Abstract
α-Pinene-modified triethoxysilane (α-PTES) was synthesized by hydrosilylation in the presence of Karstedt’s catalyst. The structure of α-PTES was determined by Fourier transform infrared spectroscopy and nuclear magnetic resonance. Under the catalysis of an organotin catalyst, α-PTES, which was the cross-linking agent, and the hydroxy-terminated poly(dimethylsiloxane) matrix were utilized to prepare the room-temperature vulcanized silicone rubber. Morphology, thermal performance, and mechanical properties of the modified silicone rubber were investigated by scanning electron microscopy, thermal gravimetric analysis, dynamic mechanical analysis, and a universal testing machine. Because of the strong rigidity of the ring structure of α-pinene, the thermal and mechanical properties of modified silicone rubber were improved greatly than those of the silicone rubber, and the cross-linking agent of which was methyltriethoxysilane. Results showed that the tensile strength and the break at elongation increased by 69.2 and 125%, respectively, and they are nearly doubled compared to the unmodified silicone rubber.
Introduction
The structure of the polysiloxane contains inorganic and organic groups, which make polysiloxane have the function of inorganic and organic materials.1 Room-temperature vulcanized (RTV) silicone rubber is a polymer elastic material, which is synthesized from polysiloxane, and shows heat resistance, weather resistance, biocompatibility, ozone resistance, electrical insulation, chemical stability, and so on.2−5 In addition, RTV silicone rubber is widely applied in the electrical, petroleum, chemical, construction, munition, aerospace, and space exploration fields due to its unique properties.6,7 However, the poor mechanical properties cause a weak intermolecular force of the RTV silicone rubber, limiting the practical application.8 Thus, it is highly desirable to improve the mechanical properties of the RTV silicone rubber.9
Chemical modification, such as modification of the cross-linking agent and poly(dimethylsiloxane) (PDMS) matrix, and filler incorporation could significantly improve the mechanical properties and thermostability of the silicone rubber.10−14 The utilized fillers that made of carbon nanotubes (CNTs) and iron oxide (Fe2O3) were made by Li et al., and the thermostability of the silicone rubber was improved.15 By using polymethyl(ketoxime)siloxane (PMKS) as a new cross-linking agent, Zhan et al. improved not only thermal but also mechanical properties of room-temperature vulcanized (RTV) silicone rubbers.16 Studies on the thermal degradation properties of poly(dimethyldiphenyl)siloxane copolymers, PDMS, and poly(dimethyldiphenylsiloxane) were conducted by Deshpande and Rezac, and the results showed the reduction of activation energy when phenyl groups were introduced.17,18
In recent years, with the global resource crisis and awareness of environmental protection, renewable biomass resources are good alternatives for petroleum-based compounds.19−21 The rigid structures, such as lignin, cyclodextrin, and rosin, of biomass resources have great potential to prepare polymers with high mechanical and thermal properties.22,23 Choi et al. studied the RTV silicone rubber and significantly improved its mechanical properties and thermal performance by using the rosin-grafted poly(dimethylsiloxane) (RGSO).24 The effects of acrylpimaric acid-modified aminopropyltriethoxysilane (APA-APTES), which was the cross-linking agent, on the thermostability and mechanical properties of modified silicone rubber were studied by Yang et al.25 The maleated rosin-modified fluorosilicone rubbers (MR-FSR) were synthesized by Xu et al., and the imide-containing vinyl fluorosilicone resin (MR-VFS) was the novel cross-linking agent. The results showed that the high-temperature thermostability and tearing strength of fluorosilicone rubber could be greatly increased by MR-VFS.26 Li et al. found that the rosin-modified aminopropyltriethoxysilane (RA) was the cross-linking agent, and the hydroxy-terminated poly(dimethylsiloxane) (PDMS) was the matrix; RTV silicone rubber could be modified under the catalysis of an organotin, which exhibited a significant enhancement in the mechanical properties and thermostability significantly.7 Because of the rosin’s polar hydrogenated phenanthrene ring structure and good rigidity, improvements in the mechanical properties and thermal performance of silicone rubber could be successfully achieved according to the research mentioned above.
The two main components secreted by pines are rosin and turpentine.27,28 Turpentine is mainly composed of terpene compounds such as α-pinene and β-pinene and has strong chemical reactivity for oxidation, isomerization, rearrangement, addition, polymerization, and other chemical reactions.29 In addition, turpentine has similar rigidity and chemical stability to petroleum-based cycloaliphatic compounds.30,31 These properties make turpentine have great potential in the preparation of polymers with high thermal stability and mechanical properties. Xu et al. prepared the bio-based alkyd resin by utilizing raw materials including tung oil and turpentine-based acrylate (IBOA). The results showed that when the content of IBOA was increased, the water resistance, thermostability, pencil hardness, and mechanical properties of the alkyd resins became much better.32 Wu et al. prepared a two-component aqueous terpene epoxy resin (EP)/polyurethane (PU) composite polymer by cross-linking of terpene-based epoxy resin polyol dispersion prepared from turpentine with polyisocyanate, and the composite product has good gloss, impact strength, adhesion, flexibility, stain resistance, heat resistance, and blocking resistance.33,34 Meanwhile, there was little research on the modification of silicone rubber by turpentine.
