlm.nih.gov/pubmed/29970908 10.1038/s41419-018-0790-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Easton DF, Pooley KA, Dunning AM, Pharoah PDP, Thompson D, Ballinger DG, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007. June;447(7148):1087–93. 10.1038/nature05887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West NR, Kost SE, Martin SD, Milne K, deLeeuw RJ, Nelson BH, et al. Tumour-infiltrating FOXP3+ lymphocytes are associated with cytotoxic immune responses and good clinical outcome in oestrogen receptor-negative breast cancer. Br J Cancer [Internet]. 2013. January 20 [cited 2018 Aug 11];108(1):155–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23169287 10.1038/bjc.2012.524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Won KY, Kim GY, Kim HK, Choi S Il, Kim SH, Bae GE, et al. Tumoral FOXP3 expression is associated with favorable clinicopathological variables and good prognosis in gastric adenocarcinoma: the tumor suppressor function of tumoral FOXP3 is related with the P21 expression in gastric adenocarcinoma. Hum Pathol [Internet]. 2017. October [cited 2018 Aug 14];68:112–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28882702 10.1016/j.humpath.2017.08.023 [DOI] [PubMed] [Google Scholar]
- 27.Suh J-H, Won KY, Kim GY, Bae GE, Lim S-J, Sung J-Y, et al. Expression of tumoral FOXP3 in gastric adenocarcinoma is associated with favorable clinicopathological variables and related with Hippo pathway. Int J Clin Exp Pathol [Internet]. 2015. [cited 2018 Aug 14];8(11):14608–18. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26823784 [PMC free article] [PubMed] [Google Scholar]
- 28.Wang L, Liu R, Li W, Chen C, Katoh H, Chen G-Y, et al. Somatic single hits inactivate the X-linked tumor suppressor FOXP3 in the prostate. Cancer Cell [Internet]. 2009. October 6 [cited 2018 Aug 14];16(4):336–46. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1535610809002633 10.1016/j.ccr.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tao H, Mimura Y, Aoe K, Kobayashi S, Yamamoto H, Matsuda E, et al. Prognostic potential of FOXP3 expression in non-small cell lung cancer cells combined with tumor-infiltrating regulatory T cells. Lung Cancer [Internet]. 2012. January [cited 2018 Aug 14];75(1):95–101. Available from: http://linkinghub.elsevier.com/retrieve/pii/S016950021100331X 10.1016/j.lungcan.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 30.Caragine TA, Okada N, Frey AB, Tomlinson S. A tumor-expressed inhibitor of the early but not late complement lytic pathway enhances tumor growth in a rat model of human breast cancer. Cancer Res [Internet]. 2002. February 15 [cited 2018 Aug 16];62(4):1110–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11861390 [PubMed] [Google Scholar]
- 31.Fishelson Z, Donin N, Zell S, Schultz S, Kirschfink M. Obstacles to cancer immunotherapy: expression of membrane complement regulatory proteins (mCRPs) in tumors. Mol Immunol [Internet]. 2003. September [cited 2018 Aug 16];40(2–4):109–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12914817 [DOI] [PubMed] [Google Scholar]
- 32.Teschendorff AE, Caldas C. A robust classifier of high predictive value to identify good prognosis patients in ER-negative breast cancer. Breast Cancer Res [Internet]. 2008. August 28 [cited 2018 Aug 16];10(4):R73 Available from: 10.1186/bcr2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azzato EM, Lee AJX, Teschendorff A, Ponder BAJ, Pharoah P, Caldas C, et al. Common germ-line polymorphism of C1QA and breast cancer survival. Br J Cancer [Internet]. 2010. April 13 [cited 2018 Aug 14];102(8):1294–9. Available from: http://www.nature.com/articles/6605625 10.1038/sj.bjc.6605625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulla R, Tripodo C, Rami D, Ling GS, Agostinis C, Guarnotta C, et al. C1q acts in the tumour microenvironment as a cancer-promoting factor independently of complement activation. Nat Commun [Internet]. 2016. February 1 [cited 2018 Oct 25];7:10346 Available from: http://www.ncbi.nlm.nih.gov/pubmed/26831747 10.1038/ncomms10346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winslow S, Leandersson K, Edsjö A, Larsson C. Prognostic stromal gene signatures in breast cancer. Breast Cancer Res [Internet]. 2015. February 21 [cited 2018 Oct 25];17(1):23 Available from: http://www.ncbi.nlm.nih.gov/pubmed/25848820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waters JP, Pober JS, Bradley JR. Tumour necrosis factor and cancer. J Pathol [Internet]. 2013. July [cited 2018 Aug 11];230(3):241–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23460481 10.1002/path.4188 [DOI] [PubMed] [Google Scholar]
