Abstract
The surge of resistant food pathogens is a major threat worldwide. Previous research conducted on phytochemicals has shown their antibacterial activity against pathogenic bacteria. The design of antimicrobial agents to curb pathogenic disease remains a challenge demanding critical attention. Flavonoids such as apigenin and quercetin were evaluated against Gram-positive and Gram-negative bacteria. The results indicated that the antibacterial activity of each flavonoid occurred at a different minimum inhibitory concentration. However, the antimicrobial activity results of the modified flavonoids were also reported, and it was observed that the Gram-positive bacteria were more susceptible in comparison to the Gram-negative bacteria. The cell wall structure of the Gram-positive and Gram-negative bacteria could be the main reason for the bacteria susceptibility. Modified flavonoids could be used as a suitable alternative antimicrobial agent for the treatment of infectious diseases. Our results indicated 100% inhibition of Listeria monocytogenes, Pseudomonas aeruginosa, and Aeromonas hydrophila with modified flavonoids.
Introduction
The prevalence of pathogenic microorganisms in human diets is linked to many food-borne diseases. The therapeutic interventions necessary to curb pathogenic strains among food sources have been reported in the literature.1 Phytochemicals serve as significant antimicrobial agents that can target pathogens interfering with many physiological processes. Furthermore, phytochemicals are capable of inhibiting and eliminating microorganisms both in vitro and in vivo.1 Phytochemicals such as quercetin (QCR) and apigenin (APGN) have been studied extensively and isolated as antimicrobial agents against many Gram-positive and Gram-negative bacteria.2 Quercetin’s antimicrobial activity has been shown as a potential therapy to combat resistant pathogens when used with rifampicin.3 The pharmaceutical properties of quercetin and apigenin serve as a platform to formulate a new functional class of plant-based antimicrobial agents that can target pathogenic microorganisms.
The parent structures of the modified flavonoids, which are quercetin and apigenin (Figure S1), are made of polyphenols which belong to the flavonoid group. Quercetin and apigenin possess many biological properties such as antioxidant, neuroprotection, antiviral, anticancer, cardiovascular, antimicrobial, anti-inflammatory, and anti-obesity.3−7 These phytochemicals are a natural source for treating infectious diseases.7,8 For instance, kaempferol, quercetin, and apigenin have been widely used in herbal medicine for traditional remedies for hundreds of years.1,3,9 Shu et al. have reported the antibacterial activity of quercetin on the oral pathogens in which it showed their bactericidal activity against caries-related bacteria.7 Quercetin and apigenin are sparingly soluble in water due to their hydrophobic structure. Scholtz et al. have demonstrated the attachment of different sugar moieties to quercetin to form a conjugation that improves their solubility in biological systems.10 Osonga et al. have also demonstrated the solubility of quercetin via sequential phosphorylation.11
In the field of nanotechnology, both quercetin and apigenin have the potential to chelate with many transition metal ions to form complexes with newly effective and enhanced biological properties.12 Quercetin and apigenin are used as a reducing agent in the chemical reduction technique during the synthesis of nanoparticles. The potential of quercetin to form a complex with metal ions such as Ag, Au, and Fe has been reported in many studies.12−16 The three ring structures A, B, C have functional groups for metal chelation.13 It is reported that the hydroxyl groups and the keto groups form the metal complexes.12,13,15 This knowledge has been utilized to improve the antimicrobial activity of quercetin.12,16 Nabavi et al. reported that the catechol moiety of the B ring and the OH groups present in quercetin are responsible for the antioxidant activity both in vivo and in vitro.17 Some applications of apigenin include the remediation of various cancer cell lines.18 Apigenin also mediates antitumor effects via modulating cell proliferation and/or apoptosis.18 The proposed antibacterial activities of quercetin and apigenin are via three mechanisms. The cytoplasmic membrane of the bacteria is damaged through the perforation action of the flavonoid. The inhibition of both energy metabolism and the synthesis of nucleic acids is another mechanism.1 Furthermore, Plaper et al. have demonstrated the mechanism of action of quercetin by targeting gyrase and reported the antibacterial activity from their results.19
This present study was conducted to investigate the effect of both modified quercetin and apigenin compounds as a potential antimicrobial agent against Gram-negative Pseudomonas aeruginosa ATCC 10145 and Aeromonas hydrophila ATCC 35654, also Gram-positive Listeria monocytogenes ATCC 19115. All of the bacteria that were studied are pathogenic strains. The presence of a thick peptidoglycan layer forms part of the cell wall of the Gram-positive bacteria, whereas structures such as lipopolysaccharide, phospholipids, and a thin layer of peptidoglycan constitute the outer membrane of the Gram-negative bacteria.2,20 The difference in the cell membrane plays a unique role in the bacteria susceptibility to the treatment of naturally synthesized antibacterial drugs.2
L. monocytogenes is a Gram-positive, facultative pathogen characterized by meningitis and gastroenteritis by inducing into cells that are nonphagocytic and spread via an actin-based motility process.21,22 Gram-negative bacteria such as P. aeruginosa and A. hydrophila have been epidemiologically linked to human illness. P. aeruginosa cause diseases that affect many organs by disrupting the normal physiological function of the body. P. aeruginosa infection cases can be associated with immunosuppressed patients undergoing chemotherapy treatment.23A. hydrophila is a facultative anaerobe that thrives in atmospheres containing low concentrations of oxygen and temperature (4 °C).24 Gracey et al. isolated A. hydrophila from the feces of gastroenteritis patients as evidence to the widespread of the disease.25 The widespread of the selected bacteria has led to concerns about multidrug-resistant strains. Hsueh et al. reported the spread of a single multidrug-resistant strain of P. aeruginosa and revealed that antibiotics treatment has not been effective due to the resistive nature of the bacteria.26 The modification of phytochemicals such as quercetin and apigenin is an essential study to design an antibacterial drug capable of eliminating resistant pathogenic bacteria completely.