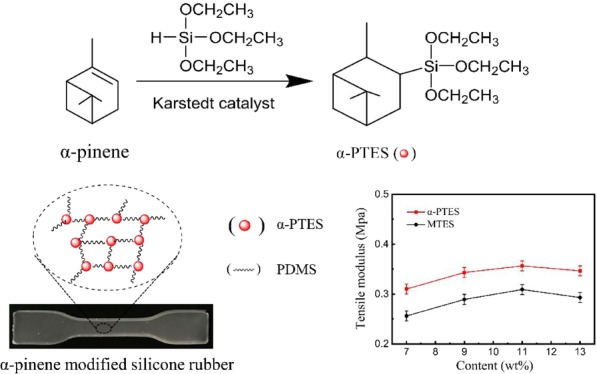
In this work, α-pinanyltriethoxysilane (α-PTES) was prepared by the hydrosilylation of α-pinene and methyltriethoxysilane in the presence of Karstedt’s catalyst. To prepare the RTV silicone rubber (Figure 1), hydroxy-terminated poly(dimethyisiloxane) (PDMS) and α-PTES, which was a new cross-linking agent, were further used. The effects of α-PTES on morphologies and mechanical and thermal properties of modified silicone rubber were investigated in this paper.
Figure 1.
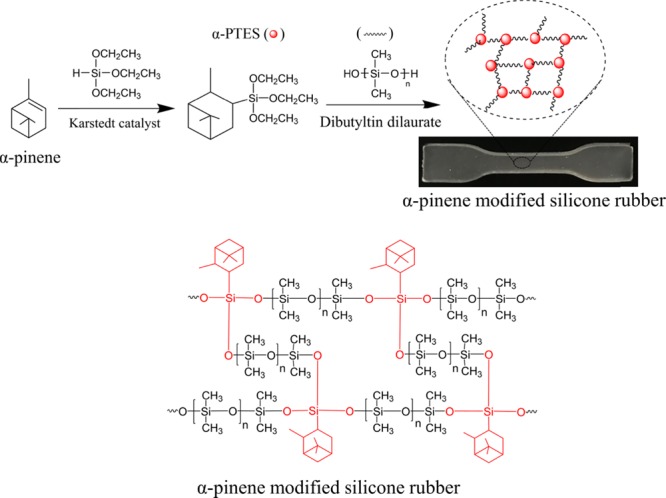
Synthetic route for α-pinene-modified RTV silicone rubber (SRαPT) and pinanyl triethoxysilane (α-PTES).
Results and Discussion
Properties of α-PTES
FT-IR Analysis
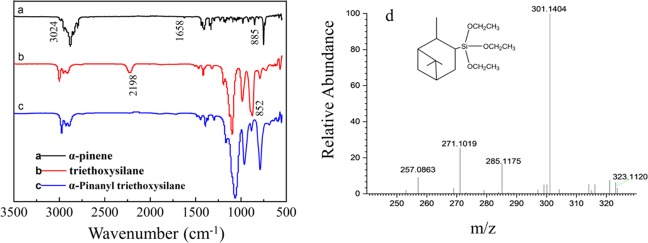
The chemical structures of α-pinene, triethoxysilane, and α-PTES were determined through FT-IR spectra. The spectrum in Figure 2a shows that the absorption peak is at 3024 cm–1 (stretching vibrations due to unsaturated C–H), 885 cm–1 (bending vibration due to unsaturated C–H), and 1658 cm–1 (stretching vibration of C=C). As shown in Figure 2, after hydrosilylation with triethoxysilane, at 1658 cm–1, the C=C stretching vibration of α-pinene disappeared in spectra of Figure 2a,c. The absorption peaks were at 2198 and 852 cm–1 (Si–H stretching) as shown in Figure 2b, which also shows that characteristic peaks of Si–H at 2198 and 852 cm–1 are almost completely disappeared, indicating the successful synthesis of α-PTES (spectra in Figure 2b,c).
Figure 2.
(a–c) FT-IR spectra of (a) α-pinene, (b) triethoxysilane, and (c) α-pinanyl triethoxysilane (α-PTES). (d) Mass spectra of α-pinanyl triethoxysilane (α-PTES).
MS Analysis
As the chemical formula of α-pinanyl triethoxysilane (α-PTES) is C16H32O3Si, the exact mass is 300.2121. As shown in Figure 2d, 285.1175 is the value of mass of α-PTES losing one methyl group, 271.1019 is the value of mass of α-PTES losing one ethyl group, and 257.0863 is the value of mass of α-PTES losing one methyl group and one ethyl group. MS m/z (CI): (M + Na)+ calcd for C16H32O3SiNa 323.2013; found, 323.1120, and this confirms that the target product has been formed.
NMR Analysis
Figure 3 shows the 1H NMR spectra in CDCl3, which further confirmed the structure of α-PTES. The protons of the C=C in α-pinene are related to the fascicles of signal peaks at 4.24 ppm. In addition, at 4.24 ppm, the clusters of signal peaks were resulted from the chemical shifts in Si–H protons. According to curves in Figure 3a,c, the protons of the Si–C for the product are associated with the fascicles of signal peaks at 1.46 and 1.1 ppm, confirming that the target product has been formed. Also, the peak at 7.26 ppm is CDCl3.
Figure 3.
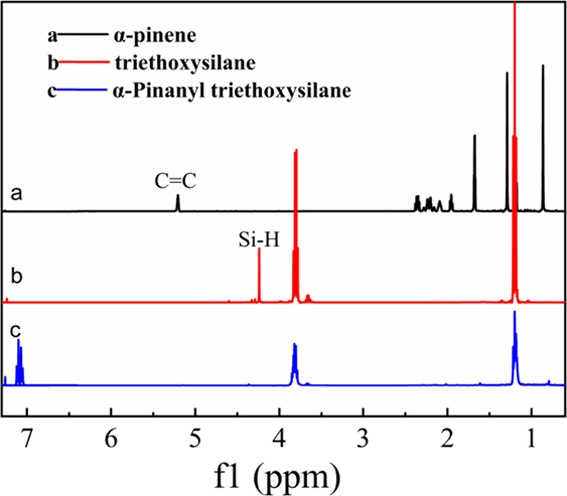
1H NMR spectra of (a) α-pinene, (b) triethoxysilane, and (c) α-pinanyl triethoxysilane (α-PTES).