- 37.Horiuchi T, Mitoma H, Harashima S, Tsukamoto H, Shimoda T. Transmembrane TNF-alpha: structure, function and interaction with anti-TNF agents. Rheumatology (Oxford) [Internet]. 2010. July 1 [cited 2018 Aug 16];49(7):1215–28. Available from: https://academic.oup.com/rheumatology/article-lookup/doi/10.1093/rheumatology/keq031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cauwels A, Brouckaert P. Survival of TNF toxicity: Dependence on caspases and NO. Arch Biochem Biophys [Internet]. 2007. June 15 [cited 2018 Aug 16];462(2):132–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17321482 10.1016/j.abb.2007.01.021 [DOI] [PubMed] [Google Scholar]
- 39.Grünhagen DJG, de Wilt JHW, van Geel AN, Verhoef C, Eggermont AMM. Isolated limb perfusion with TNF-alpha and melphalan in locally advanced soft tissue sarcomas of the extremities. Recent Results Cancer Res [Internet]. 2009. [cited 2018 Aug 16];179:257–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19230545 [DOI] [PubMed] [Google Scholar]
- 40.Deroose JP, Grünhagen DJ, van Geel AN, de Wilt JHW, Eggermont AMM, Verhoef C. Long-term outcome of isolated limb perfusion with tumour necrosis factor-α for patients with melanoma in-transit metastases. Br J Surg [Internet]. 2011. November [cited 2018 Aug 16];98(11):1573–80. Available from: http://doi.wiley.com/10.1002/bjs.7621 [DOI] [PubMed] [Google Scholar]
- 41.Alexander HR, Bartlett DL, Libutti SK, Pingpank JF, Fraker DL, Royal R, et al. Analysis of factors associated with outcome in patients undergoing isolated hepatic perfusion for unresectable liver metastases from colorectal center. Ann Surg Oncol [Internet]. 2009. July 12 [cited 2018 Aug 16];16(7):1852–9. Available from: 10.1245/s10434-009-0482-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waters JP, Pober JS, Bradley JR. Tumour necrosis factor in infectious disease. J Pathol [Internet]. 2013. June [cited 2018 Aug 16];230(2):132–47. Available from: 10.1002/path.4187 [DOI] [PubMed] [Google Scholar]
- 43.Steinman RM, Hemmi H. Dendritic Cells: Translating Innate to Adaptive Immunity In: From Innate Immunity to Immunological Memory [Internet]. Springer; Berlin Heidelberg; [cited 2018. August 14]. p. 17–58. Available from: http://link.springer.com/10.1007/3-540-32636-7_2 [DOI] [PubMed] [Google Scholar]
- 44.Lucas M, Schachterle W, Oberle K, Aichele P, Diefenbach A. Dendritic cells prime natural killer cells by trans-presenting interleukin 15. Immunity [Internet]. 2007. April [cited 2018 Aug 14];26(4):503–17. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1074761307001860 10.1016/j.immuni.2007.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bourquin C, Schmidt L, Lanz A-L, Storch B, Wurzenberger C, Anz D, et al. Immunostimulatory RNA oligonucleotides induce an effective antitumoral NK cell response through the TLR7. J Immunol [Internet]. 2009. November 15 [cited 2018 Aug 14];183(10):6078–86. Available from: 10.4049/jimmunol.0901594 [DOI] [PubMed] [Google Scholar]
- 46.Wang D, Precopio M, Lan T, Yu D, Tang JX, Kandimalla ER, et al. Antitumor Activity and Immune Response Induction of a Dual Agonist of Toll-Like Receptors 7 and 8. Mol Cancer Ther [Internet]. 2010. June 1 [cited 2018 Aug 10];9(6):1788–97. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20515950 10.1158/1535-7163.MCT-09-1198 [DOI] [PubMed] [Google Scholar]
- 47.Zoglmeier C, Bauer H, Norenberg D, Wedekind G, Bittner P, Sandholzer N, et al. CpG Blocks Immunosuppression by Myeloid-Derived Suppressor Cells in Tumor-Bearing Mice. Clin Cancer Res [Internet]. 2011. April 1 [cited 2018 Aug 14];17(7):1765–75. Available from: 10.1158/1078-0432.CCR-10-2672 [DOI] [PubMed] [Google Scholar]