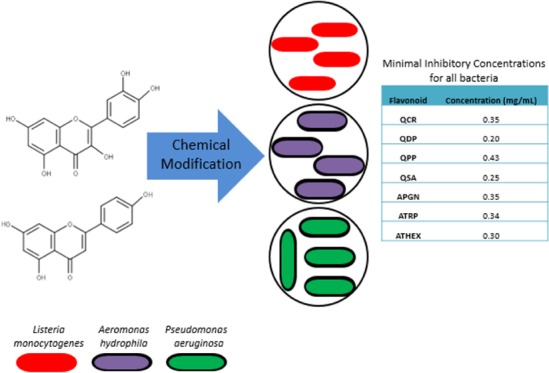
The modified compounds synthesized include quercetin 4′,5-diphosphate (QDP), quercetin 3′,4′,3,5,7-pentaphosphate (QPP), quercetin 5′-sulfonic acid (QSA), apigenin 4′,5,7-triphosphate (ATRP), and apigenin 4′,5,7-trihexanoyl (ATHEX) (Figures 1 and S1). The phosphorylation of polyphenols such as quercetin and apigenin has been investigated and reported.11 Quercetin phosphate derivatives play a vital role in sustaining many physiological processes. Wei et al. showed that the phosphorylation of the OH groups leads to an increased solubility of quercetin.27 The phosphorylation method utilizes benzyl phosphites and pyridine-N-oxide catalyst as the active reagents for sequential phosphorylation. However, they result in a low yield.11 In this study, both quercetin and apigenin were modified with benzyl phosphite following the procedure by Osonga et al.11 The synthesis of QSA was followed by using the previously reported literature.28 The addition of the sulfonate group helps to increase the solubility as well as increasing the acidity of the compound.28 The synthesis of ATHEX will be reported elsewhere. The antibacterial activities of the modified quercetin and apigenin molecules were investigated against the three selected microorganisms. Our results depicted a promising antibacterial activity of the modified quercetin and apigenin. The modified quercetin and apigenin are highly soluble, biocompatible, and possess a tremendous antibacterial activity.
Figure 1.
General structure of flavonoids showing the locations and identities of the various derivatives in the parent compounds.
Results
The antimicrobial activities of QCR against L. monocytogenes, A. hydrophila, P. aeruginosa were determined by OD measurements, and the viable counting. L. monocytogenes showed no inhibition with the addition of QCR (Figure 2). Once the viability was decreased (Figures 3 and 4), the bacterial culture was diluted 5 times, and 100 μL of 10–5 cultures was spread on LB plates. The growths of L. monocytogenes, A. hydrophila, and P. aeruginosa are presented in (Figures 5, 6, and 7), and the adverse effect of QCR on the growth of the three bacteria on the agar plate is clearly shown. QCR is bacteriostatic since there is no decrease in the OD. It thus inhibits the growth of the bacteria on the agar plates of L. monocytogenes. In comparison, results showed that QCR is bactericidal to the other two bacteria, namely, A. hydrophila and P. aeruginosa based on the decrease in OD. It is worth stating that depending on the type of bacteria involved, QCR could act in one of two different ways.
Figure 2.

Relative viability of L. monocytogenes after 16 h growth with test compounds. The error bars show +1 standard deviation for each measurement with three replicates. *p < 0.05. One-way ANOVA followed by Dunnett’s multiple comparisons test was performed using the GraphPad Prism 8.0, GraphPad Software.
Figure 3.
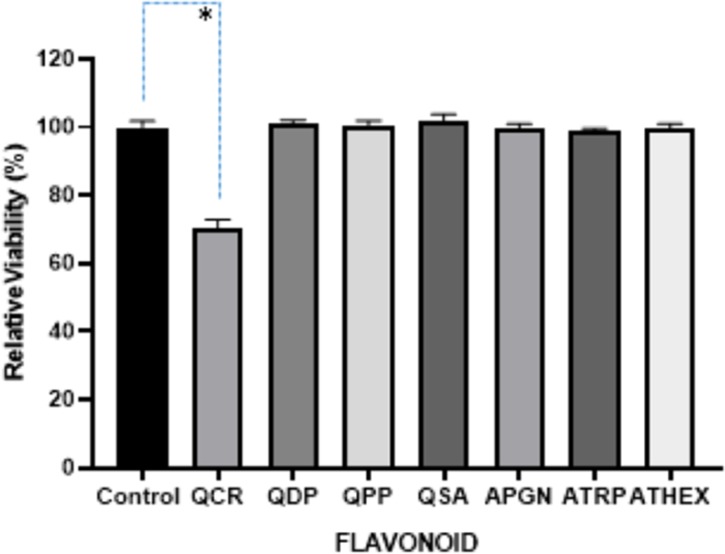
Relative viability of A. hydrophila after 16 h growth with test compounds. The error bars showed +1 standard deviation for each measurement with three replicates. *p < 0.0001. One-way ANOVA followed by Dunnett’s multiple comparisons test was performed using the GraphPad Prism, 8.0 GraphPad Software.