Characterization of SRαPT and SRTE
Under the condensation reaction of cross-linking agents and poly(dimethylsiloxane) (PDMS), SRαPT and SRTE were synthesized at room temperature using organotin as a catalyst. The components and conditions, such as the cross-linking agent, curing catalyst, environmental conditions, PDMS, and temperature, could affect the cross-linking process. Dibutyltin dilaurate catalyst (180 μL) and PDMS (5000 mPa·s, 30 g) in this experiment were utilized to all the samples. We used the α-PTES instead of MTES as the cross-linking agent to study the effects of α-PTES on the thermal performance and mechanical properties of modified RTV silicone rubber.
Morphologies
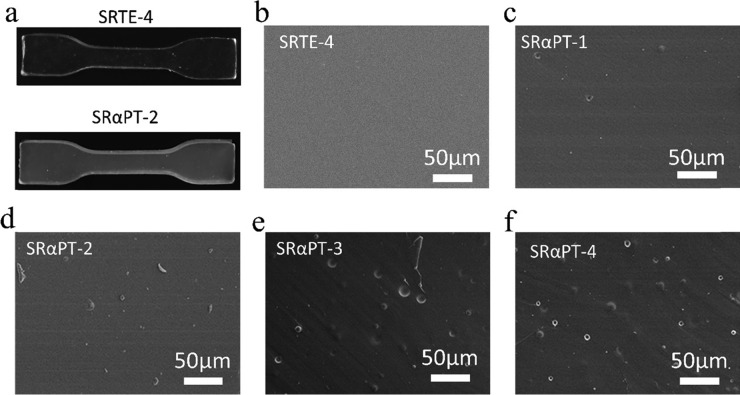
As shown in Figure 4a (SRTE-4), the RTV silicone rubber is colorless and transparent when the cross-linker is MTES. However, when the cross-linking agent is α-PTES instead of MTES, the modified RTV silicone rubber has low transparency (Figure 4a, SRαPT-2). There may be a change in the microstructure. As shown in Figure 4b–f, the morphologies of modified RTV silicone rubbers are characterized by using scanning electron microscopy. Rigid ring structures of α-PTES would increase the molecular chain rigidity of the polymer matrix. Therefore, as the α-PTES increases, the modified RTV silicone rubber becomes rougher than the unmodified silicone rubber.36 In addition, the microphase in RTV silicone rubber would be separated because of the aggregation of the rigid ring groups in α-pinene.13,37
Figure 4.
Microstructure and morphology of modified silicone rubber. (a) Photographs of SRTE-4 and SRαPT-2. (b) SEM image of SRTE-4. (c) SEM image of SRαPT-1. (d) SEM image of SRαPT-2. (e) SEM image of SRαPT-3. (f) SEM image of SRαPT-4.
Thermal Properties
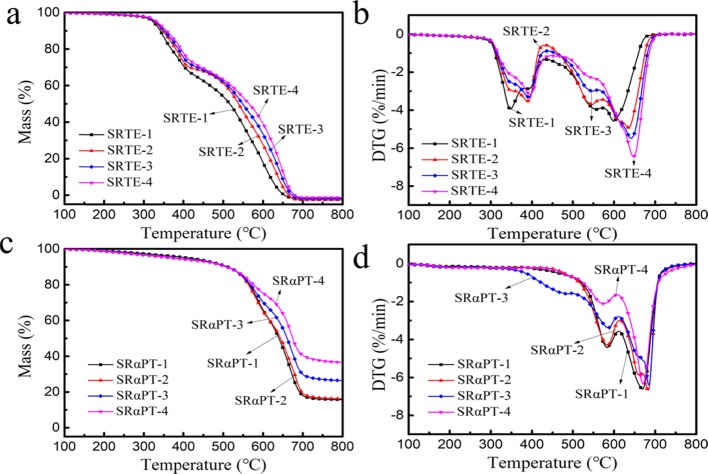
By conducting the TGA, we investigated the influence of different cross-linker contents on the thermal properties of modified silicone rubber. There is obvious three-stage weightlessness in the RTV silicone rubber samples. The first stage is mainly the decomposition of RTV silicone rubber side chain groups from 300 to 500 °C, for example, methyl collapses to methylene radicals. The second stage is the RTV silicone rubber’s main chain fracture process that generates low molecular rings primarily from 500 to 600 °C. The third stage is a carbonization process from 600 to 800 °C (Figure 5).
Figure 5.
(a) TG curves of SRTE. (b) DTG curves of SRTE. (c) TG curves of SRαPT. (d) DTG curves of SRαPT.
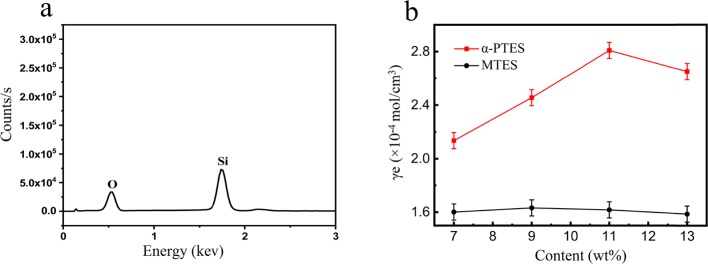
We have tested the energy dispersive spectrometry (EDS) of the RSTE-4 residue after the TGA in Figure 6a. From the diagram in Figure 6a, we can see that the elements of the RSTE-4 residue are mainly Si and O, and this is because of the restrained silicone rubber macromolecules with the increase of MTES cross-linking.
Figure 6.
(a) EDS of SRTE-4 residue. (b) Cross-linking densities of SRTE and SRαPT.