- 48.Spinetti T, Spagnuolo L, Mottas I, Secondini C, Treinies M, Rüegg C, et al. TLR7-based cancer immunotherapy decreases intratumoral myeloid-derived suppressor cells and blocks their immunosuppressive function. Oncoimmunology [Internet]. 2016. November 9 [cited 2018 Aug 10];5(11):e1230578 Available from: http://www.ncbi.nlm.nih.gov/pubmed/27999739 10.1080/2162402X.2016.1230578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anz D, Koelzer VH, Moder S, Thaler R, Schwerd T, Lahl K, et al. Immunostimulatory RNA blocks suppression by regulatory T cells. J Immunol [Internet]. 2010. January 15 [cited 2018 Aug 14];184(2):939–46. Available from: 10.4049/jimmunol.0901245 [DOI] [PubMed] [Google Scholar]
- 50.Junt T, Barchet W. Translating nucleic acid-sensing pathways into therapies. Nat Rev Immunol [Internet]. 2015. September 15 [cited 2018 Aug 14];15(9):529–44. Available from: http://www.nature.com/articles/nri3875 10.1038/nri3875 [DOI] [PubMed] [Google Scholar]
- 51.Peris K, Campione E, Micantonio T, Marulli GC, Fargnoli MC, Chimenti S. Imiquimod treatment of superficial and nodular basal cell carcinoma: 12-week open-label trial. Dermatol Surg [Internet]. 2005. March [cited 2018 Aug 14];31(3):318–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15841634 [DOI] [PubMed] [Google Scholar]
- 52.Hotz C, Treinies M, Mottas I, Rötzer LC, Oberson A, Spagnuolo L, et al. Reprogramming of TLR7 signaling enhances antitumor NK and cytotoxic T cell responses. Oncoimmunology [Internet]. 2016. November 9 [cited 2018 Aug 10];5(11):e1232219 Available from: http://www.ncbi.nlm.nih.gov/pubmed/27999742 10.1080/2162402X.2016.1232219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charrin S, Jouannet S, Boucheix C, Rubinstein E. Tetraspanins at a glance. J Cell Sci [Internet]. 2014. September 1 [cited 2018 Aug 14];127(Pt 17):3641–8. Available from: http://jcs.biologists.org/cgi/doi/10.1242/jcs.154906 [DOI] [PubMed] [Google Scholar]
- 54.van Deventer SJ, Dunlock V-ME, van Spriel AB. Molecular interactions shaping the tetraspanin web. Biochem Soc Trans [Internet]. 2017. June 15 [cited 2018 Aug 14];45(3):741–50. Available from: 10.1042/BST20160284 [DOI] [PubMed] [Google Scholar]
- 55.Beckwith KA, Byrd JC, Muthusamy N. Tetraspanins as therapeutic targets in hematological malignancy: a concise review. Front Physiol [Internet]. 2015. March 23 [cited 2018 Aug 10];6:91 Available from: http://www.ncbi.nlm.nih.gov/pubmed/25852576 10.3389/fphys.2015.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gartlan KH, Wee JL, Demaria MC, Nastovska R, Chang TM, Jones EL, et al. Tetraspanin CD37 contributes to the initiation of cellular immunity by promoting dendritic cell migration. Eur J Immunol [Internet]. 2013. May [cited 2018 Aug 14];43(5):1208–19. Available from: 10.1002/eji.201242730 [DOI] [PubMed] [Google Scholar]
- 57.Schaper F, van Spriel AB. Antitumor Immunity Is Controlled by Tetraspanin Proteins. Front Immunol [Internet]. 2018. May 29 [cited 2018 Aug 10];9:1185 Available from: http://www.ncbi.nlm.nih.gov/pubmed/29896201 10.3389/fimmu.2018.01185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kratz JR, He J, Van Den Eeden SK, Zhu Z-H, Gao W, Pham PT, et al. A practical molecular assay to predict survival in resected non-squamous, non-small-cell lung cancer: development and international validation studies. Lancet. 2012. March;379(9818):823–32. 10.1016/S0140-6736(11)61941-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Divitiis C, Nasti G, Montano M, Fisichella R, Iaffaioli RV, Berretta M. Prognostic and predictive response factors in colorectal cancer patients: between hope and reality. World J Gastroenterol. 2014. November;20(41):15049–59. 10.3748/wjg.v20.i41.15049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A Multigene Assay to Predict Recurrence of Tamoxifen-Treated, Node-Negative Breast Cancer. N Engl J Med. 2004. December;351(27):2817–26. 10.1056/NEJMoa041588 [DOI] [PubMed] [Google Scholar]