Figure 4.
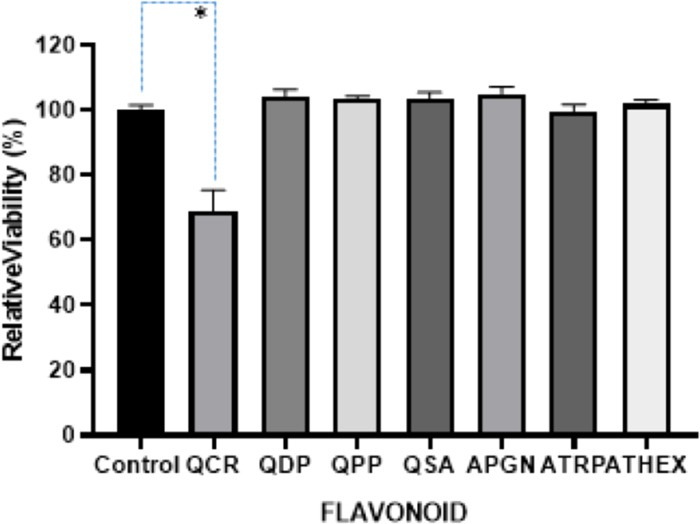
Relative viability of P. aeruginosa after 16 h growth with test compounds. The error bars showed +1 standard deviation for each measurement with three replicates. *p < 0.0001. One-way ANOVA followed by Dunnett’s multiple comparisons test was performed using the GraphPad Prism, 8.0 GraphPad Software.
Figure 5.
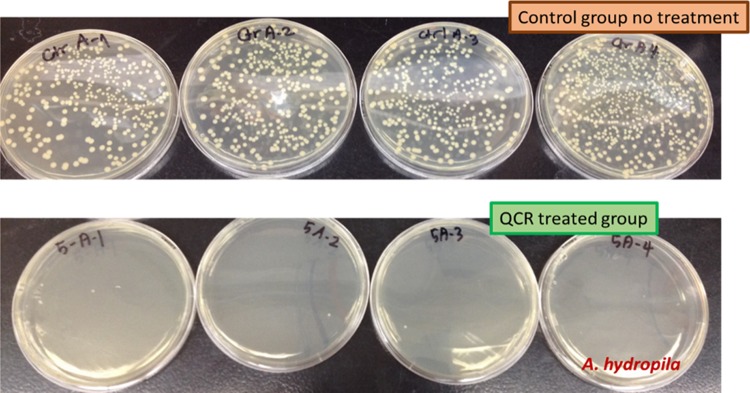
Viable counting for A. hydrophila on a non-molecule-treated plate with the 10–5 dilution of control and 0.35 mg/mL of QCR-treated LB broth. Each plate is a different replicate.
Figure 6.
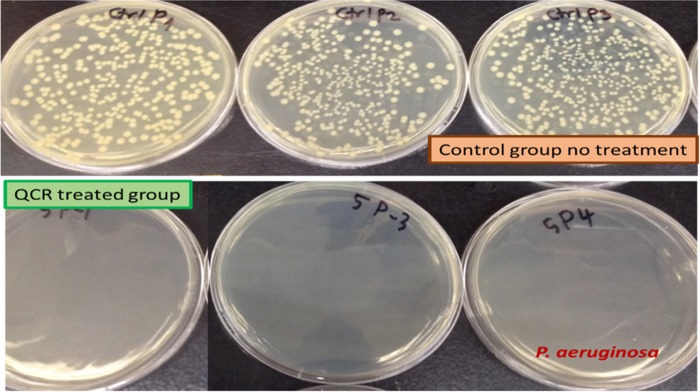
Viable counting for P. aeruginosa on a non-molecule-treated plate with the 10–5 dilution of control and 0.35 mg/mL of QCR-treated LB-broth. Each plate is a different replicate.
Figure 7.
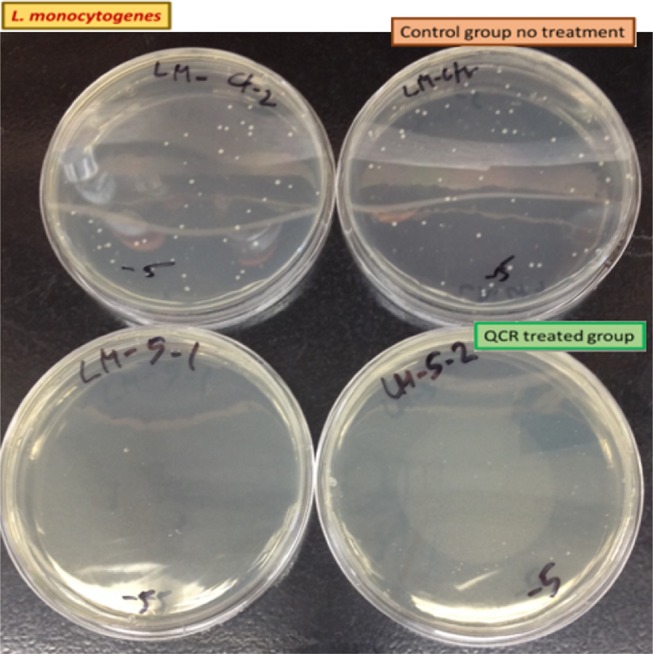
Viable counting for L. monocytogenes on a non-molecule-treated plate with the 10–5 dilution of control and 0.35 mg/mL of QCR-treated LB-broth. Total covered area by L. monocytogenes was decreased over 95%. Each plate is a different replicate.