Cross-Linking Density
The cross-linking density of RTV silicone rubber has an influence on its mechanical properties. The cross-linking density was measured by an equilibrium swelling method. As shown in Figure 6b, the α-PTES-modified silicone rubber (SRαPT) shows an increasing cross-linking density when the content of the cross-linking agent α-PTES was increased from 7 to 11 wt % and has a slightly decrease as α-PTES increased to 13%. However, the change in the cross-linking density of silicone rubber (SRTE) is not obvious. Such an increase can be caused by chain entanglement and intermolecular forces, which increase with an increasing content of α-PTES.
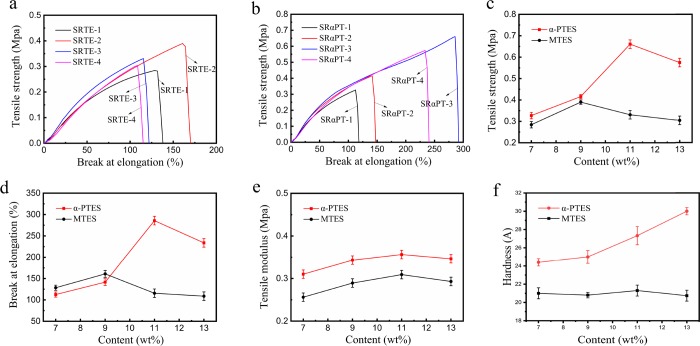
Mechanical Properties
As shown in Figure 7a,b, the α-PTES-modified RTV silicone rubber showed significantly higher mechanical properties than the unmodified silicone rubber. Figure 7c,d shows that the break at elongation and tensile strength of the α-PTES-modified silicone rubber were significantly improved. The tensile strength of SRαPT-3 was 0.66 MPa, and the break at elongation was 285.8%. They are nearly doubled compared to the SRTE-2 sample. When the elongation is 100%, the tensile moduli of all SRαPT are higher than those of all SRTE in Figure 7e. This is because the rigid ring structure of the α-PTES increased the rigid segment of the polysiloxane and improved the interaction between the molecules. Thus, it is highly desirable to improve the mechanical properties of the RTV silicone rubber.38−40
Figure 7.
(a) Stress–strain curves of SRTE. (b) Stress–strain curves of SRαPT. (c) Tensile strength of SRαPT and SRTE. (d) Break at elongation of SRαPT and SRTE. (e) Tensile moduli of SRαPT and SRTE at 100% elongation. (f) Hardness of RTV silicone rubber.
Moreover, as the α-PTES content increases from 0 to 13 wt %, the mechanical properties, tensile modulus, and the break at elongation of the RTV silicone rubber increase then decrease and reach the maximum when the α-PTES content is 11%. Although the microphase separation would occur, a lower α-PTES cross-linking agent, which could be homogeneously dispersed in the matrix, could be utilized to cure the silicone. As the α-PTES increased, the mechanical properties of the RTV silicone rubber were improved. Figure 7c–e shows that the break at elongation, mechanical properties, and tensile modulus of RTV silicone rubber begin decreasing when the α-PTES content exceeding 11 wt %. The decrease may result from the excess α-PTES, which could cause agglomeration, self-cross-linking, and microphase separation.13,41 This indicated that the mechanical properties of the silicone rubber could be positively affected by the uniform distribution of the cross-linking agent (α-PTES). The hardness of modified silicone rubber (SRαPT-4, 30 A) is better than that of silicone rubber (SRTE-4, 21 A) (Figure 7f). In addition, with increasing cross-linking agent (α-PTES), the hardness of SRαPT increased from 24 to 30 A.
Dynamic Mechanical Properties
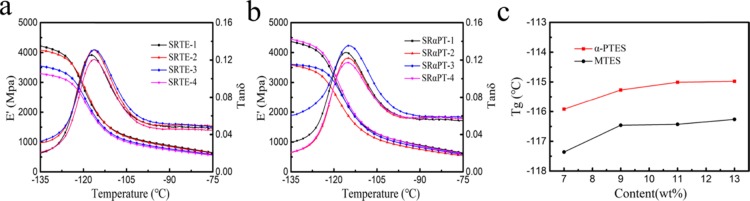
The curves of storage moduli (E′) of SRαPT and SRTE are shown in Figure 8a,b. The E′ is closely related to the chemical structure and cross-linking density of RTV silicone rubber. Compared to SRER-1 (4219 MPa), the E′ values of SRαPT-4 (4446 MPa) increased to 227 MPa. The silicone chain fluidity of the RTV silicone rubber would have significant restrictions because the increased modulus would enhance the chain entanglement and molecular chain rigidity. As the excess α-PTES would cause the self-cross-linking and agglomeration, the E′ values of SRαPT-4 decreased slightly compared to those of SRαPT-3, resulting in decreasing cross-linking density.13,38,42
Figure 8.
(a) Curves of storage modulus (E′) and tan δ of SRTE. (b) Curves of storage modulus (E′) and tan δ of SRαPT. (c) Tg curves of SRαPT and SRTE.
As shown from Figure 8a,b, there is one Tg for each sample, which is between −135 and −75 °C. The Tg of α-PTES-modified silicone rubber increased from −116.3 (SRαPT-1) to −115.0 °C (SRαPT-4), and the increase was greater than that of the unmodified silicone rubber (SRTE-4). This is because the polysiloxane chain mobility is limited due to the rigid ring structure of α-PTES and the corresponding less flexible polysiloxane chain.23,43,44Figure 8c shows that the glass-transition temperature (Tg) of each modified silicone rubber is confirmed by the peak of the tan δ curve.