- 61.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. N Engl J Med. 2011. June;364(26):2507–16. 10.1056/NEJMoa1103782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Glenn J, Steinberg WM, Kurtzman SH, Steinberg SM, Sindelar WF. Evaluation of the utility of a radioimmunoassay for serum CA 19–9 levels in patients before and after treatment of carcinoma of the pancreas. J Clin Oncol. 1988. March;6(3):462–8. 10.1200/JCO.1988.6.3.462 [DOI] [PubMed] [Google Scholar]
- 63.Chen D-T, Davis-Yadley AH, Huang P-Y, Husain K, Centeno BA, Permuth-Wey J, et al. Prognostic Fifteen-Gene Signature for Early Stage Pancreatic Ductal Adenocarcinoma. Real FX, editor. PLoS One. 2015. August;10(8):e0133562 10.1371/journal.pone.0133562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sergeant G, van Eijsden R, Roskams T, Van Duppen V, Topal B. Pancreatic cancer circulating tumour cells express a cell motility gene signature that predicts survival after surgery. BMC Cancer. 2012. December;12(1):527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van den Broeck A, Vankelecom H, Van Delm W, Gremeaux L, Wouters J, Allemeersch J, et al. Human pancreatic cancer contains a side population expressing cancer stem cell-associated and prognostic genes. Lebedeva I V, editor. PLoS One. 2013. September;8(9):e73968 10.1371/journal.pone.0073968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang G, Schetter A, He P, Funamizu N, Gaedcke J, Ghadimi BM, et al. DPEP1 Inhibits Tumor Cell Invasiveness, Enhances Chemosensitivity and Predicts Clinical Outcome in Pancreatic Ductal Adenocarcinoma. El-Rifai W, editor. PLoS One. 2012. February;7(2):e31507 10.1371/journal.pone.0031507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morrison AH, Byrne KT, Vonderheide RH. Immunotherapy and Prevention of Pancreatic Cancer. Trends in Cancer [Internet]. 2018. June [cited 2018 Jul 31];4(6):418–28. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29860986 10.1016/j.trecan.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tesfaye AA, Azmi AS, Philip PA. miRNA and Gene Expression in Pancreatic Ductal Adenocarcinoma. Am J Pathol [Internet]. 2019. January [cited 2019 Jan 16];189(1):58–70. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002944018305571 10.1016/j.ajpath.2018.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Słotwiński R, Lech G, Słotwińska SM. MicroRNAs in pancreatic cancer diagnosis and therapy. Cent J Immunol [Internet]. 2018 [cited 2019 Jan 16];43(3):314–24. Available from: https://www.termedia.pl/doi/10.5114/ceji.2018.80051 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Volcano plot displaying each gene's -log10(p-value) against log2 fold change: a) TLR7, b) TNF, c) C1QA, d) FOXP3 and e) CD37. Highly statistically significant genes fall at the top of the plot, and highly differentially expressed genes fall to either side. Genes within the selected gene set are highlighted in orange. Horizontal lines indicate various False Discovery Rate (FDR) thresholds.
(TIFF)
Box plots show levels of exhausted CD8+ cells (a) and Tregs (b) in patients with worse prognosis (group A) and patients with worse prognosis (group B). Even though scores seem overlapping, the average score for both sets of cells is higher in group B than in group A, probably due to the fact that longer-surviving PDAC patients had higher levels of intratumoral TILs.
(TIFF)
The diagram shows the abundance of exhausted CD8+ cells and Tregs compared to levels of CD8+ cells. In agreement with the previous figure, levels of exhausted CD8+ cells (green line) and Tregs (dashed orange line) are reported to be lower in the group with a worse prognosis (group A) than in the group with a better prognosis (group B) when compared with the total level of CD8+ cells.
(TIFF)
(PDF)
Data Availability Statement
The fully comprehensive gene expression data generated by the Nanostring assay have been deposited in the NCBI Gene Expression Omnibus (GEO) and are accessible via GEO accession number GSE132946. All other relevant data are within the manuscript and Supporting Information files.