QSA at 0.25 mg/mL did not affect the growth of A. hydrophila and P. aeruginosa, but it negatively affected the growth of L. monocytogenes (Figure S2). OD of A. hydrophila and P. aeruginosa and L. monocytogenes are shown in Figures 2–4. QSA seems to show bactericidal properties to L. monocytogenes and bacteriostatic to A. hydrophila and P. aeruginosa.
QPP at the minimum inhibitory concentration (MIC) of 0.43 mg/mL negatively affects the growth of L. monocytogenes, but it did not affect the growth of A. hydrophila and P. aeruginosa. The OD of A. hydrophila, P. aeruginosa, and L. monocytogenes are shown in Figures 2–4. As seen in Figure S3, over 99.9% of L. monocytogenes was killed. It is interesting that QCR and QPP had similar effects on the bacterium tested.
Discussion
The biological properties of naturally occurring flavonoids have been explored in many studies. Flavonoid such as quercetin and apigenin significantly exhibit antioxidant, antibacterial and antifungal activities.3−5 Mandalari et al., Daglia, and Wu et al. have demonstrated the antibacterial activity of flavonoid based on structural functionalities.3−5,29 The hydroxyl groups attached to flavonoid play a role in the antibacterial activity.29,30 For instance, Osawa et al. displayed that 5-hydroxyflavanones with one, two or three additional hydroxyl groups at the 7, 2′ and 4′ positions inhibited the growth of Streptococcus mutans and Streptococcus sobrinus.31
In this study, quercetin and apigenin were modified with bioactive molecules such as phosphate and sulfonic acid in an attempt to evaluate their comparative antibacterial potential against various pathogenic microorganisms. The pathogens of interest include Gram-positive L. monocytogenes, and Gram-negative A. hydrophila and P. aeruginosa. Two methods were utilized to determine the antibacterial activity of the modified quercetin and apigenin. In the first method (Figures 2–4), the data were analyzed by ANOVA with a pos-hoc test (Dunnett’s multiple comparisons test) to determine whether the viabilities were significant or not. In Figure 2, three of the viabilities were marked significant: QCR, QDP, and APGN with p values of 0.0274, 0.0130, and 0.0237, respectively. In Figures 3 and 4, only one of the viabilities was marked as significant: QCR with p values of <0.0001. In the second method, the agar plates treated with quercetin showed 100% inhibition of all three pathogens at a MIC of 0.35 mg/mL (Table 1, Figures 5–7). Vázquez-Armenta et al. have utilized a phenolic extract to inhibit the growth of the L. monocytogenes. In their investigation, the phenolic extract, which contained Rutin (quercetin-3-O-rutinoside), reduced the surface energy and adhesion of L. monocytogenes thereby inhibiting the bacterial motility.32 Rutin is a natural, modified quercetin compound containing a sugar moiety. Their results correlate to our findings that modified quercetin molecules are capable of inhibiting L. monocytogenes.
Table 1. Concentrations of MICs for the Flavonoids Used in the Study.
| flavonoid | concentration (mg/mL) |
|---|---|
| QCR | 0.35 |
| QDP | 0.20 |
| QPP | 0.43 |
| QSA | 0.25 |
| APGN | 0.35 |
| ATRP | 0.34 |
| ATHEX | 0.30 |
However, both QSA and QPP molecules were found to inhibit Gram-positive L. monocytogenes at MIC of 0.25 and 0.43 mg/mL, respectively (Table 1). There was no inhibitory effect, in the case of Gram-negative bacteria P. aeruginosa and A. hydrophila. Furthermore, it has been reported that flavonoid with MICs against the pathogenic microorganisms is highly susceptible to Gram-positive bacteria than that of Gram-negative bacteria.33 The first method of the antibacterial test was conducted in a lysogeny broth media at different MIC’s. L. monocytogenes was inhibited in lysogeny broth containing QSA, QDP, and QPP. This observation could be attributed to the adverse environment for L. monocytogenes to grow in the media. P. aeruginosa and A. hydrophila did not show any bactericidal activity to the modified compounds.