Conclusions
Under the catalysis of the Karstedt’s catalyst, we synthesized a new cross-linking agent for the preparation of RTV silicone rubber, α-PTES, through the hydrosilylation of α-pinene with triethoxysilane. 1H NMR and FT-IR confirmed the chemical structure of the cross-linking agent. The rigid ring structure and polyfunctionality of α-pinene would result in increasing cross-linking density of the modified RTV silicone rubber. Thus, the mechanical and thermal properties of the α-PTES-modified RTV silicone rubber showed significantly better than those of the unmodified silicone rubber, and the modified silicone rubber (SRαPT-3) has a tensile strength of 0.66 MPa and a break at elongation of 285.8%, which increase by 69.2 and 125%, respectively, and they are nearly doubled compared to the unmodified silicone rubber (SRTE-2). In addition, the E′ values of SRαPT-4 (4446 MPa) increased by 227 MPa compared with those of SRER-1 (4219 MPa). The increased chain entanglement and molecular chain rigidity would enhance the modulus, and the silicone chain fluidity of the silicone rubber would also have significant restrictions. This study shows that by using α-pinene as a new cross-linking agent, the improvement of the mechanical and thermal properties of the silicone rubber could be achieved.
Materials and Methods
Materials
α-Pinene (ω = 98%), dibutyltin dilaurate (ω = 95%), and methyltriethoxysilane (MTES, ω = 98%) were purchased from Shanghai Aladdin Technology Co., Ltd. Triethoxysilane (ω ≥ 99%) was purchased from Shandong Silicon Branch New Material Co., Ltd. Karstedt’s catalyst (Pt of ∼2%, in xylene) was purchased from Shanghai Neutron Star Chemical Technology Co., Ltd. Hydroxy-terminated poly(dimethylsiloxane) (PDMS, 5000 mPa·s) was purchased from Hubei New Universal Chemical Co., Ltd. The chemicals were not further purified in this study.
Synthesis of α-Pinanyl-Modified Triethoxysilane (α-PTES)
The reflux condenser, constant pressure dropping funnel, and thermometer were assembled on a 50 mL three-necked flask that was correspondingly filled with α-pinene (5 g), Karstedt’s catalyst (in xylene, Pt of ∼2 wt %, 82 μL), and triethoxysilane (9 g). The mixture was heated to 100 °C under continuous stirring for 60 h at a speed of 250 r/min. Finally, the liquid product and the unreacted raw material were distilled off under reduced pressure and normal pressure, respectively.
Preparation of Modified RTV Silicone Rubber
While using a nitrogen atmosphere as a protection, we added both the cross-linking agents (α-PTES or MTES, 2.1 g) and the PDMS (30 g) to the three-necked flask. The blend was then continuously stirred at a speed of 1500 r/min for 15 min at room temperature. Next, we added 180 μL of dibutyltin dilaurate catalyst to the same flask and stirred the mixture continuously for 15 min at a speed of 1500 r/min. Finally, we removed the bubbles and rapidly poured the mixture into a Teflon mold. To make the surface of the obtained modified silicone rubber sheet smooth, we cured the mixture for 7 days at room temperature. The ingredients of each sample are shown in Table 1 in detail.
Table 1. Ingredient Compositions That Were Used To Prepare Modified Silicone Rubber.
| sample | PDMS (g) | α-PTES (g) | MTES (g) | catalyst (μL) | α-PTES (wt %) | MTES (wt %) |
|---|---|---|---|---|---|---|
| SRTE-1 | 30 | 0 | 2.1 | 180 | 0 | 7 |
| SRTE-2 | 30 | 0 | 2.7 | 180 | 0 | 9 |
| SRTE-3 | 30 | 0 | 3.3 | 180 | 0 | 11 |
| SRTE-4 | 30 | 0 | 3.9 | 180 | 0 | 13 |
| SRαPT-1 | 30 | 2.1 | 0 | 180 | 7 | 0 |
| SRαPT-2 | 30 | 2.7 | 0 | 180 | 9 | 0 |
| SRαPT-3 | 30 | 3.3 | 0 | 180 | 11 | 0 |
| SRαPT-4 | 30 | 3.9 | 0 | 180 | 13 | 0 |
In all ingredient compositions, dibutyltin dilaurate (180 μL) and PDMS (30 g) were respectively functioned as the catalyst and the matrix. As shown in Table 1, the mass fractions of α-PTES and MTES both increase from 7 to 13 wt %.
Measurements and Characterization
FT-IR
Through attenuated total reflectance (ATR), Fourier transform infrared (FT-IR) spectra were performed on a Thermo Scientific Nicolet IS10 spectrometer (Nicolet). Each sample was analyzed through 16 scans between 500 and 3500 cm–1 at a 4 cm–1 resolution.
NMR
At two frequencies, 400.13 and 100.61 MHz, an AV400 spectrometer (Bruker, Germany) recorded the 1H NMR spectra for α-PTES. We used tetramethylsilane (TMS) and deuterochloroform (CDCl3) as the internal standard and solvent for α-PTES, respectively. The signals of TMS and CDCl3 were the chemical shift values.
Mass Spectrometry
Mass spectra were determined by a Reflex III (Bruker, Billerica, MA) time-of-flight mass spectrometer equipped with a reflectron and a 1.25 m flight tube. The sample was analyzed with the accelerating voltage (19 kV) and reflectron voltage (20 kV), and the delay times were 200 ns. All the experiments were carried out in a reflectron mode.
Density
Through a pycnometer, the samples’ densities were measured.
TG
A TG209F1 (Netzsch, Germany) was used to analyze the thermal stability of the sample. The samples were heated from 25 to 800 °C with a heating rate of 10 °C/min in an Al2O3 crucible under a nitrogen atmosphere.
Hardness
The hardness of silicone rubber was determined by an LX-A durometer (Eide fort, China) at room temperature and ∼65% relative humidity.
Mechanical Properties
To analyze the mechanical properties of samples, a UTM6502 universal testing machine (Suns) with a capacity of 500 N was used. The analysis was conducted after preparing five dumbbell-shaped specimens by using a crosshead with an RH around 50% at 23 °C and a speed of 500 mm/min. The data was expressed as the average of five measurements.