Although Bahrin et al. reported that sulfur-containing derivatives of flavonoid possess higher bacteriostatic activities against Gram-positive than Gram-negative bacteria,6 but in our case, a similar pattern was not recognized. This could be due to the fact that QSA contains a sulfonic acid group rather than just a sulfur atom in its structure like Bahrin et al. reported.6 For instance, QCR and QSA showed minimal activity on Gram-positive bacteria, QCR and QSA showed strong bacteriostatic activity on Gram-negative bacteria. Orhan et al. observed strong antibacterial activity against Gram-negative bacteria using flavonoids as well.34
The modified flavonoids showed different antibacterial activities against the tested bacteria, which were in log-phase. The turbidity results revealed QCR, QSA, QPP, and QDP showed selective bacteriostatic activity. While QDP and QPP showed their bacteriostatic activities towards Gram-positive bacteria, L. monocytogenes. QSA and QCR showed bacteriostatic activities against the Gram-negative bacteria, A. hydrophilia, and P. aeruginosa. The bacterial cultures treated with QCR, QSA, and QPP were then diluted for viable counting. While the bactericidal activity of QCR was evaluated on the three bacteria, the bactericidal activities of QSA and QPP were tested on L. monocytogenes. While QCR and QSA showed the negligible bacteriostatic effect on L. monocytogenes, they showed strong bactericidal activities. This might be related to the fact that at 16 h incubation, the bacteria exist in the stationary phase and thus can show different characteristics towards antibacterial molecules.35
The results imply that the suppression of bacterial pathogens by quercetin can be attributed to the presence of the peptidoglycan structures exposed in the Gram-positive and the peptidoglycan enclosed in an outer membrane in the Gram-negative bacteria. Our observation agrees with the investigation by Goyal et al. stating that the antibacterial activity of flavonoids is susceptible to Gram-positive in comparison to Gram-negative.33
The possible reasons behind the antibacterial character of these compounds could be attributed to flavonoid-mediated perforation, disruption of membrane integrity, interference in specific metabolic pathways including energy metabolism, nucleic acid synthesis, and co-enzyme metabolism.1,36 The pre-oxidant properties of flavonoid have been suspected as a strong player for their antibacterial roles, too.36 The position of the hydroxyl groups on flavonoids also contribute to this effect. According to Wu et al., the hydroxyl group at Carbon-3 in the C ring is important for decreasing membrane fluidity.30 In another study, the hydroxyl group at C-3 is the primary determinant for significant membrane interaction.37 The cell membrane is a direct target for antibacterial action of flavonoids, suggesting that membrane interaction could be an important mechanism of the antibacterial activities of flavonoids.38
The need to investigate and identify modified flavonoids that kill bacteria entirely rather than inhibiting their growth due to immunocompromised patients is vital, especially in neutropenic immunodepressed patients. These patients are neutropenic meaning that there is an abnormally low concentration of neutrophils in their blood. Neutrophils are a type of white blood cells that serve as the primary defense against infections. This type of immunodepression is caused by Gram-positive, and Gram-negative bacteria such as Staphylococcus aureus and P. aeruginosa.39 Bactericidal activity completely reduces the number of bacteria, and it is determined by the minimum bactericidal concentration assay. This method was used to test flavonoids and bactericidal activity. Furthermore, some investigations have been conducted to determine whether flavonoid antibacterial activity is bacteriostatic by conducting time-kill studies. Studies have reported that 3-O-octanoyl-(+)-catechin were depicted to significantly reduce the viable counts of S. aureus NCTC 6571 and EMRSA-16, respectively.38 Hence it was concluded that flavonoids are capable of rate-dependent bactericidal activity.38
Mori et al. reported and suggested that the hydrogen bonding or intercalation of the B ring of the flavonoid may play a role with the stacking of nucleic acid bases which may explain the inhibitory action on DNA and RNA synthesis.40 According to Ohemeng et al., DNA gyrase of E. coli and S. aureus were inhibited by different structural flavonoids revealing their antibacterial via this mechanism. The flavonoids used in this experiment include quercetin, apigenin and 3,6,7,3′,4′-pentahydroxyflavone and the antibacterial activity of the tested flavonoid was due to the inhibition of DNA gyrase.41
The size and functional groups that were attached to the test compounds seem to play a role in the antimicrobial testing. Two of the modified quercetin compounds had the lowest MIC being 0.20 mg/mL (QDP) and 0.25 mg/mL (QSA). The most probable reason for these compounds working better than APGN or QCR is due to the addition of the phosphate groups and the sulfonic acid group. The most abundant compound that was used in this test was QPP. Interestingly enough, QPP had the highest MIC of all the compounds (Table 1). The rich literature detailing the mechanism of action by modified flavonoid against Gram-positive pathogens supports our results that quercetin and apigenin are more effective against Gram-positive while the Gram-negative bacteria is less susceptible and this difference in bacteria susceptibility is majorly attributed to the bacterial cell membrane.
Conclusions
Quercetin and apigenin derivatives showed strong antibacterial properties against Gram-negative and lesser to Gram-positive bacteria. Our goal was to develop and understand the antibacterial effects of flavonoid derivatives as a platform for formulating effective plant-based drugs that target commonly known pathogens associated with food. The antimicrobial mechanism of bioactive flavonoids takes place in the hydrophilic region of phospholipids on the cell membrane where the membrane integrity is disrupted. It can be concluded from our results that flavonoids are susceptible to Gram-positive bacteria in comparison to Gram-negative. The MICs for the microorganisms were within the range of 0.025–0.43 mg/mL (Table 1). This knowledge could be utilized to design antimicrobial drugs that are specific for both Gram-positive and Gram-negative bacteria. However, in-depth biochemical research is required to investigate the mechanism of flavonoid selectivity on both Gram-negative and Gram-positive bacteria in order to thoroughly conclude whether the selectivity occurs during bacteriostatic in the log-phase while non-selective bactericidal agents target bacteria during the stationary phase.