SEM
At a voltage of 10 kV, the morphologies of all samples were observed through a scanning electron microscope (QUANTA 200, FEI, Holland). After coating the fractured surface of the samples with gold, an analysis was conducted.
EDS
The element composition of silicone rubber was determined by X-ACT energy dispersive X-ray spectroscopy (Oxford, England).
DMA
A DMA Q800 (TA) was used to analysis the dynamic mechanical property of the sample. Under the stretching mode, at a heating rate of 3 °C/min and with a 1 Hz frequency, the samples were analyzed with a scanning temperature range from −135 to −75 °C.
Cross-Linking Density
An equilibrium swelling method was used to recalculate the cross-linking densities of SRαPT and SRTE. Before analysis, at a temperature of 25 °C, SRαPT or SRTE (around 0.2 g) was immersed into toluene (25 mL) that was in a sealed container for 48 h. After taking the samples out of toluene and weighing them after removing the excess toluene by a filter paper, the samples were immersed into toluene for another time. We repeated the above steps every 3 h until reaching the swelling balance. According to eqs 1 and 2, the cross-linking density was obtained.8,35
| 1 |
| 2 |
In the above formula, φ is the volume fraction, m0 is the original sample weight, ρ is the modified silicone rubber density, ms is the swelling weight of the modified silicone rubber, ρ1 (0.87 g/cm3) is the toluene density, and ν0 (106.54 cm3/mol) is the toluene molar volume. χ1 (0.465) is the interaction parameter of the solvent and polymer, MC is the mean molecular weight of the cross-linking points, and γe represents the cross-linking density.
Acknowledgments
This work was supported by National Natural Science Foundation of China (31600462) and Mudanjiang Normal University National Project Cultivation Project (GP2018002).
The authors declare no competing financial interest.
References
- Pouget E.; Tonnar J.; Lucas P.; Lacroix-Desmazes P.; Ganachaud F.; Boutevin B. Well-Architectured Poly(dimethylsiloxane)-Containing Copolymers Obtained by Radical Chemistry. Chem. Rev. 2010, 110, 1233–1277. 10.1021/cr8001998. [DOI] [PubMed] [Google Scholar]
- Tong Y.; Liu H.; Chen A.; Guan H.; Kong J.; Liu S.; He C. Effect of Surface Chemistry and Morphology of Silica on the Thermal and Mechanical Properties of Silicone Elastomers. J. Appl. Polym. Sci. 2018, 135, 46646. 10.1002/app.46646. [DOI] [Google Scholar]
- Deng S.; Binauld S.; Mangiante G.; Frances J. M.; Charlot A.; Bernard J.; Zhou X.; Fleury E. Microcrystalline Cellulose as Reinforcing Agent in Silicone Elastomers. Carbohydr. Polym. 2016, 151, 899–906. 10.1016/j.carbpol.2016.06.035. [DOI] [PubMed] [Google Scholar]
- Feng L.; Li S.; Feng S. Preparation and Characterization of Silicone Rubber with High Modulus via Tension Spring-Type Crosslinking. RSC Adv. 2017, 7, 13130–13137. 10.1039/C7RA00293A. [DOI] [Google Scholar]
- Sun Z.; Huang Q.; Wang Y.; Zhang L.; Wu Y. Structure and Properties of Silicone Rubber/Styrene-Butadiene Rubber Blends with in Situ Interface Coupling by Thiol-ene Click Reaction. Ind. Eng. Chem. Res. 2017, 56, 1471–1477. 10.1021/acs.iecr.6b04146. [DOI] [Google Scholar]
- Wei C.-S.; Lu A.; Sun S.-M.; Wei X.-W.; Zho X.-Y.; Sun J. Establishment of Constitutive Model of Silicone Rubber Foams Based on Statistical Theory of Rubber Elasticity. Chin. J. Polym. Sci. 2018, 36, 1077–1083. 10.1007/s10118-018-2125-8. [DOI] [Google Scholar]
- Li Q.; Huang X.; Liu H.; Shang S.; Song Z.; Song J. Properties Enhancement of Room Temperature Vulcanized Silicone Rubber by Rosin Modified Aminopropyltriethoxysilane as a Cross-linking Agent. ACS Sustainable Chem. Eng. 2017, 5, 10002–10010. 10.1021/acssuschemeng.7b01943. [DOI] [Google Scholar]
- Xu Q.; Pang M.; Zhu L.; Zhang Y.; Feng S. Mechanical Properties of Silicone Rubber Composed of Diverse Vinyl Content Silicone Gums Blending. Mater. Des. 2010, 31, 4083–4087. 10.1016/j.matdes.2010.04.052. [DOI] [Google Scholar]
- Chen W.; Liu Y.; Xu C.; Liu Y.; Wang Q. Synthesis and Properties of an Intrinsic Flame Retardant Silicone Rubber Containing Phosphaphenanthrene Structure. RSC Adv. 2017, 7, 39786–39795. 10.1039/C7RA06798D. [DOI] [Google Scholar]
- Shang S.; Gan L.; Yuen M. C.-w.; Jiang S.-x.; Luo N. M. Carbon Nanotubes Based High Temperature Vulcanized Silicone Rubber Nanocomposite with Excellent Elasticity and Electrical Properties. Composites, Part A 2014, 66, 135–141. 10.1016/j.compositesa.2014.07.014. [DOI] [Google Scholar]
- Shi Y.; Gao X.; Zhang D.; Liu Y.; Huang G. Synthesis and Thermal Properties of Modified Room Temperature Vulcanized (RTV) Silicone Rubber Using Polyhedral Oligomeric Silsesquioxane (POSS) as a Cross Linking Agent. RSC Adv. 2014, 4, 41453–41460. 10.1039/C4RA06706A. [DOI] [Google Scholar]
- Rezakazemi M.; Vatani A.; Mohammadi T. Synergistic interactions Between POSS and Fumed Silica and Their Effect on the Properties of Crosslinked PDMS Nanocomposite Membranes. RSC Adv. 2015, 5, 82460–82470. 10.1039/C5RA13609A. [DOI] [Google Scholar]
- Xu T.; Liu H.; Song J.; Shang S.; Song Z.; Zou K.; Yang C. Synthesis and Characterization of Novel Fluorosilicone Rubber Using Imide Modified Vinyl-Containing Fluorosilicone Resin as Cross-Linker. J. Polym. Sci., Part A: Polym. Chem. 2015, 53, 1769–1776. 10.1002/pola.27619. [DOI] [Google Scholar]
- Meng Y.; Chu J.; Xue J.; Liu C.; Wang Z.; Zhang L. Design and Synthesis of Non-Crystallizable, Low-Tg Polysiloxane Elastomers with Functional Epoxy Groups Through Anionic Copolymerization and Subsequent Epoxidation. RSC Adv. 2014, 4, 31249–31260. 10.1039/C4RA02293A. [DOI] [Google Scholar]
- Li H.; Tao S.; Huang Y.; Su Z.; Zheng J. The Improved Thermal Oxidative Stability of Silicone Rubber by Using Iron Oxide and Carbon Nanotubes as Thermal Resistant Additives. Compos. Sci. Technol. 2013, 76, 52–60. 10.1016/j.compscitech.2012.12.019. [DOI] [Google Scholar]
- Zhan X.; Cai X.; Zhang J. A Novel Crosslinking Agent of Polymethyl(ketoxime)siloxane for Room Temperature Vulcanized Silicone Rubbers: Synthesis, Properties and Thermal Stability. RSC Adv. 2018, 8, 12517–12525. 10.1039/C7RA13375H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G.; Rezac M. E. The Effect of Phenyl Content on the Degradation of Poly(dimethyl diphenyl) Siloxane Copolymers. Polym. Degrad. Stab. 2001, 74, 363–370. 10.1016/S0141-3910(01)00186-0. [DOI] [Google Scholar]
- Deshpande G.; Rezac M. E. Kinetic Aspects of the Thermal Degradation of Poly(dimethyl siloxane) and Poly(dimethyl diphenyl siloxane). Polym. Degrad. Stab. 2002, 76, 17–24. 10.1016/S0141-3910(01)00261-0. [DOI] [Google Scholar]
- Qin J.; Liu H.; Zhang P.; Wolcott M.; Zhang J. Use of Eugenol and Rosin as Feedstocks for Biobased Epoxy Resins and Study of Curing and Performance Properties. Polym. Int. 2014, 63, 760–765. 10.1002/pi.4588. [DOI] [Google Scholar]
- Yan X.; Zhai Z.; Song Z.; Shang S.; Rao X. Synthesis and Properties of Polyester-Based Polymeric Surfactants from Diterpenic Rosin. Ind. Crops Prod. 2017, 108, 371–378. 10.1016/j.indcrop.2017.06.060. [DOI] [Google Scholar]
- Baroncini E. A.; Yadav S. K.; Palmese G. R.; Stanzione J. F. III Recent Advances in Bio-Based Epoxy Resins and Bio-Based Epoxy Curing Agents. J. Appl. Polym. Sci. 2016, 133, 44103. 10.1002/app.44103. [DOI] [Google Scholar]
- Zhuo X.; Liu C.; Pan R.; Dong X.; Li Y. Nanocellulose Mechanically Isolated from Amorpha fruticosa Linn. ACS Sustainable Chem. Eng. 2017, 5, 4414–4420. 10.1021/acssuschemeng.7b00478. [DOI] [Google Scholar]
- Xin J.; Li M.; Li R.; Wolcott M. P.; Zhang J. Green Epoxy Resin System Based on Lignin and Tung Oil and Its Application in Epoxy Asphalt. ACS Sustainable Chem. Eng. 2016, 4, 2754–2761. 10.1021/acssuschemeng.6b00256. [DOI] [Google Scholar]
- Choi S. J.; Yim T.; Cho W.; Mun J.; Jo Y. N.; Kim K. J.; Jeong G.; Kim T.-H.; Kim Y.-J. Rosin-Embedded Poly(acrylic acid) Binder for Silicon/Graphite Negative Electrode. ACS Sustainable Chem. Eng. 2016, 4, 6362–6370. 10.1021/acssuschemeng.6b00920. [DOI] [Google Scholar]
- Yang X.; Li Q.; Li Z.; Xu X.; Liu H.; Shang S.; Song Z. Preparation and Characterization of Room-Temperature-Vulcanized Silicone Rubber Using Acrylpimaric Acid-Modified Aminopropyltriethoxysilane as a Cross-Linking Agent. ACS Sustainable Chem. Eng. 2019, 7, 4964–4974. 10.1021/acssuschemeng.8b05597. [DOI] [Google Scholar]
- Xu T.; Liu H.; Song J.; Shang S.-b.; Song Z.; Zou K.; Yang C. Synthesis and Characterization of Maleated Rosin-Modified Fluorosilicone Resin and Its Fluorosilicone Rubber. J. Appl. Polym. Sci. 2015, 132, 41888. 10.1002/app.41888. [DOI] [Google Scholar]
- El-Ghazawy R. A.; El-Saeed A. M.; Al-Shafey H. I.; Abdul-Raheim A. R. M.; El-Sockary M. A. Rosin Based Epoxy Coating: Synthesis, Identification and Characterization. Eur. Polym. J. 2015, 69, 403–415. 10.1016/j.eurpolymj.2015.06.025. [DOI] [Google Scholar]