Experimental Section
Materials
High Purity flavonoids, quercetin and apigenin (Indofine Chemical Company, Hillsborough, NJ), sulfonic acid, dibenzyl phosphite, agar plates, Corning centrifuge tubes, lysogeny broth, and brain heart infusion agar were purchased from Sigma-Aldrich (St. Louis, MO). The following microorganisms; Pseudomonas aeruginosa ATCC 10145, Aeromonas hydrophila ATCC 35654, Listeria monocytogenes ATCC 19115 were purchased from American Type Culture Collection (ATCC) (Manassas, VA).
Methods
The flavonoid derivatives ATRP, QPP, and QDP were synthesized and as reported in our work.11 QSA was synthesized as reported42,43 while ATHEX was synthesized with modification as reported.44
The selected microorganisms were Gram-negative as Pseudomonas aeruginosa, Aeromonas hydrophila, and Gram-positive Listeria monocytogenes microorganism. L. monocytogenes was grown in a rich medium, brain heart infusion (BHI). Lysogeny broth (LB), a nutritionally rich medium agar, was used for the maintenance of the tested P. aeruginosa and A. hydrophila. The microorganisms were taken from the culture collection unit −80 °C refrigerator at the Department of Basic Science, College of Veterinary Medicine, Mississippi State University, Mississippi. All microorganisms were propagated overnight, and at the log-phase stage, the microorganisms were harvested to determine the potential antibacterial activities of the test compounds. Broth and Agar-based tests were conducted in order to investigate the difference associated with the antibacterial properties of the derived flavonoids.
Antibacterial Assessment of Flavonoid Derivatives
The antibacterial activities of the modified flavonoid were tested using a two-step approach which includes (I) incubating bacteria in flavonoid-contained broth and then (II) counting the survived bacteria on agar. For the initial procedure, the optical density (OD) of 3-replicates of 16 h incubated bacterial cultures were measured with a spectrophotometer. The change in turbidity as a result of flavonoid inhibition was used to analyze the bacteriostatic activity of the microorganism. The second approach utilized the following day was serially diluting the bacterial cultures in broth media for plate counting. In this step, the agar was solely used without adding any of the modified flavonoids. The selected bacteria in this study were grown overnight at 37 °C incubator. With regards to the second sequential step, the remaining live bacteria were counted via the hanging-drop colony forming unit method on the plates. All tests were repeated 2 times by 3 replicates of plates. This step was performed in order to determine the bactericidal activity of the flavonoid within a specific time duration.
Determination of the Minimum Inhibitory Concentrations (MICs)
The MIC is the lowest concentration of flavonoid that inhibited the visible growth of a microorganism after 24 h incubation. The minimum inhibitory concentrations (MICs) of each flavonoid were measured using the modified version of micro broth dilution method.4 The capability of different flavonoid derivatives to inhibit and eliminate bacterial growth was tested via two sequential steps stated above. All tests were repeated 2 times by 3 replicates of plates.
Statistics
The relative viabilities from the first method were analyzed by ANOVA with a post-hoc test. One-way ANOVA followed by Dunnett’s multiple comparisons test was performed using GraphPad Prism version 8.0.0, GraphPad Software, San Diego, California.
This was performed for each of the compounds for all three bacteria to determine if the results were considered significant or not. Data were considered statistically significant if the p-value was <0.05.
Acknowledgments
The authors acknowledge the National Science Foundation Grant # IOS-1543944 and Bill & Melinda Gates Foundation for funding.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00077.
Figure S1, parent and derivatives of parent compounds structures and molar masses; Figure S2, Agar plating of L. monocytogenes after addition of QSA; Figure S3, Agar plating of L. monocytogenes after addition of QPP (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Ahmad A.; Kaleem M.; Ahmed Z.; Shafiq H. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—A review. Food Res. Int. 2015, 77, 221–235. 10.1016/j.foodres.2015.06.021. [DOI] [Google Scholar]
- Nakamura K.; Ishiyama K.; Sheng H.; Ikai H.; Kanno T.; Niwano Y. Bactericidal activity and mechanism of photoirradiated polyphenols against Gram-positive and-negative bacteria. J. Agric. Food Chem. 2015, 63, 7707–7713. 10.1021/jf5058588. [DOI] [PubMed] [Google Scholar]
- Daglia M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Mandalari G.; Bennett R. N.; Bisignano G.; Trombetta D.; Saija A.; Faulds C. B.; Gasson M. J.; Narbad A. Antimicrobial activity of flavonoids extracted from bergamot (Citrus bergamia Risso) peel, a byproduct of the essential oil industry. J. Appl. Microbiol. 2007, 103, 2056–2064. 10.1111/j.1365-2672.2007.03456.x. [DOI] [PubMed] [Google Scholar]
- Wu T.; Li H.; Chen J. C.; Cao Y.; Fu W.; Zhou P.; Pang J. Apigenin, a novel candidate involving herb-drug interaction (HDI), interacts with organic anion transporter 1 (OAT1). Pharmacol. Rep. 2017, 69, 1254–1262. 10.1016/j.pharep.2017.06.012. [DOI] [PubMed] [Google Scholar]
- Bahrin L. G.; Apostu M. O.; Birsa L. M.; Stefan M. The antibacterial properties of sulfur-containing flavonoids. Bioorg. Med. Chem. Lett. 2014, 24, 2315–2318. 10.1016/j.bmcl.2014.03.071. [DOI] [PubMed] [Google Scholar]
- Shu Y.; Liu Y.; Li L.; Feng J.; Lou B.; Zhou X.; Wu H. Antibacterial activity of quercetin on oral infectious pathogens. Afr. J. Microbiol. Res. 2011, 5, 5358–5361. [Google Scholar]
- Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem. Pharmacol. 1983, 32, 1141–1148. 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Cushnie T. T.; Lamb A. J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtz S.; Williamson G. Interactions affecting the bioavailability of dietary polyphenols in vivo. Int. J. Vitam. Nutr. Res. 2007, 77, 224–235. 10.1024/0300-9831.77.3.224. [DOI] [PubMed] [Google Scholar]
- Osonga F. J.; Onyango J. O.; Mwilu S. K.; Noah N. M.; Schulte J.; An M.; Sadik O. Synthesis and characterization of novel flavonoid derivatives via sequential phosphorylation of quercetin. Tetrahedron Lett. 2017, 58, 1474–1179. 10.1016/j.tetlet.2017.02.085. [DOI] [Google Scholar]
- Mittal A. K.; Kumar S.; Banerjee U. C. Quercetin and gallic acid-mediated synthesis of bimetallic (silver and selenium) nanoparticles and their antitumor and antimicrobial potential. J. Colloid Interface Sci. 2014, 431, 194–199. 10.1016/j.jcis.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Leopoldini M.; Russo N.; Chido S.; Toscano M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. 10.1021/jf060986h. [DOI] [PubMed] [Google Scholar]
- Das S.; Roy P.; Mondal S.; Bera T.; Mukherjee A. One-pot synthesis of gold nanoparticles and application in chemotherapy of wild and resistant type visceral leishmaniasis. Colloids Surf., B 2013, 107, 27–34. 10.1016/j.colsurfb.2013.01.061. [DOI] [PubMed] [Google Scholar]
- Bukhari S. B.; Memon S.; Mahroof-Tahir M.; Bhanger M. I. Synthesis, characterization and antioxidant activity copper-quercetin complex. Spectrochim. Acta, Part A 2009, 71, 1901–1906. 10.1016/j.saa.2008.07.030. [DOI] [PubMed] [Google Scholar]
- Krishnaraj C.; Ramachandran R.; Mohan K.; Kalaichelvan P. T. Optimization for rapid synthesis of silver nanoparticles and its effect on phytopathogenic fungi. Spectrochim. Acta, Part A 2012, 93, 95–99. 10.1016/j.saa.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Nabavi S. M.; Nabavi S. F.; Eslami S.; Moghaddam H. A. In vivo protective effects of quercetin against sodium fluoride-induced oxidative stress in the hepatic tissue. Food Chem. 2012, 132, 931–935. 10.1016/j.foodchem.2011.11.070. [DOI] [Google Scholar]
- Salmani J. M. M.; Xiao-Ping Z.; Jacob J. A.; Bao-An C. Apigenin’s anticancer properties and molecular mechanisms of action: recent advances and future perspectives. Chin. J. Nat. Med. 2017, 15, 321–329. 10.1016/S1875-5364(17)30052-3. [DOI] [PubMed] [Google Scholar]
- Plaper A.; Golob M.; Hafner I.; Oblak M.; Šolmajer T.; Jerala R. Characterization of quercetin binding site on DNA gyrase. Biochem. Biophys. Res. Commun. 2003, 306, 530–536. 10.1016/S0006-291X(03)01006-4. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J.; Kahne D.; Walker S. The bacterial cell envelope. Cold Spring Harbor Perspect. Biol. 2010, 2, a000414 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussurget O.; Pizarro-Cerda J.; Cossart P. Molecular determinants of Listeria monocytogenes virulence. Annu. Rev. Microbiol. 2004, 58, 587–610. 10.1146/annurev.micro.57.030502.090934. [DOI] [PubMed] [Google Scholar]
- Kathariou S. Listeria monocytogenes virulence, and pathogenicity, a food safety perspective. J. Food Prot. 2002, 65, 1811–1829. 10.4315/0362-028X-65.11.1811. [DOI] [PubMed] [Google Scholar]