- Qu J.; Cao C. Y.; Dou Z. F.; Liu H.; Yu Y.; Li P.; Song W. G. Synthesis of Cyclic Carbonates: Catalysis by an Iron-Based Composite and the Role of Hydrogen Bonding at the Solid/Liquid Interface. ChemSusChem 2012, 5, 652–655. 10.1002/cssc.201100839. [DOI] [PubMed] [Google Scholar]
- Miyata T.; Matsumoto K.; Endo T.; Yonemori S.; Watanabe S. Synthesis and Radical Polymerization of Styrene-Based Monomer Having a Five-Membered Cyclic Carbonate Structure. J. Polym. Sci., Part A:-Polym. Chem. 2012, 50, 3046–3051. 10.1002/pola.26090. [DOI] [Google Scholar]
- Wilbon P. A.; Chu F.; Tang C. Progress in Renewable Polymers from Natural Terpenes, Terpenoids, and Rosin. Macromol. Rapid Commun. 2013, 34, 8–37. 10.1002/marc.201200513. [DOI] [PubMed] [Google Scholar]
- Wu G. M.; Liu D.; Liu G. F.; Chen J.; Huo S. P.; Kong Z. W. Thermoset Nanocomposites from Waterborne Bio-Based Epoxy Resin and Cellulose Nanowhiskers. Carbohydr. Polym. 2015, 127, 229–235. 10.1016/j.carbpol.2015.03.078. [DOI] [PubMed] [Google Scholar]
- Xu X.; Chen L.; Guo J.; Cao X.; Wang S. Synthesis and Characteristics of Tung Oil-Based Acrylated-Alkyd Resin Modified by Isobornyl Acrylate. RSC Adv. 2017, 7, 30439–30445. 10.1039/C7RA02189E. [DOI] [Google Scholar]
- Wu G. M.; Kong Z. W.; Chen C. F.; Chen J.; Huo S. P.; Jiang J. C. Crosslinking Reaction and Properties of Two-Component Waterborne Polyurethane from Terpene-Maleic Ester Type Epoxy Resin. J. Appl. Polym. Sci. 2013, 128, 132–138. 10.1002/app.38130. [DOI] [Google Scholar]
- Wu G.-m.; Kong Z.-w.; Chen J.; Huo S.-p.; Liu G.-f. Preparation and Properties of Waterborne Polyurethane/epoxy Resin Composite Coating from Anionic Terpene-Based Polyol Dispersion. Prog. Org. Coat. 2014, 77, 315–321. 10.1016/j.porgcoat.2013.10.005. [DOI] [Google Scholar]
- Pal K.; Rajasekar R.; Kang D. J.; Zhang Z. X.; Pal S. K.; Das C. K.; Kim J. K. Effect of Fillers on Natural Rubber/high Styrene Rubber Blends with Nano Silica: Morphology and Wear. Mater. Des. 2010, 31, 677–686. 10.1016/j.matdes.2009.08.014. [DOI] [Google Scholar]
- Huang K.; Zhang J.; Li M.; Xia J.; Zhou Y. Exploration of the Complementary Properties of Biobased Epoxies Derived from Rosin Diacid and Dimer Fatty Acid for Balanced Performance. Ind. Crops Prod. 2013, 49, 497–506. 10.1016/j.indcrop.2013.05.024. [DOI] [Google Scholar]
- Kang D. W.; Kim Y. M. Preparation and Properties of Poly(methyltrifluoropropylsiloxane-b-imide) Copolymer. I. J. Appl. Polym. Sci. 2002, 85, 2867–2874. 10.1002/app.10885. [DOI] [Google Scholar]
- Rahman M. A.; Lokupitiya H. N.; Ganewatta M. S.; Yuan L.; Stefik M.; Tang C. Designing Block Copolymer Architectures toward Tough Bioplastics from Natural Rosin. Macromolecules 2017, 50, 2069–2077. 10.1021/acs.macromol.7b00001. [DOI] [Google Scholar]
- Ganewatta M. S.; Ding W.; Rahman M. A.; Yuan L.; Wang Z.; Hamidi N.; Robertson M. L.; Tang C. Biobased Plastics and Elastomers from Renewable Rosin via ″Living″ Ring-Opening Metathesis Polymerization. Macromolecules 2016, 49, 7155–7164. 10.1021/acs.macromol.6b01496. [DOI] [Google Scholar]
- Dai X.; Zhang Y.; Gao L.; Bai T.; Wang W.; Cui Y.; Liu W. A Mechanically Strong, Highly Stable, Thermoplastic, and Self-Healable Supramolecular Polymer Hydrogel. Adv. Mater. 2015, 27, 3566–3571. 10.1002/adma.201500534. [DOI] [PubMed] [Google Scholar]
- Han Y.; Zhang J.; Shi L.; Qi S.; Cheng J.; Jin R. Improvement of Thermal Resistance of Polydimethylsiloxanes with Polymethylmethoxysiloxane as Crosslinker. Polym. Degrad. Stab. 2008, 93, 242–251. 10.1016/j.polymdegradstab.2007.09.010. [DOI] [Google Scholar]
- Chen D.; Yi S.; Wu W.; Zhong Y.; Liao J.; Huang C.; Shi W. Synthesis and Characterization of Novel Room Temperature Vulcanized (RTV) Silicone Rubbers Using Vinyl-POSS Derivatives as Cross Linking Agents. Polymer 2010, 51, 3867–3878. 10.1016/j.polymer.2010.06.028. [DOI] [Google Scholar]
- Liu X.; Xin W.; Zhang J. Rosin-Derived Imide-Diacids as Epoxy Curing Agents for Enhanced Performance. Bioresour. Technol. 2010, 101, 2520–2524. 10.1016/j.biortech.2009.11.028. [DOI] [PubMed] [Google Scholar]
- Wang H.; Wang H.; Zhou G. Synthesis of Rosin-Based Imidoamine-Type Curing Agents and Curing Behavior with Epoxy Resin. Polym. Int. 2011, 60, 557–563. 10.1002/pi.2978. [DOI] [Google Scholar]