- Lyczak J. B.; Cannon C. L.; Pier G. B. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist1. Microbes Infect. 2000, 2, 1051–1060. 10.1016/S1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- Berrang M.; Brackett R.; Beuchat L. Growth of Aeromonas hydrophila on fresh vegetables stored under a controlled atmosphere. Appl. Environ. Microbiol. 1989, 55, 2167–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracey M.; Burke V.; Robinson J. Aeromonas-associated gastroenteritis. Lancet 1982, 320, 1304–1306. 10.1016/S0140-6736(82)91510-0. [DOI] [PubMed] [Google Scholar]
- Hsueh P.-R.; Liu C.-Y.; Luh K.-T. Current status of antimicrobial resistance in Taiwan. Emerging Infect. Dis. 2002, 8, 132–137. 10.3201/eid0802.010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y.; Peng A.; Wang B.; Ma L.; Peng G.; Du Y.; Tang J. Synthesis and biological evaluation of phosphorylated flavonoids as potent and selective inhibitors of cholesterol esterase. Eur. J. Med. Chem. 2014, 74, 751–758. 10.1016/j.ejmech.2013.03.025. [DOI] [PubMed] [Google Scholar]
- Kopacz M. Quercetin- and morinsulfonates as analytical reagents. J. Anal. Chem. 2003, 58, 225–229. 10.1023/A:1022630319311. [DOI] [Google Scholar]
- Wu T.; He M.; Zang X.; Zhou Y.; Qiu T.; Pan S.; Xiaoyun X. A structure-activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim. Biophys. Acta, Biomembr. 2013, 1828, 2751–2756. 10.1016/j.bbamem.2013.07.029. [DOI] [PubMed] [Google Scholar]
- Xiao J. Dietary flavonoid aglycones and their glycosides: which show better biological significance?. Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. 10.1080/10408398.2015.1032400. [DOI] [PubMed] [Google Scholar]
- Osawa K.; Yasuda H.; Maruyama T.; Morita H.; Takeya K.; Itokawa H. Isoflavonones from the heartwood of Swartzia polphylla and their antibacterial activity against cariogenic bacteria. Chem. Pharm. Bull. 1992, 40, 2970–2974. 10.1248/cpb.40.2970. [DOI] [PubMed] [Google Scholar]
- Vazquez-Armenta F.; Bernal-Mercado A.; Lizardi-Mendoza J.; Silva-Espinoza B.; Cruz-Valenzuela M.; Gonzalez-Aguilar G.; Nazzaro F.; Fratianni F.; Ayala-Zavala J. F. Phenolic extracts from grape stems inhibit Listeria monocytogenes motility and adhesion to food contact surfaces. J. Adhes. Sci. Technol. 2017, 32, 1–19. [Google Scholar]
- Goyal P.; Aggarwal B. K.; Garg S. A study on combinatorial effects of various flavonoids for their antibacterial potential against clinically significant bacterial species. Hacettepe J. Biol. Chem. 2010, 38, 255–258. [Google Scholar]
- Orhan D. D.; Özçelik B.; Özgen S.; Ergun F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010, 165, 496–504. 10.1016/j.micres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Anderl J. N.; Zahler J.; Roe F.; Stewart P. S. Role of nutrient limitation and stationary-phase existence in Klebsiella pneumonia biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 2003, 47, 1251–1256. 10.1128/AAC.47.4.1251-1256.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushnie T. P. T.; Lamb A. J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Tsuchiya H. Structure-dependent membrane interaction of flavonoids associated with their bioactivity. Food Chem. 2010, 120, 1089–1096. 10.1016/j.foodchem.2009.11.057. [DOI] [Google Scholar]
- Stapleton P. D.; Shah S.; Hamilton-Miller J. M.; Hara Y.; Nagaoka Y.; Kumagai A.; Uesato S.; Taylor P. W. Anti-Staphylococcus aureus activity and oxacillin resistance modulating the capacity of 3-O-acyl-catechins. Int. J. Antimicrob. Agents 2004, 24, 374–380. 10.1016/j.ijantimicag.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Godet C.; Elsendoorn A.; Roblot R. Benefit of CT scanning for assessing pulmonary disease in the immunodepressed patient. Diagnostic Interventional Imaging 2012, 93, 425–430. 10.1016/j.diii.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Mori A.; Nishino C.; Enoki N.; Tawata S. Antibacterial activity, and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry 1987, 26, 2231–2234. 10.1016/S0031-9422(00)84689-0. [DOI] [Google Scholar]
- Ohemeng K. A.; Schwender C. F.; Fu K. P.; Barrett J. F. DNA gyrase inhibitory and antibacterial activity of some flavones(1). Bioorg. Med. Chem. Lett. 1993, 3, 225–230. 10.1016/S0960-894X(01)80881-7. [DOI] [Google Scholar]
- Kopacz M. Quercetin and morinsulfonates as analytical reagents. J. Anal. Chem. 2003, 58, 225–229. 10.1023/A:1022630319311. [DOI] [Google Scholar]
- Okello V. A.; Zhou A.; Chong J.; Knipfing M. T.; Doetschman D.; Sadik O. A.; et al. Reduction of Hexavalent Chromium using Naturally-derived Flavonoids. Environ. Sci. Technol. 2012, 46, 10743–10751. 10.1021/es301060q. [DOI] [PubMed] [Google Scholar]
- Gao Q.; Lian G.; Lin F. The first total synthesis of 7-< i> O</i>-β-d-glucopyranosyl-4′-< i> O</i>-α-l-rhamnopyranosyl apigenin via a hexanoyl ester-based protection strategy. Carbohydr. Res. 2009, 344, 511–515. 10.1016/j.carres.2008.12.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.